Abstract
The myelin-associated protein Nogo-A has received more research attention than any other inhibitor of axonal regeneration in the injured central nervous system (CNS). Circumvention of its inhibitory effect, by using antibodies specific to Nogo-A, has been shown to promote axonal regrowth. Studies in our laboratory have demonstrated that active or passive immunization of CNS-injured rats or mice with myelin-associated peptides induces a T-cell-mediated protective autoimmune response, which promotes recovery by reducing posttraumatic degeneration. Here, we show that neuronal degeneration after incomplete spinal-cord contusion in rats was substantially reduced, and hence recovery was significantly promoted, by posttraumatic immunization with p472, a peptide derived from Nogo-A. The observed effect seemed to be mediated by T cells and could be reproduced by passive transfer of a T cell line directed against the Nogo-A peptide. Thus, it seems that after incomplete spinal-cord injury, immunization with a variety of myelin-associated peptides, including those derived from Nogo-A, can be used to evoke a T cell-mediated response that promotes recovery. The choice of peptide(s) for clinical treatment of spinal-cord injuries should be based on safety considerations; in particular, the likelihood that the chosen peptide will not cause an autoimmune disease or interfere with essential functions of this peptide or other proteins. From a therapeutic point of view, the fact that the active cellular agents are T cells rather than antibodies is an advantage, as T cell production commences within the time window required for a protective effect after spinal-cord injury, whereas antibody production takes longer.
Substantial progress has been made in understanding why the injured central nervous system (CNS) fails to regenerate and why the damage caused by the primary insult is propagated rapidly and irreversibly beyond the initial lesion. In the search for answers to these questions, considerable attention has been directed to myelin-associated inhibitors (1). One such inhibitor is Nogo-A (2–6), which was recently cloned and characterized (7). Monoclonal antibodies to Nogo-A were reported to facilitate axonal regrowth and some functional recovery when injected into the cerebrospinal fluid of rats after partial transection of their spinal cords (8, 9).
Studies of animal models of partial and complete CNS lesions in our laboratory have shown that after CNS injury, the white blood cells of the immune system (macrophages and T lymphocytes) can facilitate processes of protection, repair, and regeneration (10−13). Axonal injury to the spinal cord evidently activates an anti-self (autoimmune) response mediated by T cells directed against myelin-associated antigens. This response was found to be beneficial for the injured nerve, as it can reduce the posttraumatic degeneration, an otherwise inevitable consequence of the injury (14). The ability to exhibit a protective autoimmune T cell response is genetically controlled and is inversely related to the inherent resistance of the individual or strain to the development of an autoimmune disease, such as experimental autoimmune encephalomyelitis (EAE), when challenged with myelin-associated antigens (15). Moreover, the neuroprotective T cell response is amenable to boosting by passive or active immunization. Passive transfer of T cells reactive to myelin basic protein (MBP) significantly improves recovery from spinal-cord contusion in rats (10, 16).
In seeking a way to convert the experimental immunization into an effective posttraumatic therapy, we have been testing peptides that are “safe” (i.e., do not induce autoimmune disease) and are derived from or crossreact with self-proteins (17−19). We found that vaccination with MBP or a spinal-cord homogenate containing a variety of myelin proteins promotes morphological and functional recovery of the injured spinal cords of both EAE-resistant and EAE-susceptible rats (17). This finding raised another question: would vaccination with myelin antigens that inhibit axonal outgrowth be similarly (or more) effective, or is the effect unique to a specific type of myelin protein?
In this study, we examined whether posttraumatic immunization with a peptide derived from the myelin-associated growth-inhibitory protein Nogo-A (p472) can promote recovery from spinal-cord injury. If so, we were interested in finding out whether such peptides can reduce the spread of damage without risk of inducing an autoimmune disease, and whether the effect is mediated by antibodies or by T cells.
Materials and Methods
Animals.
Inbred adult Sprague–Dawley (SPD) and Lewis rats (10–12 weeks old, 200–250 g) were supplied by the Animal Breeding Center of The Weizmann Institute of Science. The rats were matched for age and weight in each experiment and housed in a light- and temperature-controlled room.
Antigens.
MBP and ovalbumin (OVA) were purchased from Sigma. Altered (nonencephalitogenic) MBP peptide (Ala-91) was derived from an encephalitogenic peptide, amino acids 87–99 of MBP, by replacing the lysine residue 91 with alanine (VHFFANIVTPRTP) (20). Both Ala-91 and the Nogo-A-derived peptide p472 (SYDSIKLEPENPPPYEEA) (7) were synthesized at the Weizmann Institute of Science (Rehovot, Israel). Antigens were emulsified in equal volumes of complete Freund's adjuvant (CFA; Difco) supplemented with Mycobacterium tuberculosis (1 mg/ml; Difco).
Spinal-Cord Contusion or Transection.
Rats were anesthetized by i.p. injection of Rompun (xylazine, 10 mg/kg; Vitamed, Benyamina, Israel) and Vetalar (ketamine, 50 mg/kg; Fort Dodge Laboratories, Overland Park, KS), and their spinal cords were exposed by laminectomy at the level of T9. One hour after induction of anesthesia, a 10-g rod was dropped onto the laminectomized cord from a height of 25 mm or 50 mm with the New York University (N.Y.U.) Impactor, a device shown to inflict a well calibrated contusive injury of the spinal cord (21). The spinal cords of another group of rats were transected completely, as described (13).
Active Immunization.
Rats were immunized s.c. in the base of the tail on a random basis with 150 μg of p472 or Ala-91 or were injected with PBS, each emulsified in an equal volume of CFA containing 1 mg/ml bacteria. Seven days later, each rat was injected s.c. with a booster of 100 μg of the same antigen emulsified in incomplete Freund's adjuvant (Difco). The first immunization was given not later than 1 h after contusion. Control rats were sham-operated (laminectomized but not contused), immunized, and examined daily for symptoms of EAE, which were scored on a scale of 1 to 5 (22).
SPD T Cell Line Specific to p472.
T cell line was generated from draining lymph-node cells obtained from immunized rats as, described (23). Cells were grown in propagation medium for 4–10 days before being restimulated with their antigen (10 μg/ml) in the presence of irradiated (2,000 rad) thymus cells (1 × 107 cells per ml) in proliferation medium. The T cell line was expanded by repeated stimulation and propagation.
Animal Care.
In contused rats, bladder expression was assisted by massage at least twice a day (and three times a day during the first 48 h after injury) until the end of the second week, by which time SPD rats had recovered automatic voidance. Lewis rats required this treatment throughout the experiment. All rats were carefully monitored for evidence of urinary tract infection or other signs of systemic disease. During the first week after contusion and in any case of hematuria thereafter, they received a course of sulfamethoxazole (400 mg/ml) and trimethoprim (8 mg/ml) (Resprim; Teva Pharmaceutical Industries, Petah Tikva, Israel), administered orally by syringe (0.3 ml of solution per day). Daily inspections included examination of the laminectomy site for evidence of infection and assessment of the hind limbs for signs of autophagia or pressure.
Proliferation Assay.
Female SPD rats were immunized with p472. Ten days later, lymph nodes were excised, dissociated, and the washed lymphocytes (2 × 106 cells per ml) were cultured in 0.2 ml of proliferation medium (10). The cells were activated for 72 h at 37 C°, 90% relative humidity, and 7% CO2 in the presence of irradiated thymocytes (2,000 rad, 2 × 106 cells per ml), together with p472 (10 μg/ml), Ala-91 in CFA (10 μg/ml), the nonself antigen OVA (10 μg/ml), Con A (1.25 μg/ml), or no antigen. The proliferative response was determined by measuring the incorporation of 3[H]thymidine (1 μCi per well; 1 Ci = 37 GBq), which was added for the last 16 h of a 72-h culture.
ELISA for Antibody Detection.
For this assay, 96-well flat-bottomed microtiter plates treated with glutaraldehyde (0.2%) were coated with 100 μl of p472 or Ala-91 (10 μg/ml) in PBS, by overnight incubation at 4°C. The plates then were blocked with PBS containing 1% BSA (Sigma) for 2 h at 37°C and washed with PBS containing Tween 20 (0.5%). Duplicate samples of the sera to be tested were added to the plates at different dilutions in PBS containing 1% BSA (1:500, 1:1000, 1:5,000, or 1:10,000). The plates were incubated for 2 h at 37°C, then washed five times in PBS containing Tween 20 (0.5%). Peroxidase-conjugated AffiniPure goat anti-mouse IgG (Jackson ImmunoResearch) or alkaline phosphatase goat anti-mouse IgM (Jackson ImmunoResearch) diluted 1:2,500 in PBS and 1% BSA were added, and the plates were incubated for 2 h at 37°C. They were then washed five times in PBS containing Tween 20 (0.5%) and alkaline phosphatase substrate solution (100 μl per well) was added. The reaction was stopped by the addition of 1 M phosphoric acid (100 μl per well). The plates were read with an ELISA plate reader (Anthos Labec Instruments, Salzburg, Austria) OD at 405 nm (in wells with anti-IgM antibody) or OD at 450 nm (in wells with anti-IgG antibody). Background OD was subtracted from the test OD values.
Assessment of Recovery from Spinal-Cord Contusion.
Behavioral recovery was scored in an open field with the Basso, Beattie, and Bresnahan locomotor rating scale (BBB), where a score of 0 registers complete paralysis and a score of 21 registers complete mobility (24). Blind scoring ensured that observers were not aware of the treatment received by each rat, as described (17). Before each evaluation, the rats were examined carefully for perineal infection, wounds in the hind limbs, and tail and foot autophagia.
Retrograde Labeling of Rubrospinal Neurons.
Four months after spinal-cord contusion, rats were reanesthetized, and the dye rhodamine dextran amine (Fluoro-ruby; Molecular Probes) was applied below the site of contusion at T12. After 5 days, the rats again were anesthetized, and their brains were excised, processed, and cryosectioned. Sections taken through the red nucleus were inspected and analyzed qualitatively and quantitatively by fluorescence and confocal microscopy.
Partial Crush Injury of the Rat Optic Nerve.
The optic nerve was subjected to crush injury, as described (14).
Measurement of Secondary Degeneration of the Rat Optic Nerve by Retrograde Labeling of Retinal Ganglion Cells (RGCs).
Secondary degeneration of the optic nerve axons and their attached RGCs was measured after postinjury application of the fluorescent lipophilic dye 4-[4-(didecylamino)styryl]-N-methylpyridinium iodide (4-Di-10-Asp; Molecular Probes) distally to the lesion site 2 weeks after crush injury (25).
Results
Posttraumatic Vaccination with a Nogo-A-Derived Peptide Reduces Degeneration After Incomplete Spinal-Cord Partial Injury.
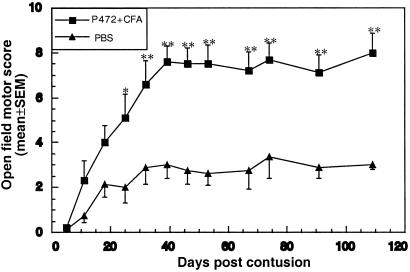
We synthesized an 18-aa peptide (SYDSIKLEPENPPPYEEA) derived from Nogo-A (p472) and used it to vaccinate rats immediately after partial injury (contusion) of the spinal cord. The choice of peptide was based on previous observations that antibodies directed against p472 were as effective as the original IN-1 antibodies in blocking the myelin-inhibitory effect on neuronal growth, suggesting that this peptide might be the physiologically significant epitope within the Nogo-A protein (7, 26, 27). Rats were injected with p472 or PBS emulsified in CFA. Fig. 1 shows the recovery of spinally contused female and male SPD rats after immunization with p472. Female rats immunized with p472 after injury recovered significantly better than females that were untreated or injected with PBS in CFA. The effect was discernible less than 2 weeks after immunization and was stronger after 4 weeks (Fig. 1). The highest behavioral score (mean ± SEM) obtained in the p472-vaccinated group (n = 5) was 8.0 ± 0.8, compared with 3.4 ± 0.9 in the untreated group (n = 5). Control rats injected with PBS in CFA scored 5.5 ± 0.5, which is higher than the untreated rats but still significantly lower than the p472-immunized rats. The above findings confirm that the endogenous immune response to a CNS insult can be boosted with myelin antigens and that the boosting has a beneficial effect (14). In all subsequent experiments, rats immunized with PBS in CFA were included as controls to verify that the effect induced by the peptide-specific immunization was more pronounced than the CFA-induced boosting of the endogenous response. In the males, improvement in recovery after p472 immunization also was significant, although less substantial than in the females: behavioral scores were 5.1 ± 0.6 with p472 in CFA and 3.6 ± 0.2 with PBS in CFA (Fig. 2A). The better recovery in the female rats is in line with a recent finding from our laboratory (17). These two experiments raised a key question: how does the observed effect of the Nogo-A-derived peptide in reducing posttraumatic immunization compare with that of MBP-derived peptides?
Figure 1.
Active posttraumatic vaccination with the Nogo-derived peptide p472 reduces paralysis after incomplete spinal-cord injury in SPD rats. Female SPD rats were subjected to spinal contusion at T8 inflicted by the N.Y.U. Impactor with a 10-g weight dropped from a height of 50 mm. Immediately after contusion, rats in one group (n = 6) were vaccinated with p472 emulsified in CFA and rats in another group (n = 6) were injected with PBS only. Motor behavior was assessed weekly in an open field by observers blinded to the treatment received by the rat. Recovery was best in rats vaccinated with p472. Results are mean values of the motor score ± SEM. Significance of the difference between the groups was determined by using a two-tailed Student's t test (*, P < 0.05; **, P < 0.01).
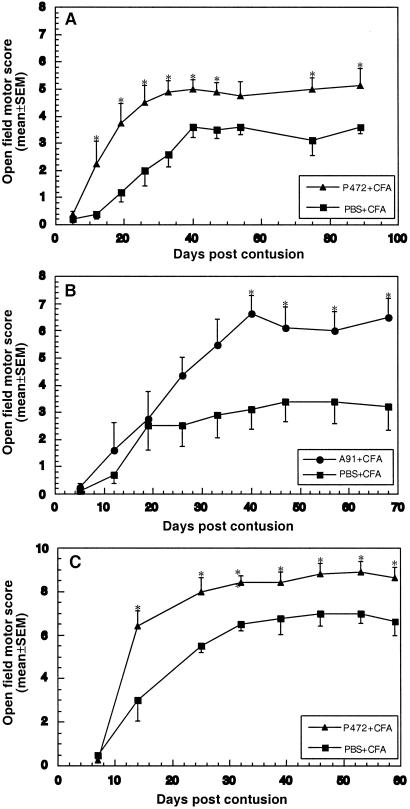
Figure 2.
Comparison between vaccination with the Nogo-derived peptide p472 and the MBP-derived altered peptide Ala-91 in male SPD rats. (A) Male SPD rats were subjected to spinal contusion as described for Fig. 1. Immediately after the contusion, the animals were randomly divided into two groups (n = 6 in each group). Rats in the first group were immunized with p472 in CFA, and rats in the second group were injected with PBS in CFA. Motor behavior was assessed as described for Fig. 1. Results are mean values of the motor score ± SEM. (B) Male SPD rats were injured and grouped as in A and were immunized with Ala-91 in CFA or injected with PBS in CFA. Motor recovery in the Ala-91-treated group, assessed as in A, was at least as good as with p472 treatment. Results are mean values of the motor score ± SEM. (C) Assessment of recovery from a milder spinal contusion inflicted by dropping the 10-g weight from a height of 25 mm (*, P < 0.05; **, P < 0.01).
We recently reported that active posttraumatic vaccination of rats with an MBP-derived peptide (p87−99; Ala-91) altered by one amino acid replacement (Lys-91 by Ala) leads to significant neuroprotection (17). Here, we compared the effects of Ala-91 and p472 (both emulsified in CFA). The results obtained with the peptide Ala-91 were better (Fig. 2B). The highest locomotor score obtained was 6.6 ± 0.6 in rats immunized with Ala-91 in CFA compared with 3.4 ± 0.7 in rats injected with PBS in CFA. These results, and the small differences between control values in the two experiments (Fig. 2 A vs. B), indicate that both peptides are effective but do not permit definite conclusions about differences in their efficacy.
In a second set of experiments, SPD males with a milder contusive injury (caused by a weight drop from a height of 25 mm) were vaccinated with p472. As before, the effect was evident from day 14 on and was then significant at all times of assessment, according to a two-tailed Student's t test (Fig. 2C). The highest behavioral score in these rats was 8.9 ± 0.5, compared with 7.0 ± 0.45 after vaccination with PBS in CFA.
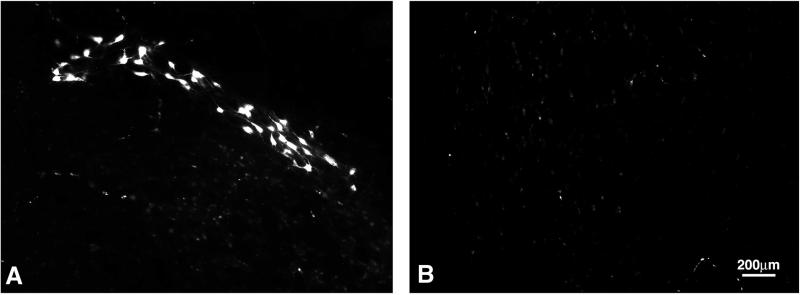
The behavioral recovery scores of p472-immunized rats after a weight drop from 25 mm reached plateau values that exceeded the threshold values correlated with sparing of the rubrospinal tracts needed for weight support. To confirm that the better recovery seen in these rats is related to a better survival of the rubrospinal tracts, we compared the integrity of the rubrospinal tracts in p472-immunized rats and rats injected with CFA in PBS (Fig. 2C). In rats with a BBB score around 8 (indicative of weight support), retrograde labeling gave an “all-or-none” indication of rubrospinal tract survival (28−30). The examined control rats (average BBB score = 6.5 ± 0.5) showed almost no labeled red nuclei. In the examined p472-immunized rats (BBB score = 9.4 ± 0.3; n = 3), 142 ± 46 red nuclei were labeled (Fig. 3).
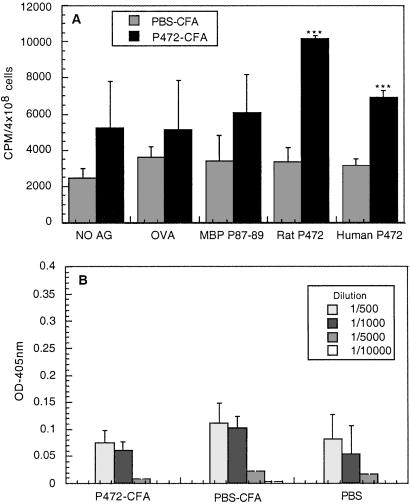
Figure 3.
Presence of proliferating T cells specific to p472 and absence of anti-p472 antibodies 1 week after vaccination with p472. (A) The proliferative response of splenocytes prepared from rats injected with p472 in CFA or with PBS in CFA to their specific antigen was compared with their proliferative response to the nonspecific antigen (OVA) in CFA or in the absence of antigen. Results are expressed as the mean cpm values of quadruplicate samples obtained from three different animals ± SEM. (B) Antibodies in the treated rats were assayed by ELISA. The amount of antibodies detected in the rats immunized with p472 in CFA was not significantly greater than in those injected with PBS in CFA. Results are expressed by mean values (expressed in arbitrary units) ± SEM obtained from three rats, each tested in triplicate at each of the indicated dilutions.
To determine whether the immunity directed against Nogo-A (yielding the improved recovery observed here) was of cellular or humoral origin, we examined the immunized rats for specific anti-p472 T cells in lymph nodes and for antibodies in the serum. T cell proliferation assay revealed proliferating T cells in rats immunized with p472 in CFA when tested with the p472-derived peptide. The same lymphocytes were incubated with MBP-derived peptide (87−99) or OVA as controls. No p472-specific T cells were found in control rats injected with PBS in CFA (Fig. 4A). Antibody assays revealed no significant differences in IgM antibody titer between the rats injected with p472 in CFA and those injected with PBS in CFA (Fig. 4B).
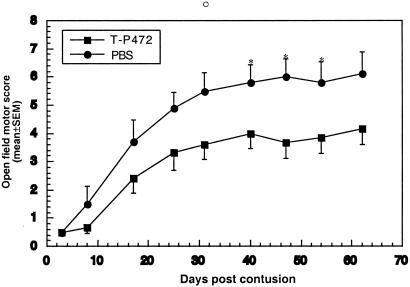
Figure 4.
Passive transfer of p472-specific T cells into spinally contused rats promotes recovery. Male SPD rats were subjected to spinal contusion as described for Fig. 1. Immediately after contusion, the rats were divided into two groups (n = 5 in each group). Rats in one group were injected intraperitoneally with p472-specific T cells (1 × 107 cells) in PBS, and rats in other groups were injected with PBS only. Motor behavior was assessed in an open field by observers blinded to the treatment received. Results are mean values of the motor score ± SEM. Significance of the differences between the groups at each time point was determined by using a two-tailed Student's t test (*, P < 0.05).
None of the immunized rats developed symptoms of EAE. Because SPD rats are resistant to EAE induction, we examined whether p472 immunization of the EAE-susceptible Lewis rats would induce the disease. No clear signs of EAE were observed. It is possible, however, that infiltrating Nogo-specific T cells caused silent clinical changes in the CNS, as reported with other myelin-derived autoantigens, for example, myelin-associated glycoprotein (31, 32).
T Cells Directed Against Nogo-A-Derived Peptide Are Neuroprotective When Passively Transferred into SPD Rats.
The above results suggest that the neuroprotection observed after active vaccination with p472 is mediated by T cells specific to that peptide. To verify this suggestion, we prepared a T cell line specific to p472. Passive transfer of anti-p472 T cells after spinal contusion in rats had a protective effect, which became apparent as early as 8 days after the injury; the highest behavioral score was 6 ± 0.6 for rats treated with the T cells and 4 ± 0.5 for untreated controls (Fig. 5). The efficacy of different routes of immunization (active and passive; compare Fig. 2A and Fig. 5) should be compared with caution because of the difference in their numbers of available T cells at the lesion site during the therapeutic time window. Also, the efficacy of passive T-cell transfer is critically dependent on matching between the MHC-II characteristics of donor and recipient, meaning that inbred strains and homologous cells must be used. The SPD strain, although not inbred, is stably heterogeneous. It derives from a hybrid of Hooded males and Wistar females, with subsequent lines developed into a stable stock. The rats used in our studies were obtained from Harlan–Sprague–Dawley, and all were descended from the same original stock. We created a primary T cell line by in vitro propagation of lymphocytes from immunized SPD rats by using antigen-presenting cells (APCs) from other SPD rats. Thus, despite the heterogeneity, the rats evidently share enough homology in their expressed MHC-II for this purpose (for the in vitro propagation in this strain, we always used twice as many thymocytes as APCs than when working with inbred strains). Because of the heterogeneity, the passively transferred T cells might be less effective than in inbred rats using homologous cells; nevertheless, our results confirmed that the protection is mediated by T cells.
Figure 5.
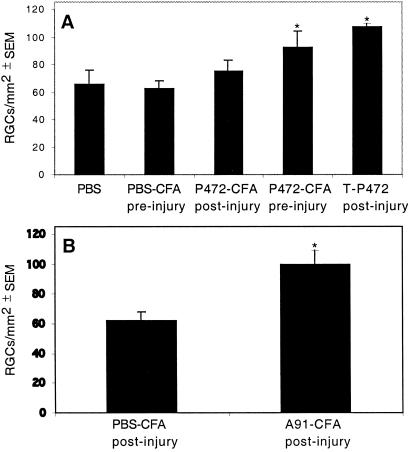
Immunization with p472 reduces RGC loss after partial crush injury of the rat optic nerve. (A) Rats were subjected to a calibrated crush injury of the optic nerve immediately before or 5 days after injection with p472 in CFA, PBS in CFA, or T cells directed to p472. (B) Rats were immunized with Ala-91 in CFA or PBS in CFA immediately after optic-nerve crush. In both A and B, the optic nerves were re-exposed 2 weeks later, and a dye was injected distally to the primary lesion. After 5 days, the retinas were excised and whole-mounted, and the RGCs were counted by observers blinded to the treatment received by the rats. Results are expressed as the mean numbers of RGCs per mm2 ± SEM.
Immunization with Nogo-A Reduces Loss of RGCs After Optic-Nerve Injury.
The posttraumatic loss of RGCs can be significantly reduced by passive transfer of T cells directed against myelin antigens or by active immunization with these antigens (12, 18, 19, 33). To determine whether active immunization is as effective with p472 as with other myelin antigens, we immunized SPD rats with p472 emulsified in CFA. Control injured rats were injected with PBS emulsified in CFA. When the rats were immunized before the insult, the number of surviving RGCs, determined by retrograde labeling 2 weeks after the insult, was significantly larger in the p472-immunized rats than in the controls (Fig. 6A). Passive transfer of p472-specific T cells immediately after the insult had a beneficial effect on recovery, like that seen in our studies with T cells that are specific to myelin peptides or crossreactive with Cop-1 (10, 18). Active immunization with Ala-91 immediately after optic-nerve injury (Fig. 6B), like passive transfer of the T cells specific to p472 (Fig. 6A), yielded a better RGC survival rate than that seen in the control (Fig. 6 A and B). This finding suggests that that both p472 and Ala-91 are potentially neuroprotective antigens, and that the possible difference in their immunogenicity is manifested by how promptly they induce a response. No such difference is observed when the T cells are passively transferred.
Figure 6.
Retrograde labeling of cell bodies in the red nucleus. Three months after spinal contusion, rats from each group were reanesthetized, and the dye rhodamine dextran amine (Fluoro-ruby) was applied below the site of contusion. Five days later, the rats were killed, and their brains were excised, processed, and cryosectioned. Sections taken through the red nucleus were inspected and analyzed by fluorescence confocal microscopy. The photographs are of p472-immunized rats and of the control rats shown in Fig 2C.
Discussion
Recovery from spinal-cord injury depends on the severity of the insult (location of injury and the number of spared neurons), the amount of secondary degeneration, the ability to regenerate, and the ability to activate local mechanisms of plasticity. Whereas the question of regeneration is still largely unsolved, much more is now known about neuroprotection in general, especially in the context of the spinal cord. In particular, it is now clear why neuronal degeneration continues to progress after the primary insult and how the damage is propagated.
The design of therapeutic strategies has been hampered by some apparent contradictions in the findings of research, especially concerning the question of whether the recovery process is advanced or impeded by inflammation (34, 35). Anti-inflammatory drugs were reported to be beneficial after spinal-cord injury, leading to the tentative conclusion that an inflammatory reaction is bad for recovery (36). However, recent studies (14, 37, 38) indicate that the adaptive immune response, which is spontaneously evoked by a spinal-cord injury, is beneficial for recovery in some strains, depending on their inherent susceptibility to EAE (15). The T cell-dependent protective autoimmunity was found to be characterized also by sexual dimorphism, with female rats recovering better than males after spinal-cord contusion (E.H. et al., unpublished work). The injury-evoked, T cell-mediated protective response was found to be boosted in EAE-resistant strains (or induced in EAE-susceptible strains) by passive transfer of anti-MBP T cells or by active vaccination with certain myelin-associated self-antigens (17).
In this study, we show that a peptide derived from Nogo-A, a myelin component known to inhibit posttraumatic regeneration, can activate the immune system in a beneficial way. This peptide had a neuroprotective effect in the same model and with the same therapeutic time window as that shown by us to accommodate a T cell-based therapy (10). Thus, after partial crush injury of the rat optic nerve or spinal cord, prompt vaccination (within 1 h after the injury) with Nogo-A-derived peptide emulsified in CFA evoked a T cell-mediated protective autoimmunity. Within this time window, we detected p472-specific T cells in the immunized injured rats, but no anti-p472 antibodies. Similar results were obtained after passive transfer of T cells specific to the Nogo-A-derived peptide. Therefore, we considered it important to determine whether a peptide derived from Nogo-A has advantages over other myelin-associated peptides as a potential vaccine. Based on the present comparison with the effect of an MBP-derived peptide, the answer is that it does not.
Recent studies reveal that, for protective autoimmunity, both autoimmune T cells and regulatory T cells are essential constituents of this network, but neither of them on its own suffices to bring about neuroprotection (39). When considering the use of a particular myelin-associated peptide for therapy, it is important to ascertain that the T cell response it elicits falls within the therapeutic window, and that it will confer the desired benefit without risking the development of an autoimmune disease. Also, such peptides should not elicit any immune activity that interferes with their essential physiological activities, if any. In susceptible rat strains, a modified encephalitogenic peptide was found to be beneficial (17). This study demonstrates that in EAE-susceptible rat strains, Nogo-A-derived peptides were not encephalitogenic. However, we cannot rule out the possibility that vaccination with Nogo-A-derived peptides might evoke, in addition to the observed T cell response, a delayed antibody response. Although apparently not needed for neuroprotection, these antibodies might interfere with the essential activities of Nogo-A in maintaining the integrity of the adult CNS. Such interference might be a potential side effect of the use of anti-Nogo-A antibodies for regeneration (26). In view of this possible drawback, it might be preferable to choose a myelin-associated peptide that does not play a role in neuronal plasticity. Alternatively, it might be possible to exploit the neuroprotective property of Nogo-derived peptides in a way that avoids the appearance of antibodies by using passive vaccination with anti-Nogo T cells rather than active immunization with the peptides themselves.
T cell-mediated active or passive vaccination is a promising treatment for posttraumatic treatment of the injured spinal cord, because it is based on harnessing the immune system. Because the active players are immune cells and not antibodies, this type of therapy is likely to provide multifactorial protection.
Acknowledgments
We thank S. Smith for editing the manuscript. M.S. holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology. The work was supported in part by Proneuron Biotechnologies, Ltd., Israel.
Abbreviations
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- SPD
Sprague–Dawley
- OVA
ovalbumin
- CFA
complete Freund's adjuvant
- RGC
retinal ganglion cells
References
- 1.Schwab M E, Bartholdi D. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Bandtlow C E, Schwab M E. Glia. 2000;29:175–181. doi: 10.1002/(sici)1098-1136(20000115)29:2<175::aid-glia11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Brittis P A, Flanagan J G. Neuron. 2001;30:11–14. doi: 10.1016/s0896-6273(01)00258-6. [DOI] [PubMed] [Google Scholar]
- 4.Fournier A E, Strittmatter S M. Curr Opin Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 5.Huber A B, Schwab M E. Biol Chem Hoppe-Seyler. 2000;381:407–419. [Google Scholar]
- 6.Qiu J, Cai D, Filbin M T. Glia. 2000;29:166–174. [PubMed] [Google Scholar]
- 7.Chen M S, Huber A B, van der Haar M E, Frank M, Schnell L, Spillmann A A, Christ F, Schwab M E. Nature (London) 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 8.Bregman B S, Kunkel-Bagden E, Schnell L, Dai H N, Gao D, Schwab M E. Nature (London) 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 9.Brosamle C, Huber A B, Fiedler M, Skerra A, Schwab M E. J Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, et al. J Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarov-Spiegler O, Solomon A S, Zeev-Brann A B, Hirschberg D L, Lavie V, Schwartz M. FASEB J. 1996;10:1296–1302. doi: 10.1096/fasebj.10.11.8836043. [DOI] [PubMed] [Google Scholar]
- 12.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen I R, Schwartz M. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 13.Rapalino O, Lazarov-Spiegler O, Agranov E, Velan G J, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, et al. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 14.Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen I R, Weiner H, Schwartz M. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauben E, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen I R, Schwartz M. Lancet. 2000;355:286–287. doi: 10.1016/s0140-6736(99)05140-5. [DOI] [PubMed] [Google Scholar]
- 17.Hauben E, Agranov E, Gothilf A, Nevo U, Cohen A, Smirnov I, Steinman L, Schwartz M. J Clin Invest. 2001;108:591–599. doi: 10.1172/JCI12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kipnis J, Yoles E, Porat Z, Cohen A, Mor F, Sela M, Cohen I R, Schwartz M. Proc Natl Acad Sci USA. 2000;97:7446–7451. doi: 10.1073/pnas.97.13.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schori H, Kipnis J, Yoles E, WoldeMussie E, Ruiz G, Wheeler L A, Schwartz M. Proc Natl Acad Sci USA. 2001;98:3398–3403. doi: 10.1073/pnas.041609498. . (First Published March 6, 2001; 10.1073/pnas.041609498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaur A, Boehme S A, Chalmers D, Crowe P D, Pahuja A, Ling N, Brocke S, Steinman L, Conlon P J. J Neuroimmunol. 1997;74:149–158. doi: 10.1016/s0165-5728(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 21.Young W. Science. 1996;273:451. doi: 10.1126/science.273.5274.451. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Nun A, Cohen I R. J Immunol. 1982;129:303–308. [PubMed] [Google Scholar]
- 23.Gillis S, Ferm M M, Ou W, Smith K A. J Immunol. 1978;120:2027–2032. [PubMed] [Google Scholar]
- 24.Basso D M, Beattie M S, Bresnahan J C. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 25.Yoles E, Schwartz M. Exp Neurol. 1998;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- 26.Raineteau O, Fouad K, Noth P, Thallmair M, Schwab M E. Proc Natl Acad Sci USA. 2001;98:6929–6934. doi: 10.1073/pnas.111165498. . (First Published May 29, 2001; 10.1073/pnas.111165498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merkler D, Metz G A S, Raineteau O, Dietz V, Schwab M E, Fouad K. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehlings M G, Tator C H. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 29.Midha R, Fehlings M G, Tator C H, Saint-Cyr J A, Guha A. Brain Res. 1987;410:299–308. doi: 10.1016/0006-8993(87)90328-3. [DOI] [PubMed] [Google Scholar]
- 30.Theriault E, Tator C H. J Comp Neurol. 1994;342:249–258. doi: 10.1002/cne.903420208. [DOI] [PubMed] [Google Scholar]
- 31.Weerth S, Berger T, Lassmann H, Linington C. J Neuroimmunol. 1999;95:157–164. doi: 10.1016/s0165-5728(99)00004-1. [DOI] [PubMed] [Google Scholar]
- 32.Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu H C, Lassmann H, Wekerle H. Eur J Immunol. 1993;23:1364–1372. doi: 10.1002/eji.1830230627. [DOI] [PubMed] [Google Scholar]
- 33.Fisher J, Yoles E, Levkovitch-Verbin H, Kaye J F, Ben-Nun A, Schwartz M. J Neurosci. 2001;21:136–142. doi: 10.1523/JNEUROSCI.21-01-00136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bethea J R, Nagashima H, Acosta M C, Briceno C, Gomez F, Marcillo A E, Loor K, Green J, Dietrich W D. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 35.Bethea J R. Prog Brain Res. 2000;128:33–42. doi: 10.1016/S0079-6123(00)28005-9. [DOI] [PubMed] [Google Scholar]
- 36.Mautes A E, Weinzierl M R, Donovan F, Noble L J. Phys Ther. 2000;80:673–687. [PubMed] [Google Scholar]
- 37.Serpe C J, Kohm A P, Huppenbauer C B, Sanders V M, Jones K J. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serpe C J, Sanders V M, Jones K J. J Neurosci Res. 2000;62:273–278. doi: 10.1002/1097-4547(20001015)62:2<273::AID-JNR11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz M, Kipnis J. Trends Mol Med. 2001;7:252–258. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]