Abstract
Objective
Inflammatory bowel disease (IBD) is a chronic, autoimmune, gastrointestinal disorder. Canada has one of the highest prevalence and incidence rates of IBD in the world. Diagnosis is challenging due to the similarity of symptoms to functional gastrointestinal disorders. Faecalcalprotectin (FC) is a biomarker for active mucosal inflammation and has proven effective in the diagnosis of IBD. Our study objective was to assess the cost-effectiveness of adding an FC test compared with standard practice (blood test) in primary care among adult patients presenting with gastrointestinal symptoms.
Design
We constructed a decision analytic tree with a 1-year time horizon. The cut-off level of 100 µg/g was used for FC testing. Probabilistic analyses were conducted for the base case and all scenarios.
Setting
Canadian health sector perspective.
Population
A hypothetical cohort of adult patients presenting with gastrointestinal symptoms in the primary care setting.
Interventions
FC test compared with blood test.
Main outcome measures
Costs, quality-adjusted life years (QALYs), incremental cost-effectiveness ratio (ICER) of FC test expressed as cost per QALY gained compared with blood test and time to IBD diagnosis.
Results
FC testing is expected to cost more ($C295.1 vs $C273.9) than standard practice but yield little higher QALY (0.751vs0.750). The ICER of FC test was $C20 323 per QALY. Probabilistic analysis demonstrated that at a willingness-to-pay threshold of $C50 000 per QALY, there was 81.3% probability of FC test being cost-effective. The use of FC test in primary care reduced the time to IBD diagnosis by 40.0 days (95% CI 16.3 to 65.3 days), compared with blood testing alone.
Conclusions
Based on this analysis of short-term outcomes, screening adult patients in primary care using FC test at a cut-off level of 100 µg/g is expected to be cost-effective in Canada.
Keywords: fecal calprotectin, inflammatory bowel disease, cost-effectiveness
Strengths and limitations of this study.
This paper presents a cost-effectiveness analysis (CEA) comparing a faecal calprotectin (FC) test with blood test in diagnosis of inflammatory bowel disease (IBD) in the primary care setting.
This was the first CEA of FC test in the Canadian context and one of few CEAs of FC test in the primary care setting in literature.
We also compared the average time to IBD diagnosis between using FC test and blood test in primary care and estimated the reduced time to IBD diagnosis by using FC test.
The analysis was from the Canadian health sector perspective and did not consider costs (eg, productivity losses) from a societal perspective.
The main limitation was the short-term time horizon of the analysis and thus there is outstanding uncertainty over the long-term impact of FC testing in this setting.
Introduction
Inflammatory bowel disease (IBD), of which the two main subtypes are Crohn’s disease (CD) and ulcerative colitis (UC), is characterised by mucosal inflammation and ulceration of the gastrointestinal tract. During the course of the disease, patients often present with symptoms such as diarrhoea, abdominal pain and fatigue, which significantly impact the quality of life of patients with IBD.1 Canada has one of the highest reported prevalence and incidence rates of IBD in the world.2 The prevalence of IBD in Canada was estimated at 0.67% (129 000 individuals with CD and 104 000 with UC) in 2012, with approximately 10 200 incidents occurring annually.3 The corresponding annual economic costs of IBD were estimated at $C2.8 billion.3
IBD shares similar presenting symptoms with functional gut disorders. One of the most common function gut disorders that is difficult to distinguish from IBD is irritable bowel syndrome (IBS), which affects around 11% of the population in Canada and globally.4 While IBS can be safely managed within primary care setting, the risk of serious complications associated with IBD (such as bowel obstruction and toxic megacolon) necessitates specialist care management. In order to distinguish IBD from functional gut disorders, the conventional diagnostic pathway in primary care includes initial blood tests, such as erythrocyte sedimentation rate (ESR) and C reactive protein (CRP), which are used to determine whether patients should be referred to gastroenterologists for further investigation including imaging studies and/or endoscopy.5 However, these blood tests lack accuracy. They may delay IBD diagnosis in the case of false negatives, and lead to unnecessary endoscopies in the case of false positives.6 7 Due to limited resources, endoscopy is not readily accessible in many areas of Canada and unnecessary endoscopies can have further impacts on healthcare resources and costs.
Recently, the detection of faecal calprotectin (FC), the most extensively studied faecal marker of IBD, has been shown to be an accurate and useful screening tool for identifying patients who need further investigation through endoscopy.6–9 The majority of studies that assessed the accuracy of FC testing to date have been in the secondary care setting.6–8 Based predominantly on secondary care data using the standard cut-off of 50 µg/g, Waugh et al have shown that FC testing is cost-effective for distinguishing between IBD and non-IBD in adults in primary care in the UK.7 10 The National Institute for Health and Care Excellence (NICE) in the UK therefore recommends FC testing as an option to help clinicians distinguish between IBD and non-IBD in adults with recent onset of gastrointestinal symptoms.10 A recent prospective primary care cohort study conducted in the UK demonstrated that FC testing using the cut-off of 100 µg/g accurately distinguishes IBD from functional gut disorder in primary care and reduces secondary care referrals as well as diagnostic healthcare costs.11 More recently, Turvill et al have also demonstrated that repeating FC testing among those with a first FC test ≥100 µg/g in primary care is cost-saving compared with CRP/ESR testing or single FC testing using the standard cut-off of 50 µg/g.12 NICE has subsequently endorsed this repeated testing algorithm, using the higher 100 µg/g cut-off, within a recent consensus document.13
In Canada, however, FC tests are currently only covered by provincial health plans in Alberta and Quebec, as well as some extended health insurance plans.14 There is still no cost-effectiveness evidence within primary care in Canada. The objective of this study, therefore, is to determine the cost-effectiveness of FC testing in the diagnosis of adult cases of IBD in primary care from the Canadian healthcare sector perspective.
Materials and methods
Comparison groups
A higher 100 µg/g cut-off in primary care has been advocated and demonstrated to increase the positive predictive power of the test and counter the high false positive rate observed at the lower 50 µg/g cut-off.11–13 15 Therefore, we chose the 100 µg/g cut-off for FC testing in primary care setting as the intervention for our analysis. Referrals based on standard care CRP/ESR testing in primary care were used as the comparator. This assumes that patients with a normal CRP/ESR would not be referred initially but would subsequently be referred if they have ongoing symptoms. This is a simplification of real-world practice—clinicians are known, for example, to refer patients with normal CRP/ESR to secondary care. Nevertheless, there is currently a lack of reliable data on the accuracy of real-world primary care referral practices in the literature particularly in Canada. Thus, we based the comparator on CRP/ESR testing, in line with previous cost-effectiveness analyses.12 16 An alternative estimate of primary care referral accuracy was based on the study of Waugh et al,7 which estimated a high sensitivity (=1) and specificity (=0.788). Since the reliability of these estimates has been previously questioned,12 they were used as a scenario analysis only.
Decision model
A decision analytic model was built to estimate the cost-effectiveness of using FC test as compared with the current practice using blood test, in the screening for IBD in the primary care setting. The patient population in the model was a hypothetical cohort of adult patients aged 19–64 years, who present with gastrointestinal symptoms suggestive of IBD in a primary care setting but are not suspected of having cancer (which requires urgent specialist referral). A decision tree was developed in Microsoft Excel where the hypothetical cohort of adult patients underwent certain pathways. The associated cost and effectiveness of each pathway was captured in the model and the expected cost and effectiveness was estimated.
Effectiveness was measured using quality-adjusted life years (QALYs). The time horizon for the cost-effectiveness analysis was 1 year as this was a reasonable length of time for a patient to reach a confirmed diagnosis of either IBD or non-IBD. Due to the brief time horizon, discounting was not applied to either costs or benefits in this analysis. Time to IBD diagnosis was also estimated from the model. The analysis perspective was the Canadian health sector.
The clinical pathways of patients presenting with gastrointestinal symptoms in primary care were established from published literature7 12 16–18 as well as input by two gastroenterologists from St. Paul’s Hospital, Vancouver. Established clinical pathways were consistent with the best-practice clinical care pathway for management of IBS in primary care as outlined by the Canadian Association of Gastroenterology19 and local primary care guidelines on the use of FC in the UK.13 15
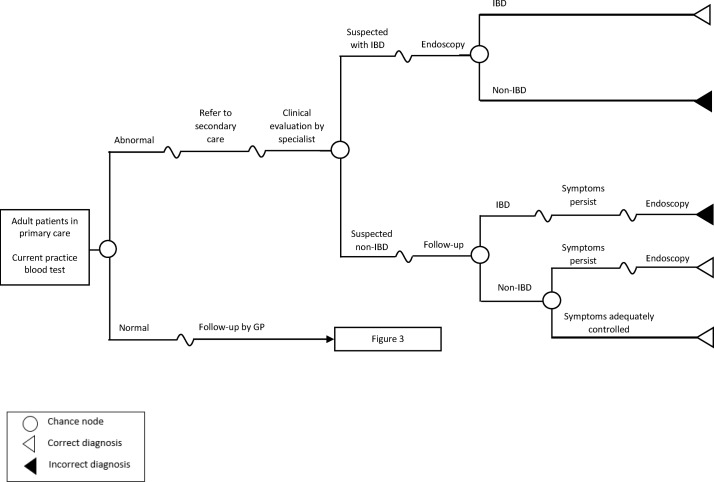
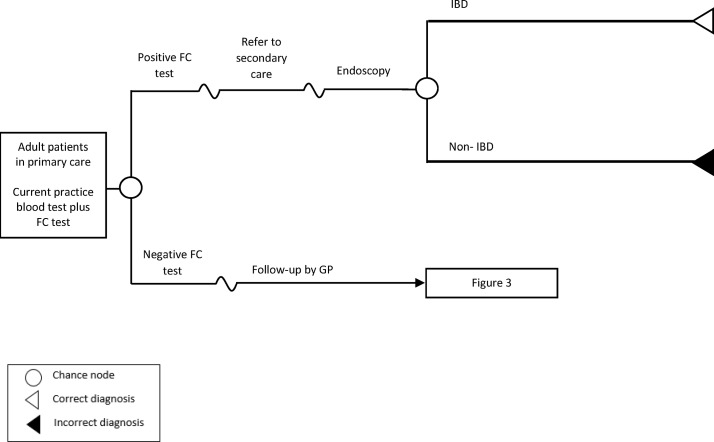
Figure 1 illustrates the current practice using the standard blood test, whereas figure 2 depicts the proposed strategy of adding FC test as a diagnostic support tool for general practitioners (GPs). Under the current practice (figure 1), based on results of the blood investigation (ESR and CRP), a GP will make a decision on whether to refer patients to specialist care or not. Patients with abnormal blood results will be referred to gastroenterology for specialist assessment. The specialist may then order an endoscopy as necessary to confirm IBD diagnosis or follow-up with patients unlikely to have IBD and monitor their symptoms accordingly. If symptoms are still persistent after 3 months (assumed and same as Waugh et al 7), an endoscopy may be ordered at the specialist follow-up visit to confirm diagnosis of IBD. Under the FC testing strategy (figure 2), patients with positive FC test results will be referred to specialist care and an endoscopy will be ordered for them at the specialist visit to confirm diagnosis of IBD.
Figure 1.
Overview of the model structure for standard practice using blood test. IBD, inflammatory bowel disease; GP, general practitioner.
Figure 2.
Overview of the model structure for faecal calprotectin testing strategy. FC, faecal calprotectin; IBD, inflammatory bowel disease; GP, general practitioner.
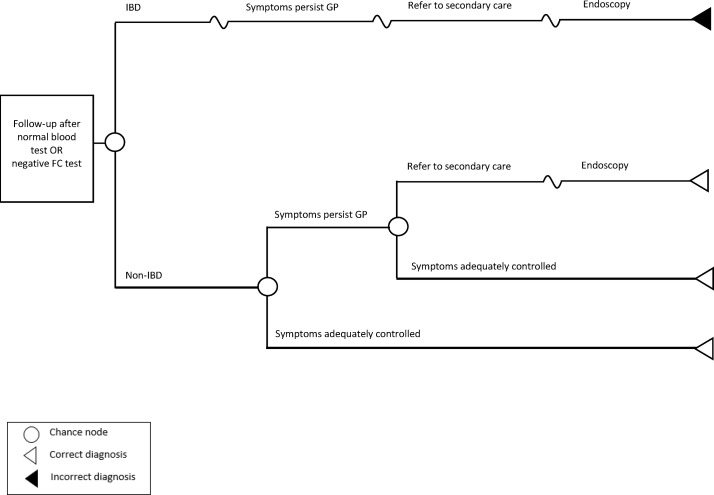
Patients with normal blood results or negative FC test results will be followed-up by the GP and receive lifestyle and dietary advice with appropriate medication to treat symptoms for 3 months (assumed) (figure 3). Those with symptoms inadequately controlled will receive more intensive management (different medication) from their GP for another 4 weeks (assumed). If symptoms are still persistent, further assessment by a gastroenterologist and endoscopy will be performed.
Figure 3.
Overview of the decision branch for normal blood test or negative faecal calprotectin test results. FC, faecal calprotectin; IBD, inflammatory bowel disease; GP, general practitioner.
Model parameters
The model parameters (table 1) were obtained from literature or based on assumptions. The parameters include sensitivity and specificity for FC testing at the 100 µg/g cut-off and ≥15 mm/hour for ESR and ≥5 mg/L for CRP blood testing; prevalence of IBD in primary care; the ratio of UC and CD; patients with no IBD with negative test results; costs; utilities and waiting time.
Table 1.
Model input parameters
| Parameter | Estimate | Distribution | Distribution parameters | Data source |
| IBD prevalence, % | 6.8 | Beta | Alpha=50 Beta=689 |
Walker et al 11 |
| UC proportion, % | 44.6 | Fixed | Rocchi et al 3 | |
| Test accuracy | ||||
| Sensitivity | ||||
| Blood test | 0.649 | Normal, logit transformation | Logit estimate=0.613 Logit SE=0.199 |
Meta-analysis based on a systematic review of three studies6 20–22 |
| FC test, at 100 µg/g cut-off | 0.860 | Beta | Alpha=43 Beta=7 |
Walker et al 11 |
| Specificity | ||||
| Blood test | 0.866 | Normal, logit transformation | Logit estimate=1.867 Logit SE=0.196 |
Meta-analysis based on a systematic review of three studies6 20–22 |
| FC test, at 100 µg/g cut-off | 0.901 | Beta | Alpha=621 Beta=68 |
Walker et al 11 |
| Model probabilities, % | ||||
| Proportion of patients with abnormal blood test with endoscopy ordered in the initial gastroenterologist consultation | 88.3 | Beta | Alpha=7.520 Beta=0.993 |
Expert opinion |
| Proportion of patients with no IBD with persistent symptoms after the initial management by GPs | 47.0 | Log-normal | 95% CI 33 to 57 | Waugh et al 7 |
| Proportion of patients with no IBD with symptoms after further intensive management by GPs that need further investigation by specialist and endoscopy | 15.0 | Fixed | Expert opinion | |
| Cost estimates ($C) | ||||
| FC test | 40.00 | Fixed | Local clinic cost, Waugh et al 7 and Yang et al 18 | |
| Initial GP visit | 68.64 | Fixed | BC MSC payment schedule26 | |
| Follow-up GP visit | 30.92 | Fixed | BC MSC payment schedule26 | |
| Initial gastroenterologist consultation | 160.25 | Fixed | BC MSC payment schedule26 | |
| Follow-up gastroenterologist consultation | 97.39 | Fixed | BC MSC payment schedule26 | |
| Surgical pathology | 85.52 | Fixed | BC MSC payment schedule26 | |
| Colonoscopy, with biopsy | 427.70 | Fixed | Sharara et al 27 | |
| Utilities | ||||
| Non-IBD | ||||
| a) With adequately controlled symptoms | 0.78 | Beta | Alpha=5.367 Beta=1.514 |
Spiegel et al 29 |
| b) With persistent symptoms | 0.73 | Calculated from a/c | Spiegel et al 29 | |
| c) Fixed ratio for utility of adequately controlled over persistent symptoms | Fixed | 1.068 | ||
| Weighted IBS utility | 0.76 | Calculated from a), b) and proportion of patients with no IBD with persistent symptoms above | ||
| IBD | ||||
| Active UC | 0.71 | Beta | Alpha=3.802 Beta=1.553 |
Stark et al 30 |
| Active CD | 0.61 | Beta | Alpha=1.116 Beta=0.713 |
Stark et al 30 |
| Monthly utility decrement for UC | 0.017 | Beta | Alpha=1.601 Beta=94.443 |
Stark et al 30 |
| Monthly utility decrement for CD | 0.023 | Beta | Alpha=1.647 Beta=68.958 |
Stark et al 30 |
| Wait time | ||||
| Time taken to undergo blood test and/or FC test after presenting with symptoms in primary care | 7 days | Fixed | Expert opinion | |
| Time taken to obtain results of blood test and FC test | 7 days | Fixed | Expert opinion | |
| Time taken to follow-up by GP first time | 3 months | Fixed | Expert opinion | |
| Time taken to follow-up by GP second time | 4 weeks | Fixed | Expert opinion | |
| Time taken to a specialist consultation for patients with IBD | 86.50 | Normal | SE=17.602 | Leddin et al 31 |
| Time taken to a specialist consultation for patients with no IBD | 122.00 | Normal | SE=9.694 | Leddin et al 31 |
| Time taken to endoscopy after seeing a specialist | 63.50 | Normal | SE=18.622 | Leddin et al 31 |
| Time taken to follow-up by a specialist | 3 months | Fixed | Expert opinion |
CD, Crohn’s disease; FC, faecal calprotectin; GP, general practitioner; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; MSC, Medical Services Commission; UC, ulcerative colitis.
Sensitivity and specificity
As mentioned above, the majority of studies measuring FC testing accuracy were conducted in the secondary care setting. As such, we used the sensitivity and specificity of FC testing at the 100 µg/g cut-off from the recent UK study conducted with the prospective primary cohort.11 For blood testing, we chose the cut-offs of ≥15 mm/hour for ESR and ≥5 mg/L for CRP. Three studies using these ESR and CRP cut-offs were identified from a published systematic review.6 20–22 Following this, a meta-analysis was conducted to synthesise the logit-transformation of sensitivity and specificity and the details can be found in the online supplementary file 1.
bmjopen-2018-027043supp001.pdf (141KB, pdf)
Prevalence of IBD in primary care
Very few studies have estimated the prevalence of IBD in primary care,7 11 23–25 with most estimates originating from UK studies. To be consistent with the sensitivity and specificity estimates used in our model, we used the prevalence of IBD (=6.8%) in primary care from the same study.11 Among IBD cases, 45% were UC and 55% were CD.3
Patients with no IBD with negative test results
Based on expert opinions, previous studies estimated that the probability of patients with no IBD still having persistent symptoms after the initial management by GPs was 50% or 60%.16–18 In our study, we applied the 47% probability used in the cost-effectiveness analysis conducted by Waugh et al.7 We also assumed that 15% of these who have persistent symptoms after initial management by GP (based on expert advice) would subsequently experience uncontrolled symptoms after further intensive management by GPs, be referred to a specialist and undergo endoscopy.
Costs
Only the diagnosis-related costs, including the costs for diagnostic testing (FC, endoscopy and pathology) and physician and gastroenterologist visits, were considered. All costs were reported in 2017 Canadian dollars. Cost data were obtained from the British Columbia Ministry of Health Medical Services Commission Payment Schedule (1 July 2017 version),26 which is comparable with other provinces in Canada; literature review for colonoscopy cost in Canada27 adjusted to 2017 cost using total healthcare implicit price index28 and literature review and a local gastroenterology clinic for FC testing cost.7 18 Costs of managing complications associated with colonoscopy such as bleeding and perforation were not considered in this analysis due to the unavailability of data.
Utilities
Our utility estimates for IBS were taken from a study conducted among 257 patients in USA using EuroQol-5D.29 The utilities of 0.78 for patients with IBD with adequate relief of symptoms or 0.73 for those with persistent symptoms were applied to patients with no IBD in our analysis.29 A weighted IBS utility of 0.76 was calculated based on the proportion (47% assumed above) of patients with no IBD with persistent symptoms and the remaining 53% with adequately controlled symptoms. In our model, patients with adequately controlled symptoms started with a weighted utility of 0.76 until the time of diagnosis, wherein a weighted utility of 0.78 (utility for adequately controlled) was applied for the rest of the 1-year time horizon. Patients with persistent symptoms started with 0.73 (utility for persistent symptoms) until the time of diagnosis followed by 0.78 if symptoms were eventually controlled or 0.76 if they had to undergo endoscopy.
Similar to Waugh et al,7 our utility estimates of IBD were taken from a study conducted among 225 patients with CD and 219 patients with UC in Germany using the EuroQol-5D.30 This study had a reasonably large sample size and reported utility estimates for active disease compared with remission for both UC and CD. The utility estimates of 0.71 for active UC and 0.61 for active CD were chosen to represent the utility of patients with IBD when they visited GP for the first time. We assumed that their utilities would then decrease by a certain amount every month due to disease progression until diagnosis was made, at which point the utility value at the time of diagnosis would be maintained throughout the rest of the 1-year time horizon. Following the method of Waugh et al by taking the utility difference between active disease and remission and dividing it by 12, we derived a monthly utility decrement of 0.0167 for UC and 0.023 for CD.7
Waiting time
The median time a patient with IBD was first referred to specialist until consultation by a specialist was 72 days (95% CI 52 to 121) and the median time from the first specialist consultation to endoscopy was 44 days (95% CI 27 to 100) in Canada.31 The median time for patients with no IBD from the first referral to specialist consultation was 126 days (95% CI 103 to 141).31 Other wait times were assumed to be fixed according to the guidelines.
Analyses
We performed probabilistic analyses to estimate means and 95% CI of total costs, QALYs and incremental cost-effectiveness ratios (ICERs) to reflect the underlying parameter uncertainty. Additionally, the time to the diagnosis of IBD among patients with IBD was calculated. A total of 5000 Monte Carlo simulations were generated from the parameter probability distributions. The base-case results were presented in a cost-effectiveness plane (online supplementary file 1) and as the cost-effectiveness acceptability curve, which demonstrates the probability of the FC testing strategy being cost-effective compared with the standard care across a range of willingness-to-pay thresholds.
To explore the sensitivity of results to specific parameter uncertainty, alternative assumptions and sources of data, we also conducted a series of scenario analyses. (1) IBD prevalence was varied from 5% to 20% in 5% increments. (2) FC testing accuracy was changed using an alternative data source. The sensitivity and specificity for repeating FC testing among the first FC testing ≥100 µg/g in the study by Turvill et al 12 were used in the model. (3) The sensitivity and specificity of the primary care practice in the study by Waugh et al 7 was used. (4) We increased the proportion of patients with abnormal blood test for whom an endoscopy was ordered in the initial gastroenterologist consultation from 83% to 100%. (5) We changed the proportion of patients with no IBD with symptoms after further intensive management by GPs that needed further investigation by specialist and endoscopy from 5% (same as Waugh et al 7 and Whitehead and Hutton16) to 30% with 5% increments. (6) Different FC test costs and an increase or decrease in other costs by 20% were implemented. (7) We changed the source of utility decrement estimates from Stark et al 30 to that of Gregor et al 32and Poole et al.33 (8) Time taken to the first follow-up by GP and time taken to follow-up by a specialist were changed from 1 to 4 months with 1-month increments. (9) We applied our model to a patient population without gastrointestinal alarm symptoms described by Walker et al.11
Patient and public involvement
Patients and/or public were not involved in our study. A hypothetical cohort of adult patients has been simulated.
Results
Base case
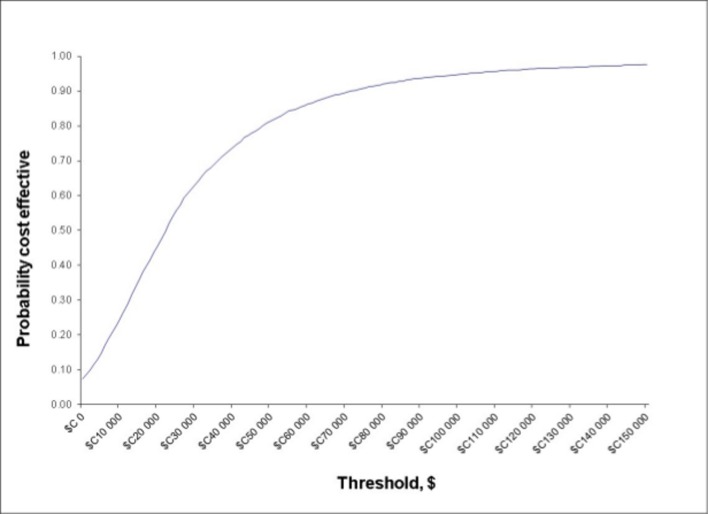
For the base case, the probabilistic analysis based on 5000 Monte Carlo simulations showed that the FC testing strategy was about $C21 more expensive on average than the standard practice using blood test ($C295.12 vs $C273.93) but yielded a slightly higher QALY (0.751 vs 0.750, respectively) (table 2). Thus, the ICER was $C20 323.35 per QALY gained. The time to diagnosis for patients with IBD was 39.96 days (95% CI 16.34 to 65.29) shorter under the FC testing strategy (192.39 days [95% CI 143.10 to 239.74]) than standard practice (232.36 days [95% CI 186.02 to 277.92]). There was an 81.3% probability that the FC testing strategy was cost-effective at the willingness-to-pay threshold of $C50 000/QALY (figure 4).
Table 2.
Results of base-case analysis and scenario analyses
| Scenario | FC testing strategy | Standard practice (blood test) |
Incremental Cost | Incremental QALY | ICER ($C/QALY) |
Probability of FC being cost-effective(%)* | ||
| Cost to $C | QALY | Cost to $C | QALY | |||||
| Base case | 295.12(274.49 to 317.53) | 0.751 (0.431 to 0.939) | 273.93 (245.40 to 306.05) | 0.750 (0.430 to 0.938) | 21.19 (−7.50 to 46.57) | 0.001 (0.0003 to 0.002) | 20 323.35 | 81.3 |
| Scenario analyses IBD prevalence to % | ||||||||
| 5 | 286.17 (268.43 to 306.09) | 0.757 (0.427 to 0.943) | 264.65 (238.41 to 294.96) | 0.756 (0.426 to 0.942) | 21.52 (−7.75 to 46.72) | 0.001 (0.0002 to 0.002) | 24 440.81 | 75.5 |
| 10 | 312.60 (295.98 to 331.11) | 0.743 (0.434 to 0.928) | 291.21 (267.12 to 319.28) | 0.742 (0.433 to 0.927) | 21.39 (−5.67 to 45.80) | 0.001 (0.0004 to 0.003) | 15 594.08 | 89.3 |
| 15 | 339.26 (323.00 to 357.86) | 0.740 (0.448 to 0.916) | 318.14 (294.04 to 345.63) | 0.738 (0.447 to 0.915) | 21.12 (−5.02 to 43.94) | 0.002 (0.0004 to 0.005) | 11 515.23 | 93.8 |
| 20 | 365.81 (350.40 to 383.68) | 0.728 (0.442 to 0.907) | 344.93 (322.74 to 371.08) | 0.725 (0.440 to 0.904) | 20.88 (−3.94 to 41.96) | 0.002 (0.0006 to 0.006) | 8843.74 | 96.7 |
| FC test accuracy (Turvill et al 12) | ||||||||
| Sensitivity=0.94 (95% CI 0.85 to 0.98) Specificity=0.92 (95% CI 0.90 to 0.94) |
285.36 (265.56 to 306.98) | 0.755 (0.431 to 0.939) | 274.16 (245.10 to 306.04) | 0.754 (0.430 to 0.937) | 11.21 (−16.20 to 35.83) | 0.001 (0.0005 to 0.003) | 8012.69 | 96.5 |
| Primary care practice accuracy (Waugh et al 7) | ||||||||
| Sensitivity=1 (7/7) Specificity=0.79 (82/104) |
295.55 (275.41 to 317.36) | 0.753 (0.446 to 0.938) | 312.85 (270.56 to 359.49) | 0.752 (0.445 to 0.937) | −17.30 (−62.90 to 22.76) | 0.001 (−0.0001 to 0.002) | N/A | 93.6 |
| Proportion of patients with abnormal blood test with endoscopy ordered in the initial gastroenterologist consultation to % | ||||||||
| 100 | 295.38 (274.60 to 317.32) | 0.751 (0.430 to 0.938) | 276.23 (248.77 to 307.54) | 0.750 (0.429 to 0.937) | 19.15 (−10.31 to 44.69) | 0.001 (0.0002 to 0.002) | 22 007.50 | 76.9 |
| Proportion of patients with no IBD with symptoms after further intensive management by GPs that need investigation by specialist and endoscopy to % | ||||||||
| 5 | 268.69 (251.37 to 286.92) | 0.754 (0.444 to 0.940) | 248.96 (222.27 to 278.72) | 0.753 (0.444 to 0.939) | 19.73 (−10.67 to 46.48) | 0.001 (0.0003 to 0.003) | 17 988.04 | 83.5 |
| 10 | 281.84 (263.26 to 301.20) | 0.754 (0.447 to 0.938) | 261.12 (234.23 to 290.74) | 0.753 (0.446 to 0.937) | 20.72 (−8.35 to 46.16) | 0.001 (0.0002 to 0.002) | 19 504.34 | 82.4 |
| 20 | 308.68 (286.39 to 332.03) | 0.751 (0.426 to 0.938) | 286.82 (257.72 to 318.88) | 0.750 (0.426 to 0.938) | 21.85 (−5.70 to 45.83) | 0.001 (0.0002 to 0.002) | 21 405.41 | 81.2 |
| 25 | 322.23 (297.17 to 350.17) | 0.749 (0.423 to 0.937) | 300.26 (268.29 to 334.99) | 0.748 (0.422 to 0.936) | 21.97 (−5.25 to 45.94) | 0.001 (0.0003 to 0.002) | 22 040.22 | 79.5 |
| 30 | 335.85 (308.40 to 366.80) | 0.750 (0.432 to 0.934) | 313.02 (280.31 to 348.54) | 0.749 (0.431 to 0.933) | 22.84 (−3.46 to 45.44) | 0.001 (0.0002 to 0.002) | 23 221.90 | 78.8 |
| Cost of FC to $C | ||||||||
| 20 | 275.24 (254.13 to 297.02) | 0.755 (0.446 to 0.940) | 273.98 (246.12 to 304.75) | 0.754 (0.445 to 0.939) | 1.26 (−27.32 to 25.62) | 0.001 (0.0002 to 0.002) | 1206.34 | 94.9 |
| 30 | 285.21 (264.91 to 307.09) | 0.753 (0.436 to 0.940) | 274.13 (246.69 to 306.58) | 0.752 (0.435 to 0.939) | 11.08 (−17.29 to 36.28) | 0.001 (0.0003 to 0.002) | 10 567.51 | 89.8 |
| 50 | 305.42 (284.54 to 327.76) | 0.751 (0.428 to 0.941) | 274.12 (246.34 to 305.69) | 0.750 (0.428 to 0.940) | 31.29 (2.93 to 55.78) | 0.001 (0.0003 to 0.002) | 29 789.72 | 71.7 |
| 60 | 315.60 (295.76 to 337.54) | 0.751 (0.430 to 0.936) | 274.19 (246.49 to 305.45) | 0.750 (0.430 to 0.936) | 41.40 (13.49 to 66.07) | 0.001 (0.0002 to 0.002) | 39 243.50 | 59.8 |
| 70 | 325.29 (305.29 to 347.98) | 0.753 (0.428 to 0.938) | 274.15 (246.63 to 305.86) | 0.751 (0.427 to 0.936) | 51.14 (22.70 to 75.99) | 0.001 (0.0002 to 0.002) | 48 712.48 | 47.4 |
| All cost estimates except FC test cost to $C | ||||||||
| +20% | 346.68 (321.97 to 372.92) | 0.752 (0.430 to 0.940) | 329.42 (295.89 to 367.82) | 0.751 (0.429 to 0.939) | 17.26 (−16.39 to 48.03) | 0.001 (0.0003 to 0.002) | 16 191.86 | 83.4 |
| −20% | 244.18 (227.92 to 262.28) | 0.752 (0.433 to 0.936) | 219.14 (196.94 to 244.50) | 0.751 (0.432 to 0.935) | 25.04 (2.13 to 44.91) | 0.001 (0.0003 to 0.003) | 23 509.13 | 79.8 |
| Utility decrement | ||||||||
| CD=0.006 (Gregor et al
32
UC=0.014 (Poole et al 33 |
295.11 (274.59 to 316.66) | 0.755 (0.427 to 0.941) | 274.24 (246.79 to 304.96) | 0.755 (0.427 to 0.940) | 20.87 (−6.50 to 45.47) | 0.001 (0.0002 to 0.001) | 30 136.89 | 68.6 |
| Time taken to follow-up by GP first time | ||||||||
| 1 month | 294.97 (274.80 to 316.36) | 0.756 (0.422 to 0.945) | 274.09 (245.92 to 306.40) | 0.755 (0.421 to 0.944) | 20.89 (−8.13 to 46.10) | 0.001 (0.0002 to 0.002) | 18 830.57 | 81.9 |
| 2 months | 295.36 (274.91 to 317.69) | 0.758 (0.437 to 0.943) | 274.07 (246.25 to 306.46) | 0.757 (0.436 to 0.942) | 21.29 (−7.90 to 45.83) | 0.001 (0.0002 to 0.002) | 19 650.08 | 81.7 |
| 4 months | 295.28 (275.08 to 317.76) | 0.749 (0.442 to 0.940) | 274.03 (245.76 to 304.35) | 0.748 (0.441 to 0.939) | 21.25 (−6.75 to 45.57) | 0.001 (0.0002 to 0.002) | 21 451.73 | 80.8 |
| Time taken to follow-up by a specialist | ||||||||
| 1 month | 295.47 (275.10 to 317.87) | 0.747 (0.425 to 0.937) | 274.37 (246.13 to 305.91) | 0.746 (0.424 to 0.936) | 21.10 (−7.54 to 46.45) | 0.001 (0.0002 to 0.002) | 23 213.73 | 76.1 |
| 2 months | 295.35 (275.19 to 318.36) | 0.757 (0.435 to 0.939) | 274.19 (247.23 to 305.55) | 0.756 (0.434 to 0.937) | 21.16 (−7.75 to 45.96) | 0.001 (0.0002 to 0.002) | 21 587.69 | 79.6 |
| 4 months | 295.49 (274.69 to 317.09) | 0.751 (0.430 to 0.940) | 274.42 (246.23 to 305.94) | 0.750 (0.429 to 0.939) | 21.07 (−7.51 to 46.49) | 0.001 (0.0003 to 0.003) | 18 991.77 | 83.4 |
| Patient population without gastrointestinal alarm symptoms (Walker et al 11) | ||||||||
| Prevalence=4% (18/447) Sensitivity=0.84 (15/18) Specificity=0.91 (390/429) |
276.29 (253.94 to 299.86) | 0.760 (0.429 to 0.948) | 258.90 (230.11 to 291.19) | 0.760 (0.429 to 0.947) | 17.40 (−13.70 to 44.78) | 0.001 (0.0002 to 0.002) | 21 608.85 | 75.6 |
95% CI values in brackets.
*At $C50 000/QALY threshold.
CD, Crohn’s disease; FC, faecal calprotectin; GP, general practitioner; IBD, inflammatory bowel disease; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; UC, ulcerative colitis.
Figure 4.
Cost-effectiveness acceptability curve.
Scenario analyses
Our analyses showed that the cost-effectiveness of FC testing strategy was sensitive to the prevalence of IBD among the patients presenting with gastrointestinal symptoms in primary care, the FC cost and the value of utility decrements (table 2). When the prevalence was increased to 20%, the probability of the FC testing strategy being cost-effective would increase to 96.7% at the threshold of $C50 000/QALY. The probability of FC testing being cost-effective became 96.5% when using the sensitivity and specificity estimates for repeating FC testing strategy in the study by Turvill et al. The maximum price at which the FC testing strategy would still be cost-effective was about $C70. At $C70, the probability of FC testing being cost-effective was 47.4% at the willingness-to-pay threshold of $C50 000/QALY. When applying a much lower utility monthly decrement especially for CD (from 0.023 to 0.006 for CD and from 0.017 to 0.014 for UC), the probability of FC testing strategy was 68.6% at the threshold of $C50 000/QALY.
Discussion
Based on cost-effectiveness models built in previous studies,7 16–18 current practice guidelines in Canada19 and clinical expertise from gastroenterologists, we constructed a decision analytic model to evaluate the cost-effectiveness of adding FC testing to current practice compared with the current practice of blood test only in the diagnosis of adult patients with IBD in the Canadian primary care setting. To our knowledge, this is the first cost-effectiveness analysis of FC testing in primary care in Canada. Our base-case analysis suggested that the FC test was cost-effective. Probabilistic analysis showed that at a willingness-to-pay threshold of $C50 000 per QALY, there was an 81.3% chance of the FC testing strategy being cost-effective. Scenario analysis demonstrated that the cost-effectiveness was most sensitive towards prevalence of IBD, monthly utility decrement of IBD and cost of FC test.
A 6.8% prevalence of IBD was applied in our base-case analysis. This estimate was based on a prospective UK primary care cohort of patients aged between 18 and 46 years.11 The prevalence was very similar to the one used in the cost-effectiveness analysis conducted by Waugh et al.7 Among our model population (aged 19–64 years), the prevalence would likely be higher. Unfortunately, Canadian estimates were not found in published literature. Thus, we conducted scenario analysis by varying the prevalence from 5% to 20%. Although the cost-effectiveness of the FC testing strategy was highly sensitive to the prevalence of IBD in the adult patient population presented in the primary care setting, our study has shown it is still cost-effective when the prevalence is as low as 5%.
The ICER of the FC testing strategy compared with blood testing increased when the monthly utility decrement for IBD was lower. This finding is consistent with the assumption made in the calculation of QALYs for patients with IBD. A delay in diagnosis would cause patients to reach a lower utility value before diagnosis. Therefore, a higher utility decrement for IBD increased the difference in QALYs gained between the two strategies and caused a decrease in ICER and vice versa.
We used the current FC test cost, $C40, in our base case, which was consistent with the cost used in previous cost-effectiveness analyses conducted in the UK and the USA.7 18 When the cost of FC testing was under $C70, the FC testing strategy had the potential to be cost-effective. The wider implementation of FC testing across Canada may drive the cost down. Laboratory-based FC testing has been shown to be cost-effective when conducted in batches.7 10
One of the strengths of our study is that we used the FC testing accuracy in primary care11 instead of the secondary care setting. The test accuracy in the secondary care setting was found to be higher than that of primary care setting. According to the most recent meta-analysis conducted by Waugh et al,7 all of studies included were for secondary setting and the synthesised sensitivity (0.93) and specificity (0.94) of FC testing at the 50 µg/g cut-off were both higher than the estimates (0.86 and 0.90) for the 100 µg/g cut-off we used for the primary care setting. However, the sensitivity and specificity values of CRP/ESR in our study were derived from secondary care sources20–22 and thus might differ in primary care setting.
Additionally, we estimated the benefit of using FC testing in primary care in terms of reducing the time to IBD diagnosis (by about 40 days). The average times to IBD diagnosis among patients with IBD were 192.4 days with FC testing and 232.4 days for standard practice. The time to diagnosis under the standard practice was reasonably consistent with a Canadian study that reported the mean time to diagnosis for CD and UC to be 255.5 and 202.3 days, respectively.34 Delayed diagnosis is a common problem in IBD. A study involving 1591 patients with IBD from the Swiss IBD cohort reported a diagnostic delay of 9 and 4 months for CD and UC.35 The delay was due to similarities in symptoms among patients with mild IBD and those with IBS. A literature review on natural history studies of CD reported that at time of diagnosis, one-third of patients already had intestinal complications such as ileitis, colitis or ileocolitis.36 In UC, an early diagnosis and identification of patients with a high risk of developing complicated disease, is crucial for choosing appropriate treatment and prevention of colectomies.37 The FC testing strategy has the potential to speed up diagnosis and reduce the wait time for a specialist and endoscopy by avoiding the unnecessary referrals.
Our study has several limitations. First, there was a lack of data for certain parameter inputs of the model. For example, costs and utility decrements of complications associated with colonoscopy such as bleeding and perforation could not be identified and were therefore not considered in this analysis. In Canada, the pooled rates of colonoscopy-related bleeding, perforation and mortality were 1.64/1000, 0.85/1000 and 0.074/1000, respectively.38 While the rates of complications associated with colonoscopy may be low, the impact on the overall costs and outcomes may be significant if the time horizon of the analysis was longer, especially when deaths occur. As the number of colonoscopies were expected to be reduced by FC testing, we took a more conservative approach by not considering the impact of the complications associated with colonoscopies. Data on the utility decrement of IBD due to delayed diagnosis were also unavailable. Therefore, we adopted the approach used by Waugh et al,7 assuming the annual utility decrement of IBD due to delayed diagnosis as the difference between active disease and remission of UC. While our CEA was limited to costs from a health sector perspective, considering costs from a societal perspective, for example, productivity losses due to colonoscopy, would further make FC testing more cost-effective.
Second, we did not consider a longer time horizon. In long term, because of the earlier diagnosis, we expect FC to generate more benefits, for example, by avoiding mortality/risk resulting from reduced unnecessary colonoscopies or bowel perforations/surgeries. Therefore, we expect our study to provide a relatively conservative cost-effectiveness estimate for FC. Nevertheless, further research on the long-term impact of early diagnosis of IBD and IBS is needed to validate this claim. Adopting a long-term horizon would likely produce more favourable results for FC and hence our finding that FC is cost-effective should hold in the long run.
Third, the model assumed that FC would be used as a single test applying a fixed cut-off of 100 µg/g. Alternative two-stage testing strategies may also be used. Turvill et al, for example, recently evaluated such a retesting FC strategy, using a cut-off of 100 µg/g and conducting a repeat FC testing for patients with an initial test above this cut-off.12 They found this retesting FC strategy to be cost-saving in a UK primary care setting, due to saving 100–150 unnecessary colonoscopies and 140–190 gastroenterology outpatient appointments compared with CRP/ESR testing alone. The utility of the second FC test is that it can cut out a high proportion of false positive test results, resulting in overall cost-savings. The results of our scenario analysis using the sensitivity and specificity estimates from the study by Turvill et al indicate a higher cost-effectiveness of FC using the retesting strategy (a 96.5% probability of being cost-effective compared with CRP/ESR testing alone) versus the single testing base-case strategy (81.3%) when a single FC test cost was applied. Future research should focus on these kinds of confirmatory testing strategies.
Additionally, our modelling assumed 100% patient uptake for every diagnostic test, blood test, FC test and endoscopy. Given the invasive nature and set of complications associated with colonoscopies, patients may refuse this diagnostic test. The FC test may also not be widely accepted, with a variable uptake rate between primary and secondary care. Some patients might decline to produce a sample of faeces for their GP, but may possibly be willing to do so for a gastroenterologist if the alternative is colonoscopy. Recently, a home-based FC kit has been made available, allowing patients to measure the concentration of FC directly using a rapid immunochromatographic assay captured by a smartphone’s camera. The availability of this kit may increase the uptake and patient adherence of FC testing.39
It is worth noting that FC test accuracy might differ by populations with different age or in different settings. We used test sensitivity and specificity values from the study by Walker et al,11 which focused on young adults between 18 and 46 years in the UK and might not be applicable to our model population aged 19–64 years. In addition, different FC tests produced by different manufacturers and using different platforms can produce significantly different test results (ie, between-method bias).7 This means that the sensitivity and specificity values adopted in our study (based on the study by Walker et al 11 using a specific ELISA test), may not hold for different laboratories with different pre-analytical and analytical operating procedures and/or using different test kits/methods. This is potentially a significant issue for home-based FC kits since the benefits of increased uptake of testing may be negated by issues with test imprecision and bias.
Future research can be conducted to estimate the cost-effectiveness of FC test for distinguishing between IBD and non-IBD in the paediatrics population when the important model parameters are available. Furthermore, there has also been growing interest in the use of FC test in a few areas of IBD management. For example, FC test might be used to monitor disease progression, predict relapse and monitor response to treatment.40 As such, an economic model which links the diagnostic outcomes of this analysis to the management of IBD in terms of treatment and monitoring can be considered in the future.
In conclusion, using FC at the 100 µg/g cut-off in primary care in the diagnosis of IBD can be a cost-effective strategy and can speed up IBD diagnosis in adults who present with gastrointestinal symptoms in Canada.
Supplementary Material
Footnotes
Contributors: WZ and GR designed the study. All authors contributed to the cost-effectiveness model: CHW initiated the model, MC, TM and GR contributed their expertise in the model building and parameter determination and WZ modified and finalised the model. WZ and CHW drafted the manuscript and all authors significantly contributed to and reviewed the final manuscript. All authors agree to be accountable for all aspects of the work.
Funding: This work was supported by the Future Leaders in Inflammatory Bowel Disease (FLIBD) Grant.
Competing interests: None declared.
Ethics approval: A hypothetical cohort of adult patients has been simulated.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data relevant to the study are included in the article or uploaded as supplementary information. There is no additional unpublished data from the study.
Patient consent for publication: Not required.
References
- 1. Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis 2008;14:554–65. 10.1002/ibd.20301 [DOI] [PubMed] [Google Scholar]
- 2. Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 3. Rocchi A, Benchimol EI, Bernstein CN, et al. . Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol 2012;26:811–7. 10.1155/2012/984575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014;6:71–80. 10.2147/CLEP.S40245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walsham NE, Sherwood RA. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol 2016;9:21–9. 10.2147/CEG.S51902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jellema P, van der Windt DA, Schellevis FG, et al. . Systematic review: accuracy of symptom-based criteria for diagnosis of irritable bowel syndrome in primary care. Aliment Pharmacol Ther 2009;30:695–706. 10.1111/j.1365-2036.2009.04087.x [DOI] [PubMed] [Google Scholar]
- 7. Waugh N, Cummins E, Royle P, et al. . Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess 2013;17:xv-xix, 1-211 10.3310/hta17550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369 10.1136/bmj.c3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenfeld G, Greenup AJ, Round A, et al. . FOCUS: future of fecal calprotectin utility study in inflammatory bowel disease. World J Gastroenterol 2016;22:8211–8. 10.3748/wjg.v22.i36.8211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NICE. Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel | Guidance and guidelines. https://www.nice.org.uk/guidance/dg11 (Accessed 18th January 2018).
- 11. Walker GJ, Moore L, Heerasing N, et al. . Faecal calprotectin effectively excludes inflammatory bowel disease in 789 symptomatic young adults with/without alarm symptoms: a prospective UK primary care cohort study. Aliment Pharmacol Ther 2018;47:1103–16. 10.1111/apt.14563 [DOI] [PubMed] [Google Scholar]
- 12. Turvill J, Turnock D, Holmes H, et al. . Evaluation of the clinical and cost-effectiveness of the york faecal calprotectin care pathway. Frontline Gastroenterol 2018;9:285–94. 10.1136/flgastro-2018-100962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. England NHS. Faecal calprotectin in primary care as a decision diagnostic for inflammatory bowel disease and irritable bowel syndrome. https://www.pcc-cic.org.uk/sites/default/files/articles/attachments/fcp_consensus_paper_2018_0.pdf (Accessed 9th Dec 2018).
- 14. GIS. Fecal calprotectin test gastrointest. Soc. https://www.badgut.org/information-centre/a-z-digestive-topics/fecal-calprotectin-test/ (Accessed 18th Jan 2018).
- 15. Turvill J. Local primary care guidelines: use of faecal calprotectin in the assessment of patients with lower gastrointestinal symptoms. 2014. https://www.nice.org.uk/guidance/dg11/resources/primary-care-guidelines-york-teaching-hospital-pdf-4535195223
- 16. Whitehead S, Hutton J. Economic report: Value of calprotectin in screening out irritable bowel syndrome. York, UK: Centre for Evidence-based Purchasing, 2010. [Google Scholar]
- 17. Dubinsky MC, Johanson JF, Seidman EG, et al. . Suspected inflammatory bowel disease--the clinical and economic impact of competing diagnostic strategies. Am J Gastroenterol 2002;97:2333–42. 10.1111/j.1572-0241.2002.05988.x [DOI] [PubMed] [Google Scholar]
- 18. Yang Z, Clark N, Park KT. Effectiveness and cost-effectiveness of measuring fecal calprotectin in diagnosis of inflammatory bowel disease in adults and children. Clin Gastroenterol Hepatol 2014;12:253–62. 10.1016/j.cgh.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. IBS. Canadian association of gastroenterology. Enhanced primary care pathway: IBS, 2016. [Google Scholar]
- 20. Kaiser T, Langhorst J, Wittkowski H, et al. . Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut 2007;56:1706–13. 10.1136/gut.2006.113431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poullis AP, Zar S, Sundaram KK, et al. . A new, highly sensitive assay for C-reactive protein can aid the differentiation of inflammatory bowel disorders from constipation- and diarrhoea-predominant functional bowel disorders. Eur J Gastroenterol Hepatol 2002;14:409–12. 10.1097/00042737-200204000-00013 [DOI] [PubMed] [Google Scholar]
- 22. Shine B, Berghouse L, Jones JE, et al. . C-reactive protein as an aid in the differentiation of functional and inflammatory bowel disorders. Clin Chim Acta 1985;148:105–9. 10.1016/0009-8981(85)90219-0 [DOI] [PubMed] [Google Scholar]
- 23. Pavlidis P, Chedgy FJ, Tibble JA. Diagnostic accuracy and clinical application of faecal calprotectin in adult patients presenting with gastrointestinal symptoms in primary care. Scand J Gastroenterol 2013;48:1048–54. 10.3109/00365521.2013.816771 [DOI] [PubMed] [Google Scholar]
- 24. Thompson WG, Heaton KW, Smyth GT, et al. . Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 2000;46:78–82. 10.1136/gut.46.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kok L, Elias SG, Witteman BJ, et al. . Diagnostic accuracy of point-of-care fecal calprotectin and immunochemical occult blood tests for diagnosis of organic bowel disease in primary care: the Cost-Effectiveness of a Decision Rule for Abdominal Complaints in Primary Care (CEDAR) study. Clin Chem 2012;58:989–98. 10.1373/clinchem.2011.177980 [DOI] [PubMed] [Google Scholar]
- 26. British Columbia Ministry of Health. Medical services commission payment schedule. 2017. https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/medical-services-plan/msc-payment-schedule-july-2017.pdf
- 27. Sharara N, Adam V, Crott R, et al. . The costs of colonoscopy in a Canadian hospital using a microcosting approach. Can J Gastroenterol 2008;22:565–70. 10.1155/2008/854984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canadian Institute for Health Information. National health expenditure trends. 2018. https://www.cihi.ca/en/national-health-expenditure-trends (Accessed 23rd Jan 2018).
- 29. Spiegel B, Harris L, Lucak S, et al. . Developing valid and reliable health utilities in irritable bowel syndrome: results from the IBS PROOF Cohort. Am J Gastroenterol 2009;104:1984–91. 10.1038/ajg.2009.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stark RG, Reitmeir P, Leidl R, et al. . Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis 2010;16:42–51. 10.1002/ibd.20989 [DOI] [PubMed] [Google Scholar]
- 31. Leddin D, Armstrong D, Borgaonkar M, et al. . The 2012 SAGE wait times program: survey of access to gastroenterology in Canada. Can J Gastroenterol 2013;27:83–9. 10.1155/2013/143018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gregor JC, McDonald JW, Klar N, et al. . An evaluation of utility measurement in Crohn’s disease. Inflamm Bowel Dis 1997;3:265–76. 10.1097/00054725-199712000-00004 [DOI] [PubMed] [Google Scholar]
- 33. Poole CD, Connolly MP, Nielsen SK, et al. . A comparison of physician-rated disease severity and patient reported outcomes in mild to moderately active ulcerative colitis. J Crohns Colitis 2010;4:275–82. 10.1016/j.crohns.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 34. Benchimol EI, Manuel DG, Mojaverian N, et al. . Health services utilization, specialist care, and time to diagnosis with inflammatory bowel disease in immigrants to ontario, canada: a population-based cohort study. Inflamm Bowel Dis 2016;22:2482–90. 10.1097/MIB.0000000000000905 [DOI] [PubMed] [Google Scholar]
- 35. Vavricka SR, Spigaglia SM, Rogler G, et al. . Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:496–505. 10.1002/ibd.21719 [DOI] [PubMed] [Google Scholar]
- 36. Peyrin-Biroulet L, Loftus EV, Colombel JF, et al. . The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. 10.1038/ajg.2009.579 [DOI] [PubMed] [Google Scholar]
- 37. Monstad I, Hovde O, Solberg IC, et al. . Clinical course and prognosis in ulcerative colitis: results from population-based and observational studies. Ann Gastroenterol 2014;27:95–104. [PMC free article] [PubMed] [Google Scholar]
- 38. Rabeneck L, Paszat LF, Hilsden RJ, et al. . Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 2008;135:1899–906. 10.1053/j.gastro.2008.08.058 [DOI] [PubMed] [Google Scholar]
- 39. Bello C, Roseth A, Guardiola J, et al. . Usability of a home-based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig Liver Dis 2017;49:991–6. 10.1016/j.dld.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 40. Ikhtaire S, Shajib MS, Reinisch W, et al. . Fecal calprotectin: its scope and utility in the management of inflammatory bowel disease. J Gastroenterol 2016;51:434–46. 10.1007/s00535-016-1182-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027043supp001.pdf (141KB, pdf)