Abstract
Objectives
Pre-exposure prophylaxis (PrEP) users are screened bi-annual for sexually transmitted infections (STIs). A novel device, called the Colli-Pee, collects first-void urine in a standardised way and the collector tube can be easily delivered by regular post to a certified laboratory. The aim of the study was a one-to-one comparison between the STI test results obtained with the urine collected in the clinic, versus urine collected at home in a real-life setting by Men who have Sex with Men (MSM) in Belgium. The user-friendliness and acceptability of the Colli-Pee device by the users was also evaluated.
Design
A single-site nested substudy in a prospective PrEP demonstration project (Be-PrEP-ared) among MSM in Belgium.
Participants
A total of 473 home-based samples from 213 MSM were received with a mean age of 38.5 years.
Interventions
Participants were requested to collect a urine sample at home using the Colli-Pee device and to send it to the laboratory via regular mail.
Primary and secondary outcome measures
The presence of Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Mycoplasma genitalium (MG) and Trichomonas vaginalis (TV) was determined using molecular amplification assays. Agreement between test results of samples collected at the clinic and collected at home were evaluated using Cohen’s kappa statistic.
Results: TV was not detected. A very good to almost perfect agreement was found for CT, NG and MG of κ=0.75, 0.87 and 0.85, respectively. Using the Colli-Pee device only one low positive CT and two MG infections were missed, however, three additional CT, two NG and six MG infections were detected.
Conclusions
The Colli-Pee device is a feasible and convenient way to collect urine at home for STI testing. This may be particularly relevant for populations that need frequent STI testing, such as PrEP users and patients who prefer home-sampling.
Trial registration number
Keywords: neisseria gonorrhoeae, chlamydia trachomatis, screening, urine, pre-exposure prophylaxis, MSM
Strengths and limitations of this study.
The study was designed as such to provide real-world experience concerning home-based sampling for sexually transmitted infection (STI) detection including shipment by post among pre-exposure prophylaxis (PrEP) users.
Home-based and clinic-based samples were processed and analysed using the same procedures and laboratory staff was blinded for the results of the matching sample.
The study was performed among Men who have Sex with Men PrEP users who have a high prevalence of STIs.
Our main limitation is that home-based samples were not taken on the same day as clinic-based sampling and participants could have become positive during that window period.
Another limitation of the study is that the temperature during transportation of home-based samples to the clinic was not monitored.
Introduction
According to the WHO’s Global Health Sector Strategy on sexually transmitted infections (STIs) 2016–2021, early diagnosis and linkage to treatment are one of the key elements for preventing further transmission of STIs.1 Currently, first-void urine is still favoured as the sample of choice for the detection of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) in men, using nucleic acid amplification tests (NAATs).2–4 In general, a regular urine container is used to collect the first-void urine sample, but the collected volume of urine is not standardised. Furthermore, this type of container is less convenient for postal delivery to the laboratory. Another sponged-based device (UriSWAB, Copan Diagnostics, Brescia, Italy) has been suggested as an alternative for postal delivery of urine. However, this device only holds 2 mL of urine and does not guarantee that only first-void urine is collected.4 5 The (Conformité Européene (CE) labelled Colli-Pee device (Novosanis, Belgium), provides a clean and standardised solution to the above-mentioned issues as it efficiently captures first-void urine (20 mL) without interruption of the urine flow and allows the samples to be sent by post (figure 1). The Colli-Pee device is currently used for the detection of human papilloma virus and several urological cancers for which collection of first-void urine is essential.6 7 In the field of STIs, a standardised first-void urine study reported that the organism load of CT is maximal in the first 4–5 mL and that the performance of diagnostic tests improved when using only first-void urine.8 9
Figure 1.
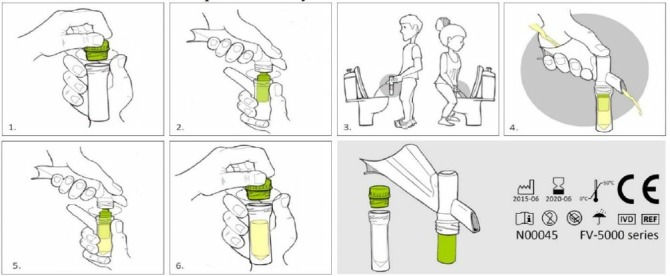
The Colli-Pee device instructions for use.
Although pre-exposure prophylaxis (PrEP) is becoming crucial in HIV prevention, recent reviews of real-world PrEP demonstration studies showed that PrEP is associated with increased diagnoses of STIs in Men who have Sex with Men (MSM).10 11 Consequently, current guidelines recommend a biannual screening of STIs in PrEP users because of their high-risk behaviour.12 13
In order to facilitate the patient flow during follow-up visits by PrEP users, and prompt treatment of STIs, home-based collection of first-void urine could be sent to the laboratory by regular mail for STI detection before the scheduled visit. STI results may then be available at the time of the physician consultation and in the case of a detected STI also immediately treated, limiting the risk of further transmission.
The objectives of this study were to compare the results of the molecular detection of several STIs using the Colli-Pee device versus a sample obtained in the clinic, the use and acceptability of the Colli-Pee device and its convenience for shipment by regular mail. To assess these objectives, a nested substudy was performed among MSM who participated in a Belgian PrEP demonstration cohort.14
Methods
The evaluation was undertaken as a substudy of Be-PrEP-ared, a PrEP demonstration study among MSM at high risk for HIV in Belgium.
The main study
The Be-PrEP-ared project (EudraCTn°: 5015–00005437) was a phase 3, single-site, open-label prospective cohort study where 200 MSM at high risk of acquiring HIV were asked to participate in the project and to take PrEP daily or event-driven. Detailed study methods are described elsewhere.14 Participants were tested for NG, CT, Mycoplasma genitalium (MG) and Trichomonas vaginalis (TV) at baseline and every 3 months. Detection of these STIs was performed at the three biological sites: urethra, anorectum and pharynx. During each study visit, participants collected urine in two urine containers at the clinic as per the following instructions: urinate in the first container up to the marked line at approximately 20 mL, afterwards complete the second cup with no restrictions. Urine in the first container (hereafter the clinic-based sample) was weighed and thereafter stored refrigerated until analysis that took place within 48 hours. Urine in the second container was used to detect proteinuria.
Laboratory procedures
In the first instance, CT/NG detection was performed using the Abbott Real Time (RT) CT/NG assay (DNA extraction and sample preparation using Abbott m2000sp and the Abbott m2000rt system for amplification and detection of CT/NG [Abbott Molecular Des Plaines, Illinois, USA]) according to manufacturer’s instructions. The remainder of the urine and DNA extracts were stored at −80°C. In the case of positivity, the same DNA extracts were tested by in-house real time (RT)-PCR assays for CT and/or NG, both based on previously published primer sets.15 16 A sample was considered positive when positive in both the Abbott and the in-house RT-PCR. An initial positive Abbott assay result followed by a negative confirmatory NAAT result was defined as ‘not confirmed’. Inhibition of the NAAT according to the Abbott assay was defined as ‘inhibition’.
The same DNA extracts were used for further testing. MG was detected and reported using an accredited in-house RT-PCR that targets the pdhD-gene17 and in addition the DiaMGTV multiplex kit (Diagenode diagnostics, Seraing, Belgium) that detects MG and TV simultaneously was used for TV detection. The results for MG of the DiaMGTV multiplex kit were not used for reporting purposes and are only provided for information only. No further confirmation of TV took place.
The Colli-Pee substudy
At the baseline visit of the Be-PrEP-ared study, participants were asked consent to participate in this substudy. After signing the informed consent form, they received a Colli-Pee device and a prepaid envelope. They were instructed to collect first-void urine the next day at home using the Colli-Pee device (the home-based sample), to document the date and time of collection and to send the collector tube filled with urine back to the laboratory by regular post, using the prepaid envelope. On receipt in the laboratory, the urine was weighed, stored refrigerated (2°C–8°C) and CT, NG, MG, TV was detected using the same NAATs within 48 hours. The urine and DNA extracts’ remnants were stored at −80°C. The quantity of human cells was measured at baseline using a human Endogenous Retrovirus-3 (ERV-3) quantitative PCR on the paired clinic- and home-based samples.18
The lab technicians were blinded for the results obtained for the clinic-based sample. In addition, the result of the home-based sample was not disclosed to the physician or participant.
During the next visit, which took place within 14 days after baseline, participants were asked to complete a small survey (five questions only) on the user-friendliness and willingness to use the Colli-Pee device (acceptability). Two questions documenting their opinion (likes-dislikes) of the Colli-Pee device were open-ended.
At follow-up month 6 and month 18 of the study, Colli-Pee devices were again distributed to those who agreed to participate and the survey was repeated at month 18 (results unreported).
Patient and public involvement
Patients were not involved in the Colli-Pee substudy. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Statistical analysis
The agreement of the results of the molecular assays using each of the two sampling methods was assessed by the use of Cohen’s kappa statistic and percent agreement. Samples that were not confirmed were coded as negative samples for the calculation of the agreement. The agreement of volume of urine collected and the agreement of concentration of human DNA in both sampling methods was assessed by using a t-test. A p-value of <0.05 was considered statistically significant. Both analyses were performed using STATA V.15.0.
A descriptive analysis was made of the results of the self-administered questionnaire on the acceptability and user-friendliness of the Colli-Pee device.
Results
Demographics
The main study took place at the Institute of Tropical Medicine, Antwerp, Belgium from September 2015 until May 2018. Of the 219 participants who were screened for eligibility into the main study, six participants did not consent to the Colli-Pee substudy. All participants who consented to the substudy were MSM and three identified themselves as transwomen.19 The mean age of the participants was 38.5 years (IQR 32–44). A total of 473 home-based samples from 213 participants were received. Two home-based samples could not be linked to the corresponding clinic-based sample and were therefore excluded, bringing the total number to 471. As shown in table 1, the number of home-based samples received at the laboratory declined over time.
Table 1.
Number of clinic and home-based samples received during the study
| Kind of visit | Clinic based | Home based (% home-based samples received) |
| Screening | 218 | 187 (85.8%) |
| Month 6 | 191 | 152 (79.6%) |
| Month 18 | 179 | 132 (73.7%) |
Although the participants were instructed to report the urine collection date and hour on the collection device, only 72.8% (343/471) were labelled with collection date. Most of the home-based samples (79.6%) were taken within 2 days after the clinic-based sample and 3.8% were taken after 20 days (13/343) (median 1 day; min-max: 0–70 days). The median time between the collection of the home-based sample and its reception at the laboratory after postal return was 5 days (min-max: 0–27 days), 72.9% arrived at the laboratory within those 5 days, an additional 25.7% within 10 days and five samples were received after 10 days (11, 13, 15, 17 and 27 days, respectively).
Comparison of weight and concentration of human material between both sampling methods
A total of 455 home-based and 423 clinic-based samples were weighed. The mean net weight of the home-based sample was 19.68g±2.14 g (95% CI 19.5 g to 19.9 g and min-max: 6.81 g- to 39.47g) vs 22.87g±13.64 g (95% CI 21.6 g to 24.2g and min-max: 2.88 g to 86.23 g) for the clinic-based sample (p<0.001).
The quantity of human cells was analysed at baseline only (n=187). In a total of five home-based and one clinic-based sample, ERV could not be detected and these samples were considered as lacking human material. After removal of the paired samples lacking ERV or containing inhibitors, 182 observations could be paired. The mean quantity of the clinic-based sample was 11.3*10³ cells/PCR (95% CI 7.4 to 15.2*10³) and for the home-based sample 14.2*10³ cells/PCR (95% CI 6.8 to 21.5*10³) (p>0.05).
STI results and agreement
Of the 471 home-based samples with a matching visit, six home-based and one clinic-based sample gave inhibition and were excluded from the analysis (n=464). The results are shown in table 2.
Table 2.
Sexually transmitted infections results of the home-based and clinic-based urine samples
| STI | Home-based urine result | Clinic-based urine results | ||
| Negative | Positive | Total | ||
|
Chlamydia trachomatis
(non-LGV) |
Negative | 454* | 1 | 455 |
| Positive | 3 | 6 | 9 | |
| Total | 457 | 7 | 464 | |
| Neisseria gonorrhoeae | Negative | 455 | 0 | 455 |
| Positive | 2 | 7 | 9 | |
| Total | 457 | 7 | 464 | |
| Mycoplasma genitalium | Negative | 431 | 2 | 433 |
| Positive | 6 | 25 | 31 | |
| Total | 437 | 27 | 464 | |
| Trichomonas vaginalis | Negative | 464 | 0 | 464 |
| Positive | 0 | 0 | 0 | |
| Total | 464 | 0 | 464 | |
*Two result were not-confirmed in the clinic-based sample.
TV was not detected. Percent agreement (Cohen’s kappa coefficient) for CT, NG and MG is 99.1% (0.75); 99.6% (0.87) and 98.3% (0.85), respectively, which indicates substantial agreement for CT and almost perfect agreement for the other two STIs.
Tables 3 and 4 show the discordant results. For some of the home-based samples, the date of collection was unknown so the time between the clinic visit and time of reception at the laboratory is depicted here. A delta cycle (DC) value of the Abbott assay of less than two indicates a low positive infection.
Table 3.
Sexually transmitted infections that were not detected in home-based urine samples
| STI | DC value CT/NG Abbott assay | Ct value in-house RT-PCR for CT, NG or MG* | Ct-value S-DiagMGTV RT-PCR (for information only) | Days between collection | Days of transport |
| CT | 1.49 | 33.19 | NA | 0 | 2 |
| MG | NA | 32.20 | Neg | 3 | 4 |
| MG | NA | 32.04 | 37.44 | 0 | 8 |
*A different in-house RT-PCR assay was used for CT, NG or MG.
Ct, cycle threshold; DC, delta cycle; Max, days between clinic visit and reception of the home-based sample at the laboratory.
Table 4.
Sexually transmitted infections that were additionally detected in home-based urine samples
| STI | DC value CT/NG Abbott assay | Ct value in-house RT-PCR for CT, NG or MG* | Ct-value S-DiagMGTV RT-PCR (for information only) |
Days between collection | Days of transport |
| CT | 3.99 | 34.26 | NA | 1 | 2 |
| CT | 2.68 | 35.31 | NA | 1 | 6 |
| CT | 0.27 | 36.06 | NA | 6 | 4 |
| NG | 2.93 | 37.58 | NA | 8 | 4 |
| NG | 10.08 | 25.26 | NA | 2 | 6 |
| MG | NA | 31.28 | Neg | Max 6 | Max 6 |
| MG | NA | 31.96 | 40.51 | 1 | 2 |
| MG | NA | 28.66 | 34.67 | Max 3 | Max 3 |
| MG | NA | 34.58 | 38.50 | 9 | 4 |
| MG | NA | 34.23 | Neg | 1 | 5 |
| MG | NA | 32.04 | 38.14 | Max 3 | Max 3 |
*A different in-house RT-PCR assay was used for CT, NG or MG.
Ct, cycle threshold; DC, delta cycle; Max, number of days between clinic visit and reception of the home-based sample at the laboratory.
Acceptability and user-friendliness of the Colli-Pee device
A total of 164 participants provided feedback regarding the use of the Colli-Pee device at baseline. On a scale of one to five, 87.8% found that the Colli-Pee device was easy to very easy to use. Instructions on how to send the Colli-Pee device were found to be easy by 90.2% of the participants. Four participants found the Colli-Pee difficult to use (2.4%) and four other participants found it difficult to follow the instructions (2.4%). Likes from participants were: the ease of use (54.9%), no interruption of the urine flow (15.9%), hygienic (11.6%) and privacy of the home-based sample collection (11.0%); the dislikes were: nothing (47.0%), not being recyclable (14.6%), not hygienic (10.4%) and being too large (6.1%).
To the question of whether they would order an online STI test, 89.0% answered positively (146/164) and 91.1% (133/146) of those individuals would use the Colli-Pee device in that case. Six participants (4.1%) would not want to use the Colli-Pee device when ordering an online STI test. Participants were also asked how much they would pay for an online STI test with self-sampling. Price indications ranged from 0€ (10 participants) to 60€. Most of the participants (89/164) were willing to pay 10–20€.
Discussion
Many studies have reported on male self-collected urine versus urethra clinician-collected sampling for STI screening, but ‘real-world’ studies, including sending of home-based urine samples for STI detection in men by post, are sparse.4 5 20 21 In this study, we showed that the Colli-Pee collection device is a valuable and reliable method for collecting first-void urine for STI detection in MSM in Belgium, and that the collector can be shipped by regular post. Compared with the clinic-based sample, a total of three STIs (one CT and two MG infections) were not detected in the home-based sample. However, 11 additional infections were found in home-based samples collected with the Colli-Pee device (3 CT, 2 NG and 6 MG infections). This high number of additional STIs could be explained by the fact that first-void urine contains more DNA/RNA than mid-stream and, as a consequence, should still be used for STI detection.9 Indeed, we showed that using the Colli-Pee device first-void urine was collected in a more standardised way compared with the clinic-based samples (p<0.001). Also, more human cells were collected in the home-based samples, however statistical significance was lacking. The fact that participants could become positive during the time in between sampling points is one of our main limitations and cannot be ignored. Preliminary data of the Be-PrEP-ared study showed high-incidence estimates after twelve months of the main Be-PrEP-ared study for urethral CT/NG and MG: 11.5, 5.1 and 6.9 incidence rate per 100 person-years, respectively.
Nevertheless, the most important observation is that only one Chlamydia positive result was missed. The DC value of the Abbott assay performed on that clinic-based sample highlighted the low bacterial load of that infection; in addition, transportation at room temperature for 2 days could have induced DNA degradation.
The WHO underlines the importance of integrating point-of-care assays (POCTs) including innovative delivery options such as self-testing.22 Unfortunately, to our knowledge, current commercial POCTs for the most important STIs such as CT and NG are still of sub-optimal quality and do not meet the ASSURED criteria that were developed by the WHO STI Diagnostics Initiative.22–25 A solution to the unavailability of qualitative POCTs could be internet-accessed STI testing (e-STI testing) which is increasingly available as an alternative to clinic testing all over the world.26 E-STI testing includes postal self-sampling test kits that are sent to a certified laboratory and web-based delivery of test results. Swab2Know, an online HIV testing project confirmed that e-HIV testing is acceptable and feasible among MSM in Belgium.27 Commercial online self-sampling services for STIs are now emerging over the internet, but evaluation of these services is lacking.
The present study is, however, subject to several limitations. First, we only enrolled Be-PrEP-ared participants and the participation level seriously declined during the study. As a result, our number of CT/NG positives is quite low, which precludes firm conclusions. Second, as mentioned above, home-based samples were not taken on the same day as clinic-based sampling and participants could have become positive during that window period. We also do not know whether participants had urinated 1 hour prior to collection. However, recent data show that the time between micturition is not crucial for the detection of Chlamydia in men.28 Third, we did not monitor the temperature of the transport of home-based samples which could also have an impact on the quality of the samples, however, outside temperature between October 2015 and May 2018 varied between −10°C and 33°C with an average of 11°C.
Fourth, we cannot exclude specimen contamination, however, participants were instructed how to correctly collect the clinic-based and home-based sample. Finally, reporting bias is also not to be excluded. Not all participants who used a Colli-Pee device completed the survey, the additional questions were included at the end of the lengthy main questionnaire of the Be-PrEP-ared study.
Besides PrEP users, e-STI testing has the potential to reach those who are most in need and a recent study showed that some higher-risk groups, such as MSM, were more likely to use online services.26 29 Many studies have shown that home-based sampling is well accepted and, in fact, is the preferred approach in these groups for STIs. Reasons for choosing home-based sampling were shorter waiting times for results, convenience and less embarrassment.30 Participants views regarding ordering an online STI test in this study were very positive, 89% would like to order such a kit. The Colli-Pee device was also found to be easy (90.2%) and although hygiene was one of the likes, it also appeared in the dislikes, probably because the need to detach the collector manually can cause leakage of urine. Participants were also concerned regarding possible ecological consequences, although the plastic material is recyclable and can be incinerated into energy.
We demonstrated that postal delivery of home-based collected urine does not influence STI detection and can be used among PrEP users. Subsequently, PrEP users will be able to send first-void urine to the laboratory with the Colli-Pee device 1–2 weeks before their routine PrEP follow-up visit. Results can then be discussed during the physician consultation and followed by treatment and future antimicrobial testing if applicable, decreasing the number of physician visits. Decreasing the number of face-to-face visits will lower the burden on staff workload and healthcare resources. However, future economic evaluations will need to be conducted to prove this statement. E-STI testing could be a promising approach in Belgium to reach patients in hard-to-reach populations and research on this topic should be stimulated. Therefore, future studies to study the acceptability and impact of postal shipment of home-collected material on the performance of STI assays requires additional assessment.
Supplementary Material
Acknowledgments
We would like to thank all the participants of the Be-PrEP-ared study who participated in this small study. We also would like to thank Be-PrEP-ared study group but especially Maureen Aerts who was crucial in the participation level of this study. Finally, we would like to thank Wendy Thys for the data entry.
Footnotes
Contributors: IDB, TC and BV designed the study. TR designed the acceptability survey. IDB performed the statistical analysis and wrote the first draft of the manuscript. HS, BDD, VC and SA performed testing. All authors read the final version of the manuscript and provided comments.
Funding: This work was supported by Novosanis who provided the Colli-PeeTM devices and partially paid for the analyses performed on the home-based samples. In addition, a grant was obtained from the Flemish Agency for Innovation and Entrepreneurship to conduct the Be-PrEP-ared study.
Competing interests: None declared.
Ethics approval: The main study was approved by the Institutional Review Board of the Institute of Tropical Medicine and theEthics Committee of the Antwerp University Hospital. In addition, aseparate approval for this substudy was obtainedby the Institutional Review Board of the Institute of Tropical Medicine (Ref:1027/15).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data will be made publicly available except for the use and acceptability data as these will be retained at the Institute of Tropical Medicine (ITM), Antwerp due to ethical and privacy concerns. According to the ITM research data sharing policy, only fully anonymised data can be shared publicly. The data can however be made available after approval of a motivated and written request to the ITM at ITMresearchdataaccess@itg.be. The ITM data access committee will verify if the dataset is suitable for obtaining the study objective and assure that confidentiality and ethical requirements are in place.
Patient consent for publication: Not required.
References
- 1. WHO. Global health sector strategy on sexually transmitted infections 2016-2021: World Heal Organ, 2016:63. [Google Scholar]
- 2. Lanjouw E, Ouburg S, de Vries HJ, et al. . 2015 European guideline on the management of Chlamydia trachomatis infections. Int J STD AIDS 2016;27:333–48. 10.1177/0956462415618837 [DOI] [PubMed] [Google Scholar]
- 3. Bignell C, Unemo M. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 2013;24:85–92. 10.1177/0956462412472837 [DOI] [PubMed] [Google Scholar]
- 4. Lunny C, Taylor D, Hoang L, et al. . Self-Collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015;10:e0132776 10.1371/journal.pone.0132776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McNicol J, Debattista J. Use of the uriswab collection device for testing of chlamydia trachomatis and neisseria gonorrhoeae: implications for a postal testing service. Int J STD AIDS 2013;24:477–80. 10.1177/0956462412472834 [DOI] [PubMed] [Google Scholar]
- 6. Vorsters A, Van den Bergh J, Micalessi I, et al. . Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur J Clin Microbiol Infect Dis 2014;33:2005–14. 10.1007/s10096-014-2147-2 [DOI] [PubMed] [Google Scholar]
- 7. Theodorescu D, Schiffer E, Bauer HW, et al. . Discovery and validation of urinary biomarkers for prostate cancer. Proteomics Clin Appl 2008;2:556–70. 10.1002/prca.200780082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wisniewski CA, White JA, Michel CE, et al. . Optimal method of collection of first-void urine for diagnosis of Chlamydia trachomatis infection in men. J Clin Microbiol 2008;46:1466–9. 10.1128/JCM.02241-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson DJ, Calderaro AC, Roberts KA. Variation in nuclear DNA concentrations during urination. J Forensic Sci 2007;52:110–3. 10.1111/j.1556-4029.2006.00329.x [DOI] [PubMed] [Google Scholar]
- 10. Traeger MW, Schroeder SE, Wright EJ, et al. . Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018;67:676–86. 10.1093/cid/ciy182 [DOI] [PubMed] [Google Scholar]
- 11. Kojima N, Davey DJ, Klausner JD. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. AIDS 2016;30:2251–2. 10.1097/QAD.0000000000001185 [DOI] [PubMed] [Google Scholar]
- 12. Salazar N. EACS HIV guidelines 8.1, 2016. [Google Scholar]
- 13. World Heal Organ. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection. Geneva: World Heal Organ, 2017. [Google Scholar]
- 14. De Baetselier I, Reyniers T, Nöstlinger C, et al. . Pre-Exposure Prophylaxis (PrEP) as an additional tool for hiv prevention among men who have sex with men in Belgium: the Be-PrEP-ared study protocol. JMIR Res Protoc 2017;6:e11 10.2196/resprot.6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen CY, Chi KH, Alexander S, et al. . A real-time quadriplex PCR assay for the diagnosis of rectal lymphogranuloma venereum and non-lymphogranuloma venereum chlamydia trachomatis infections. Sex Transm Infect 2008;84:273–6. 10.1136/sti.2007.029058 [DOI] [PubMed] [Google Scholar]
- 16. Hopkins MJ, Ashton LJ, Alloba F, et al. . Validation of a laboratory-developed real-time PCR protocol for detection of chlamydia trachomatis and neisseria gonorrhoeae in urine. Sex Transm Infect 2010;86:207–11. 10.1136/sti.2009.040634 [DOI] [PubMed] [Google Scholar]
- 17. Müller EE, Venter JM, Magooa MP, et al. . Development of a rotor-gene real-time PCR assay for the detection and quantification of Mycoplasma genitalium. J Microbiol Methods 2012;88:311–5. 10.1016/j.mimet.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 18. Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods 2001;91:109–17. 10.1016/S0166-0934(00)00244-5 [DOI] [PubMed] [Google Scholar]
- 19. Reyniers T, Nöstlinger C, Laga M, et al. . Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr 2018;79:186-194 10.1097/QAI.0000000000001791 [DOI] [PubMed] [Google Scholar]
- 20. Morré SA, van Valkengoed IG, de Jong A, et al. . Mailed, home-obtained urine specimens: a reliable screening approach for detecting asymptomatic Chlamydia trachomatis infections. J Clin Microbiol 1999;37:976–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fajardo-Bernal L, Aponte-Gonzalez J, Vigil P, et al. . Home-based versus clinic-based specimen collection in the management of Chlamydia trachomatis and Neisseria gonorrhoeae infections. Cochrane Database Syst Rev 2015:CD011317 10.1002/14651858.CD011317.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toskin I, Blondeel K, Peeling RW, et al. . Advancing point of care diagnostics for the control and prevention of STIs: the way forward. Sex Transm Infect 2017;93:S81–8. 10.1136/sextrans-2016-053073 [DOI] [PubMed] [Google Scholar]
- 23. De Baetselier I, Mwambarangwe L, Cuylaerts V, et al. . Evaluation of an enzymatic Chlamydia trachomatis point-of-care rapid assay in Rwanda: the BioChekSwab rapid test. Sex Transm Infect 2016;92:430–2. 10.1136/sextrans-2015-052202 [DOI] [PubMed] [Google Scholar]
- 24. Peeling RW, Holmes KK, Mabey D, et al. . Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect 2006;82:v1–6. 10.1136/sti.2006.024265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. European Centre for Disease Prevention and Control. Novel approaches to testing for sexually transmitted infections, including HIV and hepatitis B and C in Europe. Stockholm: European Centre for Disease Prevention and Control, 2012. [Google Scholar]
- 26. Wilson E, Free C, Morris TP, et al. . Internet-accessed sexually transmitted infection (e-STI) testing and results service: a randomised, single-blind, controlled trial. PLoS Med 2017;14:e1002479 10.1371/journal.pmed.1002479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Platteau T, Fransen K, Apers L, et al. . Swab2know: an HIV-testing strategy using oral fluid samples and online communication of test results for men who have sex with men in Belgium. J Med Internet Res 2015;17:e213 10.2196/jmir.4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathew T, O’Mahony C, Mallinson H. Shortening the voiding interval for men having chlamydia nucleic acid amplification tests. Int J STD AIDS 2009;20:752–3. 10.1258/ijsa.2009.009225 [DOI] [PubMed] [Google Scholar]
- 29. Barnard S, Free C, Bakolis I, et al. . Comparing the characteristics of users of an online service for STI self-sampling with clinic service users: a cross-sectional analysis. Sex Transm Infect 2018;94:377–83. 10.1136/sextrans-2017-053302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paudyal P, Llewellyn C, Lau J, et al. . Obtaining self-samples to diagnose curable sexually transmitted infections: a systematic review of patients' experiences. PLoS One 2015;10:e0124310 10.1371/journal.pone.0124310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


