Abstract
Background
The objective of this study was to evaluate the comparative effects of three breastfeeding promotion interventions on the duration of exclusive breastfeeding (EBF) and any breastfeeding (BF) among human immunodeficiency virus (HIV)-infected women in Uganda.
Methods
Between February 2012 and February 2013, 218 HIV-infected pregnant mothers were randomly assigned to (A) standard care (n=73), (B) enhanced family/peer support (n=72) or (C) enhanced nutrition education (n=73).
Results
The prevalence (%) of EBF/BF did not differ between intervention arms at the sixth (A, 85/92; B, 84/91; C, 87/89) and ninth (A, 17/91; B, 18/89; C, 16/87) postpartum month assessments (p>0.05). However, the risk of early BF cessation differed between intervention arms depending on the mother’s level of formal education (p=0.04). Among women with no formal education, the risk of early BF cessation was 88% (adjusted hazard ratio [aHR] 0.12 [95% confidence interval {CI} 0.05–0.30]) and 93% (aHR 0.07 [95% CI 0.03–0.18]) lower in arms B and C, respectively, than in arm A (p<0.01). HIV status disclosure to a partner was associated with a higher risk of early EBF (p=0.03) and BF (p=0.04) cessation.
Conclusions
In resource-limited settings, enhanced (vs standard care) EBF promotion interventions may not differentially influence EBF but reduce the risk of early BF cessation among women with no formal education. Targeted enhanced interventions among women with no formal education and a mother’s partner may be critical to reducing the risk of early EBF/BF cessation.
Keywords: exclusive breastfeeding, breastfeeding cessation, HIV-infected women, randomized clinical trial
Introduction
Although the negative consequences (e.g. higher risk of respiratory infections,1–3 stunted growth3,4 and infant mortality3) associated with failure to exclusively breastfeed (EBF) infants for at least 6 months postpartum are well established, mothers are often unable to reach this World Health Organization (WHO) recommendation.5 Moreover, extended periods of breastfeeding (BF) are supported by dose-dependent long-term benefits for not just the child (e.g. lower obesity and type 2 diabetes rates, increased intelligence), but also the mother (e.g. lower breast/ovarian cancer and type 2 diabetes rates).6
In resource-limited settings, some studies suggest that cultural norms and limited or lack of knowledge are barriers to the adoption of recommended EBF practices.7–9 Before the WHO 2010 recommendations and guidelines,10 among HIV-infected women, motivation to reduce the risk of mother to child transmission (MTCT) was also linked to early EBF cessation.9 Preoccupied with thoughts about unintentionally infecting the child, it is plausible that mothers may pay less attention to the competing risks to infant health and survival.9,11 Yet, with adherence to antiretroviral (ARV) treatment and appropriate BF practices, MTCT is substantially reduced (<5%) vs no treatment (range 15–45%).12 Recent results from a multisite Promoting Maternal and Infant Survival Everywhere (PROMISE) 1077BF randomized clinical trial conducted in 14 sites in east and southern African show that MTCT is even lower (<1%) with the use of either daily infant nevirapine (NVP) prophylaxis or maternal antiretroviral therapy (ART) through up to 18 months of BF.13
Considering the aforementioned benefits of EBF and emerging evidence on MTCT through BF,14 the 2016 WHO feeding guidelines recommend that HIV-infected mothers on lifelong ART should EBF for 6 months, followed by complementary feeding (i.e. introduction of non–breast milk liquids and/or solids while breastfeeding) until 24 months postpartum.15 This is different from the 2010 WHO guidelines that recommended EBF for 6 months and continued BF while introducing complementary foods until at least 12 months postpartum.10 Replacement feeding is only recommended when it is considered acceptable, feasible, affordable, sustainable and safe (AFASS).10
Mixed findings exist regarding the adoption of these ever-evolving WHO infant feeding recommendations in developing countries.14 Some countries still fall short of these goals among HIV-infected mothers,16–18 including Uganda.19 In Uganda, the low rates of EBF among HIV-infected mothers prompted renewed national efforts to increase awareness and promote the adoption of WHO recommendations through social support and education programmes.20 On a positive note, recent estimates show some parts of sub-Saharan Africa21–24 are achieving >70% adherence based on self-reported EBF rates among HIV-infected women in prevention of MTCT (PMTCT) settings. However, when validated using a deuterium oxide dilution technique that measures non-human milk and water intake among infants, EBF rates have been shown to be lower among HIV-infected and uninfected women (75% vs 43% and 60% vs 24%, respectively).22 This disparity in EBF rates between assessment methods in EBF promotion research underscores the negative role social desirability bias may play in the overestimation of EBF among HIV-infected women, suggesting a need for cautious optimism.
Globally, EBF promotion interventions (e.g. education, community health worker and peer support programmes) show promise with respect to increasing the duration of EBF in both high- and low-income countries.25,26 However, contradictory findings exist among HIV-infected women in resource-limited settings. For example, results from a recent study in South Africa27 showed EBF adherence at 3 months was 53% (adjusted risk ratio 1.53 [95% confidence interval {CI} 1.22–1.94]) higher among HIV-infected women randomized to a community health worker home visitation intervention (24%) than standard care (16%). In contrast, a different randomized trial in the same country showed there was no difference in EBF rates (p=0.67) between mothers randomized to a peer support group (45%) vs standard care (43%).28 Even wider EBF rate variations are reported for observational studies conducted in different African countries.29 Evidently the effectiveness of different EBF promotion interventions varies in different contexts; however, literature on the comparative effects of these interventions in similar settings is limited.
The objective of this randomized study was to evaluate the comparative effects of three breastfeeding promotion interventions (enhanced peer support and nutrition education vs standard care) on the duration of EBF and BF among HIV-infected women in Uganda. We hypothesized that women randomly assigned to enhanced EBF promotion interventions would have a lower risk of early EBF and BF cessation than those receiving standard care.
Materials and methods
Study population
Participants referred to the study were pregnant women who had undergone routine counselling and testing, had positive HIV rapid test results and were either on PMTCT drug regimens (zidovudine [AZT], WHO option A) or Option B+ or highly active antiretroviral therapy (HAART) as recommended at the time by the Uganda Ministry of Health (MOH).
Study sample and setting
We recruited HIV-positive pregnant women (≥18 y) in their late second or third trimester from U.S. President’s Emergency Plan for AIDS Relief (PEPFAR)-supported PMTCT sites, Mulago Hospital antenatal clinic and postnatal follow-up clinics located in Kampala, Uganda from February 2012 to February 2013. More than 33 000 pregnant women receive same-day rapid opt-out screening for HIV infection annually at Mulago Hospital antenatal clinics. These clinics identify about 3300 HIV-infected women annually (i.e. seroprevalence of 10%). All pregnant women recruited in this study continued to get routine antenatal and PMTCT follow-up clinic visits and ARV drugs as per the PMTCT schedule.
Ethical considerations
Institutional review boards and ethics committees at the Joint Clinical Research Centre and Uganda National Council for Science and Technology and Johns Hopkins Medicine in the USA approved the study and study participants provided written consent.
Eligibility and exclusion criteria
Mother–infant pairs were evaluated for study eligibility immediately after delivery. Eligible participants were HIV-positive mothers (≥18 y) who had a live birth, received ARV treatment as part of the PMTCT programme or those who planned to receive HAART as recommended by the Uganda MOH guidelines, delivered at Mulago Hospital, planned to breastfeed their children immediately after birth, resided in Kampala City or planned to stay within the Kampala district for at least 9 months postpartum, were willing to be home visited during the postpartum period and were able to bring a close family member (≥18 y) to the clinic who would be able to be in touch with the participant at least three times a week and to give support on EBF. Exclusion criteria were having a stillbirth or premature delivery and mother or infant sickness that did not allow for study participation as judged by the study staff (attending physician). Specifically, we excluded mothers if they had a breast abscess, mastitis, hepatitis B or C, psychosis or had a life-threatening illness or birth condition incompatible with life; had an infant who was unable to breastfeed due to health reasons (e.g. oral lesions); or refused to breastfeed after delivery.
Study design
This was a single-centre, parallel group, three-arm randomized trial. Participants were randomly assigned to one of three intervention arms using a 1:1:1 ratio to standard care support (arm A), enhanced family/peer support (arm B) or enhanced nutrition demonstrations (arm C).
Randomization
Eligible participants who provided both verbal and written consent for study participation completed the baseline assessment and were randomized to study arm A, B or C. Following simple randomization procedures, random assignment was programmatically determined (using random number generators) immediately after the completion of baseline assessments. Blinding participants, family/peer support and study staff to intervention arm allocation was practically impossible because of the distinct observable intervention differences during study implementation (see descriptions below). However, outcome assessors and data analysts were blinded to study arm assignments to prevent detection and interpretation bias.
Study interventions
All intervention messages were compliant with the 2010 WHO HIV and infant feeding guidelines10 and Uganda’s national guidelines.30 For detailed descriptions of study interventions, see supplementary files.
Arm A: standard messages. This group received the current MOH PMTCT messages on HIV and infant feeding promoting EBF during scheduled antenatal and postnatal follow-up clinic visits with counselling and support from PMTCT counsellors. Standard MOH counselling messages on HIV and infant feeding included group sessions and videos that emphasized the importance of EBF, appropriate breastfeeding practice and maternal nutrition. Mothers also received printed materials on how to practise EBF.
Arm B: enhanced family/peer support. In addition to all arm A intervention activities described above, this group included family members (or friends) and hospital-based peer mothers who supported a mother’s EBF practices for at least 6 months at home and in the hospital, respectively.
Arm C: enhanced nutrition education. In addition to all arm A intervention activities described above, this group received clinic-based coaching on techniques of BF and ‘hands-on’ demonstrations of safe preparation of locally available nutritious foods by a special infant feeding counsellor.
Across the intervention arms (A, B and C), training and information sessions were delivered at 2, 6, 10 and 14 weeks and at 6 months postpartum. The duration of sessions ranged from 30 to 45 min across intervention arms (but were longer in arm C than in arm A or B due to the additional hands-on demonstrations).
Primary outcome
The operational definition of EBF was based on the WHO infant feeding recommendations in the context of HIV.10 For our analyses we defined early EBF cessation as not meeting the WHO recommendations at anytime before the ninth postpartum month.
Secondary outcome
Early BF cessation was operationally defined as starting replacement feeding (i.e. infant receives no breast milk and is being fed suitable breast milk substitutes in the form of commercial infant formula or animal milk) before the ninth postpartum month.
Power and sample size
Our study sample size calculations were based on a superiority hypothesis with a 65% lower hazard (risk) of early EBF cessation among arm B or C than arm A participants (hazard ratio 0.65). To achieve 80% power, we needed at least 67 participants in each arm of the study (A, B and C) to be able to reject the null hypothesis that the experimental and control hazard rates are equal at a type I error probability of 0.05. We assumed an accrual period of 365 days and an additional 365 days for study follow-up.
Data collection
Study interviews were conducted in a local Ugandan language (Luganda) by study counsellors at baseline (i.e. delivery) with follow-up data at 2, 6, 10 and 14 weeks and 6 and 9 months using structured questionnaires at the hospital during mother–infant dyad clinic visits. Data collected included sociodemographic characteristics, HIV status disclosure and infant feeding information.
Sociodemographic characteristics included maternal age (in years), education (years of formal education), self-reported religious affiliation, marital status, occupation, type of housing, number of household members, monthly household income, whether the participant earns income by working outside the home and pregnancy/live birth history. We also assessed for HIV status disclosure to a current partner and any family member (including friends).
We collected infant feeding information using a standardized WHO infant feeding questionnaire that included the type of infant feeding and the introduction of other liquids or solids since the last visit. At the baseline and 6-month visit, study counsellors assessed each mother for AFASS criteria needed for replacement or complementary feeding and intent to continue breastfeeding after 6 months.
All completed questionnaires were reviewed by the study coordinator/designer to ensure completeness in real time before participants left the clinic. Thereafter, double data entry was employed to ensure accurate data entry on all study-enrolled mother–infant dyads.
Statistical methods
We followed study participants prospectively for 9 months postpartum to determine the time to EBF and BF cessation. Participants who did not stop EBF/BF during the study follow-up and those who dropped out of the study before EBF/BF cessation were right-censored. We compared the distribution of baseline participant characteristics between intervention arms to assess the integrity of randomization procedures. We implemented intent-to-treat–based analyses to compare intervention effects on the risk of early EBF and BF cessation.
Differences in the overall survival experiences (i.e. probability of time to EBF and BF cessation) between intervention arms and participant characteristics were examined using non-parametric Kaplan–Meier (KM) procedures with appropriate adjustments for multiple group comparisons at a 0.05 level of significance. We used Cox proportional hazards regression models to examine whether intervention effects on the risk of early EBF and BF cessation differed by study arm while controlling for differences in other participant characteristics. The adequacy of modelling assumptions (e.g. constant hazard ratio over time and a multiplicative relationship between covariates and the hazard) were tested and confirmed to be adequate in our final models. In the absence of significant two-way interactions, main and potential confounder effects were examined. We conducted confounder-adjusted analysis to control for selection bias introduced due to differential loss of follow-up related to participant characteristics. A covariate was considered a confounder if its inclusion or deletion from a model resulted in a >10% difference in the regression parameter of the variable of interest (i.e., main effect). All Cox proportional hazards models were adjusted for missing data bias under the assumption that data were missing at random, and partial likelihood was used to estimate regression parameters employed to generate hazard ratios. All statistical analyses were implemented using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Summary of study participants and follow-up
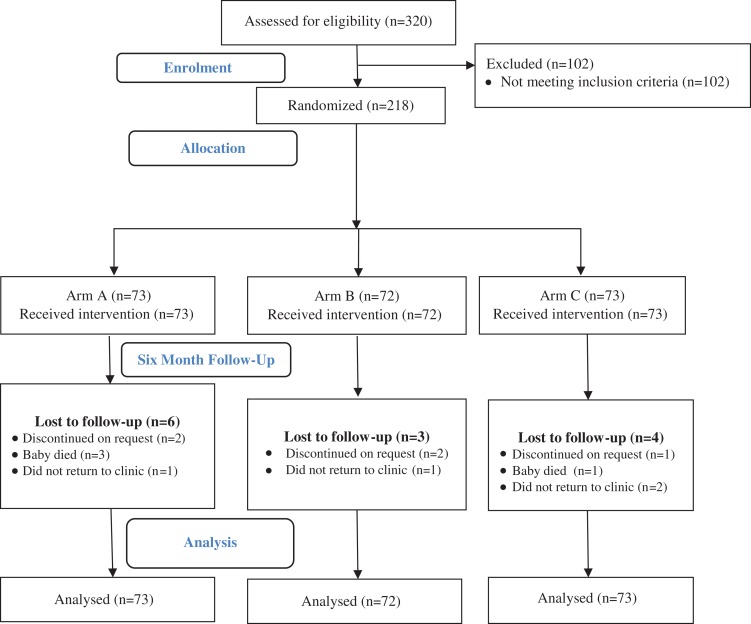
Of the 1571 referred and screened pregnant mothers, only 320 (20%) agreed to study participation; 68% (n=218) of the mothers were eligible and 32% (n=102) were excluded (Figure 1). There was an equal assignment to intervention arms (A, 73; B, 72; C, 73). The median follow-up time between intervention arms was not different (p=0.87); overall participants were followed for a median of 252 days (interquartile range 7 d). Study attrition was 7% and 21% at the 6- and 9-month follow-ups, respectively. All participants assessed at 6 months (n=203) met AFASS criteria for replacement or complementary feeding. Table 1 summarizes the distribution of study participant characteristics by intervention arm. With the exception of monthly household income (p=0.02), other baseline participant characteristics did not differ between the intervention arms (p>0.05). However, this income differential across intervention arms is confounded by the fact that 50% of the participants did not report their income.
Figure 1.
Study flow diagram showing eligibility assessment, randomized intervention arm assignments, participant follow-up and number of participants included in the analysis.
Table 1.
Baseline characteristics of the study population by study arm
| Total (N=218) | Standard (n=73) | Family/peer (n=72) | Nutrition (n=73) | p-Value | |
|---|---|---|---|---|---|
| Maternal age (y), mean (SD) | 34 (6) | 34 (6) | 34 (7) | 34 (6) | 0.635 |
| Formal level of educationa, n (%) | |||||
| None | 14 (6.5) | 3 (4.2) | 6 (8.3) | 5 (6.9) | 0.329 |
| Some primary school | 90 (41.5) | 31 (43.1) | 35 (48.6) | 24 (32.9) | |
| Some secondary school | 104 (47.9) | 35 (48.6) | 30 (41.7) | 39 (53.4) | |
| Some tertiary/university or higher | 9 (4.2) | 3 (4.2) | 1 (1.4) | 5 (6.9) | |
| Years in school, n (%) | |||||
| <5 | 27 (12) | 9 (13) | 10 (14) | 8 (11) | 0.870 |
| ≥5 | 190 (88) | 63 (87) | 62 (86) | 65 (89) | |
| Religiona, n (%) | |||||
| Protestant | 65 (29.8) | 16 (21.9) | 22 (30.6) | 27 (37) | 0.158 |
| Catholic | 75 (34.4) | 24 (32.9) | 24 (33.3) | 27 (37) | |
| Muslim | 52 (23.8) | 23 (31.5) | 18 (25) | 11 (15.1) | |
| Born again | 21 (9.6) | 9 (12.3) | 5 (6.9) | 7 (9.6) | |
| Adventist | 4 (1.8) | 1 (1.4) | 3 (4.2) | 0 (0) | |
| Other | 1 (0.5) | 0 (0) | 0 (0) | 1 (1.37) | |
| Marital statusa, n (%) | |||||
| Never married | 29 (13.3) | 9 (12.3) | 9 (12.5) | 11 (15.1) | 0.884 |
| Married | 15 (6.9) | 5 (6.9) | 4 (5.6) | 6 (8.2) | |
| Cohabiting | 161 (73.9) | 56 (76.7) | 55 (76.4) | 50 (68.5) | |
| Separated | 12 (5.5) | 3 (4.1) | 3 (4.2) | 6 (8.2) | |
| Widow | 1 (0.5) | 0 (0) | 1 (1.4) | 0 (0) | |
| Monthly household income (Ugandan shillings)a, n (%) | |||||
| ≤100 000 (<US$28) | 63 (29.0) | 14 (19.4) | 23 (31.9) | 26 (35.6) | 0.019 |
| 100 000–500 000 (US$28–138) | 44 (20.3) | 11 (15.3) | 13 (18.1) | 20 (27.4) | |
| ≥500 000 (>US$138) | 1 (0.5) | 1 (1.4) | 0 (0) | 0 (0) | |
| Didn’t know (unknown) | 109 (50.2) | 46 (63.9) | 36 (50) | 27 (37) | |
| Does mother earn income by working outside the home?b, n (%) | |||||
| Yes | 118 (53.9) | 32 (43.8) | 39 (53.4) | 47 (64.4) | 0.055 |
| No | 101 (46.1) | 41 (56.2) | 34 (46.6) | 26 (35.6) | |
| Occupationa, n (%) | |||||
| Skilled | 26 (22.2) | 4 (12.5) | 8 (21.1) | 14 (29.8) | 0.137 |
| Unskilled | 23 (19.7) | 8 (25) | 4 (10.5) | 11 (23.4) | |
| Small business | 68 (58.1) | 20 (62.5) | 26 (68.4) | 22 (46.8) | |
| Type of housing, n (%) | |||||
| Own house | 44 (20.5) | 11 (15.5) | 14 (19.4) | 19 (26.4) | 0.248 |
| Rent house | 23 (10.7) | 12 (16.9) | 8 (11.1) | 3 (4.2) | |
| Rent room/apartment | 138 (64.2) | 44 (61.9) | 47 (65.3) | 47 (65.3) | |
| Other (employment-based housing or stay with relative)c, n (%) | 10 (4.7) | 4 (5.6) | 3 (4.2) | 3 (4.2) | |
| Number of household members, median (IQR) | 4 (2) | 4 (2) | 4 (2) | 4 (2) | 0.245 |
| HIV status disclosed to current partner, n (%) | 123 (57.8) | 42 (59.2) | 42 (59.2) | 39 (54.9) | 0.841 |
| HIV status disclosed to a family member/friendd, n (%) | 152 (70.4) | 54 (75.0) | 54 (75.0) | 44 (61.1) | 0.109 |
| Type of ARVsb, n (%) | |||||
| AZT only | 31 (14.2) | 10 (13.7) | 9 (12.5) | 12 (16.4) | 0.951 |
| HAART | 112 (51.4) | 37 (50.7) | 37 (51.4) | 38 (52.1) | |
| Option B+ | 75 (34.4) | 26 (35.6) | 26 (36.1) | 23 (31.5) | |
| Previous pregnancies, median (IQR)e | 3 (2) | 3 (2) | 3 (3) | 3 (2) | 0.413 |
| Live births, median (IQR)e | 1 (2) | 2 (2) | 2 (1) | 1 (3) | 0.873 |
| Follow-up (d), median (IQR)e | 252 (3) | 252 (3) | 252 (4) | 253 (3) | 0.868 |
aFisher’s exact test.
bχ2 test.
cOther: employment-based housing (n=6) or stay with relative (n=4).
dAt least one family member knows, including parents, grandparents, siblings, aunt, uncle, mother-in-law, father-in-law, other family members and friends.
eKruskal–Wallis test.
Risk of early EBF cessation
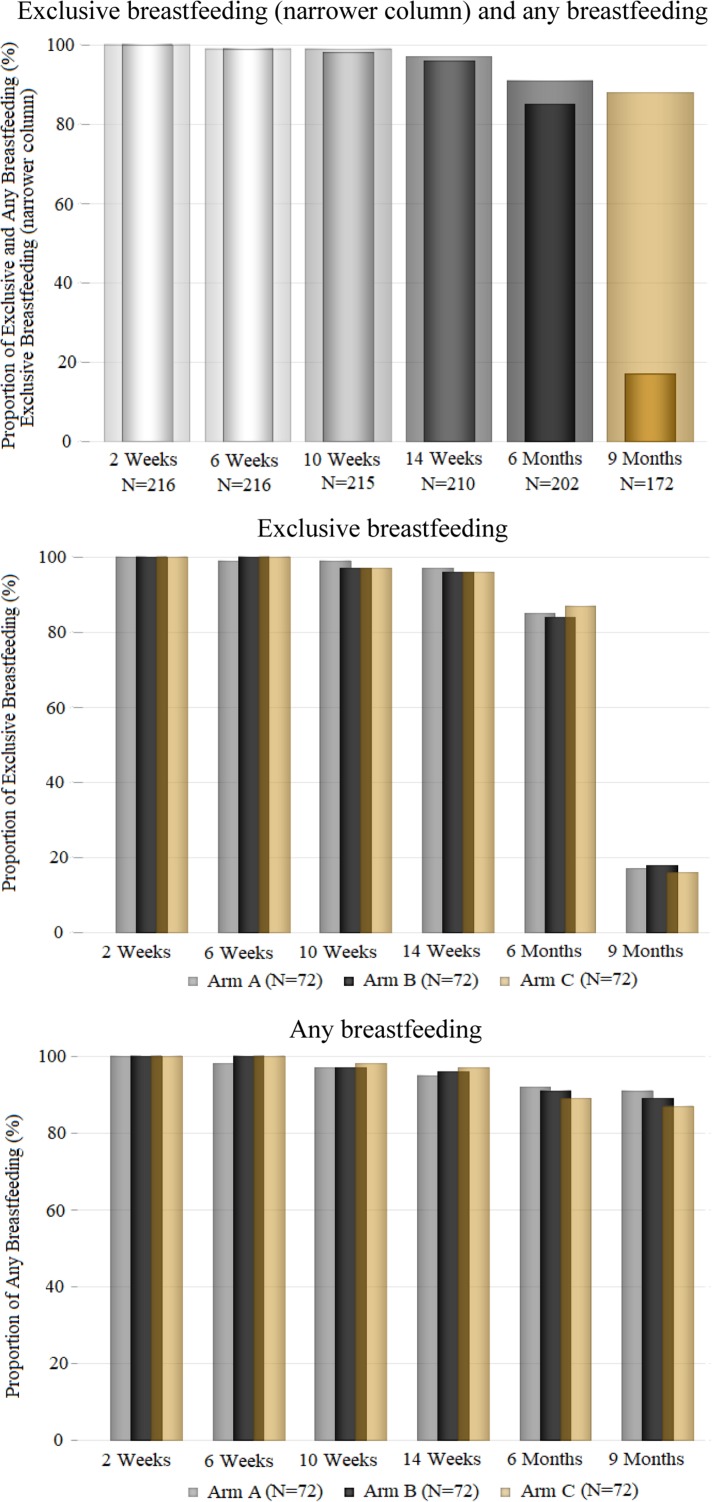
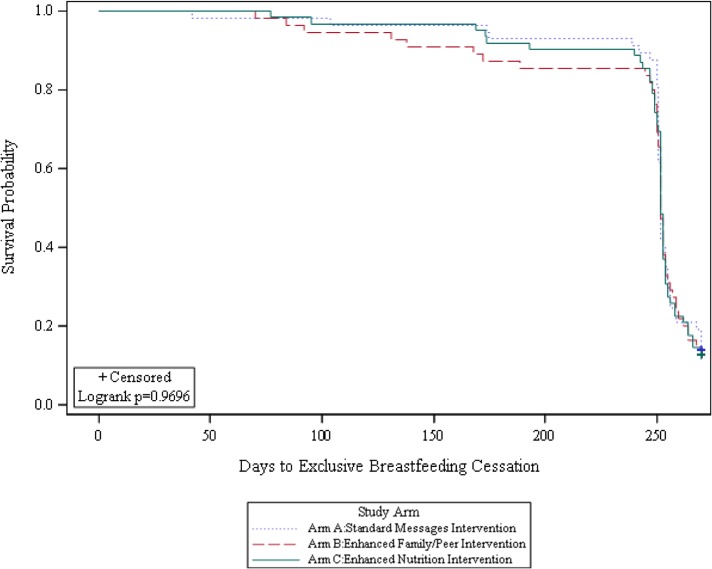
EBF rates were not different between the intervention arms at the 6-month (A, 85%; B, 84%; C, 87%; p=0.47) and 9-month (A, 17%; B, 18%; C, 16%; p=0.97) follow-ups (Figure 2). KM plots showed that the probability of time to EBF cessation did not differ between intervention arms during follow-up (Figure 3).
Figure 2.
Overall and intervention-specific rates of EBF and any BF among study participants during a 9-month follow-up, 2012–2013.
Figure 3.
Plot of the KM estimate of the survival function (probability) of EBF by intervention arm assignment.
Similar to KM analyses, multivariable Cox proportional hazards regression results (Table 2) showed that the risk of early EBF cessation did not differ between intervention arms (p=0.67) even after adjusting for household size, type of housing, HIV status disclosure to current partner and HIV treatment regimen effects. Independent of intervention effects, the risk of early EBF cessation was 56% lower among participants on AZT HIV-related treatment vs those on Option B+ treatment (adjusted hazard ratio [aHR] 0.44 [95% CI 0.20–0.96]) and 10% lower in households with one additional household member (aHR 0.90 [95% CI 0.81–0.99]). On the other hand, participants who disclosed their HIV status to current partners had a 93% higher risk of EBF cessation than those who did not (aHR 1.93 [95% CI 1.10–3.41]). The risk of early EBF cessation was 3.6 times higher among participants who lived in employment-based housing or with relatives than those who lived in houses they owned (aHR 3.62 [95% CI 1.03–12.74]). Other participant characteristics were not associated with the risk of early BF cessation (Table 2).
Table 2.
Cox proportional hazards model regression results of the association between time to EBF and BF cessation and selected covariates summarized as crude and adjusted hazard ratios (with 95% CI)
| Risk of early EBF cessation | Risk of early BF cessation | |||
|---|---|---|---|---|
| Characteristics | Crude HR (95% CI) | aHR (95% CI) | Crude HR (95% CI) | aHR (95% CI) |
| Study arm | ||||
| A: standard intervention | Reference | Reference | Reference | Reference |
| B: family/peer intervention | 1.21 (0.74–2.00) | 1.39 (0.74–2.60) | ||
| C: nutrition intervention | 0.95 (0.58–1.55) | 1.20 (0.66–2.21) | ||
| Formal education | ||||
| None (0 y) | Reference | Reference | Reference | Reference |
| Some primary school (1–7 y) | 0.97 (0.49–1.93) | 1.26 (0.56–2.82) | ||
| Some secondary school (8–13 y) | 0.89 (0.45–1.77) | 1.52 (0.64–3.58) | ||
| Some university or higher (>13 y) | 0.87 (0.27–2.78) | 1.83 (0.38–8.63) | ||
| HIV treatment indication | ||||
| Option B+ | Reference | Reference | Reference | Reference |
| AZT | 0.57 (0.29–1.13) | 0.44 (0.20–0.96) | 1.08 (0.56–2.09) | 0.97 (0.46–2.08) |
| HAART | 1.01 (0.67–1.54) | 0.74 (0.42–1.31) | 1.38 (0.89–2.14) | 1.15 (0.65–2.02) |
| Religion | ||||
| Protestant | Reference | Reference | Reference | Reference |
| Catholic | 1.10 (0.68, 1.77) | 1.21 (0.71, 2.07) | 1.83 (1.11–3.02) | 2.31 (1.27–4.20) |
| Muslim | 1.58 (0.93–2.69) | 1.94 (1.06–3.59) | 1.69 (0.99–2.92) | 1.78 (0.94–3.37) |
| Born again | 0.66 (0.29–1.50) | 0.89 (0.33–2.46) | 0.72 (0.30–1.68) | 1.25 (0.41–3.84) |
| Adventist | 3.02 (0.72–12.78) | 2.00 (0.38–10.47) | 2.58 (0.77–8.58) | 2.55 (0.62–10.42) |
| Other | 0.95 (0.13–6.96) | 1.50 (0.11–21.02) | 1.05 (0.14–7.74) | 0.21 (0.01–12.20) |
| Marital status | ||||
| Married | Reference | Reference | Reference | Reference |
| Never married | 0.92 (0.35–2.46) | 1.51 (0.45–5.04) | 0.99 (0.36–2.41) | 1.09 (0.35–3.45) |
| Cohabiting | 1.27 (0.55–2.92) | 1.22 (0.48–3.10) | 0.96 (0.42–2.21) | 1.06 (0.40–2.64) |
| Separated | 1.62 (0.54–4.82) | 1.89 (0.52–6.89) | 1.21 (0.41–3.61) | 1.03 (0.29–3.64) |
| Widow | 2.89 (0.34–24.32) | 2.18 (0.19–24.64) | 1.50 (0.18–12.49) | 0.90 (0.08–9.94) |
| Monthly household income (Ugandan shillings) | ||||
| ≤100 000 (<US$28) | Reference | Reference | Reference | Reference |
| 100 000–500 000 (US$28–$138) | 0.99 (0.65–1.51) | 0.94 (0.55–1.58) | 0.96 (0.63–1.46) | 0.89 (0.55–1.46) |
| ≥500 000 (>US$138) | – | – | – | – |
| Didn’t know (unknown) | 0.96 (0.53–1.73) | 1.24 (0.56–2.75) | 1.05 (0.59–1.89) | 1.96 (0.87–4.40) |
| Mother’s occupation | ||||
| Unskilled | Reference | Reference | Reference | Reference |
| Skilled | 1.03 (0.56–1.89) | 1.25 (0.63–2.48) | 0.86 (0.47–1.56) | 0.92 (0.40–2.11) |
| Small business | 1.02 (0.61–1.71) | 1.43 (0.75–2.73) | 0.82 (0.49–1.37) | 0.70 (0.36–1.36) |
| Type of housing | ||||
| Own house | Reference | Reference | Reference | Reference |
| Rent house | 0.72 (0.34–1.52) | 1.53 (0.58–4.05) | 0.82 (0.38–1.79) | 0.88 (0.34–2.24) |
| Rent room/apartment | 0.79 (0.47–1.30) | 1.33 (0.70–2.52) | 0.90 (0.55–1.49) | 0.83 (0.46–1.53) |
| Other (employment-based housing or stay with relative) | 1.04 (0.39–2.78) | 3.62 (1.03–12.74) | 2.52 (0.94–6.79) | 2.63 (0.82–8.36) |
| Number of household members | 0.99 (0.92–1.06) | 0.90 (0.81–0.99) | 1.02 (0.92–1.14) | 1.01 (0.88–1.17) |
| Disclosed HIV status to current partner | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 1.48 (0.99–2.21) | 1.93 (1.10–3.41) | 1.53 (1.01–2.32) | 1.66 (1.02–2.69) |
| Disclosed HIV status to at least one family member | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.85 (0.55–1.31) | 0.74 (0.42–1.28) | 0.89 (0.58–1.38) | 0.74 (0.44–1.25) |
| Number of live births | 1.17 (1.01–1.36) | 1.18 (0.95–1.48) | 1.13 (0.97–1.31) | 1.06 (0.85–1.31) |
| Number of previous pregnancies | 1.09 (0.96–1.24) | 1.10 (0.89–1.36) | 1.07 (0.94–1.23) | 1.01 (0.79–1.28) |
| Maternal age | 1.03 (0.99–1.07) | 1.01 (0.96–1.07) | 1.00 (0.96–1.04) | 0.98 (0.92–1.03) |
p<0.05 in bold.
Covariates examined as potential confounders in the final models included maternal age, HIV treatment indication, religion, marital status, personal income, occupation, type of housing, number of household members, disclosure of HIV status–current partner or any family member, number of live births and number of pregnancies.
Risk of early BF cessation
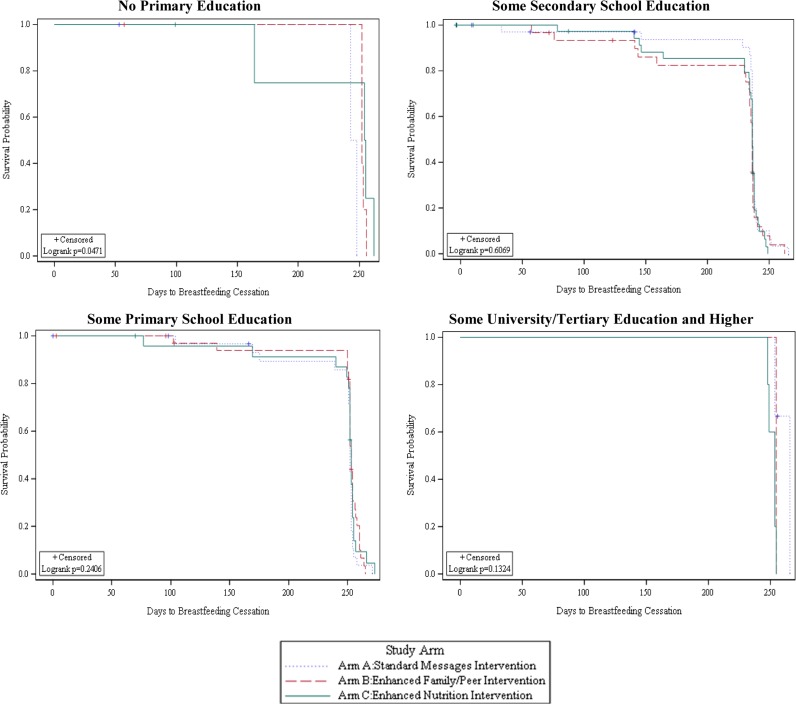
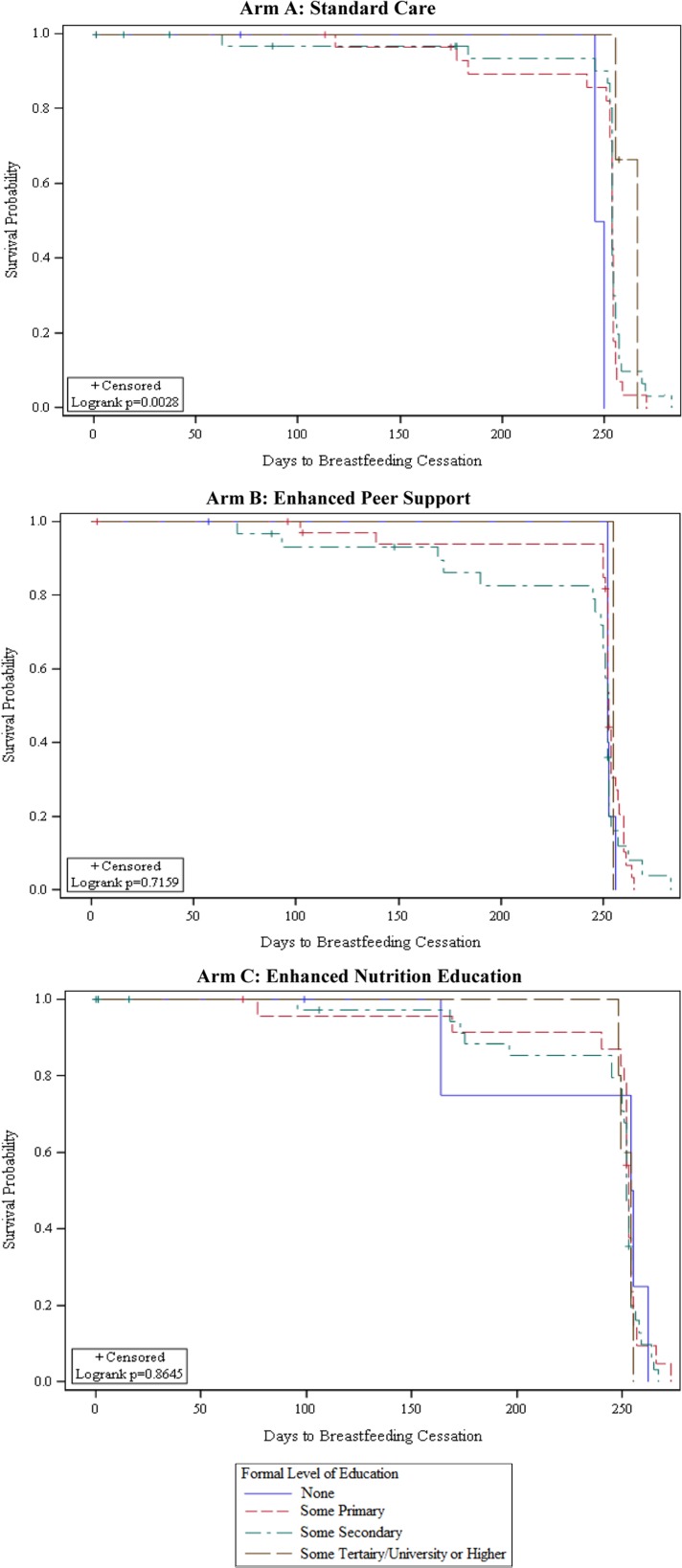
In contrast to EBF, any BF rates remained high (>87%) throughout the follow-up in all study arms (Figure 2). The probability of time to BF cessation differed between intervention arms depending on the participant’s level of formal education (Figure 4). Among participants with no formal education, the probability of longer BF duration was higher in the enhanced intervention arms (B and C) than in arm A (p=0.05). In intervention arm A, the probability of longer BF duration was higher among participants with some formal education than participants who had no formal education (Figure 5).
Figure 4.
Plot of the KM estimate of survival function (probability) of any BF by intervention arm assignment and level of education.
Figure 5.
Plot of the KM estimate of survival function (probability) of any BF by level of education within intervention arms.
Multivariable Cox proportional hazards regression results (Table 3) showed that the risk of early BF cessation differed between intervention arms depending on the mother’s level of education (intervention × education interaction; p=0.04). Among mothers with no formal education, the risk of early BF cessation was 88% (aHR 0.12 [95% CI 0.05–0.30]) and 93% (aHR 0.07 [95% CI 0.03–0.18]) lower in arm B and C than arm A, respectively, adjusted for religious affiliation and HIV status disclosure to a current partner. At higher levels of education, the risk of early BF cessation was not different between intervention arms (Table 3). After controlling for intervention effects, Catholic religious affiliation was associated with a higher risk of early BF cessation than Protestant affiliation (aHR 2.31 [95% CI 1.27–4.20]). Participants who disclosed their HIV status to their current partner had a 66% higher risk of early BF cessation than those that did not (aHR 1.66 [95% CI 1.02–2.69]).
Table 3.
Cox proportional hazards model regression results of intervention effects on risk of early BF cessation by education level summarized using aHR (95% CI)
| No formal education | Some primary school | Some secondary school | Some university | |||||
|---|---|---|---|---|---|---|---|---|
| Arm | Crude HR (95% CI) | aHR (95% CI) | Crude HR (95% CI) | aHR (95% CI) | Crude HR (95% CI) | aHR (95% CI) | Crude HR (95% CI) | aHR (95% CI) |
| A | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| B | 0.14 (0.06–0.35) | 0.12 (0.05–0.30) | 0.79 (0.38–1.71) | 0.91 (0.43–1.94) | 0.78 (0.35–1.75) | 0.89 (0.39–2.02) | 2.75 (0.17–45.47) | 1.44 (0.08–27.03) |
| C | 0.08 (0.03–0.19) | 0.07 (0.03–0.18) | 0.81 (0.39–1.73 | 0.90 (0.39–2.05) | 0.77 (0.37–1.62) | 0.79 (0.37–1.68) | 5.75 (0.50–66.62) | 17.01 (0.88–33.28) |
p<0.05 in bold.
Adjusted for confounding by religious affiliation.
Discussion
Our findings show that enhanced intervention (involving family/peer support and nutrition-based education) effects on EBF promotion are not different when compared with standard practice in PMTCT programme settings. However, these enhancements lower the risk of early BF cessation among women with no formal education. Independent of intervention effects, disclosure of HIV status to a partner and living in employment-based housing or with a relative were associated with a higher risk of early EBF cessation. The risk of early BF cessation was higher among mothers who self-reported Catholic religion affiliation and disclosed their HIV status to their partners. In contrast, the receipt of AZT HIV-related treatment and larger household size were associated with a lower risk of early EBF cessation.
Risk of early EBF cessation
The null finding regarding the effect of enhanced intervention (vs standard care) on EBF promotion is consistent with some28 but not all24,27 existing randomized trial results in sub-Saharan Africa. Moreover, intervention effect sizes examined in previous studies are different compared with our study at 6 months postpartum despite being carried out within the context of country-level PMTCT programmes. For example, intervention vs standard care EBF rates involving community health worker (CHW) home visits were 24% vs 16%,27 peer home visits were 43% vs 45%28 and peer counsellors were 73% vs 22% (in Burkina Faso), 59% vs 15% (in Uganda) and 2% vs 1% (in South Africa).24 These interventions also involved a different frequency/intensity and timing of delivery contacts. The fact that our intervention effects are different when compared with similar interventions in other countries underlines the argument for differential effects of culture and norms on EBF behaviour and promotion. For interventions that involve PMTCT counsellors, our findings suggest enhanced peer support and nutrition education do not accrue additional benefit. Future research needs to examine the comparative effects of CHW, peer support and nutrition education with and without PMTCT counsellor support, the background condition that was common to all our intervention arms. These comparisons will need to control for the potential differential effects of frequency/intensity and timing of intervention activities.29
In our study, the relatively high prevalence of EBF at 6 months across study arms (compared with recent global5 and national estimates31) may be explained by our restrictive eligibility criteria that required a self-reported intention to breastfeed after delivery, receipt of prenatal care and hospital delivery, factors known to be associated with EBF adherence.31 Moreover, all study participants were exposed to general infant feeding messages and education offered by hospital midwives/nurses and PMTCT counsellors during routine antenatal and postpartum care visits.
Independent of intervention effects, our findings also highlight the benefits of larger households on EBF adherence. Specifically, our results suggest that even after adjusting for other indicators of socio-economic status (e.g. maternal occupation, housing and education), having additional family support reflected in household size may be beneficial for EBF adherence among HIV-infected women. This benefit, however, may not be apparent in scenarios where extended family influence may function to undermine EBF adherence through misconceptions about an HIV-infected nursing mother’s breast milk (e.g. superiority of formula) and the risk to an infant.32,33
The lower risk of EBF cessation among mothers receiving AZT vs Option B+ HIV treatment regimens is a new finding that warrants further research. Existing evidence on the side effects of HIV treatment among infants born to HIV-infected mothers suggests daily NVP in an infant’s saliva may irritate a mother’s nipple.34 Additionally, other side effects such as oral lesions, conjunctivitis and blistering may contribute to the risk of early EBF cessation due to their influence on quality and frequency of breastfeeding.35 Further, because both Option A (includes AZT) and Option B+ HIV treatment regimens require NVP for 4–6 weeks or until BF cessation, it is unclear why EBF cessation would be different between these treatment groups. The potential influence of drug interactions and side effects of NVP involving Option A vs B and their effect on BF quality needs further research.
The negative effect of HIV status disclosure to a partner on the risk of early EBF (and BF) cessation is consistent with previous studies.17,21,36 Nursing mothers may have reduced autonomy in decision making about infant feeding, especially if a partner/spouse has divergent views on the need for BF or the risk it poses to an infant’s health and is the sole financial provider.9,33 If this is true, in addition to empowerment of women, EBF/BF promotion interventions should target both a nursing mother and her current partner.
Risk of early BF cessation
The enhanced intervention benefit (i.e. lower risk of early BF cessation) observed among mothers with no formal education might be explained by the additional study contacts and activities involving peers or counsellors received in the enhanced study arms (B and C). This argument is supported by findings from a previous cross-sectional study in Uganda that showed a dose–response gradient for longer BF duration as the number of counselling visits increased.36 It is also plausible that the increased health care contact as a function of the additional study contacts and activities may have mediated a longer duration of BF in the enhanced intervention arms vs standard care. The lack of enhanced intervention benefit among women with at least some formal education is consistent with previous general population studies that show higher levels of education are independently associated with a longer duration of BF.37–39
The lower risk of early BF cessation among mothers with some (vs no) formal education in the standard care arm (A) but no education effect in the enhanced intervention arms (B and C) suggests two things. One, standard care among mothers with some formal education may be sufficient to achieve desired BF goals. Two, enhancing standard care with family/peer support or nutrition education attenuates the risk of early BF cessation among mothers with no formal education. On the other hand, it is also plausible that formal education may be a proxy for other factors (e.g. culture and norms) that may be associated with the risk of early BF cessation; this should be explored further in future research.
The higher risk of early BF cessation among Catholic- vs Protestant-affiliated mothers in our study may be explained by the low rates of contraceptive use among Catholic women.40 These low rates of contraceptive use are associated with shorter birth intervals and consequently early BF cessation.41 Still, qualitative research is needed to understand why Catholic-affiliated mothers are at higher risk of early BF cessation than other religions. This information could be useful in designing targeted BF promotion strategies in this population.
Study limitations
This study has some limitations. First, EBF/BF was determined based on self-reports, which are prone to both recall and social desirability bias (misclassification). Secondly, although we had different appointment days for participants in the different arms during follow-up to decrease the likelihood of intervention mixing between study arms, we cannot rule out the potential for contamination bias due to information sharing between participants in different intervention arms. Thirdly, given that 50% of the participants did not report their monthly household income (further analyses involving income effects were not instituted), it remains unclear what effect income may have had on the risk of early EBF/BF cessation. Still, the higher risk of early EBF cessation among mothers who live in employment-based housing or with a relative points to known negative effects associated with low socio-economic status.9 Fourthly, we did not examine some factors such as spouse education, income and religion of family members (including spouse) known to influence BF behaviour.9,42 Finally, the relatively small sample sizes and low power related to some of our stratified analyses call for a cautious interpretation of study findings and warrant replication in larger-scale studies.
Conclusions
In summary, our results suggest that enhanced (vs standard care) EBF promotion interventions may not differentially influence EBF but reduce the risk of early BF cessation among women with no formal education in resource-limited PMTCT program settings. Targeted interventions among women with no formal education and a nursing mother’s partner are critical to reducing the risk of early EBF and BF cessation. Further research is needed to understand the contexts that mediate the relationships between religious beliefs, HIV treatment regimen (i.e. side effects on breast or infant health) and BF practices to inform intervention programming.
Supplementary Material
Acknowledgments
Authors’ contributions: JNM, AHO, CO, MM, PEN, MMO, PM, MN and MGF conceived the study. All authors contributed to the design of the study. AHO analysed the data. JNM, AHO, CO, MM, PEN, MMO, PM, MN and MGF interpreted the data. JNM and AHO drafted the manuscript. All authors critically revised the manuscript for intellectual content and read and approved the final manuscript. JNM and AHO are guarantors of the paper.
Acknowledgements: We thank the study-enrolled participants, study coordinators and the MU-JHU research team, whose commitment and hard work have made this research possible.
Funding: This work was supported by the Division of AIDS, National Institute of Allergy and Infectious Diseases at the National Institutes of Health (UM1-AI-069530).
Competing interests: None declared.
Ethical approval: Ethical approval for this research was received from the institutional review boards and ethics committees at the Joint Clinical Research Centre and Uganda National Council for Science and Technology and Johns Hopkins Medicine in the USA.
References
- 1. Biesbroek G, Bosch AA, Wang X et al. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am J Respir Crit Care Med 2014;190(3):298–308. [DOI] [PubMed] [Google Scholar]
- 2. Duijts L, Jaddoe VW, Hofman A et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 2010;126(1):e18–25. [DOI] [PubMed] [Google Scholar]
- 3. Black RE, Victora CG, Walker SP et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 4. Keino S, Plasqui G, Ettyang G et al. Determinants of stunting and overweight among young children and adolescents in sub-Saharan Africa. Food Nutr Bull 2014;35(2):167–78. [DOI] [PubMed] [Google Scholar]
- 5. Cai X, Wardlaw T, Brown DW. Global trends in exclusive breastfeeding. Int Breastfeed J 2012;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Victora CG, Bahl R, Barros AJD et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387(10017):475–90. [DOI] [PubMed] [Google Scholar]
- 7. Matovu JN, Onyango-Makumbi C, Namuli PE et al. WHO 2010 infant feeding guidelines in resource-limited settings: attitudes of human immunodeficiency virus-infected women and other role players in Kampala, Uganda. South Afr J Clin Nutr 2014;27(2):63–8. [Google Scholar]
- 8. Onyango-Makumbi C, Bagenda D, Mwatha A et al. Early weaning of HIV-exposed uninfected infants and risk of serious gastroenteritis: findings from two perinatal HIV prevention trials in Kampala, Uganda. J Acquir Immune Defic Syndr 2010;53(1):20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al-Mujtaba M, Sam-Agudu N, Khatri RJ. Barriers to the practice of exclusive breastfeeding among HIV-positive mothers in sub-Saharan Africa: a scoping review of counselling, socioeconomic and cultural factors. J AIDS HIV Res 2016;8(6):70–9. [Google Scholar]
- 10. World Health Organization Guidelines on HIV and infant feeding 2010: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]
- 11. Lunney KM, Jenkins AL, Tavengwa NV et al. HIV-positive poor women may stop breast-feeding early to protect their infants from HIV infection although available replacement diets are grossly inadequate. J Nutr 2008;138(2):351–7. [DOI] [PubMed] [Google Scholar]
- 12. Joint United Nations Programme on HIV and AIDS 2015 progress report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. Geneva: Joint United Nations Programme on HIV and AIDS, 2015. [Google Scholar]
- 13. Fowler MG, Qin M, Fiscus SA et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016;375(18):1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bispo S, Chikhungu L, Rollins N, Siegfried N, Newell ML. Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: a systematic review and meta-analysis. J Int AIDS Soc 2017;20(1):21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization , United Nations Children’s Fund. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
- 16. Rollins NC, Ndirangu J, Bland RM et al. Exclusive breastfeeding, diarrhoeal morbidity and all-cause mortality in infants of HIV-infected and HIV uninfected mothers: an intervention cohort study in KwaZulu Natal, South Africa. PLoS One 2013;8(12):e81307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aishat U, David D, Olufunmilayo F. Exclusive breastfeeding and HIV/AIDS: a crossectional survey of mothers attending prevention of mother-to-child transmission of HIV clinics in southwestern Nigeria. Pan Afr Med J 2015;21:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goga AE, Doherty T, Jackson DJ et al. Infant feeding practices at routine PMTCT sites, South Africa: results of a prospective observational study amongst HIV exposed and unexposed infants—birth to 9 months Int Breastfeed J 2012;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fadnes LT, Engebretsen IM, Wamani H et al. Need to optimise infant feeding counselling: a cross-sectional survey among HIV-positive mothers in eastern Uganda. BMC Pediatr 2009;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health, Republic of Uganda. Policy guidelines on infant and young child feeding. Kampala, Uganda: Ministry of Health, 2009.
- 21. Okanda JO, Borkowf CB, Girde S et al. Exclusive breastfeeding among women taking HAART for PMTCT of HIV-1 in the Kisumu Breastfeeding Study. BMC Pediatr 2014;14:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oiye S, Mwanda W, Mugambi M et al. Exclusive breastfeeding is more common among HIV-infected than HIV-uninfected Kenyan mothers at 6 weeks and 6 months postpartum. Breastfeed Med 2017;12:283–9. [DOI] [PubMed] [Google Scholar]
- 23. Bosire R, Betz B, Aluisio A et al. High rates of exclusive breastfeeding in both arms of a peer counseling study promoting EBF among HIV-infected Kenyan women. Breastfeed Med 2016;11(2):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tylleskär T, Jackson D, Meda N et al. Exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa (PROMISE-EBF): a cluster-randomised trial. Lancet 2011;378(9789):420–7. [DOI] [PubMed] [Google Scholar]
- 25. Sinha B, Chowdhury R, Sankar MJ et al. Interventions to improve breastfeeding outcomes: a systematic review and meta-analysis. Acta Paediatr 2015;104(467):114–34. [DOI] [PubMed] [Google Scholar]
- 26. Haroon S, Das JK, Salam RA et al. Breastfeeding promotion interventions and breastfeeding practices: a systematic review. BMC Public Health 2013;13(3):S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tomlinson M, Doherty T, Ijumba P et al. Goodstart: a cluster randomised effectiveness trial of an integrated, community-based package for maternal and newborn care, with prevention of mother-to-child transmission of HIV in a South African township. Trop Med Int Health 2014;19(3):256–66. [DOI] [PubMed] [Google Scholar]
- 28. Reimers P, Israel-Ballard K, Craig M et al. A cluster randomised trial to determine the efficacy of the ‘feeding buddies’ programme in improving exclusive breastfeeding rates among HIV-infected women in rural KwaZulu-Natal, South Africa. AIDS Behav 2018;22(1):212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: World Health Organization, 2016:annex 2. https://www.ncbi.nlm.nih.gov/books/NBK379869/ [accessed 1 April 2018]. [PubMed] [Google Scholar]
- 30. Ministry of Health, Republic of Uganda The new national guidelines (2010) for PMTCT and infant feeding in the context of HIV. Kampala, Uganda: Ministry of Health, 2010.
- 31. Bbaale E. Determinants of early initiation, exclusiveness, and duration of breastfeeding in Uganda. J Health Popul Nutr 2014;32(2):249–60. [PMC free article] [PubMed] [Google Scholar]
- 32. Acheampong AK, Naab F, Kwashie A. The voices that influence HIV-positive mothers’ breastfeeding practices in an urban, Ghanaian society. J Hum Lact 2018;34(1):176–83. [DOI] [PubMed] [Google Scholar]
- 33. Nabwera HM, Jepkosgei J, Muraya KW et al. What influences feeding decisions for HIV-exposed infants in rural Kenya? Int Breastfeed J 2017;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zadrozny S, Westreich D, Hudgens MG et al. Effect of postnatal HIV treatment on clinical mastitis and breast inflammation in HIV-infected breast-feeding women. Paediatr Perinat Epidemiol 2017;31(2):134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf [accessed 4 April 2018].
- 36. Matovu A, Kirunda B, Rugamba-Kabagambe G et al. Factors influencing adherence to exclusive breast feeding among HIV positive mothers in Kabarole district, Uganda. East Afr Med J 2008;85(4):162–70. [DOI] [PubMed] [Google Scholar]
- 37. Asemahagn MA. Determinants of exclusive breastfeeding practices among mothers in Azezo district, northwest Ethiopia. Int Breastfeed J 2016;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kasahun AW, Wako WG, Gebere MW, Neima GH. Predictors of exclusive breastfeeding duration among 6–12 month aged children in Gurage zone, South Ethiopia: a survival analysis. Int Breastfeed J 2017;12:20 doi: 10.1186/s13006-017-0107-z. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Motee A, Ramasawmy D, Pugo-Gunsam P, Jeewon R. An assessment of the breastfeeding practices and infant feeding pattern among mothers in Mauritius. J Nutr Metab 2013;2013:243852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kabagenyi A, Habaasa G, Rutaremwa G. Low contraceptive use among young females in Uganda: does birth history and age at birth have an influence? Analysis of 2011 Demographic and Health Survey. J Contracept Stud 2017;1(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- 41. Jayachandran S. Does contraceptive use always reduce breast-feeding? Demography 2014;51(3):917–37. [DOI] [PubMed] [Google Scholar]
- 42. Balogun OO, Dagvadorj A, Anigo KM et al. Factors influencing breastfeeding exclusivity during the first 6 months of life in developing countries: a quantitative and qualitative systematic review. Matern Child Nutr 2015; 11(4):433–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.