Significance
What forces produce and maintain social inequality, and why do society members tolerate this inequality? The “One Percent” clearly benefit from having high status, but low-status individuals have strong incentive to challenge the established pecking order and try to improve their position. This conundrum is particularly striking in the societies of many primates and spotted hyenas, where females who are born to low-status mothers rarely manage to improve their position. Here we find that females who are strongly allied with their group-mates are more likely to improve their status, and that upward social mobility is often achieved with support from their closest allies. This suggests that, much like some animals compete physically for status, these species compete through social alliances.
Keywords: dominance hierarchy, behavioral ecology, social alliances, rank reversal, social mobility
Abstract
Social hierarchies are widespread in human and animal societies, and an individual’s position in its hierarchy affects both its access to resources and its fitness. Hierarchies are traditionally thought of in terms of variation in individual ability to win fights, but many are structured around arbitrary conventions like nepotistic inheritance rather than such traits as physical strength or weapon size. These convention-based societies are perplexing because position in the hierarchy appears to be gained irrespective of individual physical ability, yet social status strongly affects access to resources and fitness. It remains unclear why individuals abide by seemingly arbitrary conventions regarding social status when they stand to benefit by ignoring these conventions and competing for top positions or access to resources. Using data from wild spotted hyenas collected over 27 y and five generations, we show that individuals who repeatedly form coalitions with their top allies are likely to improve their position in the hierarchy, suggesting that social alliances facilitate revolutionary social change. Using lifetime reproductive success as a fitness measure, we go on to demonstrate that these status changes can have major fitness consequences. Finally, we show that the consequences of these changes may become even more dramatic over multiple generations, as small differences in social rank become amplified over time. This work represents a first step in reconciling the advantages of high status with the appearance of “arbitrary” conventions that structure inequality in animal and human societies.
It has long intrigued those interested in social evolution why egalitarian societies are so rare in both humans and other animals, and why some societies appear to be so much more unequal than others. Since the identification of a pecking order in chickens in 1922 (1), we humans have recognized in nonhuman animals a reflection of the inequality that characterizes our own societies. In many societies, this inequality manifests as a dominance hierarchy, in which consistent asymmetries in the outcomes of contests between individuals produce a network of dominance relationships. This, in turn, allows for each member of the group to be classified by the degree of privilege it enjoys in its interactions with group-mates, as an individual’s position in the hierarchy usually has profound effects on its priority of access to resources during intragroup competition. Although some individuals benefit at the expense of others in these systems, all group members benefit from the stability that dominance hierarchies provide (2). Past research has revealed widespread variability among animal societies with respect to the degree of inequality, the determinants of social status, and the social mobility possible within each society (3). Although the forces underpinning variation in social inequality are not well understood, it is clear that dominance hierarchies structure many important aspects of the lives of gregarious animals.
Occupying a high-rank position in a dominance hierarchy can be tremendously beneficial with respect to both priority of resource access and fitness consequences (4). These advantages suggest that the means by which individuals secure and maintain high social status are important components of individual fitness. An obvious means for acquiring dominance status involves direct competition between individuals; indeed, the primary techniques for identifying social status use the outcomes of aggressive interactions as indicative of social dominance (5, 6). There are, however, other forces that structure dominance relationships while also obviating potentially dangerous fights. Although the determinants of social status vary among species, these forces can usually be classified as one of two main types, either individual attributes or conventions.
In attribute-based hierarchies, dominance rank depends on physical or behavioral qualities of individual group members. These attributes can be morphological traits affecting ability to win fights (7) [e.g., body size in elephant seals (Mirounga angustirostris) (8)], ability to produce a behavioral display [e.g., piping in oystercatchers (Haematopus ostralegus) (9)], or a morphological display such as status badges [e.g., face masks in paper wasps (Polistes dominulus) (10)]. Attribute-based dominance hierarchies have been well studied, and fluctuations in these attributes are associated with corresponding fluctuations in dominance status (e.g., ref. 11). Furthermore, these traits usually covary with body condition and circulating levels of testosterone (e.g., ref. 12), suggesting that they are honest indicators of the ability to win fights.
In convention-based hierarchies, dominance rank is acquired through a convention such as tenure in the group (13), age (14), or maternal rank “inheritance” (15), and these conventions appear to operate irrespective of individual quality (e.g., ref. 16). Surprisingly little is known about fluctuations in dominance status in convention-based societies, and the functioning of convention-based hierarchies is thus perplexing. If high dominance rank is desirable and the convention determining rank is not tied to individual quality, what prevents high-quality individuals from ignoring the convention and asserting dominance through other means? If rank reversals occur in these groups, how do individuals improve in rank at the expense of others?
Perhaps the most common convention-based dominance hierarchies are the “nepotistic hierarchies” found in many cercopithecine primates and spotted hyenas (Crocuta crocuta), in which dominance rank acquisition follows a pattern strikingly like genetic inheritance. Rather than a true genetic process, however, dominance rank is acquired through a behavioral inheritance process that involves learning, and follows two general rules: (i) Juveniles acquire status immediately below that of their mothers in a pattern dubbed “maternal rank inheritance,” and (ii) juveniles outrank their older siblings in a pattern called “youngest ascendency” (17). This process is dependent upon coalitionary support from kin and sometimes also from nonkin, and the mother’s presence and support during aggressive interactions play especially important roles in ensuring that her offspring acquire their ranks according to these rules (18). The fact that kin play an important role in rank acquisition in these societies suggests that individuals may gain inclusive fitness benefits by promoting rank acquisition in their relatives.
Whereas the forces underlying rank acquisition in these societies have been well studied, surprisingly little is known about how adults in nepotistic hierarchies maintain or alter their social status. Rank reversals occur rarely in these species, which is perplexing given the lack of quality-based traits structuring their hierarchies. Most documentation of rank reversals in these hierarchies comes from isolated observations of captive or seminatural populations, sometimes after demographic manipulation (19–26), or occasionally from observations of wild populations (27, 28). The only systematic naturalistic studies of rank changes in nepotistic hierarchies focus specifically on reversals between aging females and their adult female offspring, and suggest that older females allow their daughters to overtake them when the indirect fitness benefits accrued from their daughter’s reproductive potential outweigh their own reproductive value (18, 29). Aside from these specific cases of reversals between daughters and their aging mothers, no study to date has tested hypotheses suggesting forces that produce rank reversals among adults in wild nepotistic hierarchies. A common observation among studies documenting rank reversals, however, is coalitionary support during aggression among group members. Coalitionary aggression has also been implicated in rank reversals in hierarchies based on physical attributes in a variety of species (3), suggesting that polyadic aggression may also allow for cryptic competition over rank in convention-based societies. Across species, coalitions directed up the hierarchy, called “revolutionary coalitions,” are considered a means by which lower-ranking individuals can effect rank reversals (reviewed in ref. 30), and consequently may be important in driving rank reversals in nepotistic societies. In spotted hyenas, recent evidence points to social support as a driver of female dominance (31). Overall, these studies implicate coalitionary support as a mechanism underlying rank and rank reversals in convention-based societies.
Here we use a longitudinal dataset from four wild groups of spotted hyenas to study the relationship between coalitionary bonds and rank reversals among adults in nepotistic hierarchies. Spotted hyenas are highly gregarious carnivores living in large, mixed-sex groups called clans, each of which is structured by a strict matrilineal nepotistic dominance hierarchy in which rank strongly affects reproductive success (32). Rank acquisition in hyenas rigidly follows the two rules of nepotistic rank inheritance (33), making these animals excellent models for the study of nepotistic hierarchies in general. Following from these observations, our study investigates whether coalitionary alliances with group-mates allow individuals in nepotistic hierarchies to improve their status. Specifically, we test the hypothesis that individuals who have strong coalitionary bonds are more likely to support one another in challenging higher-ranked individuals, and consequently are more likely to improve their status. This hypothesis predicts that (i) up-hierarchy coalitions will occur during rank reversals, (ii) these coalitions will be more likely to occur between individuals who are more strongly bonded, and, as a result, (iii) individuals who engage in more coalitions with their top partners will be more likely than others to improve their social status. Finally, we also consider the long-term impacts of these rank reversals in terms of expected change in fitness during the lives of individual hyenas, and examine the intergenerational consequences arising from maternal rank inheritance.
Results
Identification of Rank Reversals.
We used the longitudinal hierarchy framework we advanced in a previous paper (34) to study rank reversals. In this approach, interaction data are divided into study periods spanning the length of the study, ranks are determined for each individual present in each period, and hierarchy dynamics are inferred as the difference in rank each individual occupies from one period to the next. Changes in an individual’s rank can arise from passive processes, which include demographic changes (e.g., births, immigration). Active processes produce the remainder of rank dynamics; these changes, which we call here “rank reversals,” arise when the members of a dyad reverse a previously held dominance relationship. For example, if an individual surpasses two of its group-mates, that individual will have undergone two rank reversals (because it reversed rank relationships with two individuals), and each surpassed individual will have undergone one rank reversal. Their rank change due to rank reversals associated with this event would be 2, −1, and −1, respectively. In our previous work, we found that ranking algorithms tend to overestimate the amount of active dynamics in a longitudinal hierarchy, and this shortcoming was particularly evident when used to infer simulated hierarchies with few true rank reversals.
Because spotted hyenas are reported to have highly stable hierarchies (31), we elected to use the Informed MatReorder ranking method, which we found to produce the most conservative estimates of rank reversals and to outperform other methods [Elo-rating (6), David’s Score (28), I&SI (5)] in tests on stable simulated and empirical hierarchies, including one of the hierarchies we investigate here (34). Conservative estimates of rank reversals are particularly desirable in this study because too many false positives (i.e., identification of rank reversals when there truly were none) can easily obscure an existing pattern in the true, rarely occurring cases. In prior tests of Informed MatReorder and other methods on highly stable simulated societies, all methods overestimated the amount of change in the hierarchy, but Informed MatReorder was considerably closer to estimating true hierarchy dynamics than any other method (34).
Informed MatReorder is a matrix-reordering method derived from the widely used I&SI algorithm (5). In both procedures, observations of agonistic interactions are tabulated in a sociometric matrix, which is then iteratively reordered to minimize inconsistences, or dyads in which the assigned rank positions of the two individuals are inconsistent with the outcomes of their interactions. I&SI is designed for inferring static hierarchies, and attempts to reorder all individuals to minimize the number and strength of inconsistencies. Informed MatReorder adapts I&SI to be used for inferring longitudinal hierarchies by adding two features: (i) It uses prior knowledge of dominance correlates characteristic of the study organism to inform placement of newly recruited individuals, and (ii) ranks from a given period are determined by the ranks from the previous period, updated with new information. Importantly, this “inertial tendency” for ranks to remain constant in the absence of data suggesting a change is essential to prevent overestimation of the number of rank reversals (34). Thus, Informed MatReorder minimizes both the number of inconsistencies and the number of differences between the proposed order and the order from the previous study period. In accordance with conventions of both maternal rank inheritance and youngest ascendency, when a new individual is recruited into the group, Informed MatReorder adds her to the hierarchy below her mother and above her older sisters before performing the reordering step. Because maternal rank inheritance is such a predictable pattern in this species (33), cases where the reordering step moves new individuals upward or downward from this position are interpreted as rank reversals. More details on Informed MatReorder can be found in SI Appendix, and in our previous paper (34); functions for implementing the method are available through the DynaRankR R package (35).
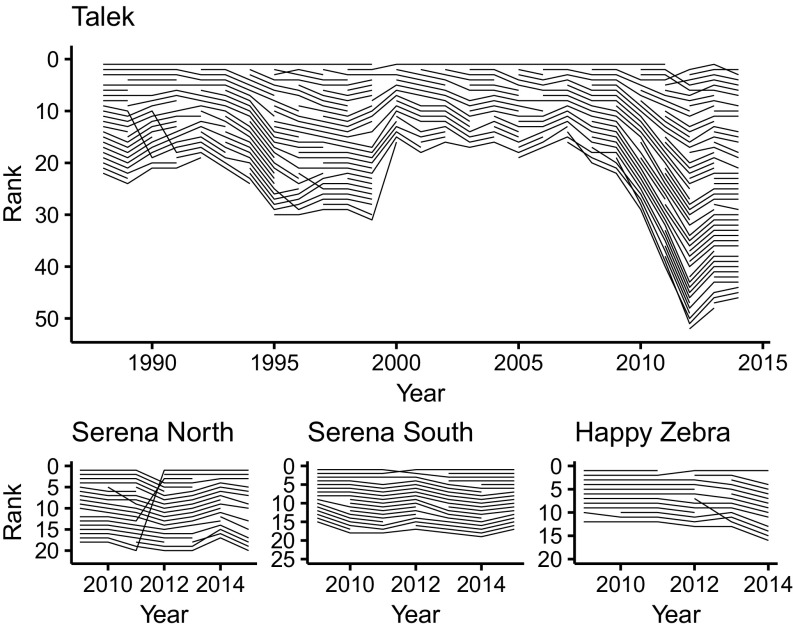
We applied Informed MatReorder to agonistic interaction data from females in four free-ranging clans of spotted hyenas in the Maasai Mara National Reserve, Kenya. We assigned ranks to each female every calendar year; this admittedly arbitrary choice of period length was selected because spotted hyenas are long-lived, nonseasonal breeders, because their interbirth intervals are seldom shorter than 1 y, and because we deemed 1 y a sufficiently long sampling period in which to summarize agonistic behavior at the individual and dyadic levels. To infer the longitudinal hierarchy, we used 12,505 aggressive interactions (mean = 11.53 interactions per individual per year, SD = 6.05, range = 1.44 to 27.21) among 249 adult females; of these, 2,966 (23.72%) interactions involved coalitionary support. These interactions include only those in which there was a clear loser, indicated by stereotyped submissive behavior (36).
In our study population, most individuals (78.1%) acquired their rank according to the previously described patterns of maternal rank inheritance and youngest ascendancy, and rank relationships were predominantly stable over time (Fig. 1), with only 13.7% of rank assignments involving a rank reversal. However, we observed a total of 141 cases of individuals changing rank due to rank reversals over the course of our study, with 43.1% of females changing rank due to rank reversal at some point in the study (which spanned the lifetime of many of the hyenas). Rank reversals did not primarily involve females overtaking their mothers; in 62 cases where females with known living mothers moved up the hierarchy, only 7 (11.3%) involved a daughter overtaking her mother.
Fig. 1.
Yearly ranks of each individual in each of four study clans. Crossing lines indicate rank reversals. By convention, lower numbers indicate higher ranks.
The Role of Coalitionary Alliances in Rank Reversals.
To measure the relationship between coalitionary support and rank change in the nepotistic hierarchies of spotted hyenas, we constructed yearly weighted nondirectional networks of coalitionary interactions for each study clan. In these networks, the strength of ties between two individuals corresponds to the number of times those individuals were observed engaging in concurrent aggression against a group-mate (n = 1,913 coalitions with allied adult females); we identified each female’s top allies as her three most frequent adult female coalition partners. To examine whether revolutionary coalitions were associated with rank reversals, we focused on 533 triadic coalitions where the two allied attackers and their target animal were all adult females. There were 33 triadic up-hierarchy coalitions, in which the target animal outranked both aggressors, and 464 triadic down-hierarchy coalitions, in which both allies outranked their target. We found that up-hierarchy coalitions occurred primarily in the context of rank reversals; 66.67% of up-hierarchy coalitions occurred during the year before or the year in which one or both aggressors surpassed the targeted individual through rank reversals. In contrast, only 0.43% of down-hierarchy coalitions occurred in the year in which or the year before the recipient surpassed one or both aggressors (χ2 = 279.87, df = 1, P < 0.0001). Logistic mixed models with random effects of clan revealed that the probability of any coalition being directed up the hierarchy increased with the strength of the coalitionary bonds between the two aggressors, and this effect was significantly more extreme than in null models generated using permutation (β = 0.187, SE = 0.038, P = 0.033; SI Appendix, Fig. S2). Because of the nonindependence of dyadic data such as those documenting coalitionary bonds, we assessed the significance of our models by comparing the observed effect of coalition bond strength to effects from null models where we ran the same regression on the data after conducting node-level permutation (37, 38). In this approach, the reported P values denote the proportion of null models in which the effect of coalitionary bond strength was as extreme as or more extreme than the effect estimated from the observed data. To further ensure that our observed effect of dyadic bond strength was not simply a function of the overall amount of coalitionary behavior in which each individual engaged, we made a similar model that included as a predictor the total number of coalitions in which two allies engaged with all adult females. This model was worse than the model using the strength of the dyadic bond as the predictor (∆AICc = 9.27), and it was not significantly better than null models generated using permutation (β = 0.029, SE = 0.007, P = 0.278). We also found that up-hierarchy coalitions were significantly more likely to occur between top allies than other allies (χ2 = 10.13, df = 1, P = 0.0015). These results suggest that, as individuals engage in more coalitions together, they become more willing to support one another in challenging dominant individuals. We lacked complete pedigree data for some subjects in this study, so we were unable to evaluate the role of kinship. However, prior work has found that spotted hyena females prefer to form coalitions with their kin (31, 39), so kinship is a likely mediator of the formation of these strong coalitionary bonds.
To examine the overall effect of social allies on rank reversals, we constructed a linear mixed model modeling the yearly number of positions each individual moved upward or downward in the adult hierarchy due to rank reversals as a function of the strength of coalitionary bonds with its top three preferred coalitionary partners in that year. Because rank reversals are nonindependent observations, we again used node-level permutation to compare the observed effect of coalitionary tie strength against the distribution of effects from null models that preserve this nonindependent structure, and calculated P values as the proportion of null models with coefficient estimates as extreme as or more extreme than our observed estimates. We found that coalitionary tie strength was strongly positively associated with the direction and magnitude of rank change, that this effect was more extreme than expected from null models generated using permutation (βcoalition ties = 8.16, SE = 1.27, P = 0.001; βcoalition ties squared = 9.72, SE = 1.19 P = 0.001; SI Appendix, Fig. S3), and that the model with coalitionary support as a predictor performed better than the model without it (∆AICc = 118.2). This relationship was still significant after removing the two individuals with the most rank reversals (βcoalition ties = 1.83, SE = 1.01, P = 0.035; βcoalition ties squared = 1.94, SE = 0.92 P = 0.016), although examination of these data suggests no reason for their exclusion.
Expected Fitness Consequences of Rank Reversals.
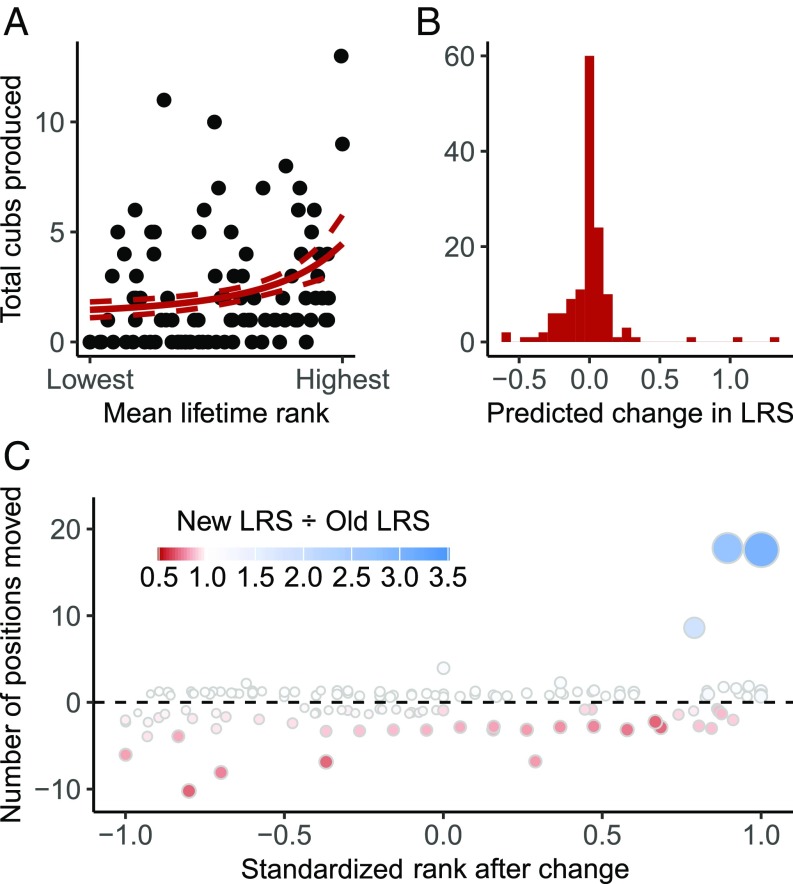
To estimate the potential fitness consequences of the observed rank reversals, we modeled the relationship between mean rank and lifetime reproductive success (LRS) for females who we observed from birth until death. LRS for each female was calculated as the total number of offspring she produced that survived to reproductive maturity (2 y old). We restricted the analysis to 96 females who survived to at least 4 y old to eliminate individuals who died soon after puberty. Mean LRS for our study population was 2.28 (SE = 0.27; range = 0 to 13), which is similar to the LRS of 2.36 ± 1.90 reported for a Tanzanian population of spotted hyenas (32). We modeled an exponential relationship between mean lifetime rank and LRS using a Poisson generalized linear mixed model, and found that rank had a significant positive effect on LRS (βrank = 0.48, SE = 0.10, P < 0.0001; Fig. 2A), which is consistent with earlier work (40). Using this model, we estimated the expected changes in LRS due to the observed rank reversals (Fig. 2B). We found that the expected fitness effects of rank reversals vary with the number of rank positions moved and where in the hierarchy they occurred. Most changes were single-position changes in the lower tiers of the hierarchy and had little effect on expected fitness (Fig. 2C). However, expected fitness consequences were larger for rank reversals among high-ranked individuals and for rank reversals amounting to large position changes regardless of hierarchy position; here, some females experienced a more than twofold change in their expected fitness.
Fig. 2.
Rank reversals are expected to have large fitness effects. (A) Data from 96 females for which we calculated LRS. Rank is a significant predictor of LRS. (B) The predicted fitness consequences of observed rank reversals, based on the model from A. (C) Large expected fitness effects result from both rank changes occurring in the upper tier of the hierarchy and rank changes of large magnitude resulting from many simultaneous rank reversals. However, single rank reversals among low-ranked individuals are predicted to have minimal fitness effects. Larger points indicate larger absolute values of predicted effects on fitness. Predicted fitness effects are colored according to the ratio of expected LRS in the new position relative to the expected LRS in the old position, with values of <1 indicating a decline in LRS and values of >1 indicating an increase in LRS.
We also examined the intergenerational effects of rank reversals. A mathematical consequence of maternal rank inheritance and higher fitness among high-ranking individuals is that individual rank declines over time as offspring born to higher-ranking females join the adult hierarchy. As a result, small differences in rank between females are expected to be amplified over time. Furthermore, this amplification is expected to continue over generations, such that descendants of two females of adjacent rank at time t could occupy rank positions separated by many individuals at future time points. Thus, a rank reversal producing a small change in rank between two hyenas at a single time point can later have large consequences for the ranks of their descendants. In this way, rank dynamics due to active processes can be amplified by rank dynamics due to passive processes.
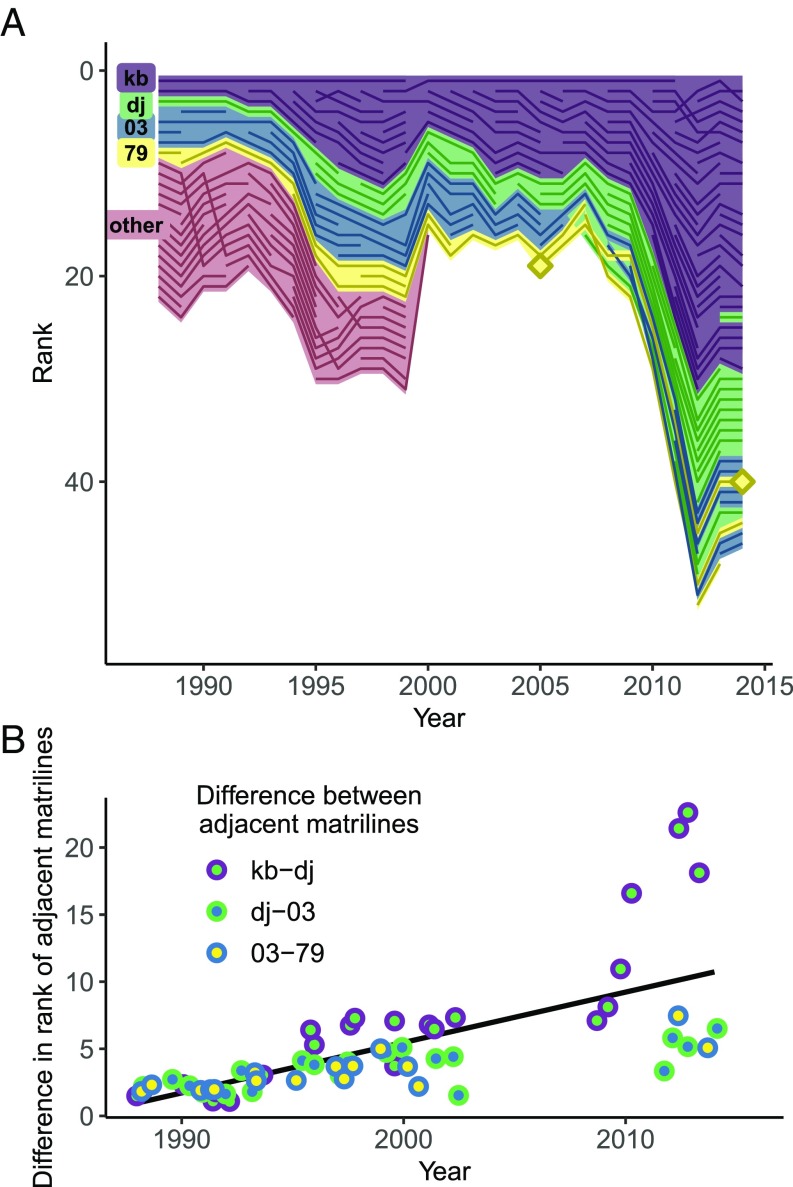
To examine this effect more closely, we calculated the average difference in rank between the female descendants of heads of four matrilines that were adjacently ranked in the first year of our study in our longest-studied group (Fig. 3A). We found that rank distance between the descendants of females from adjacently ranked matrilines increased considerably over time as a result of maternal rank inheritance and rank-related reproductive success, and this difference was most dramatic between the alpha and beta matrilines (Fig. 3B). As a result, descendants of females who were high-ranking in the first year of our study occupied very low rank positions decades later. This is consistent with the idea that the consequences of a rank reversal may become amplified over time. For example, from 2007 to 2009, a female from the “79” matriline surpassed four females from the “03” and “dj” matrilines (Fig. 3A, female marked with diamonds). By 2014, the difference in rank between her current position and where she would have been in the absence of this change had increased to six, because the females that she had surpassed successfully reared offspring that would have otherwise outranked her. Not only was the magnitude of her rank change amplified, but, because her subsequent offspring inherited her new rank, her descendants also gained from this rank reversal. Although we elected not to model the expected amplification of the observed rank reversals to avoid extrapolation from limited observations, there is a strong tendency for descendants of adjacently ranked females to occupy increasingly disparate ranks over long time scales, particularly among high-ranking matrilines.
Fig. 3.
Rank differences among females in adjacent matrilines are amplified over time due to rank-related variation in reproduction and maternal rank inheritance. (A) The ranks of descendants from four original females in four matrilines occupying adjacent rank positions from 1988 through 2014. Only these four matrilines were considered, because the others either died out or departed during clan fission events to form new clans (those females listed as “other”). (B) The difference in rank between descendants of adjacent matrilines increases over time due to the addition of newly reproductive females to the clan’s dominance hierarchy. As a result, a rank change at any given time point may become amplified into large rank differences.
Discussion
Here we provide a systematic study of rank reversals among adult females in a convention-based nepotistic society. In these societies, rank is thought to be acquired through a convention of maternal rank inheritance rather than through displays or fighting, and rank reversals are uncommon. We find that, although rare, rank reversals do occur in convention-based hierarchies, are associated with coalitionary bond strength, and can have significant fitness consequences for the individuals involved. Our results showing that differences in matrilineal rank are amplified over multiple generations suggest that the long-term fitness consequences of rank reversals may be larger than we could measure directly. The combination of female philopatry, rank-related variation in reproductive success, and maternal rank inheritance results in a large decrease in an individual’s rank over time as offspring of higher-ranking females enter the hierarchy above her. A female overtaking a group member in a rank reversal at any given time point might not only increase her LRS but also increase the average rank and fitness of her future offspring. Although we were able to demonstrate that small rank differences between females were amplified over time (Fig. 3B), we were not able to estimate the expected inclusive fitness benefits of these changes, because of currently incomplete pedigree data. An important consideration when assessing inclusive fitness effects is the kinship structure of these societies. Because female relatives occupy adjacent positions within the dominance hierarchy, a female hyena engaging in rank reversal is likely to be related to the individuals she surpasses. Thus, females engaging in rank reversals are not only gaining inclusive fitness by improving the rank of their descendants, but they are also incurring costs to their inclusive fitness by reducing the rank of more distant kin. Future studies with complete relatedness data should examine the inclusive fitness consequences of rank reversals in societies with maternal rank inheritance.
Although our models suggest that rank reversals can have important fitness benefits for individuals moving up the hierarchy, we have yet to quantify the immediate costs of attempting revolutionary coalitions. Engaging in up-hierarchy aggression, even with a coalitionary ally, has the potential to result in serious injury. In our study population, we have observed occasional extreme escalated aggression when lower-ranked females challenge higher-ranked females, although our data are currently insufficient to assess the prevalence or the consequences of this extreme aggression. However, considerable evidence from hierarchies structured by direct competition suggests that rank challenges are associated with high risk of injury or death for the combatants (41). Furthermore, there may be costs associated with rank reversals that do not result directly from escalated aggression over rank. Engaging in coalitionary aggression with social partners, even if only in low-level aggression directed down the hierarchy, is also likely to incur risk and energetic costs (30). Finally, uncertainty about the state of the hierarchy produced by rank reversals is associated with increased stress for both the individuals directly involved and other group members, suggesting that challenges over rank may incur costs for the entire group (2, 42). It remains unclear to what extent these costs offset the potential benefits of rank reversals in convention-based hierarchies.
Our results provide strong evidence of coalition-based competition resulting in rank changes in a convention-based hierarchy and are therefore consistent with recent challenges to the strict distinction between attribute-based hierarchies and convention-based hierarchies (18). These results strongly support the conclusions of a recent study investigating the importance of social support in dominance in spotted hyenas (31); evidently, in these societies, social support is a crucial component of social status and its dynamics. Coalitionary support in competition is neither an individual attribute nor a societal convention, but it may depend on both. Across convention- and attribute-based societies, individuals lending coalitionary support during aggressive interactions nearly always side with the dominant individual against the subordinate (39), which means that, in convention-based hierarchies, the convention determining rank is a good predictor of patterns of coalitionary support. However, individual social aptitude may also be important in garnering coalitionary support. Thus, both individual attributes and convention can potentially play important roles in societies where coalitionary support is a force structuring rank. Future research should investigate the role of individual attributes in predicting rank reversals in convention-based hierarchies.
Methods
Modeling Up-Hierarchy Coalitions and Rank Changes.
Spotted hyenas live in large, mixed-sex groups characterized by high degrees of fission−fusion dynamics, meaning that group members associate in subgroups that change composition frequently throughout the day. We modeled the relationship between strength of coalitionary bonds and up-hierarchy aggressive attacks with a binomial generalized linear mixed model. The dependent variable was the probability that a given coalition was directed up vs. down the hierarchy, and the predictor was the strength of the coalitionary tie between members of the allied dyad in the year of the observed interaction. Bond strength was measured as the total number of polyadic agonistic interactions in which at least two adult females were allied against another. We initially included random effects for dyad, clan, and year nested within clan, because of repeated observations at each of those levels, but all except clan were dropped because they were estimated to explain 0 variance. To account for nonindependence between observations of up- and down-hierarchy coalitions, we conducted node-level permutations and ran the model with the permuted data. In these permutations, each coalition was assigned a random direction (i.e., up- or down-hierarchy) from the pool of coalitions observed in that clan in that year. Following suggested guidelines (37, 38), we performed this permutation 1,000 times and compared our observed effect to the distribution of effects from the permuted data, to assess statistical significance.
We modeled the relationship between the yearly rank change each individual underwent and its alliance strength using a linear mixed model with random effects for individual identity, clan, and year nested within clan, although the latter two effects were dropped because they were estimated to explain 0 variance. We measured alliance strength as the sum of coalitionary ties with the three group-mates with strongest ties, akin to what has been done elsewhere (43). We included the log of the total number of observation sessions involving that individual in that year as an offset in the model to account for differences in observation effort. We elected to control for observation at the individual level rather than the dyadic level, because we wanted to control for variability in observation of individuals rather than variability in their social relationships. In the fission−fusion societies of spotted hyenas, dyadic association rates reflect the animals’ social preferences (31, 39). Observations of rank reversals are not independent of one another, so we again assessed statistical significance by permuting the observations of number of positions moved due to rank reversals among individuals within clan and year. We compared the observed effect of alliance strength on the amount and direction of rank change with the effects from 1,000 models with permuted data. In the outlier analysis, the random effect of identity explained no variance and was causing convergence problems, so we repeated the same procedure as above but without the random effect.
Assessing Fitness Effects.
Because of the rarity of rank reversals and the long lives of hyenas, we did not have the statistical power to directly measure the fitness consequences of the observed rank reversals. We estimated expected fitness effects using the LRS of 96 adult females for which we had complete lifetime reproductive data and who survived to at least 4 y of age. We modeled the total number of offspring they produced as a function of their mean rank over their lifetime. Rank was standardized to range from −1 (lowest in group) to 1 (highest in group). We modeled an exponential relationship between mean rank and LRS because model comparison with AIC revealed this model to be superior to models with linear relationship (∆AIC = 4.08) or quadratic relationships (∆AIC = 2.75).
To study the intergenerational consequences of rank reversals, we examined the relative change in ranks of descendants of four females from adjacent matrilines over time. We only considered matrilines in which descendants of original females in 1988 remained present in the group at the end of our study period. Descendants from all other matrilines had either died or had split off to form entire new clans. We did not include the other three study groups because we did not know the matrilineal kin relationships among most females at the start of the study. We also excluded individuals who changed rank in the calculation of these average rank differences because we were interested in understanding the expected change in rank in the absence of rank reversals. All data presented in this study were obtained in accordance with the Michigan State University Institutional Animal Care and Use Committee protocol (03/17-038-00).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8644.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810384116/-/DCSupplemental.
References
- 1.Schjelderup-Ebbe T. Contributions to the social psychology of the domestic chicken. Reprinted Z Psychol. 1922;88:225–252. [Google Scholar]
- 2.Beaulieu M, Mboumba S, Willaume E, Kappeler PM, Charpentier MJE. The oxidative cost of unstable social dominance. J Exp Biol. 2014;217:2629–2632. doi: 10.1242/jeb.104851. [DOI] [PubMed] [Google Scholar]
- 3.Broom M, Koenig A, Borries C. Variation in dominance hierarchies among group-living animals: Modeling stability and the likelihood of coalitions. Behav Ecol. 2009;20:844–855. [Google Scholar]
- 4.Holekamp KE, Strauss ED. Aggression and dominance: An interdisciplinary overview. Curr Opin Behav Sci. 2016;12:44–51. [Google Scholar]
- 5.de Vries. Finding a dominance order most consistent with a linear hierarchy: A new procedure and review. Anim Behav. 1998;55:827–843. doi: 10.1006/anbe.1997.0708. [DOI] [PubMed] [Google Scholar]
- 6.Neumann C, et al. Assessing dominance hierarchies: Validation and advantages of progressive evaluation with elo-rating. Anim Behav. 2011;82:911–921. [Google Scholar]
- 7.Dugatkin LA, Reeve HK. Winning, losing, and reaching out. Behav Ecol. 2014;25:675–679. [Google Scholar]
- 8.Haley MP, Deutsch CJ, Le Boeuf BJ. Size, dominance and copulatory success in male northern elephant seals, Mirounga angustirostris. Anim Behav. 1994;48:1249–1260. [Google Scholar]
- 9.Ens BJ, Goss-Custard JD. Piping as a display of dominance by wintering oystercatchers Haematopus ostralegus. Ibis. 1986;128:382–391. [Google Scholar]
- 10.Tibbetts EA, Dale J. A socially enforced signal of quality in a paper wasp. Nature. 2004;432:218–222. doi: 10.1038/nature02949. [DOI] [PubMed] [Google Scholar]
- 11.Bro Jørgensen J, Beeston J. Multimodal signalling in an antelope: Fluctuating facemasks and knee-clicks reveal the social status of eland bulls. Anim Behav. 2015;102:231–239. [Google Scholar]
- 12.Muck C, Goymann W. Throat patch size and darkness covaries with testosterone in females of a sex-role reversed species. Behav Ecol. 2011;22:1312–1319. [Google Scholar]
- 13.East ML, Hofer H. Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav Ecol. 2001;12:558–568. [Google Scholar]
- 14.Archie EA, Morrison TA, Foley CAH, Moss CJ, Alberts SC. Dominance rank relationships among wild female African elephants, Loxodonta africana. Anim Behav. 2006;71:117–127. [Google Scholar]
- 15.Kawamura S. The matriarchal social order in the minoo-B group. Primates. 1958;1:149–156. [Google Scholar]
- 16.Street SE, Cross CP, Brown GR. Exaggerated sexual swellings in female nonhuman primates are reliable signals of female fertility and body condition. Anim Behav. 2016;112:203–212. [Google Scholar]
- 17.Holekamp KE, Smale L. Dominance acquisition during mammalian social development: The ‘inheritance’ of maternal rank. Am Zool. 1991;31:306–317. [Google Scholar]
- 18.Lea AJ, Learn NH, Theus MJ, Altmann J, Alberts SC. Complex sources of variance in female dominance rank in a nepotistic society. Anim Behav. 2014;94:87–99. doi: 10.1016/j.anbehav.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chikazawa D, Gordon TP, Bean CA, Bernstein IS. Mother-daughter dominance reversals in rhesus monkeys (Macaca mulatta) Primates. 1979;20:301–305. [Google Scholar]
- 20.Chapais B. An experimental analysis of a mother-daughter rank reversal in Japanese macaques (Macaca fuscata) Primates. 1985;26:407–423. [Google Scholar]
- 21.Gouzoules H. A description of genealogical rank changes in a troop of Japanese monkeys (Macaca fuscata) Primates. 1980;21:262–267. [Google Scholar]
- 22.Oates-O’Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, Lerche NW. Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2010;49:196–201. [PMC free article] [PubMed] [Google Scholar]
- 23.Chapais B, Girard M, Primi G. Non-kin alliances, and the stability of matrilineal dominance relations in Japanese macaques. Anim Behav. 1991;41:481–491. [Google Scholar]
- 24.Anderson EJ, Weladji RB, Paré P. Changes in the dominance hierarchy of captive female Japanese macaques as a consequence of merging two previously established groups. Zoo Biol. 2016;35:505–512. doi: 10.1002/zoo.21322. [DOI] [PubMed] [Google Scholar]
- 25.Ehardt CL, Bernstein IS. Matrilineal overthrows in rhesus monkey groups. Int J Primatol. 1986;7:157–181. [Google Scholar]
- 26.Dettmer AM, Woodward RA, Suomi SJ. Reproductive consequences of a matrilineal overthrow in rhesus monkeys. Am J Primatol. 2015;77:346–352. doi: 10.1002/ajp.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels A, Silk JB, Altmann J. Continuity and change in dominance relations among female baboons. Anim Behav. 1987;35:785–793. [Google Scholar]
- 28.Balasubramaniam KN, et al. Consistency of dominance rank order: A comparison of David’s scores with I&SI and Bayesian methods in macaques. Am J Primatol. 2013;75:959–971. doi: 10.1002/ajp.22160. [DOI] [PubMed] [Google Scholar]
- 29.Combes SL, Altmann J. Status change during adulthood: Life-history by-product or kin selection based on reproductive value? Proc Biol Sci. 2001;268:1367–1373. doi: 10.1098/rspb.2001.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bissonnette A, et al. Coalitions in theory and reality: A review of pertinent variables and processes. Behaviour. 2015;152:1–56. [Google Scholar]
- 31.Vullioud C, et al. Social support drives female dominance in the spotted hyaena. Nat Ecol Evol. 2018;3:71–76. doi: 10.1038/s41559-018-0718-9. [DOI] [PubMed] [Google Scholar]
- 32.Hofer H, East ML. Behavioral processes and costs of co-existence in female spotted hyenas: A life history perspective. Evol Ecol. 2003;17:315–331. [Google Scholar]
- 33.East ML, et al. Maternal effects on offspring social status in spotted hyenas. Behav Ecol. 2009;20:478–483. [Google Scholar]
- 34.Strauss ED, Holekamp KE. Inferring longitudinal hierarchies: Framework and methods for studying the dynamics of dominance. J Anim Ecol. 2019 doi: 10.1111/1365-2656.12951. [DOI] [PubMed] [Google Scholar]
- 35.Strauss ED. 2019 DynaRankR: Inferring Longitudinal Dominance Hierarchies. R package Version 1.0.0. Available at https://CRAN.R-project.org/package=DynaRankR. Accessed February 26, 2019.
- 36.Rowell TE. The concept of social dominance. Behav Biol. 1974;11:131–154. doi: 10.1016/s0091-6773(74)90289-2. [DOI] [PubMed] [Google Scholar]
- 37.Farine DR. A guide to null models for animal social network analysis. Methods Ecol Evol. 2017;8:1309–1320. doi: 10.1111/2041-210X.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehead H. Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. Univ Chicago Press; Chicago: 2008. [Google Scholar]
- 39.Smith JE, et al. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav Ecol. 2010;21:284–303. [Google Scholar]
- 40.Swanson EM, Dworkin I, Holekamp KE. Lifetime selection on a hypoallometric size trait in the spotted hyena. Proc R Soc B. 2011;278:3277–3285. doi: 10.1098/rspb.2010.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaburu SSK, Inoue S, Newton-Fisher NE. Death of the alpha: Within-community lethal violence among chimpanzees of the mahale mountains national park. Am J Primatol. 2013;75:789–797. doi: 10.1002/ajp.22135. [DOI] [PubMed] [Google Scholar]
- 42.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 43.Silk JB, et al. Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.