Abstract
Determinants of lifetime health are complex and emphasize the need for robust predictors of disease risk. Allostatic load (AL) has become a clinical framework to estimate the cumulative biological burden associated with chronic stress. To assist knowledge translation in the developmental origins of health and disease field, clinically valid methods for reliable AL assessment in experimental models are urgently needed. Here, we introduce the rat cumulative allostatic load measure (rCALM), as a new preclinical knowledge translation tool to assess the burden of chronic stress. First, we identified an array of stress-associated physiological markers that are particularly sensitive to hypothalamic–pituitary–adrenal axis dysregulation by ancestral prenatal stress. Second, we determined which of these markers are susceptible to an intervention by environmental enrichment (EE) to mitigate AL. The markers most responsive to stress and EE therapy were assembled to become operationalized in the rCALM. Third, the new rCALM was validated for the ability to indicate future disease risks. The results show that the rCALM estimates the burden of chronic stress and serves as a proxy to estimate stress resilience and vulnerability to disease. Using the rCALM we showed that enrichment therapy can offset the adverse health outcomes linked to a high AL. Thus, the rCALM provides a model for the development of new test strategies that facilitate knowledge translation in preclinical animal models.
Keywords: allostatic load, allostasis, biomarker, generational stress, transgenerational inheritance, environmental enrichment, corticosterone, precision medicine, animal model, developmental origins of health and disease (DOHaD) scale, disease prediction
Introduction
The majority of complex diseases are determined or at least influenced by environmental and lifestyle factors. In particular, the burden of stress is felt across all major physiological systems and influences long-term health trajectories. Allostasis is defined as the ability to achieve stability through change [1]. Stress triggers the multiple and often opposing physiological systems including the immune system, metabolic system and the neuroendocrine system in the attempt to maintain homeodynamic stability [2]. If stress becomes chronic or recurrent, maintaining allostasis becomes more difficult. A concept that has been proven helpful in identifying the long-term burden on health by cumulative stress is allostatic load (AL). AL refers to the cost of chronic exposure to fluctuating or heightened neural or neuroendocrine response resulting from chronic or repeated environmental challenge [2]. The biological concept of AL incorporates elements of stress pathophysiology in one comprehensive model [3] and has become a central paradigm to predict or diagnose complex human disease within the developmental origins of health and disease framework.
The classic AL model unifies multi-systemic interactions by combining primary and secondary mediators of stress into a single index [4]. Primary mediators include stress hormones activated by the sympathetic–adrenal–medullary axis (with epinephrine, norepinephrine), the hypothalamic–pituitary–adrenal [HPA axis, with corticotropic releasing hormone, adrenocorticotropic hormone, cortisol or corticosterone (CORT)] and primary immune modulators or cytokines [e. g. interleukin (IL)-6] directly influenced by stress [5]. Secondary mediators that are a result of chronic or long-term stress responses include metabolic changes (e.g. glucose, cholesterol, fat deposition), cardiovascular alterations (e.g. blood pressure) and immune regulators (e.g. IL-1β, IL-2) [5]. The AL model has predictive capacity to detect individuals at risk of tertiary outcomes [5]. Tertiary outcomes or comorbidities from elevated primary and secondary mediators include a decline in health and cognition, accelerated ageing, metabolic diseases (i.e. diabetes), cardiovascular and immune systems diseases and also death [6, 7].
In 1997, Seeman et al. [8] proposed operationalizing AL through the use of an allostatic load index (AI). AI is measured as the sum of dysregulated physiological biomarkers [8] that in turn reflect the multi-systemic physiological toll imposed on the body for maintaining allostasis. To predict cognitive decline associated with ageing in the MacArthur Studies of Successful Aging [7–9], AI included measures of blood pressure, waist–hip ratio, serum high density lipoprotein, total cholesterol, glycosylated hemoglobin serum, dehydroepiandrosterone sulphate, overnight urinary cortisol and overnight urinary noradrenalin and adrenalin as indices of cardiovascular activity, metabolism, HPA axis activity and sympathetic nervous system activity [10]. The AI was shown to reproducibly predict adverse health outcomes such as diabetes, physical and cognitive decline and elevated mortality risk [7, 9–14]. The AI has been demonstrated to effectively predict future health risks and disease vulnerability as opposed to any biomarker individually [7, 15].
In contrast to extensive clinical studies, no measure of AL has been developed for animal models. An AI would facilitate the comprehensive interpretation of HPA axis activation, predict stress vulnerability or resilience and risk of disease or functional recovery following an insult. Thus, development of an AI is critical to enhance the translational value of preclinical animal models of disease. The purpose of this proof-of-principle study was to create an AI for laboratory rats using common biomarkers. The effectiveness of the new AI, which here has been termed the “rat cumulative allostatic load measure” (rCALM), was tested in a rat model of transgenerational stress and validated by applying it to an intervention based on environmental enrichment.
Results
Stress and Enrichment Modify Core Markers of AL
The means and standard deviations of individual biomarkers are summarized in Table 1. Corticosterone levels showed a main effect of Enrichment as enriched housing significantly reduced basal circulating CORT levels across all groups [F(1, 42) = 16.16, P < 0.001]. The elevated plus maze (EPM) revealed more risk assessment behaviour in stressed animals (P < 0.05) compared to their non-stressed counterparts. Further pairwise comparisons also revealed that enrichment in the transgenerational prenatal stress (TPS) and multigenerational prenatal stress (MPS) groups showed significantly reduced risk assessment behaviours compared to the standard housing rats (P < 0.05). Blood glucose levels showed an effect of Enrichment in the control group (P < 0.05), and an effect of TPS (P < 0.001) and MPS (P < 0.0001) compared to controls. Morris water task (MWT) revealed a significant increase in swim speed in TPS-EE (P < 0.05) and MPS-EE (P < 0.05) compared to their respective control groups (TPS, MPS) and an increase in swim speed in TPS and MPS compared non-stress controls (P < 0.05). Furthermore, MPS elevated lactate and creatine levels compared to controls (P < 0.05).
Table 1:
summary of descriptive statistics
| Biomarker | C |
C-EE |
TPS |
TPS-EE |
MPS |
MPS-EE |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | Mean | ± | Mean | ± | Mean | ± | Mean | ± | Mean | ± | |
| OF | 552.3 | 29.25 | 560.52 | 15.00 | 551.32 | 45.82 | 525.73 | 54.99 | 557.00 | 27.03 | 523.17 | 42.35 |
| EPM | 93.87 | 31.12 | 106.50 | 24.51 | 101.62 | 28.47 | 79.75# | 33.01 | 89.87 | 19.43 | 86.00# | 35.20 |
| MWT | 0.213 | 0.018 | 0.202 | 0.033 | 0.231* | 0.023 | 0.194# | 0.016 | 0.245* | 0.020 | 0.225# | 0.027 |
| CORT | 387.68 | 106.99 | 61.43# | 56.96 | 501.05 | 287.47 | 58.68# | 68.16 | 514.22 | 295.83 | 111.38# | 174.65 |
| Blood glucose | 6.33 | 0.52 | 6.00# | 0.78 | 5.34* | 0.32 | 5.48 | 0.59 | 6.58* | 0.34 | 6.35 | 0.65 |
| Body weight | 574.75 | 56.12 | 556.87 | 59.80 | 591.12 | 27.32 | 586.75 | 50.94 | 598.75 | 47.88 | 579.25 | 90.42 |
| IL-1β | 339.51 | 197.25 | 392.19 | 285.55 | 346.60 | 202.40 | 276.33 | 231.24 | 370.45 | 302.67 | 320.85 | 291.25 |
| IL-2 | 106.01 | 104.04 | 82.23 | 73.03 | 128.92 | 157.93 | 81.68 | 46.99 | 114.93 | 85.04 | 64.14 | 39.38 |
| IL-6 | 15.50 | 6.46 | 14.25 | 4.70 | 14.25 | 4.07 | 11.87 | 4.78 | 11.00 | 3.13 | 13.25 | 3.50 |
| Leptin | 3.15E4 | 2.57E3 | 1.82E4 | 1.37E3 | 2.10E4 | 3.28E3 | 1.57E4 | 1.78E3 | 1.17E4 | 4.78E3 | 9.84E3 | 2.50E3 |
| Lactate | 1.61 | 0.21 | 1.49 | 0.16 | 1.89 | 0.36 | 1.83 | 0.28 | 2.08* | 0.11 | 1.97 | 0.68 |
| Creatine | 0.27 | 0.04 | 0.25 | 0.02 | 0.31 | 0.05 | 0.31 | 0.03 | 0.34* | 0.02 | 0.33 | 0.11 |
EPM, elevated plus maze; MWT, Morris water task; CORT, corticosterone; IL, interleukin.
Denotes significant effect of stress (P < 0.05).
Denotes significant effect of EE (P < 0.05).
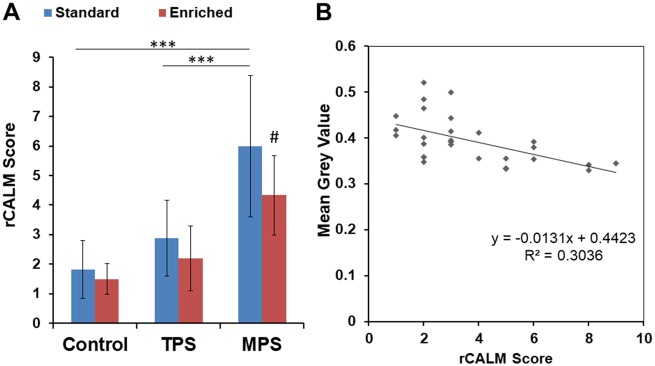
The 10th, 25th, 50th, 75th and 90th percentile distribution of each biomarker and the cut-offs used for dichotomizations are summarized in Table 2. The rCALM index was able to reveal effects of housing conditions on stress responses. Mean rCALM scores were highest in the MPS and TPS animals and were lowest in groups exposed to environmental enrichment. The summary of the average rCALM scores is shown in Fig. 1A. rCALM scores were significantly different between treatment groups (H = 19.866, P = 0.0013). Specifically, rCALM scores were significantly higher in MPS rats compared to control animals (P < 0.0001), and TPS animals (P = 0.0003). Enrichment had a significant effect on the MPS group (P = 0.0023), while decreasing the rCALM score across all groups.
Table 2:
summary of biomarker categories
| Category | Marker category | Biomarker | Percentile |
||||
|---|---|---|---|---|---|---|---|
| 10th | 25th | 50th | 75th | 90th | |||
| Primary | Neuroendocrine | Corticosterone (µg/ml) | 19.00 | 86.11 | 182.89 | 498.75 | 639.45 |
| Primary | Immune | IL-6 (µg/ml) | 8.30 | 9.50 | 11.50 | 15.00 | 20.50 |
| Secondary | Immune | IL-1β (µg/ml) | 171.31 | 222.65 | 363.13 | 585.84 | 779.32 |
| Secondary | Immune | IL-2 (µg/ml) | 21.19 | 40.59 | 81.24 | 120.54 | 156.89 |
| Secondary | Behavioural | OF (margin time) | 497.90 | 539.00 | 552.20 | 574.55 | 589.86 |
| Secondary | Behavioural | EPM (risk assessment) | 65.00 | 79.00 | 91.00 | 120.50 | 126.40 |
| Secondary | Behavioural | MWT (swim speed) | 0.18 | 0.20 | 0.22 | 0.24 | 0.26 |
| Secondary | Metabolic | Blood glucose (mmol/l) | 5.80 | 6.20 | 6.90 | 8.35 | 9.54 |
| Secondary | Metabolic | Body weight (g) | 523.70 | 558.00 | 597.00 | 624.00 | 653.60 |
| Secondary | Metabolic | Leptin (µg/ml) | 5387.03 | 8008.26 | 10 382.54 | 14 871.96 | 26 872.85 |
| Secondary | Metabolic | Lactate (mmol) | 1.34 | 1.58 | 1.87 | 2.15 | 2.35 |
| Secondary | Metabolic | Creatine (mmol) | 0.23 | 0.27 | 0.32 | 0.35 | 0.38 |
Values indicate the 10th, 25th, 50th, 75th and 90th percentile along with the high-risk cut-off level (highlighted in pink).
OF, open field; EPM, elevated plus maze; MWT, Morris water task; CORT, corticosterone; IL, interleukin.
Figure 1:
validity of the rCALM score as an indicator of AL. (A) The average rCALM scores across experimental groups in standard and enrichment housing conditions. (B) rCALM score and MGV associations. rCALM was significantly correlated with MGV. Values represent mean ± SD. ***Denotes significant effect of stress (P < 0.001), #denotes significant effect of enriched environment (P < 0.05)
The dichotomized biomarkers showed significant differences in swim speed in the MWT (H = 12.796, P = 0.0254), IL-1β (H = 11.51, P = 0.0422), lactate (H = 17.2, P = 0.0041) and creatine (H = 18.18, P = 0.0027) levels. Notably, all of the high-risk animals showing an rCALM score of 5 or higher revealed elevated IL-1β and creatine values.
Predictive Value of the rCALM Index Versus Individual and Subgroup Biomarkers
Correlations for all individual biomarkers are shown in Table 3. Values that correlated with mean grey value (MGV) include lactate (R = −0.42, P < 0.05), creatine (R=−0.41, P < 0.05) and the rCALM score (R = −0.551, R2 = 0.3036, P < 0.05; Fig. 1B). To determine if rCALM still has predictive value without the influence of lactate and creatine, both were removed from the AI to test the predictive value. rCALM scores without the influence of lactate and creatine were still significantly different between treatment groups (H = 13.110, P = 0.0224). rCALM still had the largest values in TPS and MPS animals, with enriched environment lowering rCALM. Moreover, rCALM without these biomarkers was still correlated with MGV (R = −0.490, R2 = 0.2401, P < 0.05). However, the significance factor was stronger when creatine and lactate were included in the index.
Table 3:
correlation coefficients and significances
| R | P-value | |
|---|---|---|
| OF, EPM | 2.84E-04 | 0.9989 |
| OF, corticosterone | 0.08 | 0.7051 |
| OF, blood glucose | 0.13 | 0.5237 |
| OF, IL-1β | 0.18 | 0.3652 |
| OF, IL-2 | 0.03 | 0.892 |
| OF, leptin | 0.12 | 0.5456 |
| OF, IL-6 | 0.37 | 0.0542 |
| OF, weight | 0.19 | 0.3253 |
| OF, lactate | 0.09 | 0.6412 |
| OF, creatine | 0.12 | 0.5379 |
| OF, MWT | 0.42 | 0.0265* |
| OF, neural density | 0.36 | 0.0613 |
| OF, rCALM | −0.51 | 0.0047* |
| EPM, CORT | −2.57E-03 | 0.9897 |
| EPM, blood glucose | −0.03 | 0.8766 |
| EPM, IL-1β | 0.01 | 0.9527 |
| EPM, IL-2 | 0.01 | 0.9439 |
| EPM, Leptin | 0.09 | 0.6596 |
| EPM, IL-6 | −4.40E-03 | 0.9824 |
| EPM, weight | 0.09 | 0.636 |
| EPM, lactate | −0.15 | 0.4571 |
| EPM, creatine | −0.14 | 0.4887 |
| EPM, MWT | 0.14 | 0.4829 |
| EPM, neural density | 0.23 | 0.2365 |
| EPM, rCALM | −0.33 | 0.0818 |
| Corticosterone, blood glucose | 0.2 | 0.3063 |
| Corticosterone, IL-1β | −0.08 | 0.7017 |
| Corticosterone, IL-2 | 0.2 | 0.3155 |
| Corticosterone, leptin | 0.05 | 0.7871 |
| Corticosterone, IL-6 | 0.16 | 0.4238 |
| Corticosterone weight | 0.14 | 0.4695 |
| Corticosterone, lactate | −0.06 | 0.7809 |
| Corticosterone, creatine | −0.08 | 0.6819 |
| Corticosterone, MWT | −0.08 | 0.6709 |
| Corticosterone, neural density | −0.19 | 0.3403 |
| Corticosterone, rCALM | −0.13 | 0.5063 |
| Blood glucose, IL-1β | 0.11 | 0.5856 |
| Blood glucose, IL-2 | −0.12 | 0.5535 |
| Blood glucose, leptin | 0.22 | 0.2702 |
| Blood glucose, IL-6 | 0.1 | 0.6205 |
| Blood glucose, weight | 0.03 | 0.8831 |
| Blood glucose, lactate | 0.02 | 0.9179 |
| Blood glucose, creatine | 0.02 | 0.9029 |
| Blood glucose, MWT | −0.05 | 0.8042 |
| Blood glucose, neural density | −0.17 | 0.3932 |
| Blood glucose, rCALM | 0.22 | 0.2662 |
| IL-1β, IL-2 | 0.18 | 0.2155 |
| IL-1β, leptin | −0.17 | 0.2582 |
| IL-1β, IL-6 | 0.19 | 0.1901 |
| IL-1β, weight | 0.18 | 0.2186 |
| IL-1β, lactate | 0.27 | 0.0684 |
| IL-1β, creatine | 0.27 | 0.0684 |
| IL-1β, MWT | −0.21 | 0.2911 |
| IL-1β, neural density | −0.32 | 0.0903 |
| IL-1β, rCALM | 0.46 | 0.0143* |
| IL-2, leptin | −0.11 | 0.4725 |
| IL-2, IL-6 | −0.09 | 0.5537 |
| IL-2, weight | 0.08 | 0.5932 |
| IL-2, lactate | 0.23 | 0.1162 |
| IL-2, creatine | 0.23 | 0.1162 |
| IL-2, MWT | 0.2 | 0.1799 |
| IL-2, neural density | −0.29 | 0.0879 |
| IL-2, rCALM | 0.35 | 0.0147* |
| Leptin, IL-6 | 0.47 | 0.0108* |
| Leptin, weight | 0.15 | 0.4644 |
| Leptin, lactate | −0.31 | 0.1115 |
| Leptin, creatine | −0.31 | 0.1041 |
| Leptin, MWT | 0.06 | 0.7675 |
| Leptin, neural density | 0.14 | 0.4844 |
| Leptin, rCALM | −0.12 | 0.5434 |
| IL-6, weight | −0.23 | 0.2358 |
| IL-6, lactate | −0.22 | 0.2701 |
| IL-6, creatine | −0.23 | 0.2424 |
| IL-6, MWT | 0.28 | 0.1515 |
| IL-6, neural density | 0.18 | 0.3533 |
| IL-6, rCALM | −0.12 | 0.5532 |
| Weight, lactate | 0.17 | 0.3914 |
| Weight, creatine | 0.18 | 0.3538 |
| Weight, MWT | 0.01 | 0.9568 |
| Weight, neural density | −0.25 | 0.2025 |
| Weight, rCALM | −0.15 | 0.4611 |
| Lactate, creatine | 0.98 | <0.0001* |
| Lactate, MWT | 0.27 | 0.1728 |
| Lactate, neural density | −0.26 | 0.0249* |
| Lactate, rCALM | −0.02 | 0.9171 |
| Creatine, MWT | 0.27 | 0.1702 |
| Creatine, neural density | −0.25 | 0.0329* |
| Creatine, rCALM | −0.05 | 0.8054 |
| MWT, neural density | −0.09 | 0.6548 |
| MWT, rCALM | −0.34 | 0.0769 |
| Neural density, rCALM | −0.56 | 0.0016* |
Highlighted in pink are markers that are significantly correlated with the rCALM index, in grey are markers that are significantly correlated with neural density and in blue is the correlation between the rCALM and neural density.
Denotes significance (P < 0.05).
Similarly, to determine if a subgroup of biomarkers would be better correlated with MGV, a smaller group of biomarkers was created by pooling those individual biomarkers that were most highly correlated with both rCALM and neural density [open field (OF), corticosterone, IL-1β, IL-2, lactate, creatine]. Results revealed that, although these six biomarkers when pooled were moderately correlated with MGV (R = −0.421, R2 = 0.203, P < 0.05), the rCALM index as whole had a larger r-value. Both of these representations indicate that overall, using an array of biomarkers and a multisystem approach provides the highest predictive value.
Discussion
The goal of this study was to create a proof-of-principle for a comprehensive and cumulative index that measures nonlinear effects of stress [10] and assesses the risk of chronic health impacts generated by stress in rodents. Recent evidence suggests that trans- and multigenerational stress represents a critical risk factor in complex disease etiology [16–18]. The burden of chronic stress induced by ancestral experience, however, had not been systematically quantified. While indices for AL have become a valuable tool for predicting stress-related diseases [5], the lack of prospective human cohort data spanning at least three generations emphasizes the need for modelling ancestral stress in laboratory animals. Here, we developed a novel AI for use in laboratory rodents based on guidelines by Seeman et al. [8]. The new rCALM index was developed using 12 biomarkers commonly measured in relation to stress physiology and behaviour in rat and mouse studies. The findings show that most biomarkers when analysed individually, did not predict high risk for neuronal deficits. In contrast, when all biomarkers were standardized and dichotomized to dictate high risk, rCALM was able to predict neurologic deficits. Moreover, we demonstrate that the rCALM is also an effective indicator of therapeutic benefit of life style interventions that aim to moderate AL.
Validity of this new tool was confirmed by rCALM predicting elevated AL in ancestrally stressed rats, showing that remote ancestral stress raises the risk of low neuronal density. This finding indicates a heightened cumulative burden in ancestrally stressed animals, due to increased AL and greater vulnerability to stress-induced disease [19]. In contrast, treatment with enriched environment, a powerful therapy that improves recovery in animal models of neurological disease [20, 21], reduces the cumulative burden by AL and improves neuronal density in ancestrally stressed rats particularly in the MPS group.
The rCALM index was developed to quantify the effect of cumulative stress based on the notion of primary mediators leading to secondary outcomes which culminate in tertiary outcomes that characterize tangible diseases [22]. In this study, the tertiary outcome measured was neuronal density as measured by MGV in the prefrontal cortex. The prefrontal cortex is particularly vital to higher-order executive functions, sensory perception and social reasoning [23]. Behavioural impairments characterized by alterations in neuronal density in the prefrontal cortex include anxiety, attention deficit hyperactivity disorder [24] and depression [25]. Moreover, studies investigating the relationship between prefrontal cortex function and HPA axis activation had concluded that the prefrontal cortex is a part of the regulatory circuitry involved in the stress response [26]. The present decrease in MGV may therefore contribute to HPA axis dysregulation. Notably, reduced neural density occurred in animals with greater AL.
The MPS group, with the highest AL score, seemed to benefit most from enrichment. This group is subjected to a larger cumulative burden by stress, as direct stress recurs in each generation in addition to their ancestral exposure [27]. The benefit of enrichment may therefore reach multiple physiological systems. Many studies have investigated the effect of enrichment on AL and measurement by the AI in the human population. An “enriched” environment in the human population could be interpreted as those with higher socioeconomic status and higher education, which have both been correlated with a lower AI score [28]. This study, however, more closely relates to social enrichment, which has shown specific protection against AL, thus ultimately decreasing the AI score [9, 29]. Findings of this study demonstrate that enrichment has the capacity to promote resilience against the cumulative effects of AL at both the endocrine and the neurological level.
Advantages of rCALM Over Individual Biomarkers of Ancestral Stress
Biomarkers that exhibited a significant effect of stress when comparing raw mean values include risk assessment behaviour, swim speed, blood glucose, lactate and creatine. Biomarkers that responded to enrichment include risk assessment, swim speed, CORT, blood glucose, lactate and creatine. This indicates that ancestral stress does affect behaviour, specifically hyperactivity and anxiety as measured by MWT and EPM, as well as metabolic functions, as measured by blood glucose, lactate and creatine. Moreover, enrichment can mitigate or reverse some of these adverse outcomes. The majority of these individual biomarkers, however, were not able to predict changes in neuronal density, the tertiary outcome.
Individual biomarkers that were significant at high risk while acting as predictive indicators for low neural density included lactate and creatine. Changes in metabolism, as measured by lactate and creatine, assist in illustrating the large and pertinent effects of cumulative stress on metabolism. Impaired cerebral energy metabolism, which is linked to altered neuronal plasticity, is among the leading hypotheses that explain the pathogenesis and etiology of psychiatric illness, such as major depression and bipolar disorder [30–35].
Other individual biomarkers that did not predict changes in neural density, but significantly contributed to the overall rCALM score included IL-1β, and swim speed in MWT. Notably, animals with ancestral stress had higher risk for elevated IL-1β and all animals with an rCALM score greater than 5 received an IL-1β score of 1. This score may indicate upregulated inflammation and immune responses linked to an insufficient stress response by glucocorticoids, or a response to higher metabolic rates [36]. Understanding interactions between the stress response and the immune system will shed light on the association between HPA axis dysfunction and psychopathologies such as depression and schizophrenia that are associated with altered immune status. Moreover, high risk for immune dysregulation due to stress may also shed light on vulnerability to autoimmune and inflammatory diseases [37].
A recent proposition by Juster et al. [5] and Seeman et al. [7] concerns the masking of the predictive value of individual AL components. By breaking the AI into neuroendocrine and metabolic biomarkers, previous studies found that the individual clusters did not overlap and may therefore individually contribute to health risks [5, 7]. Clustering biomarkers provide biological signatures that are vital in predicting morbidity and mortality [38]. Previous results show mixed support for the inclusion of a comprehensive AI instead of subgroups of fewer biomarkers [5]. This study addressed this issue in two ways. It was determined that rCALM was better correlated with MGV than individual biomarkers as well as a permutation of the rCALM which included a subgroup of six biomarkers. When biomarkers, which correlated highly with MGV (i.e. lactate and creatine), were removed from rCALM, the index was still significantly correlated with MGV. The overall focus of an AI should be on identifying levels of biomarkers that identify high risk which are superior to quantifying single biomarkers, for better prediction and prevention of tertiary outcomes.
Effectiveness of the rCALM Index
Stratification tools, such as the rCALM, are effective discriminating tools to dissociate stress resilience versus stress vulnerability. For example, a composite index of behavioural traits may provide a better characterization of an individual’s vulnerability to prolonged stress and stress-induced depression than a single measure [39]. Accordingly, our data show that higher multivariable rCALM scores are associated with increased risk to lower neuronal density. Interestingly, some measurements of stress such as corticosterone, did not vary much between transgenerational and multigenerational stress experiences, yet the respective overall rCALM scores were higher in the multigenerational group. This observation indicates that ancestral stress, possibly through epigenetic regulation [40], promotes adaptation to stress in some functions, while creating vulnerabilities in others.
The rCALM generates a fingerprint of a multi-level signature of the chronic burden of stress. The physiological mediators of the stress burden are interconnected, reciprocal and nonlinear in their effects [5, 41]. To better assess the cumulative effects of chronic or recurrent stress and to detect potentially sub-clinical symptoms of stress, a panel of biomarkers is more sensitive than a single marker. The value of assessing multiple biomarkers, including primary and secondary mediators, may improve high-risk detection as well as intervention strategies to promote health and well-being in humans [42] and enhance resilience to chronic stresses in wildlife [43]. Multivariable tools, such as the rCALM increase the ability to identify individuals at risk for developing complex diseases that influenced by stress. As a result, targeted prevention strategies and personalized medicine approaches that focus on high-risk individuals may be more effective than population-based strategies [44]. By determining high-risk groups, individuals may be stratified for most effective treatment strategies in precision medicine approaches.
Directions for the Future Use of rCALM
This study presents the first account of the impact of cumulative stress and AL in rats. The rCALM may be regarded as a dynamic tool in which the number and nature of variables included may vary. The individual composite variables will significantly affect the validity and reliability of rCALM to predict stress vulnerability. Present biomarkers were selected based on clinical approaches and previous literature to provide a multi-level systems approach. In general, a larger number of variables will yield more accurate results and better clinical translation. Clinical comparisons with the human AI should involve measurements such as body mass index, lipoproteins and blood pressure. Further additions to rCALM, especially when investigating experiential and environmental origins of disease, would include epigenetic signatures, such as miRNA and DNA methylation marks. Lastly, the collection of longitudinal data, sampled at multiple time points throughout development and/or ageing, along with its application to other experimental models in rats and mice, would validate the predictive power and capabilities of the rCALM index.
Conclusions
The present proof-of-principle study provides a conceptual framework for the development of a clinically relevant, translatable and comprehensive assessment of physiological burden and disease risk in preclinical animal models. For the first time we introduce an index for research in laboratory animals, the rCALM, as a valid method to estimate the physiological burden induced by chronic stress. We show that the rCALM effectively indicates stress resilience and vulnerability in terms of neurological and behavioural function. As chronic stress and intergenerational stress programming and associated diseases create a rapidly growing economic burden to our society [45], refined detection strategies such as the rCALM are of utmost importance for prediction, treatment and prevention. The present findings suggest that the rCALM provides a suitable role model for the development of test strategies that facilitate knowledge translation in preclinical precision medicine approaches.
Methods
Animals
Data used for the (rCALM) index were collected from F3 male offspring rats born to one of the following three maternal lineages: non-stress controls (n = 16), TPS (n = 16) and MPS ( n = 16). TPS rats were the F3 generation of a filial line in which only the F0 dams were stressed during gestation [46]. MPS rats were the F3 generation of a filial line in which dams from each consecutive generation (F0, F1, F2) were gestationally stressed. Maternal stress involved daily exposure of pregnant dams to restraint in a Plexiglas cylinder for 20 min and forced swimming in warm water (22°C) for 5 min from gestational days 12–18 [46, 47]. The animals received the two stress procedures each day in a semi-random order either in the morning or afternoon hours. At weaning, rats derived from the three lineages were assigned to either housing in standard cages, or housing in an enriched environment (EE). Animals assigned to EE lived in social housing, with novel objects added for additional enrichment. Thus, the following groups were tested: non-stress controls in standard (Control; n = 8) and EE (Control-EE; n = 8) housing conditions, TPS in standard (TPS; n = 8) and EE (TPS-EE; n = 8) housing and MPS in standard (MPS; n = 8) and EE (MPS-EE; n = 8) housing. The rats were housed under a 12 h light/dark cycle with lights on at 7:30 AM, room temperature set at 20°C and relative humidity of 30%, with food and water available ad libitum. All procedures were performed in accordance with the guidelines of the Canadian Council on Animal Care and approved by the University of Lethbridge Animal Welfare Committee.
Development of the rCALM
The rCALM AI was developed based on guidelines proposed by the first operationalized study of AI [8]. The index was developed using the 12 biomarkers most commonly measured in relation to stress physiology and behaviour in laboratory rodents. The rCALM was compiled of markers representing the acute (primary mediators: corticosterone, IL-6) and chronic (secondary outcomes: behavioural assessments, blood glucose, IL-1β, IL-2, leptin, weight, lactate and creatine) manifestations of stress involving multiple system levels (e.g. immune system, neuroendocrine, metabolic). The marker combination was chosen to enhance predictive capacity of the composite measure [48]. The following presents the individual biomarkers selected for rCALM.
Neuroendocrine Markers. The glucocorticoid CORT is the primary hormonal mediator of the stress response in rodents. In response to acute and chronic stress, activation of the HPA axis results in the release of CORT from the adrenal gland cortex, which through a negative feedback mechanism via glucocorticoid receptors in the brain can downregulate the stress response. This response has been shown to be programmed by prenatal stress [49, 50]. Moreover, CORT also binds to the mineralocorticoid receptor, which in the brain regulates basal and stress-induced HPA-axis activity [49]. Both receptor types and their interaction are critical for stress vulnerability and resilience [51]. Generally, CORT regulates functions such as behaviour, metabolism, immune response and plays a major role in mental health and complex diseases.
To measure CORT, blood samples (0.6 ml) were collected from the lateral tail vein under 4% isoflurane anaesthesia using a 23 gauge butterfly needle coated in heparin, between 8:00 AM and 9:00 AM on the day of collection. Blood was transferred to centrifuge tubes and plasma was obtained by centrifugation at 5000 rpm for 10 min at 4°C. The samples were stored at −80°C. Plasma CORT concentrations were determined by radioimmunoassay, run in duplicates, using commercial kits (ELISA, Abcam, Inc., ON, Canada).
Markers of Affective State. (i) Open field exploration. The OF task allows the quantification of locomotor activity, exploratory behaviour and anxiety-like behaviours [52, 53]. The OF task was conducted using the VersaMax Legacy Open Field system (Omnitech Electronics, Inc., Dartmouth, NS, Canada), which measured an animal's activity for a period of 10 min using an array of infrared sensors connected to a computer. The time spent in the margins of the OF is considered an indicator of anxiety-like behaviour [54] and was included in the rCALM. (ii) Elevated plus maze. The EPM allows the quantification of motor activity and anxiety-like behaviours [55]. A particularly robust measurement of anxiety-like behaviours in response to stress is risk assessment (i.e. stretch extend postures), which was chosen for the rCALM [56]. (iii) Morris water task. The MWT commonly assesses spatial navigation, learning and memory [57], but can also be used to assess hyperactivity by measuring swim speed [58]. The MWT was conducted over the course of 9 days using a pool filled with room temperature water. The water was made opaque by adding non-toxic white tempura paint and visual cues were placed on the walls for spatial orientation [57]. A computer-assisted tracking system (HVS Image Water 2020™, Middlesex, UK) was used to track rat position and collect data obtained from an overhead video camera. Swim speed was used as a marker for hyperactivity [58]. Behavioural tasks that required manual scoring (EPM—stretch extend postures) were video taped. Video tapes were evaluated by an experimenter blind to the experimental conditions.
Immune Markers. The major pro-inflammatory interleukins IL-1β, IL-2 and IL-6 were included as immune markers for the rCALM index. Cytokines are small glycoproteins that regulate the physiological functions of immunity and inflammatory responses [59]. IL-1β and IL-2 are mediators of immune and neuroendocrine functions during stress at both peripheral and central nervous system (CNS) levels [60]. IL-1β influences the secretion of pituitary hormones which lead to the secretion of glucocorticoids. IL-2 is present in the hypothalamus, the pituitary gland and the locus coeruleus, all of which are involved in the control of neuroendocrine axes [60]. IL-6 is the cytokine most documented in AL studies and a marker for chronic or systemic inflammation. It mediates the interaction between the immune system and CNS inflammation and is differentially affected by both acute and chronic stress responses [61]. Blood was collected between 8:00 AM and 9:00 AM and plasma was used to quantify cytokine biomarkers using a rat cytokine/chemokine array 27-plex Discovery Assay® using the Bio-Plex™ 200 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA; analyses by Eve Technologies Corp, Calgary, AB, Canada), and a Milliplex Rat Cytokine/Chemokine kit (Millipore, St. Charles, MO, USA) according to manufacturer’s protocols.
Metabolic Markers. (i) Body weight, blood glucose and leptin. Changes in body weight are a robust indicator of chronic stress and AL, and excess cortisol is found to be positively correlated with body weight [62]. Animals were weighed every other day between 7:30 AM and 9:30 AM. Body weight for the rCALM index was collected on postnatal day (P) 120. Blood glucose functions as a major source of energy and higher plasma values are positively correlated with elevated cortisol levels and weight gain [63]. Blood glucose was measured between 8:00 AM and 9:00 AM using an Ascensia Breeze Blood Glucose Meter (Bayer, Toronto, ON, Canada). Leptin serves as marker of body weight by being a fat-derived hormone with pivotal roles in the regulation of body weight and food intake [64]. Moreover, leptin also regulates neuronal and glial maturation during brain development [65]. Patients suffering from schizophrenia or major depression have normal body mass indices but reduced leptin levels [62]. Leptin assessment was performed using the cytokine assay described above [Discovery Assay® for the Bio-Plex™ 200 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)].
Lactate and Creatine. Lactate serves as a marker of sympathetic nervous system activation [66, 67], muscle activity and psychosocial stress [68], as well as liver function [69]. Plasma lactate provides an alternative energy substrate to glucose and may act as the preferred energy source for activated neurons within the CNS [70, 71]. Thus, lactate levels may represent an indicator of cerebral activity.
Creatine provides a physiological buffer in tissues with large and shifting energy demands, such as muscle and brain [72]. In the brain, creatine serves as energy shuttle and regulator of energy homeostasis [73, 74]. Deviations in creatine levels may indicate altered metabolic or mitochondrial function and energy demand [34].
Blood lactate and creatine levels were assessed with 1H Nuclear Magnetic Resonance spectroscopy. On P100, 6.0 ml of blood was collected from the lateral tail vein under 4% isoflurane anaesthesia between 8:00 AM and 9:00 AM on the day of collection. Blood was transferred to centrifuge tubes and plasma was obtained by centrifugation at 5000 rpm for 10 min at 4°C. The samples were stored at −80°C. NMR spectra were collected on a 700 MHz Bruker Avance III HD spectrometer (Bruker, UK). The 1D NOESY gradient water suppression pulse sequence noesygpr1d (Bruker, UK) was used. Each sample was run for 512 scans to a total acquisition size of 256 k. The spectra were zero filled to 512 k, automatically phased and baseline corrected, and line-broadened by 0.3 Hz. The processed spectra were then exported to MATLAB for statistical analysis. All peaks were referenced to formate (8.22δ) and a reference metabolite library was used. Concentrations of lactate and creatine were measured using the MestreNova 10.0.1 qNMR plugin, referenced to an internal standard.
Computing the Composite rCALM Index: Standardization and Measurement
To determine disease risk, the sum of individual z-scores for each biomarker was calculated based on the samples distribution. Calculation of z-scores for standardization allows each biomarker weight to differ conditionally on its own deviation from the samples mean. Once the biomarkers were standardized, each individual marker was analysed for high risk. The percentiles used for an indicator of high risk for each biomarker are listed in Table 2.
The AL index underlying rCALM is based on a clinical assessment tool in which biomarkers in the highest or lowest 25% were deemed high risk [48]. Accordingly, for this study, values falling within the high risk percentile (above 75th) were dichotomized as “1” and those within the standard ranges as “0.”
The rCALM index was calculated by summing the number of biomarkers for which the animal fell into the high-risk category, so that the overall sum value was between 0, indicating low risk, and 12, indicating the maximum risk (as the summed total of all component markers is 12). It should be noted that cut-off points could be set to the 10th percentiles (below 10 and above 90) as they could produce an even stronger predictor of health outcome [75]. However, the 75th percentile was chosen for this study as a proof-of-principle to maximize the predictive power for early signs of tertiary health outcomes.
Tertiary Outcome: Neural Density—MGV
Neural density was measured using MGV that acted as the tertiary outcome used to determine the predictive value of rCALM and individual markers. At the age of 180 days rats were euthanized with an overdose of Euthansol® (Merck, QC, Canada) and perfused transcardially with phosphate buffer solution (∼200 ml) followed by a transcardial injection of ∼200 ml of 4% paraformaldehyde (PFA; Sigma-Aldrich, MO, USA). Brains were extracted, stored in brain bottles containing 4% PFA, refrigerated for 24 h and then transferred to sucrose solution for at least 3 days.
Every third series of sections was mounted and stained with cresyl violet to detect Nissl bodies. The slides were captured using a motorized Zeiss AxioImager M1 microscope (Zeiss, Jena, Germany) at 1× magnification. The quantitative cytoarchitectonic analyses in cresyl violet-stained sections corresponding to a region of interest measuring 0.766 mm2 thick sections at Bregma level 3.70 (caudal prefrontal cortex) was performed as described by McCreary et al. [27]. The absolute grey level index was ascertained as the measured parameter [27, 76].
Statistical Analysis
Statistical computations were based on Statview software version 5.0 (SAS Institute, NC, USA). Descriptive statistics are reported where results represent means ± standard deviations. Analysis of variance (ANOVA) was used to compare the mean levels of each biomarker across all groups (C, C-EE, TPS, TPS-EE, MPS, MPS-EE), followed by Fisher’s post-hoc tests or pairwise Student t-tests. The alpha level was set to 0.05 and significant P-values were designated with an asterisk or hashtag in all tables and figures.
As rCALM is an ordinal scoring system and is not normally distributed, the non-parametric Kruskal–Wallis test was used to compare the distributions of rCALM scores across all groups. Pairwise comparisons were performed by collapsing groups and applying separate Mann–Whitney U tests. To investigate whether rCALM levels, along with individual biomarkers, predict neuronal density we computed Simple regressions (R) to determine the relationship between biomarkers, rCALM and MGV.
Ethical standards
The authors assert that all procedures contributing to this work were performed in accordance with the guidelines of the Canadian Council on Animal Care and approved by the University of Lethbridge Animal Welfare Committee.
Acknowledgements
The authors thank Erin A. Falkenberg and Teddi Reynolds for excellent assistance with the experiments.
Funding
This research was supported by the Alberta Innovates-Health Solutions Interdisciplinary Team Grant #200700595 “Preterm Birth and Healthy Outcomes” (G.M., D.M.O.), Canadian Institutes of Health Research Grant #102652 (G.M., D.M.O.), Alberta Innovates CRIO Program (G.M.) and National Sciences and Engineering Research Council of Canada DG #05519 (G.M.). J.M. was supported by NSERC CREATE #371155.
Conflict of interest statement. None declared.
References
- 1. Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Handbook of of Life Stress, Cognition and Health. New York: John Wiley & Sons, 1988, 629–49.
- 2. McEwen BS, Stellar E.. Stress and the individual. Mechanisms leading to disease. Arch Intern Med 1993;153:2093–101. [PubMed] [Google Scholar]
- 3. Nugent KL, Chiappelli J, Rowland LM, Hong LE.. Cumulative stress pathophysiology in schizophrenia as indexed by allostatic load. Psychoneuroendocrinology 2015;60:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Prog Brain Res 2000;122:25–34. [DOI] [PubMed] [Google Scholar]
- 5. Juster RP, McEwen BS, Lupien SJ.. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 2010;35:2–16. [DOI] [PubMed] [Google Scholar]
- 6. Leahy R, Crews DE.. Physiological dysregulation and somatic decline among elders: modeling, applying and re-interpreting allostatic load. Coll Antropol 2012;36:11–22. [PubMed] [Google Scholar]
- 7. Seeman TE, McEwen BS, Rowe JW, Singer BH.. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA 2001;98:4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS.. Price of adaptation—allostatic load and its health consequences. Arch Intern Med 1997;157:2259–68. [PubMed] [Google Scholar]
- 9. Seeman T, Glei D, Goldman N, Weinstein M, Singer B, Lin YH.. Social relationships and allostatic load in Taiwanese elderly and near elderly. Soc Sci Med 2004;59:2245–57. [DOI] [PubMed] [Google Scholar]
- 10. McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 2000;22:108–24. [DOI] [PubMed] [Google Scholar]
- 11. Bizik G, Picard M, Nijjar R, Tourjman V, McEwen BS, Lupien SJ, Juster RP.. Allostatic load as a tool for monitoring physiological dysregulations and comorbidities in patients with severe mental illnesses. Harv Rev Psychiatry 2013;21:296–313. [DOI] [PubMed] [Google Scholar]
- 12. Danese A, McEwen BS.. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 2012;106:29–39. [DOI] [PubMed] [Google Scholar]
- 13. Langelaan S, Bakker AB, Schaufeli WB, van Rhenen W, van Doornen LJ.. Is burnout related to allostatic load? Int J Behav Med 2007;14:213–21. [DOI] [PubMed] [Google Scholar]
- 14. McEwen BS. Allostasis, allostatic load, and the aging nervous system: role of excitatory amino acids and excitotoxicity. Neurochem Res 2000;25:1219–31. [DOI] [PubMed] [Google Scholar]
- 15. Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE.. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J Clin Epidemiol 2002;55:696–710. [DOI] [PubMed] [Google Scholar]
- 16. Metz GAS, Ng JWY, Kovalchuk I, Olson DM.. Ancestral experience as a game changer in stress vulnerability and disease outcomes. Bioessays 2015;37:602–11. [DOI] [PubMed] [Google Scholar]
- 17. Babenko O, Kovalchuk I, Metz GA.. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev 2015;48:70–91. [DOI] [PubMed] [Google Scholar]
- 18. Zucchi FCR, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, Kovalchuk I, Kovalchuk O, Metz GAS.. Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PLoS One 2013;8:e56967.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 2000;886:172–89. [DOI] [PubMed] [Google Scholar]
- 20. Knieling M, Metz GA, Antonow-Schlorke I, Witte OW.. Enriched environment promotes efficiency of compensatory movements after cerebral ischemia in rats. Neuroscience 2009;163:759–69. [DOI] [PubMed] [Google Scholar]
- 21. Baldini S, Restani L, Baroncelli L, Coltelli M, Franco R, Cenni MC, Maffei L, Berardi N.. Enriched early life experiences reduce adult anxiety-like behavior in rats: a role for insulin-like growth factor 1. J Neurosci 2013;33:11715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McEwen BS, Seeman T.. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci 1999;896:30–47. [DOI] [PubMed] [Google Scholar]
- 23. Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R.. Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA 2012;109(Suppl. 2):17186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brennan AR, Arnsten AF.. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci 2008;1129:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM.. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res 2010;44:799–807. [DOI] [PubMed] [Google Scholar]
- 26. Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ.. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology 2008;33:517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCreary JK, Truica LS, Friesen B, Yao Y, Olson DM, Kovalchuk I, Cross AR, Metz GA.. Altered brain morphology and functional connectivity reflect a vulnerable affective state after cumulative multigenerational stress in rats. Neuroscience 2016;330:79–89. [DOI] [PubMed] [Google Scholar]
- 28. Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB.. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med 2004;58:1985–97. [DOI] [PubMed] [Google Scholar]
- 29. Brooks KP, Gruenewald T, Karlamangla A, Hu P, Koretz B, Seeman TE.. Social relationships and allostatic load in the MIDUS study. Health Psychol 2014;33:1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, Delmastro KK, Shi X, Renshaw PF.. Review: magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depress Res Treat 2011;2011:650450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stork C, Renshaw PF.. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry 2005;10:900–19. [DOI] [PubMed] [Google Scholar]
- 32. Wood SJ, Yucel M, Pantelis C, Berk M.. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann Acad Med Singapore 2009;38:396. [PubMed] [Google Scholar]
- 33. Yildiz-Yesiloglu A, Ankerst DP.. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res 2006;147:1–25. [DOI] [PubMed] [Google Scholar]
- 34. Allen PJ. Creatine metabolism and psychiatric disorders: does creatine supplementation have therapeutic value? Neurosci Biobehav Rev 2012;36:1442–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koene S, Kozicz TL, Rodenburg RJT, Verhaak CM, de Vries MC, Wortmann S, van de Heuvel L, Smeitink JA, Morava E.. Major depression in adolescent children consecutively diagnosed with mitochondrial disorder. J Affect Disord 2009;114:327–32. [DOI] [PubMed] [Google Scholar]
- 36. Johnson AR, Milner JJ, Makowski L.. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 2012;249:218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marques AH, Silverman MN, Sternberg EM.. Glucocorticoid dysregulations and their clinical correlates. From receptors to therapeutics. Ann N Y Acad Sci 2009;1179:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH.. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA 2006;103:14158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Castro JE, Diessler S, Varea E, Marquez C, Larsen MH, Cordero MI, Sandi C.. Personality traits in rats predict vulnerability and resilience to developing stress-induced depression-like behaviors, HPA axis hyper-reactivity and brain changes in pERK1/2 activity. Psychoneuroendocrinology 2012;37:1209–23. [DOI] [PubMed] [Google Scholar]
- 40. Zucchi FC, Yao Y, Metz GA.. The secret language of destiny: stress imprinting and transgenerational origins of disease. Front Genet 2012;3:96.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McEwen BS, Gianaros PJ.. Stress- and allostasis-induced brain plasticity. Annu Rev Med 2011;62:431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metab Clin Exp 2003;52:10–6. [DOI] [PubMed] [Google Scholar]
- 43. Edes AN, Wolfe BA, Crews DE.. Evaluating allostatic load: a new approach to measuring long-term stress in wildlife. J Zoo Wildl Med 2018;49:272–82. [DOI] [PubMed] [Google Scholar]
- 44. Zulman DM, Vijan S, Omenn GS, Hayward RA.. The relative merits of population-based and targeted prevention strategies. Milbank Q 2008;86:557–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Skinner MK. Environmental stress and epigenetic transgenerational inheritance. BMC Med 2014;12:153.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yao Y, Robinson AM, Zucchi FC, Robbins JC, Babenko O, Kovalchuk O, Kovalchuk I, Olson DM, Metz GA.. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med 2014;12:121.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ambeskovic M, Soltanpour N, Falkenberg EA, Zucchi FC, Kolb B, Metz GA.. Ancestral exposure to stress generates new behavioral traits and a functional hemispheric dominance shift. Cereb Cortex 2017;27:2126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seplaki CL, Goldman N, Glei D, Weinstein M.. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Exp Gerontol 2005;40:438–49. [DOI] [PubMed] [Google Scholar]
- 49. Harris A, Seckl J.. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 2011;59:279–89. [DOI] [PubMed] [Google Scholar]
- 50. Glover V, O'Connor TG, O'Donnell K.. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev 2010;35:17–22. [DOI] [PubMed] [Google Scholar]
- 51. ter Heegde F, De Rijk RH, Vinkers CH.. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology 2015;52:92–110. [DOI] [PubMed] [Google Scholar]
- 52. Jadavji NM, Supina RD, Metz GA.. Blockade of mineralocorticoid and glucocorticoid receptors reverses stress-induced motor impairments. Neuroendocrinology 2011;94:278–90. [DOI] [PubMed] [Google Scholar]
- 53. Smith LK, Jadavji NM, Colwell KL, Katrina Perehudoff S, Metz GA.. Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson's disease. Eur J Neurosci 2008;27:2133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Korte SM, De Boer SF.. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol 2003;463:163–75. [DOI] [PubMed] [Google Scholar]
- 55. Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. [DOI] [PubMed] [Google Scholar]
- 56. Roy V, Chapillon P.. Further evidences that risk assessment and object exploration behaviours are useful to evaluate emotional reactivity in rodents. Behav Brain Res 2004;154:439–48. [DOI] [PubMed] [Google Scholar]
- 57. Faraji J, Metz GA, Sutherland RJ.. Characterization of spatial performance in male and female Long-Evans rats by means of the Morris water task and the ziggurat task. Brain Res Bull 2010;81:164–72. [DOI] [PubMed] [Google Scholar]
- 58. Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L.. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res 2005;163:78–90. [DOI] [PubMed] [Google Scholar]
- 59. Khan M. Role of Cytokines, Immunopharmacology. Boston, MA: Springer US, 2008, 33–59. [Google Scholar]
- 60. Tanebe K, Nishijo H, Muraguchi A, Ono T.. Effects of chronic stress on hypothalamic lnterleukin-1beta, interleukin-2, and gonadotrophin-releasing hormone gene expression in ovariectomized rats. J Neuroendocrinol 2000;12:13–21. [DOI] [PubMed] [Google Scholar]
- 61. Segerstrom SC, Miller GE.. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 2004;130:601–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kiess W, Meidert A, Dressendörfer RA, Schriever K, Kessler U, Köunig A, Schwarz HP, Strasburger CJ.. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res 1995;37:502–6. [DOI] [PubMed] [Google Scholar]
- 63. Eigler N, Sacca L, Sherwin RS.. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J Clin Invest 1979;63:114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Friedman JM, Halaas JL.. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. [DOI] [PubMed] [Google Scholar]
- 65. Ahima RS, Bjorbaek C, Osei S, Flier JS.. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology 1999;140:2755–62. [DOI] [PubMed] [Google Scholar]
- 66. Hamann JJ, Kelley KM, Gladden LB.. Effect of epinephrine on net lactate uptake by contracting skeletal muscle. J Appl Physiol 2001;91:2635–41. [DOI] [PubMed] [Google Scholar]
- 67. Meyer C, Saar P, Soydan N, Eckhard M, Bretzel RG, Gerich J, Linn T.. A potential important role of skeletal muscle in human counterregulation of hypoglycemia. J Clin Endocrinol Metab 2005;90:6244–50. [DOI] [PubMed] [Google Scholar]
- 68. Kubera B, Hubold C, Otte S, Lindenberg AS, Zeiß I, Krause R, Steinkamp M, Klement J, Entringer S, Pellerin L.. et al. Rise in plasma lactate concentrations with psychosocial stress: a possible sign of cerebral energy demand. Obes Facts 2012;5:384–92. [DOI] [PubMed] [Google Scholar]
- 69. Phypers B, Pierce JT.. Lactate physiology in health and disease. Contin Educ Anaesth Crit Care Pain 2006;6:128–32. [Google Scholar]
- 70. Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA.. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab 2003;23:658–64. [DOI] [PubMed] [Google Scholar]
- 71. Glenn TC, Martin NA, Horning MA, McArthur DL, Hovda DA, Vespa P, Brooks GA.. Lactate: brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J Neurotrauma 2015;32:820–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wyss M, Schulze A.. Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience 2002;112:243–60. [DOI] [PubMed] [Google Scholar]
- 73. Brosnan JT, Brosnan ME.. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 2007;27:241–61. [DOI] [PubMed] [Google Scholar]
- 74. Mak CS, Waldvogel HJ, Dodd JR, Gilbert RT, Lowe MT, Birch NP, Faull RL, Christie DL.. Immunohistochemical localisation of the creatine transporter in the rat brain. Neuroscience 2009;163:571–85. [DOI] [PubMed] [Google Scholar]
- 75. Seplaki CL, Goldman N, Weinstein M, Lin YH.. How are biomarkers related to physical and mental well-being? J Gerontol A Biol Sci Med Sci 2004;59:201–17. [DOI] [PubMed] [Google Scholar]
- 76. Zilles K, Zilles B, Schleicher A.. A quantitative approach to cytoarchitectonics. VI. The areal pattern of the cortex of the albino rat. Anat Embryol 1980;159:335–60. [DOI] [PubMed] [Google Scholar]