Abstract
Objectives
To describe (i) the trend in oral anticoagulant (OAC) use following the introduction of non-vitamin K antagonist oral anticoagulant (NOAC) therapy for stroke prevention in atrial fibrillation (AF) patients and (ii) the current patterns of use of NOAC therapy in new users with AF in France.
Design
(i) Repeated cross-sectional study and (ii) population-based cohort study.
Setting
French national healthcare databases (50 million beneficiaries).
Participants
(i) Patients with identified AF in 2011, 2013 and 2016 and (ii) patients with AF initiating OAC therapy in 2015–2016.
Primary and secondary outcome measures
(i) Trend in OAC therapy use in patients with AF and (ii) patterns of use of NOAC therapy in new users with AF.
Results
Between 2011 and 2016, use of OAC therapy moderately increased (+16%), while use of antiplatelet therapy decreased (−22%) among all patients with identified AF. In 2016, among the 1.1 million AF patients, 66% used OAC therapy and were more likely to be treated by vitamin K antagonist (VKA) than NOAC therapy, including patients at higher risk of stroke (63.5%), while 33% used antiplatelet therapy. Among 192 851 new users of OAC therapy in 2015–2016 with identified AF, NOAC therapy (66.3%) was initiated more frequently than VKA therapy, including in patients at higher risk of stroke (57.8%). Reduced doses were prescribed in 40% of NOAC new users. Several situations of inappropriate use at NOAC initiation were identified, including concomitant use of drugs increasing the risk of bleeding (one in three new users) and potential NOAC underdosing.
Conclusions
OAC therapy use in patients with AF remains suboptimal 4 years after the introduction of NOACs for stroke prevention in France and improvement in appropriate prescribing regarding NOAC initiation is needed. However, NOAC therapy is now the preferred drug class for initiation of OAC therapy in patients with AF, including in patients at higher risk of stroke.
Keywords: anticoagulation, dabigatran, rivaroxaban, apixaban, claims database, France
Strengths and limitations of this study.
This study is the first to report both the 2011–2016 trend in oral anticoagulant (OAC) coverage in patients with atrial fibrillation (AF) following the introduction of non-vitamin K antagonist oral anticoagulant (NOAC) therapy in France and the current patterns of use of NOACs in new users including assessment of potential inappropriate use.
This study is based on reimbursement data for 50 million beneficiaries with access to all OAC prescriptions filled in the ambulatory setting.
As indications for treatment are not available in the databases, AF was mostly identified on the basis of discharge and long-term disease diagnosis codes and an algorithm previously validated in the French healthcare databases that helped to further identify AF in outpatients.
Introduction
Non-vitamin K antagonist oral anticoagulants (NOACs) have been gradually introduced over the past decade as a more convenient, fixed-dose alternative to vitamin K antagonists (VKAs), the only oral anticoagulant (OAC) therapy available up until now for long-term stroke prevention in patients with non-valvular atrial fibrillation (AF).1 Compared with VKA, NOAC therapy avoids the need for regular laboratory monitoring of patients by international normalised ratio (INR) testing due to a wider therapeutic window, allows once (rivaroxaban, edoxaban) or twice (dabigatran, apixaban) daily intake and is associated with fewer drug–drug interactions to date.2–6 NOACs have been demonstrated to have similar or superior efficacy to warfarin for stroke prevention in patients with non-valvular AF, and these findings have recently been implemented in the 2016 European Society of Cardiology (ESC) guidelines that recommended NOAC over VKA therapy in this indication.7 Moreover, antiplatelet therapy is no longer recommended in these patients.7 8 The use of NOAC therapy, massively adopted worldwide including in France,9–15 is expected to overcome the suboptimal use of OAC therapy extensively reported with VKA therapy, including underprescribing and high discontinuation rates.16 17
Despite their improved ease of use, NOAC prescribed dose needs to be adjusted to the patient’s clinical profile with regards to age, renal function, weight and risks of bleeding and drug–drug interactions. Two dose regimens are therefore proposed for each NOAC: standard dose regimen (ie, dabigatran 150 mg/12 hours, rivaroxaban 20 mg/24 hours; apixaban 5 mg/12 hours) and reduced-dose regimen (ie, dabigatran 75 mg or 110 mg/12 hours, rivaroxaban 10 mg or 15 mg/24 hours; apixaban 2.5 mg/12 hours). The reasons for prescribing a reduced-dose regimen are listed in the summary of product characteristics (SmPCs) of each NOAC with differences across NOACs as well as in the ESC7 8 and European Heart Rhythm Association guidelines.18 Recent publications have suggested that the frequency of use of reduced-dose NOACs in clinical practice could largely exceed that expected on the basis of the conditions summarised in these guidelines.11 19–21 However, national data are lacking concerning the current French patterns of NOAC use, including the potential issue of NOAC underdosing.
A steady increase in the initiation of NOAC therapy has already been reported in patients with AF in France,14 but trends in OAC coverage of patients with AF following the introduction of NOACs and a description of the current national patterns of NOAC use have not yet been reported. This study, based on the French nationwide healthcare databases, therefore had a twofold objective: (1) to describe the trends in OAC use following the introduction of NOAC for stroke prevention in patients with AF during the 2011–2016 period and (2) to describe the current patterns of use of OAC therapies with particular focus on NOAC use in new users with AF.
Methods
Data source
French national health insurance (Assurance Maladie) covers the entire French population by means of several specific schemes according to the beneficiary’s occupational sector, the largest scheme being the ‘Régime général’ (around 50 million beneficiaries).
This study was conducted using data from the French health insurance system database (Système national d’information inter-régimes de l’Assurance maladie, SNIIRAM) linked to the French hospital discharge database (Programme de médicalisation des systèmes d’information, PMSI).22 23 The SNIIRAM database contains individualised, anonymous and comprehensive data on health spending reimbursements. Demographic data include date of birth, gender and vital status. Drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification. Each packaging of each product is identified by means of a national-specific pack identifier code providing information on the name of the product, active ingredient and dose per unit, number of units and route of administration; but, the exact dosage prescribed and the indication are not available. However, for most drugs, particularly OACs, each dispensing of the prescribed drug cannot exceed the quantity necessary for 1 month of treatment. The PMSI database provides detailed information about discharge diagnoses and medical procedures related to all hospitalisations in France. This information regarding morbidities is completed by diagnoses corresponding to patient eligibility for 100% reimbursement of severe and costly long-term diseases (LTD) and disability, such as AF, coronary heart disease, certain debilitating diseases (such as multiple sclerosis or rheumatoid arthritis), HIV infection, cancer, etc. All information concerning medical diagnosis is encoded according to the International Classification of Diseases, 10th revision (ICD-10). Finally, the SNIIRAM-PMSI databases also indicate medical procedures performed in the ambulatory setting, including information about the type and date of all laboratory tests performed, but not including their results.
The French healthcare databases have been previously described and used in epidemiological and pharmacoepidemiological studies.22 24–26
Study populations and study designs
Two study populations were defined; one for each objective.
To answer the first objective, a repeated cross-sectional study was performed to describe the trends in OAC use following the introduction of NOAC in patients with AF. Patients with AF were identified in 2011 (as none of the NOACs was available for stroke prevention in France) and 2016 (the most recent data available at the time of writing the study protocol; dabigatran, rivaroxaban and apixaban were all reimbursed for stroke prevention in France, as apixaban was reimbursed from January 2014 onwards). OAC coverage was also calculated for year 2013 as this year represented the first calendar year for which the first two NOACs were available in France, that is a pivotal year for the pharmacological management of AF by OACs. For each of these calendar years, a patient was considered to have AF when at least one diagnosis of AF (ICD-10 code I48) was identified from discharge and LTD diagnoses in the SNIIRAM-PMSI database in the calendar year considered or during the previous 5 years. Patients with no continuous ‘Régime général’ health insurance coverage for at least 6 years before the calendar year considered were excluded.
To answer the second objective, a population-based cohort study was performed including patients with AF initiating OAC therapy in 2015–2016. First, OAC new users were identified among patients with continuous ‘Régime général’ health insurance coverage as those with at least one reimbursement for OAC therapy in 2015–2016 and no reimbursement for any OAC (VKA or NOAC) in the previous 24 months. The patient’s index date was the date of first OAC reimbursement identified during the 2015–2016 period. Second, the cohort of NOAC news users was restricted to those treated for AF: (i) patients treated for other OAC indications that is, patients treated for deep vein thrombosis/pulmonary embolism (DVT/PE) or with lower limb orthopaedic procedures were excluded; (ii) OAC new users treated for AF were identified from the resulting cohort as the sum of ‘OAC new users with confirmed AF’ for those with a diagnosis of AF (ICD-10 code I48) or specific AF management procedures identified from LTD or hospitalisation discharge data during a 6-year preindex period, and ‘OAC new users with probable AF’ for outpatients identified using an algorithm discriminating AF from DVT/PE with 95% specificity.27 The remaining patients were not classified as probable patients with AF and were excluded. Codes used for identification of AF and all of the patient characteristics considered, including comorbidities, are displayed in online supplementary table 1.
bmjopen-2018-026645supp001.pdf (737.9KB, pdf)
Patient and public involvement
Patients and or public were not involved.
Exposure
NOAC (dabigatran, rivaroxaban and apixaban) and VKA therapies (fluindione, warfarin and acenocoumarol) were identified using ATC codes; edoxaban was not available in France during the period considered.
Outcomes
Trends in OAC therapy use in patients with AF
The proportion of patients with AF treated by OAC therapy was assessed before and after approval of NOAC therapies for stroke prevention in France. Trends in the use of antiplatelet agents were also assessed in patients with AF over the same timeframe.
Patterns of use of NOAC therapy in new users with AF
The description of patterns of NOAC use in new users treated for AF in 2015–2016 included comparison of the baseline characteristics among NOAC new users and compared with those of VKA new users and potential inappropriate use of NOAC therapy was then investigated by identifying:
(i) NOAC off-label use or non-approved indication/dose: contraindications to NOAC therapy according to SmPCs (concomitant coagulopathy, purpura and other haemorrhagic conditions, liver fibrosis and cirrhosis, recent gastrointestinal ulceration or intracranial haemorrhage), valvular AF (NOAC are only approved for non-valvular AF), prosthetic heart valve (contraindicated for dabigatran), cancer (NOAC are not approved for prevention of thromboembolism in patients with cancer) and prescription of NOAC doses not approved for stroke prevention in Europe (dabigatran 75 mg and rivaroxaban 10 mg are not approved for stroke prevention in Europe and are therefore off-label doses in patients with AF); (ii) non-compliance with guidelines with respect to follow-up and clinical work-up of patients during the first year following NOAC initiation: no monitoring of patients’ renal function (renal function should be assessed at initiation and annually during NOAC therapy),28 discontinuation of NOAC therapy (OAC therapy is recommended as lifetime treatment in most patients with AF); (iii) clinically relevant drug–drug interactions at initiation increasing the bleeding risk at initiation and (iv) potential inappropriate underdosing, that is, patients in whom the NOAC dose prescribed at initiation was inappropriately reduced in view of their individual stroke and bleeding risks.
Data analysis
Descriptive analyses are expressed as mean and SD for continuous variables, and numbers and percentages for categorical variables.
Trends in OAC therapy use in patients with AF
For each calendar year, the proportion of patients treated by a drug was defined by the number of patients with at least one reimbursement for this drug in the calendar year considered over the total number of patients identified as having AF in the same year. Proportions are reported according to the type of OAC first reimbursed in the year considered. Antiplatelet drugs and OAC therapies were considered to be coprescribed when they were reimbursed at least once on the same day during the calendar year studied. Analyses were replicated in subgroups of patients with AF: (i) aged 75 years and over; (ii) female and male, separately; (iii) with a history of hospitalisation for arterial thromboembolic events (ATE) and (iv) with concomitant ischaemic heart disease or prosthetic heart valve.
Patterns of use of NOAC therapy in new users with AF
Baseline characteristics of NOAC new users with AF included sociodemographic data, including deprivation index of the patient’s municipality of residence,29 type of initial prescriber, clinical scores predicting the risk of stroke (CHA2DS2-VASc score) or bleeding (HAS-BLED score),30 31 adapted to claims data and the other main comorbidities and comedications, including proxies of frailty. A negative binomial regression analysis for each NOAC therapy and each baseline characteristic was performed to assess the association between these characteristics and the choice of NOAC therapy versus VKA therapy, while adjusting for age and sex.
Compliance with guidelines regarding renal function monitoring and treatment persistence patterns were assessed in new users for whom data for at least 1 year of follow-up were available, that is, patients included in 2015 and who had not died and had not been hospitalised for 3 months or longer. Compliance with renal function monitoring was assessed at NOAC initiation (no reimbursement for renal function monitoring during the 3 months before and the 3 months after NOAC initiation) and during the first year following treatment initiation. OAC non-persistence patterns were assessed over the 1-year period following the index date by calculating proxies of OAC discontinuation: number of patients with only one reimbursement and 1-year crude discontinuation rates.
Drugs increasing the risk of bleeding were those responsible for clinically relevant pharmacodynamic interactions with OAC therapy, that is, concomitant reimbursement of other parenteral and oral antithrombotic drugs, non-steroidal anti-inflammatory drugs; selective serotonin reuptake inhibitors and selective serotonin-norepinephrine reuptake inhibitors.7 18 32 Patients taking concomitant drugs with OAC therapy were defined as those with a reimbursement for the drug of interest during the period corresponding to the index date and the following 45 days. Analyses were replicated in VKA new users for descriptive purposes.
Finally, potential inappropriate underdosing with NOACs was defined as initiation of NOAC therapy in patients at risk of stroke in whom reduced doses of NOAC were prescribed with no identified justification. As this study was based on claims data and as, up until 2016, ESC guidelines recommended prescribing reduced-dose NOAC in patients with HAS-BLED ≥3,8 the proportion of AF patients initiating reduced-dose NOAC with an HAS-BLED score <3 among all NOAC new users with a CHA2DS2-VASc score ≥2 was used to quantify potential inappropriate underdosing in NOAC new users. Analyses were replicated in patients (i) with CHA2DS2-VASc score ≥4 and (ii) aged 75 and over with a history of ATE.
Results
Trends in OAC therapy use in patients with AF
The number of patients identified from French healthcare databases as having AF increased between 2011 (n=853 440) and 2016 (n=1 098 657). A high proportion of these patients were identified exclusively by hospitalisation discharge diagnosis and this proportion decreased only slightly over this time interval from 90.2% (n=770 002) to 86.2% (n=946 657).
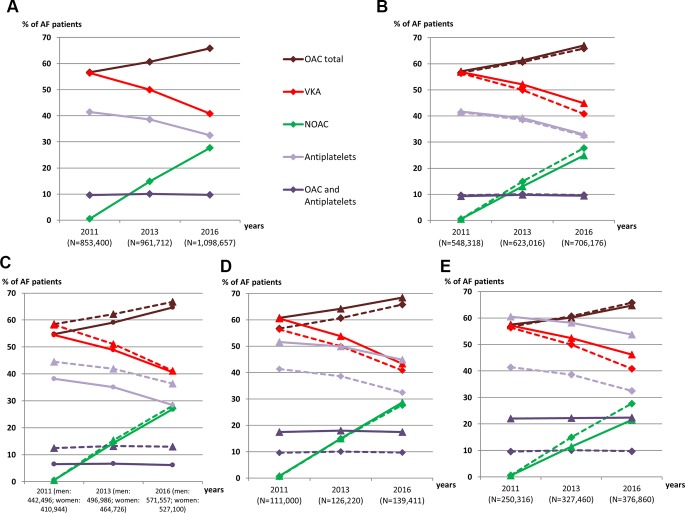
Between 2011 and 2016, the proportion of patients with at least one reimbursement for OAC therapy among all patients with AF moderately increased (+16%) from 56.7% to 65.8%, corresponding to a steady decrease in VKA use (from 56.6% to 40.8% of all patients with AF) associated with the introduction of NOACs (from 0.6% to 27.7%). In 2016, among patients with identified AF, VKA therapy remained the preferred OAC therapy (62.0%), including in patients aged 75 years and over (67.0%) and in those with a history of ATE (63.5%).
Between 2011 and 2016, the use of antiplatelet therapy decreased in patients with AF (−22%), but the proportion of patients with concomitant OAC and antiplatelet therapy remained stable (9.7% in 2016). In 2016, 32.5% of patients with AF had at least one reimbursement for antiplatelet therapy during the year.
Similar trends were observed in the subgroup analyses. A slightly higher rate of OAC therapy was observed in patients with a history of ATE (68.4% vs 65.8% in the total cohort in 2016). However, OAC coverage was lower in women than in men with AF (64.7% vs 66.8% in 2016) (figure 1).
Figure 1.
Time trends in the use of oral antithrombotic therapy between 2011 and 2016 in patients with AF in France. (A) Total population: patients with AF. (B) Patients aged 75 years and over. (C) Female patients (solid line) and male patients (dashed line). (D) Patients with history of arterial thrombo-embolicevents. (E) Patients with IHD or with prosthetic heart valve. For Figures B, D and E, estimates from all AF patients already presented in figure A. are indicated by dashed lines for the purposes of comparison. AF, atrial fibrillation; IHD, ischaemic heart diseases; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant; VKA, vitamin K antagonist.
Patterns of use of NOAC therapy in new users with AF
Baseline characteristics of OAC new users
Among 540 914 patients initiating OAC therapy in 2015–2016, a total of 192 851 (35.7%) patients were included in the study population, corresponding to 127 841 NOAC new users and 65 010 VKA new users with AF. The mains reasons for ineligibility were other indications or uncertain identification of the indication for NOAC (figure 2).
Figure 2.
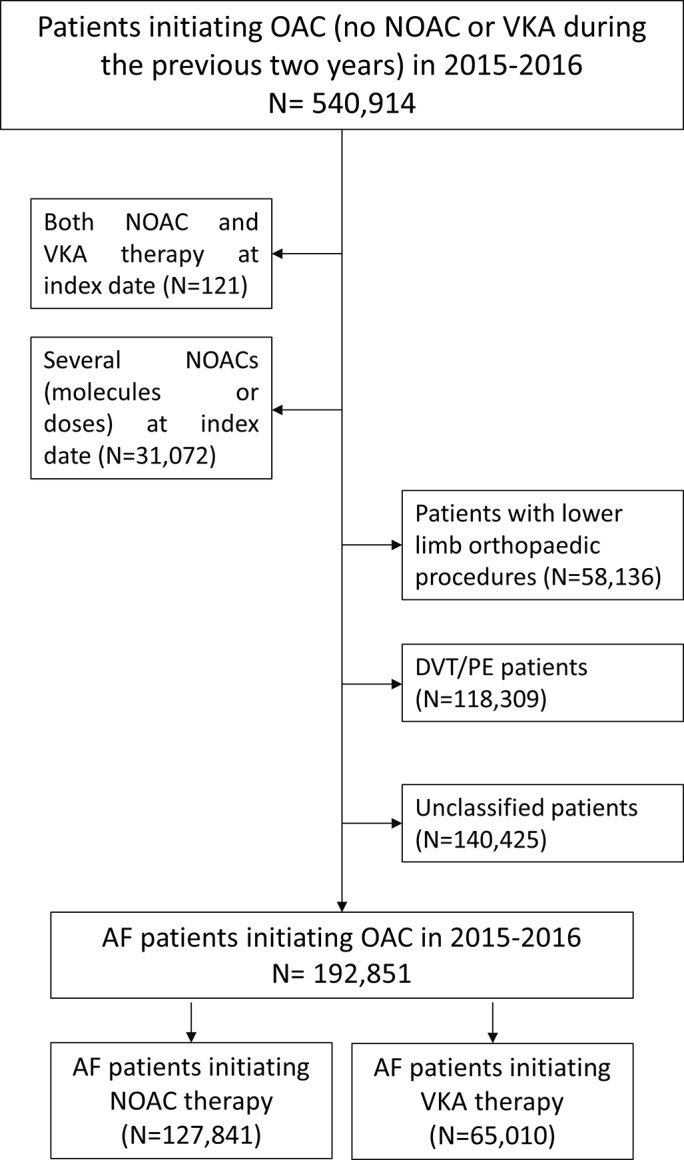
Patient flow chart. AF, atrial fibrillation; DVT/PE, deep vein thrombosis/pulmonary embolism; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant; VKA, vitamin K antagonist.
The mean age of the NOAC new users cohort was 74.1±11.6 years; patients over the age of 80 represented 37.3% of the total cohort. One-half of the NOAC new users were women, and 40.0% received a reduced dose at initiation (62.2% for dabigatran new users). Apixaban was the NOAC most commonly initiated; apixaban new users were older and had more comorbidities than the other two groups of NOAC new users. NOAC therapy was more likely to be initiated than VKA therapy among patients aged 75 years and older (59.4%) and in patients with a history of ATE (57.8%) (table 1).
Table 1.
Baseline characteristics of anticoagulant-naive patients with atrial fibrillation initiating oral anticoagulants in 2015–2016
| Characteristics (N; %*) | NOAC | VKA n=65 010 | |||
| Dabigatran n=9085 |
Rivaroxaban n=54 456 |
Apixaban n=64 300 |
Total NOAC n=1 27 841 |
||
| NOAC: reduced doses | 5652 (62.2) | 19 429 (35.7) | 26 003 (40.4) | 51 084 (40.0) | NA |
| Female sex | 4546 (50.0) | 26 147 (48.0) | 33 375 (51.9) | 64 068 (50.1) | 33 865 (52.1) |
| Age (years), mean (SD) | 74.1 (11.6) | 72.8 (11.9) | 75.3 (11.3) | 74.1 (11.6) | 78.0 (11.3) |
| 18–54 | 548 (6.0) | 4007 (7.4) | 3172 (4.9) | 7727 (6.0) | 2361 (3.6) |
| 55–64 | 1117 (12.3) | 7651 (14.0) | 7117 (11.1) | 15 885 (12.4) | 5680 (8.7) |
| 65–74 | 2514 (27.7) | 16 057 (29.5) | 16 645 (25.9) | 35 216 (27.5) | 12 969 (19.9) |
| 75–79 | 1554 (17.1) | 9053 (16.6) | 10 692 (16.6) | 21 299 (16.7) | 9587 (14.7) |
| ≥80 | 3352 (36.9) | 17 688 (32.5) | 26 674 (41.5) | 47 714 (37.3) | 34 413 (52.9) |
| ≥90 | 493 (5.4) | 2559 (4.7) | 4654 (7.2) | 7706 (6.0) | 8399 (12.9) |
| Deprivation index | |||||
| Quintile 1 (least deprived) | 1394 (15.3) | 10 265 (18.9) | 11 266 (17.5) | 22 925 (17.9) | 10 263 (15.8) |
| Quintile 2 | 1586 (17.5) | 10 678 (19.6) | 12 496 (19.4) | 24 760 (19.4) | 11 884 (18.3) |
| Quintile 3 | 1780 (19.6) | 10 701 (19.7) | 12 799 (19.9) | 25 280 (19.8) | 12 811 (19.7) |
| Quintile 4 | 1917 (21.1) | 10 794 (19.8) | 13 142 (20.4) | 25 853 (20.2) | 14 272 (22.0) |
| Quintile 5 (most deprived) | 2113 (23.3) | 11 172 (20.5) | 13 825 (21.5) | 27 110 (21.2) | 14 699 (22.6) |
| Overseas departments | 295 (3.2) | 846 (1.6) | 772 (1.2) | 1913 (1.5) | 1081 (1.7) |
| First prescriber’s specialty | |||||
| Hospital practitioner | 3720 (40.9) | 22 905 (42.1) | 29 316 (45.6) | 55 941 (43.8) | 39 083 (60.1) |
| General practitioner | 2062 (22.7) | 11 145 (20.5) | 11 590 (18.0) | 24 797 (19.4) | 15 539 (23.9) |
| Private cardiologist | 3093 (34.0) | 18 978 (34.9) | 21 843 (34.0) | 43 914 (34.4) | 8511 (13.1) |
| Private orthopaedic surgeon | 16 (0.2) | 100 (0.2) | 95 (0.1) | 211 (0.2) | 73 (0.1) |
| Other private specialist | 168 (1.8) | 1149 (2.1) | 1276 (2.0) | 2593 (2.0) | 1583 (2.4) |
| CHA2DS2-VASc score† | |||||
| Mean score (SD) | 3.7 (1.6) | 3.5 (1.6) | 3.9 (1.6) | 3.7 (1.6) | 4.5 (1.6) |
| 0 | 183 (2.0) | 1294 (2.4) | 847 (1.3) | 2324 (1.8) | 309 (0.5) |
| 1 | 635 (7.0) | 4854 (8.9) | 3637 (5.7) | 9126 (7.1) | 1607 (2.5) |
| ≥2 | 8267 (91.0) | 48 308 (88.7) | 59 816 (93.0) | 1 16 391 (90.1) | 63 094 (97.0) |
| C (heart failure) | 2849 (31.4) | 17 805 (32.7) | 23 548 (36.6) | 44 202 (34.6) | 32 727 (50.3) |
| H (antihypertensive drugs) | 7547 (83.1) | 44 260 (81.3) | 54 596 (84.9) | 106 403 (83.2) | 59 139 (91.0) |
| D(iabetes) | 1959 (21.6) | 11 279 (20.7) | 14 087 (21.9) | 27 325 (21.4) | 18 806 (28.9) |
| S(troke: ATE) | 1207 (13.3) | 4930 (9.1) | 8448 (13.1) | 14 585 (11.4) | 10 638 (16.4) |
| V(ascular diseases) | 2242 (24.7) | 13 924 (25.6) | 18 766 (29.2) | 34 932 (27.3) | 28 894 (44.4) |
| Age ≥75 and arterial thromboembolic events† | 769 (8.5) | 3126 (5.7) | 5608 (8.7) | 9503 (7.4) | 7762 (11.9) |
| Age <65 and no arterial thromboembolic events† | 1510 (16.6) | 11 074 (20.3) | 9396 (14.6) | 21 980 (17.2) | 7035 (10.8) |
| HAS-BLED score† | |||||
| Mean score (SD) | 2.0 (0.9) | 1.9 (0.9) | 2.1 (0.9) | 2.0 (0.9) | 2.7 (1.0) |
| ≥3 | 2169 (23.9) | 11 388 (20.9) | 16 834 (26.2) | 30 391 (23.8) | 36 417 (56.0) |
| A(bnormal) | |||||
| Renal function | 345 (3.8) | 2426 (4.5) | 3822 (5.9) | 6593 (5.2) | 14 260 (21.9) |
| Liver function | 169 (1.9) | 967 (1.8) | 1106 (1.7) | 2242 (1.8) | 2372 (3.6) |
| B(leeding) | |||||
| Predisposition | 182 (2.0) | 1161 (2.1) | 1605 (2.5) | 2948 (2.3) | 5873 (9.0) |
| Major bleeding | 692 (7.6) | 3729 (6.8) | 5134 (8) | 9555 (7.5) | 9348 (14.4) |
| D(rug/alcohol) | |||||
| Alcohol abuse‡ | 272 (3.0) | 1698 (3.1) | 1730 (2.7) | 3700 (2.9) | 2923 (4.5) |
| Drug–drug interactions | 947 (10.4) | 5838 (10.7) | 7570 (11.8) | 14 355 (11.2) | 23 451 (36.1) |
| Parenteral anticoagulant (heparin) | 64 (0.7) | 331 (0.6) | 361 (0.6) | 756 (0.6) | 9824 (15.1) |
| Antiplatelet drugs | 826 (9.1) | 5225 (9.6) | 6951 (10.8) | 13 002 (10.2) | 15 433 (23.7) |
| NSAIDs | 72 (0.8) | 365 (0.7) | 344 (0.5) | 781 (0.6) | 212 (0.3) |
| Other comorbidities† | |||||
| Ischaemic heart disease | 1821 (20.0) | 11 321 (20.8) | 15 439 (24.0) | 28 581 (22.4) | 23 657 (36.4) |
| Frailty (proxies) | 1666 (18.3) | 8971 (16.5) | 12 730 (19.8) | 23 367 (18.3) | 24 175 (37.2) |
| Dementia or Parkinson’s disease | 524 (5.8) | 3204 (5.9) | 4125 (6.4) | 7853 (6.1) | 7437 (11.4) |
| Psychiatric disorders | 1722 (19.0) | 10 593 (19.5) | 12 844 (20.0) | 25 159 (19.7) | 16 598 (25.5) |
| Smoking‡ | 1024 (11.3) | 6481 (11.9) | 7442 (11.6) | 14 947 (11.7) | 11 434 (17.6) |
| Comedications§ | |||||
| Antiarrhythmics or cardiac glycosides | 5996 (66.0) | 35 761 (65.7) | 41 031 (63.8) | 82 788 (64.8) | 35 600 (54.8) |
| Lipid-lowering agents | 3913 (43.1) | 22 250 (40.9) | 28 812 (44.8) | 54 975 (43.0) | 31 903 (49.1) |
| Oral corticosteroids | 1105 (12.2) | 6964 (12.8) | 8079 (12.6) | 16 148 (12.6) | 8967 (13.8) |
| Antiulcer agents | 4295 (47.3) | 24 842 (45.6) | 31 469 (48.9) | 60 606 (47.4) | 39 842 (61.3) |
| Polymedication (≥5 ATC classes) | 3750 (41.3) | 21 725 (39.9) | 28 196 (43.9) | 53 671 (42.0) | 45 153 (69.5) |
| Polymedication (≥10 ATC classes) | 738 (8.1) | 4653 (8.5) | 6246 (9.7) | 11 637 (9.1) | 14 947 (23.0) |
*Unless otherwise stated.
†Comorbidities were defined using a rolling 1-year period following the initiation of OAC therapy.
‡Smoking or alcohol data: measured by using proxies such as reimbursements for specific therapy or hospitalisations related to smoking or alcohol consumption/diseases.
§Comorbidities were defined using a rolling 4-month period preceding the initiation of OAC therapy.
ATC, Anatomical Therapeutic Chemical classification; ATE, arterial thromboembolic events (ischaemic stroke, arterial systemic embolism or transient ischaemic attack); NOAC, non-vitamin K antagonist oral anticoagulant; NSAIDs, non-steroidal anti-inflammatory drugs; VKA, vitamin K antagonist.
Characteristics associated with bleeding risk, such as older age, renal impairment, history of bleeding or bleeding predisposition and treatment with a concomitant drug increasing the risk of bleeding at OAC initiation, were strong predictors of being treated with VKA therapy versus NOAC therapies (online supplementary table 2).
Potential inappropriate use of NOAC therapy
About 15% of NOAC new users with AF were considered to be using NOAC off-label or for a non-approved indication. In particular, 8.5% of NOAC new users with AF had valvular heart disease (8.5%), including prosthetic heart valve (1.5%) and 4.6% had a recently or currently treated cancer (table 2).
Table 2.
Potential inappropriate use of NOAC therapy in oral anticoagulant-naïve patients with AF in 2015–2016
| Characteristics (N; %) | NOAC | VKA n=65 010 |
|||
| Dabigatran n=9085 | Rivaroxaban n=54 456 | Apixaban n=64 300 | Total NOAC n=1 27 841 |
||
| Contraindications or non-approved indication/dose | 1457 (16.0) | 8614 (15.8) | 9542 (14.8) | 19 613 (15.3) | NA |
| Any valvular heart disease | 649 (7.1) | 4146 (7.6) | 6122 (9.5) | 10 917 (8.5) | 16 461 (25.3) |
| Prosthetic heart valve (mechanical or bioprosthetic valves) | 106 (1.2) | 665 (1.2) | 1096 (1.7) | 1867 (1.5) | 6726 (10.3) |
| Recently hospitalised for coagulopathy, purpura and other haemorrhagic conditions* | 90 (1.0) | 479 (0.9) | 596 (0.9) | 1165 (0.9) | 2142 (3.3) |
| Liver fibrosis and cirrhosis* | 50 (0.6) | 282 (0.5) | 367 (0.6) | 699 (0.5) | 1174 (1.8) |
| Recent gastrointestinal ulceration or intracranial haemorrhage† | 40 (0.4) | 120 (0.2) | 248 (0.4) | 408 (0.3) | 350 (0.5) |
| Recently or currently treated cancer* | 417 (4.6) | 2531 (4.6) | 2898 (4.5) | 5846 (4.6) | 4252 (6.5) |
| Reduced-dose NOAC not approved for stroke prevention in patients with AF in Europe | 357 (3.9) | 1844 (3.4) | NA | 2201 (3.5) | NA |
| Inappropriate use during follow-up‡ | |||||
| No monitoring of renal function at initiation | 637 (16.8) | 3804 (15.5) | 3642 (14.5) | 8083 (15.1) | 5401 (17.0) |
| No monitoring of renal function in the year postinitiation | 378 (10.0) | 2347 (9.6) | 2138 (8.5) | 4863 (9.1) | 2984 (9.4) |
| Non-persistence patterns, N (%) | |||||
| One reimbursement only | 605 (15.9) | 2666 (10.9) | 1771 (7.1) | 5042 (9.4) | 2426 (7.6) |
| One-year treatment discontinuation rates§ | 984 (25.9) | 6210 (25.4) | 4524 (18,0) | 11 718 (21,9) | 8399 (26.4) |
| Concomitant use of drug increasing the risk of bleeding¶ | 2639 (29.3) | 15 797 (29.3) | 18 556 (29.2) | 36 992 (29.3) | 33 025 (52.3) |
| Antiplatelet agents or parenteral anticoagulants | 1728 (19.2) | 10 386 (19.3) | 12 382 (19.5) | 24 496 (19.4) | 28 112 (44.5) |
| Parenteral anticoagulants | 287 (3.2) | 1577 (2.9) | 1520 (2.4) | 3384 (2.7) | 12 078 (19.1) |
| Antiplatelet agents | 1490 (16.6) | 9179 (17.0) | 11 170 (17.6) | 21 839 (17.3) | 19 710 (31.2) |
| Aspirin | 1336 (14.8) | 8215 (15.2) | 10 026 (15.8) | 19 577 (15.5) | 17 770 (28.1) |
| NSAIDs | 375 (4.2) | 2111 (3.9) | 2017 (3.2) | 4503 (3.6) | 1030 (1.6) |
| SSRIs and SSNRIs | 836 (9.3) | 4852 (9.0) | 5880 (9.2) | 11 568 (9.1) | 7317 (11.6) |
*Comorbidities identified using hospitalisation and/or LTD data, and/or specific procedures during a rolling 1-year period preceding the initiation of OAC therapy.
†Comorbidities identified using hospitalisation data during a rolling 6-week period preceding the initiation of OAC therapy.
‡Data on patients with at least a 1 year of follow-up ie, patients initiating OAC in 2015 after excluding patients who died and those hospitalised for 3 months or longer (n=3796; 24 483; 25 118; 53 397 and 31 777 for dabigatran, rivaroxaban, apixaban, total NOAC and total VKA new users, respectively); period considered (unless otherwise stated): rolling 1-year period following the initiation of OAC therapy (index date included).
§1-year crude discontinuation rate for patients initiating OAC in 2015 who died and those hospitalised for 3 months or longer, defined as prolonged treatment discontinuation ie, 90-day gap with no medication coverage after the 30-day coverage period of a refill.
¶Data for new users still alive after a 45-day period following the index date (n=9001; 53 885; 63 578; 126 464 and 63 180 for dabigatran, rivaroxaban, apixaban, total NOAC and total VKA new users, respectively); period considered: rolling 6-week period following the initiation of OAC therapy (index date included).
AF, atrial fibrillation; ATE, arterial thromboembolic events; NA, not applicable; NOAC, non-vitamin K antagonist oral anticoagulant; NSAIDs, non-steroidal anti-inflammatory drugs; SSNRIs, selective serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; VKA, vitamin K antagonist.
About 15% and 9% of NOAC new users had no reimbursement for renal function tests at initiation and during the 1-year period postinitiation, respectively. Discontinuation during the 1-year period following initiation was frequent, as more than 20% of patients had five or less reimbursements (table 2).
Nearly 30% of NOAC new users were using at least one concomitant drug increasing the risk of bleeding (52% in VKA new users). The most common concomitant drugs concerned at initiation were antiplatelet agents or parenteral anticoagulants (table 2).
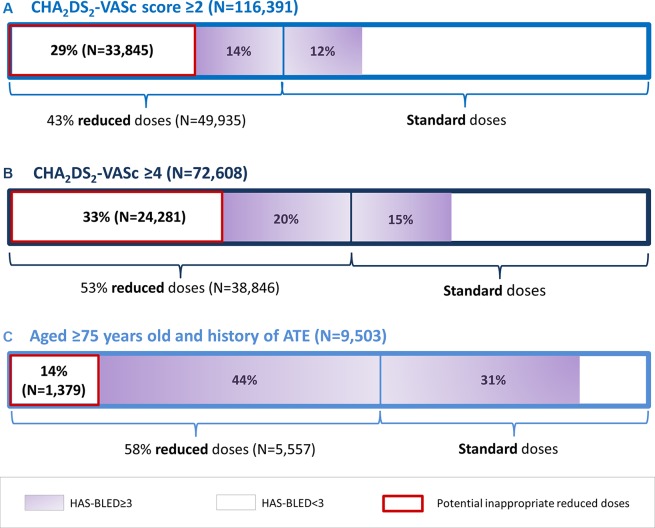
Among the 116 391 NOAC new users with AF with a CHA2DS2-VASc score ≥2, 29.1% (n=33 845) were prescribed a reduced dose although they had an HAS-BLED score <3. This meant that nearly 1 in 3 NOAC new users with AF and at risk of stroke were therefore potentially prescribed an inappropriately reduced dose of NOAC at initiation. This proportion was 33% (n=24 281) and 14.5% when defining patients at risk of stroke as patients with a CHA2DS2-VASc score ≥4 and aged 75 and over with a history of ATE, respectively (figure 3).
Figure 3.
Potential NOAC underdosing in new users with AF. NOAC, non-vitamin K antagonist oral anticoagulant; AF, atrial fibrillation; ATE, arterial thromboembolic events.
Differences in baseline characteristics were observed in patients with HAS-BLED <3 according to the type of NOAC dose prescribed, for example, patients with reduced-dose NOAC were older and frailer than those with standard-dose NOAC (online supplementary table 3).
Discussion
Main findings
OAC therapy use among patients with AF improved in France between 2011 and 2016, but remained suboptimal with about 66% of the 1.1 million patients with identified AF treated by OAC therapy in 2016. Use of antiplatelet therapy in patients with AF decreased over the same period, but still concerned 33% of all patients with AF in 2016. Patients with AF were more likely to be treated by VKA than NOAC therapy, including older patients and those at higher risk of stroke.
Nearly 193 000 patients with AF were identified as OAC new users in 2015–2016: patients were more likely to be treated by NOAC than VKA therapy, including older patients and those at higher risk of stroke. Results on current patterns of use of NOAC therapy in new users with AF suggest several situations of inappropriate use, including frequent concomitant use of drugs increasing the risk of bleeding and potential inappropriate underdosing.
Comparison with postmarketing literature and clinical implications
The overall improvement of management of patients with AF observed with regards to OAC therapy after the introduction of NOACs has been reported in many countries.33–36 The same applies to the steady decrease in VKA use in favour of NOAC therapies,12 37 38 and the gaps remaining in optimal OAC coverage and high antiplatelet drug use.39–41
This study demonstrated channelling of NOAC therapy towards patients at lower risk of stroke and bleeding when considering all patients with AF, in line with what has become a common feature reported worldwide in clinical practice by many observational studies on the current patterns of use of NOACs.15 42–45 In particular, data from the ESC-sponsored ‘EURObservational Research Programme on AF’ General Long-Term Registry showed that younger age, having fewer risk factors or a history of non-valvular heart diseases were also found to be clinical predictors for being treated with NOACs versus VKAs.46
However, NOAC therapy is now the preferred OAC therapy at initiation in the oldest patients and those at higher risk of stroke. This French pattern of OAC use has never been previously reported and contrasts with published results concerning earlier periods.47 This emerging pattern is encouraging for AF management, as older and high-risk patients are those who should derive most benefit from NOAC versus VKA therapy.48
Reduced doses were often prescribed in OAC new users, including dabigatran 75 mg and rivaroxaban 10 mg, which are not approved for stroke prevention in Europe.49 In addition to the overall channelling mentioned above, these findings may reflect a ‘bleeding avoidance’ strategy of prescribers (ie, overestimation of the potential bleeding risk versus the likely benefit of stroke reduction) and the differential perception of the comparative safety of NOACs versus VKA and between NOACs. The early safety alert on bleeding in dabigatran-treated patients, followed by the contraindication of this NOAC in patients with prosthetic heart valves, may have reinforced the fears of prescribers in relation to the safety of dabigatran, which would explain the difference in reduced-dose prescription rates between the three NOACs in this study, despite the intermediate stroke and bleeding risk profile of dabigatran compared with that of rivaroxaban and apixaban in new users. Similarly, among NOAC new users, apixaban was prescribed to the oldest and most severe patients. Apixaban was the only NOAC found to be superior to warfarin for all types of bleeding outcome and all-cause mortality, which would also illustrate the tendency of physicians to prescribe OAC therapies according to bleeding risk.5 This may also explain the potential inappropriate underdosing observed in NOAC users in this study. This pattern of NOAC use has been previously reported, but mostly in field and registry studies based on small sample sizes. The reported inappropriate underdosing rate varies according to studies and the definition used. NOAC underdosing concerned 30.4% of Turkish patients in the RAMSES study (n=2086),50 18.4% of Japanese patients of the KiCS AF registry (n=1284),51 52 between 19.7% and 27.6% of patients in the SAKURA AF registry (n=3266)53 and 9.4% to 16% of patients in the ORBIT-AF II registry (n=7925).21 54 In the subgroup of Dutch patients (n=899) enrolled in the XANTUS registry, 33% of patients were also treated with reduced-dose rivaroxaban despite presenting normal renal function.55 Using a large US administrative database, Yao et al found that 13.3% of the 13 392 NOAC new users with no renal indication for dose reduction were potentially underdosed.56 Taken together with our results, these data suggest that inappropriate underdosing might be a common issue in NOAC new users that should be systematically assessed when studying NOAC patterns of use. This is of particular concern, as recent data have suggested a relationship between NOAC dose and clinical outcomes.57 In particular, NOAC underdosing has been shown to be associated with increased risk for adverse outcomes.21 56
These patterns of NOAC use contrast with the other patterns concomitantly observed in this study, such as the high level of concomitant prescription of antiplatelet agents and parenteral anticoagulants or, to a lesser extent, NOAC use in non-approved indications such as prosthetic heart valves that are both associated with an increased risk of bleeding.58–60
Strengths and limitations
This study is the first to report the improved trend in OAC coverage in French patients with AF over the last 5 years as well as the recent patterns of use of OAC therapy in new users, particularly including a nationwide assessment of the growing issue of NOAC underdosing, based on health data for more than 50 million beneficiaries. Moreover, all OAC prescriptions filled in the ambulatory setting are captured in the databases and are reimbursed with no restriction of coverage: selection bias related to the access of patients to more expensive NOAC therapy is therefore not an issue with the use of French healthcare databases.22 61
However, several limitations related to the nature of the data used should be underlined. First of all, it cannot be verified whether patients actually took the drugs for which they were reimbursed. Second, as the indication for treatment is not available in the databases, and despite the use of an algorithm to identify AF among outpatients in the French healthcare databases, identification of AF was mostly based on non-validated discharge and LTD diagnoses recorded in the databases. Moreover, it cannot be excluded that the increase in the identified number of patients with AF over the 2011–2016 period could be partially explained by changes in LTD legislation in 2011 (eg, hypertension was removed from the list of LTD, while access to LTD was facilitated for patients with severe arrhythmia and valvular heart diseases) which could have helped to identify patients with AF. Third, identification of inappropriate underdosing at NOAC initiation was also indirectly assessed by using stroke and bleeding risk scores computed from claims data. Important medical data such as patient’s weight, glomerular filtration rate and exact alcohol consumption are not available in the French healthcare databases, which may have led to underestimation of the HAS-BLED score and therefore to overestimation of the proportion of patients potentially underdosed at initiation. These missing clinical data may also explain the prescription of reduced-dose NOAC at treatment initiation in clinical practice. Furthermore, the agreement between these empirical scores in patients with AF and the prescriber-assessed stroke and bleeding risk is a subject of discussion.62 Consequently, the rate of inappropriate underdosing should be interpreted with caution and must be confirmed by further studies. However, NOAC misuse and underdosing have also been reported in a French prospective field study based on patients’ medical charts.63 Of note, as INR values were not available in the databases, underdosing with VKA therapy was not assessed in this study, but has been frequently reported and must not be overlooked.53 64 In addition, as stated in the 2016 ESC guidelines,7 HAS-BLED score is not designed to evaluate prescription of NOAC type and dosage, and no longer must be used for this purpose in clinical practice.
Finally, the results for NOAC and VKA new users are difficult to compare, as they were not adjusted for significant differences in baseline characteristics, and this comparison was not the purpose of this study.
Conclusion
OAC therapy use has modestly increased after the introduction of the NOACs for stroke prevention in patients with AF in France, and NOAC therapy is now the preferred OAC therapy at initiation in older patients at higher risk of stroke. However, results from this nationwide drug utilisation study suggest the need for improvement in appropriate prescription of OAC therapy in these patients, especially regarding the use of concomitant interacting drugs and the choice of initial NOAC dose.
Supplementary Material
Acknowledgments
The authors thank Dr Saul, medical translator, for assistance in writing the manuscript.
Footnotes
Contributors: GM, AP, CB, AN: designed the study. All authors have contributed substantially to the interpretation of results. GM, AP, AN: drafted the article. CB, JD, AN: conducted the statistical analysis. GM, AP, AN, CB, JD, AW: provided critical revision of the manuscript for important intellectual content. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: This observational study based on the French healthcare databases was approved by the French Data Protection Agency (Commission Nationale de l’Informatique et des Libertés, Cnil) and did not require patient consents or ethics committee approval.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Permanent access to the French healthcare databases is automatically granted to certain government agencies, public institutions and public service authorities. Temporary access for studies and research is possible upon request from the National Health Data Institute (INDS). All databases used in this study only contained anonymous patient records.
Patient consent for publication: Not required.
References
- 1. Steinberg BA, Piccini JP. Anticoagulation in atrial fibrillation. BMJ 2014;348:g2116 10.1136/bmj.g2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verheugt FW, Granger CB. Oral anticoagulants for stroke prevention in atrial fibrillation: current status, special situations, and unmet needs. Lancet 2015;386:303–10. 10.1016/S0140-6736(15)60245-8 [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 5. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 6. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 7. Kirchhof P, Benussi S, Kotecha D, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 8. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. 10.1093/eurheartj/ehs253 [DOI] [PubMed] [Google Scholar]
- 9. Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation- quality and cost implications. Am J Med 2014;127:1075–82. 10.1016/j.amjmed.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 10. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med 2015;128:1300–5. 10.1016/j.amjmed.2015.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haastrup SB, Hellfritzsch M, Rasmussen L, et al. Use of Non-Vitamin K antagonist oral anticoagulants 2008-2016: a Danish Nationwide Cohort Study. Basic Clin Pharmacol Toxicol 2018;123:452–63. 10.1111/bcpt.13024 [DOI] [PubMed] [Google Scholar]
- 12. Kjerpeseth LJ, Ellekjær H, Selmer R, et al. Trends in use of warfarin and direct oral anticoagulants in atrial fibrillation in Norway, 2010 to 2015. Eur J Clin Pharmacol 2017;73:1417–25. 10.1007/s00228-017-2296-1 [DOI] [PubMed] [Google Scholar]
- 13. Staerk L, Fosbøl EL, Gadsbøll K, et al. Non-vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: Temporal trends 2011-2015 in Denmark. Sci Rep 2016;6:31477 10.1038/srep31477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huiart L, Ferdynus C, Renoux C, et al. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open 2018;8:e018180 10.1136/bmjopen-2017-018180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urbaniak AM, Strøm BO, Krontveit R, et al. Prescription patterns of Non-Vitamin K oral anticoagulants across indications and factors associated with their increased prescribing in atrial fibrillation between 2012-2015: a study from the Norwegian prescription database. Drugs Aging 2017;34:635–45. 10.1007/s40266-017-0476-4 [DOI] [PubMed] [Google Scholar]
- 16. Broderick JP, Bonomo JB, Kissela BM, et al. Withdrawal of antithrombotic agents and its impact on ischemic stroke occurrence. Stroke 2011;42:2509–14. 10.1161/STROKEAHA.110.611905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fang MC, Go AS, Chang Y, et al. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circ Cardiovasc Qual Outcomes 2010;3:624–31. 10.1161/CIRCOUTCOMES.110.937680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467–507. Europace 10.1093/europace/euv309 [DOI] [PubMed] [Google Scholar]
- 19. Dillinger JG, Aleil B, Cheggour S, et al. Dosing issues with non-vitamin K antagonist oral anticoagulants for the treatment of non-valvular atrial fibrillation: Why we should not underdose our patients. Arch Cardiovasc Dis 2018;111:85–94. 10.1016/j.acvd.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 20. Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 2017;69:2475–84. 10.1016/j.jacc.2017.03.540 [DOI] [PubMed] [Google Scholar]
- 21. Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin k antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol 2016;68:2597–604. 10.1016/j.jacc.2016.09.966 [DOI] [PubMed] [Google Scholar]
- 22. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: From the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Revue d'Épidémiologie et de Santé Publique 2017;65:S149–67. 10.1016/j.respe.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 23. Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: Powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2017;26:954–62. 10.1002/pds.4233 [DOI] [PubMed] [Google Scholar]
- 24. Maura G, Blotière PO, Bouillon K, et al. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation 2015;132:1252–60. 10.1161/CIRCULATIONAHA.115.015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ 2016;353:i2002 10.1136/bmj.i2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neumann A, Maura G, Weill A, et al. Clinical events after discontinuation of β-Blockers in patients without heart failure optimally treated after acute myocardial infarction: a cohort study on the french healthcare databases. Circ Cardiovasc Qual Outcomes 2018;11:e004356 10.1161/CIRCOUTCOMES.117.004356 [DOI] [PubMed] [Google Scholar]
- 27. Billionnet C, Alla F, Bérigaud É, et al. Identifying atrial fibrillation in outpatients initiating oral anticoagulants based on medico-administrative data: results from the French national healthcare databases. Pharmacoepidemiol Drug Saf 2017;26:535–43. 10.1002/pds.4192 [DOI] [PubMed] [Google Scholar]
- 28. Haute autorité de santé (French National Authority for Health). Guide parcours de soins Fibrillation atriale. 2014. https://www.has-sante.fr/portail/jcms/c_1741768/fr/guide-parcours-de-soins-fibrillation-atriale (Accessed 18 Jul 2018).
- 29. Rey G, Jougla E, Fouillet A, et al. Ecological association between a deprivation index and mortality in France over the period 1997 - 2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health 2009;9:33 10.1186/1471-2458-9-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124 10.1136/bmj.d124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 32. Renoux C, Vahey S, Dell’Aniello S, et al. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol 2017;74:173 10.1001/jamaneurol.2016.4529 [DOI] [PubMed] [Google Scholar]
- 33. Cowan JC, Wu J, Hall M, et al. A 10 year study of hospitalized atrial fibrillation-related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J 2018;39:2975–83. 10.1093/eurheartj/ehy411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steinberg BA, Gao H, Shrader P, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: Results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J 2017;194:132–40. 10.1016/j.ahj.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 35. Brown JD, Shewale AR, Dherange P, et al. A Comparison of Oral Anticoagulant Use for Atrial Fibrillation in the Pre- and Post-DOAC Eras. Drugs Aging 2016;33:427–36. 10.1007/s40266-016-0369-y [DOI] [PubMed] [Google Scholar]
- 36. Apenteng PN, Gao H, Hobbs FR, et al. Temporal trends in antithrombotic treatment of real-world UK patients with newly diagnosed atrial fibrillation: findings from the GARFIELD-AF registry. BMJ Open 2018;8:e018905 10.1136/bmjopen-2017-018905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alalwan AA, Voils SA, Hartzema AG. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am J Health Syst Pharm 2017;74:1237–44. 10.2146/ajhp160756 [DOI] [PubMed] [Google Scholar]
- 38. Olesen JB, Sørensen R, Hansen ML, et al. Non-vitamin K antagonist oral anticoagulation agents in anticoagulant naïve atrial fibrillation patients: Danish nationwide descriptive data 2011-2013. Europace 2015;17:187–93. 10.1093/europace/euu225 [DOI] [PubMed] [Google Scholar]
- 39. Lacoin L, Lumley M, Ridha E, et al. Evolving landscape of stroke prevention in atrial fibrillation within the UK between 2012 and 2016: a cross-sectional analysis study using CPRD. BMJ Open 2017;7:e015363 10.1136/bmjopen-2016-015363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Averlant L, Ficheur G, Ferret L, et al. Underuse of Oral Anticoagulants and Inappropriate Prescription of Antiplatelet Therapy in Older Inpatients with Atrial Fibrillation. Drugs Aging 2017;34:701–10. 10.1007/s40266-017-0477-3 [DOI] [PubMed] [Google Scholar]
- 41. Alamneh EA, Chalmers L, Bereznicki LR. Suboptimal Use of Oral Anticoagulants in Atrial Fibrillation: Has the Introduction of Direct Oral Anticoagulants Improved Prescribing Practices? Am J Cardiovasc Drugs 2016;16:183–200. 10.1007/s40256-016-0161-8 [DOI] [PubMed] [Google Scholar]
- 42. Douros A, Renoux C, Coulombe J, et al. Patterns of long-term use of non-vitamin K antagonist oral anticoagulants for non-valvular atrial fibrillation: Quebec observational study. Pharmacoepidemiol Drug Saf 2017;26:1546–54. 10.1002/pds.4333 [DOI] [PubMed] [Google Scholar]
- 43. Komen J, Forslund T, Hjemdahl P, et al. Factors associated with antithrombotic treatment decisions for stroke prevention in atrial fibrillation in the Stockholm region after the introduction of NOACs. Eur J Clin Pharmacol 2017;73:1315–22. 10.1007/s00228-017-2289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel PA, Zhao X, Fonarow GC, et al. Novel oral anticoagulant use among patients with atrial fibrillation hospitalized with ischemic stroke or transient ischemic attack. Circ Cardiovasc Qual Outcomes 2015;8:383–92. 10.1161/CIRCOUTCOMES.114.000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lauffenburger JC, Farley JF, Gehi AK, et al. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol 2015;115:1095–101. 10.1016/j.amjcard.2015.01.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boriani G, Proietti M, Laroche C, et al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. Europace 2018;20:747–57. 10.1093/europace/eux301 [DOI] [PubMed] [Google Scholar]
- 47. Maura G, Billionnet C, Alla F, et al. Comparison of treatment persistence with dabigatran or rivaroxaban versus Vitamin K antagonist oral anticoagulants in atrial fibrillation patients: a competing risk analysis in the french national health care databases. Pharmacotherapy 2018;38:6–18. 10.1002/phar.2046 [DOI] [PubMed] [Google Scholar]
- 48. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 49. Sørensen HT, Riis AH, Lash TL, et al. Statin use and risk of amyotrophic lateral sclerosis and other motor neuron disorders. Circ Cardiovasc Qual Outcomes 2010;3:413–7. 10.1161/CIRCOUTCOMES.110.936278 [DOI] [PubMed] [Google Scholar]
- 50. Başaran Ö, Dogan V, Beton O, et al. Suboptimal use of non-vitamin K antagonist oral anticoagulants: Results from the RAMSES study. Medicine 2016;95:e4672 10.1097/MD.0000000000004672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ono T, Kohsaka S, Takatsuki S, et al. Inconsistent dosing of non–Vitamin K oral anticoagulants. J Am Coll Cardiol 2017;70:118 10.1016/j.jacc.2017.03.609 [DOI] [PubMed] [Google Scholar]
- 52. Hsu JC, Akao M, Abe M, et al. International Collaborative Partnership for the Study of Atrial Fibrillation (INTERAF): rationale, design, and initial descriptives. J Am Heart Assoc 2016;5:e004037 10.1161/JAHA.116.004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okumura Y, Yokoyama K, Matsumoto N, et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: Findings from the SAKURA AF Registry. J Arrhythm 2017;33:289–96. 10.1016/j.joa.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Steinberg BA, Shrader P, Pieper K, et al. Frequency and outcomes of reduced dose Non-Vitamin K antagonist anticoagulants: results from ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). J Am Heart Assoc 2018;7:e007633 10.1161/JAHA.117.007633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pisters R, van Vugt SPG, Brouwer MA, et al. Real-life use of Rivaroxaban in the Netherlands: data from the Xarelto for Prevention of Stroke in Patients with Atrial Fibrillation (XANTUS) registry. Neth Heart J 2017;25:551–8. 10.1007/s12471-017-1009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yao X, Shah ND, Sangaralingham LR, et al. Non-Vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol 2017;69:2779–90. 10.1016/j.jacc.2017.03.600 [DOI] [PubMed] [Google Scholar]
- 57. Reilly PA, Lehr T, Haertter S, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients. J Am Coll Cardiol 2014;63:321–8. 10.1016/j.jacc.2013.07.104 [DOI] [PubMed] [Google Scholar]
- 58. Bouillon K, Bertrand M, Boudali L, et al. Short-term risk of bleeding during heparin bridging at initiation of Vitamin K antagonist therapy in more than 90 000 patients with nonvalvular atrial fibrillation managed in outpatient care. J Am Heart Assoc 2016;5:e004065 10.1161/JAHA.116.004065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang SH, Chou IJ, Yeh YH, et al. Association between use of Non-Vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 2017;318:1250 10.1001/jama.2017.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van de Werf F, Brueckmann M, Connolly SJ, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: the randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J 2012;163:931–7. 10.1016/j.ahj.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 61. Steffen M. Universalism, responsiveness, sustainability--regulating the French health care system. N Engl J Med 2016;374:401–5. 10.1056/NEJMp1504547 [DOI] [PubMed] [Google Scholar]
- 62. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation 2014;129:2005–12. 10.1161/CIRCULATIONAHA.114.008643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lafon T, Vallejo C, Hadj M, et al. Mésusage et iatrogénie des anticoagulants oraux directs (AOD): étude observationnelle dans le service des urgences du CHU de Limoges (Misuse and adverse effects of new direct oral anticoagulants: A prospective observational study in patients admitted to an emergency unit of a French university hospital). Thérapie 2018;73:209–15. 10.1016/j.therap.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 64. Pokorney SD, Simon DN, Thomas L, et al. Stability of international normalized ratios in patients taking long-term warfarin therapy. JAMA 2016;316:661–3. 10.1001/jama.2016.9356 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026645supp001.pdf (737.9KB, pdf)