Abstract
Objectives
To quantify population-level health and economic consequences of sick leave among workers with influenza symptoms.
Interventions
Compared with current sick leave practice (baseline), we evaluated the health and cost consequences of: (1) increasing the proportion of workers on sick leave from 65% (baseline) to 80% or 90%; (2) shortening the maximum duration from symptom onset to sick leave from 4 days (baseline) to 2 days, 1.5 days, 1 day and 0.5 days; and (3) combinations of 1 and 2.
Methods
A dynamic compartmental influenza model was developed using Norwegian population data and survey data on employee sick leave practices. The sick leave interventions were simulated under 12 different seasonal epidemic and 36 different pandemic influenza scenarios. These scenarios varied in terms of transmissibility, the proportion of symptomatic cases and illness severity (risk of primary care consultations, hospitalisations and deaths). Using probabilistic sensitivity analyses, a net health benefit approach was adopted to assess the cost-effectiveness of the interventions from a societal perspective.
Results
Compared with current sick leave practice, sick leave interventions were cost-effective for 31 (65%) of the pandemic scenarios, and 11 (92%) of the seasonal scenarios. Economic benefits from sick leave interventions were greatest for scenarios with low transmissibility, high symptomatic proportions and high illness severity. Overall, the health and economic benefits were greatest for the intervention involving 90% of sick workers taking sick leave within one-half day of symptoms. Depending on the influenza scenario, this intervention resulted in a 44.4%–99.7% reduction in the attack rate. Interventions involving sick leave onset beginning 2 days or later, after the onset of symptoms, resulted in economic losses.
Conclusions
Prompt sick leave onset and a high proportion of sick leave among workers with influenza symptoms may be cost-effective, particularly during influenza epidemics and pandemics with low transmissibility or high morbidity.
Keywords: human influenza, infection control, epidemiology, public health, health economics
Strengths and limitations of this study.
Although national recommendations for influenza management often advise sick leave from work, no systematic studies of health and cost consequences of such recommendations have been published, and no studies have evaluated the effects of sick leave interventions in detail.
This study uses mathematical modelling to compare current sick leave practice with 14 alternative sick leave interventions, related to the proportion of ill employees taking sick leave and the timeliness of sick leave relative to symptoms, to investigate the epidemiological effects of these interventions and their economic consequences.
Some of the parameters used in the modelling and evaluation are not influenza-specific, such as the above current sick leave practice, but rather based on influenza-like illness, being derived from interviews unaccompanied by test results.
All interventions were assessed for a variety of potential epidemic and pandemic influenza scenarios with varying characteristics.
We have studied population-wide effects for the Norwegian setting and our findings may not be directly transferrable to other settings or subgroups.
Introduction
Seasonal influenza affects 5%–15% of the world’s population annually. Globally, influenza epidemics are responsible for 250 000–500 000 deaths and 3–5 million cases of severe illness per year.1 During an influenza pandemic, the disease burden may increase substantially. The disease also imposes a considerable cost burden on the healthcare system, but the greatest proportion of costs are indirect costs resulting from lost workdays.2
When influenza-infected workers report to work, their coworkers are at risk of becoming infected. We recently conducted a literature review on influenza transmission in the workplace and assessed sick leave recommendations during influenza in 18 European countries.3 We found that while pandemic preparedness plans of many European countries officially advise sick workers to be absent from work, only one study was identified that had assessed the effectiveness of sick leave interventions during seasonal influenza.3 This was a modelling study indicating that liberal sick leave policies and increased payment compensations during sick leave would reduce workplace transmission up to 39%.3 4 Norway is a western European society with generous social welfare programmes, so few workers lose income as a result of sick leave due to influenza-like symptoms.5–7 No studies to date have ascertained whether sick leave during influenza is a cost-effective way of reducing the spread of influenza. In addition, countries that advise workers with influenza to take sick leave recommend diverse sick leave strategies.3
Influenza transmission depends on a complex interaction between the host, pathogen and the environment. Characteristics, such as the attack rate (AR) and disease severity of a particular influenza season, may affect which sick leave strategies are most cost-effective to implement. The effectiveness of sick leave as a mitigation intervention is limited by asymptomatic transmission. The proportion of asymptomatic cases reported in the literature varies between 25% and 75%,8–11 and asymptomatic cases may shed reduced amounts of the virus.12 Moreover, in symptomatic individuals, virus shedding may begin 1–2 days prior to the onset of symptoms.9 10 During the symptomatic phase, workers can either choose to be present at work while feeling ill (‘presenteeism’) or to remain at home (‘absenteeism’). Studies have suggested that workplace presenteeism during influenza infection is widespread.13 14 From a public health and socioeconomic perspective, incentivising sick leave during influenza infection may reduce disease transmission enough to reduce the overall costs to society.15 From the perspective of an employer, however, the burden of work absenteeism may be considerable, as the value of the work employees would have produced is lost.16 17
Using a model framework, we attempted to quantify the costs and health consequences of increasing sick leave among workers with influenza symptoms. In our study, we define sick leave as the period of time a worker is absent from paid work due to influenza symptoms. We simulated the effect of implementing different sick leave policies during an influenza outbreak in the Norwegian population. We conducted a survey to inform the model with local data on current influenza-related sick leave behaviour in Norway, and compared different sick leave interventions with current practice.
Material and methods
Modelling assumptions
We developed a model to quantify the number of mild, moderate and severe influenza cases. A scenario-based approach was applied to account for the fact that influenza, particularly pandemic influenza, varies in terms of transmissibility, likelihood of symptomatic infections and illness severity (ie, risk of primary care visits, hospitalisations and death). We differentiated between interventions (variation in sick leave behaviour) and scenarios (variations in influenza characteristics), and studied each sick leave intervention given each distinct influenza scenario. In total, we analysed current sick leave practice (baseline), and 14 alternative sick leave interventions combined with 48 influenza scenarios. The health outcomes from the disease model were used in an economic model to estimate costs and quality adjusted life years (QALYs). Because the parameters of the economic model (listed in Table SMM1, online supplementary file 1) were uncertain, we used Monte Carlo simulations to explore the consequences of the uncertainty. In this paper, we outline the main characteristics of the models and their input parameters. A detailed description of the survey and models is provided in online supplementary file 1.
bmjopen-2018-027832supp001.pdf (1.3MB, pdf)
Influenza-related sick leave
During epidemics, Norwegian health authorities advise that workers with symptoms of influenza remain at home until feeling well enough to work. During pandemics, sick leave is recommended until at least 24 hours following defervescence.3 18 Lacking data on influenza-related absences, we conducted a web-based survey in a convenience sample of 490 Norwegian employees. In total, 46% (224/490) of the participants reported having experienced influenza-like symptoms during the previous influenza season. Based on expert opinion, influenza-like symptoms, for the purposes of the survey, included: fever, cough, sore throat, headache, fatigue, muscle pain and/or stuffy nose. Among participants reporting influenza-like-symptoms, 74% had taken sick leave. The duration of absence varied from 1 to 13 workdays (mean of 2.4 days) and individuals waited from 1 to 8 days (mean of 2.7 days) after the onset of symptoms to take leave (Figure SMM1, online supplementary file 1). Among those who took sick leave, 24% began on the first day that they experienced symptoms, 43% on the second day, 19% on the third day, while 14% waited at least 4 days before taking sick leave (Figure SMM2, online supplementary file 1).
The survey respondents were mostly public sector employees who have high job security. There is evidence that workers with lower job security are more likely to attend work despite feeling ill,19 therefore we lowered the baseline sick leave rate in our model to 65% to make the results more representative of the general working population in Norway.
In the baseline sick leave setting, we assumed that symptomatic workers would stay at home for an average of 3.5 calendar days for seasonal influenza, adjusting for a working week of 5 days. For pandemic influenza, we increased this period to 6.5 calendar days, in line with the Norwegian national guidelines during the 2009 H1N1 pandemic that suggested 1 week of absence from the onset of symptoms. Consistent with the survey, we assumed that among those workers who take sick leave because of influenza, 24%, 43%, 19% and 14% would initiate sick leave on the first, second, third and fourth day relative to symptom onset, respectively. We found no data in the literature on the proportion of children absent from school or day-care due to influenza-like illness. Therefore, we assumed that 90% of children with influenza would remain at home, with cumulative withdrawal rates of 33%, 67% and 100% on the first, second and third day relative to symptom onset, respectively.
Interventions
We considered all combinations of the following interventions aimed at increasing the proportion of workers taking sick leave and/or reducing the delay from symptom onset to withdrawal from the workplace: (1) proportion of symptomatic workers taking sick leave: 65%, 80% and 90%, and (2) maximum time from symptom onset to sick leave: 0.5 days, 1 day, 1.5 days, 2 days and 4 days. These interventions were chosen based on the results from our survey on sick-leave behaviour, and on perceived feasibility. Interventions were compared with the baseline sick leave practice, defined as 65% of ill workers taking sick leave after a maximum of 4 days with symptoms. In children, the baseline pattern of sick leave was kept constant.
We simulated interventions with <4 days of maximum delay from symptoms onset to sick leave using a truncated variant of the baseline daily withdrawal proportions. For example, in the case of a maximum of 2 days delay, 24% would initiate sick leave when symptoms first appeared, 43% on the following day and the remaining 33% on the next day.
Main features of the influenza model and the economic model
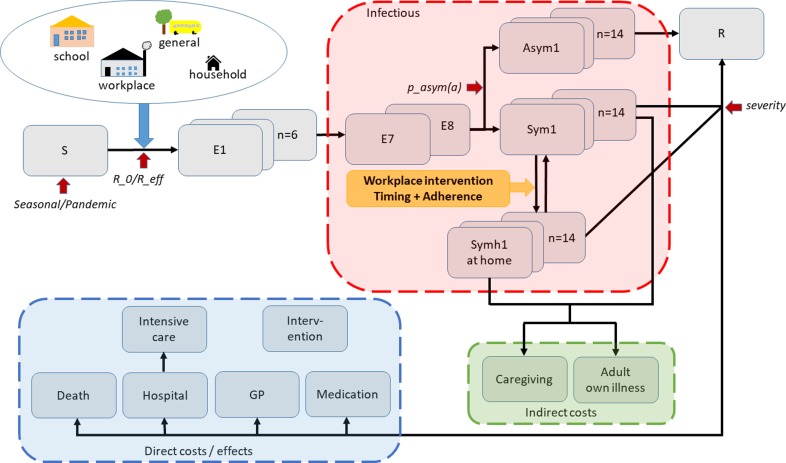
We developed an age-structured, deterministic simulation model (figure 1) for the spread of influenza in Norway (population: 5.05 million in January 2013). The social mixing structure, representing mixing within households, schools, workplaces and general society, was reconstructed from simulations based on real demographic data (Figure SMM3, online supplementary file 1). People at home with influenza illness were assumed to not mix with other people at work/school, or in the general population. We calibrated the model to a broad spectrum of seasonal and pandemic influenza scenarios: seasonal epidemics at an effective reproductive number (R_eff) of 1.2, 1.3 and 1.4, assuming 35% of children and 25% of adults would develop symptoms (low symptomatic proportions), or that 65% of children and 55% of adults would develop symptoms (high symptomatic proportions). For pandemic influenza, we constructed scenarios at a basic reproductive number (R_0) of 1.4, 1.6 and 1.8, also assuming low or high symptomatic proportions as described above. The reproductive number is defined as the number of secondary cases that one influenza case would produce, and can be regarded as a measure of transmissibility.
Figure 1.
Schematic representation of our model. An age-stratified SEIR model is used to model influenza spread; following infection, susceptible individuals (S) enter the incubation state, divided into pre-infectious (E1–E6) (E) and infectious (E7–E8) compartments. A proportion of individuals develop asymptomatic infection (Asym1-14) followed by recovery; the remainder develop symptomatic infection, categorised into people at school/work (Sym1-14), or people at home (Symh1-Symh14). Infectious individuals (red box, collectively denoted by I) mix with susceptible individuals in school, workplace, general community and household settings; people at home during illness experience reduced mixing outside their households. When the infectious period ends, individuals are moved to the Removed class (R), not participating in disease spread anymore. Influenza scenarios are defined by: initial proportions of susceptible persons, transmissibility, proportions of asymptomatic individuals and severity (red arrows). Influenza interventions are modelled by varying the timing and proportion of workers who take sick leave (yellow arrow). Healthcare utilisation and deaths were estimated based on the age-specific incidence of symptomatic infections. Direct costs and effects include healthcare/medication costs, and quality of life detriments due to morbidity and mortality (blue box). People who work during illness and people who stay home from work due to own illness or to provide caregiving incur indirect costs due to lost productivity (green box). See online supplementary file 1 for further details about the model structure.
We assumed that individuals become infectious prior to the onset of symptoms, and that their infectivity would peak approximately on the first day of symptoms and would last for 7 days, according to a given infectivity profile (Figure SMM4, online supplementary file 1). Individuals with asymptomatic infection were assumed to be half as infectious as those with symptoms, but with a similar contour of infectivity.
We developed a probabilistic health economic model to translate the output from the infection model into costs of healthcare, costs of sick leave (productivity losses) and the intervention costs for each intervention. Productivity losses are highly relevant in sick leave intervention studies, and therefore we assessed cost-effectiveness from a societal perspective. To ease comparison between the interventions and scenarios, we used a net health benefit (NHB) approach assuming that the value of a QALY (λ) was Norwegian kroner (NOK) 570 807 (US$98 06020 in line with Norwegian guidelines).21 By definition, NHB=QALY gains – (cost of intervention/ λ). This means that an intervention is cost-effective if NHB expressed as QALYs is >0. All costs were measured in 2012 NOK (US$1.00=NOK 5.82).20
The age-specific incidence of symptomatic influenza from simulations of the dynamic model was used as input data for the economic analyses. We used the estimates adopted in the 2014 Norwegian pandemic preparedness plan for the proportion of clinical cases that would require healthcare (visit to a general practitioner (GP), hospitalisation or intensive care treatment), and used estimates of mortality from the same source.22 The plan includes three distinct morbidity/mortality estimates for moderate, severe and very severe pandemics. The morbidity during seasonal influenza was assumed to be similar to that observed during a moderate pandemic.
The dynamic influenza model was developed in Matlab version R2013a using the ode45 solver. The economic model was developed in STATA V.13 and Excel 2010.
Patient and public involvement
Public health officials were involved in the development of the study design and outcome measures. Patients were not involved in study development, and study findings were not disseminated to study participants, as these were anonymous.
Results
This section is organised as follows: first, we present the baseline disease burden and baseline economic costs for each of the main scenarios. Second, we describe the health impacts of the sick leave interventions. Third, we present the results of the cost-effectiveness analyses. Lastly, we present results from the sensitivity analyses, in which we have assumed extra mixing in the household and general population in individuals who are absent from work. We present the epidemiological results by reporting relative changes in the clinical AR, which is defined as the proportion of the population that acquire a clinical infection. The comparative changes in GP visits, hospitalisations and mortalities closely mimicked changes in the AR. We report the cost-effectiveness results in terms of mean NHB. Complete tables for all results related to the epidemiologic outcomes, direct and indirect costs in the economic model, including probabilistic variation, are available on request from the authors.
Baseline scenarios
Table 1 shows the key epidemiologic and economic results for each of the baseline scenarios for seasonal and pandemic influenza. In the absence of any intervention, the model produced clinical ARs ranging from 3.2% to 16.9% for seasonal influenza at an R_eff of 1.2–1.4, and 9.4%–34.8% for pandemic influenza at an R_0 of 1.4–1.8. Visits to a GP and hospitalisations ranged from 478 to 2521 and 23 to 122 per 100 000 people for seasonal epidemics, and from 1398 to 8688 and 67 to 1207 per 100 000 for pandemics. The corresponding mortality ranged from 5 to 26 expected deaths per 100 000 people for seasonal influenza, and from 15 to 243 deaths for pandemic influenza.
Table 1.
Key population baseline epidemiological and economic outcomes for seasonal epidemics and severe pandemics in each of the scenarios considered
| Baseline outcomes in the total population | Seasonal influenza | Pandemic influenza severe (moderate; very severe)* | ||||
| R_eff† | R_0‡ | |||||
| 1.2 | 1.3 | 1.4 | 1.4 | 1.6 | 1.8 | |
| Low symptomatic proportions | ||||||
| Clinical attack rate, AR (%) | 3.2 | 5.3 | 7.0 | 9.4 | 13.0 | 15.6 |
| Median number of GP visits per 100 000 population | 478 | 789 | 1053 | 1866 (1398; 2334) | 2587 (1939; 3236) | 3115 (2334; 3896) |
| Median number of hospitalisations (per 100 000 population) | 23 | 38 | 51 | 184 (67; 325) | 255 (93; 450) | 307 (112; 541) |
| Median number of deaths (per 100 000 population) | 5 | 8 | 11 | 21 (15; 65) | 30 (20; 90) | 35 (24; 109) |
| Mean total costs (million US$) | 94 | 155 | 205 | 473 (401; 569) | 656 (557; 789) | 790 (670; 950) |
| Productivity losses (% of total costs) | 83 | 83 | 83 | 75 (88; 62) | 75 (88; 62) | 75 (88; 62) |
| High symptomatic proportions | ||||||
| Clinical attack rate, AR (%) | 9.0 | 13.3 | 16.9 | 22.3 | 29.5 | 34.8 |
| Median number of GP visits per 100 000 population | 1342 | 1983 | 2521 | 3329 (4442; 5557) | 5892 (4415; 7370) | 6946 (5205; 8688) |
| Median number of hospitalisations (per 100 000 population) | 65 | 96 | 122 | 438 (160; 772) | 581 (212; 1024) | 685 (251; 1207) |
| Median number of deaths (per 100 000 population) | 14 | 20 | 26 | 50 (34; 155) | 66 (44; 1024) | 78 (53; 243) |
| Mean total costs (million US$) | 257 | 378 | 479 | 1134 (963; 1363) | 1503 (1276; 1807) | 1770 (1503; 2128) |
| Productivity losses (% of total costs) | 82 | 82 | 82 | 75 (88; 62) | 75 (88; 62) | 75 (88; 62) |
*Moderate (severe; very severe) refers to illness severity in the influenza scenario.
†Effective reproductive number.
‡Basic reproductive number; cd, 35% of children aged <16 years, and 25% of adults aged 16+ years develop symptoms, e, 65% of children aged <16 years, and 55% of adults aged 16+ years develop symptoms.
The mean total costs of influenza in Norway, including productivity losses and healthcare resource use ranged from US$94 to US$479 million for seasonal epidemics, US$401 to US$1503 million for moderate pandemics, US$473 to US$1770 million for severe pandemics and US$569 to US$2128 million for very severe pandemics. Production losses made up the majority of the total costs. The proportion of the total costs owing to productivity losses was 82%–83% during seasonal influenza, and 62%–82% during pandemic influenza. The proportion was lowest during very severe pandemic influenza, where the healthcare costs increased substantially (online supplementary figure S1).
bmjopen-2018-027832supp002.pdf (2MB, pdf)
Epidemiological impact of sick leave interventions in workplaces
Figures 2 and 3 display the intervention effects on the AR, the epidemic peak delay and changes in the epidemic curves when compared with the baseline scenarios.
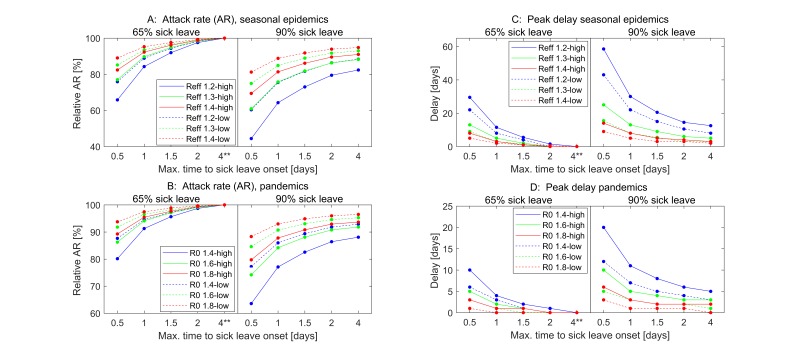
Figure 2.
Impact of workplace-based interventions on clinical attack rate and timing of peak for seasonal epidemics (panels A and C) and for pandemics (panels B and D). Scenarios assuming low symptomatic proportions (35% children, 25% adults develop symptoms) are depicted with stippled lines; scenarios assuming high symptomatic proportions (65% children, 55% adults develop symptoms) are depicted with solid lines. Each level of transmissibility has a unique colour (blue=lowest transmissibility, green=medium transmissibility and red=highest transmissibility). The figure shows sick leave interventions with 65% and 90% adherence combined with absence onset within 0.5, 1, 1.5, 2 and 4 days. The baseline intervention (65% adherence and sick leave onset within 4 days of symptom onset) is indicated by **.
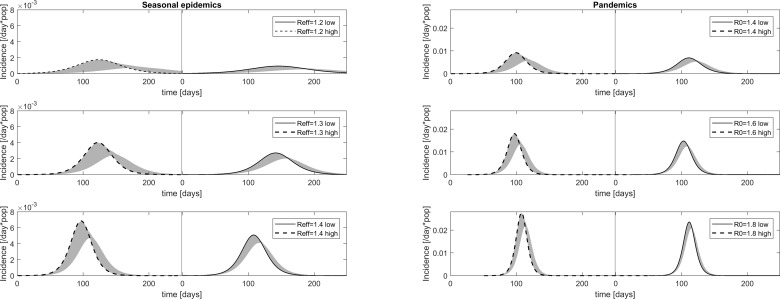
Figure 3.
Impact of workplace-based interventions on the epidemic and pandemic curves in all main scenarios. Daily incidence in baseline scenarios are depicted for seasonal epidemics at R_eff=1.2, 1.3 and 1.4 (left column) and for pandemic influenza at R0=1.4, 1.6 and 1.8 (right column). In each panel, the solid lines depict the baseline scenario (65% adherence and sick leave onset within 4 days of symptom onset) assuming low symptomatic proportions (35% children, 25% adults develop symptoms), and the stripled lines depict the baseline scenario assuming high symptomatic proportions (65% children, 55% adults develop symptoms). The shaded grey regions illustrate the range of curves obtained when introducing the 14 different workplace-based interventions.
For the seasonal influenza scenarios, the AR was reduced by 44.4%–98.8% (mean value of 85.4%) compared with the baseline values (figure 2A). The interventions achieved the highest reduction at the lowest transmissibility of R_eff=1.2 (blue) and at high symptomatic proportions (solid lines); the relative minimum AR was 60.3% assuming low symptomatic proportions (stippled lines). As expected, the interventions with a high proportion of workers on sick leave (90%) and early withdrawal from work/school (0.5 days) had the greatest effect. General trends in the pandemic scenarios were similar to those obtained in the seasonal epidemics. However, as the transmissibility in these scenarios was higher on average, the interventions were less effective. Overall, the interventions reduced the AR by 63.6%–99.7% (mean AR of 91.0%) relative to their baseline values (figure 2B). Pandemic scenarios with low symptomatic proportions had a relative minimum AR of 77.3%.
In the seasonal influenza scenarios, the interventions delayed the epidemic peak by 0–58 days. The delay was particularly pronounced at R_eff=1.2 (figure 2C and figure 3, left column top panel). The scenarios assuming low symptomatic proportions had a maximum time delay of 43 days, and most cases exhibited a delay of 1–2 weeks. Pandemic scenarios resulted in shorter peak time delays than the seasonal scenarios, ranging from 0 to 20 days (figure 2D and figure 3, right column); the delay of time to peak was at most 10 days in scenarios with low symptomatic proportions.
The median age among avoided clinical cases was similar within each scenario, ranging from 26.7 to 33.6 years for the seasonal scenarios, and from 33.6 to 38.1 years for the pandemic scenarios (online supplementary figures S2 and 3). More infections were avoided in younger individuals when transmissibility or symptomatic proportions were low.
Cost-effectiveness of sick leave interventions in workplaces
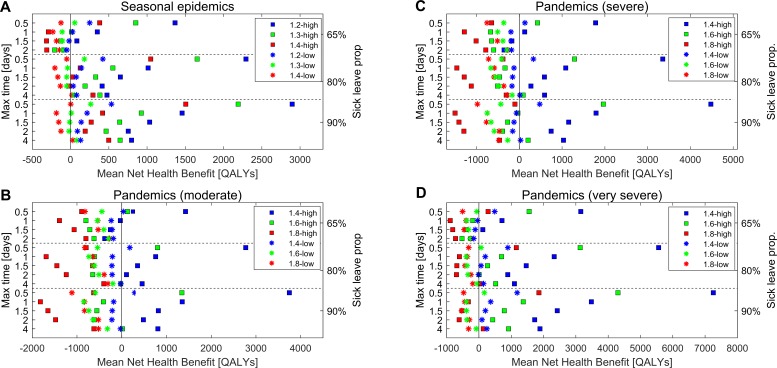
Figure 4 summarises the results of the cost-effectiveness analyses for seasonal influenza (figure 4A), and for pandemics assuming moderate, severe and very severe illness (figure 4B–D, respectively).
Figure 4.
Mean net health benefit of workplace-based interventions for all main scenarios; seasonal epidemics (A), moderate pandemics (B), severe pandemics (C) and very severe pandemics (D). Scenarios assuming low symptomatic proportions (35% children, 25% adults develop symptoms) are depicted with crosses, and scenarios assuming high symptomatic proportions (65% children, 55% adults develop symptoms) are depicted with squares. Each level of transmissibility has a unique colour (blue=lowest transmissibility, green=medium transmissibility and red=highest transmissibility).
In total, for 100% (6/6) of seasonal influenza scenarios, sick leave interventions were cost-effective compared with current sick leave practice; cost-effective interventions were obtained for 50% (3/6) of moderate, 50% (3/6) of severe and 87% (5/6) of very severe pandemic scenarios. In general, the mean NHB was higher at low transmissibility (blue) compared with high transmissibility (red), assuming that all other factors remained equal (figure 4). The mean NHB was larger at high symptomatic proportions (squares) compared with low symptomatic proportions (crosses), for similar transmissibility.
In the pandemic scenarios assuming low symptomatic proportions, interventions were cost-effective for R_0<1.6, except in the case of a very severe pandemic where interventions were also cost-effective for R_0=1.6 (figure 4B–D). For pandemic influenza with high symptomatic proportions, all scenarios at R_0<1.8 produced cost-effective interventions. For very severe pandemic scenarios, cost-effective interventions were also found for R_0=1.8.
In 16 of the 17 scenarios for which interventions were cost-effective, the superior intervention was for 90% of ill workers to take sick leave within one-half day of the onset of symptoms (figure 4 and online supplementary figure S1). While in one scenario, a seasonal epidemic at R_eff=1.4 with low symptomatic proportions, 90% of symptomatic workers taking sick leave at the baseline delay from symptom onset, was the most cost-effective intervention. In this particular case, the combination of 90% of symptomatic workers taking sick leave and sick leave onset within 0.5 days ranked third in terms of cost effectiveness. Generally, when symptomatic proportions were low, the only cost-effective interventions were those in which sick leave onset occurred within 0.5 days, or interventions solely increasing the adherence. In contrast, scenarios with high symptomatic proportions produced cost saving results for a variety of different interventions.
Among the cost-effective interventions, the largest mean NHB was in the range 31–535 QALYs for low symptomatic proportions and 1506–2898 QALYs for high symptomatic proportions in the seasonal scenarios. For pandemic scenarios with low symptomatic proportions, interventions were cost-effective for moderate and severe scenarios with low transmissibility (R_0=1.4), and for very severe scenarios with low and moderate transmissibility (R_0=1.4 and R_0=1.6). The largest mean NHBs were 292, 477 and 170–1185 QALYs for assumptions of moderate, severe and very severe morbidity/mortality, respectively. For high symptomatic proportions, the QALY value varied from 345 to 3749, 1966 to 4481 and 1859 to 7256 for moderate, severe and very severe morbidity/mortality, respectively.
Notably, interventions that focused exclusively on increasing the proportion of symptomatic workers taking sick leave, had comparatively high probabilities of being cost-effective, as shown by the stochastic simulations and illustrated in acceptability curves (online supplementary figure S4). Conversely, interventions with sick leave starting later than 1 day after the onset of symptoms were generally not cost-effective, except for scenarios with high symptomatic proportions, or when combined with an increased proportion of symptomatic workers taking sick leave.
Sensitivity analyses: assuming extra mixing for individuals absent from work
In the sensitivity analyses where additional mixing in the household and the general population was assumed, the effectiveness of sick leave interventions was somewhat diminished compared with the main scenarios (online supplementary figure S5). However, on the whole, the cost-effectiveness and ranking of the different interventions under the various scenarios were retained (online supplementary figures S6 and 7). The reduction in the AR relative to the baseline varied from 52.7% to 99.4% in the seasonal scenarios, and 69.1% to 99.7% in the pandemic scenarios (online supplementary figure S5). In total, 83% (5/6) of seasonal scenarios, and 33% (2/6) of moderate, 50% (3/6) of severe and 67% (4/6) of very severe pandemic scenarios produced cost-saving interventions. Consistent with the results obtained in the main analyses, the best intervention for the scenarios with cost-effective results was 90% of symptomatic workers taking sick leave with withdrawal at 0.5 days after the onset of symptoms. For this intervention, the mean NHB varied from 101 to 2192 QALYs for seasonal epidemics, and from 168 to 2414, 131 to 3019 and 388 to 5314 QALYs for moderate, severe and very severe pandemics, respectively.
Discussion
We have shown that the effectiveness of sick leave during influenza on reducing the spread of the disease is dependent on: (1) timing of absence onset, (2) the proportion of ill workers leaving work and (3) the characteristics of the influenza epidemic (transmissibility, influenza severity, etc). The results of our study indicate that the earlier the absence and the greater the proportion leaving work, the greater the effectiveness. Leaving work >2 days after onset of symptoms has minimal impact on the spread of the disease. Even when taking costs of lost production into account, early absence among high proportions of workers is cost-effective in most disease scenarios. Exceptions are pandemics with low transmissibility and general epidemics with low symptomatic proportions.
The modelling approach allowed us to simulate population level effects of different sick leave interventions under a range of possible influenza scenarios, providing information that would not readily be observed in real-life studies. The scenarios presented are largely consistent with those proposed in a recent review on pandemic influenza scenarios in Europe, in which the authors argued for the use of multiple scenarios based on the recent experience from the 2009 H1N1 pandemic.23 Other studies address the effects of expanding the right to sick leave,4 24 but since access to paid sick leave is more or less universal in Norway, we have focused specifically on different sick leave interventions. Our study is the first to investigate epidemiological and economic outcomes of workplace-based interventions on a population level. We are also the first, to our knowledge, to investigate the effects of the timeliness of sick leave initiation relative to symptom onset during influenza.
Our results indicate that early withdrawal is important for cost-effectiveness, but this result may depend on the ability to differentiate influenza from other illnesses with similar symptoms. Because influenza symptoms are non-specific, and it is unknown whether sick leave interventions are cost-effective for illnesses with influenza-like symptoms, for example, respiratory syncytial virus, early withdrawal may not be as cost-effective in practice. Influenza surveillance data, which is available in many countries, could be used to restrict recommendations to apply only in geographic regions where influenza activity is rising. Another central question is how these sick leave recommendations can be communicated effectively to the working population and the costs of achieving the sick leave behaviours described. In our study, the cost-effective interventions were also assumed to be the most costly to implement, with a mean cost of US$5.6 million; but the true cost is uncertain. A pilot study could be initiated to assess costs and feasibility of earlier sick leave and increased proportion of symptomatic workers taking sick leave.
Our study has several limitations. The profile of infectiousness assumed in our model was an influential variable. Although it was based on data from a household study, we acknowledge that there is uncertainty related to how infectiousness changes over time, and to the relative infectivity of an asymptomatic infection. The proportion of GP visits and hospital admissions, and the case-fatality rate assumed under different influenza scenarios were based on estimates proposed by Norwegian experts, and were not age-specific. A recent review reported lower estimates in other European countries,23 but these values are likely country-specific. Another limitation of this study was that influenza illness has been shown to reduce productivity at work,25 however, this may vary depending on occupation. We assumed that 8% of workers would continue to work from home during their illness and while taking care of sick children, but information on this topic is scarce. A study from Sweden found that 60% of parents work from home when their children are sick26 thus our assumption may underestimate the economic benefit of the intervention. The economic benefits from earlier onset of sick leave may also have been underestimated. It seems plausible that earlier sick leave onset could lead to a quicker recovery, however, we could not find any evidence of this in the literature; therefore, we assumed the recovery period to be constant, and independent of sick leave onset. Finally, influenza cases and workplace absences were modelled to occur randomly on a population level. In reality, absences may cluster in specific workplaces, which may cause understaffing for critical functions and a subsequent increase in cost.
We assumed that the number of days of sick leave was 3.5 calendar days for seasonal influenza and 6.5 calendar days for pandemic influenza. Because we found that the epidemiological benefits of sick leave were limited after 2 days of symptoms, we also explored the effect of assuming the same number of total absence days during pandemics as during epidemics (3.5 calendar days). This resulted in higher economic benefits for interventions involving early onset within 1 day, but lower benefits for other interventions.
Current recommendations on sick leave during influenza are typically focused on the duration of sick leave, but the present results suggest that recommendations may be improved by encouraging prompt initiation of sick leave. However, although sick leave can reduce the spread of influenza, our findings indicate that this effect is insufficient to offset an ongoing epidemic or pandemic so, ideally, sick leave interventions should be implemented in conjunction with existing strategies. Economic evaluations of mitigation interventions such as vaccines, antivirals and school closuresare common in the literature.27–29 In contrast, studies on sick leave interventions are limited,28 29 which is somewhat surprising considering that this is a widespread recommendation in national pandemic preparedness plans.3 Moreover, pharmaceutical interventions are limited by availability30; therefore, non-pharmaceutical interventions can be considered as viable backup strategies. As a result, there is a need for quantitative modelling for policy planning and decision-making purposes. The present economic results are based on Norwegian demographic and economic assumptions, and several factors would need to be recalculated for use in other countries. Nevertheless, our model provides a structure for analysing this problem and provides a method, which could be applied in other settings.
The findings in this paper indicate that there are epidemiological and economic benefits from sick leaves during influenza; however, further studies are needed to assess these effects in more detail and in other settings. Future studies should consider collecting additional data on influenza transmission pathways, sick leave practice and the behaviour of workers during sick leave. Ideally, such studies should also aim to test for influenza to establish aetiology, rather than relying on self-reported influenza status. Moreover, it is of importance to conduct studies to explore the effects of sick leave interventions within specific occupational groups. For example, influenza has been found to be less prevalent in janitors and technicians compared with other occupations.31 Likewise, some workers may be more likely to spread influenza (eg, a waiter in a restaurant), or be more likely to spread influenza to high-risk persons (eg, healthcare workers). Finally, investigations into the cost-effectiveness of sick leave interventions for other communicable diseases, perhaps especially those with high illness severity or low transmissibility, are warranted.
Conclusion
Recommending early absence from work among all workers with influenza symptoms represents an effective intervention during influenza epidemics and pandemics. The intervention is also cost-effective in most influenza scenarios.
Supplementary Material
Acknowledgments
The authors wish to thank Siri Helene Hauge and Olav Hungnes of the Norwegian Institute of Public Health for their insightful comments and guidance on influenza and influenza-related policies. We would also like to thank Arna Desser for her assistance with proofreading the article.
Footnotes
Contributors: The study was designed by BFdB, CHE and ISK. The mathematical model was designed by BFdB and GST, and the economic model was developed by CHE, ISK and RW. The data analysis was performed by CHE and BFdB. The manuscript was prepared by CHE and BFdB. All authors revised and accepted the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: Informed consent was obtained from all survey participants. The study was reviewed by the Data Protection Official at the University of Oslo, and it was considered that approval from an ethical committee was not required due to the nature/content of the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The main sources of data have been provided in the text of the main article or in the supplementary files, however, additional information can be provided by the authors on request.
Patient consent for publication: Not required.
References
- 1. World Health Organization. Influenza (seasonal) factsheet No211: World Health Organization, 2009. [Google Scholar]
- 2. Xue Y, Kristiansen IS, de Blasio BF. Modeling the cost of influenza: the impact of missing costs of unreported complications and sick leave. BMC Public Health 2010;10:724 10.1186/1471-2458-10-724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edwards CH, Tomba GS, de Blasio BF. Influenza in workplaces: transmission, workers' adherence to sick leave advice and European sick leave recommendations. Eur J Public Health 2016;26:478–85. 10.1093/eurpub/ckw031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar S, Grefenstette JJ, Galloway D, et al. . Policies to reduce influenza in the workplace: impact assessments using an agent-based model. Am J Public Health 2013;103:1406–11. 10.2105/AJPH.2013.301269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Insurance Act. Act 1997-02-28-19. Act relating to national insurance Avd I 1997 Nr. 5LOV-1997-02-28-19. https://lovdata.no/dokument/NL/lov/1997-02-28-19.
- 6. Intensjonsavtale om et mer inkluderende arbeidsliv 4. Intensjonsavtale om et mer inkluderende arbeidsliv 4. mars 2014 - 31. desember 2018 (IA-avtalen). 2014. https://www.regjeringen.no/globalassets/upload/asd/dokumenter/2014/ia_20142018/signert_ia_avtale.pdf.
- 7. Ose SO, Dyrstad K, Slettebak R, et al. . Evaluering av IA-avtalen (2010-2013). 2013. http://www.sintef.no/prosjekter/evaluering-av-ia-avtalen-2010-2013/.
- 8. Elder AG, O’Donnell B, McCruden EA, et al. . Incidence and recall of influenza in a cohort of Glasgow healthcare workers during the 1993-4 epidemic: results of serum testing and questionnaire. BMJ 1996;313:1241–2. 10.1136/bmj.313.7067.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carrat F, Vergu E, Ferguson NM, et al. . Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008;167:775–85. 10.1093/aje/kwm375 [DOI] [PubMed] [Google Scholar]
- 10. Lau LL, Cowling BJ, Fang VJ, et al. . Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis 2010;201:1509–16. 10.1086/652241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suess T, Remschmidt C, Schink SB, et al. . Comparison of shedding characteristics of seasonal influenza virus (sub)types and influenza A(H1N1)pdm09; Germany, 2007-2011. PLoS One 2012;7:e51653 10.1371/journal.pone.0051653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patrozou E, Mermel LA. Does influenza transmission occur from asymptomatic infection or prior to symptom onset? Public Health Rep 2009;124:193–6. 10.1177/003335490912400205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nichol KL, D’Heilly SJ, Greenberg ME, et al. . Burden of influenza-like illness and effectiveness of influenza vaccination among working adults aged 50-64 years. Clin Infect Dis 2009;48:292–8. 10.1086/595842 [DOI] [PubMed] [Google Scholar]
- 14. Carrat F, Sahler C, Rogez S, et al. . Influenza burden of illness: estimates from a national prospective survey of household contacts in France. Arch Intern Med 2002;162:1842-8. [DOI] [PubMed] [Google Scholar]
- 15. Liao S, Ma Y, Chen J, et al. . Paid sick-leave: is it a good way to control epidemics? : Glass K, Colbaugh R, Ormerod P, Complex sciences: second international conference, COMPLEX 2012, S, December 5-7, 2012, Revised Selected Papers. Santa Fe, NM, USA: ChamSpringer International Publishing, 2013:213–27. [Google Scholar]
- 16. Keech M, Scott AJ, Ryan PJJ. The impact of influenza and influenza-like illness on productivity and healthcare resource utilization in a working population. Occup Med 1998;48:85–90. 10.1093/occmed/48.2.85 [DOI] [PubMed] [Google Scholar]
- 17. Keech M, Beardsworth P. The impact of influenza on working days lost. Pharmacoeconomics 2008;26:911–24. 10.2165/00019053-200826110-00004 [DOI] [PubMed] [Google Scholar]
- 18. Norwegian Institute of Public health. Reviderte råd for anbefalinger om sykefravær ved influensaliknende sykdom [Revised advice for recommendations on absenteeism due to influenza-like illness]. https://www.fhi.no/globalassets/migrering/dokumenter/pdf/rapport-om-anbefalinger-om-fravar-ved-sykdom-2009-11-18.pdf.pdf.
- 19. Johns G. Presenteeism in the workplace: a review and research agenda. J Organ Behav 2010;31:519–42. 10.1002/job.630 [DOI] [Google Scholar]
- 20. Central bank of Norway.. Exchange rate for USD. 2013. http://www.norges-bank.no/Statistikk/Valutakurser/
- 21. Norwegian Directorate of Health. Helseeffekter i samfunnsøkonomiske analyser. 2007. https://helsedirektoratet.no/lists/publikasjoner/attachments/643/helseeffekter-i-samfunnsokonomiske-analyser-is-1435.pdf
- 22. Ministry of Health and Care Services. Norwegian national influenza pandemic preparedness plan. 2006. https://www.regjeringen.no/no/dokumenter/nasjonal-beredskapsplan-for-pandemisk-in/id102132/
- 23. Napoli C, Fabiani M, Rizzo C, et al. . Assessment of human influenza pandemic scenarios in Europe. Euro Surveill 2015;20:29–38. 10.2807/1560-7917.ES2015.20.7.21038 [DOI] [PubMed] [Google Scholar]
- 24. Kumar S, Quinn SC, Kim KH, et al. . The impact of workplace policies and other social factors on self-reported influenza-like illness incidence during the 2009 H1N1 pandemic. Am J Public Health 2012;102:134–40. 10.2105/AJPH.2011.300307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brouwer WB, van Exel NJ, Koopmanschap MA, et al. . Productivity costs before and after absence from work: as important as common? Health Policy 2002;61:173–87. 10.1016/S0168-8510(01)00233-0 [DOI] [PubMed] [Google Scholar]
- 26. Novus. Unionen – Privata tjänstemän om att vobba 2015: Unionen. 2015. http://www.unionenopinion.se
- 27. Lee VJ, Lye DC, Wilder-Smith A. Combination strategies for pandemic influenza response - a systematic review of mathematical modeling studies. BMC Med 2009;7:76 10.1186/1741-7015-7-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aledort JE, Lurie N, Wasserman J, et al. . Non-pharmaceutical public health interventions for pandemic influenza: an evaluation of the evidence base. BMC Public Health 2007;7:208 10.1186/1471-2458-7-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crabtree A, Henry B. Non-pharmaceutical measures to prevent influenza transmission: the evidence for individuals protective measures: national collaborating center for infectious diseases. 2011.
- 30. Bell D, Nicoll A, Fukuda K, et al. . Non-pharmaceutical interventions for pandemic influenza, international measures. Emerg Infect Dis 2006;12:81–7. 10.3201/eid1201.051370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson NJ, Bonauto DK, Fan ZJ, et al. . Distribution of influenza-like illness (ILI) by occupation in Washington State, September 2009-August 2010. PLoS One 2012;7:e48806 10.1371/journal.pone.0048806 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027832supp001.pdf (1.3MB, pdf)
bmjopen-2018-027832supp002.pdf (2MB, pdf)