Significance
The generalization of fear responses toward neutral stimuli is a highly prevalent and debilitating dimension of trauma- and anxiety-related disorders. Understanding the neural circuits that underlie the ability to suppress fear generalization is of significant translational interest. Recent studies have largely focused on examining fear generalization in the context of traditionally discussed fear-related circuitry like the amygdala, prefrontal cortex, and hippocampus, which monitor and detect threatening stimuli. However, very little is understood about the role of thalamic and subthalamic regions in modulating fear generalization. Combining discriminative auditory fear conditioning in mice with C-FOS mapping and chemogenetic manipulation of neuronal activity, we demonstrate a role for the subthalamic zona incerta in suppressing fear generalization.
Keywords: trauma, posttraumatic stress disorder, subthalamic, fear inhibition, anxiety
Abstract
Fear expressed toward threat-associated stimuli is an adaptive behavioral response. In contrast, the generalization of fear responses toward nonthreatening cues is a maladaptive and debilitating dimension of trauma- and anxiety-related disorders. Expressing fear to appropriate stimuli and suppressing fear generalization require integration of relevant sensory information and motor output. While thalamic and subthalamic brain regions play important roles in sensorimotor integration, very little is known about the contribution of these regions to the phenomenon of fear generalization. In this study, we sought to determine whether fear generalization could be modulated by the zona incerta (ZI), a subthalamic brain region that influences sensory discrimination, defensive responses, and retrieval of fear memories. To do so, we combined differential intensity-based auditory fear conditioning protocols in mice with C-FOS immunohistochemistry and designer receptors exclusively activated by designer drugs (DREADDs)-based manipulation of neuronal activity in the ZI. C-FOS immunohistochemistry revealed an inverse relationship between ZI activation and fear generalization: The ZI was less active in animals that generalized fear. In agreement with this relationship, chemogenetic inhibition of the ZI resulted in fear generalization, while chemogenetic activation of the ZI suppressed fear generalization. Furthermore, targeted stimulation of GABAergic cells in the ZI reduced fear generalization. To conclude, our data suggest that stimulation of the ZI could be used to treat fear generalization in the context of trauma- and anxiety-related disorders.
Expressing fear toward cues that have previously been associated with trauma is adaptive (conditioned fear). Equally adaptive is the expression of fear toward stimuli that closely resemble traumatic cues (fear generalization). Such generalization of fear allows the organism to be “better safe than sorry.” However, fear generalization can diminish quality of life and is a highly debilitating dimension of trauma- and anxiety-related disorders like posttraumatic stress disorder and generalized anxiety disorder (1–4). Reducing fear generalization, while maintaining adaptive fear responses, will reduce the daily burden experienced by individuals living with these disorders and requires identifying neural circuitry that could modulate fear generalization.
Brain regions such as the lateral amygdala (5–7), central amygdala (8, 9), prefrontal cortex (10, 11), hippocampus (3, 12), and bed nucleus of the stria terminalis (13, 14) have been implicated in fear generalization. More importantly, these regions play crucial roles in detecting threats and assigning valence to environmental stimuli (15–18). Therefore, while manipulating these regions could potentially reduce fear generalization, doing so might compromise threat detection, conditioned fear, and survival. In this study, we set out to ask whether targeting brain regions outside of the aforementioned canonical fear-related circuitry could reduce fear generalization.
Thalamic and subthalamic brain regions are ideal candidates to modulate fear generalization because they serve as hubs relaying information from sensory cortices to limbic, midbrain, and brainstem nuclei; are involved in stimulus discrimination; and gate behavioral states (12, 19–26). While the auditory and paraventricular thalamus has been shown to influence fear generalization (27, 28), less is known about the contributions of subthalamic brain regions to fear generalization. Most recently, the zona incerta (ZI), a subthalamic region, has received attention for its role in modulating defensive responses and retrieval of fear-related memories (29, 30). Notably, studies in rodents have highlighted that the ZI influences sensory discrimination (31, 32) and that stimulation of the ZI in humans facilitates discrimination of fearful from nonfearful stimuli (33). Motivated by this literature, we hypothesized that the ZI might be able to modulate fear generalization. Combining discriminative auditory fear conditioning in mice with C-FOS staining and chemogenetic manipulation of neuronal activity, our findings bring to light nuanced contributions of this subthalamic region to fear generalization and suggest that stimulating the ZI may be of therapeutic value in reducing fear generalization.
Results
Decreased Neuronal Activity in the ZI Accompanies Fear Generalization That Manifests After Conditioning with High-Intensity Foot-Shocks.
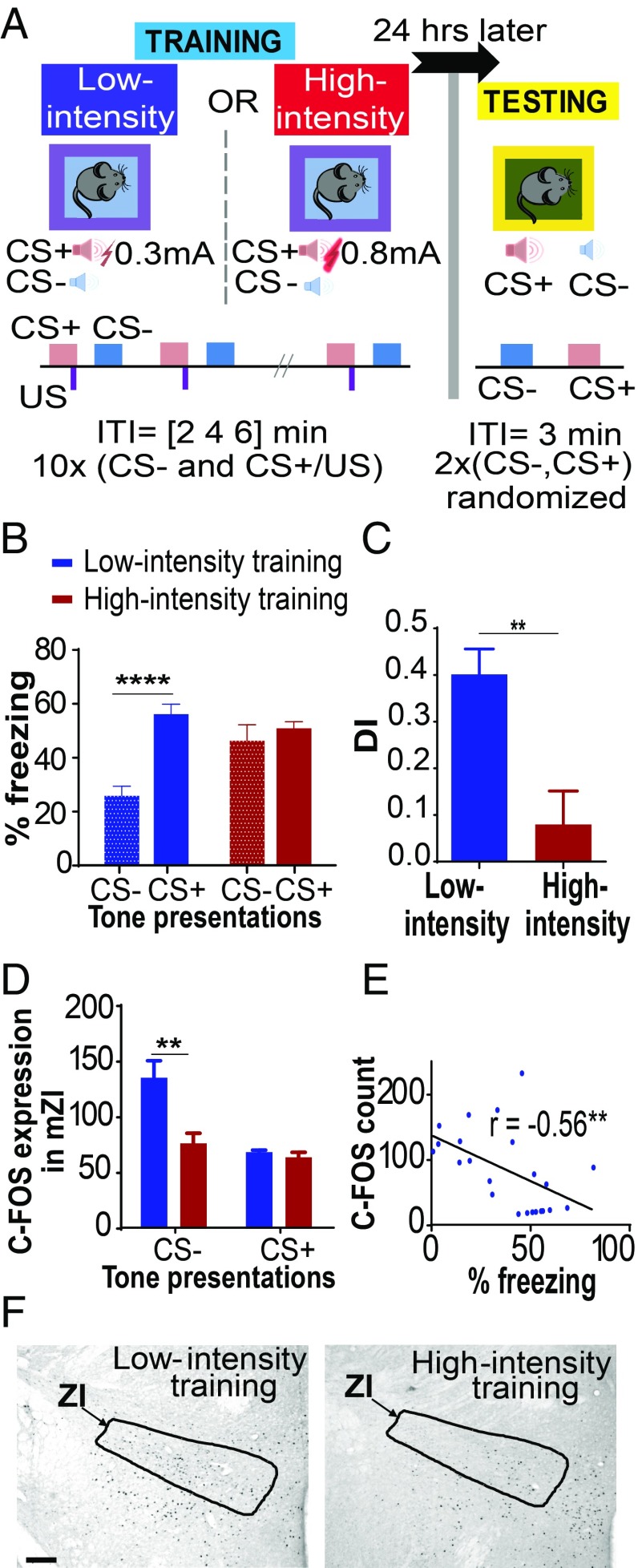
Wild-type mice trained under low-threat conditions (0.3-mA foot-shocks) exhibited increased freezing to CS+ (conditioned auditory stimulus) and reduced freezing to CS− (neutral auditory stimulus) (Fig. 1 A and B). Under high-threat conditions (0.8-mA foot-shocks), wild-type mice exhibited fear generalization, as indicated by increased freezing to both the CS+ and CS− tones [Fig. 1 A and B; low-intensity training group (n = 14), high-intensity training group (n = 10); training × tone interaction: F(1,22) = 19.17, P < 0.0001; post hoc tests: low-intensity training:CS− vs. low-intensity training:CS+, P < 0.0001; low-intensity training:CS− vs. high-intensity training:CS−, P < 0.01]. Animals trained under high-threat conditions showed poor discrimination in their fear response to the CS+ and CS− and increased generalization, as noted by their lower discrimination index compared with animals trained under low-threat conditions (Fig. 1C; P < 0.01, t = 3.640, df = 22). Both groups showed low and indistinguishable freezing to the testing context (context B) (SI Appendix, Fig. S1), demonstrating a specificity of freezing responses to the tones.
Fig. 1.
Fear generalization is associated with decreased neuronal activation in the ZI. (A) Outline of the discriminative auditory fear conditioning protocol. On day 1, one group of mice received CS+ tone presentations paired with 0.3-mA foot-shocks (low-threat intensity) and unpaired CS− tone presentations. Another group of mice received CS+ tone presentations paired with 0.8-mA foot-shocks (high-threat intensity) and unpaired CS− tone presentations. On day 2, freezing responses were recorded for the CS+ and CS− tone presentations. ITI, intertrial interval. (B) Animals trained under low-threat conditions show a low freezing response to CS− and a high freezing response to CS+ (no fear generalization). In contrast, animals trained under high-threat conditions show an increased freezing response to both CS− and CS+ (fear generalization). (C) Discrimination indices (DIs) reveal significant fear generalization in the animals trained under high-threat conditions. (D) Decreased C-FOS expression was observed in the ZI of animals that showed increased fear to CS− presentations on the testing day. (E) Significant correlation was found between C-FOS expression in the ZI and behavioral fear responses. (F) Representative images of C-FOS expression in the ZI in response to tone presentations during the testing day after training under low-threat or high-threat conditions. (Scale bar: 100 μm.) **P < 0.01; ****P < 0.0001. Blue bars, low-intensity training; red bars, high-intensity training. Data are represented as mean ± SEM.
To examine neuronal activation of the ZI in the context of fear generalization, we counted the number of cells expressing the immediate early gene, C-FOS, in the ZI after exposing animals to either CS− or CS+ tone presentations. These animals had been previously trained under low-threat or high-threat conditions (SI Appendix, Fig. S2). Animals trained under high-threat conditions expressed increased fear to CS− on the day of testing, accompanied by lower numbers of C-FOS–positive cells in the ZI (Fig. 1 D and F and SI Appendix, Figs. S2 and S3). We did not find any significant differences between groups in the numbers of C-FOS–positive cells in the ZI after exposure to the CS+ [training × tone interaction: F(1,19) = 4.944, P < 0.05; post hoc tests: low-intensity training:CS− vs. high-intensity training:CS−, P < 0.01; CS−: low-intensity shock group (n = 7), high-intensity shock group (n = 8); CS+: low-intensity shock group (n = 4), high-intensity shock group (n = 4)]. In general, higher levels of fear expression (as measured by the freezing responses) were associated with lower numbers of C-FOS–expressing cells in the ZI (Fig. 1E; n = 21 animals; P < 0.01, r = −0.5563).
Decreasing Cellular Activity in the ZI Results in Fear Generalization After Conditioning with Low-Intensity Foot-Shocks.
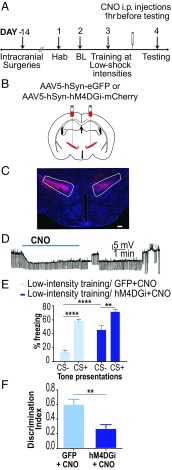
We utilized Gi-coupled designer receptors exclusively activated by designer drugs (DREADDs) to decrease activity of cells in the ZI (Fig. 2 A–D). Bath application of 20 μM clozapine-N-oxide (CNO) in vitro hyperpolarized hM4DGi-expressing neurons in the ZI, reducing cellular activity (Fig. 2D). Fourteen days before training animals under low-threat conditions, we injected adeno-associated virus (AAV)-containing human synapsin (hSyn) promoter driven transgenes: AAV5-hSyn-hM4D(Gi)-mCherry or AAV5-hSyn-EGFP bilaterally into the ZI of wild-type mice (SI Appendix, Fig. S4), and CNO was then administered i.p. 1 h before testing fear generalization. Decreasing activity of the ZI resulted in fear generalization in animals trained under low-threat conditions (Fig. 2E). Specifically, the low-intensity training hM4D(Gi)+CNO animals exhibited significantly higher freezing responses to CS− compared with freezing responses to the CS− of the low-intensity training GFP+CNO animals [low-intensity training GFP+CNO group (n = 6), low-intensity training hM4DGi+CNO group (n = 7); DREADD × tone interaction: F(1,11) = 6.335, P < 0.05; DREADD treatment main effect: F(1,11) = 26.73, P < 0.001; tone main effect: F(1,11) = 91.91, P < 0.0001; post hoc tests: low-intensity training GFP+CNO:CS− vs. low-intensity training GFP+CNO:CS+, P < 0.0001; low-intensity training hM4DGi+CNO:CS− vs. low-intensity training hM4DGi+CNO:CS+, P < 0.01; low-intensity training GFP+CNO:CS− vs. low-intensity training hM4DGi+CNO:CS−, P < 0.0001]. Low-intensity training hM4DGi+CNO animals showed an impaired ability to discriminate between the CS+ and CS−, as noted by their lower discrimination index compared with low-intensity training GFP+CNO animals (Fig. 2F; P < 0.01, t = 3.572, df = 14). Both groups showed low and indistinguishable freezing to the testing context (context B) (SI Appendix, Fig. S5), suggesting a specificity of freezing responses to the tones. Chemogenetic inhibition of cells in the ZI was not accompanied by alterations in locomotor activity or anxiety-like behavior (SI Appendix, Fig. S6).
Fig. 2.
Decreasing cellular activity in the ZI results in fear generalization. (A) Experimental protocol for chemogenetic inhibition. Two weeks after intracranial injection of the control or DREADD virus, animals were conditioned to low-threat intensities. The next day, CNO was administered i.p. 1 h before testing fear generalization. BL, baseline; Hab, habituation. (B) Wild-type animals were injected with either the control virus (AAV5-hSyn-EGFP) or inhibitory DREADDs (AAV5-hSyn-hM4DGi-mCherry) at −1.52 mm posterior to the bregma. (C) Representative image of the ZI targeted with intracranial infusions of DREADD-expressing mCherry viruses (in red) and Hoechst-stained nuclei (in blue). (Scale bar: 100 μm.) (D) Patch-clamp recording of hSyn-hM4DGi-mCherry–expressing cells in the ZI showing membrane hyperpolarization during CNO exposure. (E) Chemogenetic inhibition of the ZI (hM4DGi+CNO) resulted in a significant increase in fear response to CS− compared with controls (GFP+CNO). (F) Chemogenetic inhibition of the ZI (hM4DGi+CNO) resulted in an impaired ability to discriminate between the CS+ and the CS−. **P < 0.01; ****P < 0.0001. Data are represented as mean ± SEM.
Increasing Cellular Activity in the ZI Reduces Fear Generalization That Manifests After Conditioning with High-Intensity Foot-Shocks.
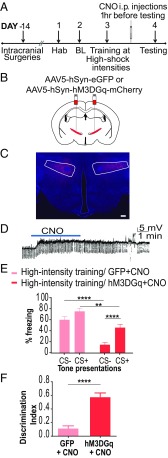
We utilized Gq-coupled DREADDs to increase activity of cells in the ZI (Fig. 3 A–D). Bath application of 20 μM CNO in vitro depolarized hM3DGq-positive neurons in the ZI, increasing cellular activity (Fig. 3D). Fourteen days before training animals under high-threat conditions, we injected AAV5-hSyn-hM3D(Gq)-mCherry or AAV5-hSyn-EGFP bilaterally into the ZI of wild-type mice, and CNO was then administered i.p. 1 h before testing fear generalization. Increasing activity of the ZI reduced fear generalization in animals trained under high-threat conditions (Fig. 3E). Specifically, the high-intensity training hM3D(Gq)+CNO animals exhibited significantly lower freezing responses to CS− than to CS+, compared with the high-intensity training GFP+CNO animals [high-intensity training GFP+CNO group (n = 7), high-intensity training hM3DGq+CNO (n = 10); DREADD × tone interaction: F(1,15) = 20.16, P < 0.001; DREADD treatment main effect: F(1,15) = 19.47, P < 0.001; tone main effect: F(1,15) = 136.6, P < 0.0001; post hoc tests: high-intensity training GFP+CNO:CS− vs. high-intensity training hM3DGq+CNO:CS−, P < 0.0001; high-intensity training hM3DGq+CNO:CS+ vs. high-intensity training hM3DGq+CNO:CS−, P < 0.0001; high-intensity training GFP+CNO:CS+ vs. high-intensity training hM3DGq+CNO:CS+, P < 0.01]. High-intensity training hM3DGq+CNO animals showed better discrimination in their fear response to the CS+ and CS−, as noted by their higher discrimination index compared with high-intensity training GFP+CNO animals (Fig. 3F; P < 0.0001, t = 5.931, df = 17). Both groups showed low and indistinguishable freezing to the testing context (context B) (SI Appendix, Fig. S7), suggesting a specificity of freezing responses to the tones. Chemogenetic activation of the ZI was not accompanied by alterations in locomotor activity or anxiety-like behavior (SI Appendix, Fig. S8).
Fig. 3.
Increasing cellular activity in the ZI prevents fear generalization. (A) Experimental protocol for chemogenetic activation. Two weeks after intracranial injection of the control or DREADD virus, animals were conditioned to high-threat intensities. The next day, CNO was administered i.p. 1 h before testing fear generalization. BL, baseline; Hab, habituation. (B) Wild-type animals were injected with either the control virus (AAV5-hSyn-EGFP) or excitatory DREADDs (AAV5-hSyn-hM3DGq-mCherry) at −1.52 mm posterior to the bregma. (C) Representative image of the ZI targeted with intracranial infusions of DREADD-expressing mCherry viruses (in red) and Hoechst-stained nuclei (in blue). (Scale bar: 100 μm.) (D) Patch-clamp recording of hSyn-hM3DGq-mCherry–expressing cells in the ZI showing membrane depolarization during CNO exposure. (E) Training using high-intensity foot-shock causes fear generalization, as seen by high freezing to both CS+ and CS−. Chemogenetic activation of the ZI (hM3DGq+CNO) resulted in a significant decrease in the fear response to CS+ as well as CS− compared with controls (GFP+CNO). (F) Chemogenetic activation of the ZI (hM3DGq+CNO) resulted in a better ability to discriminate between the CS+ and the CS−. **P < 0.01; ****P < 0.0001. Data are represented as mean ± SEM.
Increasing Activity of GABAergic Cells in the ZI Reduces Fear Generalization That Manifests After Conditioning with High-Intensity Foot-Shocks.
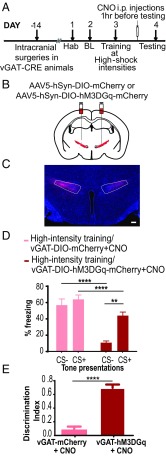
GABAergic cells in the ZI have been implicated in defensive responses like freezing and avoidance, as well as in retrieval of aversive memories (29, 30). We tested whether stimulating GABAergic cells in the ZI can reduce fear generalization (Fig. 4A). Fourteen days before training animals under high-threat conditions, we injected AAV5-DIO-hSyn-hM3D(Gq)-mCherry or AAV5-DIO-hSyn-mCherry bilaterally into the ZI of vesicular GABA transporter (vGAT)-CRE mice (Fig. 4 B and C). CNO was administered i.p. 1 h before testing fear generalization. Increasing activity of GABAergic cells in the ZI alone drastically reduced fear generalization observed in animals trained under high-threat conditions (Fig. 4D). Specifically, vGAT-CRE:DIO-hM3D(Gq)-mCherry+CNO animals exhibited significantly lower freezing responses to CS− than to CS+, compared with vGAT-CRE:DIO-mCherry+CNO animals [vGAT-CRE:DIO-mCherry+CNO group (n = 9), vGAT-CRE:DIO-hM3D(Gq)-mCherry+CNO (n = 11); DREADD × tone interaction: F(1,18) = 21.48, P < 0.0001; DREADD treatment main effect: F(1,18) = 26.03, P < 0.0001; tone main effect: F(1,18) = 50.84, P < 0.0001; post hoc tests: high-intensity training DIO-hM3DGq+CNO:CS+ vs. high-intensity training DIO-hM3DGq+CNO:CS−, P < 0.0001; high-intensity training DIO-GFP+CNO:CS− vs. high-intensity training DIO-hM3DGq+CNO:CS−, P < 0.0001; high-intensity training DIO-GFP+CNO:CS+ vs. high-intensity training DIO-hM3DGq+CNO:CS+, P < 0.05]. High-intensity training DIO-hM3DGq+CNO animals showed better discrimination in their fear response to the CS+ and CS−, as noted by their higher discrimination index compared with high-intensity training DIO-GFP+CNO animals (Fig. 4E; P < 0.0001, t = 9.151, df = 18). Both groups showed low and indistinguishable freezing to the testing context (context B) (SI Appendix, Fig. S9), suggesting a specificity of freezing responses to the tones. Such chemogenetic stimulation of GABAergic cells in the ZI was not accompanied by alterations in locomotor activity or anxiety-like behavior (SI Appendix, Fig. S10).
Fig. 4.
Targeted chemogenetic activation of GABAergic cells in the ZI can reduce fear generalization. (A) Experimental design: vGAT-CRE animals received intracranial injections of CRE-dependent control or DREADD virus, and after 2 weeks, they were conditioned to high-threat intensities. One day posttraining, CNO was administered i.p. 1 h before testing for fear generalization. BL, baseline; Hab, habituation. (B) vGAT-CRE animals were injected with either the control virus (AAV5-hSyn-DIO-mCherry) or excitatory DREADDs (AAV5-hSyn-DIO-hM3DGq-mCherry) at −1.52 mm posterior to the bregma. (C) Representative image of the GABAergic cells within the ZI infected with mCherry-expressing excitatory DREADDs (in red) and Hoechst-stained nuclei (in blue). (Scale bar: 100 μm.) (D) Animals with expression of DIO-hM3DGq virus in vGAT-CRE–expressing GABAergic cells in the ZI and injected with CNO (DIO-hM3DGq+CNO) 1 h before testing for fear generalization showed a significant decrease in fear response to CS− compared with animals that were infused with the DIO-mCherry virus in vGAT-CRE–expressing GABAergic cells in the ZI and injected with CNO (DIO-mCherry+CNO). (E) Chemogenetic activation of GABAergic cells in the ZI (DIO-hM3DGq+CNO) resulted in a better ability to discriminate between the CS+ and the CS−. **P < 0.05; ****P < 0.0001. Data are represented as mean ± SEM.
Discussion
Our results demonstrate a role for the ZI in modulating fear generalization. First, we found reduced C-FOS activation in the ZI associated with increased fear toward a neutral auditory stimulus. Next, we found that reducing cellular activity in the ZI resulted in fear generalization in animals trained under low-threat conditions. Chemogenetic stimulation of cellular activity in the ZI and targeted stimulation of GABAergic cells in the ZI reduced generalized fear responses normally observed after training animals under high-threat conditions.
Increasing threat intensities broaden generalization gradients in humans (34). In accordance with these data, we found that animals trained under high-threat conditions (0.8-mA foot-shocks) generalized fear to both conditioned (CS+) and neutral (CS−) tones, whereas animals trained under low-threat conditions (0.3-mA foot-shocks) did not demonstrate such generalization. These observations agree with previous reports that conditioning using increasing shock intensities promotes cue-related fear generalization in rodents (5, 35). While others have reported fear generalization to occur across contexts using similar protocols (13, 36–38), we do not observe the generalization of fear in testing context B after high-intensity fear conditioning in context A. This lack of contextual fear generalization after high-intensity training could be attributed to differences in study organism and experimental design. First, most studies that have demonstrated context-related fear generalization have used rats, and there may be species differences in cued- and contextual-fear generalization. Second, context A and context B were made easily distinguishable in our experiment with the use of distinct floors, odors, and lighting, which are changes that a recent study in mice demonstrated were sufficient to prevent contextual fear generalization (39).
To test whether the ZI is potentially involved in fear generalization, we first sought to compare C-FOS staining in the ZI of animals trained under low-threat and high-threat conditions. Excitingly, we found fewer C-FOS–positive cells in the ZI of animals that had been trained under high-threat conditions and generalized fear to the CS− than in the ZI of animals that had been trained under low-threat conditions and did not generalize fear. Could the ZI modulate fear generalization? The ZI is ideally positioned to convey information regarding the salience of specific sensory stimuli and to orchestrate appropriate fear-related behavioral responses. First, the ZI receives projections from sensory cortices (including the auditory cortex) and can coordinate activity across cortical networks according to attentional demands (40, 41). Additionally, the ZI innervates midbrain regions like the periaqueductal gray, which plays an important role in orchestrating fearful behaviors (15, 42, 43). Second, the ZI has been implicated in sensory discrimination and can modulate incoming sensory information (44). Finally, stimulating the ZI in humans facilitates the discrimination of fearful faces from nonfearful ones (33, 45). It is possible that stressful states like those created by high-intensity threat conditioning directly perturb cellular function in the ZI, rendering fear generalization as a behavioral outcome. Alternatively, generalized fear responses could arise indirectly due to amygdala→ZI connectivity (30, 42). Loss of cue specificity and widening of the memory trace in the amygdala occurring during fear generalization (5) could alter the ZI’s influence on modulating fear responses. Future experiments will need to examine if and how stress and amygdala function impact cellular and molecular niches in the ZI to cause fear generalization.
Building on our observations from the C-FOS study, we used DREADD-based strategies to test whether manipulating cellular activity in the ZI affected fear generalization. Reducing cellular activity in the ZI resulted in animals trained under low-threat conditions showing fear generalization. Conversely, increasing the activity of cells in the ZI of animals trained under high-threat conditions distinctly reduced fear generalization. Given the complex neurochemical profile of the ZI (46), we wanted to determine the cell populations responsible for suppressing fear generalization. The GABAergic neurons of the ZI were of particular interest, since they have been shown to gate ascending sensory information by fast feed-forward inhibition of higher order thalamic nuclei (19, 44, 47, 48). More recently, GABAergic cells in the ZI have been shown to be important for defensive responses as well as acquisition and retrieval of fear memories (29, 30). We found that stimulating GABAergic cells in the ZI reduced fear generalization in animals conditioned under high-threat intensities. We did not observe any significant differences in total distance traveled and velocity during open-field tests performed after any of our chemogenetic manipulations of the ZI (SI Appendix, Figs. S6, S8, and S10). These data collected in the open-field test ruled out the possibility that alterations in locomotor behavior could explain our results, which is a possibility that needed to be excluded, given that the caudal ZI is targeted via deep brain stimulation to ameliorate motor symptoms in patients with Parkinson’s disease (33, 42, 45). These data are not surprising, given that we targeted the medial, and not the caudal, subdivision of the ZI (SI Appendix, Fig. S4). Furthermore, freezing to the testing context (context B) remained unaltered after manipulating cellular activity in the ZI (SI Appendix, Figs. S5, S7, and S9), further emphasizing that the observed effects on fear generalization were specific to the CS+ and CS− tones presented.
Stimulation of GABAergic cells and the parvalbumin-expressing cells in the ZI has been demonstrated to reduce fearful behavior (29, 30). In line with these findings, we find that stimulating GABAergic cells within the ZI results in reduced fear responses toward the conditioned stimuli (DREADD treatment main effects are reported in Figs. 3 and 4). However, it should be pointed out that the reduction in fear responses to the CS− that we observe after stimulating GABAergic cells in the ZI is of a qualitatively greater magnitude than the decrease in fear responses to the CS+ after such stimulation. Moreover, animals continue to show a high fear response to the CS+ even after activation of the ZI, suggesting that our results are not a consequence of all fear responses being pulled off from the ceiling levels of fear observed after training with high-intensity foot-shocks. Further support for this perspective comes from the significant differences in the discrimination index between the control and DREADD-treated groups. Therefore, stimulating the ZI still leaves room for adaptive (albeit lower) fear responses to the CS+ to be expressed, while reducing fear to the neutral CS−. Our anterograde tracing of projections from GABAergic cells in the ZI (SI Appendix, Fig. S11) to regions like the nucleus reuniens (implicated in fear generalization), as well as the PAG (implicated in conditioned and unconditioned fear), are in accordance with recent findings (29, 30, 49). These tracing data suggest the potential for dissociating independent roles of the ZI in titrating fear generalization to the CS− and conditioned fear to the CS+.
With the ZI being chemoarchitecturally diverse, it would be informative to know the neurotransmitter/neuromodulator profiles of the ZI cells that are activated in animals that do not generalize fear. While blocking synaptic transmission in the ZI has been shown to alter anxiety-related measures (30), we did not find similar effects with chemogenetic manipulations of the ZI (SI Appendix, Figs. S6C, S8C, and S10C), a discrepancy possibly explained by cell type-specific influences on behavior. Future studies that target specific subpopulations of activated cells in the ZI will be required to achieve a finer grained resolution of how the ZI influences distinct dimensions of fear and anxiety.
Our experimental results bolster the recently demonstrated link between ZI activity and fearful behavior, and its role in calibrating fearful behavior toward environmental stimuli (29, 30). Our study makes a contribution to this body of work by demonstrating a role for the ZI in fear generalization. To conclude, our work suggests that stimulating the ZI in the clinic during exposure therapy could potentially reduce fear generalization, while leaving adaptive fear responses intact.
Materials and Methods
Animals.
Adult female and male C57BL/6J and vGAT-CRE mice were bred in the vivarium and group-housed under a 14:10-h light/dark cycle with ad libitum food and water. All experiments were approved by the Emory Institutional Animal Care and Use Committee and followed NIH standards.
Auditory Fear Conditioning to Test Fear Generalization.
Differential intensity-based auditory fear conditioning was used to test fear generalization as described (50) (outlined in Fig. 1A). Details are provided in SI Appendix.
Stereotaxic Surgeries.
We infused AAV5-hSyn-hM4DGi-mCherry (to reduce activity), AAV5-hSyn-hM3DGq-mCherry (to stimulate activity), and AAV5-hSyn-EGFP (control) viruses into the ZI of C57BL/6J animals. To stimulate cellular activity in the ZI of vGAT-CRE mice, we used AAV5-hSyn-DIO-hM3DGq-mCherry and AAV5-hSyn-DIO-mCherry (control) viruses. The AAVs were bilaterally injected into the ZI via stereotaxic surgery at −1.52 mm AP, ±0.73 mm ML, and−4.79 mm DV relative to bregma. Details are provided in SI Appendix.
C-FOS Immunohistochemistry and Cell Counting.
C-FOS expression was detected 90 min after exposure to either the CS+ or the CS− on the testing day (SI Appendix, Fig. S2) in 35-μm brain sections of animals trained under low-threat or high-threat conditions using rabbit polyclonal anti–C-FOS antibody (1:6,000 dilution, ABE 457; Millipore) and MCID Core Imaging software. Details are provided in SI Appendix.
Histology.
To verify placement of intracranial virus injections, brains were sectioned at 35 μm, stained with Hoechst nuclear stain (1:1,000), mounted on slides using SlowFade Gold Antifade mountant (Life Technologies), and visualized using a Nikon Eclipse E800 fluorescent microscope. Details are provided in SI Appendix.
Open-Field Test.
The open-field arena (50 × 50 × 50 cm3) was illuminated by red lights, and animals were acclimated to the red light in the testing room for 1 h after i.p. CNO injections (1 mg/kg). The mice were then placed in the center of the arena and allowed to explore for 5 min. Video data were analyzed using TopScan 2.0 (CleverSys, Inc.).
Electrophysiology.
At 4 to 6 wk after viral injections, 300-μm mouse brain slices containing the ZI were obtained as previously reported (51). Standard whole-cell patch-clamp recordings from fluorescent neurons in the ZI were performed. Details are provided in SI Appendix.
Statistical Analysis.
GraphPad Prism was used to analyze the data. Unpaired t tests were used for datasets containing only two groups and one dependent variable (C-FOS immunohistochemistry). Repeated-measures two-way ANOVA was used to analyze datasets with more than one independent variable (behavior experiments). Post hoc tests were only performed when interaction effects between the independent variables were significant, and Sidak’s correction was applied to account for multiple comparisons. Significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank the Yerkes Veterinary and Animal Care staff for animal husbandry. Funding was provided to B.G.D. by the Department of Psychiatry and Behavioral Sciences, the Brain Health Center, the Yerkes National Primate Research Center (YNPRC), a Canadian Institute for Advanced Research (CIFAR) Azrieli Global Scholar Award, and the Catherine Shopshire Hardman Fund. Additional funding was provided to the YNPRC by the Office of Research Infrastructure Programs (Grant ODP51OD11132).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820541116/-/DCSupplemental.
References
- 1.Dunsmoor JE, Paz R. Fear generalization and anxiety: Behavioral and neural mechanisms. Biol Psychiatry. 2015;78:336–343. doi: 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D. Fear generalization in humans: Systematic review and implications for anxiety disorder research. Behav Ther. 2015;46:561–582. doi: 10.1016/j.beth.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Jasnow AM, Lynch JF, 3rd, Gilman TL, Riccio DC. Perspectives on fear generalization and its implications for emotional disorders. J Neurosci Res. 2017;95:821–835. doi: 10.1002/jnr.23837. [DOI] [PubMed] [Google Scholar]
- 4.Kaczkurkin AN, et al. Neural substrates of overgeneralized conditioned fear in PTSD. Am J Psychiatry. 2017;174:125–134. doi: 10.1176/appi.ajp.2016.15121549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci. 2015;18:112–120. doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- 6.Jones GL, et al. A genetic link between discriminative fear coding by the lateral amygdala, dopamine, and fear generalization. eLife. 2015;4:e08969. doi: 10.7554/eLife.08969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajbhandari AK, Zhu R, Adling C, Fanselow MS, Waschek JA. Graded fear generalization enhances the level of cfos-positive neurons specifically in the basolateral amygdala. J Neurosci Res. 2016;94:1393–1399. doi: 10.1002/jnr.23947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciocchi S, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 9.Sanford CA, et al. A central amygdala CRF circuit facilitates learning about weak threats. Neuron. 2017;93:164–178. doi: 10.1016/j.neuron.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozeske RR, et al. Prefrontal-periaqueductal gray-projecting neurons mediate context fear discrimination. Neuron. 2018;97:898–910.e6. doi: 10.1016/j.neuron.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 11.Zelikowsky M, et al. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci USA. 2013;110:9938–9943. doi: 10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lissek S, et al. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Soc Cogn Affect Neurosci. 2014;9:1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duvarci S, Bauer EP, Paré D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebow MA, Chen A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- 16.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 17.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 19.Barthó P, Freund TF, Acsády L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- 20.Do Monte FH, Quirk GJ, Li B, Penzo MA. Retrieving fear memories, as time goes by…. Mol Psychiatry. 2016;21:1027–1036. doi: 10.1038/mp.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyll S, Budinger E, Noesselt T. Thalamic influences on multisensory integration. Commun Integr Biol. 2011;4:378–381. doi: 10.4161/cib.4.4.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antunes R, Moita MA. Discriminative auditory fear learning requires both tuned and nontuned auditory pathways to the amygdala. J Neurosci. 2010;30:9782–9787. doi: 10.1523/JNEUROSCI.1037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: Effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7:157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- 24.Heldt SA, Falls WA. Posttraining lesions of the auditory thalamus, but not cortex, disrupt the inhibition of fear conditioned to an auditory stimulus. Eur J Neurosci. 2006;23:765–779. doi: 10.1111/j.1460-9568.2006.04604.x. [DOI] [PubMed] [Google Scholar]
- 25.Halassa MM, Acsády L. Thalamic inhibition: Diverse sources, diverse scales. Trends Neurosci. 2016;39:680–693. doi: 10.1016/j.tins.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resnik J, Sobel N, Paz R. Auditory aversive learning increases discrimination thresholds. Nat Neurosci. 2011;14:791–796. doi: 10.1038/nn.2802. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara NC, Cullen PK, Pullins SP, Rotondo EK, Helmstetter FJ. Input from the medial geniculate nucleus modulates amygdala encoding of fear memory discrimination. Learn Mem. 2017;24:414–421. doi: 10.1101/lm.044131.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han JH, et al. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn Mem. 2008;15:443–453. doi: 10.1101/lm.993608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou XL, et al. Inhibitory gain modulation of defense behaviors by zona incerta. Nat Commun. 2018;9:1151. doi: 10.1038/s41467-018-03581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M, et al. A central amygdala to zona incerta projection is required for acquisition and remote recall of conditioned fear memory. Nat Neurosci. 2018;21:1515–1519. doi: 10.1038/s41593-018-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legg CR. Visual discrimination impairments after lesions in zona incerta or lateral terminal nucleus of accessory optic tract. Brain Res. 1979;177:461–478. doi: 10.1016/0006-8993(79)90464-5. [DOI] [PubMed] [Google Scholar]
- 32.Thompson R, Bachman MK. Zona incerta - link between the visual cortical sensory system and the brain-stem motor system. Physiol Psychol. 1979;7:251–253. [Google Scholar]
- 33.Burrows AM, et al. Limbic and motor function comparison of deep brain stimulation of the zona incerta and subthalamic nucleus. Neurosurgery. 2012;70(Suppl 1):125–130; discussion 130–131. doi: 10.1227/NEU.0b013e318232fdac. [DOI] [PubMed] [Google Scholar]
- 34.Dunsmoor JE, Kroes MC, Braren SH, Phelps EA. Threat intensity widens fear generalization gradients. Behav Neurosci. 2017;131:168–175. doi: 10.1037/bne0000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laxmi TR, Stork O, Pape HC. Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 36.Poulos AM, et al. Conditioning- and time-dependent increases in context fear and generalization. Learn Mem. 2016;23:379–385. doi: 10.1101/lm.041400.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem. 2004;81:162–166. doi: 10.1016/j.nlm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 39.Huckleberry KA, Ferguson LB, Drew MR. Behavioral mechanisms of context fear generalization in mice. Learn Mem. 2016;23:703–709. doi: 10.1101/lm.042374.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barthó P, et al. Cortical control of zona incerta. J Neurosci. 2007;27:1670–1681. doi: 10.1523/JNEUROSCI.3768-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitrofanis J, Mikuletic L. Organisation of the cortical projection to the zona incerta of the thalamus. J Comp Neurol. 1999;412:173–185. [PubMed] [Google Scholar]
- 42.Mitrofanis J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Mota-Ortiz SR, Sukikara MH, Felicio LF, Canteras NS. Afferent connections to the rostrolateral part of the periaqueductal gray: A critical region influencing the motivation drive to hunt and forage. Neural Plast. 2009;2009:612698. doi: 10.1155/2009/612698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trageser JC, et al. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol. 2006;96:1456–1463. doi: 10.1152/jn.00423.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blomstedt P, et al. Unilateral caudal zona incerta deep brain stimulation for Parkinsonian tremor. Parkinsonism Relat Disord. 2012;18:1062–1066. doi: 10.1016/j.parkreldis.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Kolmac C, Mitrofanis J. Distribution of various neurochemicals within the zona incerta: An immunocytochemical and histochemical study. Anat Embryol (Berl) 1999;199:265–280. doi: 10.1007/s004290050227. [DOI] [PubMed] [Google Scholar]
- 47.Lavallée P, et al. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J Neurosci. 2005;25:7489–7498. doi: 10.1523/JNEUROSCI.2301-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trageser JC, Keller A. Reducing the uncertainty: Gating of peripheral inputs by zona incerta. J Neurosci. 2004;24:8911–8915. doi: 10.1523/JNEUROSCI.3218-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aizenberg M, Geffen MN. Bidirectional effects of aversive learning on perceptual acuity are mediated by the sensory cortex. Nat Neurosci. 2013;16:994–996. doi: 10.1038/nn.3443. [DOI] [PubMed] [Google Scholar]
- 51.Daniel SE, Guo J, Rainnie DG. A comparative analysis of the physiological properties of neurons in the anterolateral bed nucleus of the stria terminalis in the Mus musculus, Rattus norvegicus, and Macaca mulatta. J Comp Neurol. 2017;525:2235–2248. doi: 10.1002/cne.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.