Abstract
Objective
Fibromyalgia (FM) and migraine are common pain disorders that tend to coexist. This study determined whether these two conditions exhibited any mutual influences.
Setting
Cohort study.
Participants
A retrospective, longitudinal cohort study was conducted using data obtained from a nationwide healthcare database. This study had two arms. Arm 1 comprised 33 216 patients with FM and arm 2 consisted of 7420 patients with migraine; all of these patients were diagnosed between 2000 and 2010. Using the aforementioned database, control subjects who had neither FM nor migraine and were matched with the FM and migraine patients by sex, age and index date of diagnosis were recruited. Each control cohort was four times the size of the corresponding study cohort. Follow-up for the control and study cohorts was conducted until the end of 2011.
Results
The incidence rates of FM and migraine were calculated in arms 1 and 2, respectively. The overall incidence of migraine was greater in the FM cohort than in the corresponding control cohort (4.39 vs 2.07 per 1000 person-years (PY)); crude HR=2.12, 95% CI=1.96 to 2.30; adjusted HR (aHR)=1.89, 95% CI=1.75 to 2.05). After adjustment for sex, age and comorbidities, the overall incidence of FM in the migraine cohort was 1.57 times greater than that in the corresponding control cohort (7.01 vs 4.49 per 1000 PY; aHR=1.52, 95% CI=1.39 to 1.65).
Conclusions
The present study revealed a bidirectional link between FM and migraine.
Keywords: fibromyalgia, migraine, bidirectional analysis, retrospective cohort
Strengths and limitations of this study.
Our study contained a large sample size because of its population-based design.
We based our study solely on information from diagnoses in patient files and included no information on patients whose cases were unidentified.
This study was naturally highly prone to observational bias because patients with migraine and those with fibromyalgia are generally more likely to seek medical attention for other conditions than are those with neither.
Health claims information in the Longitudinal Health Insurance Database mainly comprises documentation on diseases recorded according to the International Classification of Diseases, Ninth Revision, Clinical Modification but lacks descriptions of clinical subsets for disease manifestation or progression such as episodic or chronic migraine and migraine with or without aura.
The selection process of two study cohorts and two control cohorts was based solely on inclusion and exclusion criteria and did not involve subjective patient omission.
Introduction
A major symptom of fibromyalgia (FM) is headache. Migraine is a type of headache and some migraines are severe enough to be debilitating. Notably, similarities have been observed between migraines and FM, and many instances of overlapping symptoms, causes and treatments were noted in the present study, where the two conditions were considered in the same context.1 Several studies have reported that high proportions (20%–36%) of patients with migraine also have FM.2–5 Similarly, the frequency of migraine occurrence in patients with FM is 45%–80%, suggesting that migraine is common in patients with FM.6 7 Despite reports that the prevalence of FM is higher among migraine patients and vice versa,8–13 no explanations have been provided for this high rate of co-occurrence.
Migraine is a complex, recurrent disorder that manifests as a throbbing headache and is frequently associated with nausea, allodynia and sensitivity to sound or light. Migraines may develop into a chronic condition or disability.14 15 Migraine pain is believed to be caused by the nociceptive activation of the trigeminovascular system, including sensory neurons from the trigeminal ganglion and upper cervical nerve roots, which modulate central signals to numerous subcortical sites.16 The combination of tonic nociceptive input and central disinhibition may also play a role in the development of FM. Many migraineurs experience a condition referred to as ‘allodynia’ during migraine attacks. Typically, allodynia is confined to the head and neck but may involve other areas of the body.17 Increasing evidence indicates that peripheral tissues are relevant contributors to painful impulse input and can initiate or maintain central sensitisation, thereby contributing to the progression of FM.18 Migraine is believed to trigger FM. Repeated headaches in patients with migraine may increase the neuronal response to both nociceptive and non-nociceptive stimulation and induce spontaneous neuronal activity, which may concurrently increase patient sensitivity to FM.19 Several studies have highlighted the role of the hypothalamus in migraines.17 Evidence indicates the direct and indirect anatomical connections of the hypothalamus to the thalamus and autonomic brainstem nuclei, thereby supporting the role of the hypothalamus in nociceptive and autonomic modulation in patients with migraine.20 However, brain mechanisms common in patients with FM result in the central sensitisation of pain neurons, leading to the evolution of a complex syndrome.21
Early in the course of FM, widespread musculoskeletal pain often appears in the neck or shoulder region.22 Neck pain may activate local nociceptors and transmit pain impulses through upper cervical spinal nerves such as the greater occipital nerve to the trigeminal nucleus caudalis, thereby inducing a migraine attack.23 Some experts believe that FM and migraine headaches both involve defects in the systems that regulate certain chemical messengers in the brain, including serotonin and epinephrine (epinephrine).1 These defects may be reflected in the similar psychological comorbidities of the two conditions, including depression, anxiety, interpersonal sensitivity and somatisation.9 Psychosocial distress or abnormalities commonly occur in patients with migraine and those with FM.
Although studies have reported high comorbidity rates for migraine and FM, the following crucial concerns must be addressed. (1) Most such studies were conducted at tertiary care centres. Patients are often referred to tertiary clinics when they present with extreme pain, disability or medication overuse. Therefore, such sample populations may differ from patients treated in general practice. (2) Most such studies used a cross-sectional design to investigate prevalence rather than incidence of migraine or FM. (3) Whether a significant association exists, suggesting that people with migraine are more likely to develop FM than the general population or vice versa, remains unknown. Therefore, our population-based longitudinal cohort was employed to investigate the link between migraine and FM.
Methods
Data source
Data for this research were obtained from the Longitudinal Health Insurance Database (LHID). The LHID comprises data of insurance claims filed by 1 million patients under Taiwan’s National Health Insurance (NHI) programme, which covers 99% of Taiwan’s 23 million citizens with single-payer health insurance. According to a government report, no differences between the LHID and Taiwan’s NHI programme exist with respect to demographic characteristics. The health claims information in the LHID includes general patient information (eg, birthdate, sex, occupation), documentation of diseases (recorded according to the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]) and other data related to medical services.
Study cohorts
A bidirectional cohort study design was used to interpret the longitudinal association between FM and migraine.
Figure 1 displays the procedure for establishing the two arms of this study. For arm 1, we identified patients with FM (ICD-9-CM code 729.1) aged ≥20 years and newly diagnosed ≥3 times consecutively within 3 months from 2000 to 2010. The first diagnosis date was designated as the index date for entry into the FM cohort. Patients with a history of migraine (ICD-9-CM code 346) were excluded from this arm. For each patient with FM, we randomly selected four individuals without FM or migraine from the population of the LHID2000 who were frequency-matched by sex, age (in 5-year increments) and entry date of the patient with FM; these subjects were recruited into the non-FM (control) cohort.
Figure 1.
Flow chart illustrating the selection of study subjects. FM, fibromyalgia.
A similar procedure was used for arm 2 to establish a cohort of patients with migraine who had no history of FM, were aged ≥20 years and were newly diagnosed ≥3 times consecutively within 3 months from 2000 to 2010.
Subjects in both arms were followed until diagnosis of migraine or FM, withdrawal from the NHI programme, death or 31 December 2011. The patients in the two cohorts presented with some baseline comorbidities: diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), hyperlipidaemia (ICD-9-CM code 272), depression (ICD-9-CM codes 296.2, 296.3, 296.5, 300.4 309 and 311), anxiety (ICD-9-CM codes 300.0, 300.2, 300.3, 308.3 and 309.81), sleep disorder (ICD-9-CM codes 307.4 and 780.5), coronary artery disease (CAD; ICD-9-CM codes 410–414), chronic fatigue syndrome (CFS; ICD-9-CM code 780.71) and irritable bowel syndrome (IBS; ICD-9-CM code 564.1).
Statistical analyses
The characteristics of the study cohorts are expressed as means and corresponding SD for age and as numbers and percentages for sex and comorbidities. Age difference was assessed using a t test, and sex and comorbidity distributions were tested using a χ2 test. The incidence density for each cohort was calculated as the total event number divided by the sum of follow-ups (per 1000 person-years (PY)). The cumulative incidence curve for each cohort was measured using the Kaplan-Meier method and the curve difference was calculated using the log-rank test. To determine the risks of migraine and FM in arms 1 and 2, respectively, HRs and corresponding 95% CIs were estimated using single-variable and multivariable Cox proportional hazard models. Data management and all statistical analyses were performed using SAS for Windows V.9.4 (SAS Institute) and incidence curves was plotted using R software. All significance levels were set as two-sided p<0.05.
Public and Patient involvement
None.
Results
Table 1 presents the demographic characteristics of the FM and non-FM cohorts. The age-matched and sex-matched cohorts exhibited differences in comorbidity distribution. The prevalence of comorbidities was significantly higher in the FM cohort than in the non-FM cohort (p<0.001).
Table 1.
Demographic characteristics and comorbidities in patients with and without FM
| Variable | FM | P value | |
| No | Yes | ||
| n=132 863 | n=33 216 | ||
| Sex | n (%) | n (%) | 0.99 |
| Female | 71 880 (54.1) | 17 970 (54.1) | |
| Male | 60 983 (45.9) | 15 246 (45.9) | |
| Age, mean (SD) | 50.9 (16.9) | 51.4 (16.7) | <0.001* |
| Stratify age | 0.99 | ||
| ≤49 | 64 292 (48.4) | 10 673 (48.4) | |
| 50–65 | 36 820 (27.7) | 9205 (27.7) | |
| 65+ | 31 751 (23.9) | 7938 (23.9) | |
| Comorbidity | |||
| Diabetes | 10 485 (7.89) | 3193 (9.61) | <0.001 |
| Hypertension | 37 284 (28.1) | 11 287 (34.0) | <0.001 |
| Hyperlipidaemia | 22 446 (16.9) | 7301 (22.0) | <0.001 |
| Depression | 4690 (3.53) | 1804 (5.43) | <0.001 |
| Anxiety | 10 494 (7.90) | 4214 (12.7) | <0.001 |
| Sleep disorder | 21 095 (15.9) | 8121 (24.5) | <0.001 |
| CAD | 17 918 (13.5) | 5821 (17.5) | <0.001 |
| CFS | 199 (0.15) | 93 (0.28) | <0.001 |
| IBS | 5125 (3.86) | 1870 (5.63) | <0.001 |
Χ2 test; *two-sample t-test.
CAD, coronary artery disease; CFS, chronic fatigue syndrome; FM, fibromyalgia; IBS, irritable bowel syndrome.
Table 2 indicates that the migraine incidences were 4.39 and 2.07 per 1000 PY in the FM and non-FM cohorts, respectively. Figure 2 reveals a higher incidence curve for the FM cohort than for the non-FM cohort (log-rank test=371.4, p<0.001). After adjustment for age, sex and comorbidities, the patients with FM exhibited a 1.89-times higher risk of migraine compared with the non-FM subjects (HR=1.89, 95% CI=1.75 to 2.05). Among women, the relative risk of migraine was 1.76-times higher in patients with FM compared with non-FM subjects (HR=1.76, 95% CI 1.60 to 1.93), whereas among men, the risk was 2.29-times higher in patients with FM than in non-FM subjects (HR=2.29, 95% CI=1.97 to 2.67). Regarding age, the HRs for migraine in the FM cohort were 2.06 (95% CI=1.85 to 2.29), 1.66 (95% CI=1.43 to 1.92) and 1.69 (95% CI=1.39 to 2.05) for ≤50, 51–65 and ≥65 years, respectively.
Table 2.
Comparison of the incidence and HRs of migraine stratified by sex and age between patients with and without FM
| Variable | Without FM | With FM | ||||||||
| Event | PY | Rate† | Crude HR (95% CI) | Adjusted HR‡ (95% CI) | Event | PY | Rate† | Crude HR (95% CI) | Adjusted HR‡ (95% CI) | |
| All | 1810 | 8 76 077 | 2.07 | 1(Reference) | 1(Reference) | 954 | 2 17 386 | 4.39 | 2.12 (1.96 to 2.30)*** | 1.89 (1.75 to 2.05)*** |
| Sex | ||||||||||
| Female | 1373 | 4 87 506 | 2.82 | 1(Reference) | 1(Reference) | 669 | 1 20 773 | 5.54 | 1.97 (1.79 to 2.16)*** | 1.76 (1.60 to 1.93)*** |
| Male | 437 | 3 88 571 | 1.12 | 1(Reference) | 1(Reference) | 285 | 96 613 | 2.95 | 2.62 (2.26 to 3.05)*** | 2.29 (1.97 to 2.67)*** |
| Stratify age | ||||||||||
| ≤50 | 922 | 4 44 710 | 2.07 | 1(Reference) | 1(Reference) | 548 | 1 10 557 | 4.96 | 2.39 (2.15 to 2.66)*** | 2.06 (1.85 to 2.29)*** |
| 50–65 | 564 | 2 45 579 | 2.30 | 1(Reference) | 1(Reference) | 258 | 60 603 | 4.26 | 1.85 (1.60 to 2.15)*** | 1.66 (1.43 to 1.92)*** |
| 65+ | 324 | 1 85 788 | 1.74 | 1(Reference) | 1(Reference) | 148 | 46 226 | 3.20 | 1.83 (1.51 to 2.23)*** | 1.69 (1.39 to 2.05)*** |
| Comorbidity§ | ||||||||||
| No | 774 | 5 08 879 | 1.52 | 1(Reference) | 1(Reference) | 311 | 95 605 | 3.25 | 2.14 (1.88 to 2.44)*** | 2.13 (1.87 to 2.43)*** |
| Yes | 1036 | 3 67 197 | 2.82 | 1(Reference) | 1(Reference) | 643 | 1 21 780 | 5.28 | 1.88 (1.71 to 2.08)*** | 1.80 (1.63 to 1.98)*** |
***P<0.001.
†Incidence rate per 1000 PY.
‡Multivariable analysis including sex, age, and the comorbidities of diabetes, hypertension, hyperlipidaemia, depression, anxiety, sleep disorder, coronary artery disease (CAD), chronic fatigue syndrome (CFS) and irritable bowel syndrome (IBS).
§Patients with any of the comorbidities of diabetes, hypertension, hyperlipidaemia, depression, anxiety, sleep disorder, CAD, CFS or IBS were classified as the comorbidity group.
FM, fibromyalgia; PY, person-years.
Figure 2.

Comparison of cumulative incidence of migraine between patients with and without fibromyalgia using the Kaplan-Meier method.
Table 3 presents the influence of factors associated with migraine occurrence in the FM cohort. Male sex, hyperlipidaemia, depression, anxiety, sleep disorder, CAD, CFS and IBS were all associated with higher risk of migraine (all p<0.05).
Table 3.
Cox model with HRs and 95% CIs for migraine associated with FM and covariates
| Variable | Crude | Adjusted† | ||
| HR | (95% CI) | HR | (95% CI) | |
| FM | 2.12 | (1.96 to 2.30)*** | 1.89 | (1.74 to 2.04)*** |
| Sex (Women vs Men) | 2.28 | (2.09 to 2.48)*** | 2.08 | (1.91 to 2.27)*** |
| Age, years | 1.00 | (0.99 to 1.00)** | 0.99 | (0.99 to 1.00)*** |
| Baseline comorbidities (yes vs no) | ||||
| Diabetes | 0.82 | (0.70 to 0.96)* | 0.73 | (0.61 to 0.860*** |
| Hypertension | 1.06 | (0.97 to 1.15) | – | – |
| Hyperlipidaemia | 1.30 | (1.19 to 1.43)*** | 1.14 | (1.03 to 1.27)* |
| Depression | 2.37 | (2.06 to 2.72)*** | 1.20 | (1.03 to 1.39)* |
| Anxiety | 2.68 | (2.44 to 2.95)*** | 1.64 | (1.47 to 1.84)*** |
| Sleep disorder | 2.63 | (2.43 to 2.85)*** | 1.97 | (1.80 to 2.15)*** |
| CAD | 1.30 | (1.18 to 1.44)*** | 1.10 | (0.98 to 1.23) |
| CFS | 2.24 | (1.01 to 4.99)* | 1.45 | (0.65 to 3.22) |
| IBS | 1.98 | (1.71 to 2.29)*** | 1.36 | (1.17 to 1.58)*** |
*P<0.05; **P<0.01; ***P<0.001.
†Multivariable analysis including sex, age and the comorbidities of diabetes, hyperlipidaemia, depression, anxiety, sleep disorder, CAD, CFS and IBS.
CAD, coronary artery disease; CFS, chronic fatigue syndrome; FM, fibromyalgia; IBS, irritable bowel syndrome.
Table 4 lists the comorbidities as well as the age and sex-matched comparisons in the migraine cohort which exhibited a higher prevalence of comorbidities than the non-migraine cohort.
Table 4.
Demographic characteristics and comorbidities in patients with and without migraine
| Variable | Migraine | P value | |
| No | Yes | ||
| n=69 680 | n=17 420 | ||
| Sex | n (%) | n (%) | 0.99 |
| Female | 51 176 (73.4) | 12 794 (73.4) | |
| Male | 18 504 (26.6) | 4626 (26.6) | |
| Age, mean (SD) | 44.2 (15.6) | 44.5 (15.3) | 0.04* |
| Stratify age | 0.99 | ||
| ≤49 | 46 768 (67.1) | 11 692 (67.1) | |
| 50–65 | 14 940 (21.4) | 3735 (21.4) | |
| 65+ | 7972 (11.4) | 1993 (11.4) | |
| Comorbidity | |||
| Diabetes | 3567 (5.12) | 975 (5.60) | 0.01 |
| Hypertension | 12 563 (18.0) | 4551 (26.1) | <0.001 |
| Hyperlipidaemia | 8278 (11.9) | 3187 (18.3) | <0.001 |
| Depression | 2019 (2.90) | 1851 (10.6) | <0.001 |
| Anxiety | 4366 (6.27) | 3724 (21.4) | <0.001 |
| Sleep disorder | 9469 (13.6) | 6976 (40.1) | <0.001 |
| CAD | 5560 (7.98) | 2449 (14.1) | <0.001 |
| CFS | 71 (0.10) | 41 (0.24) | <0.001 |
| IBS | 2106 (3.02) | 1224 (7.03) | <0.001 |
Χ2 test. *two-sample t-test.
CAD, coronary artery disease; CFS, chronic fatigue syndrome; IBS, irritable bowel syndrome.
Table 5 and figure 3 reveals that the incidence of FM was significantly higher in patients with migraine than in those without (7.01 vs 4.49 per 1000 PY; log-rank test=116.7, p<0.001). After adjustment for age, sex and comorbidities, patients with migraine exhibited a 1.52-times higher risk of FM compared with those without migraine (HR=1.52, 95% CI=1.39 to 1.65). Among female patients, those with migraine exhibited a 1.43-times higher risk of FM compared with non-migraine subjects (HR=1.43, 95% CI=1.29 to 1.59), whereas among male patients, those with migraine exhibited a 1.78-times higher risk of FM compared with non-migraine subjects (95% CI=1.50 to 2.11). Regarding age, the HRs for FM were 1.64 (95% CI=1.46 to 1.84), 1.30 (95% CI=1.09 to 1.53) and 1.28 (95% CI=1.03 to 1.58) in patients with migraine aged <50, 50–64, and ≥65 years, respectively.
Table 5.
Comparison of the incidence and HRs of fibromyalgia stratified by sex and age between patients with and without migraine
| Variable | Without migraine | With migraine | ||||||||
| Event | PY | Rate† | Crude HR (95% CI) | Adjusted HR‡ (95% CI) | Event | PY | Rate† | Crude HR (95% CI) | Adjusted HR‡ (95% CI) | |
| All | 2034 | 4 53 130 | 4.49 | 1(Reference) | 1(Reference) | 800 | 1 14 070 | 7.01 | 1.57 (1.44 to 1.70)*** | 1.52 (1.39 to 1.65)*** |
| Sex | ||||||||||
| Female | 1556 | 3 35 328 | 4.64 | 1(Reference) | 1(Reference) | 568 | 84 606 | 6.71 | 1.45 (1.32 to 1.60)*** | 1.43 (1.29 to 1.59)*** |
| Male | 478 | 1 17 802 | 4.06 | 1(Reference) | 1(Reference) | 232 | 29 464 | 7.87 | 1.94 (1.66 to 2.27)*** | 1.78 (1.50 to 2.11)*** |
| Stratify age | ||||||||||
| ≤50 | 1060 | 3 10 621 | 3.41 | 1(Reference) | 1(Reference) | 470 | 78 131 | 6.02 | 1.77 (1.58 to 1.97)*** | 1.64 (1.46 to 1.84)*** |
| 50–65 | 608 | 96 607 | 6.29 | 1(Reference) | 1(Reference) | 207 | 24 189 | 8.56 | 1.36 (1.16 to 1.59)*** | 1.30 (1.09 to 1.53)** |
| 65+ | 366 | 45 902 | 7.97 | 1(Reference) | 1(Reference) | 123 | 11 751 | 10.5 | 1.3291.07 to 1.61)** | 1.28 (1.03 to 1.58)* |
| Comorbidity§ | ||||||||||
| No | 1082 | 3 09 229 | 3.50 | 1(Reference) | 1(Reference) | 255 | 43 664 | 5.84 | 1.67 (1.46 to 1.92)*** | 1.79 (1.56 to 2.06)*** |
| Yes | 952 | 1 43 901 | 6.62 | 1(Reference) | 1(Reference) | 545 | 70 406 | 7.74 | 1.18 (1.06 to 1.31)** | 1.2991.16 to 1.44)*** |
*P<0.05; **P<0.01; ***P<0.001.
†Incidence rate per 1000 PY.
‡Multivariable analysis including sex, age, and the comorbidities of diabetes, hypertension, hyperlipidaemia, depression, anxiety, sleep disorder, CAD, IBS.
§Patients with any of the comorbidities of diabetes, hypertension, hyperlipidaemia, depression, anxiety, sleep disorder, CAD, chronic fatigue syndrome or IBS were classified as the comorbidity group.
PY, person years.
Figure 3.
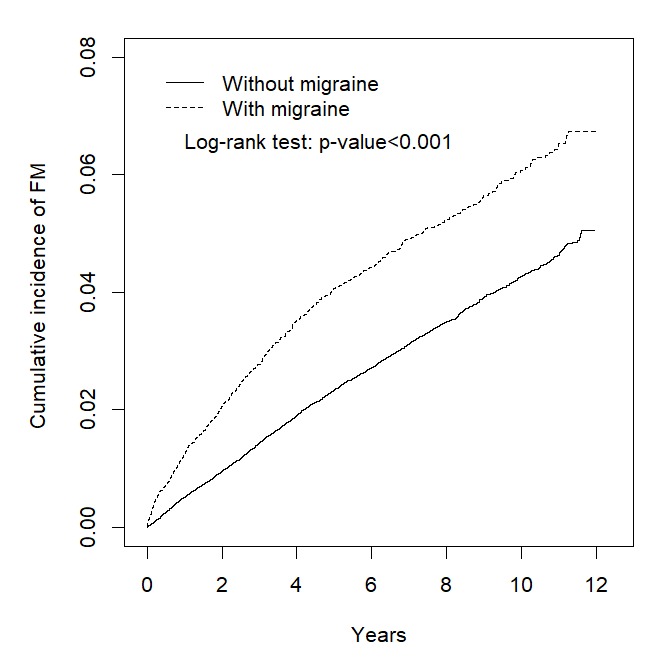
Comparison of the cumulative incidence of fibromyalgia between patients with and without migraine, using the Kaplan-Meier method.
Table 6 presents the associations of sex, age and comorbidities with risk of FM. The variables, including age, migraine, hypertension, hyperlipidaemia, depression, sleep disorder and CAD, were all associated with lower risk of FM.
Table 6.
Cox model with HRs and 95% CIs for fibromyalgia associated with migraine and covariates
| Variable | Crude | Adjusted† | ||
| HR | (95% CI) | HR | (95% CI) | |
| Migraine | 1.57 | (1.44 to 1.70)*** | 1.51 | (1.38 to 1.65)*** |
| Sex (Women vs Men) | 1.05 | (0.97 to 1.15) | – | – |
| Age, years | 1.02 | (1.02 to 1.03)*** | 1.02 | (1.01 to 1.02)*** |
| Baseline comorbidities (yes vs no) | ||||
| Diabetes | 1.58 | (1.36 to 1.82)*** | 0.99 | (0.85 to 1.16) |
| Hypertension | 1.81 | (1.67 to 1.96)*** | 1.10 | (0.99 to 1.22) |
| Hyperlipidaemia | 1.69 | (1.54 to 1.85)*** | 1.15 | (1.03 to 1.28)* |
| Depression | 1.38 | (1.17 to 1.63)*** | 1.06 | (0.89 to 1.26) |
| Anxiety | 1.34 | (1.19 to 1.51)*** | 0.92 | (0.80 to 1.05) |
| Sleep disorder | 1.45 | (1.33 to 1.58)*** | 1.09 | (0.98 to 1.20) |
| CAD | 1.74 | (1.57 to 1.94)*** | 1.01 | (0.89 to 1.14) |
| CFS | 2.11 | (0.79 to 5.62) | – | – |
| IBS | 1.28 | (1.06 to 1.53)** | 0.94 | (0.78 to 1.13) |
*P<0.05; **P<0.01; ***P<0.001.
†Multivariable analysis including age and the comorbidities of diabetes, hypertension, hyperlipidaemia, anxiety, sleep disorders, stroke, and peptic ulcer disease and use of non-steroidal anti-inflammatory drugs.
CAD, coronary artery disease; CFS, chronic fatigue syndrome; IBS, irritable bowel syndrome.
Discussion
The results of comparing the two cohort arms suggested a bidirectional risk of migraine and FM in patients with FM and those with migraine, respectively. The analysis of arm 1 revealed incidence rates for migraine of 4.39 and 2.07 per 1000 PY in patients with and without FM, respectively [adjusted HR (aHR)=1.89, 95% CI=1.75 to 2.05 in patients with FM]. The analysis of arm 2 revealed incidence rates for FM of 7.01 and 4.49 per 1000 PY in patients with and without migraine, respectively (aHR=1.52, 95% CI=1.39 to 1.65 in patients with migraine). These results indicated that FM had stronger predictive power for the onset of migraine than did migraine for the onset of FM.
The Kaplan-Meier plots demonstrated that incidence of migraine in the FM cohort and that of FM in the migraine cohort increased steadily during the 12-year follow-up period. Moreover, similar patterns were observed in the two corresponding comparison cohorts. The cumulative incidence measured by the Kaplan-Meier plots revealed greater risk of migraine among patients with FM than risk of FM among patients with migraine.
Our predictive analytics have the potential to guide diagnosis and treatment. For example, a subsequent diagnosis of FM may result from failure of antimigraine treatment to alleviate fatigue.24 Because migraine is often more effectively managed than FM, the authors hypothesised that patients with FM are more likely to be treated for migraine than are patients with migraine for FM. Therefore, clinical trials of patients with migraine in the future have the potential to evaluate the effects of FM on health outcomes and the efficacy of FM treatment.10
Cohort analysis for the association between FM and risk of new-onset migraine
This study revealed a positive association between FM diagnosis and the risk of migraine. Adjusting for hypertension, CAD and CFS had no strong influence on this association. However, sex, age (particularly in patients aged <49 years), diabetes, hyperlipidaemia, depression, anxiety, sleep disorder and IBS continued to demonstrate statistically significant effects.
Because “high frequency and chronic migraine increase sensitivity to pain in patients with FM,25 such heightened pain sensitivity may be attenuated by comorbid diabetes. There is also a documented report showing a significant positive association between migraine frequency and intensity with total and [low-density lipoprotein] cholesterol, independent of diet and lifestyle.26” Several hypotheses have been proposed to explain the development of chronic widespread pain and episodic throbbing or pulsating pain across the head and neck regions as possible effects of comorbidities such as depression and anxiety. Depression and anxiety disorders have been identified as crucial secondary symptoms of FM.11 27 28 The pain associated with FM may initiate the development of mood disorders as a result of stress imposed on the body. Furthermore, according to multiple evidence-based studies, depression and anxiety may induce the onset or present as a prodrome of migraine.29 30 Research has indicated that serotonin levels might be related to interconnections between anxiety and migraine.31 A lower level of serotonin may be central to the dysregulation of descending antinociceptive systems, leading to FM and migraine.31 32
Poor sleep quality or sleep deprivation in healthy individuals can induce symptoms of FM,33 suggesting that sleep abnormalities may be a pathological characteristic of FM rather than merely a result of pain.34 Relevant literature has reported the advantages of targeting sleep conditions to relieve the symptoms of migraine.35 As the prevalence of sleep disorders increases in both patients with FM and those with migraine, appreciation of the strong links between FM and migraine also increases.
IBS frequently coexists with both FM and migraine36 37; however, the underlying mechanisms for the association of FM with increased risks of IBS and migraine are unclear. FM, migraine, and IBS may be distinct manifestations of a common pathophysiological process affecting the gastrointestinal tract. These disorders are referred to as ‘central sensitivity syndrome’ and are mutually associated.38 A growing amount of evidence indicates that central sensitisation phenomena play a role in the pathogenesis of FM and that of migraine. Central sensitisation at the levels of the spinal dorsal horn and trigeminal nucleus may also be involved in the progression of migraine attacks, and prolonged nociceptive inputs may result in the maintenance of supraspinal sensitisation and central neuroplastic changes, causing episodic headaches to become chronic.39 Notably, increased intestinal permeability may be observed in IBS.40 Altered intestinal permeability with overgrowth of intestinal bacteria may trigger the development of FM41 and that of migraine.42 The microbiome–gut–brain axis—a bidirectional communication route of the central and enteric nervous systems with microbiome through the neural, humoral, endocrine and immune pathways36 37 43 — has been proposed as a multifaceted pathophysiological mechanism underlying IBS,43 FM44 45 and migraine.42 46 In addition, mutual interaction has been established between gut microbiota and the central, autonomic and enteric nervous systems through the hypothalamic–pituitary–adrenal axis.43
Cohort analysis for the association between migraine and risk of new-onset FM
This study revealed higher risk of FM in patients with migraine than in those without in every factor-based subset of the cohorts. Notably, patients with hyperlipidaemia had higher risk of FM. Moreover, adverse lipid profiles occurred more frequently in patients with migraine who had a higher body mass index.47 48 Although lack of exercise may precipitate the development of an adverse lipid profile, exercise may trigger acute migraine attacks49 and some patients may avoid exercise to prevent migraines. This hypothesis could be supported by the results of one study that revealed that patients with headache had lower aerobic endurance and flexibility than did healthy controls.50 Aerobic exercise could relieve depression and anxiety and prevent the negative effects of stress.51 Furthermore, avoiding exercise may exacerbate mood distress and, thus, could be related to the development of FM.
Increased migraine frequency, as a result of migraines becoming chronic, intensifies the sensitivity to pain in somatic areas outside of the cephalic region and may predispose patients to FM.6 Hypothalamic neuroendocrine dysfunction has been proposed as a brain mechanism common to both FM and migraine.52 Both conditions also share the mechanism of central sensitisation of pain neurons. Magnesium, which is often used as an agent for relieving migraine headaches, is also beneficial for treating FM. Low magnesium levels can exacerbate symptoms of FM and are also implicated in migraines.53 Researchers have discovered that people who do not respond to standard migraine treatments often also have FM.17 Considering the high comorbidity rates of migraine and FM, many professionals assume that the central nervous system is responsible for pain-processing abnormalities, including central sensitisation and inadequate pain inhibition, alongside repeated headache episodes. Moreover, tonic peripheral nociceptive input is associated with augmented windup in response to neurotransmitters, immunomodulation, vascular changes and hormone influence, which may increase the risk of FM.1 6 36 37 43 52
Our study contained a large sample because of our population-based design. Moreover, we were careful to minimise selection bias during analysis and our ample documentation of medical profiles allowed for minimal effects from confounding factors among the subjects. However, this study had limitations. We based our study solely on information from diagnoses in patient files and included no information from patients whose cases were unidentified. Poor categorisation of a patient’s symptoms may have affected the discernibility between migraine and FM. Because many crucial variables are not retrievable and various methods are used to diagnose FM and the numerous subtypes of migraines, our data provides merely a glimpse of these two conditions. Furthermore, assessing treatment responses in our large database analysis was impossible, rendering the identification of ‘diagnosis by exclusion’ difficult in this study. Future studies are recommended to further delineate ‘diagnosis by exclusion.’ Furthermore, this study did not consider the severity of FM and migraines in patients; therefore, no definitive statement can be made regarding the intensity of FM and subsequent risk of developing migraine conditions or vice versa. Moreover, this study was naturally highly prone to observational bias because patients with migraine and those with FM are generally more likely to seek medical attention for other conditions than are those with neither.
Conclusion
This study was the first to reveal a population-based bidirectional association between onset of FM and that of migraine in patients with migraine and those with FM, respectively. The risk of migraine was reportedly greater than that of FM. The incidence rates of FM in the migraine cohort and migraine in the FM cohort increased with age in both directions. However, the HRs relative to the corresponding comparison cohorts were attenuated with increases in age.
Supplementary Material
Footnotes
Contributors: Conceptualisation: I-WP, C-HK. Methodology: C-LL, C-HK. Software: C-LL, C-HK. Validation: I-WP, EC, T-YC, C-LL, C-HK. Formal analysis: I-WP, EC, T-YC, C-LL, C-HK. Investigation: C-LL, C-HK. Resources: C-LL, C-HK. Data curation: IWP, EC, TYC, CLL, CHK. Writing (original draft preparation): I-WP, EC, T-YC, C-LL, C-HK. Writing (review and editing): I-WP, EC, T-YC, C-LL, C-HK. Visualisation: I-WP, EC, T-YC, C-LL, C-HK. Supervision: C-HK. Project administration: C-HK. Funding acquisition: C-HK.
Funding: This work was supported by grants from the MOHW, Taiwan (MOHW108-TDU-B-212-133004), China Medical University Hospital (DMR-107-192), Academia Sinica Stroke Biosignature Project (BM10701010021), the Ministry of Science and Technology (MOST) Clinical Trial Consortium for Stroke (MOST 107-2321-B-039 -004-), the Tseng-Lien Lin Foundation, Taichung, Taiwan and the Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript. No additional external funding was received for this study.
Competing interests: None declared.
Ethics approval: This study was approved for exemption by the Institutional Review Board of China Medical University (CMUH104-REC2-115-CR3).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The dataset used in this study was obtained from Taiwan’s Ministry of Health and Welfare (MOHW), from which we were required to obtain approval to access the data. Any researcher interested in accessing this dataset can submit an application form to the MOHW requesting access. Please contact the staff of the MOHW (email: stcarolwu@mohw.gov.tw) for further assistance. Taiwan MOHW Address: No. 488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan (ROC). Phone: +886-2-8590-6848. All relevant data are provided in this manuscript.
Patient consent for publication: Not required.
References
- 1. de Tommaso M. Prevalence, clinical features and potential therapies for fibromyalgia in primary headaches. Expert Rev Neurother 2012;12:287–96. 10.1586/ern.11.190 [DOI] [PubMed] [Google Scholar]
- 2. Evans RW, de Tommaso M. Migraine and fibromyalgia. Headache 2011;51:295–9. 10.1111/j.1526-4610.2010.01835.x [DOI] [PubMed] [Google Scholar]
- 3. de Tommaso M, Federici A, Serpino C, et al. Clinical features of headache patients with fibromyalgia comorbidity. J Headache Pain 2011;12:629–38. 10.1007/s10194-011-0377-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Küçükşen S, Genç E, Yılmaz H, et al. The prevalence of fibromyalgia and its relation with headache characteristics in episodic migraine. Clin Rheumatol 2013;32:983–90. 10.1007/s10067-013-2218-2 [DOI] [PubMed] [Google Scholar]
- 5. Marcus DA, Bhowmick A. Fibromyalgia comorbidity in a community sample of adults with migraine. Clin Rheumatol 2013;32:1553–6. 10.1007/s10067-013-2310-7 [DOI] [PubMed] [Google Scholar]
- 6. Giamberardino MA, Affaitati G, Martelletti P, et al. Impact of migraine on fibromyalgia symptoms. J Headache Pain 2015;17:28 10.1186/s10194-016-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vij B, Whipple MO, Tepper SJ, et al. Frequency of migraine headaches in patients with Fibromyalgia. Headache 2015;55:860–5. 10.1111/head.12590 [DOI] [PubMed] [Google Scholar]
- 8. Centonze V, Bassi A, Cassiano MA, et al. Migraine, daily chronic headache and fibromyalgia in the same patient: an evolutive "continuum" of non organic chronic pain? About 100 clinical cases. Neurol Sci 2004;25:s291–2. 10.1007/s10072-004-0314-4 [DOI] [PubMed] [Google Scholar]
- 9. Ifergane G, Buskila D, Simiseshvely N, et al. Prevalence of fibromyalgia syndrome in migraine patients. Cephalalgia 2006;26:451–6. 10.1111/j.1468-2982.2005.01060.x [DOI] [PubMed] [Google Scholar]
- 10. Peres MF, Zukerman E, Young WB, et al. Fatigue in chronic migraine patients. Cephalalgia 2002;22:720–4. 10.1046/j.1468-2982.2002.00426.x [DOI] [PubMed] [Google Scholar]
- 11. Whealy M, Nanda S, Vincent A, et al. Fibromyalgia in migraine: a retrospective cohort study. J Headache Pain 2018;19:61 10.1186/s10194-018-0892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Tommaso M, Sciruicchio V, Delussi M, et al. Symptoms of central sensitization and comorbidity for juvenile fibromyalgia in childhood migraine: an observational study in a tertiary headache center. J Headache Pain 2017;18:59 10.1186/s10194-017-0764-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Tommaso M. Migraine and fibromyalgia. J Headache Pain 2015;16(Suppl 1):A45 10.1186/1129-2377-16-S1-A45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. 10.1177/0333102413485658 [DOI] [PubMed] [Google Scholar]
- 15. Dodick DW. Clinical practice. Chronic daily headache. N Engl J Med 2006;354:158–65. 10.1056/NEJMcp042897 [DOI] [PubMed] [Google Scholar]
- 16. Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013;154:S44–53. 10.1016/j.pain.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 17. Lolignier S, Eijkelkamp N, Wood JN. Mechanical allodynia. Pflügers Archiv 2015;467:133–9. 10.1007/s00424-014-1532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Staud R. Peripheral pain mechanisms in chronic widespread pain. Best Pract Res Clin Rheumatol 2011;25:155–64. 10.1016/j.berh.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain 2001;89:107–10. 10.1016/S0304-3959(00)00478-4 [DOI] [PubMed] [Google Scholar]
- 20. Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol 2017;264:2031–9. 10.1007/s00415-017-8434-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol 2007;26:465–73. 10.1007/s10067-006-0433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55. 10.1001/jama.2014.3266 [DOI] [PubMed] [Google Scholar]
- 23. Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain 2003;126:1801–13. 10.1093/brain/awg190 [DOI] [PubMed] [Google Scholar]
- 24. Marcus DA, Bernstein C, Rudy TE. Fibromyalgia and headache: an epidemiological study supporting migraine as part of the fibromyalgia syndrome. Clin Rheumatol 2005;24:595–601. 10.1007/s10067-005-1121-x [DOI] [PubMed] [Google Scholar]
- 25. Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol 2011;25:141–54. 10.1016/j.berh.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tana C, Santilli F, Martelletti P, et al. Correlation between migraine severity and cholesterol levels. Pain Pract 2015;15:662–70. 10.1111/papr.12229 [DOI] [PubMed] [Google Scholar]
- 27. González-Roldán AM, Bomba IC, Diesch E, et al. Controllability and hippocampal activation during pain expectation in fibromyalgia syndrome. Biol Psychol 2016;121:39–48. 10.1016/j.biopsycho.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 28. Santos DM, Lage LV, Jabur EK, et al. The influence of depression on personality traits in patients with fibromyalgia: a case-control study. Clin Exp Rheumatol 2017;35(3):13–19. [PubMed] [Google Scholar]
- 29. Amouroux R, Rousseau-Salvador C. [Anxiety and depression in children and adolescents with migraine: a review of the literature]. Encephale 2008;34:504–10. 10.1016/j.encep.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 30. Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am 2001;85:911–41. 10.1016/S0025-7125(05)70351-5 [DOI] [PubMed] [Google Scholar]
- 31. Panconesi A. Serotonin and migraine: a reconsideration of the central theory. J Headache Pain 2008;9:267–76. 10.1007/s10194-008-0058-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Becker S, Schweinhardt P. Dysfunctional neurotransmitter systems in fibromyalgia, their role in central stress circuitry and pharmacological actions on these systems. Pain Res Treat 2012;2012:1–10. 10.1155/2012/741746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McBeth J, Lacey RJ, Wilkie R. Predictors of new-onset widespread pain in older adults: results from a population-based prospective cohort study in the UK. Arthritis & Rheumatology 2014;66:757–67. 10.1002/art.38284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choy EH. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol 2015;11:513–20. 10.1038/nrrheum.2015.56 [DOI] [PubMed] [Google Scholar]
- 35. Moldofsky H. Sleep and pain. Sleep Med Rev 2001;5:385–96. 10.1053/smrv.2001.0179 [DOI] [PubMed] [Google Scholar]
- 36. Chen X, D’Souza R, Hong ST. The role of gut microbiota in the gut-brain axis: current challenges and perspectives. Protein Cell 2013;4:403–14. 10.1007/s13238-013-3017-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 2011;23:187–92. 10.1111/j.1365-2982.2010.01664.x [DOI] [PubMed] [Google Scholar]
- 38. Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 2008;37:339–52. 10.1016/j.semarthrit.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 39. Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol 2012;8:89–99. 10.3988/jcn.2012.8.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009;146:41–6. 10.1016/j.pain.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goebel A, Buhner S, Schedel R, et al. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology 2008;47:1223–7. 10.1093/rheumatology/ken140 [DOI] [PubMed] [Google Scholar]
- 42. van Hemert S, Breedveld AC, Rovers JM, et al. Migraine associated with gastrointestinal disorders: review of the literature and clinical implications. Front Neurol 2014;5:241 10.3389/fneur.2014.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28:203–9. [PMC free article] [PubMed] [Google Scholar]
- 44. Berstad A, Valeur J. Gut gateway to generalized pain. Scand J Pain 2016;13:164–5. 10.1016/j.sjpain.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 45. Mu C, Yang Y, Zhu W. Gut microbiota: the brain peacekeeper. Front Microbiol 2016;7:345 10.3389/fmicb.2016.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dai YJ, Wang HY, Wang XJ, et al. Potential beneficial effects of probiotics on human migraine headache: a literature review. Pain Physician 2017;20:251–5. [PubMed] [Google Scholar]
- 47. Saberi A, Hatamian HR, Kazemnejad E, et al. Hyperlipidemia in migraine: Is it more frequent in migraineurs? Iran J Neurol 2011;10:46–50. [PMC free article] [PubMed] [Google Scholar]
- 48. Salmasi M, Amini L, Javanmard SH, et al. Metabolic syndrome in migraine headache: a case-control study. J Res Med Sci 2014;19:13–17. [PMC free article] [PubMed] [Google Scholar]
- 49. Koppen H, van Veldhoven PL. Migraineurs with exercise-triggered attacks have a distinct migraine. J Headache Pain 2013;14:99 10.1186/1129-2377-14-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neusüss K, Neumann B, Steinhoff BJ, et al. Physical activity and fitness in patients with headache disorders. Int J Sports Med 1997;18:607–11. 10.1055/s-2007-972689 [DOI] [PubMed] [Google Scholar]
- 51. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev 2001;21:33–61. 10.1016/S0272-7358(99)00032-X [DOI] [PubMed] [Google Scholar]
- 52. Valença MM, Medeiros FL, Martins HA, et al. Neuroendocrine dysfunction in fibromyalgia and migraine. Curr Pain Headache Rep 2009;13:358–64. 10.1007/s11916-009-0058-1 [DOI] [PubMed] [Google Scholar]
- 53. Assarzadegan F, Asgarzadeh S, Hatamabadi HR, et al. Serum concentration of magnesium as an independent risk factor in migraine attacks: a matched case-control study and review of the literature. Int Clin Psychopharmacol 2016;31:287–92. 10.1097/YIC.0000000000000130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



