Abstract
Digital data generated in the course of clinical care are increasingly being leveraged for a wide range of secondary purposes. Researchers need to develop governance policies that can assure the public that their information is being used responsibly. Our aim was to develop a generalisable model for governance of research emanating from health data repositories that will invoke the trust of the patients and the healthcare professionals whose data are being accessed for health research. We developed our governance principles and processes through literature review and iterative consultation with key actors in the research network including: a data governance working group, the lead investigators and patient advisors. We then recruited persons to participate in the governing and advisory bodies. Our governance process is informed by eight principles: (1) transparency; (2) accountability; (3) follow rule of law; (4) integrity; (5) participation and inclusiveness; (6) impartiality and independence; (7) effectiveness, efficiency and responsiveness and (8) reflexivity and continuous quality improvement. We describe the rationale for these principles, as well as their connections to the subsequent policies and procedures we developed. We then describe the function of the Research Governing Committee, the majority of whom are either persons living with diabetes or physicians whose data are being used, and the patient and data provider advisory groups with whom they consult and communicate. In conclusion, we have developed a values-based information governance framework and process for Diabetes Action Canada that adds value over-and-above existing scientific and ethics review processes by adding a strong patient perspective and contextual integrity. This model is adaptable to other secure data repositories.
Keywords: information governance, research governance, participatory governance
Strengths and limitations of this study.
The governance framework is built on values-based principles designed to gain the trust of patients and healthcare providers.
Half of the research governing committee members are people living with diabetes or their caregivers.
While this is a case study, we believe the governing principles are generalisable to other health research data repositories, and the operational model is adaptable to other settings.
Background
Digital data generated in the course of clinical care are increasingly being leveraged for a wide range of secondary purposes. These include health research by both public and private sector researchers. Recent events involving questionable uses of these records have shaken the confidence of the public regarding potential misuse of their personal information.1 2 As the number and size of health information platforms grow, and data linkages continue to become more extensive, researchers need to develop governance policies that can assure the public that their information is being used ethically, securely and with a clear public interest. In this paper, we present the conceptual and operational governance frameworks developed for Diabetes Action Canada—a pan-Canadian research consortium funded by the Canadian Institutes of Health Research’s Strategy for Patient-Oriented Research (SPOR) Program in chronic disease.3
Diabetes Action Canada’s mandate is to improve the lives of Canadians living with diabetes and its related complications. It facilitates connections between patients, their primary healthcare providers, specialists and health researchers with the goals of improving healthcare and reducing costs to the healthcare system. A key component of its mandate is to conduct patient-oriented research to help achieve these goals.4
To support its research activities, Diabetes Action Canada has developed a national diabetes repository—a secure analytical research environment situated at the Centre for Advanced Computing at Queen’s University in Kingston, Ontario—where analyses can be conducted securely in a virtual environment.5 The data in the repository originate from the electronic medical records (EMRs) from the practices of family physicians who contribute to the Canadian Primary Care Sentinel Surveillance Network (CPCSSN).6
The CPCSSN extracts de-identified EMRs from the practices of consenting primary care providers. Structured data from the chart are included as well as selected free-text terms. This includes data from the summary health profile such as health conditions, allergies and immunisations. CPCSSN also extracts selected laboratory data, vital signs, medications prescribed, dates of encounters, dates and types of referrals and risk factors (smoking status, alcohol use) and patient demographics.6
Patients are notified of the collection for research purposes through posted notices in the physicians’ offices. Patients can opt out at any time by contacting a member of the practice-based research network in their region. Notices advising patients of this are posted in the offices of participating primary care providers.7
The data extracted from these patients’ records are de-identified at the source. Prior to de-identification, a pseudonymous variable is generated and a key code file allowing reidentification is generated at the site of care and left there. This permits linkage with other records and reidentification of records at source. Only the subset of records of persons living with type 1 or type 2 diabetes is imported into the repository.
Other systems internationally use similar methods to extract, transform and manage primary care EMR data for purposes of clinical research, epidemiology and the study of health systems. As an example, the UK’s Clinical Practice Research Datalink (CPRD) has been in existence for over 30 years.8 The CPRD extracts de-identified data that are similar to those in CPCSSN and manages a growing list of research services based on these data. It has been part of more than 2000 peer-reviewed publications on a range of topics including medication use and safety, health policy and chronic disease management. The CPCSSN has now been in existence for a decade; its pattern of growth and development as Canada’s primary care EMR repository is following a path similar to the CPRD’s.
During this developmental phase, access to the data in the repository is restricted to researchers within Diabetes Action Canada. In future, the intention is for this to be open to outside researchers.
Early on, the need to develop a process to govern access to the data was recognised. While there was a considerable body of literature addressing information governance within the business literature, at the outset of this project, we were aware of relatively little literature in the context of health data repositories.9–13
In this paper, we describe the conceptual and operational models that were developed for the Diabetes Action Canada research governance process, with the hope that it may provide a model for other researchers who are also addressing similar issues over governance of the research in their research network.
Aim
To develop a generalisable model for governance of research emanating from health data repositories that will invoke the trust of the patients and the healthcare professionals whose data are being accessed for health research.
Methods
Our work was informed by three sources of literature:
Basic business texts in data governance.14 15
A database of 32 articles gathered from the authors’ existing library and recommendations from our data governance working group.
A scoping review of the literature using Ovid Medline from 2000 to 2017, with the assistance of a health sciences research librarian.
The full scoping review process and resulting analysis are the subject of a forthcoming publication. Search terms for the scoping review combined the topics of biobank and EMRs, governance and regulation, and social licence and trust. This returned 1075 articles, which were combined with the earlier database of 32 articles. On screening of abstracts of the 1075 papers, 122 articles were identified for coding in NVivo by the two authors. The initial coding scheme was developed based on guidance from the business texts and input from the data governance working group. The coding scheme was amended following the initial pilot coding of the first five papers. The results of this analysis informed the development of the conceptual and operational models for information governance models described in this paper.
The draft conceptual model was developed first. This was vetted through face-to-face meetings, initially with the data governance working group members, which included a patient representative. Feedback largely consisted of requests for clarification or elaboration on the principles selected. After a couple of iterations, the draft was then presented to the executive director and lead investigators of the network for their feedback, and with the general patient advisory circle, which has patient representatives from several of the more specialised patient advisory circles associated with the network. At the executive level and in the patient circle, there was strong endorsement, particularly for the participatory component being advocated.
The operational framework was developed in conjunction with both the data governance working group and the technical working group that was responsible for developing the operational model for the repository. The names and affiliations of the data governance and technical working group members may be found in online supplementary appendix 1. The technical working group was fortunate to have a patient representative with a strong systems background. The operational framework was designed to address the oversight process for requests to access the data in the repository, as opposed to the technical and procedural security aspects. A similar process was used for vetting the operational model as was done for the conceptual model. As with the conceptual model, revisions consisted more of refining and clarification.
bmjopen-2018-026828supp001.pdf (288.5KB, pdf)
Once the models were endorsed by these groups, we recruited patients, healthcare professionals, researchers and an individual with content knowledge in research ethics to participate in the governing and advisory bodies. Patients were recruited through the network partners who were responsible for recruiting participants in the patient advisory circles. Healthcare professionals were recruited through our partners in the Canadian Primary Care Sentinel Surveillance System. The two researchers were selected from within the network on the basis of their expertise in observational and clinical trials research.
In the next section, we describe the relevant literature that informed our models, the models we developed and the initial operation of the governance process.
Results
Conceptual model
Considerations
There are many definitions of information governance. We started with Smallwood’s definition: ‘…the overarching polices and processes to optimize and leverage information while keeping it secure and meeting legal and privacy obligations, in alignment with stated organizational business objectives.’14 From this definition, we abstract three core goals of information governance:
To optimise data use to meet one’s business objectives.
To keep the data secure.
To meet legal and privacy obligations.
While this definition works well for private sector data holdings and uses, in the context of research using data generated in the course of healthcare, additional considerations come into play. In the business model, the business entity usually owns the data and leverages the data to meet its business objectives. Hence, the individual firm is responsible for its information governance policies and practices.
In the context of a public sector health research network, data are often drawn from multiple parties where there is often no clear single owner of the data. Indeed, privacy legislation in Canada does not discuss ownership of data. It is framed in the language of custody and control over data, and to duties and obligations of those holding the data. Similarly, in the UK, the revised Caldicott principles delineate six principles for the secure management of personal health information. The updated version added a seventh principle: the duty to share information can be as important as the duty to protect patient confidentiality.16
Consequently, we suggest that, for health research, it is more appropriate to refer to stewardship rather than ownership of data. In addition, contributors to the research enterprise should carry a collective responsibility for information governance and the business objective must also meet a public interest test.17
Further, for use of data in the public sector, it is now recognised that, to ensure social licence for use of the data, the information governance objectives may need to go beyond mere compliance with formal regulations.1 Laurie and Sethi argue that ‘a good governance framework needs to include an overt statement of the values and standards according to which activity will be assessed. This must be accessible and sufficiently adaptable to be adopted and implemented across all levels of decision-making and by all actors involved in the process.’13 Similarly, Barocas and Nissenbaum state that ‘procedural approaches cannot replace policies based on substantive moral and political principles that serve specific contextual goals and values.’18
Based on these considerations, we added a fourth objective to Smallwood’s three core goals of information governance:
Earn and maintain the trust of patients, partners, data providers and the public for use of data for research in the public interest.
Trust is, in fact, a linchpin in the public acceptability of the research enterprise. Carter et al argue that: ‘… individuals’ cooperation with specific research studies is usually secured through three principal mechanisms: their expectations about how research is conducted and regulated; their trust in the institutions and individuals who recruit them; and their beliefs in the wholesomeness and public value of the research endeavour.’ 1
Elsewhere, they expand on the trust element: ‘the public’s support and tolerance for research, and its associated risks, often depends far more on an often fragile set of cues about the safety and social good of research participation, and on institutional and professional credentials, than it does on the formal architecture of research regulation, or on rational assessment of the detail of information sheets or other documents aimed at gaining ‘informed consent’.’ That does not negate the importance of attention to details around regulation and good communications. It does, however, point to the fragile dependence of the research enterprise on care taken by all researchers to ensure that their work is conducted with high integrity and that the public interest in the research is clearly articulated.
Trust assumes some level of uncertainty and, consequently, vulnerability.19 We recognised that much of the information use being planned would take us into ‘grey zones’ of research use: the indistinct interface between research and clinical practice, the healthcare system and management of the health of populations of people living with diabetes. Consequently, we identified the need to incorporate reflexivity into our research governance process. That is, the governance process has to critically assess common regulatory assumptions and practices in the context of new research circumstances and test alternative assumptions and practices.20
Particularly when the individual does not have an opportunity to exercise control over the use of their data, it is important to ensure that the public or patients, as appropriate, be involved at multiple stages in the governance process. The importance of stakeholder involvement in governance has been widely recognised.21–25
Finally, we needed to consider how the governance process we developed would complement the existing scientific and ethics review processes to which any research protocol would also be subjected. Given the focus on trust of both patients and the healthcare professionals whose data were being used, we chose to focus on how best to account for the patient’s perspective throughout all stages of the research process.
Guiding principles
Based on the considerations above, we identified eight principles that would guide our governance process:
Transparency.
Accountability.
Follow rule of law.
Integrity.
Participation and inclusiveness.
Impartiality and independence.
Effectiveness, efficiency and responsiveness.
Reflexivity and continuous quality improvement.
While these principles have drawn from a wide cross-section of literature, the model has been particularly influenced by the conceptual work of Laurie and Sethi, who called for values based—as opposed to technical—principles and the incorporation reflexivity to proceed in the face of uncertainty.11–13 26 Smallwood’s definition of information governance informed the first three principles14 and Carter et al, who highlighted the importance of public trust and social licence inspired the introduction of the integrity principle.1
Below, we provide a brief description of how these broad principles inform our operational governance process, and how these principles map to the four goals of information governance described above. A more detailed explanation of the principles may be found in online supplementary appendix 2.
bmjopen-2018-026828supp002.pdf (312.9KB, pdf)
Transparency
All decisions, policies and practices regarding data use are freely accessible to those affected by the decisions and to the public. These shall be available in an easily understandable format. (maps to: earn and maintain trust; meet privacy obligations)
Accountability
A governing body is accountable to those who will be affected by its decisions or actions. This is enforced through transparency and the rule of law. (maps to: earn and maintain trust, meet legal obligations)
Following the rule of law
The governance framework should follow all appropriate legal frameworks and the governing body should ensure compliance with applicable laws, regulations, standards and organisational policies across jurisdictions and institutions. (meet legal obligations, earn and maintain public trust)
Integrity
The governing process should ensure that uses of the data:
Have a clear patient/public interest that is consistent with the intended purpose of the repository?
Are of high scientific and ethical integrity? Ethical integrity includes: respect for persons, beneficence/non-maleficence and justice. Justice includes concern for equity.
-
Are maintained in a secure and private manner?
(Meet business objectives; meet legal and privacy obligations; earn and maintain trust; keep data secure).
Participation and inclusiveness
Patients and their families, healthcare professionals, and researchers should participate in governance over data use—through ongoing communication between the research governing committee (RGC) and the three patient advisory circles (general, francophone and immigrant, and Indigenous), and other stakeholder advisory groups.
The governing bodies responsible for access to data in the repository should account for differing interests to reach a broad consensus on what is in the best interest of those with diabetes and their families. Participation in governance should be inclusive, equitable, informed and organised. The full range of positions of the advisory groups should be considered. Ongoing, two-way engagement between the governing body and advisory groups is best. (Earn and maintain trust)
Impartiality and independence
As described above, the goal in deliberations is to reach a broad consensus on what is in the best interest of those living with diabetes and their families. All members in the process must look beyond their personal interests as either patients, healthcare providers or researchers.
In addition, the governance process must be able to operate in a zone of bounded independence27 from management, to ensure that its decisions are free from institutional conflicts of interest. (Earn and maintain trust)
Effectiveness, efficiency and responsiveness
Governance over the data repository should ensure the objectives of the organisation are being met in an effective and efficient fashion. The governing processes should serve all within a reasonable time frame. (Earn and maintain trust; meet business objectives)
Reflexivity and continuous quality improvement
Information governance should include processes that: allow research to proceed in the face of uncertainty; and incorporate continuous learning and quality improvement from prior experiences with data use. It should promote a culture of reflexivity, and responsiveness among researchers and those governing access to the data.26 (Earn and maintain trust).
Operational model
Structure
Building on these governance principles, we then formulated an operational model for our governance process. In this section and the next, we make explicit links to these guiding principles.
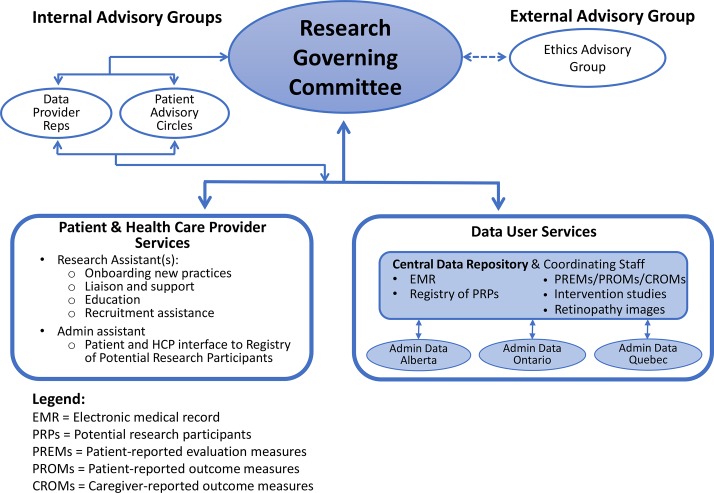
Our operational model is summarised in figure 1. Below, we focus on the roles of the RGC and its internal and external advisory groups.
Figure 1.
Diabetes Action Canada research governance structure.
Research governing committee
The RGC is the overall authority for governance over any research—observational studies or clinical trials—that are conducted involving data or patients in the network. It has decision-making authority regarding individual studies. The committee is accountable to the steering council, the highest authority in Diabetes Action Canada. (Principle 2: accountability; Principle 6: impartiality and independence.)
In its early stages, the committee is reviewing all applications. This will help it work through and document the important issues in approving applications and to develop standardised approval policies so that, in future when volumes increase and processes become routine, it will only have to review studies that have been flagged by the repository manager as requiring committee input.
There are two ways in which the RGC adds value over and above scientific and ethics review. First, it ensures contextual integrity of the research, through an intimate understanding of the data and the healthcare settings in the system being studied. Equally important, it ensures a patient-centred perspective of the research, by checking that the research:
includes patient-relevant outcomes;
has taken into adequate account benefits and burdens/risks among people living with diabetes;
is engaging in good communication practices with research participants, particularly around approaching and consenting to participate in research and in communicating about use of their health information for research? (Principle 4: integrity of purpose).
Half of the committee members (n=6) are people who are living with diabetes or their caregivers. These people were identified chiefly through the network partners who were responsible for creating the patient advisory circles, from the same pool of patients used to recruit the patient advisory circle members. Another two members are representatives from the data provider advisory group, described below. Currently, these are physicians who are members of CPCSSN, a subset of whose de-identified EMRs reside in Diabetes Action Canada’s secure data repository. Another two members are researchers, whose roles are to be technical advisors around scientific validity and merit of the research proposal. The other two members are individuals with expertise in research ethics or law. The committee may draw in outside experts if required. One of the two co-chairs of the committee is a patient representative. The other co-chair is drawn from the rest of the members of the committee. (Principle 5: participation and inclusiveness).
Data provider advisory group
Currently, the main data source for research activities of the network consists of the de-identified EMRs of physicians participating in CPCSSN. The data provider advisory group was developed to ensure that the perspectives of these data providers are represented at the RGC, through two members that group participate on the RGC. Three of the seven members of the group are front-line family physicians (ie, not academics). Current members were suggested by CPCSSN executive. In future, as the sources of research data grow, other healthcare professionals and data providers will be added to this advisory group.
This group provides advice on research applications, considering: logistics of conducting the research in the practice setting (particularly if a clinical trial); design considerations, as they relate to practice-level decisions and interpretation of findings. They also serve as liaisons with the larger group of practices that are providing data to the repository. (Principle 5: participation and inclusiveness).
Patient circles
Patient circles were developed at the outset of Diabetes Action Canada.28 Patient circle members either have diabetes themselves or are caregivers for a person living with diabetes. They are called on individually and collectively for advice on multiple aspects of the network endeavours.
Currently, there are three patient circles:
The general patient circle (10–15 people).
The francophone and immigrant patient circle (6–8 people).
The indigenous patient advisory circle (8–15 people).
Members of the patient advisory circles have been drawn from multiple sources, including: an online survey, snowball sampling and from community organisations. Members are selected to maximise diversity in age, gender and geographical location. In addition, candidates are interviewed to identify those with good group skills and a desire to contribute to a goal that exceeds his/her own health situation. They are then offered training in patient-oriented research.
The six patient representatives on the RGC have been identified from the general patient circle and from a list of potential candidates for the circles maintained by Diabetes Action Canada. The patient co-chair of the RGC provides reports to the general patient circle, apprising them of the activity of the RGC and soliciting their input, should there be any controversial issues with which they are grappling. The general patient circle is the liaison point because there is representation from the francophone and immigrant, and the indigenous patient circles in the general patient circle. (Principle 5: participation and inclusiveness). Further, there will be a separate governance process developed for research involving indigenous people.
External ethics advisory group
This committee will act as a ‘critical friend’ to advise on issues that cannot be resolved through deliberations among RGC members and the internal advisory groups described above. This advisory group provides one more instance of the governing principle of reflexivity. It will be at arm’s length to the RGC. It carries no formal authority, but has the freedom to go public if it is concerned about some particular policy direction taken by Diabetes Action Canada. Members will be drawn from ethics and legal scholars outside Diabetes Action Canada, both nationally and internationally, with expertise in: governance over secondary use of data; privacy and access to data; registry-based clinical trialsand practice-based research. (Principle 8: Reflexivity and continuous quality improvement).
Process
Standard operating procedures, including application forms, have been developed. A summary of the application process for research use of the data is provided in online supplementary appendix 3.
In the application form, several questions focus on the patient orientation of the research. For example, the researcher is asked to indicate:
The patient outcomes being measured.
How the research will benefit those living with diabetes or the public more generally.
The potential research-related risks of the study to research participants/data subjects and potential adverse social implications of the research.
The ways in which people living with diabetes have been involved in the planning of the research. (Principle 4: integrity of purpose, scientific integrity, ethical integrity).
The repository manager reviews the application for completeness. If the project has not received scientific review, the protocol is sent to a scientific advisory group for their approval prior to review by the RGC. Researchers are encouraged to submit prior to research ethics board (REB) approval to ensure the feasibility and appropriateness of the proposed protocol from the perspective of Diabetes Action Canada. In that way, rework at the level of the REB is minimised. (Principle 4: scientific and ethical integrity; Principle 7: effectiveness, efficiency and responsiveness).
Applications for research use of the data are circulated to RGC members at least 2 weeks in advance, to provide an opportunity for patient and data provider members of the committee to identify issues requiring deliberation with their respective advisory group, in advance of the RGC meeting. (Principle 5: participation and inclusiveness).
At the committee meeting, when vetting a particular protocol, patient and data provider representatives are invited to comment first. Concerns raised by the researchers and ethics people follow thereafter. The committee members aim for a consensus-based resolution to any concerns. When committee members fail to come to consensus, even subsequent to consultation with the patient advisory circles and the data provider advisory group, The RGC may turn to the ethics advisory group for guidance on how to proceed with an application or to seek general policy direction. (Principle 5: participation and inclusiveness; Principle 6: impartiality and independence; Principle 8: reflexivity and continuous quality improvement).
For applications in which concerns have been raised that there is insufficient patient or healthcare provider input into the research, the committee may exercise the option to assign a patient or healthcare professional member of the committee (or one of the advisory groups) to become a collaborator on the project to provide advice and the patient’s or HCP’s perspective on the research, throughout the project. They also retain the option to review a draft report prior to publication of findings. (Principle 4: scientific integrity (to ensure adequate inputs) and Principle 5: participation and inclusiveness).
The repository manager will monitor the time required for protocols to pass various checkpoints in the system, to identify any unnecessary bottlenecks in the system and make recommendations for process improvement. (Principle 7: effectiveness, efficiency and responsiveness; Principle 8: reflexivity and continuous quality improvement.)
Finally, Diabetes Action Canada is in the process of posting:
Its policies around data collection, access, use and retention of data.
Its business processes and governance activities; so they can be readily accessible to partners and the public. In future, it will also perform regular audits of its data use practices. (Principle 1: transparency; Principle 2: accountability).
Implementation
In January of 2018, a daylong training workshop was convened for the RGC. A training manual was produced for that purpose, and will be posted on the Diabetes Action Canada website. Topics covered in the workshop included:
An explanation of the types of studies that they would be encountering (data studies; studies making direct contact with patients and hybrid studies).
The stages of the research process and how the RGC fits into this.
What are research governance and information governance and how will they be applied in the context of Diabetes Action Canada’s secure data repository?
Diabetes Action Canada’s governing principles, and how these may apply when reviewing protocols.
What is the ‘added value’ of the RGC vis-à-vis scientific and ethic review.
The structure and function of the governing process and their specific contributions.
Participants were then led through two case studies to test out the application and review process.
Evaluation
At the time of writing, the Diabetes Action Canada secure data repository has been available for research for only a few months. We are in the early days of implementing the governance process and we are still refining those processes—both the internal functioning of the RGC and the consultative processes. We are also continuing to address learning needs of RGC members.
Similarly, our plans for the evaluation of the governance process are in the formative stages. Drawing from relevant SPOR29 and the Patient-Centered Outcomes Research Institute (PCORI)30 evaluation frameworks, key issues that we will address in the evaluation include:
Outcome measures, such as: the proportion of projects reviewed in which changes were recommended and the nature of the changes recommended, including: (1) addition of more patient-relevant outcomes, (2) improvements in participant communications materials (eg, consent forms and information materials) and (3) reductions in risks and burdens to patient participants.
Process measures, such as: patient representatives’ sense of empowerment in the process; and the timeliness of the reviews—both objectively and from the perspective of researchers who submit applications.
Periodic review of Diabetes Action Canada’s information governance processes and procedures on to ensure that they conform to and are congruent with the objectives and principles enunciated in this paper.
Discussion
In Canada, governance over research involving humans, their data, and their samples focuses on the scientific and ethical integrity of the research. Scientific integrity is largely addressed though peer-review processes at the funding and publication stages of the research life-cycle, much like research in other jurisdictions. Ethical integrity is formally addressed through review of research protocols prior to study commencement by research ethics boards at the researchers’ institution(s). Ethics guidance is provided by the Tri-Council Policy Statement on Ethical Conduct for Research Involving Humans, second edition, which addresses research involving human participants, their tissue, or their data.31 For database research, one still needs to consider relevant privacy laws, which are a provincial jurisdiction. These provincial privacy laws have provisions for secondary research use of data without consent. While they are substantively similar, founded on the Canadian version of the Organization for Economic Cooperation and Development (OECD) Privacy Principles,32 33 there are notable inconsistencies across provinces. Some have health-specific privacy legislation, others legislation that covers all sectors; some have multiple legislation to consider. In some cases, small differences in wording and interpretation of legislation present challenges to cross-national data studies.
Most legislation also requires the review and approval of the research protocol by the institution that is the legal data custodian or steward of the data. While Diabetes Action Canada does not currently manage personal—that is, identifiable—health information, the data it holds are of sufficient granularity as to make it possible to indirectly reidentify individuals, should the data be linked or manipulated. Therefore, data in its custody are not released to researchers. Instead, the researcher must apply for permission to gain secure remote access to the data for analyses.
Within this research governance landscape, Diabetes Action Canada has developed and implemented an information governance process designed to foster public trust in the responsible use of the data in their custody. The operational model has been designed to complement the scientific and ethics review processes that research already receives, and is adaptable to other settings.
We believe the principles in the conceptual model we developed are generalisable to many other settings. That being said, we advise that any organisation that considers adopting these principles critically analyse whether they are consonant with the values of the organisation, as it is these core principles to which they will repeatedly return when making difficult or controversial decisions.
While all eight governing principles enunciated are important in fostering public trust, the integrity and participation principles are particularly relevant. The integrity principle establishes the criterion that the research must have a clear patient or public interest, and be of high scientific and ethical integrity. The participation principle ensures the substantive participation of patients and other relevant stakeholders, which helps to achieve the integrity principle.
Over the past decade, there have been many studies examining the public’s or patients’ attitudes towards the conditions under which data studies may be acceptable.34 Much less common is the involvement of patients or the public in an ongoing fashion in the governance over programmes of data-intensive research. The closest exemplar we were able to find in the area of data-intensive research is the consumer panel for data linkage research, associated with the Secure Anonymized Information Linkage (SAIL) databank.35 Their panel is advisory in nature, addressing both access policy and individual projects and representatives of that panel sit on an independent Information Governance Review Panel.
The governance process developed for Diabetes Action Canada goes one step further. It gives people living with diabetes and data providers majority representation in the key decision-making body in the governance process. We are unaware of any other research governance structures that have instilled as strong a role for patients and healthcare professionals in a research network. We are not suggesting that all research networks should choose as radical a path. However, we believe strong lay participation in policy-making and governance is an increasingly important approach to securing the trust of the public.
As our research platforms grow in size and scope, the need for public trust in the uses of these datasets also grows. We believe our model for governance over health information platforms adds substantively to the conceptual and methodological foundations for information governance to help address this need.
bmjopen-2018-026828supp003.pdf (574.7KB, pdf)
Acknowledgments
We wish to acknowledge the contribution and assistance of Conrad Pow and members of the data governance working group and the technical working group. Pow assisted with the development and refinement of the operational protocol for the application and review process for requests to access to the data. The members of the data governance working group were charged with setting the terms of reference for the governance process and reviewing successive drafts of the conceptual and operational models of the governance process. The Technical Working Group reviewed the drafts of the operational model.
Footnotes
Contributors: DJW and JT conducted the literature review and developed the initial and successive conceptual and operational governance models. MG, KK and FS reviewed and suggested revisions to the models. DJW developed the training workshop syllabus and content. MG and DM reviewed and suggested revisions to the workshop material. DJW wrote the initial draft of the manuscript and made subsequent revisions, in response to feedback from JT, MG, KK, DM and FS.
Funding: This work was supported by Diabetes Action Canada, which is funded, in part, through a Canadian Institutes of Health Research chronic disease network grant under the Strategy for Patient-Oriented Research (Funding Reference number: SCA 145101).
Competing interests: The Institute of Health Policy, Management and Evaluation received funds from Diabetes Action Canada SPOR Network towards a partial secondment of DJW time for the development of the governance process for the diabetes data repository. The Department of Family and Community Medicine, University of Toronto, received funds from Diabetes Action Canada SPOR Network towards a partial secondment of MG time for the development of the Diabetes data repository. KK received personal fees from InfoClin during the conduct of the study towards designing the architecture of the Diabetes data repository. In addition, KK has a patent ‘Prediction of Diabetes Mellitus Type 2 Using Biomarkers in Electronic Health Records and Differential Calculus’, pending.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Carter P, Laurie GT, Dixon-Woods M. The social licence for research: why care.data ran into trouble. J Med Ethics 2015;41:404–9. 10.1136/medethics-2014-102374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powles J, Hodson H. Google DeepMind and healthcare in an age of algorithms. Health Technol 2017;7:351–67. 10.1007/s12553-017-0179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous. Strategy for patient-oriented research. 2018. http://www.cihr-irsc.gc.ca/e/41204.html.
- 4. Anonymous. Strategy for patient-oriented research. Patient engagement framework. Ottawa, Ontario: Canadian Institutes of Health Researach, 2014. [Google Scholar]
- 5. Anonymous. Launch of the diabetes action canada national data repository: Diabetes action canada. 2018. https://diabetesaction.ca/launch-of-the-diabetes-action-canada-national-diabetes-repository/.
- 6. Garies S, Birtwhistle R, Drummond N, et al. Data resource profile: National electronic medical record data from the Canadian Primary Care Sentinel Surveillance Network (CPCSSN). Int J Epidemiol 2017;46:1091–2. 10.1093/ije/dyw248 [DOI] [PubMed] [Google Scholar]
- 7. Birtwhistle R, Keshavjee K, Lambert-Lanning A, et al. Building a pan-Canadian primary care sentinel surveillance network: initial development and moving forward. J Am Board Fam Med 2009;22:412–22. 10.3122/jabfm.2009.04.090081 [DOI] [PubMed] [Google Scholar]
- 8. Clinical practice research datalink: medicines and healthcare products regulatory agency, UK. https://www.cprd.com/ [DOI] [PMC free article] [PubMed]
- 9. Burton PR, Murtagh MJ, Boyd A, et al. Data Safe Havens in health research and healthcare. Bioinformatics 2015;31:3241–8. 10.1093/bioinformatics/btv279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lea NC, Nicholls J, Dobbs C, et al. Data safe havens and trust: Toward a common understanding of trusted research platforms for governing secure and ethical health research. JMIR Med Inform 2016;4:e22 10.2196/medinform.5571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laurie G, Sethi N. Towards principles-based approaches to governance of health-related research using personal data. Eur J Risk Regul 2013;4:43–57. 10.1017/S1867299X00002786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sethi N, Laurie GT. Delivering proportionate governance in the era of eHealth: Making linkage and privacy work together. Med Law Int 2013;13(2-3):168–204. 10.1177/0968533213508974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laurie G, Sethi N. Information Governance of Use of Health-Related Data in Medical Research in Scotland: Towards a Good Governance Framework. Edinburgh: University of Edinburgh, 2012. [Google Scholar]
- 14. Smallwood R. Information governance: Concepts, strategies, and best practices. Hoboken, NJ: Wiley, 2014. [Google Scholar]
- 15. Ladley J. Data governance: How to design, deploy, and sustain an effective data governance program. Waltham, MA: Morgan Kauffman, 2012. [Google Scholar]
- 16. Department of Health. Information: To share or not to share? The information governance review. 2013. https://www.gov.uk/government/publications/the-information-governance-review (accessed 25 Sep 2018).
- 17. Simm K. The concepts of common good and public interest: from Plato to biobanking. Camb Q Healthc Ethics 2011;20:554–62. 10.1017/S0963180111000296 [DOI] [PubMed] [Google Scholar]
- 18. Barocas S, Nissenbaum H. Big Data’s End Run around Anonymity and Consent. Privacy, Big Data, and the Public Good. New York, NY: Cambridge University Press, 2014. [Google Scholar]
- 19. Nissenbaum H. Securing trust online: Wisdom or oxymoron?. Boston University Law Review 2001;81:635–64. [Google Scholar]
- 20. Laurie GT, Dove ES, Ganguli-Mitra A, et al. Charting regulatory stewardship in health research: Making the invisible visible. Camb Q Healthc Ethics 2018;27:333–47. 10.1017/S0963180117000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Doherty KC, Burgess MM, Edwards K, et al. From consent to institutions: designing adaptive governance for genomic biobanks. Soc Sci Med 2011;73:367–74. 10.1016/j.socscimed.2011.05.046 [DOI] [PubMed] [Google Scholar]
- 22. Greenhalgh T, Morris L, Wyatt JC, et al. Introducing a nationally shared electronic patient record: case study comparison of Scotland, England, Wales and Northern Ireland. Int J Med Inform 2013;82:e125–e138. 10.1016/j.ijmedinf.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 23. Geissbuhler A, Safran C, Buchan I, et al. Trustworthy reuse of health data: a transnational perspective. Int J Med Inform 2013;82:1–9. 10.1016/j.ijmedinf.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 24. Allen C, Des Jardins TR, Heider A, et al. Data governance and data sharing agreements for community-wide health information exchange: lessons from the beacon communities. EGEMS 2014;2:5 10.13063/2327-9214.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaushal R, Hripcsak G, Ascheim DD, et al. Changing the research landscape: the New York City Clinical Data Research Network. J Am Med Inform Assoc 2014;21:587–90. 10.1136/amiajnl-2014-002764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laurie G. Reflexive governance in biobanking: on the value of policy led approaches and the need to recognise the limits of law. Hum Genet 2011;130:347–56. 10.1007/s00439-011-1066-x [DOI] [PubMed] [Google Scholar]
- 27. Beecher JA. The prudent regulator: politics, independence, ethics, and the public interest. Energy Law Journal 2008;29:577. [Google Scholar]
- 28. Anonymous. How partient-parters are involved. 2018. https://diabetesaction.ca/patient-partners-involved/.
- 29. Anonymous. Evaluation of the Strategy for Patient-Oriented Research. Ottawa, Canada: KPMG, 2016. [Google Scholar]
- 30. Esmail L, Moore E, Rein A. Evaluating patient and stakeholder engagement in research: moving from theory to practice. J Comp Eff Res 2015;4:133–45. 10.2217/cer.14.79 [DOI] [PubMed] [Google Scholar]
- 31. Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada. Tri-council policy statement: ethical conduct for research involving humans. Ottawa: Interagency Secretariat on Research Ethics, 2014. [Google Scholar]
- 32. Organisation for Economic Co-operation and Development. The OECD Privacy Framework. Paris, France 2013. [Google Scholar]
- 33. Canadian Standards Association. Model code for the protection of personal information. A national standard of Canada. Ontario 1996. [Google Scholar]
- 34. Aitken M, de St Jorre J, Pagliari C, et al. Public responses to the sharing and linkage of health data for research purposes: a systematic review and thematic synthesis of qualitative studies. BMC Med Ethics 2016;17:1–24. 10.1186/s12910-016-0153-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones KH, McNerney CL, Ford DV. Involving consumers in the work of a data linkage research unit. Int J Consum Stud 2014;38 45–51. 10.1111/ijcs.12062 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026828supp001.pdf (288.5KB, pdf)
bmjopen-2018-026828supp002.pdf (312.9KB, pdf)
bmjopen-2018-026828supp003.pdf (574.7KB, pdf)