Abstract
Obesity increases the risk of multiple gastrointestinal cancers and worsens disease outcomes. Conversely, strong inverse associations have emerged between physical activity and colon cancer and possibly other gastrointestinal malignancies. The effect of weight loss interventions — such as modifications of diet and/or physical activity or bariatric surgery — remains unclear in patients who are obese and have gastrointestinal cancer, although large clinical trials are underway. Human intervention studies have already shed light on potential mechanisms underlying the energy balance-cancer relationship, with preclinical models supporting emerging pathway effects. Central to interventions that reduce obesity or increase physical activity are pluripotent cancer-preventive effects (including reduced systemic and adipose tissue inflammation and angiogenesis, altered adipokine levels and improved insulin resistance) that directly interface with the hallmarks of cancer. Other mechanisms, such as DNA repair, oxidative stress and telomere length, immune function, effects on cancer stem cells and the microbiome, could also contribute to energy balance effects on gastrointestinal cancers. Although some mechanisms are well understood (for instance, systemic effects on inflammation and insulin signalling), other areas remain unclear. The current state of knowledge supports the need to better integrate mechanistic approaches with preclinical and human studies to develop effective, personalized diet and exercise interventions to reduce the burden of obesity on gastrointestinal cancer.
Chronic excessive caloric intake and physical inactivity lead to energy imbalance, which over time results in overweight and obesity development1,2. The rise in obesity constitutes a continuing global health epidemic and affects both developed and developing countries. In the USA alone, >70% of adults aged 20 years and over are considered overweight (BMI 25.0–29.9 kg/m2), with nearly 38% of that population categorized as obese (BMI ≥30 kg/m2)3. These rates have consistently increased since 1988 (REF3) and have prompted a plethora of studies investigating the causes, effects and prevention of the obesity epidemic. An expert panel convened by the International Agency for Research on Cancer (IARC) has concluded based on evidence from epidemiological and translational studies that 16 types of cancer are now probably or convincingly associated with excessive body weight, making obesity the second leading cause of cancer after smoking4 (TABLE 1). The World Cancer Research Fund (WCRF) reaches similar conclusions about obesity as a risk factor for a multitude of cancer types5, including cancers of the gastrointestinal tract (including colorectal, oesophageal, hepatic, pancreatic and possibly gastric cancers). In addition, physical activity — a complex behaviour in which bodily movement produced by skeletal muscle contraction increases energy expenditure — ‘consistently’ and ‘convincingly’ (per WCRF rating guidelines) reduces the risk of colon cancer by an estimated 30%6. Although increased physical activity alone is insufficient to achieve substantial weight loss, it is a central factor in weight maintenance and an integral component of energy balance7. In addition, physical activity seems to directly affect some cancer-preventive pathways independent of weight loss, as detailed later. However, it has been a challenge to increase physical activity levels in the general population8,9. In the absence of effective interventions that reduce obesity on a population level, it is of utmost importance to understand the biological mechanisms that underlie the associations between obesity and cancer. Elucidating factors that mediate the energy balance-cancer link increases the ability to alter this association in meaningful ways. The targeting of genetic factors mediating this link facilitates the effective design and optimal delivery of pharmacological interventions. Further, understanding these mechanisms also enables tailored interventions to reduce or eliminate the factors that most strongly drive the association between energy balance and gastrointestinal carcinogenesis.
Table 1 |.
Level of evidence for obesity and physical activity as risk factors for gastrointestinal cancer
| Cancer site | WCRFa |
IARCb |
||
|---|---|---|---|---|
| Level of evidence | Risk | Level of evidence | Risk | |
|
Obesity | ||||
| Colorectum | Strong convincing | Increased | Sufficient | Increased |
| Liver | Strong convincing | Increased | Sufficient | Increased |
| Oesophagus | Strong convincing | Increased | Sufficient | Increased |
| Pancreas | Strong convincing | Increased | Sufficient | Increased |
| Stomach | • Strong probable (non-cardia) | • Increased (non-cardia) | Sufficientc | Increasedc |
| • Limited (cardia) | • No conclusion (cardia) | |||
|
Physical activity | ||||
| Colorectum | Strong convincing | Decreased | NA | NA |
| Liver | Limited-suggestive | Decreased | NA | NA |
| Oesophagus | Limited-suggestive | Decreased | NA | NA |
| Pancreas | Limited | No conclusion | NA | NA |
| Stomach | Limited | No conclusion | NA | NA |
IARC, International Agency for Research on Cancer; NA, not available; WCRF, World Cancer Research Fund.
Definitions by the WCRF are as follows: strong convincing evidence is defined as judgement of a convincing causal relationship, which is highly unlikely to be modified in the near future as new evidence accumulates; strong probable evidence is defined as a judgement of a probable causal relationship, which would generally justify goals and recommendations designed to reduce the incidence of a disease; limited-suggestive evidence indicates that there is too limited evidence to allow a judgement of a probable or a convincing causal relationship, but there is evidence suggestive of a direction of effect; limited evidence indicates that there is insufficient evidence to allow a firm conclusion.
Sufficient evidence (as defined by the IARC Working Group on Energy Balance and Cancer) indicates the presence of a preventive relationship in humans between the absence of body fatness and the risk of cancer that has been observed in studies where chance, bias and confounding factors could be ruled out with confidence.
IARC assessments do not distinguish between cardia and non-cardia stomach cancer.
As part of this Review (which complements the Review by Gunter et al.10, focusing on the epidemiological evidence of the obesity-cancer link), we describe the results of energy balance interventions on gastrointestinal cancer risk and on clinical end points among patients with gastrointestinal cancer — specifically cancers of the colon, rectum, oesophagus, stomach, liver and pancreas (we do not provide information on gallbladder cancer but would like to highlight the need for intervention studies with respect to this cancer type, as to date, there is very limited evidence11). Subsequently, we focus on the results from human energy balance interventions, specifically with physical activity or weight loss. These studies help establish a mechanistic understanding from translational and clinical perspectives by testing the effects of energy balance interventions on biomarkers related to the hallmarks of cancer. Finally, we provide evidence from preclinical studies, as available.
Interventions for cancer prevention
Chronic positive energy balance resulting in obesity can increase the risk of 16 types of cancer, including cancers of the colorectum, oesophagus, liver, pancreas and possibly stomach2. Health behaviour interventions, such as dietary and exercise approaches to maintain or lose weight, seek to improve health and probably concurrently reduce obesity-driven gastrointestinal tumour development and progression. Exercise is defined as a subset of physical activity that is structured, repetitive and planned, with an objective to improve or maintain physical fitness. Although well-designed physical activity interventions have investigated biological mechanisms, no large-scale physical activity trials with the end point of gastrointestinal cancer incidence or adenoma recurrence (as a surrogate measure) have been undertaken to date.
The effects of a dietary intervention on cancer risk were tested in the Women’s Health Initiative (WHI) Dietary Modification (WHI-DM) trial, a randomized controlled trial (RCT) of 48,835 postmenopausal women aged 50–79 years in the USA, in which a dietary intervention sought to reduce total fat intake and increase intake of grains, vegetables and fruits12. Women enrolled in the intervention group lost a mean of 2.2 kg of weight in the first year and maintained lower weight during 7.5 years of follow-up than women in the control group13. Although low-fat dietary intake did not reduce long-term colorectal cancer risk among participants in the intervention versus control group (HR 1.08, 95% CI 0.90–1.29)12, findings published in 2018 show that the low-fat dietary intervention led to reduced incidence of pancreatic cancer among individuals who were overweight or obese (HR 0.71, 95% CI 0.53–0.96)14. Observed discrepancies between cancer types might be explained by the limited achieved weight loss (mean 2.2 kg) among members of the study population over a long study period. Further, participants were restricted to postmenopausal women (50–79 years old), a study population with altered hormonal status and older age. Colorectal cancer develops over multiple decades, and thus, an intervention after menopause will probably have limited effects.
Clinical trials among individuals at high risk of gastrointestinal cancer, including individuals who are obese or those diagnosed with Barrett oesophagus, have reported results in the past few years15,16. For example, the effect of an exercise intervention on biomarkers of oesophageal cancer risk was evaluated in the Exercise and the Prevention of Oesophageal Cancer (EPOC) study17. This exploratory trial with a 6-month aerobic and resistance exercise intervention (60 minutes for 5 days per week) versus a control group (45 minutes stretching for 5 days per week) was conducted in 33 men with Barrett oesophagus who were inactive and overweight or obese18. Results from this study reported that the intervention reduced waist circumference (−4.5 cm; P < 0.01), but there were no statistically significant effects on relevant biomarkers of oesophageal cancer risk (such as leptin, adiponectin, IL-6 and C-reactive protein (CRP)) between groups. Probable explanations for this lack of effect include the brevity of the intervention and the exploratory nature of the study, with a small sample size and limited statistical power. The bodyweight and physical activity intervention (BeWEL) trial tested the effect of a combined exercise and dietary intervention among 329 adults diagnosed with a histopathologically confirmed colorectal adenoma who were overweight or obese, and the study demonstrated substantial weight loss after 12 months (P < 0.001)19. Although outcome measures did not include risk of colorectal cancer, these types of studies illustrate the potential for energy balance interventions targeted at high-risk populations to affect future cancer risk19.
Surgical interventions, another class of energy balance interventions, can not only reduce body weight but also alter biomarkers of cancer risk20. Observational studies have evaluated patterns of cancer incidence after bariatric surgery. For example, a comparison of 6,596 patients who were severely obese (BMI ≥35 kg/m2) and underwent Roux-en-Y gastric bypass surgery matched (by gender, age and BMI) to 9,442 individuals who did not undergo surgery reported a decrease in total cancer incidence among the surgical group compared with the no surgery group, which was primarily as a result of decreased incidence of regional to distant stage cancers (HR 0.76, 95% CI 0.65–0.89; P = 0.0006)21. In particular, incidence of several obesity-related cancers (including cancers of the oesophagus, pancreas and liver) was found to be 38% lower in the gastric bypass group relative to those in the control group (HR 0.62, 95% CI 0.49–0.78; P < 0.0001)21. Not all cancers are equally affected by bariatric surgeries, as one study suggests that bariatric surgery is associated with an increase in colorectal cancer risk over time22. A population-based cohort study among individuals with an obesity diagnosis showed a worse rectal cancer prognosis among those who had undergone bariatric surgery; however, weaknesses of the study include the limited sample size (n = 131 patients who were obese and underwent bariatric surgery with colorectal cancer) and the lack of adjustment for tumour stage23. Another emerging issue is that alterations in gastrointestinal anatomy resulting from bariatric surgery can lead to different presentation of symptoms, thus making diagnosis of cancer more challenging24.
In addition to strategies that aim to modify gastrointestinal cancer risk, clinical trials that evaluate specific mechanisms of gastrointestinal cancer risk have been conducted or launched. For example, as referred to later, the A Program Promoting Exercise and an Active Lifestyle (APPEAL) study tested the effects of an exercise intervention on proliferation, apoptosis and prostaglandin levels in the rectal mucosa25–27. Of interest is also the ongoing Microbiome, Exercise Tracking Study (METS) (NCT02780284), which seeks to assess the effect of physical activity interventions on the gut microbiome among 20 individuals at high risk of colorectal cancer. Overall, it is challenging to conduct clinical trials for cancer prevention, as generally, they need to be of a very long duration, are expensive and might need to be targeted to populations with early-stage carcinogenesis. For example, the dietary modification used in the WHI trial showed some, albeit limited, efficacy and was targeted at an older population, in which there might be limited success in disrupting ongoing cancer development. Interventions in high-risk groups (such as those with Barrett oesophagus) are underway yet need to be expanded to achieve sufficient statistical power. Trials testing the effects of bariatric surgeries provide an important line of evidence; however, the studies to date have had major limitations in their study design. Thus, clinical trials that investigate the role of energy balance interventions on obesity-driven cancers of the gastrointestinal tract are still needed to better understand the full potential of dietary and/or exercise weight loss interventions on gastrointestinal cancer prevention.
Interventions after cancer diagnosis
Energy balance interventions, such as caloric restriction or exercise, have demonstrated benefits both during and after treatment for patients with gastrointestinal cancer28–34. The importance of physical activity and diet after colorectal cancer diagnosis has been reviewed by Van Blarigan and Meyerhardt35. Although no RCT has been completed to date, there is consistent epidemiological evidence among survivors of colorectal cancer that physical activity substantially decreases risk of mortality by ~50%35. In addition, physical activity before and after diagnosis consistently and convincingly reduces the risk of colon cancer-specific mortality by an estimated 25% and 26%, respectively36. A large RCT is currently underway to test whether these effects are causative or possibly attributable to other confounding factors (NCT00819208)37. Highlights of ongoing and completed RCTs that seek to test the effects of intentional weight loss via diet and/or exercise interventions on clinical end points among patients with colorectal, oesophageal, gastric or pancreatic cancer are summarized in TABLE 2 (a more detailed table is provided in Supplementary table 1). Particularly important in the cancer setting is the distinction between intentional weight loss and unintentional weight loss, as the latter is generally associated with disease progression. With regard to unintentional weight loss, a study published in 2014 demonstrated that >10% body mass lost before oesophagectomy was associated with worse survival outcomes among patients with oesophageal cancer38. Similarly, postoperative unintentional weight loss (≥15%) among patients diagnosed with stage II or stage III gastric cancer was associated with decreased overall survival outcomes39. These studies underscore the need to understand the causes of weight loss in studies of clinical outcomes in patients with cancer.
Table 2 |.
Selected randomized trials with energy balance interventions
| Study name (status) | Study population | Intervention groups | Intervention time | Outcome measures | ClimcalTrials gov identifie |
|---|---|---|---|---|---|
| CHALLENGE (ongoing) | 962 patients who had survived stage II/III colon cancer | • Health education promoting physical activity and healthy nutrition • Health education promoting physical activity and healthy nutrition plus an exercise guidebook and physical activity consultations |
3 years | • Primary outcome: 10-year disease free survival • Secondary outcomes: 10-year overall survival (and others) |
NCT00819208 |
| PERFECT (ongoing) | 150 patients with oesophageal cancer undergoing surgery | • Supervised aerobic and resistance exercise programme • Usual care |
12 weeks | • Primary outcome: quality of life • Secondary outcomes: recurrence and survival (and others) |
NTR5045 |
| PREHAB (ongoing) | 120 patients with gastro-oesophageal cancer undergoing perioperative chemotherapy | • Supervised total-body exercise up to 1 hour ≥3 days per week for 18 sessions • Usual care |
3 months | • Primary outcome: complete oncological treatment • Secondary outcomes: disease-free survival, overall survival, 30-day and 90-day mortality |
NCT02780921 |
| PancFit (ongoing) | 128 patients with pancreatic cancer undergoing preoperative therapy (chemotherapy, radiation or chemoradiation) | • Multi-modal exercise (aerobic and strength) and nutrition programme, 3–6 weeks and 3–7 months post-surgery • Usual care |
7 months | • Primary outcome measure: 6-minute walk test | NCT03187951 |
| EXERT (ongoing) | 60 patients with rectal cancer undergoing neoadjuvant chemotherapy followed by total mesorectal excision | • 3 supervised high-intensity aerobic interval training sessions during chemotherapy and ≥150 min per week of unsupervised aerobic exercise • Usual care |
12 weeks | • Primary outcome: cardiorespiratory fitness • Secondary outcomes: functional fitness, quality of life, symptom management |
NCT03082495 |
| SUPPORT (ongoing) | 65 patients with pancreatic cancer | • Progressive resistance training • Home-based resistance training • Usual care |
6 months | • Primary outcomes: cardiorespiratory fitness, muscle strength | NCT01977066 |
| RENEW (completed) | 641 patients who had survived colorectal, breast or prostate cancer (91 colorectal) | • Telephone counselling and mailed material-based diet plan plus exercise intervention • Wait-listed for 12 months (control) |
12 months | • Intervention group: PSF36 mean change −2.15 ± 0.9 • Control group: PSF36 mean change −4.84 ±0.9 |
NCT00303875 |
| Effects of a “Walk, Eat, & Breathe” Nursing Intervention For Patients With Esophageal Cancer: A Randomized Controlled Trial (completed) | 59 patients with oesophageal cancer, stage IIb or higher, undergoing neoadjuvant chemoradiotherapy | • Walk-and-eat intervention • Usual care |
4–5 weeks | • Intervention group: 6-minute walk test mean change −18.0 m ± 75.3 m • Usual care group: 6-minute walk test mean change −118.0 m ± 160.6 m |
NCT02850172 |
PSF36, Physical Functioning Scale of the Short-Form 36.
Patients with gastrointestinal cancer can experience substantial treatment-related toxicities and deleterious sequelae (such as cancer-related fatigue, cachexia, functional decline and decreased quality of life (QOL)) when undergoing surgery, chemotherapy, radiation or other treatment modalities32. However, consistent evidence has demonstrated the positive benefits of exercise and weight loss interventions on patient treatment and clinical outcomes. A systematic review and meta-analysis analysed the efficacy of resistance training among individuals who had survived cancer and concluded that clinical benefits on body composition and muscular function were positively associated with exercise interventions32. The analysis further showed a marginally significant (P = 0.05) effect on reduced cancer-related fatigue measured by the Functional Assessment of Cancer Therapy (FACT) index32. Similar improvements in muscle strength and endurance capacities were among the clinical benefits associated with exercise training during colorectal cancer treatment40 and with survivors of metastatic cancer28,33. In a pilot RCT of 59 patients with oesophageal cancer undergoing neo-adjuvant chemoradiotherapy, walk-and-eat interventions — supervised walking sessions three times per week and weekly nutrition advice — preserved functional walking capacity and nutritional status29. Moreover, individuals in the intervention group reported greater weight loss and improvements in physical function, QOL, dietary behaviours and levels of physical activity than those in the control group29. For 102 patients with resected pancreatic cancer, RCT results demonstrated that participation in a home-based walking programme for 6 months conferred improvements in fatigue and pain scores, as well as overall physical functioning and health-related QOL, compared with the usual care control group30. No differences in overall survival were reported30. Clinical trials exploring the effect of intentional weight loss or physical activity interventions in patients diagnosed with primary gastric and/or oesophageal tumours who underwent surgery with curative intent (for example, the Physical Exercise Following Esophageal Cancer Treatment (PERFECT) study; NTR5045)41 are underway but have not yet been published.
Lifestyle interventions might positively affect postoperative recovery and length of hospital stay for patients with gastrointestinal cancer. A postoperative inpatient exercise study demonstrated that twice daily exercise interventions (including stretching, balance, core and low-intensity resistance exercises) reduced the length of hospital stay for patients with colon cancer in the intervention group (7.82 ± 1.07 days) compared with those who received usual care (9.86 ± 2.66 days)42. In particular, patients with high BMI and the metabolic syndrome are at increased risk of postoperative complications. The Adjuvant Exercise for General Elective Surgery (AEGES) prospective case-control study sought to test the effectiveness of an exercise programme in 72 patients with stage I gastric cancer who were diagnosed with the metabolic syndrome34. Cho and colleagues34 reported that patients who participated in a preoperative exercise programme were less likely than patients who did not participate in the programme to experience post-surgical complications (1 patient versus 22 patients; P = 0.008) and had shorter hospital stays (median 9 days versus 10 days; P = 0.038).
Overall, although several studies among patients with gastrointestinal cancer have demonstrated positive associations between energy balance interventions during and/or after treatment and physical function, QOL and other patient-reported outcomes (TABLE 2; Supplementary table 1), there is a dearth of RCTs evaluating these interventions in relation to patient survival and recurrence of disease. Clinical trials are needed to further establish effect size and causality. The ongoing Colon Health and Life-Long Exercise Change (CHALLENGE) trial (NCT00819208)37 compares a combined physical activity and general health education programme with a general health education programme only and is expected to provide important answers. Initial results of the programme on physical activity and fitness have been reported and show a substantial increase in recreational physical activity levels and improvement in fitness 1 year after enrolment began43. Results from the overall trial are anticipated in 2029. Further, clinical trials among patients with gastrointestinal cancer that evaluate intervention effects on physical functioning, QoL, cancer-related fatigue and cachexia, among other clinical end points, are needed to better tailor interventions and help create the evidence base for linking energy balance interventions to improved clinical outcomes. Given the rising number of cancer survivors worldwide, there is an increasing need to better address the effect of weight loss interventions in long-term cancer survivorship studies. With regards to bariatric surgery, research studies that focus on the effect of surgical procedures on cancer-specific outcomes, such as survival and recurrence, are warranted.
Biological mechanisms
How the biological mechanisms of interventional approaches, such as intentional weight loss, physical activity, diet, exercise and bariatric surgery, modulate gastrointestinal tumorigenesis is not entirely understood. However, over the past decades, a substantial body of work has defined components that are clear mediators of the energy balance-cancer link, and many of these mediators interact with one or more of the cancer hallmarks44 (FIG. 1). Particularly informative are clinical trials that independently and jointly test the effects of caloric restriction and physical activity (for example, the Nutrition and Exercise in Women (NEW) trial, NCT00470119 (REF45); and the Sex Hormones and Physical Exercise 2 (SHAPE-2) study, NCT01511276 (REF46)). In addition to clinical trials and evidence from nonrandomized intervention studies, mechanistic-based and intervention-based studies in animal models have also provided invaluable insight into the mediators underlying the obesity and gastrointestinal cancer link. Preclinical studies have leveraged experimental animal models of cancer, including genetically engineered mouse and rat models, carcinogen-induced or orthotopically transplanted models and, more recently, patient-derived xenograft models, to elucidate the effects of disrupting the obesity-gastrointestinal cancer link via caloric restriction and exercise interventions47.
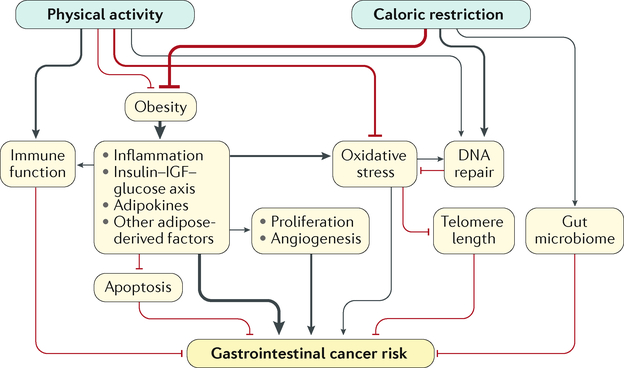
Fig. 1 |. Mechanisms underlying the association between energy balance and obesity-related gastrointestinal cancers.
Line thickness corresponds to the current evidence for the strength of the effect or association. Black lines represent positive or enhancing effects, and red lines represent negative or inhibiting effects. IGF, insulin-like growth factor.
One consideration in the exploration of mechanisms is that malignancies of the gastrointestinal tract arise from distinct tissue origins and therefore represent a diverse set of diseases. However, as molecular subtyping and metabolic characterization of these cancers have advanced, several common as well as tissue-specific features have emerged. These findings inform our collective understanding of the effect of obesity on these cancers and could reveal potential common targets for prevention or treatment. For example, each organ-specific form of gastrointestinal cancer seems to have subtypes that reflect mesenchymal gene signatures, immune cell infiltration or metabolic dysregulation, with additional gene signatures reflective of the epithelial tissue of origin within each distinct subtype (REF48). Likewise, some metabolic features are shared among malignancies of the gastrointestinal tract, whereas others are distinct to the tissue of origin. For instance, increased uptake of glucose and the conversion of glucose to lactate is a common feature of nearly all gastrointestinal cancers despite their differences in epithelial tissue of origin or oncogenic drivers49. However, pancreatic tumours exhibit a unique dependency on metabolic pathways for branched-chain amino acids50. Finally, studies of colorectal cancer also show that the carcinogenesis-promoting effect of excess energy balance might differ between different tumour subtypes, with findings suggesting that a high BMI has a stronger influence on tumour subtypes that maintain better intestinal differentiation51. Together, these features have informed our collective understanding of the effect of obesity on these cancers and might also reveal potential common targets for cancer prevention or control.
Tumour-promoting inflammation
Inflammation is considered an enabling characteristic for promoting tumour progression, as nearly all malignant lesions of the gastrointestinal tract contain an immune cell infiltrate44. Consistent with this notion, biomarkers of inflammation have been associated with obesity and gastrointestinal cancer in a large number of epidemiological, clinical and preclinical studies52–54. In addition, as described here, evidence from humans and mice supports the premise that intentional weight loss via behavioural interventions, bariatric surgery or pharmacological approaches can reverse some of the obesity-associated changes in certain inflammatory factors, particularly circulating levels of CRP — the most commonly reported inflammatory marker that changes with weight loss. However, it is also clear that the underlying causes, the cellular contributors and the molecular and metabolic factors involved in the obesity-inflammation-gastrointestinal cancer triad are extremely complex.
Adipose tissue has a central role in inflammatory processes. The increased white adipose tissue (WAT) mass (BOX 1) associated with obesity drives chronic inflammation through at least three established and interacting mechanisms: altered production of secreted inflammatory factors from adipose and other tissues; increased tissue inflammation as measured by crownlike structures and other measures of immune cell infiltration; and adipose tissue remodelling. Results of the effects of interventions modulating these processes are discussed here.
Box 1 |. Adipose tissue compartments and their role in disease.
The components of adipose tissue are specific to the species, developmental stage and anatomic location. Broadly, adipose tissue compartments can be classified into white adipose tissue (WAT), brown adipose tissue (BAT) and beige (or brite) adipose tissue.
BAT and beige adipose tissue
Thermogenesis is a central metabolic function of these tissues
BAT functions as a defence against obesity and obesity-associated disorders, such as type 2 diabetes
WAT
WAT is the key adipose tissue compartment with respect to energy storage
Increased WAT mass (obesity) perturbs the immune balance of adipose tissue and increases chronic systemic inflammation
WAT can be further subdivided into visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT)
VAT and SAT
VAT is bioenergetically and lipolytically more active than SAT owing to higher mitochondrial density and activity, particularly among individuals who are overweight or obese
Disease risk and prognosis is most strongly correlated with WAT and/or VAT across multiple tumour types, including cancers of the oesophagus and colon
VAT and SAT differ by cellular structure, molecular composition and secretome
VAT and SAT have differential effects on gastrointestinal cancer risk because of their distinct body compartment localization
Circulating biomarkers of inflammation
Dietary and exercise interventions can disrupt the energy balance–cancer association and potentially decrease circulating levels of inflammatory biomarkers as a cancer-preventive approach55. Human studies have contributed largely by testing effects on circulating levels of CRP, IL-6 or other established and stable biomarkers of inflammation25,56–63. Initially, a 12-month moderate-to-vigorous aerobic exercise intervention conducted among 202 sedentary individuals by Campbell and colleagues reported no changes in CRP levels56. By contrast, a subsequent RCT among 115 postmenopausal women found that exercise interventions yielded an increased reduction in CRP levels relative to controls57. Improved CRP levels were also reported for women who were overweight or obese enrolled in a dietary weight loss intervention programme58. In the most informative trial, Imayama et al. sought to investigate the effects of both exercise and/or a caloric restriction weight loss diet on inflammatory markers among 439 postmenopausal women who were overweight or obese enrolled in the Nutrition and Exercise for Women (NEW) trial45,59. Both dietary and dietary plus exercise interventions led to substantially decreased levels of CRP, serum amyloid A protein (SAA) and IL-6 and a decreased neutrophil count relative to control subjects59. For the combined diet and exercise intervention, a 41.7% reduction in CRP was achieved — a decline similar to or even stronger than effects induced by anti-inflammatory pharmacological agents (such as NSAIDs)59. Changes in inflammatory markers were consistently seen with greater weight loss, independent of the study arm.
Results from a randomized dose comparison trial of an exercise intervention in 400 healthy postmenopausal women who were inactive did not find differences in circulating inflammatory biomarkers between the two different doses in the exercise arms (300 minutes per week versus 150 minutes per week)60. A further post hoc analysis showed a 22% reduction in CRP levels among participants with a self-selected exercise volume of >245 minutes per week versus those exercising <110 minutes per week (P = 0.04)60. Additional analyses restricted to exercise time in the target heart rate zone noted similar trends for CRP and IL-6 blood levels60. In accordance with these results, findings from the SHAPE-2 study46 demonstrated that the exercise intervention decreased CRP levels in women (treatment effect ratio (TER) 0.59) compared with the diet intervention (TER 0.77) or controls63.
IL-6 and TNF are potent inducers of pro-inflammatory and pro-tumorigenic cyclooxygenase 2 (COX2; also known as PTGS2) expression, leading to the production of prostaglandin E2 (PGE2)62. Consequently, COX2 upregulation in tumours is associated with poor clinical outcomes across multiple obesity-driven gastrointestinal cancers61,64,65. Given this clinical significance and the potential protective effects of exercise on the colon via modulation of prostaglandin production, a year-long RCT evaluated the effects of aerobic exercise intervention among 185 healthy participants25. Results from the APPEAL study showed no changes in prostaglandin concentrations (PGE2 and PGF2α) directly in the colonic mucosa with the intervention; however, the assay to measure prostaglandin levels was technically challenging25.
In addition to caloric restriction and exercise interventions, the literature suggests that surgical weight loss methods can consistently and strongly reduce levels of systemic inflammatory biomarkers66–69. Taken together, the results from these clinical and preclinical studies55,70,71 provide proof of principle that weight loss interventions following chronic obesity can modulate tumour-promoting systemic inflammation.
Adipose tissue tumour microenvironment
The interactions between an evolving tumour and its microenvironment, such as adipose tissue, are known to involve a complex interplay among multiple cells, local and systemic secreted mediators and other components72–74. Cross-sectional studies have investigated the adipose tumour microenvironment, its effects on tumour progression and characteristics in patients with cancer who are overweight or obese74. Adipose-driven processes, including increased inflammation and the production and secretion of adipokines, are hypothesized to alter cancer cell metabolism and affect the cancer-promoting processes of invasion, metastasis and immune clearance74. The role of adipose tissue as an organ that drives carcinogenesis in a paracrine, as well as endocrine, function is just beginning to be untangled. The importance of adipose tissue in cancer development and progression depends on its type and related function (FIG. 2). Visceral adipose tissue (VAT) is generally more bioenergetically active than subcutaneous adipose tissue (SAT), and obesity-driven excess of VAT has been associated with an increased risk of several gastrointestinal cancers74 (BOX 1).
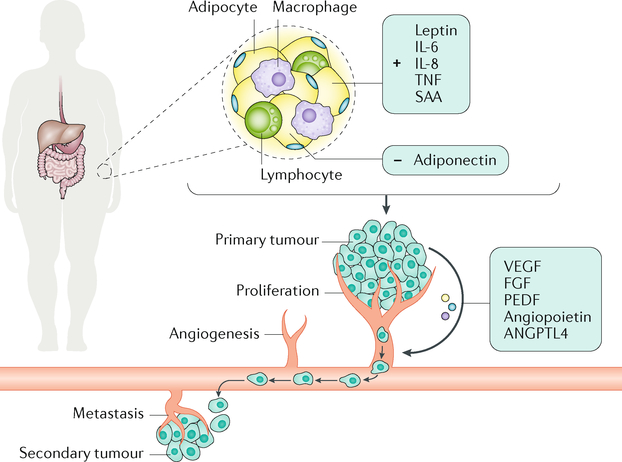
Fig. 2 |. Adipokines and cytokines secreted by adipose tissue and their effects on cancer-related processes.
The increased secretion of adipokines (leptin and adiponectin) and cytokines (C-reactive protein, TNF, IL-6, IL-8 and serum amyloid A protein (SAA)) driven by adipose tissue in obesity can alter the expression and secretion of factors positioned by the primary tumour (vascular endothelial growth factor (VEGF), pigment epithelium-derived factor (PEDF), angiopoietin-related protein 4 (ANGPTL4) and fibroblast growth factor (FGF)) to promote tumour proliferation, invasion, angiogenesis and metastasis.
Energy balance interventions have been increasingly focused on investigating the effects of either weight loss or exercise directly on abdominal SAT as a target tissue75. A review published in 2017 summarized the result of 19 studies: eight modified dietary intake, five altered exercise levels, and six studies tested a combination of both types of intervention75. Changes in SAT were most prevalent among studies testing dietary weight loss75. The most commonly observed changes were reductions in gene expression levels of leptin (which can be considered a positive control, as leptin levels are positively linked to fat mass)76, TNF and IL-6. Simultaneously, adiponectin expression was increased75. For exercise-only studies, there were limited changes in SAT gene expression75. An ongoing study is testing the effects of different types of dietary weight loss (continuous caloric restriction versus intermittent fasting) on SAT gene expression among individuals who are overweight (HELENA trial; NCT02449148)77.
An intervention study evaluated RNA biomarkers of brown-like adipocyte (brite, or beige) cellular formation from subcutaneous WAT biopsy samples from 79 women who were overweight or obese and demonstrated that caloric restriction and weight loss were associated with SAT remodelling towards a whiter adipocyte phenotype78. In one of the first studies among cancer survivors (n = 39 individuals who had survived non-metastatic colon cancer), Brown et al.79 observed that aerobic exercise led to a dose-response reduction in VAT levels, an emerging driver of tumorigenesis. Adipose tissue gene expression was not evaluated.
Overall, a clear picture emerges that inflammation is a central pathway driving gastrointestinal carcinogenesis and that energy balance interventions, particularly those that result in weight loss or exercise at the target rate, can have a substantial positive effect on an individual’s inflammatory state. Thus, this pathway has been central to elucidating clear mediators of the energy balance-cancer link (FIG. 1). Adipose tissue is emerging as a key player with biological responsiveness to weight loss, and it will be important to discover whether both the paracrine and endocrine functions of adipose tissue are drivers in gastrointestinal carcinogenesis74.
Adipokines and steroid hormones
Obesity-driven dysregulation of adipose tissue results in adverse interactions between various adipokines and sex and steroid hormones. Although the effects of diet and/or exercise interventions on hormonal regulation have been well studied in sex-specific cancers (such as tumours of the breast and prostate), few studies have investigated these effects in gastrointestinal cancers80–82.
Adipokines are adipose-derived peptide hormones (such as leptin or adiponectin) and cytokines (such as IL-6 or TNF)83. Leptin regulates appetite, food intake and body weight76. The development of obesity seems to lead to leptin resistance in peripheral tissues that is similar to insulin resistance in type 2 diabetes, which then promotes appetite and increases food intake76. Adiponectin acts as the counterpart of leptin and plays an important role in energy homeostasis and lipid and glucose metabolism76. For example, the genetic ablation of adiponectin in mice can increase apoptosis, whereas the overproduction of adiponectin can decrease caspase 8-mediated cell death84. In addition to promoting cell survival, systemic effects of adiponectin include decreasing inflammation and promoting insulin sensitivity84. Obesity-related dysregulation of adipokines has been shown to promote tumour development, progression, angiogenesis and metastasis85. Although leptin seems to promote tumour-related processes, including those brought about by the production of reactive oxygen species (discussed later) and altered immune and inflammatory responses, adiponectin seems to inhibit these same pathways and therefore prevents cancer development and progression85 (FIG. 2). The secretion of adipokines and cytokines by adipose tissue creates a paracrine loop between adipocytes and inflammatory cells (such as macrophages and lymphocytes) that contributes to obesity-related, chronic low-grade inflammation (described earlier). In patients with colorectal cancer, leptin has been shown to promote mitogenic and anti-apoptotic pathways that are involved in carcinogenic processes, although a clear picture of the mechanistic role of leptin in tumour angiogenesis has not been fully established83. The protective role of adiponectin might be exerted either by directly affecting signalling pathways involved in cancer cell growth and proliferation or by indirectly regulating whole body insulin sensitivity through altered hormone and cytokine levels83.
Several studies have investigated the effect of weight loss interventions on circulating levels of adipokines, particularly leptin86–92. In the NEW trial, circulating levels of adiponectin increased by 9.5% in the diet group (P ≤ 0.0001) and 6.6.% in the combined diet and exercise group (P ≤ 0.0001) versus the control group86. Relative to the control group, all intervention groups experienced substantial decreases in circulating leptin levels86. For adiponectin, these results are consistent with another intervention study that compared the effects of caloric restriction only with an exercise plus caloric restriction intervention92. After 20 weeks, the two intervention groups had different changes in levels of plasma adiponectin (P = 0.014). Plasma adiponectin levels were significantly increased (P = 0.0001) among the 48 participants in the combined intervention group but were unchanged among the 22 individuals in the caloric restriction-only group92. Overall, studies have consistently shown that adiponectin is inversely associated, and leptin is directly associated, with the degree of weight or fat mass loss86,87,89,90. Systemic changes in adipokine levels as a result of weight loss interventions among patients with gastrointestinal cancer might be linked to cancer outcomes. Direct evidence testing these effects is being generated in clinical trials.
Limited research is available on the effect of weight loss interventions on the expression of adipokines in adipose tissue75. Studies in individuals who are obese have shown that diet-only interventions lead to reduced leptin expression with weight loss in SAT93–96. With regard to exercise-only interventions, a mixed aerobic and resistance intervention surprisingly led to an increased expression of leptin compared with decreased leptin expression in the control group97 or the aerobic-only group93. No changes in leptin expression levels were reported in a study of aerobic exercise94 or another study of resistance exercise only98. Overall, most results indicate that diet-only, exercise-only and combined diet plus exercise interventions trigger increased circulating levels of adiponectin75.
As demonstrated by Sjostrom and colleagues in studies of Swedish individuals who were obese, bariatric surgery is associated with reduced overall cancer mortality and has a sustained effect on circulating adipokines99,100. Other studies have corroborated this evidence that bariatric surgery can decrease circulating leptin levels and correspondingly increase levels of circulating adiponectin101,102. However, exploratory studies that have evaluated the effect of bariatric surgery on adipose tissue gene expression have yielded inconsistent findings103,104. Results from these studies have demonstrated either increases in adiponectin expression levels in SAT 2 weeks after surgery (P = 0.007)104 or have reported no statistically significant changes in adiponectin expression in SAT (P = 0.069)103. Inconsistent results might be due to the inclusion of patients undergoing different types of bariatric surgery (such as gastric sleeve versus laparoscopic band surgery), heterogeneity among patients in their weight loss and adipose tissue distribution following the procedure and differences in sample collection procedures105. In addition, although some patients undergoing bariatric surgery experience a substantial overall weight reduction, this effect does not necessarily correspond to loss of excess fat from adipose tissue compartments.
Sex hormones, a subgroup of steroid hormones, have been reported to modify the risk of several cancers, particularly sex-specific tumour types. Interestingly, for colorectal cancer, oestrogen has been consistently shown to be antitumorigenic, particularly in postmenopausal women106–109. Data from 16,808 postmenopausal women enrolled in the WHI clinical trial showed that combined oestrogen-progestin hormonal therapy significantly reduced the incidence of colorectal cancer by 44% (P = 0.014)109,110. Results for gastric cancer are inconclusive but tend to show that oestrogens might also decrease gastric cancer risk111. At the same time, there is limited evidence from clinical trials that sex hormones (such as oestrogen, progestin or testosterone) have a role in the development of other gastrointestinal cancers, including cancers of the oesophagus, pancreas or liver112–115; a possible explanation is that these cancer types occur predominantly in men.
Multiple studies have shown that intentional weight loss reduces sex hormone levels, including oestrone, oestradiol, androstenedione and dihydrotestosterone116–118, and increases sex hormone-binding globulin levels119–121. These studies have been predominantly conducted in cohorts of postmenopausal women. However, considering that oestrogen is preventive against colorectal cancer, this mechanistic pathway is unlikely to have a role in explaining an increased risk of obesity-driven colorectal cancer or the reverse protective association with physical activity and is not further considered here.
Experimental studies in rodent models of gastrointestinal cancer report inconsistent results, as data show both inhibition and promotion of cancer cell growth with hormonal therapies122–124. Findings from a mouse model published in 2017 suggest that individual differences in oestrogen receptor-α (ERα; also known as ESR1) and ERβ expression affect whether oestradiol has proinflammatory or anti-inflammatory effects in the colon, with this effect also probably occurring in other gastrointestinal tract tissues123. The strain of mouse or rat used and their underlying genetic differences in sex hormone metabolism might also contribute importantly to the observed differences in response to hormonal therapies124.
Obesity-driven metabolic reprogramming
The deregulation of cellular energetics is an emerging hallmark of cancer. Reduced dependence on mitochondrial respiration and increased aerobic glycolysis to generate substrate for the demands of rapid cell proliferation are common characteristics of gastrointestinal cancer cells49. Associated with this shift, which is termed the Warburg effect, is also an increased dependence on adequate systemic levels of glucose and other nutrients, similar to obesity-driven metabolic reprogramming49. Thus, the systemic metabolic alterations that result from obesity or energy balance-related interventions, such as caloric restriction or exercise, can influence cancer cell-intrinsic metabolism. Findings also suggest concomitant metabolic changes in stromal cells in the microenvironment, particularly cancer-associated fibroblasts, that contribute energy-rich fuels (such as pyruvate, fatty acids and lactate) to drive mitochondrial oxidative phosphorylation of cancer cells during periods of high ATP demand125. This two-compartment coupling of cancer cell metabolism, in which signals from cancer cells drive aerobic glycolysis in neighbouring stromal cells for the generation of substrate used by the cancer cells for efficient energy production, is termed the reverse Warburg effect; systemic metabolic perturbations also influence this effect126. Efforts are underway to exploit vulnerabilities associated with these cancer-associated metabolic alterations, and interventions aimed at limiting glucose availability, such as ketogenic diets or pharmacologic approaches such as glycolytic inhibitors and mitochondrial-targeted therapeutics, are under investigation125,126.
Changes in systemic metabolic factors, including high levels of circulating insulin and insulin-like growth factor 1 (IGF1), are probably key players in colorectal, pancreatic and other gastrointestinal cancers. In overall results from the NHANES, BMI and total circulating IGF1 levels were found to be inversely related127. However, IGF1 bioavailability is complicated by the presence of at least seven IGF-binding proteins, and thus levels of bioavailable IGF1 were not assessed. An early study noted reduced IGF1 levels associated with physical activity and dietary interventions128, and these findings were corroborated by Nemet et al.129. Circulating insulin levels were also found to be inversely associated with negative energy balance via exercise training88,130. Insulin also induces fatty acid synthase (FAS) expression, a key enzyme of de novo lipogenesis, in adipocytes131,132. Ortega and colleagues reported that the expression of main lipogenic enzymes, including FAS, was downregulated in VAT from 188 patients who were obese and undergoing elective surgical procedures133. However, the link between lipid anabolism pathways in adipose tissues and metabolic status, particularly among obesity-related gastrointestinal cancers, remains incompletely understood. In the study by Friedenreich and colleagues including 320 postmenopausal women, aerobic exercise interventions did not change circulating levels of IGF1 and IGF-binding protein 3 (IGFBP3)88. These findings are consistent with those from the combined dietary and/or exercise weight loss interventions in the NEW trial, although the IGF1-IGFBP3 ratio increased in the diet (5.0%) and combined diet and exercise (5.4%) groups relative to the control group134. In preclinical models, increased circulating levels of insulin and decreased levels of IGFBP1 via excess energy balance have been shown to promote carcinogenic cell growth, as these growth factors have a central role in cell proliferation and apoptotic processes135 (FIG. 3).
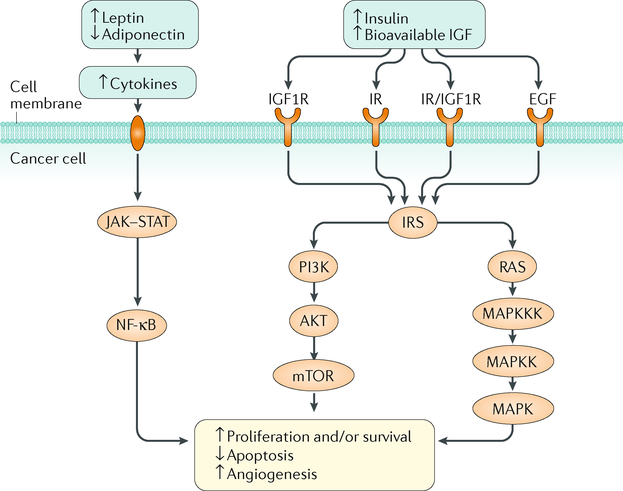
Fig. 3 |. The insulin-insulin-like growth factor axis and adipose tissue-derived adipokine signalling in cancer.
Excess energy balance promotes cell proliferation, angiogenesis and an anti-apoptotic environment by increasing circulating levels of insulin and bioavailable insulin-like growth factor (IGF) (via the mechanistic target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) signalling pathways) and dysregulating adipokine expression (via nuclear factor-κB (NF-κB) signalling). AKT, RAC serine/threonine-protein kinase; EGF, epidermal growth factor; IGF1R, insulin-like growth factor 1 receptor; IR, insulin receptor; IRS, insulin receptor substrate; JAK, Janus kinase; MAPKK, dual specificity mitogen-activated protein kinase kinase; MAPKKK, mitogen-activated protein kinase kinase kinase; PI3K, phosphoinositide 3-kinase; RAS, GTPase RAS; STAT, signal transducer and activator of transcription.
Preclinical weight loss studies have also reported reduced epithelial cell proliferation and formation of new blood vessels, accompanied by a pro-apoptotic environment that is modulated via suppression of the mechanistic target of rapamycin (mTOR) signalling network136 (FIG. 3). Strong evidence from preclinical studies has emerged to demonstrate that caloric restriction reduces obesity-related hyperactivation of mTOR complex 1 (mTORC1) in multiple tissues, including colon, pancreas and liver, in obese mice55, with diet and/or exercise interventions in mice and rats supporting these findings137,138. Exercise interventions in mice also demonstrated decreased kinase activity of mTOR in tumours of the liver139, suggesting that energy balance interventions have the potential to decrease tumour cell proliferation via the mTOR signalling pathway.
Results from the NEW RCT also demonstrated that, relative to individuals in the control group, whole body insulin resistance (measured using the homeostatic model assessment (HOMA) index) decreased an additional 22% and 24%, respectively, among individuals who were overweight or obese and who underwent either dietary weight loss and/or exercise interventions, regardless of age140,141. These findings are corroborated by several studies, including work by Friedenreich and colleagues, who found that a year-long aerobic exercise intervention among 320 postmenopausal women who were inactive and cancer-free was associated with a decrease in insulin resistance88. Similarly, Le and colleagues also reported improvement in insulin sensitivity among 245 women who were overweight or obese after a year-long dietary weight loss RCT58. In particular, women who were insulin sensitive were those with the greatest changes in weight loss58,142.
Mechanisms linking the insulin-IGF1 axis to improved survival among physically active patients with colorectal cancer have also begun to emerge35. In an exploratory analysis, Hanyuda et al. reported that the association of post-diagnosis physical activity with survival in patients with colorectal carcinoma might differ by tumour insulin receptor substrate 1 (IRS1) expression level143. Further, Brown and colleagues reported in 2017 that aerobic exercise interventions reduced fasting insulin concentrations and insulin resistance levels in patients who had survived colon cancer144. Taken together, the evidence presented here positions the insulin-IGF1 axis as a central link between obesity and gastrointestinal cancers. Research in this area will continue to shed light on the positive mediating effects of dietary and weight loss interventions on cancer risk and outcomes.
Proliferation, apoptosis and angiogenesis
The mechanisms of cellular homeostasis, including proliferation, apoptosis and angiogenesis, are intricately regulated in the gastrointestinal tract. Imbalances between cell division, growth and death mechanisms are directly related to energy availability and obesity and can affect both adipose tissue formation and accumulation of cell mass for a clinically detectable cancer74 (FIGS 1,2). The few existing interventional studies related to gastrointestinal cancer that include markers of proliferation, apoptosis and/or angiogenesis are further elaborated upon below.
Cellular proliferation
Given that insufficient apoptosis or increased proliferation can shift the colon crypt cell equilibrium, leading to polyp and tumour formation, a 12-month exercise intervention RCT with 202 healthy, sedentary participants evaluated the effects of exercise-induced colon crypt proliferation via quantification of Ki-67-stained cells in colonic mucosal crypts. The aerobic exercise intervention of moderate-to-vigorous intensity led to a reduction in colon crypt cell proliferation among men, whereas no corresponding proliferative changes were detected among women. Preclinical studies to date have corroborated a reduction in proliferation associated with interventions, as negative associations between caloric restriction and cell proliferation have been reported in murine models of colon carcinogenesis and in murine liver cell models145–148. Bariatric surgical procedures (such as Roux-en-Y gastric bypass or jejunoileal bypass) have been found to lead to increased levels of cellular proliferation in the rectum145,149–151. However, sleeve gastrectomy has not been associated with hyperproliferation in the rectal mucosa among individuals who are morbidly obese149. These differential effects of surgery type on rectal cell hyperproliferation have been linked to differences in sustained expression of cell proliferation-related and inflammatory genes in the rectal mucosa149.
Apoptosis
A large trial with more than 200 sedentary participants tested the effects of moderate-to-vigorous intensity exercise for 12 months on expression of the apoptosis-regulating proteins apoptosis regulator BCL-2 and apoptosis regulator BAX26. This unique investigation showed that the exercise intervention resulted in increased expression of these proteins at the bottom of colon crypts in men (P = 0.05) and decreased expression of these proteins in the middle of crypts among women (P = 0.03)26. For both men and women, weekly minutes of exercise were inversely associated with BAX staining density in the middle of colon crypts (P-trend = 0.03)26. There is no good explanation for these sex-specific results at this time. Rates of apoptosis have also consistently been reported to be related to dietary restriction in preclinical colon and breast cancer models, as in vivo experiments evaluating energy restriction and tumour growth have noted the induction of a pro-apoptotic state mediated by the mitochondrial pathway of caspase activation146,147,152.
Angiogenesis
The formation of new blood vessels is required to supply cells with oxygen and nutrients and to remove products of metabolism153. To evaluate the effects of energy balance interventions on circulating levels of angiogenic factors, Duggan and colleagues randomized 439 postmenopausal women who were overweight or obese to diet and/or exercise regimens or a control group154. Both exercise and diet interventions led to a reduction in circulating concentrations of the angiogenic protein vascular endothelial growth factor (VEGF) at a 30-month follow-up point, with greatest effects observed among participants in the exercise intervention group relative to the control group (−19.7% versus −4.5%). Consistent with previously published results, women who maintained weight loss (>10% of baseline body weight) had markedly reduced circulating levels of VEGF and increased circulating levels of pigment epithelium-derived factor (PEDF), an antiangiogenic protein155,156. Among 79 individuals who were obese but otherwise healthy, diet-induced and/or exercise-induced weight loss was associated with decreased levels of VEGFA and angiopoietin and increased concentrations of angiopoietin-related protein 4 (ANGPTL4)157. Sabater and colleagues reported that weight loss via a dietary regimen led to significantly decreased circulating PEDF (P < 0.0001) and that insulin sensitivity independently contributed to 14% of the variance in PEDF levels (after controlling for BMI, age and fasting triglycerides)158. Reduced caloric intake can also result in antiangiogenic gene expression patterns in adipose tissue157. Mouse models have largely reflected clinical findings, in which caloric restriction has been shown to relate to decreased vascularization of tumours159,160. Together, substantial evidence has emerged from clinical and preclinical studies to support the axiom that the fundamental processes of cellular proliferation, death and angiogenesis are important mediators of the obesity-gastrointestinal carcinogenesis axis that can be perturbed via exercise and dietary weight loss interventions.
Oxidative stress, DNA repair and telomere length
Gastrointestinal cancer-driven metabolic reprogramming affects the manner by which nutrients are taken up into cells, as well as the propensity for particular energy substrates161. Key mediator pathways found to be linked to both energy imbalance and gastrointestinal carcinogenesis include oxidative stress, DNA repair and telomere length162 (FIG. 1). Intervention studies have reported that obesity is linked to genomic integrity via mechanisms of oxidative stress163. Moreover, physically active adults have reduced exercise-induced oxidative stress and improved antioxidant defence capacities164. Many studies have reported that exercise interventions either reduce165–169 or have no effect170,171 on levels of oxidative stress markers (such as 8-hydroxy-2′-deoxyguanosine, 8-iso-prostaglandin F2α and F2-isoprostane) among individuals who are overweight and obese and have reported decreased levels of oxidative stress measured among individuals who survived cancer172.
Although increased BMI is also known to be related to deficiencies in DNA mismatch repair173, a causal link between dietary and exercise interventions and effects on DNA repair machinery has not been well established. Results from intervention studies on DNA repair capacity are limited to the NEW trial, which observed no changes in DNA repair capacity with dietary and/or exercise interventions174.
The length of telomeres is an important criterion of proliferative potential in tissue, and shorter telomeres are associated with increased adiposity175. Intervention studies within the NEW trial reported no changes in telomere length with dietary and/or exercise interventions. However, limitations of the assay used to measure telomere length were noted174,176. Nevertheless, earlier studies reported that caloric restriction among adolescents who were overweight or obese was associated with increased telomere length177. Preclinical models support these findings178, suggesting that energy balance interventions can increase telomerase activity and the expression of telomere-stabilizing proteins to protect against apoptosis (FIG. 1). Thus, these pathways are emerging as potential links between energy balance and gastrointestinal carcinogenesis.
Other emerging factors
Obesity is associated with reduced gut bacterial diversity179, including decreased butyrate-producing bacteria180 and decreased production of anti-inflammatory short-chain fatty acids, including butyrate181. Such changes in microbiome diversity and metabolites can contribute to pro-inflammatory signals in the gastrointestinal tract and possibly in distant tissues182. Moreover, consumption of a high-fat diet can impair gut barrier function, resulting in higher plasma levels of lipopolysaccharide (LPS), a component of Gram-negative bacteria183. LPS induces metabolic endotoxaemia, which is associated with increased pro-inflammatory cytokine expression and infiltration of macrophages in adipose tissue184. In a randomized crossover trial of 50 Danish adults, two 8-week dietary intervention periods consisting of a refined grain diet and a whole grain diet (separated by a washout period of >6 weeks) did not alter the gut microbiome nor insulin sensitivity but did reduce systemic inflammation, as measured by serum IL-6 and CRP levels185. Therefore, although evidence to date is insufficient, obesity-related gut microbial dysbiosis and impaired barrier function might contribute to chronic systemic and adipose tissue inflammation, as well as to mechanisms of gastrointestinal carcinogenesis186. Further, this increased metabolic potential of the microbiome is transmissible between individuals. A case report published in 2015 observed that a patient with a Clostridium difficile infection who was successfully treated with faecal microbiota transplantation from a healthy donor who was overweight developed new-onset obesity (pre-treatment BMI 26 kg/m2 versus 16-month post-treatment BMI 33 kg/m2)187. Preclinical evidence supports this hypothesis, as colonization of a germ-free mouse with the microbiota of an obese (versus lean) mouse leads to greater fat mass gain independent of calorie consumption179.
Gut hormones are also emerging mediators of the obesity-gastrointestinal cancer association, as ghrelin, a hormone produced in the fundic glands of the stomach, can inhibit the production and/or expression of pro-inflammatory cytokines in addition to promoting fat storage and mediating fatty acid metabolism188. Prospective studies have demonstrated that decreased ghrelin expression can increase oesophageal and gastric cancer risk189,190. A cross-sectional study of 159 healthy, inactive, postmenopausal women recruited for an exercise intervention observed that frequent intentional weight loss of >10 lb (>4.5 kg; indicative of weight cycling) was associated with increased ghrelin concentrations191. Results from an RCT support these associations, as a combined dietary weight loss and exercise intervention resulted in the strongest increase (7.4%) in fasting plasma ghrelin levels among postmenopausal women who were overweight or obese compared with exercise or diet intervention only91. Bi-directional crosstalk between ghrelin and hormones involved in energy regulation (such as adiponectin, leptin and insulin) further emphasizes the importance of this pathway in the role of obesity-related gastrointestinal carcinogenesis192.
An emerging hallmark of cancer is the ability of tumour cells to evade immunological destruction, particularly by macrophages, natural killer (NK) cells and B and T cells44. Exercise can directly alter several cell subsets that are involved in innate and adaptive immune function via structural damage in muscle fibres, and this damage can trigger a pro-inflammatory immune response that is mediated by pro-inflammatory cytokines (such as TNF and IL-1β) and characterized by increased monocyte and macrophage counts193–195. Exercise might alter important steps of the immune system-tumour interaction, although short-term and long-term effects need to be distinguished193. Particularly challenging in this field is the limited availability of meaningful biomarkers of immune function that can be reliably measured. An RCT among 115 postmenopausal women who were overweight or obese reported no effect of 12 months of aerobic exercise on in vitro immune function, as measured via immune markers (NK cell cytotoxicity, T cell proliferation, immune cell counts and phenotypes, and serum immunoglobulins)196. A dietary intervention RCT of 91 women who were obese demonstrated that weight loss was associated with a decrease in mitogen-stimulated lymphocyte proliferation, a component of innate and adaptive immunity, but not NK cell cytotoxicity197. Similar effects were noted in preclinical studies, as dietary interventions altered the peripheral immune system, as measured by the percentage of lymphocyte subpopulations in peripheral blood198.
Mechanisms linking immunity to obesity include the role of adipose tissue-driven inflammatory response, as discussed earlier, given that leukocytes are found in adipose tissues199,200. Exercise-mediated normalization of vasculature in the tumour microenvironment might improve immune infiltration201. In preclinical models, the presence of infiltrated macrophages in the adipose tissue of diet-induced obese mice has generated interest into the molecular dynamics of immunometabolism202. Sufficient evidence does not yet exist to fully understand the complex and multifaceted interaction between exercise, the inflammation-immune axis and tumour progression193.
Nonalcoholic fatty liver disease (NAFLD), a chronic disease in which free fatty acids and triglycerides accumulate in the liver, is a risk factor for hepatocellular carcinoma203. Systemic and liver-specific molecular mechanisms involved in NAFLD and hepatocellular carcinogenesis include alterations in cellular lipid metabolism and insulin resistance203, which might be altered by dietary interventions (such as caloric restriction or the Dietary Approaches to Stop Hypertension (DASH) diet) and aerobic or resistance exercise weight loss interventions204–208. For example, an RCT of 220 patients with central obesity and NAFLD reported statistically significant reductions in body weight and intrahepatic triglyceride content in the vigorous-moderate exercise group (150 minutes per week jogging at 65–80% maximum heart rate for 6 months and 150 minutes per week brisk walking at 45–55% maximum heart rate for another 6 months) and in the moderate exercise (150 minutes per week brisk walking for 12 months) group relative to the control group at both 6-month and 12-month followup time points209. Systematic reviews and meta-analyses have consistently reported that intervention trials of diet and/or exercise led to reductions in steatosis and/or markers of NAFLD activity, including serum levels of liver enzymes, with combination trials demonstrating the greatest efficacy204,210–212. The mechanisms that are responsible for the perturbation of the obesity-NAFLD link via exercise and diet interventions are continuing to be explored.
The cancer stem cell (CSC) hypothesis postulates that tumours originate through dysregulation of the normal cell self-renewal process213, resulting in the aberrant replication and differentiation characteristic of a variety of gastrointestinal tumours214–217. Although not fully understood, evidence suggests an association between CSCs, also known as tumour-initiating cells, and obesity218,219. Obesity might promote the mobilization and recruitment of circulating progenitor cells to pathological sites; a study by Bellows et al. of peripheral blood mononuclear cells from individuals who were disease-free showed that 14 individuals who were obese had a fivefold higher frequency of circulating progenitor cells (P = 0.0019) and a tenfold higher frequency of circulating mesenchymal stromal progenitor cells (P = 0.0021) than 12 individuals who were not obese219. Clinical and preclinical diet and/or exercise intervention studies are needed to further unravel the CSC-obesity link.
Conclusions
In light of the vast challenge of the obesity epidemic worldwide and an increasing number of gastrointestinal cancers being attributed to obesity, there is a continued need for research in this area to understand the distinctions between fitness versus fatness and effective mechanisms for disrupting the obesity-cancer link. Physical activity has emerged as a factor that might be effective in the prevention of gastrointestinal tumours independent of effects on body weight, although large-scale intervention studies that evaluate the effect of physical activity in relation to primary gastrointestinal tumour incidence or recurrence are still needed. Similarly, with increasing numbers of cancer survivors worldwide, and the need to provide evidence-based recommendations to clinicians and patients, we need to continue progress on long-term cancer survivorship studies that test the effect of physical activity and/or dietary weight loss interventions on patient-reported outcomes and physical functioning, as well as on survival and recurrence of disease. For example, although physical activity is emerging as a powerful means to improve cancer-related fatigue, we do not yet have interventions that are easily implemented within the clinic flow and that will be reimbursed by insurance — both of which are factors that are instrumental for clinical translation. In addition, with bariatric surgery having an increasing role in obesity reduction, further research should focus on the effects of different types of surgical procedures and their effect on biomarkers of cancer risk and incidence.
Finally, sex-specific differences of the effects of energy balance interventions on gastrointestinal cancers need to be defined. Cumulative data from meta-analyses of prospective studies highlight stronger associations between BMI and gastrointestinal cancer in men than in women, specifically for colorectal cancer220. However, there is a dearth of data on the different effects of weight loss interventions on males and females with regard to gastrointestinal cancer risk, as well as post-diagnosis outcomes.
Many biological mechanisms have a role in mediating the effects of energy balance on gastrointestinal cancer. We have illustrated above how human intervention studies have shed light on the ‘black box’ that links the broad concept of energy balance to cancer risk, with preclinical models supporting these mechanistic relationships. Strong evidence implicates systemic inflammation and insulin resistance pathways, whereas adipose tissue-dependent effects, changes in immune function, effects on CSCs and changes in the microbiome might also mediate the effects of energy balance on gastrointestinal cancers. Although we have strong evidence for the effect of weight loss interventions on certain cancer-associated pathways including insulin signalling, adipokines and inflammation, the crosstalk between these pathways is not clear. Multi-omic investigations discerning pathways and interrelationships with proofs of principle (via clinical and/or preclinical testing) on how to effectively disrupt these effects are probably needed to advance the field. As bariatric surgery continues to develop as a possible cancer prevention and control intervention, a comprehensive understanding of the mechanisms underscoring the mechanistic perturbations associated with surgical interventions for weight loss is a pre-requisite to evaluating whether those effects can be mirrored via non-surgical energy balance interventions.
In terms of new areas of research, adipose tissue is emerging as a paracrine driver of obesity-associated tumours; understanding the role of the adipose tissue-tumour microenvironment and crosstalk between the two could provide novel opportunities for intervention. Similarly, we are just beginning to understand the role of the gut microbiota in energy balance and cancer risk and avenues for intervention, and as such, evaluable biospecimens (such as faecal samples) should be collected in human studies — and appropriate animal models developed — to advance the field. Finally, there is tremendous heterogeneity across cancers, and most of the mechanisms discussed as part of this Review are probably effective for only a subset of gastrointestinal tumours. We are also just beginning to understand the potential interplay between tumour characteristics and energy balance mechanisms, and we need to build on this emerging evidence by stringent molecular characterization.
Supplementary Material
Key points.
Energy balance interventions, such as physical activity and caloric restriction, can reduce individual cancer risk and prevent gastrointestinal carcinogenesis.
Intervention trials are instrumental in fully understanding the effects of energy balance on cancer risk, clinical outcomes and underlying biological mechanisms.
The main mechanistic pathways underlying the obesity-gastrointestinal cancer link include systemic inflammation, metabolic reprogramming and adipose tissue-dependent effects.
Additional pathways that might have a role are oxidative stress and DNA repair, proliferation, apoptosis and angiogenesis, the gut microbiome and immune function.
Preclinical studies using both diet-induced and genetically induced obesity models provide supporting evidence to clinical findings.
Acknowledgements
C.M.U. was supported by grants from the US National Institutes of Health (NIH)-US National Cancer Institute (NCI) (R01 CA189184, R01 CA207371 and U01 CA206110). C.M.U. and C.H. were also supported by NIH-NCI grant R01 CA211705. A.N.H. was supported by the NIH under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the US National Human Genome Research Institute. S.D.H. was supported by NIH-NCI grant R35 CA197627.
Footnotes
Competing interests
The authors declare no competing interests.
Reviewer information
Nature Reviews Gastroenterology & Hepatology thanks N. Berger and other anonymous reviewer(s) for their contribution to the peer review of this work
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41575-018-0053-2.
References
- 1.Demark-Wahnefried W et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol. Biomarkers Prev 21, 1244–1259 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romieu I et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control 28, 247–258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health (National Center for Health Statistics, Hyattsville, MD, 2017). [PubMed] [Google Scholar]
- 4.Lauby-Secretan B et al. Body fatness and cancer — viewpoint of the IARC Working Group. N. Engl. J. Med 375, 794–798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund (WCRF)/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. (AICR, Washington DC, 2018). [Google Scholar]
- 6.Wolin KY, Yan Y, Colditz GA & Lee IM Physical activity and colon cancer prevention: a meta-analysis. Br. J. Cancer 100, 611–616 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly JE et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc 41, 459–471 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control & Prevention. Facts about Physical Activity. Centers for Disease Control & Prevention; https://www.cdc.gov/physicalactivity/data/facts.htm (2016). [Google Scholar]
- 9.Reis RS et al. Scaling up physical activity interventions worldwide: stepping up to larger and smarter approaches to get people moving. Lancet 388, 1337–1348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy N, Jenab M & Gunter MJ Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat. Rev. Gastroenterol. Hepatol 10.1038/s41575-018-0038-1 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Emmons KM & Colditz GA Realizing the potential of cancer prevention - the role of implementation science. N. Engl. J. Med 376, 986–990 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson CA et al. Cancer incidence and mortality during the intervention and postintervention periods of the Women’s Health Initiative dietary modification trial. Cancer Epidemiol. Biomarkers Prev 23, 2924–2935 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard BV et al. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative dietary modification trial. JAMA 295, 39–49 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Jiao L et al. Low-fat dietary pattern and pancreatic cancer risk in the Women’s Health Initiative dietary modification randomized controlled trial. J. Natl Cancer Inst 110, djx117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spechler SJ & Goyal RK Barrett’s esophagus. N. Engl. J. Med 315, 362–371 (1986). [DOI] [PubMed] [Google Scholar]
- 16.Reid BJ, Li X, Galipeau PC & Vaughan TL Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat. Rev. Cancer 10, 87–101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winzer BM, Paratz JD, Reeves MM & Whiteman DC Exercise and the Prevention of Oesophageal Cancer (EPOC) study protocol: a randomized controlled trial of exercise versus stretching in males with Barrett’s oesophagus. BMC Cancer 10, 292 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winzer BM, Paratz JD, Whitehead JP, Whiteman DC & Reeves MM The feasibility of an exercise intervention in males at risk of oesophageal adenocarcinoma: a randomized controlled trial. PLOS One 10, e0117922 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson AS et al. The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ 348, g1823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchwald H The evolution of metabolic/bariatric surgery. Obes. Surg 24, 1126–1135 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Adams TD et al. Cancer incidence and mortality after gastric bypass surgery. Obesity 17, 796–802 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derogar M et al. Increased risk of colorectal cancer after obesity surgery. Ann. Surg 258, 983–988 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Tao W et al. Colorectal cancer prognosis following obesity surgery in a population-based cohort study. Obes. Surg 27, 1233–1239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tse WHW, Kroon HM & van Lanschot JJB Clinical challenges in upper gastrointestinal malignancies after bariatric surgery. Dig. Surg 35, 183–186 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrahamson PE et al. No effect of exercise on colon mucosal prostaglandin concentrations: a 12-month randomized controlled trial. Cancer Epidemiol. Biomarkers Prev 16, 2351–2356 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Campbell KL et al. Effect of a 12-month exercise intervention on the apoptotic regulating proteins bax and bcl-2 in colon crypts: a randomized controlled trial. Cancer Epidemiol. Biomarkers Prev 16, 1767–1774 (2007). [DOI] [PubMed] [Google Scholar]
- 27.McTiernan A et al. Effect of a 12-month exercise intervention on patterns of cellular proliferation in colonic crypts: A randomized controlled trial. Cancer Epidemiol. Biomarkers Prev 15, 1–10 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Morey MC et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA 301, 1883–1891 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu YJ et al. A walk-and-eat intervention improves outcomes for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy. Oncologist 20, 1216–1222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo TP et al. A progressive postresection walking program significantly improves fatigue and health-related quality of life in pancreas and periampullary cancer patients. J. Am. Coll. Surg 214, 463–477 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Meyerhardt JA et al. Physical activity and survival after colorectal cancer diagnosis. J. Clin. Oncol 24, 3527–3534 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Strasser B, Steindorf K, Wiskemann J & Ulrich CM Impact of resistance training in cancer survivors: a meta-analysis. Med. Sci. Sports Exerc 45, 2080–2090 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Friedenreich CM, Neilson HK, Farris MS & Courneya KS Physical activity and cancer outcomes: a precision medicine approach. Clin. Cancer Res 22, 4766–4775 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Cho H et al. Matched pair analysis to examine the effects of a planned preoperative exercise program in early gastric cancer patients with metabolic syndrome to reduce operative risk: the Adjuvant Exercise for General Elective Surgery (AEGES) study group. Ann. Surg. Oncol 21, 2044–2050 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Van Blarigan EL & Meyerhardt JA Role of physical activity and diet after colorectal cancer diagnosis. J. Clin. Oncol 33, 1825–1834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Je Y, Jeon JY, Giovannucci EL & Meyerhardt JA Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int. J. Cancer 133, 1905–1913 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Courneya KS et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr. Oncol 15, 279–285 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Schaaf MK et al. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J. Thorac Cardiovasc. Surg 147, 490–495 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Aoyama T et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int. J. Clin. Oncol 22, 476–483 (2017). [DOI] [PubMed] [Google Scholar]
- 40.van Rooijen SJ et al. Systematic review of exercise training in colorectal cancer patients during treatment. Scand. J. Med. Sci. Sports 28, 360–370 (2018). [DOI] [PubMed] [Google Scholar]
- 41.van Vulpen JK et al. Physical ExeRcise Following Esophageal Cancer Treatment (PERFECT) study: design of a randomized controlled trial. BMC Cancer 17, 552 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn KY et al. The effects of inpatient exercise therapy on the length of hospital stay in stages I-III colon cancer patients: randomized controlled trial. Int. J. Colorectal Dis 28, 643–651 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Courneya KS et al. Effects of a structured exercise program on physical activity and fitness in colon cancer survivors: one year feasibility results from the CHALLENGE trial. Cancer Epidemiol. Biomarkers Prev 25, 969–977 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Foster-Schubert KE et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity 20, 1628–1638 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Gemert WA et al. Design of the SHAPE-2 study: the effect of physical activity, in addition to weight loss, on biomarkers of postmenopausal breast cancer risk. BMC Cancer 13, 395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teicher BA Tumor Models in Cancer Research (Springer, 2011). [Google Scholar]
- 48.Bijlsma MF, Sadanandam A, Tan P & Vermeulen L Molecular subtypes in cancers of the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol 14, 333–342 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Sawayama H et al. Clinical impact of the Warburg effect in gastrointestinal cancer (review). Int. J. Oncol 45, 1345–1354 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Mayers JR et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353, 1161–1165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanyuda A et al. Body mass index and risk of colorectal carcinoma subtypes classified by tumor differentiation status. Eur. J. Epidemiol 32, 393–407 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berriel Diaz M, Herzig S & Schafmeier T Biological mechanisms for the effect of obesity on cancer risk: experimental evidence. Recent Results Cancer Res. 208, 219–242 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Westbrook AM, Szakmary A & Schiestl RH Mouse models of intestinal inflammation and cancer. Arch. Toxicol 90, 2109–2130 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Crusz SM & Balkwill FR Inflammation and cancer: advances and new agents. Nat. Rev. Clin. Oncol 12, 584–596 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Lashinger LM, Ford NA & Hursting SD Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J. Nutr 144, 109–113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell KL et al. No reduction in C-reactive protein following a 12-month randomized controlled trial of exercise in men and women. Cancer Epidemiol. Biomarkers Prev 17, 1714–1718 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell PT et al. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med. Sci. Sports Exerc 41, 1533–1539 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le T et al. Effects of diet composition and insulin resistance status on plasma lipid levels in a weight loss intervention in women. J. Am. Heart Assoc 5, e002771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imayama I et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 72, 2314–2326 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedenreich CM et al. Inflammatory marker changes in postmenopausal women after a year-long exercise intervention comparing high versus moderate volumes. Cancer Prev. Res 9, 196–203 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Lin PC, Lin YJ, Lee CT, Liu HS & Lee JC Cyclooxygenase-2 expression in the tumor environment is associated with poor prognosis in colorectal cancer patients. Oncol. Lett 6, 733–739 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maihofner C et al. Expression of cyclooxygenase-2 parallels expression of interleukin-1beta, interleukin-6 and NF-kappaB in human colorectal cancer. Carcinogenesis 24, 665–671 (2003). [DOI] [PubMed] [Google Scholar]
- 63.van Gemert WA et al. Effect of weight loss with or without exercise on inflammatory markers and adipokines in postmenopausal women: the SHAPE-2 trial, a randomized controlled trial. Cancer Epidemiol. Biomarkers Prev 25, 799–806 (2016). [DOI] [PubMed] [Google Scholar]
- 64.de Maat MF et al. Epigenetic silencing of cyclooxygenase-2 affects clinical outcome in gastric cancer. J. Clin. Oncol 25, 4887–4894 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Peng L, Zhou Y, Wang Y, Mou H & Zhao Q Prognostic significance of COX-2 immunohistochemical expression in colorectal cancer: a meta-analysis of the literature. PLOS One 8, e58891 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forsythe LK, Wallace JM & Livingstone MB Obesity and inflammation: the effects of weight loss. Nutr. Res. Rev 21, 117–133 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Santos J et al. Effect of bariatric surgery on weight loss, inflammation, iron metabolism, and lipid profile. Scand. J. Surg 103, 21–25 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Lasselin J et al. Adipose inflammation in obesity: relationship with circulating levels of inflammatory markers and association with surgery-induced weight loss. J. Clin. Endocrinol. Metab 99, E53–E61 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Kelly AS et al. Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int. J. Obes 40, 275–280 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazur-Bialy AI et al. Beneficial effect of voluntary exercise on experimental colitis in mice fed a high-fat diet: the role of irisin, adiponectin and proinflammatory biomarkers. Nutrients 9, E410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Q et al. Differential effect of weight loss with low-fat diet or high-fat diet restriction on inflammation in the liver and adipose tissue of mice with diet-induced obesity. Atherosclerosis 219, 100–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen F et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 13, 45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Hernandez AI, Catalan V, Gomez-Ambrosi J, Rodriguez A & Fruhbeck G Mechanisms linking excess adiposity and carcinogenesis promotion. Front. Endocrinol 5, 65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Himbert C et al. Signals from the adipose microenvironment and the obesity-cancer link-a systematic review. Cancer Prev. Res 10, 494–506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell KL, Landells CE, Fan J & Brenner DRA Systematic review of the effect of lifestyle interventions on adipose tissue gene expression: implications for carcinogenesis. Obesity 25 (Suppl. 2), S40–S51 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Koerner A, Kratzsch J & Kiess W Adipocytokines: leptin — the classical, resistin—the controversical, adiponectin — the promising, and more to come. Best Pract. Res. Clin. Endocrinol. Metab 19, 525–546 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Schubel R et al. The effects of intermittent calorie restriction on metabolic health: Rationale and study design of the HELENA Trial. Contemp. Clin. Trials 51, 28–33 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Nakhuda A et al. Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss. Am. J. Clin. Nutr 104, 557–565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown JC et al. Dose-response effects of aerobic exercise on body composition among colon cancer survivors: a randomised controlled trial. Br. J. Cancer 117, 1614–1620 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nuri R, Moghaddasi M, Darvishi H & Izadpanah A Effect of aerobic exercise on leptin and ghrelin in patients with colorectal cancer. J. Cancer Res. Ther 12, 169–174 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Lee MK et al. Effect of home-based exercise intervention on fasting insulin and adipocytokines in colorectal cancer survivors: a randomized controlled trial. Metabolism 76, 23–31 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Wallace AM, Sattar N & McMillan DC Effect of weight loss and the inflammatory response on leptin concentrations in gastrointestinal cancer patients. Clin. Cancer Res 4, 2977–2979 (1998). [PubMed] [Google Scholar]
- 83.Riondino S et al. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J. Gastroenterol 20, 5177–5190 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holland WL et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med 17, 55–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J, Morley TS, Kim M, Clegg DJ & Scherer PE Obesity and cancer-mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol 10, 455–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbenhardt C et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J. Intern. Med 274, 163–175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rokling-Andersen MH et al. Effects of long-term exercise and diet intervention on plasma adipokine concentrations. Am. J. Clin. Nutr 86, 1293–1301 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Friedenreich CM et al. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr. Relat. Cancer 18, 357–369 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kelly KR et al. Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Med. Sci. Sports Exerc 46, 920–926 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thompson HJ et al. Impact of weight loss on plasma leptin and adiponectin in overweight-to-obese post menopausal breast cancer survivors. Nutrients 7, 5156–5176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mason C et al. The effects of separate and combined dietary weight loss and exercise on fasting ghrelin concentrations in overweight and obese women: a randomized controlled trial. Clin. Endocrinol 82, 369–376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, You T, Murphy K, Lyles MF & Nicklas BJ Addition of exercise increases plasma adiponectin and release from adipose tissue. Med. Sci. Sports Exerc 47, 2450–2455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB & Richelsen B Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am. J. Physiol. Endocrinol. Metab 298, E824–E831 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Campbell KL et al. Gene expression changes in adipose tissue with diet- and/or exercise-induced weight loss. Cancer Prev. Res 6, 217–231 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaittinen M, Kolehmainen M, Schwab U, Uusitupa M & Pulkkinen L Microfibrillar-associated protein 5 is linked with markers of obesity-related extracellular matrix remodeling and inflammation. Nutr. Diabetes 1, e15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walhin JP, Richardson JD, Betts JA & Thompson D Exercise counteracts the effects of short-term overfeeding and reduced physical activity independent of energy imbalance in healthy young men. J. Physiol 591, 6231–6243 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sjogren P et al. Functional changes in adipose tissue in a randomised controlled trial of physical activity. Lipids Health Dis 11, 80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phillips MD et al. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med. Sci. Sports Exerc 44, 2099–2110 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Sjostrom L et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 10, 653–662 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Herder C et al. Adiponectin and bariatric surgery: associations with diabetes and cardiovascular disease in the Swedish Obese Subjects study. Diabetes Care 37, 1401–1409 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Serra A et al. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity: 1-year follow-up. Clin. Nutr 25, 400–408 (2006). [DOI] [PubMed] [Google Scholar]
- 102.Frikke-Schmidt H, O’Rourke RW, Lumeng CN, Sandoval DA & Seeley RJ Does bariatric surgery improve adipose tissue function? Obes. Rev 17, 795–809 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hagman DK et al. The short-term and long-term effects of bariatric/metabolic surgery on subcutaneous adipose tissue inflammation in humans. Metabolism 70, 12–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sams VG et al. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surg. Endosc 30, 3499–3504 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Fruhbeck G Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat. Rev. Endocrinol 11, 465–477 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Falk RT et al. Estrogen metabolites are not associated with colorectal cancer risk in postmenopausal women. Cancer Epidemiol. Biomarkers Prev 24, 1419–1422 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rudolph A et al. Colorectal cancer risk associated with hormone use varies by expression of estrogen receptor-beta. Cancer Res. 73, 3306–3315 (2013). [DOI] [PubMed] [Google Scholar]
- 108.Murphy N et al. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J. Natl Cancer Inst 107, djv210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Limsui D et al. Postmenopausal hormone therapy and colorectal cancer risk by molecularly defined subtypes among older women. Gut 61, 1299–1305 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prentice RL et al. Colorectal cancer in relation to postmenopausal estrogen and estrogen plus progestin in the Women’s Health Initiative clinical trial and observational study. Cancer Epidemiol. Biomarkers Prev 18, 1531–1537 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Camargo MC et al. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol. Biomarkers Prev 21, 20–38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sukocheva OA, Li B, Due SL, Hussey DJ & Watson DI Androgens and esophageal cancer: what do we know? World J. Gastroenterol 21, 6146–6156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petrick JL et al. Association between circulating levels of sex steroid hormones and esophageal adenocarcinoma in the FINBAR Study. PLOS One 13, e0190325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Andren-Sandberg A & Johansson J Influence of sex hormones on pancreatic cancer. Int. J. Pancreatol 7, 167–176 (1990). [DOI] [PubMed] [Google Scholar]
- 115.Lukanova A et al. Prediagnostic plasma testosterone, sex hormone-binding globulin, IGF-I and hepatocellular carcinoma: etiological factors or risk markers? Int. J. Cancer 134, 164–173 (2014). [DOI] [PubMed] [Google Scholar]
- 116.Campbell KL et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J. Clin. Oncol 30, 2314–2326 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McTiernan A et al. Effect of exercise on serum androgens in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol. Biomarkers Prev 13, 1099–1105 (2004). [PubMed] [Google Scholar]
- 118.McTiernan A et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 64, 2923–2928 (2004). [DOI] [PubMed] [Google Scholar]
- 119.Kim C et al. Racial/ethnic differences in sex hormone levels among postmenopausal women in the diabetes prevention program. J. Clin. Endocrinol. Metab 97, 4051–4060 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hawkins VN et al. Effect of exercise on serum sex hormones in men: a 12-month randomized clinical trial. Med. Sci. Sports Exerc 40, 223–233 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Friedenreich CM et al. Effects of exercise dose on endogenous estrogens in postmenopausal women: a randomized trial. Endocr. Relat. Cancer 22, 863–876 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Longnecker DS Hormones and pancreatic cancer. Int. J. Pancreatol 9, 81–86 (1991). [DOI] [PubMed] [Google Scholar]
- 123.Armstrong CM, Allred KF, Weeks BR, Chapkin RS & Allred CD Estradiol has differential effects on acute colonic inflammation in the presence and absence of estrogen receptor beta expression. Dig. Dis. Sci 62, 1977–1984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roshan MH, Tambo A & Pace NP The role of testosterone in colorectal carcinoma: pathomechanisms and open questions. EPMA J. 7, 22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilde L et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin. Oncol 44, 198–203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu Y et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget 8, 57813–57825 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Faupel-Badger JM, Berrigan D, Ballard-Barbash R & Potischman N Anthropometric correlates of insulin-like growth factor 1 (IGF-1) and IGF binding protein-3 (IGFBP-3) levels by race/ethnicity and gender. Ann. Epidemiol 19, 841–849 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith AT, Clemmons DR, Underwood LE, Ben-Ezra V & McMurray R The effect of exercise on plasma somatomedin-C/insulinlike growth factor I concentrations. Metabolism 36, 533–537 (1987). [DOI] [PubMed] [Google Scholar]
- 129.Nemet D et al. Negative energy balance plays a major role in the IGF-I response to exercise training. J. Appl. Physiol (1985) 96, 276–282 (1985) (2004). [DOI] [PubMed] [Google Scholar]
- 130.Frank LL et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes. Res 13, 615–625 (2005). [DOI] [PubMed] [Google Scholar]
- 131.Wang Y et al. The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J. Nutr 134, 1032–1038 (2004). [DOI] [PubMed] [Google Scholar]
- 132.Claycombe KJ et al. Insulin increases fatty acid synthase gene transcription in human adipocytes. Am. J. Physiol 274, R1253–R1259 (1998). [DOI] [PubMed] [Google Scholar]
- 133.Ortega FJ et al. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity 18, 13–20 (2010). [DOI] [PubMed] [Google Scholar]
- 134.Mason C et al. Effects of dietary weight loss and exercise on insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in postmenopausal women: a randomized controlled trial. Cancer Epidemiol. Biomarkers Prev 22, 1457–1463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Margolis LM et al. Calorie restricted high protein diets downregulate lipogenesis and lower intrahepatic triglyceride concentrations in male rats. Nutrients 8, E571 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moore T et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev. Res 1, 65–76 (2008). [DOI] [PubMed] [Google Scholar]
- 137.Bae JY et al. Exercise and dietary change ameliorate high fat diet induced obesity and insulin resistance via mTOR signaling pathway. J. Exerc. Nutr. Biochem 20, 28–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harvey AE et al. Calorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-kappaB activation, and inflammation-related gene expression in an insulin-like growth factor-1-dependent manner. PLOS One 9, e94151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Piguet AC et al. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J. Hepatol 62, 1296–1303 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Weiss EP, Reeds DN, Ezekiel UR, Albert SG & Villareal DT Circulating cytokines as determinants of weight loss-induced improvements in insulin sensitivity. Endocrine 55, 153–164 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mason C et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am. J. Prev. Med 41, 366–375 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rock CL et al. Effects of diet composition on weight loss, metabolic factors and biomarkers in a 1-year weight loss intervention in obese women examined by baseline insulin resistance status. Metabolism 65, 1605–1613 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hanyuda A et al. Survival benefit of exercise differs by tumor IRS1 expression status in colorectal cancer. Ann. Surg. Oncol 23, 908–917 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brown JC et al. Dose-response effects of aerobic exercise among colon cancer survivors: a randomized phase II trial. Clin. Colorectal Cancer 17, 32–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sainsbury A et al. Increased colorectal epithelial cell proliferation and crypt fission associated with obesity and roux-en-Y gastric bypass. Cancer Epidemiol. Biomarkers Prev 17, 1401–1410 (2008). [DOI] [PubMed] [Google Scholar]
- 146.Zhu Z, Jiang W & Thompson HJ Effect of energy restriction on tissue size regulation during chemically induced mammary carcinogenesis. Carcinogenesis 20, 1721–1726 (1999). [DOI] [PubMed] [Google Scholar]
- 147.Olivo-Marston SE et al. Effects of calorie restriction and diet-induced obesity on murine colon carcinogenesis, growth and inflammatory factors, and microRNA expression. PLOS One 9, e94765 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bruss MD, Thompson AC, Aggarwal I, Khambatta CF & Hellerstein MK The effects of physiological adaptations to calorie restriction on global cell proliferation rates. Am. J. Physiol. Endocrinol. Metab 300, E735–E745 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kant P et al. Mucosal biomarkers of colorectal cancer risk do not increase at 6 months following sleeve gastrectomy, unlike gastric bypass. Obesity 22, 202–210 (2014). [DOI] [PubMed] [Google Scholar]
- 150.Kant P et al. Rectal epithelial cell mitosis and expression of macrophage migration inhibitory factor are increased 3 years after Roux-en-Y gastric bypass (RYGB) for morbid obesity: implications for long-term neoplastic risk following RYGB. Gut 60, 893–901 (2011). [DOI] [PubMed] [Google Scholar]
- 151.Appleton GV, Wheeler EE, Al-Mufti R, Challacombe DN & Williamson RC Rectal hyperplasia after jejunoileal bypass for morbid obesity. Gut 29, 1544–1548 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thompson HJ, Zhu Z & Jiang W Identification of the apoptosis activation cascade induced in mammary carcinomas by energy restriction. Cancer Res. 64, 1541–1545 (2004). [DOI] [PubMed] [Google Scholar]
- 153.Bouis D, Kusumanto Y, Meijer C, Mulder NH & Hospers GA A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol. Res 53, 89–103 (2006). [DOI] [PubMed] [Google Scholar]
- 154.Duggan C, Tapsoba JD, Wang CY, Foster-Schubert KE & McTiernan A Long-term effects of weight loss & exercise on biomarkers associated with angiogenesis. Cancer Epidemiol. Biomarkers Prev 26, 1788–1794 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duggan C, Xiao L, Wang CY & McTiernan A Effect of a 12-month exercise intervention on serum biomarkers of angiogenesis in postmenopausal women: a randomized controlled trial. Cancer Epidemiol. Biomarkers Prev 23, 648–657 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duggan C, Tapsoba Jde D, Wang CY & McTiernan A Dietary weight loss and exercise effects on serum biomarkers of angiogenesis in overweight postmenopausal women: a randomized controlled trial. Cancer Res. 76, 4226–4235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cullberg KB et al. Effect of weight loss and exercise on angiogenic factors in the circulation and in adipose tissue in obese subjects. Obesity 21, 454–460 (2013). [DOI] [PubMed] [Google Scholar]
- 158.Sabater M et al. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight loss. J. Clin. Endocrinol. Metab 95, 4720–4728 (2010). [DOI] [PubMed] [Google Scholar]
- 159.Kurki E, Shi J, Martonen E, Finckenberg P & Mervaala E Distinct effects of calorie restriction on adipose tissue cytokine and angiogenesis profiles in obese and lean mice. Nutr. Metab 9, 64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mukherjee P, Abate LE & Seyfried TN Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin. Cancer Res 10, 5622–5629 (2004). [DOI] [PubMed] [Google Scholar]
- 161.Pavlova NN & Thompson CB The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marseglia L et al. Oxidative stress in obesity: a critical component in human diseases. Int. J. Mol. Sci 16, 378–400 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Furukawa S et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest 114, 1752–1761 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Meijer EP, Goris AH, van Dongen JL, Bast A & Westerterp KR Exercise-induced oxidative stress in older adults as a function of habitual activity level. J. Am. Geriatr. Soc 50, 349–353 (2002). [DOI] [PubMed] [Google Scholar]
- 165.Campbell PT et al. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med. Sci. Sports Exerc 42, 1448–1453 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duggan C et al. Dietary weight loss, exercise, and oxidative stress in postmenopausal women: a randomized controlled trial. Cancer Prev. Res 9, 835–843 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Buchowski MS et al. Effect of modest caloric restriction on oxidative stress in women, a randomized trial. PLOS One 7, e47079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gutierrez-Lopez L et al. Hypocaloric diet and regular moderate aerobic exercise is an effective strategy to reduce anthropometric parameters and oxidative stress in obese patients. Obes. Facts 5, 12–22 (2012). [DOI] [PubMed] [Google Scholar]
- 169.Chae JS et al. Mild weight loss reduces inflammatory cytokines, leukocyte count, and oxidative stress in overweight and moderately obese participants treated for 3 years with dietary modification. Nutr. Res 33, 195–203 (2013). [DOI] [PubMed] [Google Scholar]
- 170.Meydani M, Das S, Band M, Epstein S & Roberts S The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: results from the CALERIE Trial of Human Caloric Restriction. J. Nutr. Health Aging 15, 456–460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Friedenreich CM et al. Effects of exercise on markers of oxidative stress: an ancillary analysis of the Alberta Physical Activity and Breast Cancer Prevention Trial. BMJ Open Sport Exerc. Med 2, e000171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Repka CP & Hayward R Oxidative stress and fitness changes in cancer patients after exercise training. Med. Sci. Sports Exerc 48, 607–614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shimizu I, Yoshida Y, Suda M & Minamino T DNA damage response and metabolic disease. Cell Metab. 20, 967–977 (2014). [DOI] [PubMed] [Google Scholar]
- 174.Habermann N et al. No effect of caloric restriction or exercise on radiation repair capacity. Med. Sci. Sports Exerc 47, 896–904 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mundstock E et al. Effect of obesity on telomere length: systematic review and meta-analysis. Obesity 23, 2165–2174 (2015). [DOI] [PubMed] [Google Scholar]
- 176.Mason C et al. Independent and combined effects of dietary weight loss and exercise on leukocyte telomere length in postmenopausal women. Obesity 21, E549–E554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Garcia-Calzon S et al. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: the EVASYON study. PLOS One 9, e89828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Werner C et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 120, 2438–2447 (2009). [DOI] [PubMed] [Google Scholar]
- 179.Turnbaugh PJ & Gordon JI The core gut microbiome, energy balance and obesity. J. Physiol 587, 4153–4158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aguirre M, Bussolo de Souza C & Venema K The gut microbiota from lean and obese subjects contribute differently to the fermentation of arabinogalactan and inulin. PLOS One 11, e0159236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jakobsdottir G, Xu J, Molin G, Ahrne S & Nyman M High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLOS One 8, e80476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Le Chatelier E et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013). [DOI] [PubMed] [Google Scholar]
- 183.Cani PD, Delzenne NM, Amar J & Burcelin R Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol. Biol 56, 305–309 (2008). [DOI] [PubMed] [Google Scholar]
- 184.Cani P D. et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50, 2374–2383 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Roager HM et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut 10.1136/gutjnl-2017-314786 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Djuric Z Obesity-associated cancer risk: the role of intestinal microbiota in the etiology of the host proinflammatory state. Transl Res. 179, 155–167 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alang N & Kelly CR Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis 2, ofv004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Higgins JA Resistant starch and energy balance: impact on weight loss and maintenance. Crit. Rev. Food Sci. Nutr 54, 1158–1166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Murphy G et al. Serum ghrelin is inversely associated with risk of subsequent oesophageal squamous cell carcinoma. Gut 61, 1533–1537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.de Martel C et al. Serum ghrelin levels and risk of subsequent adenocarcinoma of the esophagus. Am. J. Gastroenterol 102, 1166–1172 (2007). [DOI] [PubMed] [Google Scholar]
- 191.Hooper LE et al. Frequent intentional weight loss is associated with higher ghrelin and lower glucose and androgen levels in postmenopausal women. Nutr. Res 30, 163–170 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cui H, Lopez M & Rahmouni K The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol 13, 338–351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koelwyn GJ, Wennerberg E, Demaria S & Jones LW Exercise in regulation of inflammationimmune axis function in cancer initiation and progression. Oncology (Williston Park) 29, 908–922 (2015). [PMC free article] [PubMed] [Google Scholar]
- 194.Walsh NP et al. Position statement. Part one: immune function and exercise. Exerc. Immunol. Rev 17, 6–63 (2011). [PubMed] [Google Scholar]
- 195.Walsh NP et al. Position statement. Part two: maintaining immune health. Exerc. Immunol. Rev 17, 64–103 (2011). [PubMed] [Google Scholar]
- 196.Campbell P T. et al. Effect of exercise on in vitro immune function: a 12-month randomized, controlled trial among postmenopausal women. J. Appl. Physiol 104, 1648–1655 (2008). [DOI] [PubMed] [Google Scholar]
- 197.Nieman DC et al. Immune response to exercise training and/or energy restriction in obese women. Med. Sci. Sports Exerc 30, 679–686 (1998). [DOI] [PubMed] [Google Scholar]
- 198.Martinez-Carrillo BE, Jarillo-Luna RA, Campos-Rodriguez R, Valdes-Ramos R & Rivera-Aguilar V Effect of diet and exercise on the peripheral immune system in young Balb/c mice. Biomed. Res. Int 2015, 458470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pollard JW Trophic macrophages in development and disease. Nat. Rev. Immunol 9, 259–270 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Catalan V, Gomez-Ambrosi J, Rodriguez A & Fruhbeck G Adipose tissue immunity and cancer. Front. Physiol 4, 275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Koelwyn GJ, Quail DF, Zhang X, White RM & Jones LW Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer 17, 620–632 (2017). [DOI] [PubMed] [Google Scholar]
- 202.Duffaut C, Galitzky J, Lafontan M & Bouloumie A Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem. Biophys. Res. Commun 384, 482–485 (2009). [DOI] [PubMed] [Google Scholar]
- 203.Michelotti GA, Machado MV & Diehl AM NAFLD. NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol 10, 656–665 (2013). [DOI] [PubMed] [Google Scholar]
- 204.Smart NA, King N, McFarlane JR, Graham PL & Dieberg G Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta-analysis. Br. J. Sports Med 52, 834–843 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Goodpaster BH et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 304, 1795–1802 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hallsworth K et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60, 1278–1283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Larson-Meyer DE et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity 16, 1355–1362 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Razavi Zade M et al. The effects of DASH diet on weight loss and metabolic status in adults with nonalcoholic fatty liver disease: a randomized clinical trial. Liver Int. 36, 563–571 (2016). [DOI] [PubMed] [Google Scholar]
- 209.Zhang HJ et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern. Med 176, 1074–1082 (2016). [DOI] [PubMed] [Google Scholar]
- 210.Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB & Kontogianni MD Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism 68, 119–132 (2017). [DOI] [PubMed] [Google Scholar]
- 211.Kenneally S, Sier JH & Moore JB Efficacy of dietary and physical activity intervention in nonalcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 4, e000139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Orci LA et al. Exercise-based Interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin. Gastroenterol. Hepatol 14, 1398–1411 (2016). [DOI] [PubMed] [Google Scholar]
- 213.Quante M & Wang TC Stem cells in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol 6, 724–737 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zeuner A, Todaro M, Stassi G & De Maria R Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell 15, 692–705 (2014). [DOI] [PubMed] [Google Scholar]
- 215.Lee CJ, Dosch J & Simeone DM Pancreatic cancer stem cells. J. Clin. Oncol 26, 2806–2812 (2008). [DOI] [PubMed] [Google Scholar]
- 216.Takaishi S, Okumura T & Wang TC Gastric cancer stem cells. J. Clin. Oncol 26, 2876–2882 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sell S & Leffert HL Liver cancer stem cells. J. Clin. Oncol 26, 2800–2805 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zheng Q et al. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr. Relat. Cancer 18, 491–503 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bellows CF, Zhang Y, Simmons PJ, Khalsa AS & Kolonin MG Influence of BMI on level of circulating progenitor cells. Obesity 19, 1722–1726 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Colditz GA & Peterson LL Obesity and cancer: evidence, impact, and future directions. Clin. Chem 64, 154–162 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.