Abstract
Introduction
Non-communicable diseases (NCDs) are the leading cause of death globally. Even though NCD disproportionally affects low-to-middle income countries, these countries including South Africa, often have limited capacity for the prevention and control of NCDs. The standard evidence-based care for the long-term management of NCDs includes rehabilitation. However, evidence for the effectiveness of rehabilitation for NCDs originates predominantly from high-income countries. Despite the disproportionate disease burden in low-resourced settings, and due to the complex context and constraints in these settings, the delivery and study of evidence-based rehabilitation treatment in a low-resource setting is poorly understood. This study aims to test the design, methodology and feasibility of a minimalistic, patient-centred, rehabilitation programme for patients with NCD specifically designed for and conducted in a low-resource setting.
Methods and analysis
Stable patients with cancer, cardiovascular disease, chronic respiratory disease and/or diabetes mellitus will be recruited over the course of 1 year from a provincial day hospital located in an urban, low-resourced setting (Bishop Lavis, Cape Town, South Africa). A postponed information model will be adopted to allocate patients to a 6-week, group-based, individualised, patient-centred rehabilitation programme consisting of multimodal exercise, exercise education and health education; or usual care (ie, no care). Outcomes include feasibility measures, treatment fidelity, functional capacity (eg, 6 min walking test), physical activity level, health-related quality of life and a patient-perspective economic evaluation. Outcomes are assessed by a blinded assessor at baseline, postintervention and 8-week follow-up. Mixed-method analyses will be conducted to inform future research.
Ethics and dissemination
This study has been approved by the Health Research and Ethics Council, Stellenbosch University (M17/09/031). Information gathered in this research will be published in peer-reviewed journals, presented at national and international conferences, as well as local stakeholders.
Trial registration number
PACTR201807847711940; Pre-results.
Keywords: rehabilitation, developing countries, noncommunicable diseases, randomised controlled trial, feasibility studies, neoplasms, diabetes mellitus, cardiovascular diseases, pulmonary disease, chronic obstructive
Strengths and limitations of this study.
This is the first feasibility study of patient-centred rehabilitation for non-communicable disease, specifically tailored to the context of an urban, low-resource setting.
This study uses a postponed information randomisation model to avoid randomising patients to usual care.
This study will inform feasibility and cost–benefits to upscale rehabilitation for non-communicable disease in low-resource settings.
The experimental group size is dependent on the patient’s willingness to participate in the rehabilitation programme.
Generalisation of results to other low-resourced settings needs to be explored.
Introduction
Non-communicable diseases (NCDs) are the leading cause of death globally. Almost three quarters of NCD-related deaths occur in low-income and middle-income countries (LMICs).1 Moreover, approximately 60% of NCD deaths occur before the age of 70 with 82% of these ‘premature’ deaths occurring in LMICs.1 Cardiovascular diseases account for most NCD deaths (17.5 million annually), followed by cancer (8.2 million), respiratory diseases (4 million) and diabetes (1.5 million). These four groups of diseases account for 82% of all NCD deaths and 54% of loss in disability-adjusted life years; however, they share important commonalities in terms of modifiable risk factors.1
South Africa is facing evolving healthcare needs moving from a predominantly communicable disease profile towards a NCD profile. This cannot be contributed solely to the remarkable improvements concerning the prevention and control of HIV/AIDS and tuberculosis, but also to increased urbanisation and economic growth.2 3 Accordingly, as of 2011, NCDs are the leading cause of death in South Africa, which makes the prevention and control of NCD paramount. Even though NCD disproportionally affects LMICs, these countries, including South Africa, often have limited capacity for the prevention and control of NCD.1 The rapid rise in NCDs is predicted to impede poverty reduction initiatives in low-income countries, particularly by increasing household costs associated with healthcare. Not much is known about the true economic and societal costs of NCDs in South Africa. The WHO recently estimated the loss of economic output associated with chronic diseases in 23 LMICs. It was estimated that in South Africa between 2006 and 2015, cumulative gross domestic product losses due to heart disease, stroke and diabetes alone amounted to US$1.88 billion.4
Rehabilitation can be defined as the ‘sum of activities required to influence favourably the underlying cause of the disease, as well as the best possible physical, mental and social conditions, so that they (patients) may by their own efforts, preserve or resume when lost, as normal a place as possible in the society’.5 The core components of rehabilitation for patients with NCD include baseline patient assessment, educational interventions, risk factor modification, psychosocial interventions, physical activity counselling and exercise training.5–11 However, the unmet need for rehabilitation globally, and especially in LMICs is profound,12 13 and thought to be a direct function of the lack of reimbursement and governmental funding. The reasons are complex, and include healthcare budgetary issues (particularly for lower-income countries), inadequate legislation, lack of trained healthcare providers and a dearth of evidence from randomised controlled trials (RCTs) evaluating the effects of rehabilitation in LMICs such as those that are available in high-income countries.14 While there is substantial evidence for the benefits of exercise-based rehabilitation in high-resource settings,15–18 the study, delivery and implementation of evidence-based rehabilitation in low-resourced settings are poorly understood. Hence, it is important to determine a minimalistic yet effective rehabilitation intervention and accompanying research methodology to optimise the (cost) benefits and sustainability of rehabilitation services in a low-resource setting.19 An effective, evidence-based, rehabilitation paradigm, specifically for resource-limited settings, is essential in terms of attaining United Nations’ sustainable development goal 3 ‘Ensure healthy lives and promote well-being for all at all ages’ in the context of an NCD epidemic.20 The role of rehabilitation is instrumental for effective implementation of a variety of global action plans including the Global Strategy and Action Plan on Ageing and Health (2016–2020), and Framework on Integrated People-centred Health Services.12
A particularly important aspect regarding rehabilitation in a low-resource setting, and in specifically in South Africa, is the influence of multiple comorbidities on the outcome of rehabilitation; that is, ‘quadruple burden of disease’ (communicable, non-communicable, perinatal and maternal, and injury-related disorders).2 21–23 Despite the widespread development of clinical practice guidelines, comorbidity remains a known barrier to the application of such guidelines in various settings and across conditions.24 25 The robust evidence on which most clinical practice guidelines are founded is primarily based on short-term RCTs, which exclude those with comorbid conditions.23 26 This limits the ability to generalise their results to settings with a high disease burden. For instance, a patient may present herself with simultaneous chronic obstructive pulmonary disorder and diabetes in the presence of an HIV infection with secondary cardiomyopathy as a side effect of HIV treatment. Such complex patients argue against a ‘disease’-specific rehabilitation approach (eg, cardiac rehabilitation and pulmonary rehabilitation). Thus, there is a clear need for a patient-centred approach, incorporating the complexity of multiple comorbidities in a single-personalised rehabilitation programme. A study by Derman and colleagues on patient-centred rehabilitation for patients with NCD in a high-resource LMIC setting found significant improvements in a variety of outcomes including lipid profile, muscle strength and walking capacity.27 However, the translation of this programme to a low-resource setting is limited due to the aforementioned quadruple burden of disease, but also setting-specific barriers and facilitators for treatment adherence (patient) and treatment fidelity (therapist). The transition to a patient-centred approach has been identified as the crux to the reimagined future of 2030 by the WHO through their #REHAB2030 call for action.12
The aim of this study is therefore to (i) test the feasibility28 and key characteristics of a minimalistic patient-centred rehabilitation intervention that is designed specifically for the low-resource setting and (ii) inform the research methodology and study design for a full-scale randomised clinical trial on the effectiveness of patient-centred rehabilitation for NCD in a low-resource setting.
These aims can be structured according to the following objectives:
To assess the feasibility and acceptance of a minimalistic patient-centred rehabilitation programme in a low-resource setting.
To assess recruitment processes including attrition, retention and study uptake to inform a definitive RCT.
To assess the feasibility of using a postponed information randomisation model in the context of a low-resource setting.
To assess barriers and facilitators for treatment adherence (patient) and fidelity (therapist and physician).
To assess the clinical relevance and validity of various outcomes in a low-resource community to inform the selection of primary and secondary outcomes, and sample size calculations for a full-scale RCT.
To assess the feasibility of a patient-perspective economic evaluation in the context of a low-resource setting.
To demonstrate proof of principle by gathering information about the process of change between the two treatment arms.
Methods and analysis
Design
This is a randomised pilot study28 with blinded assessments to evaluate the feasibility of a patient-centred lifestyle rehabilitation programme in addition to usual care, compared with usual care alone, in a low-resource setting over the course of a 1-year timespan (2019).
Setting
Bishop Lavis is a densely populated, urban area—home to ~54 000 people living mostly in formal dwellings.29 Only 66% of the economically active population (aged 15–65 years) of this community is employed. Approximately half of these (47%) earn between 0 and 544$ (purchase power parity) per household (average ~4.4 dependents per household) per month.30 In contrast, the gross average monthly household wage in South Africa is ~3231$. The dominant types of occupation in Bishop Lavis are those classified as elementary occupations, for example, machine operators and assemblers, craft and related trades workers, and clerks. Crime rates in the area are high, with Bishop Lavis being in the top 10 of neighbourhoods in terms of murders, attempted murder, robbery and drug-related or gang-related crimes.31
The Bishop Lavis Rehabilitation Centre (BLRC) is a university-driven service learning centre that provides physiotherapy, occupational therapy, dietetics, as well as speech and language therapy to the community of Bishop Lavis and its surroundings. However, no structural patient-centred rehabilitation programme is in place for people with NCD. The BLRC was opened in January 1994 as a collaboration between the University of Stellenbosch, the Provincial Administration of the Western Cape and the Bishop Lavis local authority.
Patient and public involvement
Patients were not directly involved in the design of this study; however, their input was voiced through the >25 year experience of the BLRC staff (see acknowledgements) working in this environment. All components of this study, including intervention and assessments, have been tested using volunteers at the BLRC. Feedback with respect to study findings will be provided during a patient-information day on completion of this study.
Participants
Inhabitants of the Bishop Lavis community diagnosed with at least one of the four major NCDs, namely cardiovascular diseases (eg, heart failure and stroke), cancers, chronic respiratory diseases (including chronic obstructive pulmonary disorder) and diabetes will be recruited through the Bishop Lavis Day Care hospital physician and nursing staff for the study. This study will take place over 1 year between January 2019 and December 2019. Overseen by the family physician (MA) at the Bishop Lavis clinic, physician and nursing staff will determine eligibility of the patient based on the following eligibility criteria, as well as verify contact details.
Cardiovascular disease, cancer, diabetes and/or chronic respiratory disease.
Stable medical condition.
Agree to be contacted by research team.
The eligible patient will subsequently be contacted by the assessor (BLF), who will provide an oral explanation of the study and if interested, invite the eligible patient for a baseline assessment. During baseline assessment, the assessor (BLF) will determine inclusion/exclusion based on the following criteria, and obtain written informed consent for the observational study at this stage (consent 1) prior to any outcome measure testing.
Inclusion criteria
At least 18 years of age or older (ie, adult).
Able to perform some weight-bearing or non-weight-bearing exercise.
Minimal of one confirmed diagnosis according to the WHO classification32 of cardiovascular disease (ICD: I0-99), chronic respiratory disease (ICD: J30-98), malignant neoplasms (ICD: C00-97) or diabetes (E10-E14; excluding those with complications [E10.2-E10.29, E11.2-E11.29, E12.2, E13.2-E13.29 and E14.2]).
Exclusion criteria
No generic contraindications for exercise training or disease-specific contraindications for exercise training (table 1).33
Other contraindications for exercise prescription as determined by the physiotherapist.
Structured exercise training at regular intervals (more than once per week) at a moderate-to-vigorous intensity in the previous 3 months.
Psychiatric concerns, substance abuse or known history of violence that would jeopardise the safe conduct of this programme.
Pregnancy.
Table 1.
Generic and disease-specific contraindications to be considered during enrolment
| Generic | Disease specific | ||||
| ICD: C00-97 | ICD: E10-14 | ICD: I0-99 | ICD: J30-98 | ||
| Factors related to treatment |
|
||||
| Haematologic |
|
|
|||
| Musculoskeletal |
|
|
|||
| Systemic |
|
|
|
|
|
| Gastrointestinal |
|
||||
| Cardiovascular |
|
|
|
||
| Pulmonary |
|
|
|
||
| Neurologic |
|
|
|||
The absolute contraindications to exercise participation and direct exclusion are highlighted in bold. All other criteria are reviewed on a case-by-case basis by the medical practitioner at the time of inclusion if applicable. BP, Blood Pressure; bpm, beat per minute; DBP, Diastolic Blood Pressure; HR, Heart Rate; ICD, International Classification of Disease; SBP, Systolic Blood Pressure
*While endurance training can start within 2 days of a cardiac event, the guidelines for resistance training indicate a minimum of 2–3 weeks following transcatheter procedures, and a minimum of 5 weeks after myocardial infarction or cardiac surgery.
Data management and randomisation logistics
Data collection and randomisation are facilitated through http://www.castoredc.com. Castor EDC is an intuitive and secure cloud-based electronic data capture platform that facilitates defined user roles, advanced monitoring, participant management and powerful calculations. Data storage is compliant with all relevant regulations including good clinical practice.
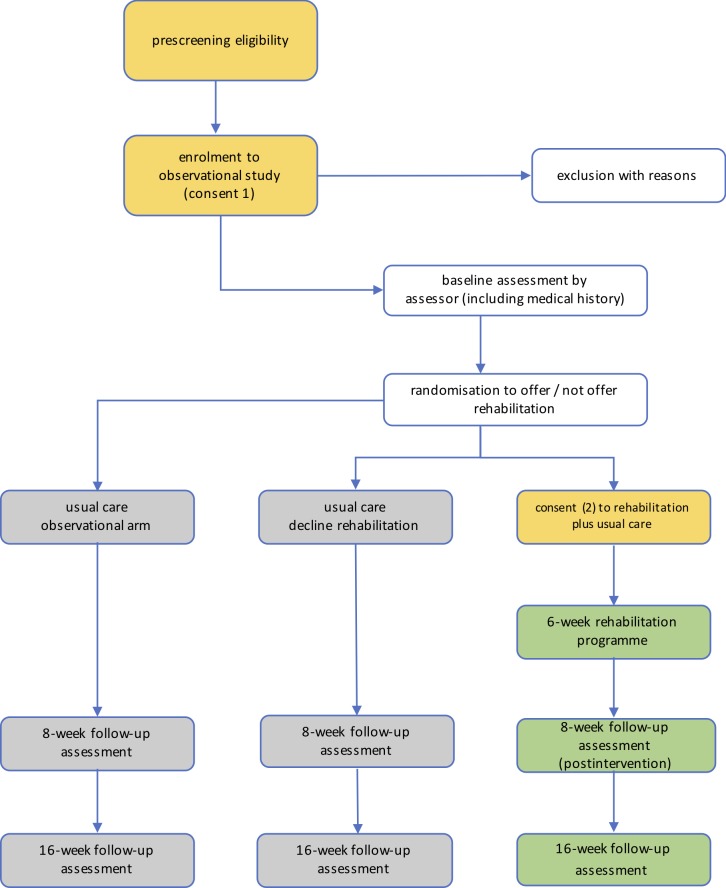
Randomisation is conducted using a postponed-information model (figure 1).34 35 After inclusion in the observational cohort study, an appointment is made for baseline assessment (1) and followed by the extended (additional to the inclusion screening) medical history. Demographics and outcomes will be evaluated by the blinded assessor at the initial assessment. At this time, also follow-up assessments will be scheduled. Subsequently, the participant will be ‘silently’ (ie, unknowingly) randomised to being offered or not being offered the rehabilitation programme (consent 2) using a 3:1 centralised and concealed allocation scheme. The assessor (BLF) will trigger randomisation through Castor EDC online (but will not have access to the outcome), and the coordinating physiotherapist (AR) will contact the patient with the randomisation outcome to ensure blinding of the assessor. This procedure entails three potential outcomes:
The participant is not offered the rehabilitation programme, and as such is unaware of its existence. The participant will remain in the observational arm.
The participant is offered the rehabilitation programme but declines to consent to the rehabilitation programme. The participant will continue the study in the observational arm.
The participant is offered the rehabilitation programme and agrees to participate (consent 2). The participant will provide the second informed consent and is subsequently contacted by the physiotherapist to initiate the rehabilitation programme (based at the BLRC).
Figure 1.
Study flow chart of postponed information model. Blinded follow-up assessments of all outcomes at 8 weeks, 16 weeks postrandomisation. The 6-week rehabilitation programme starts ~2 weeks after randomisation to allow for logistical arrangements.
It is hypothesised that the postponed information model reduces the ethical boundaries to allocate patients to a control condition (as is the case in this specific setting, where structured rehabilitation for NCDs is non-existent), while maintaining the recruitment efficiency and robustness of a conventional RCT.34 35 This model also ensures that a participant makes an informed decision to participate in an intervention, without the risk of being randomised to usual care, and therefore resembles clinical practice more closely. It can be hypothesised that patients who provide the second consent to participate in the rehabilitation programme are subsequently more motivated to engage in the intervention. It can be postulated that by resembling clinical practice more closely, translation from research into clinical practice is more likely. Finally, this model also allows the assessment of patients who decline to participate or discontinue the intervention, providing additional insights into the feasibility of the intervention. The present study will therefore inform, through qualitative (eg, focus group interviews) and quantitative (eg, retention rate, acceptance rate) research techniques, whether or not a postponed information model is a viable randomisation strategy and reduces some of the methodological constrains for conducting an RCT in a low-resource setting. Participants will be informed about the full extent of this model during a patient-information day on completion of the study.
Outcomes and participant characteristics
Due to the feasibility nature of this RCT, no a-priori primary outcome is identified or power-analysis conducted. Outcomes have been selected based on their clinical relevance, pragmatic implementation in a low-resource setting and expected lack of dependency on the health-literacy of the patient. All outcomes will be assessed at baseline, 8 weeks postrandomisation (ie, postintervention) and 16 weeks postrandomisation by an assessor blinded to treatment allocation (see table 2 for the assessment schedule).
Table 2.
Assessment and treatment schedule
| Weeks | 0 | 1–2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11–16 | 17 | 18 | |
| Phase | Inclusion | Baseline | Scheduling | Treatment/usual care | Postintervention | Follow-up | ||||||||
| Inclusion/exclusion criteria | X | |||||||||||||
| Medical history | X | |||||||||||||
| Outcomes | ||||||||||||||
| Physical examination | X | X | X | |||||||||||
| Lifestyle risk factors | X | X | X | |||||||||||
| IPAQ | X | X | X | |||||||||||
| PSQI | X | X | X | |||||||||||
| 6MWT | X (n=2) | X | X | |||||||||||
| TUG | X | X | X | |||||||||||
| SSST | X | X | X | |||||||||||
| HRQOL | X | X | X | |||||||||||
| Economic evaluation | X | X | X | |||||||||||
| Treatment type | Treatment/usual care | |||||||||||||
| Exercise | X | X | X | X | X | X | ||||||||
| Education | X | X | X | |||||||||||
| Adherence | X | X | X | X | X | X | ||||||||
| Treatment fidelity | Continuous evaluation | |||||||||||||
6MWT, six-minute walk test (assessed twice at baseline to correct for a learning effect); HRQOL, health-related quality of life; IPAQ, International Physical Activity Questionnaire; PSQI, Pittsburgh Sleep Quality Index; SSST, six-spot step test; TUG, timed up and go test.
Participants’ characteristics
The following participants’ characteristics will be recorded to describe the study sample: demographics (eg, age), socioeconomic status and lifestyle-related factors (eg, smoking).
Medical history
A qualified physiotherapist (BLF) will take a detailed medical history, which is double checked offline against exclusion criteria by the family physician (MA). A disease severity classification is included in the medical history for cardiovascular disease,36 cancer (https://cancerstaging.org) and diabetes (type 1 and type 2).37 Disease severity for chronic respiratory disease is determined after inclusion, during the physical examination (according to the patient’s forced expiratory volume [FEV1]).
Physical examination and lifestyle inventory
Each participant will undergo a basic physical examination by the assessor who is blinded to treatment allocation during follow-up assessments. The examination includes the measurement and recording of height (m), weight (kg), hip and waist circumference (cm), resting blood pressure (mm Hg), lung spirometry (FEV and Force Vital Capacity) and resting heart rate (beats per minute). Lifestyle risk factors including tobacco consumption (one selected item), alcohol consumption (one selected item), diet (four items), and selected items from the violence module (two items), will be assessed using components of the WHO STEPS instrument.38 Separate questionnaires are included for physical activity and quality of sleep.
Physical activity
The International Physical Activity Questionnaire (IPAQ) is a 27-item self-reported measure of physical activity for use with individual adult patients aged 15–69 years old. Duration (minutes) and frequency (days) of physical activity in the last 7 days is measured in domains of job-related, transportation, housework, house maintenance, caring for family, recreation, sport and leisure-time, and time spent sitting. The IPAQ has acceptable psychometric properties relative to other self-report measures.39
Quality of sleep
Quality of sleep is assessed using the Pittsburgh Sleep Quality Index (PSQI). The PSQI differentiates ‘poor’ from ‘good’ sleep quality by measuring seven areas (components): subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications and daytime dysfunction over the last month. The PSQI has shown moderate to excellent psychometric properties in clinical and non-clinical samples.40
Functional capacity
Six-minute walk test
The primary mode of transport for most people living in a low-resource setting (eg, Bishop Lavis) is walking.29 It is evident that having one or more NCDs has a severe impact on mobility, and therefore daily life and participation. The six-minute walk test (6MWT) has been shown to be a valid, reliable and responsive measure across various patient groups.41 The 6MWT is a functional walking test that requires the participant to walk around a measured and demarcated (eg, pylons or coloured tape to mark turning points) 30 m track for 6 min continuously when conducted in accordance with published guidelines.42 Due to resource-constraints (space), a 10 m lap distance will be used instead. The 6MWT will be conducted twice during baseline testing to reduce the learning effect, which has been reported to be as large as 27–35 m in patients with chronic heart failure.43
Timed up and go test
The timed up and go (TUG) test is a measure of function which closely corresponds with balance and fall risk.44 The participant is asked to stand up from an armed chair, walk 3 m and return to the chair as quickly as they feel safe and comfortable. Each participant will get one practice run, and two runs that count. The best run is used as the outcome. The test has shown excellent test–retest reliability (Intra-class Correlation[ICC} Coefficient=0.93), and moderate concurrent validity with the 6MWT (r=−0.81).45
Six-spot step test
The six-spot step test (SSST) is a relatively new quantitative test of ambulation with components of coordination, dynamic balance and lower-limb function.46 47 The SSST is performed in a 5 m rectangular field with five marked circles (diameter 20 cm) which contain a wooden block (4×8 cm2, 140 g).47 From the starting line, the participant is instructed to walk to the other side as quickly as safe and comfortable, kicking the wooden blocks out of the circles in the process. The assessor first provides a demonstration, after which the participant does two runs with the dominant and two with the non-dominant leg. The SSST combines straight walking with bouts of single-leg standing (during kicking), making it unique from other common walking tests (including 6MWT and TUG).
Health-related quality of life (EQ-5D-5L)
The EQ-5D-5L is a 5-item, self-report questionnaire to assess self-care, mobility, pain/discomfort, anxiety/depression and usual activities on a 1–5 scale of perceived problems in these domains. In addition, general health is scored using a visual-analogue-scale. Combined, these six items form a profile of health-related quality of life. The EQ-5D-5L is essential in terms of the economic evaluation. The EQ-5D-5L is widely used and validated in a surplus of chronic medical conditions, most recently in a large cohort of the elderly.48
Cost–benefits
While the provider-perspective economic evaluation is generally feasible in a low-resource setting (eg, clinical record review), most studies conducted in a low-resource setting refrain from economic evaluations from a patient-perspective. In the present study, we aim to test the feasibility of a patient-perspective economic evaluation in addition to key provider statistics (personnel, equipment, inpatient visits, outpatient visits and drug use). To that extent, the following outcomes will be included: direct costs related to transportation (patient or caregiver), direct medical costs (ie, over the counter drugs and supplements), strategies to pay for out-of-pocket expenses (ie, medical poverty trap)49 and patient-reported productivity costs based on the Productivity Cost Questionnaire.50 51
Treatment adherence and fidelity
Each patient will keep a paper and pen-based exercise diary/file in which the moderate-to-vigorous physical activity and resistance exercises are logged, if applicable photos are added to illustrate proper execution of the exercises and patients keep note of the extent (frequency, duration, intensity and repetitions) to which they have completed their physical activity targets and prescribed resistance exercises (see programme description below). These records will then be reviewed during each supervised session, and where necessary, the patient will be encouraged to improve his/her adherence. Barriers that limit adherence will be recorded.
Treatment fidelity practices are related to study design, training providers and delivery of treatment.52 53 To optimise fidelity of treatment provision, all treatment providers will receive a 1-day training, which will cover the study protocol, and considerations for exercise-based rehabilitation in cardiovascular disease, cancer, chronic respiratory disease and diabetes. The standardised training will reduce the likelihood of a provider×treatment interaction. Adherence to the prescribed intervention from a patient perspective is recorded during the intervention (frequency, dose and intensity). Participants will receive a ‘graduation diploma’ if they complete and adhere to 90% of the supervised exercise and education sessions. During the conduct of the intervention, providers will sign-off on the delivered components of the intervention following each session. An independent physiotherapist will review 10% of the therapy sessions, convene with the therapy provider to ensure protocol adherence and address potential provider differences due to level of education, skill level or background.
The patient-centred rehabilitation programme
There is no consensus as to the minimum duration of an exercise-based rehabilitation programme to lead to clinically relevant improvements. However, rehabilitation programmes as short as 3–5 weeks have shown clinical relevant improvements walking capacity (mean difference=30.9 m, 95% CI 9.4 to 52.4, p=0.005).54 55 Rather than the duration per se, the effectiveness of exercise-based rehabilitation should mostly be attributed to the extent the exercises are specific for the desired goal, and to the extent in which the exercises and dose are individualised to the patients’ functional capacity at baseline and progresses over time. In the present study, we aim to develop a treatment paradigm that, on the one hand, potentially results in clinically relevant and sustained improvements in body function, activity and participation, while, on the other hand, keeping the cost–benefits optimal. To that end, the rehabilitation programme for the present feasibility study has been limited to 6 weeks, designed with respect to the anticipated difficulties related to the low-resource setting,56 while still addressing the core components of rehabilitation in terms of risk factor analysis, exercise and patient-education.57
Exercise component
The rehabilitation programme will start ~2 weeks after randomisation to allow for appropriate scheduling. The supervised exercise prescription will be tailored to each patient’s initial functional capacity, profile of comorbidities, use of medication and active disease status and consist of an aerobic and resistance component. The exercise component of the intervention will consist of one 60 min supervised group session (max five per group) per week, and two 30 min home-based sessions, and will progress in terms of intensity throughout the 6 weeks according to the patient’s (increasing) ability. Each group session will entail a 10 min group-based warm-up, 20 min aerobic-type training with a specific educational component (see below) and 30 min of resistance training. Even though the supervised sessions are group-based, each patient will follow his or her own individualised, patient-centred, exercise programme. Group sessions will be offered once daily. Participants need to sign-up at which timeslot they wish to attend the following week. It is hypothesised that by giving the participant this flexibility, and given anticipated barriers related to the low-resource setting, adherence to the supervised sessions will be higher.
The primary exercise component is to enable the patient to be health-enhancing, moderately-to-vigorously active, five times a week for 30 min or a combined minimum of 150 min/week in a home environment, at completion of the 6-week intervention, in accordance with the American College of Sports Medicine (ACSM) guidelines for physical activity.33 To ascertain this goal, each supervised 60 min practical exercise sessions has a specific theme (see box 1).
Box 1. Six different themes addressed during the supervised exercise sessions.
Exercise and safety; recognising body responses to exercise and safety warnings.
Home-based exercise options.
What entails moderate intensity exercise (individualised moderate intensity reference).
What entails vigorous intensity exercise (individualised upper intensity reference).
Alternative community exercise modalities.
Long-term goal setting–continuing a physically active lifestyle.
It is hypothesised that by introducing an educational theme to the supervised aerobic exercise component, this relatively short rehabilitation programme is more likely to result in sustainable benefits.
The secondary exercise component will entail the participants engaging in two-to-three progressive resistance type exercises, involving large muscle groups for improving specific muscle and/or gait function. This is in line with recent suggestions for a stronger focus on resistance training (compared with higher intensity aerobic exercise-based rehabilitation) might be a more viable paradigm to improve health outcomes.58 Resistance training exercises can be general exercises to improve stability, balance or muscle strength or can be more specified to the health condition, for instance in patients with hemiplegia or respiratory muscle weakness. Progression and intensity of exercise are based on the aim (eg, muscle strength, endurance and power) in accordance with the ACSM guidelines for resistance training (table 3). Each participant will be requested to keep a paper-based exercise diary during the intervention phase. All prescribed exercises, both aerobic as well as resistance type exercises, should be viable with no or minimal equipment.59 Leaflets will be handed out to the participants with preferred, key exercises to promote proper conduct of the exercise in a home-environment.
Table 3.
Different types of resistance training schemes according to the ACSM (https://www.acsm.org/docs/brochures/resistance-training.pdf)
| Muscle strength | Muscle power | Muscle endurance |
|
|
|
1RM, one-repetition maximum; ACSM, American College of Sports Medicine.
Educational component
Each patient will be requested to enrol in each of the three educational sessions through the course of the 6-week programme to facilitate informed healthy choices.57 Topic one will be presented during week 1 of the intervention, topic two during week 3 and topic three during week 6. Each topic will be presented daily throughout that week and patients can sign-up according to their availability. It is hypothesised that providing this flexibility, adherence will be higher. Each session consisted of a 15–30 min standardised educational part, and a 15–30 min group discussion to enable vicarious learning (ie, learning by the experiences of peers)60 and address perceived facilitators and barriers with respect to the subject at hand.
NCDs of lifestyle.
Heart-health behaviour (eg, tobacco-use and nutrition).
Health benefits of physical activity.
Usual care
Usual care at the Bishop Lavis Day Clinic is directed mainly towards ongoing medical management of community members with chronic disease. Referral to the (in-house) rehabilitation centre is limited, and not standardised. An optional education session for patients with NCD is hosted monthly, with shifting themes.
Sample size
There are no precise estimates on the prevalence of NCD in Bishop Lavis per se. However, results from the Global Health Action indicate a prevalence of ~52% NCDs in South Africa.61 Approximately 22% of these patients reported the presence of ≥2 chronic conditions. Among others, cultural background and living in an urban area are considered risk factors for a higher prevalence of NCD. If we translate these numbers to the Bishop Lavis community (54 006 inhabitants), one may estimate that the population of people with NCD is roughly 28 083. It is hypothesised that using the 3:1 allocation (offer vs non-offer) ratio, this will approximately result into a 1:1 group allocation; in other words, for every three patients who will be offered the rehabilitation programme, two will consent and one will decline. The study will be conducted over the course of 1 year, with the final group starting in week 40. As such, recruitment, reasons for non-participation or adherence can be evaluated within the context of an entire year (eg, seasonal changes). The theoretical maximum capacity of the programme is 25 patients per week, five complete treatment cycles of 8 weeks within 40 weeks, leading to 125 patients in the experimental group.
Data analysis
The feasibility of the postponed-information model and recruitment strategy in a low-resource setting will be evaluated quantitatively based on the eligible patients, participant and retention rate, group-allocation ratio, drop-out rate and treatment adherence.
Treatment fidelity is assessed by reviewing 10% of the provided treatment sessions against the study protocol by an independent rehabilitation specialist.
Feasibility of the different treatment components is assessed by reviewing the training dairies and adherence rates for both the supervised exercise sessions, as well as education sessions.
Feasibility of the various endpoints is assessed by performing a preliminary longitudinal data-analysis (ie, random-coefficient analysis or generalised estimating equations) to determine the time-by-group interaction for each outcome measure and based on an intention-to-treat principle. It has been shown that both these longitudinal data techniques are robust to missing data in the analysis of continuous outcomes.62 63 If appropriate, analyses will be adjusted for patient characteristics that differ between the two groups. Independent variables (covariates) can be added to the model to assess and estimate their impact on the dependent variable. Among others, this may include the overall treatment adherence to estimate the extent in which protocol deviations may bias the results. The longitudinal analysis will be performed blinded to treatment allocation.
Acceptance of the programme is evaluated using group-based focus interviews with both participants of the intervention and participants that declined the intervention.
Ethics and dissemination
All of the participants will be recruited through voluntary participation, and written informed consent forms from all trial participants will be obtained by researchers in accordance with the Declaration of Helsinki.64 Each participant will receive a unique identifier to ensure confidentiality before, during and after the trial.
Safety
Patients will be asked to report any adverse events (AEs) during the home-based training at each supervised session. All AEs that occur during testing or rehabilitation treatment will be recorded and reviewed by the medical practitioner to determine seriousness and relation to the provided treatment. Patients will be asked to report any AEs during the home-based training at each supervised session. Muscle soreness and increased levels of a fatigue are only reported as AEs if lasting >48 hours. Serious AEs will be expedited to the medical ethics committee as per good clinical practice. This study is covered by Stellenbosch University’s no-fault study insurance, a medical doctor is on the study team, and both testing and supervised treatment are conducted in a hospital environment, ensuring prompt and adequate treatment of any issues or injuries arising during the conduct of the study.
Reimbursement of participants
Each participant will receive a monetary token for participating in this study to the value of R100 per completed assessment visit (R300 in total per participant). There are a number of arguments to justify the amount per visit. First, given the low-resource environment (average income of ZAR1600/month) of the Bishop Lavis community, a higher reimbursement will substantially increase the likelihood of undue influence in signing informed consent. Second, all visits (assessment and treatment) will take place within the Bishop Lavis community, substantially reducing the time, inconvenience and travel requirements. Third, the inconvenience of the assessment battery is reduced to a minimum and does not entail invasive procedures. No reimbursement will be provided for the supervised treatment visits (n=6[exercise]+3[education]). First, this will increase the undue influence for patients to sign consent based on the monetary revenue it would entail. But more importantly, this will significantly limit the ecological validity, sustainability and implementation of the rehabilitation model studied into clinical practice, if shown feasible.
Supplementary Material
Acknowledgments
The authors would like to thank Mrs Marlie Enright (PT), and Mrs Maatje Kloppers (OT) for their instrumental advice during the drafting of this protocol.
Footnotes
Contributors: MH, WD and SH were involved in the conception and design of the study. AR and BLF will be coordinating the intervention and assessments, respectively, and are anticipated to obtain an MSc in Physiotherapy on the basis of this study. MA is the family physician at the community clinic, and will oversee the recruitment and safety of participants. MH obtained ethical approval from the Stellenbosch University Health and Research Council. MH secured funding for this study. All authors edited and revised the manuscript. All authors approved the final version of the manuscript.
Funding: This work is supported by the AXA Research fund, grant number S005459. The funder has no role in the study design; collection, management, analysis and interpretation of data; writing of the report and the decision to submit the report for publication.
Competing interests: None declared.
Ethics approval: The study protocol has been reviewed by the Stellenbosch University Health Research and Ethics Committee and has been approved on 16 May 2018 (reference number: M17/09/031).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. World Health Organization. Global status report on noncommunicable diseases, 2014. http://www.who.int/nmh/publications/ncd-status-report-2014/en/ (accessed 25 Jun 2018). [DOI] [PubMed]
- 2. Mayosi BM, Flisher AJ, Lalloo UG, et al. The burden of non-communicable diseases in South Africa. Lancet 2009;374:934–47. 10.1016/S0140-6736(09)61087-4 [DOI] [PubMed] [Google Scholar]
- 3. Pillay-van Wyk V, Msemburi W, Laubscher R, et al. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. Lancet Glob Health 2016;4:e642–53. 10.1016/S2214-109X(16)30113-9 [DOI] [PubMed] [Google Scholar]
- 4. Abegunde DO, Mathers CD, Adam T, et al. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007;370:1929–38. 10.1016/S0140-6736(07)61696-1 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Rehabilitation after cardiovascular diseases, with special emphasis on developing countries. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1993;831:1-122. [PubMed] [Google Scholar]
- 6. Balady GJ, Williams MA, Ades PA, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2007;115:2675–82. 10.1161/CIRCULATIONAHA.106.180945 [DOI] [PubMed] [Google Scholar]
- 7. Buckley JP, Furze G, Doherty P, et al. BACPR scientific statement: British standards and core components for cardiovascular disease prevention and rehabilitation. Heart 2013;99:1069–71. 10.1136/heartjnl-2012-303460 [DOI] [PubMed] [Google Scholar]
- 8. Grace SL, Turk-Adawi KI, Contractor A, et al. Cardiac Rehabilitation Delivery Model for Low-Resource Settings: An International Council of Cardiovascular Prevention and Rehabilitation Consensus Statement. Prog Cardiovasc Dis 2016;59:303–22. 10.1016/j.pcad.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 9. Piepoli MF, Corrà U, Adamopoulos S, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol 2014;21:664–81. 10.1177/2047487312449597 [DOI] [PubMed] [Google Scholar]
- 10. Woodruffe S, Neubeck L, Clark RA, et al. Australian Cardiovascular Health and Rehabilitation Association (ACRA) core components of cardiovascular disease secondary prevention and cardiac rehabilitation 2014. Heart Lung Circ 2015;24:430–41. 10.1016/j.hlc.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 11. Kachur S, Chongthammakun V, Lavie CJ, et al. Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Prog Cardiovasc Dis 2017;60:103–14. 10.1016/j.pcad.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization. Rehabilitation 2030: A Call for Action: WHO; http://www.who.int/rehabilitation/rehab-2030/en/ (accessed 22 Mar 2018). [DOI] [PubMed] [Google Scholar]
- 13. Pesah E, Supervia M, Turk-Adawi K, et al. A Review of Cardiac Rehabilitation Delivery Around the World. Prog Cardiovasc Dis 2017;60:267–80. 10.1016/j.pcad.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 14. Babu AS, Lopez-Jimenez F, Thomas RJ, et al. Advocacy for outpatient cardiac rehabilitation globally. BMC Health Serv Res 2016;16:471 10.1186/s12913-016-1658-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson L, Thompson DR, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016:CD001800 10.1002/14651858.CD001800.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Long L, Anderson L, Dewhirst AM, et al. Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev 2018;2:CD012786 10.1002/14651858.CD012786.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease : The Cochrane Library: John Wiley & Sons, Ltd, 2016;59 10.1002/14651858.CD005305.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris NR, Kermeen FD, Holland AE. Exercise‐based rehabilitation programmes for pulmonary hypertension : The Cochrane Library: John Wiley & Sons, Ltd, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavie CJ, Kachur S, Milani RV. Making cardiac rehabilitation more available and affordable. Heart 2019;105:94–95. 10.1136/heartjnl-2018-313762 [DOI] [PubMed] [Google Scholar]
- 20. United Nations - Sustainable development goals. United Nations Sustainable Development. https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed 25 Jun 2018).
- 21. Nelson ML, Grudniewicz A, Albadry S. Applying Clinical Practice Guidelines to the Complex Patient: Insights for Practice and Policy from Stroke Rehabilitation. Healthc Q 2016;19:38–43. 10.12927/hcq.2016.24697 [DOI] [PubMed] [Google Scholar]
- 22. Arokiasamy P, Uttamacharya U, Jain K, et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal? BMC Med 2015;13:178 10.1186/s12916-015-0402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glynn LG, Buckley B, Reddan D, et al. Multimorbidity and risk among patients with established cardiovascular disease: a cohort study. Br J Gen Pract 2008;58:488–94. 10.3399/bjgp08X319459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fortin M, Contant E, Savard C, et al. Canadian guidelines for clinical practice: an analysis of their quality and relevance to the care of adults with comorbidity. BMC Fam Pract 2011;12:74 10.1186/1471-2296-12-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005;294:716–24. 10.1001/jama.294.6.716 [DOI] [PubMed] [Google Scholar]
- 26. Jadad AR, To MJ, Emara M, et al. Consideration of multiple chronic diseases in randomized controlled trials. JAMA 2011;306:2670–2. 10.1001/jama.2011.1886 [DOI] [PubMed] [Google Scholar]
- 27. Derman W, Schwellnus M, Hope F, et al. Description and implementation of U-Turn Medical, a comprehensive lifestyle intervention programme for chronic disease in the sport and exercise medicine setting: pre-post observations in 210 consecutive patients. Br J Sports Med 2014;48:1316–21. 10.1136/bjsports-2014-093814 [DOI] [PubMed] [Google Scholar]
- 28. Eldridge SM, Lancaster GA, Campbell MJ, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One 2016;11:e0150205 10.1371/journal.pone.0150205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De la Cornillere W-L. Participants’ experience of the Bishop Lavis Rehabilitation Centre stroke group, 2007. [Google Scholar]
- 30. PPP conversion factor, GDP (LCU per international $). Data. https://data.worldbank.org/indicator/PA.NUS.PPP (accessed 25 Jun 2018).
- 31. Crime Stats SA - Crime Stats Simplified. http://crimestatssa.co.za/ (accessed 25 Jun 2018).
- 32. World Health Organization. WHO methods and data sources for global burden of disease estimates 2000-2011. Geneva: Department of Health Statistics and Information Systems, 2013. [Google Scholar]
- 33. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 34. Gallo C, Perrone F, De Placido S, et al. Informed versus randomised consent to clinical trials. Lancet 1995;346:1060–4. 10.1016/S0140-6736(95)91741-1 [DOI] [PubMed] [Google Scholar]
- 35. Young-Afat DA, Verkooijen HA, van Gils CH, et al. Brief Report: Staged-informed Consent in the Cohort Multiple Randomized Controlled Trial Design. Epidemiology 2016;27:389–92. 10.1097/EDE.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 36. New York Heart Association. Diseases of the heart and blood vessels: nomenclature and criteria for diagnosis: Little, Brown, 1964. [Google Scholar]
- 37. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62–S69. 10.2337/dc10-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization. The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS). Geneva: World Health Organization, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 40. Mollayeva T, Thurairajah P, Burton K, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev 2016;25:52–73. 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 41. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 2017;23:377–81. 10.1111/jep.12629 [DOI] [PubMed] [Google Scholar]
- 42. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 43. Uszko-Lencer N, Mesquita R, Janssen E, et al. Reliability, construct validity and determinants of 6-minute walk test performance in patients with chronic heart failure. Int J Cardiol 2017;240:285–90. 10.1016/j.ijcard.2017.02.109 [DOI] [PubMed] [Google Scholar]
- 44. Hafsteinsdóttir TB, Rensink M, Schuurmans M. Clinimetric properties of the Timed Up and Go Test for patients with stroke: a systematic review. Top Stroke Rehabil 2014;21:197–210. 10.1310/tsr2103-197 [DOI] [PubMed] [Google Scholar]
- 45. Hwang R, Morris NR, Mandrusiak A, et al. Timed up and go test: a reliable and valid test in patients with chronic heart failure. J Card Fail 2016;22:646–50. 10.1016/j.cardfail.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 46. Kreutzfeldt M, Jensen HB, Ravnborg M, et al. The six-spot-step test - a new method for monitoring walking ability in patients with chronic inflammatory polyneuropathy. J Peripher Nerv Syst 2017;22:131–8. 10.1111/jns.12210 [DOI] [PubMed] [Google Scholar]
- 47. Nieuwenhuis MM, Van Tongeren H, Sørensen PS, et al. The six spot step test: a new measurement for walking ability in multiple sclerosis. Mult Scler 2006;12:495–500. 10.1191/1352458506ms1293oa [DOI] [PubMed] [Google Scholar]
- 48. Lutomski JE, Krabbe PF, Bleijenberg N, et al. Measurement properties of the EQ-5D across four major geriatric conditions: Findings from TOPICS-MDS. Health Qual Life Outcomes 2017;15:45 10.1186/s12955-017-0616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McIntyre D, Thiede M, Dahlgren G, et al. What are the economic consequences for households of illness and of paying for health care in low- and middle-income country contexts? Soc Sci Med 2006;62:858–65. 10.1016/j.socscimed.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 50. Bouwmans C, Krol M, Severens H, et al. The iMTA Productivity Cost Questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health 2015;18:753–8. 10.1016/j.jval.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 51. Bouwmans C, Krol M, Brouwer W, et al. IMTA Productivity Cost Questionnaire (IPCQ). Value Health 2014;17:A550 10.1016/j.jval.2014.08.1791 [DOI] [PubMed] [Google Scholar]
- 52. Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443–51. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- 53. Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:S52–S63. 10.1111/j.1752-7325.2011.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bellet RN, Adams L, Morris NR. The 6-minute walk test in outpatient cardiac rehabilitation: validity, reliability and responsiveness—a systematic review. Physiotherapy 2012;98:277–86. 10.1016/j.physio.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 55. Sherrington C, Pamphlett PI, Jacka JA, et al. Group exercise can improve participants’ mobility in an outpatient rehabilitation setting: a randomized controlled trial. Clin Rehabil 2008;22:493–502. 10.1177/0269215508087994 [DOI] [PubMed] [Google Scholar]
- 56. Baumann LC. Insights on conducting research in low-resource settings: examples from Vietnam and Uganda. Transl Behav Med 2011;1:299–302. 10.1007/s13142-011-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grace SL, Turk-Adawi KI, Contractor A, et al. Cardiac rehabilitation delivery model for low-resource settings. Heart 2016;102:1449–55. 10.1136/heartjnl-2015-309209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Steele J, Fisher J, Skivington M, et al. A higher effort-based paradigm in physical activity and exercise for public health: making the case for a greater emphasis on resistance training. BMC Public Health 2017;17:300 10.1186/s12889-017-4209-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alison JA, McKeough ZJ. Pulmonary rehabilitation for COPD: are programs with minimal exercise equipment effective? J Thorac Dis 2014;6:1606–14. 10.3978/j.issn.2072-1439.2014.07.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roberts D. Vicarious learning: a review of the literature. Nurse Educ Pract 2010;10:13–16. 10.1016/j.nepr.2009.01.017 [DOI] [PubMed] [Google Scholar]
- 61. Phaswana-Mafuya N, Peltzer K, Chirinda W, et al. Self-reported prevalence of chronic non-communicable diseases and associated factors among older adults in South Africa. Glob Health Action 2013;6:20936 10.3402/gha.v6i0.20936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Twisk JW. Longitudinal data analysis. a comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol 2004;19:769–76. 10.1023/B:EJEP.0000036572.00663.f2 [DOI] [PubMed] [Google Scholar]
- 63. Twisk J, de Boer M, de Vente W, et al. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol 2013;66:1022–8. 10.1016/j.jclinepi.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 64. General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 2014;81:14–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.