Abstract
Introduction
Population ageing is accelerating rapidly in Israel as well as worldwide, necessitating adaptation of the healthcare system and consideration of new approaches that serve the specific needs of older adults. In addition to cognitive function, frailty is one of the most challenging expressions of physical and mental ageing, a multidimensional syndrome of increased vulnerability. Several studies have shown that low intake of certain micronutrients and protein is associated with higher risk of frailty and cognitive impairment. However, whether global diet quality is involved in the aetiology of the latter outcomes is unclear.
Methods and analysis
We are conducting, among older adult subjects who took part in ‘Mabat Zahav’ (Israeli National Health and Nutrition Survey of Older Adults) in 2005–2006 (T0, n=1852), an extensive follow-up interview (T1) that includes comprehensive geriatric assessment and evaluation of general health and quality of life. Diet quality is evaluated using the Healthy Eating Index (HEI) 2010, based on 24-hour diet recall measured at T0 and T1. Frailty is assessed using two different approaches: the phenotype framework and the accumulation of deficits model. Cognitive function is assessed by Mini-Mental State Examination (MMSE) and cognitive decline is assessed by the difference between repeated MMSE measurements. Different analytic methods will be applied to evaluate the role of diet quality in development of frailty and cognitive decline with inverse probability weighting used to minimise attrition bias. About 600 subjects are expected to be interviewed between May 2017 and December 2019.
Ethics and dissemination
Ethical approval was obtained from the Helsinki Committee of Sheba Medical Center, Tel Hashomer, Israel and the Ethical Committee of Tel-Aviv University. All participants sign an informed consent form. The findings of the study will be published in peer-reviewed journals.
Keywords: diet quality, older adults, healthy aging, longitudinal studies, epidemiology
Strengths and limitations of this study.
The study transforms a large national survey of older adults with a broad-spectrum data into a cohort study with a specific age-related questionnaire including comprehensive geriatric assessment.
Obtaining data at two points in time, more than a decade apart, will allow us to evaluate long-term changes in older adult population and examine adverse clinical outcomes.
Selection bias due to death, loss to follow-up and non-response.
Misclassification bias due to self-report data and nutritional assessment that is based on a single 24-hour dietary recall.
Background
Healthy ageing
Population ageing is accelerating rapidly in Israel as well as worldwide, necessitating adaptation of the healthcare systems and consideration of new approaches that serve the specific needs of older adults.1 According to current forecasts, the percentage of persons 65 years and older will increase from 8.5% in 2015 to 19.0% by 2030.2 The concept of healthy ageing is generally described as optimising opportunities for improving and preserving health and physical, social and mental health and enhancing successful life-course transitions.3 While this definition depicts healthy ageing (also termed successful ageing) as a complex process of adaptation to changes across the lifespan, the concept needs to be looked at in terms of a measurable outcome that can be empirically validated.4 Despite the differences in healthy ageing definitions, there is some consensus in the studies that ‘successful ager’ outcome should measure function in domains of cognitive, physical and mental well-being.4 In our study, we intend to transform a large national survey of older adults into a cohort study with specific age-related questionnaires including general health, functional status, quality of life, social support, depression and cognitive function. Healthy ageing will be assessed by various measurements, with emphasis on frailty state and cognitive function. The predictive role of diet quality in the development of the latter outcomes will be evaluated, as described in detail in the following sections.
Frailty
Frailty is recognised as an important medical syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiological systems.5 Frail older persons are at high risk of accelerated physical and cognitive functional decline, disability and death.6 The concept of frailty adopts an integrative approach that represents general properties of ageing and health rather than particular functional deficiency or decline.7
Assessment of frailty has implications both for the individual and on society at large, forecasting healthcare use8 9 and providing opportunities for preventive intervention,10 11 thus making it a key issue in chronic disease management and healthy ageing.12 13
Methods to measure frailty vary throughout the literature,14–17 with two principal models of frailty emerging: (1) the Fried and colleagues’ Biological Phenotype5 framework, which conceptualises frailty as a biological syndrome characterised by a decline in overall function and loss of resistance to stressors. This model is composed of five physical indicators including low physical activity, weak grip strength, slow walking speed, exhaustion and unintentional weight loss. (2) The Rockwood and colleagues’ Accumulation of Deficits Index,18 which defines frailty as the cumulative effect of individual deficits. Under this model, frailty is measured by ~40 parameters of disease states, functional status, cognitive function and psychosocial status, collectively referred to as deficits. The index is a calculation of the presence or absence of each deficit as a proportion of total.8 Frailty and successful ageing models share common aspects of ageing.19 Frailty is recognised as an independent determinant of successful ageing, supporting the idea that successful agers might be non-frail individuals.19 20
Cognitive function
Another important aspect of successful ageing is the maintenance of cognitive function.21 Cognitive function is a predictor of independence and quality of life.22 Cognitive function assessed repeatedly is important because it is possible for an elderly person to have a normal cognitive score that still represents a significant decline for that individual. Mild cognitive impairment (MCI) is a syndrome that is currently thought of as a transition phase between healthy cognitive ageing and dementia.23 MCI is defined as cognitive decline greater than expected for an individual’s age and education level but that does not interfere notably with activities of daily life (ADLs).24 The estimated prevalence of MCI in population-based studies ranges from 10% to 20% in people older than 65 years of age. Clinical studies indicate that older adults with MCI will progress to Alzheimer’s disease (AD) at a rate of 10%–15% per year, compared with healthy control subjects who convert at a rate of 1% to 2% per year,25 making it an area of intense interest for theoretical and practical reasons. A widely recognised instrument for detection of cognitive impairment is the Mini-Mental State Examination (MMSE).26 27 The MMSE consists of 30 questions and has a maximum score of 30 points. MCI will be assessed according to poor performance on MMSE (ie, a score of 1.5 SD below the age-specific and education-specific mean) and preserved independence in functional abilities.27 28
Nutrition
Nutrition is an important element that affects and is affected by the ageing process.29 30 Malnutrition is highly prevalent among older adults and associated with a general decline in physical and mental functioning, higher hospitalisation rate and increased mortality.29 Eating patterns of various cultures around the world have been associated with risk for chronic diseases.31 However, examining the intake of a single nutrient or food group does not account for the complexity of dietary intake, as food and nutrients are not eaten in isolation. Consequently, indices of dietary quality, patterns and variety are increasingly used by nutritional epidemiologists.32 The Healthy Eating Inde (HEI)-201033 is such an index, originally released in 1995 and then updated in 2010 by the US Department of Agriculture (USDA) as a measure of diet quality. The concept of diet quality as a determinant of frailty development is not new, but whether it is a predictor or consequence of frailty has not been investigated adequately.34 Several studies have shown that low intake of certain micronutrients and protein is associated with a higher risk of developing frailty. However, very few studies have assessed the effect of overall diet quality on frailty.35 Two studies have suggested that increasing adherence to Mediterranean diet (MD; a diet characterised by high intake of fish, vegetables, legumes, fruits, cereals and unsaturated fatty acids36) is associated with decreasing risk of frailty among community-dwelling older adults in Spain and Italy.37 38 In addition, it was recently demonstrated that higher adherence to MD is associated with lower AD risk.39 40 However, in most studies, the MD score was defined from sample-specific scores; thus, only the relative but not the absolute effect of MD was assessed, and the results of these studies are difficult to compare across populations.
Research objective
General objective
Investigate the relationship between diet quality and healthy ageing.
Specific aims
Develop a frailty index (FI) based on ‘Mabat Zahav’ data (T0) and evaluate its prevalence and association with subsequent survival.
Examine the predictive role of diet quality in development of frailty, cognitive changes and other healthy ageing aspects among study participants.
Investigate long-term changes of dietary consumption and nutritional status among study participants.
Hypothesis
We hypothesise that diet quality in older adults is predictive of successful ageing as measured by a variety of clinical outcomes.
Methods/design
Research design
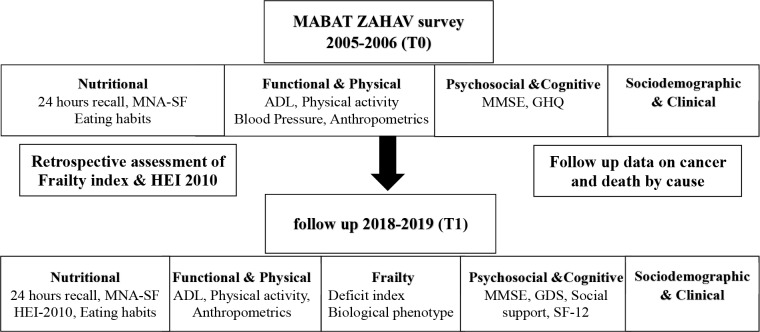
The study employs a cohort study design. It constitutes the second interview of Mabat Zahav study.41 The First National Health and Nutrition Survey of Older Adults Aged 65 and Over in Israel (‘Mabat Zahav’) was carried out in 2005–2006 by the Israel Center for Disease Control and the Nutrition Department of the Israel Ministry of Health. The data collected on the survey included information regarding health and nutrition status, health behaviours (physical activity, alcohol consumption, medication use and use of nutrition supplements), knowledge and attitudes regarding nutrition and utilisation of health services. The survey framework and population is further described in the following section. The current study questionnaire (T1) duplicates most parts of the original (T0) interview (figure 1). In addition, measurements pertaining to frailty status and cognitive function are performed, as well as psychosocial assessments including the Multidimensional Scale of Perceived Social Support (MSPSS),42 Geriatric Depression Scale (GDS)43 and the Short Form of Health-Related Quality of Life (SF-12).44 The added psychosocial questionnaires are in order to enable a more comprehensive analysis of the concept of healthy ageing and well-being. The HEI-2010, a measure of diet quality retrospectively assessed at T0, will serve as the exposure variable and be assessed prospectively at T1 in order to evaluate general changes in diet quality and composition. Frailty at T0 is retrospectively assessed through the Rockwood and colleagues’ Deficit Index.18 The index will be developed according to published criteria in order to identify frail participants at study entry. Frailty will be assessed prospectively at T1 by both the Deficit Index and the Fried and colleagues’ Biological Phenotype framework.5 Cognitive changes and MCI will be assessed prospectively. Mortality follow-up will be conducted among T0 participants through linkage to the nationwide database of causes of death (compiled by the Central Bureau of Statistics) via their national identification numbers.
Figure 1.
Study design sketch. ADL, activities of daily life; GDS, Geriatric Depression Scale; GHQ, General Health Questionnaire; HEI, Healthy Eating Index; MMSE, Mini-Mental State Examination; MNA-SF, Mini Nutritional Assessment-Short Form; SF-12, Short Form of Health-Related Quality of Life.
‘Mabat Zahav’ survey: study population
The Mabat Zahav survey population was a random sample of Israeli citizens aged ≥65 years old. The survey included 1852 community-dwelling participants (1536 Jews and 316 Arabs) residing in Israel, who had lived in the country for at least 1 year in urban and rural settlements with more than 20 000 residents. Exclusion criteria of Mabat Zahav survey included: significant cognitive reduction (MMSE <17) and hospitalisation at the time of the study. Survey methods included a personal interview in the interviewees’ homes or sheltered accommodation using a structured questionnaire.
Sampling frame: adults aged 65 years and over insured by the two major HMO in Israel, Clalit Health Services and Maccabi Health Services, representing 86.3% of all of the elderly in Israel, were sampled. Oversampling was carried out in the Arab population, because of the small percentage of elderly in the Arab population (6.3%), in order to ensure a sample large enough for statistical analyses and comparisons with the Jewish sector. The overall sample size target was 1800 participants: 1500 Jews and 300 Arabs.
Sampling method: lists of insured older adults from each of the two HMO were combined and divided into population groups (Jews and Arabs). Sampling was carried out in two stages due to low response rate at the first stage, in order to meet the sample size target. First stage: 5100 people were randomly sampled, with 4250 from the Jewish list and 850 from the Arab list. Interviewing of individuals from the first sample commenced in July 2005. A total of 1081 individuals were interviewed, of which 1051 questionnaires met inclusion criteria (909 Jews and 142 Arabs). Second stage: an additional sample was drawn in January 2006, including 4250 Jews and 2500 Arabs, since the initial lists were exhausted. A total of 771 individuals were interviewed, of which 748 questionnaires met inclusion criteria (590 Jews and 158 Arabs). The interviews were held in multiple languages, and the questionnaires were translated accordingly: 1277 (69%) in Hebrew, 316 (17%) in Arabic, 257 (14%) in Russian and 2 in English.41 All data collected are available online at the ministry of health government website.45
Exclusion criterion in current research stage (T1)
Significant cognitive reduction as measured by a MMSE score of less than 1726 46 or inability to communicate.
Sample size
Among T0 initial participants (1852), 1799 (1499 Jews and 300 Arabs) questionnaires were included in the final survey analysis. Forty-six participants (29 Jews and 17 Arabs) had a MMSE score of less than 17 (after adjustment for age and education), and seven questionnaires were only partially completed and therefore excluded from the statistical analysis. According to the mortality registry of the Ministry of Health, 1115 participants were alive in February 2017. We assume that 25% of candidates will be unable to participate due to either exclusion criteria or severe medical condition and another 20% could not be contacted due to address or telephone number changes. We expect a response rate of 55%–60% among the remaining candidates for T1 interview, and so about 600 subjects are expected to be reinterviewed (figure 2). Our efforts to maximise recruitment include the following steps:
Figure 2.
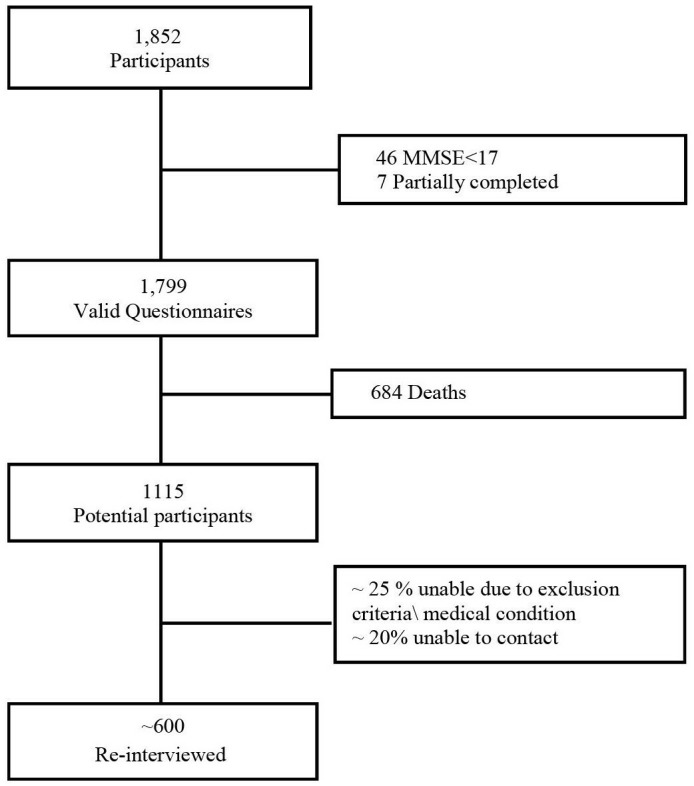
Sample size flow chart. MMSE, Mini-Mental State Examination.
Disconnected phone numbers and no response: (A) locating address changes via the Ministry of Interior database. (B) Searching by the Israeli non-commercial telephone directory according to city of residence and family name only (in case of incorrect street name). (C) Conducting ten attempts to contact each non-respondent.
Refusal: we are trying to encourage cooperation by: (A) offering to conduct interviews 7 days a week, morning times and afternoons. (B) Offering to divide the interview into two separate times, in case the length of the interview is a concern for the participant.
Data collection
A personal interview is conducted in the interviewees’ homes by trained interviewers using a structured questionnaire. Anthropometric measurements are performed using standardised protocols as described in a subsequent section. Interviews are conducted in Hebrew, Arabic or Russian. Estimated time of an interview is an hour and a half. In case the participant is unable to complete the questionnaire by himself or herself, but still meets inclusion criteria, information from a proxy is obtained regarding dietary intake, chronic diseases, ADL, sociodemographic status and medication use. The proxy interview does not include the following assessments: GDS, SF-12, MSPSS and self-rated health. All data (except the 24 hours dietary recall) are collected using KoBotoolbox47 software, which is a freely available application to design surveys for data collection through smart devices and run on Android-based platforms. The data are exported into a password-protected Excel file on a daily basis. All responses are typed directly during the interview through Lenovo TAB2 A10-30 tablet. The 24-hours dietary recall is handwritten before being typed to ‘Tzamert’ program,48 an Israeli nutrient data program, which enables recording of food intake and calculation of nutrient intake. In case of any technical difficulties, the questionnaire is completed manually by the interviewer. A pilot study (n=30) was conducted, after which questionnaires undertook minor adjustments.
Exposure variable: the HEI-2010 score at baseline (T0): dietary data from the 24-hour dietary recall questionnaire was entered into the ‘Tzamert’ program. The program uses the nutrient data in the BINAT program—the Israeli nutrient database that is maintained and updated by the Nutrition Department of the Ministry of Health. An HEI-2010 score32 will be calculated for T0 and T1 interviews separately. The HEI-2010 has 12 components, 9 of which assess adequacy of the diet, including: (1) total fruit; (2) whole fruit; (3) total vegetables; (4) greens and beans; (5) whole grains; (6) dairy; (7) total protein foods; (8) seafood and plant proteins; and (9) fatty acids. The remaining three: refined grains, sodium and empty calories assess dietary components that should be consumed in moderation. For each component, the respondents receive a minimum score of 0 and a maximum score of 5 or 10 (for perfect adherence to recommendations); intermediate degrees of adherence are calculated proportionately. Thus, the overall index has a range from 0 (worst) to 100.32
Nutritional status assessments
Dietary recall: the multiple-pass 24-hour dietary recall questionnaire is administrated. The method was originally developed by the USDA in order to limit the extent of under-reporting that occurs with self-reported food intake.49 The interviewer uses three distinct passes to gather information about a subject’s food intake during the preceding 24 hours. The first pass is termed the quick list; here the interviewees are asked to recall all they had eaten and drunk in the 24 hours period that preceded the interview. The second pass is termed the detailed description. In this pass, the interviewees are asked to clarify any foods mentioned in the quick list. The third pass is termed the review. The interviewer reviews the list of foods mentioned and probes for additional eating occasions and clarifies food portion sizes.49 In order to assist the interviewees in identifying food types and quantities during the interview, the interviewers use the ‘Food and Food Quantities Guide’, which is partially based on the Food Guide of the USDA. The guide includes detailed questions on foods, as well as many photographs of Israeli foods. In order to facilitate quantification of amounts consumed, the interviewers use, in addition to the guide, identification aids such as a measuring cup, tablespoon and teaspoon.
Food security: household food security is defined as a situation whereby all household members have access at all times to a food supply which is adequate for a healthy active life. Food security is assessed using the short six-item food security USDA questionnaire.50
Malnutrition risk: modified Mini Nutritional Assessment-Short Form (MNA-SF).51 The six-item questionnaire is a nutritional screening tool that assesses malnutrition risk.
Primary outcomes
Frailty assessment: frailty at T0 and T1 is assessed by the Deficit Index model.18 Under this model, frailty is measured by ~40 parameters of symptoms, signs, disease states and disabilities, collectively referred to as deficits. Adapting the Rockwood index of accumulation of deficits method7 with T0 data, an FI was developed comprising 33 variables. The FI at T1 will comprise the same variables as T0 FI and will serve as the outcome measure. The FI is a calculation of the presence or absence of each deficit as a proportion of the total. Dichotomous items are coded as 0 if the deficit is absent and as 1 if it is present, while ordinal variables are graded into a score between 0 and 1 (0 representing no impairment, 0.5 for minor impairment and 1 for major impairment). Scores are then summed up and divided by the total number of variables, yielding an FI between 0 and 1, with 1 representing the greatest frailty (a threshold of ≥0.25 is typically used to define frailty.52 Frailty at T1 is additionally assessed by the Biological Phenotype model.5 Frailty using this instrument is identified by the presence of three or more of the following components: (1) shrinking: weight loss, unintentional, of more than 4.5 kg, or more than 5% of body weight, in the previous year; (2) weakness: grip strength in the lowest 20% (adjusted for sex and body mass index); (3) poor endurance and energy: as indicated by self-report of exhaustion; (4) slowness: the slowest 20% of the participants in the sample, based on time of a 5 m walk (adjusted for sex and standing height); and (5) low physical activity level: a weighted score of kilocalories expended per week will be calculated based on a Physical Activity Scale for the Elderly Questionnaire.53 The lowest quintile of physical activity will be identified for each gender.
Cognitive assessment: cognitive status is evaluated using the MMSE.26 The questions are grouped into seven categories, each representing a different cognitive domain or function: orientation to time (5 points); orientation to place (5 points); registration of three words (3 points); attention and calculation (5 points); recall of three words (3 points); language (8 points) and visual construction (1 point).54 The MMSE scores (maximum, 30 points) will be education and age standardised.46 Some participants cannot complete test items due to physical disability. The MMSE in these subjects will be scored out of the items that can be tested.55 Cognitive impairment will be defined as a score <24.26 Cognitive decline will be calculated as the MMSE score difference between 2005 and 2017 and will be defined by the 10% of the sample who declined the most (ie, the 90th percentile of decline). MCI will be assessed according to poor performance on MMSE and preserved functional independence.28 Poor performance on MMSE will be defined by a score of 1.5 SD below the age-specific and education-specific mean. Preserved functional independence will be defined according to Katz et al scale of ADL score.27 28
Secondary outcomes
Health status evaluation: the questionnaire includes data on self-rated health (current status and recent changes) and chronic diseases (eg, cardiovascular diseases, Parkinson’s disease, respiratory diseases, renal disease, cancer, glaucoma and cataract, diabetes mellitus, osteoporosis and hypertension). In addition, the questionnaire includes demographic details, alcohol consumption and smoking habits information.
Assessment of disabilities: function is assessed by the Katz scale of ADL56 based on ability to dress, shower/bathe, sit down and rise from a chair, eat and go to the bathroom. The maximum score is 15, with a score of 5 indicating ‘no functional limitations’, a score of 6–10 indicating some functional limitations and a score of 11 or more indicating several functional limitations.
Psychosocial assessments: assessments include depression, perceived social support and health-related quality of life. Depression is evaluated via a five-item short form of the Yesavage GDS.43 A score of 2 or higher indicates possible depression. Social support is assessed through the MSPSS,57 a 12-item questionnaire designed to measure perceptions of support from three sources: family, friends and a significant other (four items for each source). Answers are given on a 1–7 scale. Weighted scores are calculated by averaging the specific items, each scale (source) individually and the entire questionnaire. A high score represents a high level of perceived social support. Health-related quality of life is evaluated via SF-12,44 physical component score and mental component score will be constructed from SF-12, using standard (US) and country-specific scoring algorithms.
Drugs: the participants are asked about any medication use on a regular basis (prescription as well as over-the-counter drugs). In the preliminary letter, the participants are asked to prepare their regular medication list. Medications are coded using the Anatomical Therapeutical and Chemical system developed by the WHO.41
Anthropometric measurements: include standing height and weight, ulna length (to calculate height, using recognised formulae58) and waist and midupper arm circumference. Weight measurements are carried out using an analogue scale suitable for weighing up to 130 kg, with accuracy to 0.5 kg. The scales are placed on an uncarpeted floor and calibrated before weighing. Height is measured using a spring coil measuring tape. Waist circumference is measured using a flexible tape, with the ability to measure up to 150 cm, at the narrowest part of the torso, where a ‘fold’ is created when bending sideways.41 Midupper arm circumference is measured at the midpoint between the tip of the shoulder and the tip of the elbow (olecranon process and the acromion) using a flexible tape.59
Blood pressure and pulse measurements: the interviewers conduct blood pressure and pulse measurements using an electronic monitor. The measurements are carried out according to a protocol based on recommendations of the American Heart Association.60 Sitting blood pressure and pulse are measured in the right arm and is carried out twice, with a minute rest in-between. In case of a difference of 10% or more between measurements of either systolic or diastolic pressure, a third measurement is carried out. The final value will be the mean of measurements.
Mortality and cause of death: original participants were linked to the nationwide database of causes of death (compiled by the Central Bureau of Statistics) via their national identification numbers. Mortality information is managed by the Ministry of Health. Since 1999, deaths are coded according to the International Classification of Diseases, 10th Edition.
Quality assurance
Quality assurance is carried out in various ways: (1) a pilot study (n=30) was conducted, after which questionnaires and research tools were finalised; (2) interviewer training: a 2-day seminar was designed and included standard procedures of administrating research questionnaires, performing anthropometric measurements and handling data in general; (3) all interviews (under interviewee consent) are recorded; (4) the study coordinator randomly monitor 5%–10% of all interviews; and (5) dietary data quality assurance includes: (A) a food recall check: time sequence, completeness of information, matching of the items in the ‘Quick List’ with those in the ‘Comprehensive List’ and (B) following data entry into the Tzamert program, testing will be performed for outliers, in appropriate quantities, lack of correlation between meal times and types and missing quantities and incorrect coding.
Statistical analysis according to specific aims
Analyses will be performed using SAS V.9.4, IBM SPSS V.25 and R version 3.4.4 (R Development Core Team). When appropriate, the sampling approach will be accounted for through weighting.
Frailty categories assessed at baseline (T0; frail vs robust) will serve as the exposure variable. Baseline characteristics across FI categories will be compared by χ2 test for categorical variables and Student’s t-test for continuous variables. Cox proportional hazards regression models61 will be fitted to evaluate the HRs for death. Several adjustment methods will be applied including traditional multivariable adjustment and propensity score adjustment.62 The incremental discriminatory ability of FI over demographic and SES variables in predicting death during a 12-year follow-up will be evaluated by the c-statistic. Assessing the c-statistic and its corresponding SE from Cox proportional hazards models will be performed with methods proposed by Harrell et al.63
Baseline characteristics across HEI categories as measured at T0 will be compared by χ2 test for categorical variables and analysis of variance for continuous variables. The predictive role of nutritional indices in the long-term incidence of frailty, as assessed by two methods, cognitive decline and other outcomes, will be assessed using logistic regression models.64 Adjustment will be made for sociodemographic, clinical and psychosocial variables, via either multivariable adjustment or propensity score.65 Of the 1799 participants in the initial survey, many are no longer able to participate in the T1 interview (death, loss to follow-up and non-response). Because frailty status could not be assessed among the latter group, selection bias is introduced.66 This bias will be addressed through an adaptation of a marginal structural model, applying inverse probability weights.66 67 Accordingly, the probability of original participants to take part in the second interview will be estimated. Each observation will then be weighted by the reciprocal (ie, the inverse) of the predicted probability of participating at T1.
Nutrient intake will be calculated using the ‘Tzameret’ program, as described previously. Data of nutrient consumption among current study participants will be compared with international recommendation, that is, Dietary Reference Intake.68 69 Prevalence of malnutrition risk at T1 will be assessed by MNA-SF.51 Changes in nutrient consumption will be evaluated by descriptive statistics, and paired t-test will be used to evaluate the mean difference between HEI scores at T0 and T1.
Approach to missing data
The distribution of missing values will be examined. Depending on the extent of the problem, several approaches will be considered. In case of a low rate of missing data, a complete case analysis will be considered, that is, removal of subjects where any of the predictor variables are missing. Otherwise, we will employ multiple imputation methodology.70 For this purpose, the number of complete (imputed) datasets will be defined by the following formula: (1+λ/m)−1=efficiency, where λ is the fraction of missing information and m the number of datasets to impute. We will assume an efficiency of 0.975. Missing values will be replaced by imputed values based on models incorporating demographic, socioeconomic, psychosocial and clinical variables. The results of these datasets will then be combined using Rubin’s rules.70
Estimated statistical power
Among 1800 initial participants, some 1115 survived so far. About 600 subjects are expected to be reinterviewed, assuming a response rate of 55% for T1 interview. Considering frailty prevalence of over 35% at the estimated average age of 84 years in T1 interview,71 about 250 frail subjects can be expected. This sample size is sufficient for detecting an adjusted OR for frailty of ≤0.60 between the upper and lower HEI score tertiles (significance level at 5% and power of 80%). Association of this magnitude was previously reported. For example, a previous 6-year follow-up study showed that adherence to MD diet was associated with lower odds of developing frailty (OR=0.30 [95% CI 0.14 to 0.66]).38
Patient and public involvement
Patients and or public are not involved.
Ethical aspect
Potential participants receive a preliminary letter with a description of the study, a request to participate and an announcement that telephone contact would be made in the near future. In addition, the letter provides the telephone number of the research coordinator for further questions. After a minimum of 2 weeks, potential participants are contacted by telephone in order to set an interview appointment for those who agree to participate. The interview does not involve clinical procedures, and no human biological specimens are collected. Therefore, participants’ burden is minimal. Each interviewee is asked to sign an informed consent form.
Dissemination
The findings of the study will be published in peer-reviewed journals and will be presented at national and international conferences.
Discussion
The Israeli Longitudinal Study on Aging sets out to transform a large national survey of older adults with a broad-spectrum data into a cohort study with a specific age-related questionnaire including comprehensive geriatric assessment, evaluation of general health and quality of life. Obtaining data at two points in time, more than a decade apart, will allow us to evaluate long-term changes in older adults population and examine dietary role in the context of healthy ageing and adverse clinical outcomes. Participants’ estimated current mean age of 84 years old, defined as the ‘oldest-old’, have over past decades been the most rapidly expanding segment of the population in developed countries and also the most susceptible to disease and disability.72 Only few studies have explicitly examined the concept of robust ageing among the oldest-old and investigated its heterogeneity in functioning, cognitive abilities, diet quality and nutritional status changes. Both frailty and cognitive decline are at the core definition of healthy ageing19 20 73–75 and are highly prevalent in older people; still, as their status varies considerably among older adults, important issues such as how they develop, are they preventable and can they be detected reliably have yet to be defined. Obviously, our study has several limitations. Of the 1799 participants in the original survey, many are no longer able to participate in T1 interview (death, loss to follow-up and non-response). Because frailty status and cognitive state could not be assessed among the latter group, selection bias is introduced. This bias will be addressed through applying inverse probability weights based on estimated propensity score. Another limitation is the fact that dietary quality assessment (exposure variable) is based on a single 24-hour dietary recall. Although evaluation of the HEI score is suitable for a single 24-hour dietary recall intake,76 77 individual diets can vary greatly from day to day. Furthermore, we cannot preclude that participants may have changed their dietary habits during the follow-up. The 24 hours dietary recall tool is widely used to assess dietary intake in population studies since 1965,78 with studies indicating its accuracy for estimating energy intake.49 79 In addition, the multiple-pass 24-hour dietary recall technique, which is used in our study, manage to limit the extent of under-reporting that occurs with single self-reported food intake.49 80 Like most similar studies, self-report information and information from a proxy can lead to misclassification bias that may lead to under or overestimation of dietary recall, frailty and other measures. Nevertheless, examining the role of diet quality in the context of healthy ageing and adverse clinical outcomes may help to broaden our knowledge regarding the older adults population, provide a scientific basis on which policy makers can rely and pave the way for early therapeutic interventions.
Supplementary Material
Acknowledgments
We are indebted to all those who agree to participate in the study for their cooperation and patience answering our comprehensive questionnaire and their willingness to let us into their homes. We are grateful for our professional and dedicated study team: Osnat Fried, BSc; Michal Weber, BSc, RD; Rana Younis, BSc, RD and Polina Pokrass, BA, RN. This work was performed in partial fulfilment of the requirements for a PhD degree of Abigail Goshen, Sackler Faculty of Medicine, Tel Aviv University, Israel.
Footnotes
Contributors: AG: study coordinator of the ILSA, drafted the manuscript, assisted in the conception of the study and led field activities and study monitoring; UG: coinvestigator for the ILSA, conceived of the study concept and design and supervised the study; TSho: coinvestigator of the ILSA and physician-in-charge of the study; LK-B: principal investigator of MABAT ZAHAV survey; TShi: coordinator of MABAT ZAHAV survey; YG: principal investigator of the ILSA, conceived of the study concept and design and codrafted the manuscript. All authors revised, reviewed and approved the final paper.
Funding: This study is supported by grant no. 3-12787 from the Chief Scientist Office, Ministry of Health (principal investigator: YG) and The Bircher-Benner Foundation, Tel Aviv University (principal investigator: YG).
Competing interests: None declared.
Ethics approval: Ethical approval for the study was obtained from the Helsinki Committee of Chaim Sheba Medical Center at Tel Hashomer and the Ethical Committee of Tel-Aviv University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Keehan SP, Lazenby HC, Zezza MA, et al. Age estimates in the National Health Accounts. Health Care Financ Rev 2004;26:1–16. [PMC free article] [PubMed] [Google Scholar]
- 2. U.S. Census Bureau. Population projections of the united states by age, sex, race, and hispanic origin: 1995 to 2050: U.S. Census Bureau, 2004. [Google Scholar]
- 3. Peel NM, McClure RJ, Bartlett HP. Behavioral determinants of healthy aging. Am J Prev Med 2005;28:298–304. 10.1016/j.amepre.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 4. Peel N, Bartlett H, McClure R. Healthy ageing: how is it defined and measured? Australas J Ageing 2004;23:115–9. 10.1111/j.1741-6612.2004.00035.x [DOI] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M157. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 6. Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc 2004;52:625–34. 10.1111/j.1532-5415.2004.52174.x [DOI] [PubMed] [Google Scholar]
- 7. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001;1:323–36. 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. García-González JJ, García-Peña C, Franco-Marina F, et al. A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr 2009;9:47 10.1186/1471-2318-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci 2004;59:1310–7. 10.1093/gerona/59.12.1310 [DOI] [PubMed] [Google Scholar]
- 10. Freiheit EA, Hogan DB, Eliasziw M, et al. Development of a frailty index for patients with coronary artery disease. J Am Geriatr Soc 2010;58:1526–31. 10.1111/j.1532-5415.2010.02961.x [DOI] [PubMed] [Google Scholar]
- 11. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010;58:681–7. 10.1111/j.1532-5415.2010.02764.x [DOI] [PubMed] [Google Scholar]
- 12. Lee DH, Buth KJ, Martin BJ, et al. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010;121:973–8. 10.1161/CIRCULATIONAHA.108.841437 [DOI] [PubMed] [Google Scholar]
- 13. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 2013;68:62–7. 10.1093/gerona/gls119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008;168:382–9. 10.1001/archinternmed.2007.113 [DOI] [PubMed] [Google Scholar]
- 16. Hubbard RE, O’Mahony MS, Woodhouse KW. Characterising frailty in the clinical setting--a comparison of different approaches. Age Ageing 2009;38:115–9. 10.1093/ageing/afn252 [DOI] [PubMed] [Google Scholar]
- 17. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc 2011;59:2129–38. 10.1111/j.1532-5415.2011.03597.x [DOI] [PubMed] [Google Scholar]
- 18. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007;62:738–43. 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 19. Cosco TD, Armstrong JJ, Stephan BC, et al. Successful aging and frailty: mutually exclusive paradigms or two ends of a shared continuum? Can Geriatr J 2015;18:35–6. 10.5770/cgj.18.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woo J, Leung J, Zhang T, et al. Successful aging and frailty: opposite sides of the same coin? J Am Med Dir Assoc 2016;17:797–801. 10.1016/j.jamda.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 21. Rowe JW, Kahn RL. Successful aging. Gerontologist 1997;37:433–40. 10.1093/geront/37.4.433 [DOI] [PubMed] [Google Scholar]
- 22. Fiocco AJ, Yaffe K. Defining successful aging: the importance of including cognitive function over time. Arch Neurol 2010;67:876–80. 10.1001/archneurol.2010.130 [DOI] [PubMed] [Google Scholar]
- 23. DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003;2:15–21. 10.1016/S1474-4422(03)00262-X [DOI] [PubMed] [Google Scholar]
- 24. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet 2006;367:1262–70. 10.1016/S0140-6736(06)68542-5 [DOI] [PubMed] [Google Scholar]
- 25. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–92. 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- 26. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 27. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133–42. 10.1212/WNL.56.9.1133 [DOI] [PubMed] [Google Scholar]
- 28. Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 2014;312:2551–61. 10.1001/jama.2014.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmed T, Haboubi N. Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging 2010;5:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shahar D, Shai I, Vardi H, et al. Dietary intake and eating patterns of elderly people in Israel: who is at nutritional risk? Eur J Clin Nutr 2003;57:18–25. 10.1038/sj.ejcn.1601523 [DOI] [PubMed] [Google Scholar]
- 31. World Heart Organization. Diet, Nutrition, and the Prevention of Chronic Diseases. Geneva, Switzerland: World Heart Organization, 1990. [Google Scholar]
- 32. Patterson RE, Haines PS, Popkin BM. Diet quality index: capturing a multidimensional behavior. J Am Diet Assoc 1994;94:57–64. 10.1016/0002-8223(94)92042-7 [DOI] [PubMed] [Google Scholar]
- 33. Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013;113:569–80. 10.1016/j.jand.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deckelbaum RJ, Fisher EA, Winston M, et al. Summary of a scientific conference on preventive nutrition: pediatrics to geriatrics. Circulation 1999;100:450–6. 10.1161/01.CIR.100.4.450 [DOI] [PubMed] [Google Scholar]
- 35. Bollwein J, Diekmann R, Kaiser MJ, et al. Distribution but not amount of protein intake is associated with frailty: a cross-sectional investigation in the region of Nürnberg. Nutr J 2013;12:109 10.1186/1475-2891-12-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995;61:1402S–6. 10.1093/ajcn/61.6.1402S [DOI] [PubMed] [Google Scholar]
- 37. León-Muñoz LM, Guallar-Castillón P, López-García E, et al. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014;15:899–903. 10.1016/j.jamda.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 38. Talegawkar SA, Bandinelli S, Bandeen-Roche K, et al. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J Nutr 2012;142:2161–6. 10.3945/jn.112.165498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scarmeas N, Stern Y, Mayeux R, et al. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol 2006;63:1709–17. 10.1001/archneur.63.12.noc60109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol 2004;3:579–87. 10.1016/S1474-4422(04)00878-6 [DOI] [PubMed] [Google Scholar]
- 41. ICDC. Mabat Zahav National Health and Nutrition Survey age 65 and over 2005-6. 2011. https://www.health.gov.il/PublicationsFiles/Mabat_2005-2006-a.pdf.
- 42. Zimet GD, Powell SS, Farley GK, et al. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess 1990;55:610–7. 10.1080/00223891.1990.9674095 [DOI] [PubMed] [Google Scholar]
- 43. Hoyl MT, Alessi CA, Harker JO, et al. Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc 1999;47:873–8. 10.1111/j.1532-5415.1999.tb03848.x [DOI] [PubMed] [Google Scholar]
- 44. Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 45. Ministry of Health Israel. Mabat Zahav Survey - National Health and Nutrition Survey for People Age 65 and Over, 2005-2006. https://www.health.gov.il/UnitsOffice/ICDC/mabat/Pages/Mabat_Gold.aspx.
- 46. Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993;269:2386–91. 10.1001/jama.1993.03500180078038 [DOI] [PubMed] [Google Scholar]
- 47. KoBotoolbox software. http://www.kobotoolbox.org/
- 48. Tzamert software: Nutrition Department of the Ministry of Health. https://www.health.gov.il/Subjects/FoodAndNutrition/Nutrition/professionals/Pages/Tzameret.aspx
- 49. Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc 1996;96:1140–4. 10.1016/S0002-8223(96)00293-3 [DOI] [PubMed] [Google Scholar]
- 50. Blumberg SJ, Bialostosky K, Hamilton WL, et al. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health 1999;89:1231–4. 10.2105/AJPH.89.8.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bauer JM, Kaiser MJ, Anthony P, et al. The Mini Nutritional Assessment--its history, today’s practice, and future perspectives. Nutr Clin Pract 2008;23:388–96. 10.1177/0884533608321132 [DOI] [PubMed] [Google Scholar]
- 52. Myers V, Drory Y, Gerber Y, et al. Clinical relevance of frailty trajectory post myocardial infarction. Eur J Prev Cardiol 2014;21:758–66. 10.1177/2047487312462828 [DOI] [PubMed] [Google Scholar]
- 53. Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153–62. 10.1016/0895-4356(93)90053-4 [DOI] [PubMed] [Google Scholar]
- 54. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–35. 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- 55. Vertesi A, Lever JA, Molloy DW, et al. Standardized Mini-Mental State Examination. Use and interpretation. Can Fam Physician 2001;47:2018–23. [PMC free article] [PubMed] [Google Scholar]
- 56. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist 1970;10:20–30. 10.1093/geront/10.1_Part_1.20 [DOI] [PubMed] [Google Scholar]
- 57. Zimet GD, Dahlem NW, Zimet SG, et al. The multidimensional scale of perceived social support. J Pers Assess 1988;52:30–41. 10.1207/s15327752jpa5201_2 [DOI] [Google Scholar]
- 58. Gauld LM, Kappers J, Carlin JB, et al. Height prediction from ulna length. Dev Med Child Neurol 2004;46:475–80. 10.1111/j.1469-8749.2004.tb00508.x [DOI] [PubMed] [Google Scholar]
- 59. Tsai AC, Chang TL, Yang TW, et al. A modified mini nutritional assessment without BMI predicts nutritional status of community-living elderly in Taiwan. J Nutr Health Aging 2010;14:183–9. 10.1007/s12603-010-0046-5 [DOI] [PubMed] [Google Scholar]
- 60. Smith L. New AHA recommendations for blood pressure measurement. Am Fam Physician 2005;72:1391. [Google Scholar]
- 61. Cox DR. Regression models and life-tables. J R Stat Soc B 1972;34:187–202. 10.1111/j.2517-6161.1972.tb00899.x [DOI] [Google Scholar]
- 62. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 64. Domínguez-Almendros S, Benítez-Parejo N, Gonzalez-Ramirez AR. Logistic regression models. Allergol Immunopathol 2011;39:295–305. 10.1016/j.aller.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 65. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 66. Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004;15:615–25. 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- 67. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 68. Trumbo P, Schlicker S, Yates AA, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002;102:1621–30. 10.1016/S0002-8223(02)90346-9 [DOI] [PubMed] [Google Scholar]
- 69. Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 2001;101:294–301. 10.1016/S0002-8223(01)00078-5 [DOI] [PubMed] [Google Scholar]
- 70. Rubin DB. Multiple imputation for nonresponse in surveys: John Wiley & Sons, 2004. [Google Scholar]
- 71. Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92. 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 72. Fried LP. Epidemiology of aging. Epidemiol Rev 2000;22:95–106. 10.1093/oxfordjournals.epirev.a018031 [DOI] [PubMed] [Google Scholar]
- 73. Rosado-Artalejo C, Carnicero JA, Losa-Reyna J, et al. Cognitive performance across 3 frailty phenotypes: toledo study for healthy aging. J Am Med Dir Assoc 2017;18:785–90. 10.1016/j.jamda.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 74. Shlisky J, Bloom DE, Beaudreault AR, et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr 2017;8:17.2–26. 10.3945/an.116.013474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Allerhand M, Gale CR, Deary IJ. The dynamic relationship between cognitive function and positive well-being in older people: a prospective study using the English Longitudinal Study of Aging. Psychol Aging 2014;29:306–18. 10.1037/a0036551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guenther PM, Reedy J, Krebs-Smith SM, et al. Evaluation of the Healthy Eating Index-2005. J Am Diet Assoc 2008;108:1854–64. 10.1016/j.jada.2008.08.011 [DOI] [PubMed] [Google Scholar]
- 77. Guenther PM, Kirkpatrick SI, Reedy J, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr 2014;144:399–407. 10.3945/jn.113.183079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zimmerman TP, Hull SG, McNutt S, et al. Challenges in converting an interviewer-administered food probe database to self-administration in the National Cancer Institute automated self-administered 24-hour recall (ASA24). J Food Compos Anal 2009;22:S48–S51. 10.1016/j.jfca.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jonnalagadda SS, Mitchell DC, Smiciklas-Wright H, et al. Accuracy of energy intake data estimated by a multiple-pass, 24-hour dietary recall technique. J Am Diet Assoc 2000;100:303–11. 10.1016/S0002-8223(00)00095-X [DOI] [PubMed] [Google Scholar]
- 80. Raper N, Perloff B, Ingwersen L, et al. An overview of USDA’s Dietary Intake Data System. J Food Compos Anal 2004;17:545–55. 10.1016/j.jfca.2004.02.013 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.