Abstract
Objectives
This analysis examined the association between psoriasis severity, assessed by body surface area (BSA) and the Investigator’s Global Assessment (IGA; previously used only in clinical trials), and patient-reported outcomes (PROs) in a real-world setting.
Design
Cross-sectional analysis within the Corrona Psoriasis Registry, an independent, prospective registry.
Setting
70 dermatology practices in the USA.
Participants
1529 adult patients with psoriasis being treated with biological or non-biological systemic psoriasis treatment by 31 May 2016.
Primary and secondary outcome measures
Psoriasis severity was assessed by percentage of affected BSA (mild (0%–5%), moderate (>5%–10%), severe (>10%–15%), very severe (>15%)) and IGA scores (clear/almost clear (0–1), mild (2), moderate (3), severe (4)). PROs (pain, itch, fatigue; Dermatology Life Quality Index [DLQI]; EuroQoL Visual Analogue Scale [EQ-VAS]; Work Productivity and Activity Impairment [WPAI]) were compared across BSA and IGA levels using analysis of variance and X2 tests. The association between psoriasis severity and PROs was examined using multivariable regression models.
Results
The mean age was 50.6 years and 47% of patients were female. Consistently with more severe psoriasis, symptoms worsened, DLQI scores increased (p<0.05 for each level of BSA and IGA), EQ-VAS decreased (p<0.05 for each level of BSA and IGA) and WPAI scores increased. By BSA score, moderate to very severe psoriasis was associated with poorer outcomes for the ‘impairment while working’ and ‘daily activities impaired’ WPAI domains (all p<0.05 vs mild psoriasis). Very severe psoriasis was associated with increased ‘work hours missed’ and ‘work hours affected’ (both p<0.05 vs mild psoriasis) Findings were similar by IGA. Results were confirmed by multivariable regression analyses.
Conclusions
In a real-world setting, more severe psoriasis, assessed by BSA and IGA, was consistently associated with worse PROs.
Keywords: psoriasis, disease severity, health-related quality of life, patient-reported outcomes, work productivity
Strengths and limitations of this study.
This is the first study to explore the link between psoriasis severity measured by the Investigator’s Global Assessment and patient-reported outcomes (PROs) in a real-world setting.
Due to the cross-sectional study design, causal inferences regarding the relationship between psoriasis severity and PROs cannot be made, and changes in psoriasis severity or PROs over time were not measured.
Patients were recruited from specific dermatology practices, which may have been more focused on psoriasis therapy, and therefore, may not be representative of the general US psoriasis population.
Introduction
Psoriasis is a chronic, immune-mediated, systemic, inflammatory and often debilitating skin disease, affecting 2.6%–3.7% of the population in the USA.1 With itching, pain and scaling as its key symptoms, psoriasis can have a significant impact on patients’ health-related quality of life (HRQoL) and work productivity, depending on disease severity.2–4
A growing body of real-world evidence has shown greater psoriasis severity is associated with worse QoL and higher impairments in work productivity.4 5 Survey data from the National Psoriasis Foundation in the USA revealed patients with severe psoriasis had a greater likelihood of being unemployed than those having mild disease.5 In another US survey, Korman et al found increased psoriasis severity was associated with more itching, pain and scaling; poorer QoL; and greater productivity impairment.4
However, methods of measuring psoriasis severity are not used consistently across studies. Affected body surface area (BSA) is a widely known and used measure of psoriasis severity in clinical practice,6 7 and dermatologists prefer this tool for evaluating patient outcomes.7 Although BSA has been used in studies of psoriasis-associated QoL, BSA-defined disease severity varies across studies (eg, no/little <1%, mild 1%–2%, severe ≥3%, as used by the National Health and Nutrition Examination Survey8 vs mild 0%–<3%, moderate 3%–<10%, severe ≥10%, as used by the National Psoriasis Foundation5). In addition, using BSA alone does not capture information regarding disease location or symptoms.7
Several other severity measures exist, with their respective strengths and limitations. The Psoriasis Area and Severity Index (PASI) score is the most widely used and most thoroughly validated severity measure as a primary endpoint in clinical trials. However, it has not been employed routinely in clinical practice and tends to be poorly understood by clinicians and patients.6 9 10 In addition, it shows low sensitivity to changes in disease severity in cases with low BSA involvement (ie, <10%).6 The Physician’s Global Assessment (PGA) has been described as being easier to understand compared with the PASI and more similar to assessments of disease used in clinical practice.10 However, definitions and criteria for points within the PGA values lack standardisation, and expert consensus has not yet been reached.9 Further, a large discordance may exist between PGA and BSA, resulting in either an overestimate or underestimate of true disease severity.11
The 5-point Investigator’s Global Assessment (IGA) modified (mod) 2011 scale is typically used in clinical trials and gauges psoriasis severity according to the patient’s degree of skin redness, thickening and scaling. Its advantage over other tools (6-point IGA and PGA) is that it more narrowly defines the lowest level of disease severity.9 However, the IGA mod 2011 scale has not been examined in real-world studies of psoriasis-associated QoL.
Although the IGA mod 2011 scale provides a useful framework for the assessment of disease features, use of this scale alone and without accounting for BSA may not accurately reflect disease severity. In clinical practice, physicians may use a combination of objective assessments of psoriasis severity, such as the IGA mod 2011 scale, BSA and symptoms, and more subjective measures, such as the emotional impact of psoriasis on the patient.6
This analysis aims to define the relationship between psoriasis severity and symptom severity, QoL and work productivity among US patients with psoriasis in a real-world setting. Separate analyses were conducted, with psoriasis severity defined using both BSA and IGA.
Methods
Study design
A cross-sectional study was conducted using the enrolment data from the Corrona Psoriasis Registry to identify associations between disease severity and patient-reported outcomes (PROs).
Patient and public involvement
Patients were not involved in determining the design, the recruitment to or the conduct of this study. All patients enrolled in the Corrona Psoriasis Registry receive a patient newsletter that shares study results twice per year.
Data source
The Corrona Psoriasis Registry is an independent, prospective observational cohort launched in April 2015 in collaboration with the National Psoriasis Foundation, with a target enrolment of 10 000 patients with psoriasis from 200 sites throughout the USA. The study inclusion criteria matched those for registry enrolment: Patients must be at least 18 years old, must have been given a psoriasis diagnosis by a dermatologist and had to have begun treatment with a qualifying biological or non-biological systemic psoriasis treatment either within the 12 months preceding or on the day of the enrolment visit. Data collected from the registry launch date (April 2015) to 31 May 2016 were analysed from 70 dermatology practices for the study.
Study measures
Data related to demographics, disease severity (BSA and IGA scores), disease duration, prior and current use of systemic treatments for psoriasis, physician-reported medical history (eg, cardiovascular disease, diabetes mellitus, cardiovascular disease and diabetes risk factors, lymphoma/malignancy, Crohn’s disease, anxiety/depression) and PROs collected at registry enrolment were examined. Patients reported their levels of pain, itching and fatigue on a Visual Analogue Scale (VAS) of 0 (none) to 100 (very severe) and completed two validated and commonly used HRQoL assessment instruments: the Dermatology Life Quality Index (DLQI)12 and the visual analogue component of the EuroQoL Five Dimensions (EQ-5D) Questionnaire VAS.13 In addition, the patients completed the Work Productivity and Activity Impairment (WPAI) questionnaire.14 Dermatologists assessed disease severity in terms of the percentage of total BSA affected and/or the IGA mod 2011 scale score. BSA percentages were categorised as mild (0%–5%), moderate (>5%–10%), severe (>10%–15%) and very severe (>15%). The 5-point IGA was used to categorise levels of skin induration, scaling and redness as clear/almost clear (0–1), mild (2), moderate (3) and severe (4).
Dermatology Life Quality Index
The DLQI, which is a dermatology-specific tool to measure HRQoL, requires respondents to answer 10 questions classified within 6 domains: symptoms and feelings, daily activities, leisure activities, work and school, personal relationships and treatment. Respondents indicate the degree to which they experienced problems for a recall period of 1 week, and responses are assessed with a 4-point Likert scale: 0 (not at all/not relevant), 1 (a little), 2 (a lot) and 3 (very much). Responses are calculated for the total DLQI score, which is 0–30. Higher scores indicate worse HRQoL.
EuroQol Visual Analogue Scale
The EQ-VAS is a non-disease-specific HRQoL assessment tool in which respondents indicate their state of health on the day of assessment on a scale of 0–100, with 100 being the best imaginable state of health and 0 being the worst imaginable state of health.
Work Productivity and Activity Impairment
The WPAI questionnaire measures impairment in work hours missed, work productivity and impairment, and daily activities. Based on a scale of 1 (no effect) to 10 (completely prevented patient from working/participating), respondents report the following domains for the previous week: work hours missed, work hours affected, impairment while working and daily activities impaired. Daily activities include housework, shopping, exercise and studying. Responses for all domains, except for daily activities, are valid only if the respondent is employed.
Statistical analysis
Data regarding patient characteristics, disease characteristics, comorbidities, treatment history and PROs collected at registry enrolment were reported for the overall study population and by BSA and IGA disease severity groups. Frequency counts and percentages were reported for all categorical variables (sex, employment status, disability status, psoriatic arthritis diagnosis, treatment history and history of comorbidities). Means and SDs were reported for all continuous variables (age, body mass index [BMI], psoriasis duration, BSA and IGA). Significance testing with analysis of variance was used for continuous variables, and X2 tests of association were employed for categorical variables to investigate if any differences in values were present across the levels of BSA and IGA disease severity.
Multivariable linear regression was used to model the association between disease severity levels and PROs. To address potential confounding, the model adjusted a priori for age, gender, disease duration and BMI at enrolment. IGA and BSA were modelled separately. Ordinal regression modelling was performed as a confirmatory sensitivity analysis.
Statistical analyses included patients who had complete data on analysis variables at enrolment. To minimise the potential impact of missing data, variables of interest were specified as ‘required’ during data collection; therefore, no statistical techniques were needed to account for missing data. All analyses were performed using STATA (StataCorp LP 2015, Release 14, V.2) with significance set at the p<0.05 level.
Protection of patients
The study used blinded data to maintain patient confidentiality.
Results
Study sample characteristics
As of 31 May 2016, 1529 patients were enrolled in the registry; the mean age was 50.6 years and 47% were female. Among these patients, 1525 had complete BSA data and 1527 had complete IGA data, and the BSA and IGA patients were similar in age and gender types. No patients were omitted from the analysis, but some did not have complete data sets.
Similar proportions of patients were biological experienced and had prior non-biological systemic therapy across disease severity groups (BSA and IGA). The proportion of patients who were biological experienced ranged from 53% to 59% across BSA categories and 53% to 57% across IGA categories. Proportions of patients who had been treated with non-biological systemic therapies ranged from 45% to 49% across BSA categories, and 42% to 54% across IGA categories. The disease severity groups also had similar disease durations (table 1).
Table 1.
Baseline patient characteristics by BSA and IGA severity categories
| BSA severity groups (n=1525) | IGA severity groups (n=1527) | |||||||
| Mild: 0%–5% (n=873) |
Moderate: >5%–10% (n=316) |
Severe: >10%–15% (n=109) |
Very severe: >15% (n=227) |
Clear/almost clear: 0/1 (n=375) |
Mild: 2 (n=404) |
Moderate: 3 (n=586) |
Severe: 4 (n=162) |
|
| Patient characteristics | ||||||||
| Female, n (%) | 439 (50) | 136 (43) | 53 (49) | 88 (39) | 186 (50) | 205 (51) | 262 (45) | 64 (40) |
| Age (years), mean (SD) | 50.6 (14.4) | 50.8 (13.9) | 49.8 (14.8) | 50.4 (14.9) | 50 (14.3) | 51.5 (14.8) | 50.7 (14.1) | 49.5 (14.9) |
| Body weight (kg), mean (SD) | 87.5 (22.2) | 88.7 (24.8) | 92.3 (23.8) | 95.4 (27.3) | 85.6 (19.6) | 89.6 (24.3) | 90.0 (25.3) | 94.1 (24.9) |
| Body mass index (kg/m2), mean (SD) | 30.1 (6.8) | 30.2 (7.3) | 32.0 (8.1) | 32.2 (8.4) | 29.2 (5.7) | 31.0 (7.6) | 30.8 (7.7) | 32.0 (8.0) |
| Employed, n (%) | 575 (66) | 209 (67) | 72 (66) | 137 (61) | 261 (70) | 258 (64) | 374 (64) | 101 (63) |
| Disabled, n (%) | 59 (7) | 28 (9) | 8 (7) | 31 (14) | 20 (5) | 22 (5) | 60 (10) | 24 (15) |
| Disease characteristics | ||||||||
| Psoriasis duration (years), mean (SD) | 15.6 (13.8) | 15.1 (12.9) | 15.7 (13.7) | 17.2 (13.4) | 15.8 (13.2) | 17.0 (14.8) | 15.2 (13.2) | 14.1 (12.4) |
| Psoriatic arthritis diagnosis, n (%) | 369 (42) | 120 (38) | 37 (34) | 90 (40) | 152 (41) | 165 (41) | 223 (38) | 78 (48) |
| Treatment history | ||||||||
| Biological naïve, n (%) | 410 (47) | 148 (47) | 48 (44) | 93 (41) | 165 (44) | 188 (47) | 277 (47) | 70 (43) |
| Biological experienced, n (%) | 463 (53) | 168 (53) | 61 (56) | 134 (59) | 210 (56) | 216 (53) | 309 (53) | 92 (57) |
| Non-biological systemic therapy, n (%) | 389 (45) | 141 (45) | 52 (48) | 111 (49) | 169 (45) | 170 (42) | 268 (46) | 88 (54) |
| Psoriasis severity | ||||||||
| BSA (%), mean (SD) | 2.2 (1.7) | 8.3 (1.6) | 13.4 (1.5) | 34.8 (18.8) | 1.3 (3.3) | 5.7 (8.5) | 11.7 (12.1) | 26.6 (21.9) |
| IGA, mean (SD) | 1.7 (1.1) | 2.8 (0.6) | 3.1 (0.5) | 3.3 (0.7) | 0.6 (0.5) | 2.0 (0.0) | 3.0 (0.0) | 4.0 (0.0) |
| History of comorbidities | ||||||||
| Cardiovascular disease, n (%) | 103 (12) | 35 (11) | 13 (12) | 30 (13) | 48 (13) | 52 (13) | 66 (11) | 16 (10) |
| Coronary artery disease, n (%) | 25 (3) | 4 (1) | 2 (2) | 12 (5) | 9 (2) | 16 (4) | 12 (2) | 6 (4) |
| Congestive heart failure, n (%) | 7 (1) | 9 (3) | 0 (0) | 5 (2) | 3 (1) | 5 (1) | 11 (2) | 2 (1) |
| Stroke, n (%) | 15 (2) | 3 (1) | 2 (2) | 1 (0) | 6 (2) | 8 (2) | 6 (1) | 1 (1) |
| Cardiovascular disease/diabetes risk factors, n (%) | 413 (47) | 159 (50) | 51 (47) | 113 (50) | 167 (45) | 190 (47) | 296 (51) | 84 (52) |
| Hypertension, n (%) | 327 (38) | 126 (40) | 45 (41) | 95 (42) | 139 (37) | 152 (38) | 239 (41) | 64 (40) |
| Hyperlipidaemia, n (%) | 253 (29) | 97 (31) | 23 (21) | 60 (26) | 96 (26) | 123 (31) | 168 (29) | 46 (28) |
| Metabolic syndrome, n (%) | 13 (1) | 3 (1) | 3 (3) | 7 (3) | 5 (1) | 8 (2) | 7 (1) | 6 (4) |
| Diabetes mellitus, n (%) | 111 (13) | 50 (16) | 17 (16) | 38 (17) | 40 (11) | 55 (14) | 92 (16) | 29 (18) |
| Lymphoma/malignancy, n (%) | 40 (5) | 20 (6) | 5 (5) | 9 (4) | 18 (5) | 17 (4) | 34 (6) | 5 (3) |
| Crohn’s disease, n (%) | 4 (0) | 3 (1) | 0 (0) | 1 (0) | 2 (1) | 3 (1) | 2 (0) | 1 (1) |
| Depression, n (%) | 161 (18) | 58 (18) | 24 (22) | 48 (21) | 56 (15) | 79 (20) | 120 (20) | 36 (22) |
| Anxiety, n (%) | 154 (18) | 52 (16) | 25 (23) | 44 (19) | 69 (18) | 75 (19) | 98 (17) | 33 (20) |
History of comorbidities/medical history: cardiovascular disease: combined histories of myocardial infarction, acute coronary syndrome, coronary artery disease, congestive heart failure, peripheral artery disease, cardiac revascularisation procedure, ventricular arrhythmia, cardiac arrest, unstable angina, stroke, transient ischaemic attack, pulmonary embolism, carotid artery disease, deep vein thrombosis or other cardiovascular event; cardiovascular/diabetes risk factors: hypertension, hyperlipidaemia or metabolic syndrome; lymphoma/malignancy: lymphoma, breast, lung, skin (excluding non-melanoma skin cancer) or other.
Prior use of biologicals: adalimumab, alefacept, certolizumab, efalizumab, etanercept, golimumab, infliximab, ixekizumab, secukinumab, ustekinumab, investigational drugs and other patient-specified biologicals.
Prior use of non-biologicals: acitretin, apremilast, cyclosporine, hydroxyurea, methotrexate, mycophenolate, mofetil, sulfasalazine, tofacitinib, 6-thioguanine and other patient-specified non-biologicals.
BSA, body surface area; IGA, Investigator’s Global Assessment.
The most common comorbidities were: hypertension (BSA range: 38%–42%; IGA range: 37%–41%), hyperlipidaemia (BSA range: 21%–31%; IGA range: 26%–31%), depression (BSA range: 18%–22%; IGA range: 15%–22%) and anxiety (BSA range: 16%–23%; IGA range: 17%–20%; table 1). Across BSA and IGA groups, at least 60% of patients worked full time or part time. Increasing proportions of patients were disabled as severity increased according to BSA (range: 7%–14%) and IGA (range: 5%–15%; table 1).
PROs descriptive analysis results
Fatigue, itching and pain VAS scores worsened with disease severity as assessed by both BSA and IGA (figure 1A,B). Across BSA categories, mean fatigue scores ranged from 26.5 to 40.2, itching was 24.7 to 55.7 and pain was 15.2 to 41.7. Among IGA categories, mean fatigue scores ranged from 21.9 to 41.8, itching was 12.1 to 57.3 and pain was 7.4 to 44.3.
Figure 1.
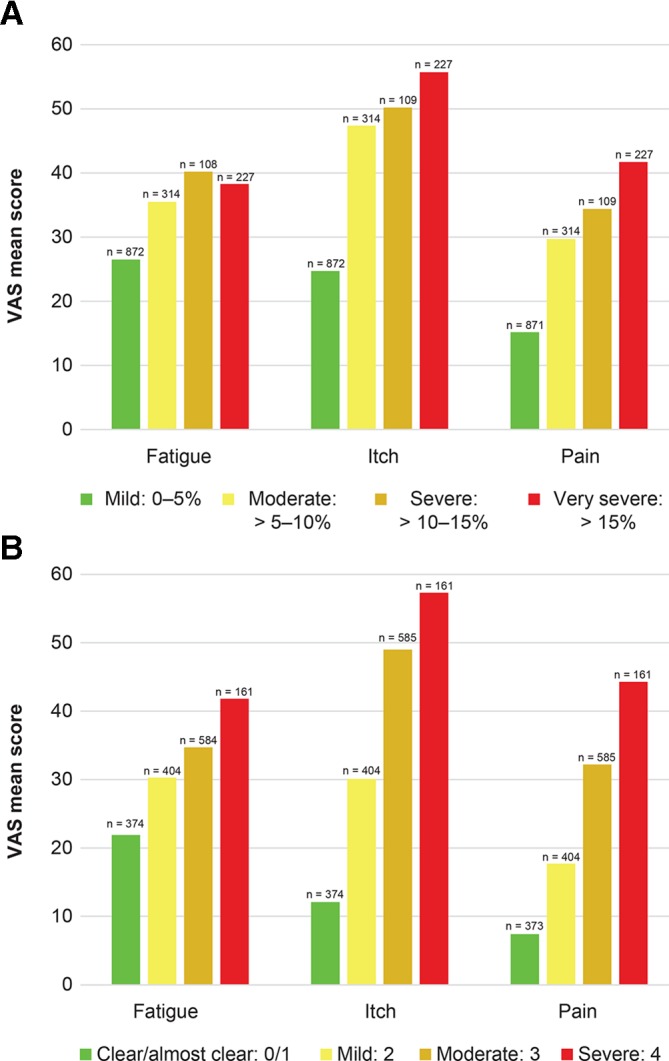
Patient-reported symptoms by BSA severity group (A) and IGA severity group (B). Fatigue, itch and pain symptom scale: 0–100. BSA, body surface area; IGA, Investigator’s Global Assessment; VAS, Visual Analogue Scale.
DLQI scores worsened and EQ-VAS health status decreased with increasing disease severity (figure 2A,B and figure 3A,B). Across BSA and IGA categories, mean DLQI scores ranged from 5.2 to 10.2 and 4.3 to 9.7, respectively. Mean EQ-VAS scores ranged from 62.9 (very severe) to 76.4 (mild) across BSA categories and 62.1 (severe) to 78.8 (clear/almost clear) across IGA categories, with higher scores indicating better health. Work productivity impairment also increased with greater disease severity (figure 4A,B). By BSA category, the ‘work hours missed’ domain was 2.3%–5.9%, ‘impairment while working’ was 8.4%–19.0%, ‘work hours affected’ was 9.5%–20.0% and ‘daily activities impaired’ was 13.1%–31.5%. By IGA category, the ‘work hours missed’ domain was 1.2%–4.2%, ‘impairment while working’ was 5.5%–19.9%, ‘work hours affected’ was 6.0%–20.9% and ‘daily activities impaired’ was 8.2%–34.1%.
Figure 2.
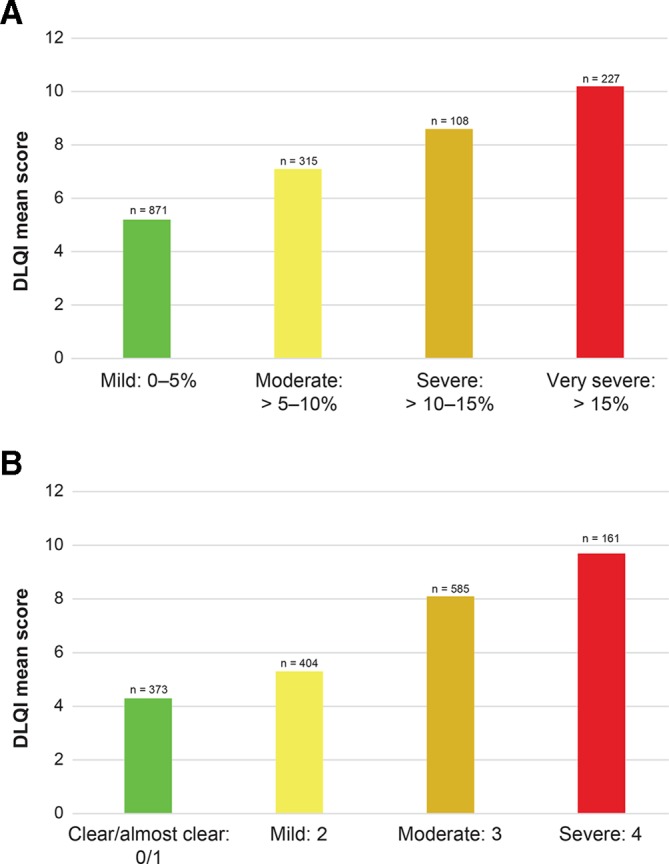
DLQI scores by BSA severity group (A) and IGA severity group (B). DLQI scale: 0–30. BSA, body surface area; DLQI, Dermatology Life Quality Index; IGA, Investigator’s Global Assessment.
Figure 3.
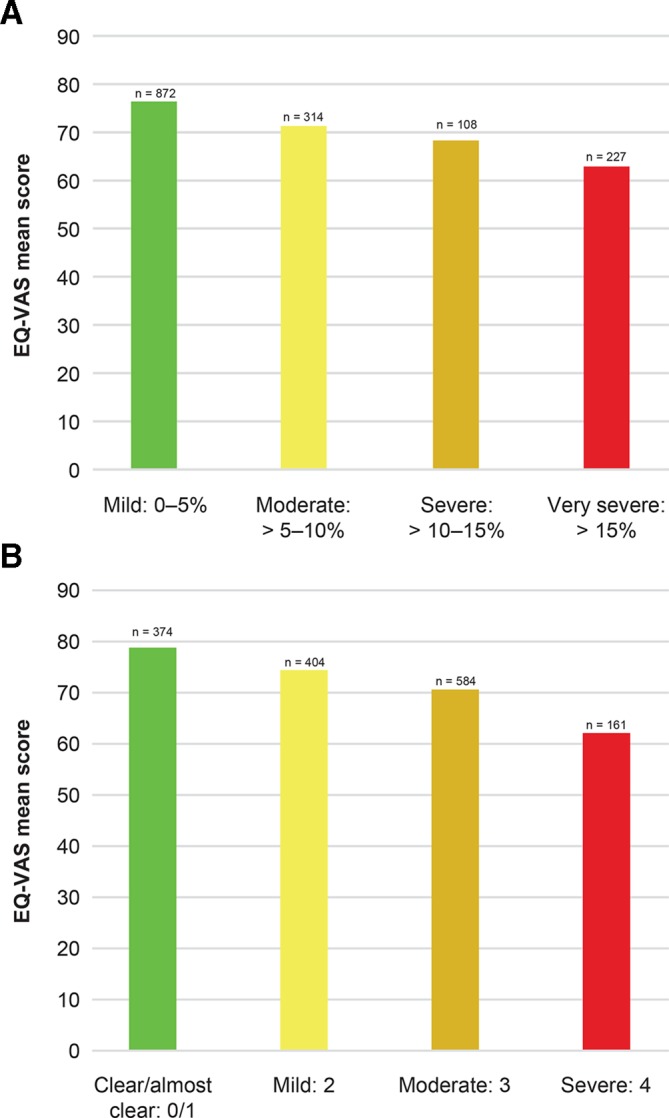
EQ-VAS by BSA severity group (A) and IGA severity group (B). EQ-VAS scale: 0–100. BSA, body surface area; DLQI, Dermatology Life Quality Index; EQ-VAS, EuroQoL Visual Analogue Scale; IGA, Investigator’s Global Assessment.
Figure 4.
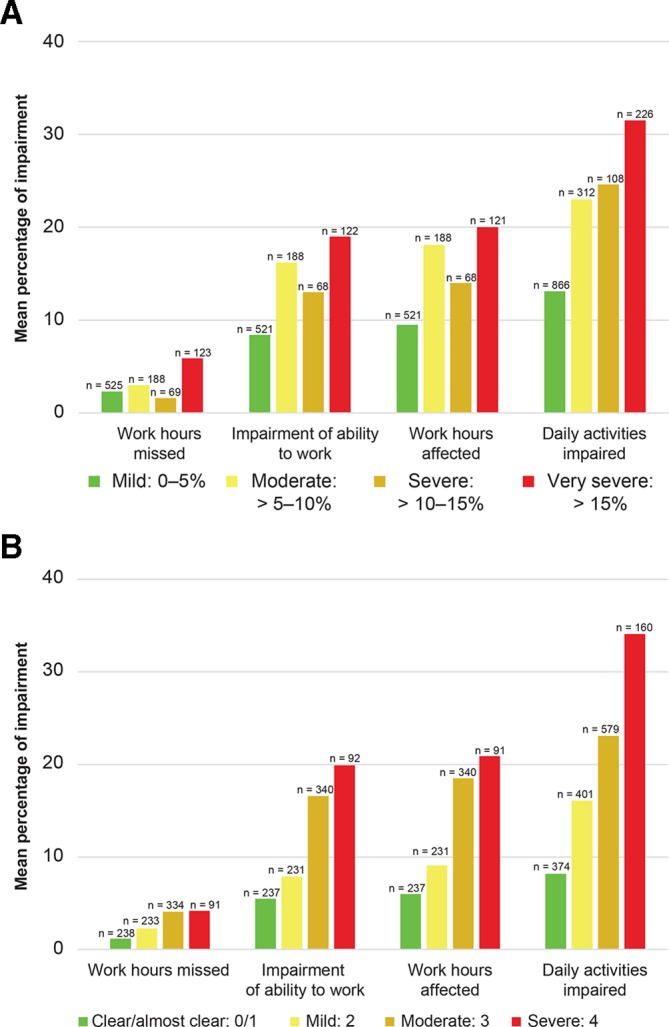
WPAI domains by BSA severity group (A) and IGA severity group (B). BSA, body surface area; IGA, Investigator’s Global Assessment; WPAI, Work Productivity and Activity Impairment.
Multivariable linear regression model
The multivariable linear regression models confirmed the overall pattern in the descriptive results, demonstrating an association between greater disease severity when assessed by BSA and IGA, and worsening symptoms, worse QoL and greater WPAI. Worsening itch, pain and fatigue were significantly associated with increases in BSA and IGA levels: p<0.001 for moderate, severe and very severe BSA (reference: mild) and for mild, moderate and severe IGA (reference: clear/almost clear). Overall DLQI and EQ-VAS scores also worsened with disease severity (p<0.05 for each level of BSA and IGA) (table 2).
Table 2.
Linear regression results by BSA and IGA severity
| BSA severity groups (reference=mild: 0%–5%) |
IGA severity groups (reference=clear/almost clear: 0/1) |
|||||
| Parameter, coefficient (95% CI) | Moderate: >5%–10% |
Severe: >10%–15% | Very severe: >15% | Mild: 2 | Moderate: 3 | Severe: 4 |
| Symptoms | ||||||
| Fatigue | 9.50 (5.87, 13.14)* |
13.13 (7.49, 18.76)* |
12.67 (8.52, 16.82)* |
7.52 (3.55, 11.49)* | 12.45 (8.79, 16.11)* | 19.69 (14.48, 24.91)* |
| Itch | 23.17 (19.27, 27.06)* |
25.42 (19.40, 31.44)* |
32.10 (27.66, 36.54)* |
18.27 (14.21, 22.33)* | 37.15 (33.41, 40.90)* | 45.70 (40.36, 51.04)* |
| Pain | 15.09 (11.46, 18.71)* |
19.37 (13.76, 24.97)* |
27.68 (23.55, 31.82)* |
10.36 (6.48, 14.24)* | 25.15 (21.57, 28.72)* | 37.37 (32.27, 42.47)* |
| DLQI | 1.91 (1.17, 2.66)* |
3.40 (2.25, 4.56)* |
5.26 (4.41, 6.11)* |
0.85 (0.04, 1.67)* | 3.76 (3.01, 4.52)* | 5.47 (4.40, 6.55)* |
| EQ-VAS | –5.14 (–7.91, –2.36)* |
–7.34 (–11.64, –3.04)* |
–12.98 (–16.14,– 9.81)* |
–3.47 (–6.51, –0.43)* | –7.35 (–10.16, –4.55)* | –15.15 (–19.15, –11.55)* |
| WPAI questionnaire | ||||||
| Work time missed | 0.58 (–1.38, 2.55) |
–0.71 (−3.68, 2.26) |
3.50 (1.17, 5.82)* |
1.13 (–1.01, 3.27) | 2.96 (1.01, 4.92)* | 2.87 (0.01, 5.73)* |
| Impairment while working | 8.00 (4.58, 11.39)* |
5.22 (0.06, 10.39)* |
11.52 (7.49, 15.55)* |
2.87 (–0.78, 6.53) | 11.56 (8.22, 14.91)* | 15.14 (10.29, 20.00)* |
| Working hours affected | 8.79 (5.20, 12.37)* |
5.17 (–0.27, 10.61) |
11.44 (7.19, 15.70)* |
3.49 (–0.35, 7.33)* | 13.05 (9.54, 16.55)* | 15.65 (10.54, 20.77)* |
| Daily activities affected | 10.28 (7.01, 13.54)* |
11.54 (6.50, 16.59)* |
19.69 (15.97, 23.40)* |
7.88 (4.34, 11.42)* | 15.04 (11.78, 18.31)* | 26.36 (21.70, 31.01)* |
All models adjusted a priori for age, gender, psoriasis duration and body mass index at registry enrolment.
DLQI scale: 0–30; EQ-VAS scale: 0–100; fatigue, itch and pain symptom scale: 0–100; WPAI scale: 0–10.
*Significant at p<0.05.
BSA, body surface area; DLQI, Dermatology Life Quality Index; EQ-VAS, EuroQoL Visual Analogue Scale; IGA, Investigator’s Global Assessment; WPAI, Work Productivity and Activity Impairment.
In BSA models, the moderate, severe and very severe psoriasis categories were significantly associated with poorer outcomes in the WPAI domains of ‘impairment while working’ and ‘daily activities impaired’ compared with mild severity (all p<0.05) (table 2). Very severe disease was significantly associated with increased ‘work hours missed’ (p<0.05), and moderate and very severe disease were associated with increased ‘work hours affected’ (both p<0.05) compared with mild disease.
In IGA models, mild, moderate and severe psoriasis categories were significantly associated with worse outcomes for the WPAI domain of ‘daily activities impaired’ compared with clear/almost clear (all p<0.05). Compared with the mild psoriasis category, moderate and severe psoriasis categories were significantly associated with poorer outcomes in the domains of ‘work hours missed’, ‘impairment while working’ and ‘work hours affected’ (all p<0.05).
Sensitivity analysis
Ordinal regression modelling was performed as a sensitivity analysis to confirm the results of the linear regression; results confirmed a consistent trend, with increasing severity of disease associated with worsening QoL and greater impairment in work productivity and activity. Results of the proportional odds models for BSA and IGA disease severity categories are shown in online supplementary table 1.
bmjopen-2018-027535supp001.pdf (75.6KB, pdf)
Discussion
In this cross-sectional analysis of the Corrona Psoriasis Registry, multivariable linear regression models showed patient-reported symptoms, QoL and work productivity worsened with increasing disease severity, as measured by BSA and IGA. The results were statistically significant across all levels of psoriasis severity for patient-reported pain, itch and fatigue; DLQI overall scores; EQ-VAS and the ‘daily activities impaired’ domain of the WPAI questionnaire. For the WPAI domains ‘work hours missed’, ‘impairment while working’ and ‘work hours affected’ outcomes were significantly worse for patients with the highest severity of psoriasis (BSA=very severe, IGA=severe). Findings were overall consistent between the BSA and IGA results.
To the authors’ knowledge, the present study was the first to explore the link between IGA and PROs in a real-world setting. PGA has been used previously in real-world settings in both postmarketing safety studies15 16 and patient registries.16–19 A multicentre, prospective study conducted in Spain found psoriasis severity was the primary factor affecting QoL. Although PGA data were collected in that study, PASI was ultimately used for the multivariate modelling.20
Of note, the BSA and IGA categories as defined in the present study differ somewhat from those used in prior research. The present study used the 5-point mod 2011 scale, which differs from the 6-point scales used in some clinical trials of biological treatments for psoriasis9 in that the ‘almost clear’ category is more narrowly defined compared with the ‘minimal’ category in PGA and the other IGA versions.9 The category cut-off points for BSA used in the present study (ie, mild: 0%–5%, moderate: >5%–10%, severe: >10%–15% and very severe: >15%) also differed from those that have been used in certain studies and referred to in guidelines and expert consensus statements (eg, mild 0% to <3%, moderate 3% to <10% and severe ≥10%5; moderate to severe >10%,21 no/little <1%, mild 1% to 2%, severe ≥3%).8 The addition of the ‘very severe’ category in the present study may shed light on specific unmet medical needs in this segment of the population with psoriasis. Further research is required to fully understand how differences in BSA categorisation may impact results across clinical trials and observational studies.
In a prior study by Korman et al of psoriasis severity and PROs, severity of symptoms, EQ-5D, DLQI and WPAI domains were assessed using BSA category (mild, moderate or severe as determined by a physician).4 Although the categorisation of psoriasis severity differed, the results were generally consistent with the findings of the present study, for which severity of fatigue, itching and pain; DLQI total scores; and WPAI domains worsened with increasing disease severity.4 In addition, lower EQ-5D summary scores were reported with increasing disease severity,4 similar to the lower EQ-VAS scores observed in the present study.
Although the present study demonstrates the association between increased psoriasis severity and worsened PROs, future research may clarify this relationship. The present analysis did not address the potential for the outcomes of interest to be highly correlated with one another. For instance, previous research by Lewis-Beck et al found an inverse relationship between itching, pain and scaling severity and work productivity.22 Further research may investigate how QoL and work productivity measures may interact with one another in the context of psoriasis severity. In addition, due to the cross-sectional study design, the results represent psoriasis severity and PROs at one time point. Future research using longitudinal data could show how changes in psoriasis severity may relate to changes over time in QoL and work productivity. In addition, particularly for a longitudinal study, the combination and interaction of BSA and IGA as a single measure of severity could prove informative.
The results of this study must be interpreted in the context of the source of the data, study design and analysis methods. First, this study was a cross-sectional analysis, which does not allow for causal inferences regarding psoriasis severity and the outcomes of interest. Second, the patients enrolled in the registry were recruited from specific dermatology practices, which may have been more focused on psoriasis therapy and, therefore, may not be representative of the general US psoriasis population. The linear regression model was robust to the non-normal distribution of the data; however, estimates at the extreme lower and upper levels of severity may have been overestimated or underestimated.
Conclusions
Increased psoriasis severity as measured by both BSA and IGA categories was associated with worsened PROs in this USA-based psoriasis registry study. Future research is warranted to understand the potential interrelationships between PROs and to understand whether longitudinal improvements in psoriasis severity are associated with improvements in PROs.
Supplementary Material
Acknowledgments
Novartis participated in the interpretation of data, review and approval of the manuscript. The authors take full responsibility for the content of the paper. They thank Jacob Willet, Karen Kurtyka and Lisa Kaspin-Powell (Oxford PharmaGenesis, Newtown, Pennsylvania, USA) for medical writing support, editorial assistance and collation and incorporation of comments from all authors. These services were funded by Novartis Pharmaceutical Corporation, East Hanover, New Jersey, USA. The authors also thank all patients who participated in this study.
Footnotes
Contributors: BS interpreted the results, critically reviewed the manuscript and agreed to be accountable for all aspects of the manuscript. JDG helped design the study, acquired the data, critically reviewed the manuscript and agreed to be accountable for all aspects of the manuscript. CK helped design the study, interpreted the results and critically reviewed the manuscript. MM helped design the study, collected the data, undertook the data analysis, interpreted the results and critically reviewed the manuscript. NG undertook the data analysis, interpreted the results and critically reviewed the manuscript. PH helped design the study, interpreted the results and critically reviewed the manuscript. YZ helped design the study, interpreted the results and critically reviewed the manuscript. VH interpreted the results and critically reviewed the manuscript. FL helped design the study, interpreted the results and critically reviewed the manuscript. ML helped design the study, acquired the data and critically reviewed the manuscript.
Funding: This study was sponsored by Corrona, LLC and the analysis was funded by Novartis. Access to study data was limited to Corrona and Corrona statisticians completed all of the analysis; all authors contributed to the interpretation of the results. Corrona has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene,Crescendo, Eli Lilly and Company, Genentech, Gilead, GSK, Janssen, Momenta Pharmaceuticals, Novartis, Pfizer, Regeneron, Roche, Merck, UCB and Valeant. Parts of this manuscript were presented as a poster 3 March to 7 March 2017 at 75th Annual Meeting of the American Academy of Dermatology in Orlando, Florida, USA.
Competing interests: BS has served as a consultant for AbbVie, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Celgene, Dermira, Galderma, GlaxoSmithKline, Eli Lilly, Janssen, LEO Pharma, Medac, Meiji Seika Pharma, Menlo Therapeutics, Novartis, Ortho Dermatologics/Valeant, Pfizer, Regeneron, Sanofi-Genzyme, Sun Pharma, UCB Pharma, as coscientific director of the Corrona Psoriasis Registry and has received grant support for the University of Connecticut fellowship programme from AbbVie and Janssen. ML is an employee of Mount Sinai and receives research funds from: Abbvie, Boehringer Ingelheim, Celgene, Eli Lilly, Incyte, Janssen/Johnson & Johnson, Leo Pharmaceuticals, Medimmune/Astra Zeneca, Novartis, Pfizer, Sciderm, Valeant and ViDac. ML is also a consultant for Allergan, Aqua, Boehringer Ingelheim, Corrona, LEO Pharma, Menlo and Promius. JDG is an employee and shareholder of Corrona, LLC and has been a consultant to Genentech, Janssen, Novartis, Pfizer and Eli Lilly. MM is an employee of Corrona, LLC and at the time of the study, was a member of the University of Delaware, Department of Behavioral Health and Nutrition Affiliate Faculty (non-remunerative position). NG is an employee of Corrona, LLC, and CK was an employee of Corrona, LLC, at the time of the study. VH and PH are employees of Novartis, and YZ and FL were employees of Novartis at the time of the study.
Ethics approval: All participating investigators were required to obtain full board approval for research involving human subjects through a central institutional review board (IntegReview). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained for the respective governing IRB, and documentation of approval was submitted to the sponsor prior to initiating any study procedures. The Corrona Psoriasis Registry was approved by both local and central review boards at the participating sites.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Patient consent for publication: Obtained.
References
- 1. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014;70:512–6. 10.1016/j.jaad.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 2. Bhosle MJ, Kulkarni A, Feldman SR, et al. Quality of life in patients with psoriasis. Health Qual Life Outcomes 2006;4:35 10.1186/1477-7525-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gelfand JM, Feldman SR, Stern RS, et al. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol 2004;51:704–8. 10.1016/j.jaad.2004.04.014 [DOI] [PubMed] [Google Scholar]
- 4. Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol 2016;41:514–21. 10.1111/ced.12841 [DOI] [PubMed] [Google Scholar]
- 5. Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PLoS One 2012;7:e52935 10.1371/journal.pone.0052935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008;58:826–50. 10.1016/j.jaad.2008.02.039 [DOI] [PubMed] [Google Scholar]
- 7. Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: Treatment targets for plaque psoriasis. J Am Acad Dermatol 2017;76:290–8. 10.1016/j.jaad.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 8. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med 2014;47:37–45. 10.1016/j.amepre.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langley RG, Feldman SR, Nyirady J, et al. The 5-point Investigator’s Global Assessment (IGA) Scale: A modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat 2015;26:23–31. 10.3109/09546634.2013.865009 [DOI] [PubMed] [Google Scholar]
- 10. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005;64 Suppl 2(suppl_2):ii65–ii68. discussion ii69–73 10.1136/ard.2004.031237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and Body Surface Area Composite Tool for Assessing Psoriasis Response to Apremilast Therapy: Results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol 2017;16:147–53. [PubMed] [Google Scholar]
- 12. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–6. 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 13. EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 14. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 15. Kimball AB, Pariser D, Yamauchi PS, et al. OBSERVE-5 interim analysis: an observational postmarketing safety registry of etanercept for the treatment of psoriasis. J Am Acad Dermatol 2013;68:756–64. 10.1016/j.jaad.2012.10.055 [DOI] [PubMed] [Google Scholar]
- 16. Menter A, Thaçi D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol 2015;73:410–9. 10.1016/j.jaad.2015.06.038 [DOI] [PubMed] [Google Scholar]
- 17. Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol 2016;861:74:851-e854 10.1016/j.jaad.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 18. Huynh DH, Boyd TA, Etzel CJ, et al. Persistence of low disease activity after tumour necrosis factor inhibitor (TNFi) discontinuation in patients with psoriatic arthritis. RMD Open 2017;3:e000395 10.1136/rmdopen-2016-000395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindström Egholm C, Krogh NS, Pincus T, et al. Discordance of global assessments by patient and physician is higher in female than in male patients regardless of the physician’s sex: data on patients with rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis from the DANBIO Registry. J Rheumatol 2015;42:1781–5. 10.3899/jrheum.150007 [DOI] [PubMed] [Google Scholar]
- 20. Daudén E, Pujol RM, Sánchez-Carazo JL, et al. Demographic characteristics and health-related quality of life of patients with moderate-to-severe psoriasis: The VACAP study. Actas Dermosifiliogr 2013;104:807–14. 10.1016/j.ad.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 21. Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 2011;303:1–10. 10.1007/s00403-010-1080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis-Beck C, Abouzaid S, Xie L, et al. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate-to-severe psoriasis using structural equation modeling. Patient Prefer Adherence 2013;7:199–205. 10.2147/PPA.S39887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027535supp001.pdf (75.6KB, pdf)


