Abstract
Background.
As silver diamine fluoride (SDF) gains popularity for caries arrest, the authors aimed to investigate the content of fluoride and silver in 38% SDF produced for the US market and its short-term stability.
Methods.
Five samples of 38% SDF were evaluated when the bottle was first opened, and at 7 and 28 days. Fluoride concentrations were determined with a fluoride ion-selective electrode, and silver concentrations were determined with a simultaneous inductively coupled plasma mass spectrometer. pH was measured with a pH probe. Weight and volume of individual drops were measured.
Results.
At day 0, 40% of individual measured values were above the expected fluoride concentration, and at day 28, 93% were above the expected fluoride concentration (P = .005). At day 0, 19% of individual measured values were below the lowest expected silver concentration, and at day 28, 93% were below (P < .001). Acidity (pH 10) was consistent over the 3 periods. Mean (standard deviation) weight of a drop was 40 (4.0) milligrams, and mean (standard deviation) volume was 32.55 (1.89) microliters, 30% more than the reported value of 25 μL.
Conclusion.
Over 28 days, the product pH is stable, whereas the fluoride content tends to increase and the silver content tends to decrease. Drops were larger than expected when dispensed from the bottle.
Practical Implications.
Drops are larger than expected, so each delivers higher than expected quantities of silver and fluoride. Clinicians should exercise caution when using this product on young children, replace the cap immediately, and use as soon as dispensed.
Keywords: Silver diamine fluoride, SDF, silver, fluoride, stability, caries
Untreated caries is a public health issue among children worldwide. Silver diamine fluoride (SDF), with the chemical formula of Ag(NH3)2F, could help provide effective, safe, accessible, and affordable control of this chronic childhood disease.1
Silver and fluoride products have been used in dentistry for over a century, but not until 1970, when SDF was developed in Japan, did a single product combine the antibacterial effects of silver and the remineralizing effects of fluoride stabilized in an alkaline ammonia solution, providing caries arrest, prevention of secondary caries, and dentin desensitization.2 Although the exact mechanism of action is still under study, SDF appears to inhibit cariogenic biofilms3 and slow the breakdown of dentin collagen4 to decrease demineralization.5
The US Food and Drug Administration approved SDF in 2014 at a 38% concentration as a device to treat dentinal hypersensitivity in patients 21 years or older.5 Advantage Arrest SDF 38% (Elevate Oral Care) is the only product available in the United States and is available in a 10-milliliter bottle with 8 mL of solution that provides approximately 250 drops, which is enough to treat 125 sites (defined as up to 5 teeth6 and as a unit-dose ampule with 0.1 mL7). According to the manufacturer, the light-sensitive liquid with ammonia odor has a specific gravity of 1.35 and contains 24% through 27% silver, 7.5% through 11% ammonia, 5% through 6% fluoride, less than 1% blue coloring, and less than or equal to 62.5% deionized water.8 SDF was originally manufactured as a clear liquid, but now it has blue tint to aid in its application.
Systematic reviews report that SDF arrests approximately 80% of carious lesions on primary teeth,1,9 suggest that twice-a-year applications are more effective than once a year, and suggest that 38% SDF is more effective than 12% SDF. Organizations like the American Academy of Pediatric Dentistry and the World Health Organization support the use of SDF for the management of caries in the primary dentition.10,11 Clinical trials, including more than 3,900 young children worldwide, indicate that SDF is safe, producing only minor transient adverse effects, like gingival irritation and metallic taste.5 All these trials used products not available in the United States.
SDF is contraindicated in patients with an allergy to silver compounds.8 Toxicity is a concern because large amounts of fluoride can lead to fluorosis, and silver can be absorbed through mucous membranes in the mouth, the nasal cavity, and dentinal and pulp tissues and accumulate in the body; therefore, it is important for clinicians to know the content and stability of silver and fluoride in commercial products, like SDF.
The aim of this study was to determine the initial content and stability of pH, fluoride, and silver concentrations of Advantage Arrest SDF 38% clear and blue solutions over 28 days. Our secondary aim was to verify the weight and volume of each drop as dispensed by means of the dropper. We hypothesized that different samples of the product would be stable over time in terms of their pH and silver and fluoride content and that the reported size of a drop would be accurate and consistent.
METHODS
Five bottles, each with 8 mL of Advantage Arrest SDF 38%, were purchased for analysis in our laboratory. The product came from 3 different lots: 1 bottle of clear solution (lot 16195), 2 bottles of blue-tinted liquid (lot 17137), and 2 bottles of blue-tinted liquid (lot 17190), which are referred to as SDF 1, SDF 2A and 2B, and SDF 3A and 3B, respectively. Variables measured included silver and fluoride content, pH, and weight per volume of individual drops dispensed from the manufacturer’s original dropper and bottle. Expected range of content was 24.4% through 28.8% silver and 5.0% through 5.9% fluoride (from literature descriptions on this product12), with a pH of 10 (from studies done on similar products13). The manufacturer’s product package insert lists the SDF total concentration in the range of 38.3% through 43.2% wt/vol, and, therefore, we calculated the acceptable range to be 45,215 through 51,000 parts per million for fluoride and 256,721 through 289,565 ppm for silver. Expected volume of a drop was 25 microliters12; no weight of drop was reported by the manufacturer or in the literature. Measurements of all variables were obtained when the bottle was first opened (day 0), at day 7, and at day 28. SDF bottles were kept at room temperature (standard deviation) (22.7°C [0.9°C]) and opened for 1 minute 3 times a week to simulate conditions of use in a clinical setting. On a separate day, 2 individual drops from each of the bottles were measured, 1 by volume and 1 by weight, every hour for 8 hours. Solutions were diluted with deionized ultrapure water for fluoride and silver analysis.
Fluoride analysis
A multi-ion meter (HD440d, HACH) equipped with a fluoride ion selective electrode (ISE) (ISEF 121, HACH) was used. The SDF samples were diluted by means of a factor of 1,000 in 2 steps. The fluoride ISE was calibrated using 3 fluoride standard solutions (HACH) at concentrations of 1.0, 10.0, and 100.0 milligrams per liter. Final diluted SDF sample or standard solution (25 mL) was placed in a 50-mL plastic beaker, and 1 fluoride ionic strength adjustment (ISA) powder pillow (HACH) was added to the solution. ISA was used in all sample and standard solutions to achieve a constant ionic strength. During constant electromagnetic stirring, the fluoride concentration in mg/L, the probe potential in millivolt, and the solution temperature in degrees C were recorded.
The 10.0 mg/L fluoride standard solution was analyzed as the control at the beginning and end of each run and after every 6 SDF samples to check the reproducibility of analysis. Three measurements were performed for each diluted SDF sample, and the coefficient of variation (CV) for each analyzed sample was calculated. The average for the replicates was used for further analysis.
Silver analysis
A simultaneous inductively coupled plasmaemass spectrometer (si-ICP-MS) (SPECTRO Analytical Instruments) was used for all silver measurements.14 Original sample solutions were diluted by a factor of 1.0 × 107 in 4 steps and were introduced via an autosampler (Teledyne CETAC Technologies) by means of pneumatic nebulization with ultra high-purity argon as carrier gas (Airgas, Randor) with a flow rate of 0.92 L per minute, using a SeaSpray nebulizer (Glass Expansion) and nickel sampler and skimmer cones (ICPMS Cones) to the si-ICP-MS.
Before performing any quantitative analysis, the si-ICP-MS was optimized and calibrated to achieve maximum sensitivity. As a routine, MERCK VI multielement standard (Merck), diluted by a factor of 500 with ultrapure water (18.2 megaohm.centimeter) (ELGA, PurelabUltrapure Water Purification Systems) and acidified with ultrapure nitric acid to 2% (volume/volume), was used to optimize and tune the si-ICP-MS. For silver analysis, the final diluted samples contained 2% (volume/volume) Suprapur 65% nitric acid (analytical-reagent grade, Merck) and Rh (Inorganic Ventures) as an internal standard at the concentration of 20 parts per billion. To consider variability in instrument conditions during the analysis, the Rhodium internal standard was introduced at the identical concentration into all solutions.
The diluted MERCK VI multielement standard and National Institute of Standards and Technology SRM1640a, containing 20 and 8.081 ppb silver, respectively, were analyzed as controls at the beginning and end of each run and after every 5 SDF samples to check the reproducibility of the analysis. Nine replicates were performed for each diluted SDF sample, and the CV for each analyzed sample was calculated. The average for the replicates was used for further analysis.
pH measurements
The ion meter described for fluoride analysis was used but otherwise equipped with a PHC729 pH probe (HACH) for pH measurements. The pH probe was calibrated using 3 buffer solutions (HACH) with pH values of 4.01, 7.00, and 10.01. Approximately 1 mL of SDF solution was placed in each well of a 24-well cell culture plate. Individual SDF samples were measured under stirring until stabilized, and their pH was recorded. The probe was rinsed with ultrapure water between measurements and dried with a lint-free cloth.
The buffer solutions with pH values of 7.00 and 10.01 were measured as controls at the beginning and end of each run and after every 6 SDF samples to check reproducibility. Three measurements were performed for each SDF sample, and the CV for each analyzed sample was calculated. The average for the replicates was used for further analysis.
Weight and volume measurements
An Accuris instrument scale (W3100A-120, analytical series, Edison) with readability of 0.0001 g was used for weighing, and an Eppendorf Research plus pipette (Eppendorf), with a range of 10 through 100 μL, was used for measuring the volume of single drops of each SDF sample. Weight and volume measurements were acquired from 2 separate drops.
Statistical analysis
Our inferential strategy focused on the number of individual samples that provided estimates of silver and fluoride ion concentration that were over or under the limits set by the manufacturer. These rates were then compared over time with the Fisher exact test. As an alternate strategy, we could have compared mean values of these measures over time, but that approach could be compromised by means of offsetting overlimit and underlimit values that appear nominal when averaged. The Fisher tests and descriptive statistics were both computed using SPSS Version 24 (IBM).
RESULTS
Five SDF samples from 3 different manufacturing lots were evaluated at 3 time points. Each cell of the table shows the mean (standard deviation [SD]) fluoride ion content, silver content, and pH over at least 3 replicate assays for that sample. The CVs for those replicate assays ranged from 0.1% through 3.6% for fluoride, from 1.5% through 5.6% for silver, and from 0.1% through 1.2% for pH. These data indicate that the assays were reliable. The average of the 3 (9 for silver) replicates was used in further analysis.
The mean fluoride concentration ranged from the higher end of the range mentioned in the product description (51,000 ppm) on day 0 and day 7 to greater than 54,000 ppm on day 28 (Table). At days 0 and 7, 40% through 50% of individual sample values were above the limit described in the package insert, and this rate increased to 93% at day 28 (P = .005). These data indicate that the product delivers the advertised concentration of fluoride when first opened and that fluoride concentrations increase 7 through 28 days after opening.
Table.
Fluoride and silver concentrations and the pH values of 5 silver diamine fluoride samples at 3 time points after first opening.*
| VARIABLES | SDF† 1 | SDF 2A | SDF 2B | SDF 3A | SDF 3B | MEAN ppm | % HIGH(> 51,000ppm) |
|---|---|---|---|---|---|---|---|
| Fluoride Concentrations | |||||||
| ppm‡ | ppm | ppm | ppm | ppm | |||
| Day 0 | 52,100 (58) | 50,800 (100) | 53,300 (656) | 49,600 (351) | 49,400 (208) | 51,000 (1,560) | 40.0 |
| Day 7 | 51,800 (231) | 51,500 (929) | 51,400 (794) | 48,900 (1,010) | 49,400 (737) | 50,600 (1,420) | 46.7 |
| Day 28 | 53,000 (379) | 56,000 (208) | 55,600 (643) | 54,400 (361) | 52,500 (1,920) | 54,300 (1,630) | 93.3§ |
|
SDF 1 |
SDF 2A |
SDF 2B |
SDF 3A |
SDF 3B |
MEAN ppm |
% LOW(< 256,721ppm) |
|
|
Silver Concentrations |
|||||||
| ppm | ppm | ppm | ppm | ppm | |||
| Day 0 | 257,000 (14,000) | 274,000 (11,900) | 259,000 (7,900) | 265,000 (10,600) | 285,000 (10,000) | 267,000 (12,900) | 19.0 |
| Day 7 | 258,000 (5,470) | 264,000 (6,350) | 267,000 (7,530) | 256,000 (6,990) | 262,000 (6,950) | 261,000 (4,800) | 13.3 |
| Day 28 | 254,000 (9,500) | 246,000 (11,200) | 244,000 (8,770) | 247,000 (11,600) | 251,000 (10,200) | 248,000 (6,510) | 93.3¶ |
|
SDF 1 |
SDF 2A |
SDF 2B |
SDF 3A |
SDF 3B |
MEAN (SD) |
||
|
pH Values |
|||||||
| pH | pH | pH | pH | pH | |||
| Day 0 | 10.08 (0.03) | 9.75 (0.03) | 9.74 (0.03) | 9.93 (0.01) | 9.91 (0.02) | 9.88 (0.13) | –# |
| Day 7 | 10.06 (0.02) | 9.87 (0.10) | 9.80 (0.01) | 9.92 (0.12) | 9.94 (0.02) | 9.92 (0.11) | – |
| Day 28 | 10.05 (0.04) | 9.84 (0.03) | 9.81 (0.03) | 9.88 (0.08) | 9.96 (0.03) | 9.91 (0.1) | – |
Data are given as mean (standard deviation) unless otherwise indicated. Expected fluoride concentrations: 45,215–51,000 ppm (calculated from percentages reported on package insert). N = 45 (3 per each of the 5 bottles for each period). Expected silver concentrations: 256,721–289,565 ppm (calculated from percentages reported on package insert). N = 135 (9 for each of the 5 bottles for each period. Expected pH values: not reported by manufacturers. N = 45 (3 per each of the 5 bottles for each period). Samples A and B indicate bottles from the same manufacturing lot.
SDF: Silver diamine fluoride.
ppm: Parts per million.
P = .005.
P < .001.
Data not available.
The mean silver concentrations ranged from within the range mentioned in the product description (256,721–289,565 ppm) on day 0 and day 7 to less than 249,000 ppm on day 28 (Table). At days 0 and 7, 13% through 19% of individual sample values were below the limit set in the package insert, and this rate increased to 93% at day 28 (P < .001). These data indicate that the product delivers the advertised concentration of silver when first opened but that concentrations decrease 7 through 28 days after opening.
Average pH levels varied within 0.5% of 10.0 (Table) and were consistent over the 3 periods (P = .21).
Figures 1A and 1B show the samples’ different color presentations and their drop size immediately after dispensing and after 30 minutes. Note the rapid oxidation of silver. There was wide variation in weight and volume of drops (2) from each bottle measured at the 3 time points. Figures 2A and 2B show the variation in weight (mg) and volume (μL) in 8 drops dispensed from each bottle every hour for 8 hours. From the latter repeated measurements, the drops had a mean (SD) weight of 40 (4.0) mg and a mean (SD) volume of 32.55 (1.89) μL. In terms of accuracy, the average dropper volume measure was approximately 30% higher than expected. Both measures indicated precision within 10% of the mean. These measures showed a similar distribution on days 0, 7, and 28 (that is, there was no effect of open time on the weight or volume of each drop).
Figure 1.
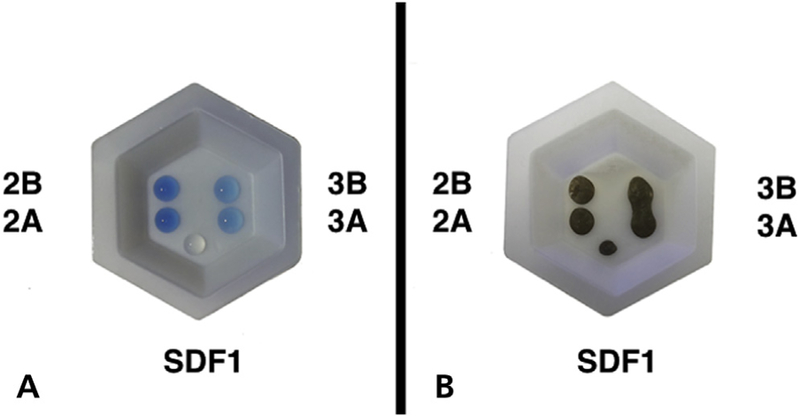
Sample drops of 5 investigated silver diamine fluoride (SDF) solutions: fresh (A) and after 30 minutes (B), showing the rapid oxidation of silver.
Figure 2.
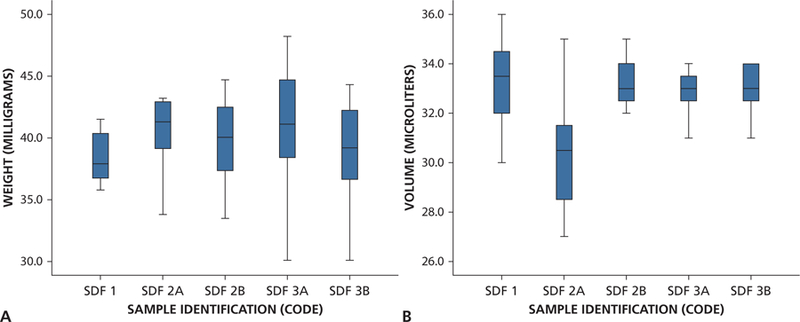
Box and whisker plots showing distribution of weight (A) and volume (B) measured by the hour for 8 hours for each bottle. SDF: Silver diamine fluoride.
DISCUSSION
Almost one-half of the individual fluoride concentrations were as described in the package insert when bottles were first opened, and almost all were above those levels at day 28. The mean fluoride concentration at day 28 was 3.2% higher than at day 0 and was 3.2% higher than the upper limit shown in the package insert. Furthermore, most individual measures of silver concentration were initially as described in the package insert, but almost all were below those levels at day 28. The mean silver concentration at day 28 was 6.8% lower than at day 0 and was also 3.2% lower than the lower limit in the package insert. pH levels were stable among samples and over time. The comparison of content and stability of the blue-tinted liquid to the original clear product is important because the latter has been used for studies of safety and pharmacokinetics.
Thus, when the bottles are first opened, the concentrations of fluoride and silver are close to the manufacturer’s reported expected levels within lots and between lots and comparable to the original clear product, up to 7 days after opening. After 28 days, the fluoride concentration increases and the silver concentration decreases. This could be owing to precipitation of silver because the bottle is opened repeatedly; fluoride concentration could increase with time owing to water evaporation. These changes in fluoride and silver concentration, however, would have little clinical consequence because the changes are not large, but further studies are required to see if this trend continues after 28 days. On the basis of our results, it is important to follow the manufacturer’s storage precautions, which include storing in original packaging in a cool, dark place; replacing the cap immediately after use; and using as soon as dispensed. It would be expected that single-use unit-dose ampules with 0.1 mL (comparable to 3 drops dispensed from the bottle) would contain the amounts of components we report at baseline, when bottles are first opened. Further studies would be required to corroborate this.
Mei and colleagues13 also studied the stability of fluoride and silver and the acidity of 12%, 30% (Caristop, Biodinamica), and 38% (Saforide, Toyo Seiyaku Kasei) products over days. Their results on the 38% product also show a reduction in the concentration of silver, from 249,000 through 241,000 ppm at day 28, whereas the fluoride concentration was highest at day 0 (56,000 ppm), which they cited as 25% higher than the expected 44,800 ppm advertised by the manufacturer. The mean difference score used by Mei and colleagues13 might underestimate the risk of a discrepancy in any particular sample by means of averaging positive and negative deviations from zero. Our analytical approach now shows also that almost all samples of both fluoride and silver deviated from baseline after 28 days. Thus, our results are not only consistent with those of Mei and colleagues13 but provide a better insight of the changes of fluoride and silver levels in SDF over time.
Methods and procedures used for analysis of the samples determine the precision of the measurements. Therefore, we used the best recognized methods. For the fluoride analysis, we used a fluoride ISE, which is the reference standard for fluoride analysis.15 Similar methods have been used in other studies of content and stability of other preparations of SDF,13 which allow us to draw direct comparisons between products. To eliminate most potential interferences and to have a constant ionic strength, a fluoride ionic strength adjustor known as ISA was used in all fluoride calibration standards and samples.16 For the silver analysis, ICP-MS combines an ICP ion source and a mass spectrometer, which separates and detects the ions produced by means of the ICP, according to their mass-to-charge ratio, and measures the analyte concentration by means of mass fractionation based on the obtained signals (counts per second). It provides a highly sensitive and reliable method for analyte detection.17
Our study indicates that volume and weight of drops of the same solution are not consistent between or within bottles, time, or both, even when temperature is constant. Because a drop is an empirical measure, it is not possible to get the same size drop in every single application. A drop size is determined by means of the equipment, technique used, and experience to create a drop of a specific solution. Additional factors that contribute to the size are the temperature and viscosity, density, surface tension, and specific gravity of the agent.18 Methods used to measure weight are inherently more precise than methods used to measure volume. Our results are consistent with those of Vasquez and colleagues,19 who reported that the average total weight of SDF 38% (Saforide) was 7.57 mg for the calculated volume of 6.04 mL used in their pharmacokinetics study. However, the only measure of a drop mentioned by the manufacturer is volume: approximately 25 μL,12,20 consistent with other studies that base their safety recommendations on that figure.
Manufacturers report the fluoride content of a single application is within safety margins established for fluoride varnish.12,20 The US Environmental Protection Agency (EPA) suggests levels of silver in drinking water not to exceed 1.142 mg/L for 1 through 10 days, and a total dose exposure limit of 1 g (or 0.014 mg per kilogram per day) to avoid argyria because the long-term effects of repeated exposure to silver result in gradual and permanent accumulation of silver in different parts of the body.21 According to our results, an average (SD) drop measuring 32.5 μL would have approximately 1.64 (0.04) to 1.76 (0.05) mg of fluoride, and 8.08 (0.13) to 8.71 (0.38) mg of silver, which is consistent with the figures reported by Vasquez and colleagues19: 1.50 mg of silver in 6.04 mL of SDF 38% used in their study. They applied SDF on 3 tooth surfaces of each of 6 adults, with a mean weight of 63 kg, and measured serum concentrations of fluoride and silver at baseline and 4 hours after application. They concluded that fluoride exposure was below the EPA oral reference dose. Silver exposure exceeded the EPA oral reference dose for cumulative daily exposure over a lifetime, but for occasional use was well below concentrations associated with toxicity, and stated that SDF should pose little toxicity risk when used in adults. The amount of SDF used was 6.04 mL; this was less than one-fifth of the average drop found in our study.
The ammonia component stabilizes the solution in an alkaline environment that showed no variation over the time studied. The ammonia is not available as free ammonia, so the possibility of toxic effects from it would be minimal.
Manufacturers’ indications are to use 1 through 2 drops on adults.6 Guidelines for use in children recommend using 1 drop.10 Because the average size of a drop in our study was 30% more than that used for safety calculations, and because of the high concentrations of silver and fluoride (fluoride was higher than expected), clinicians should exercise caution when using this product on young children.
Strengths of this study include the use of precise analytical techniques to corroborate the content and stability of the product. Limitations of the study are that we only tested 5 bottles from 3 different manufacturing lots and over only 28 days.
CONCLUSIONS
Over 28 days, the product’s pH is stable, whereas the fluoride content tends to increase and the silver content tends to decrease. Drops were larger than expected when dispensed from the bottle, so each delivers higher than expected quantities of silver and fluoride. Clinicians should exercise caution when using this product on young children, replace the cap immediately, and use as soon as dispensed.
SDF is a valuable tool for the management of caries when traditional treatment is not an option and helps clinicians and families avoid more-involved procedures that may increase the risk to the patient. As with all other products used on young children, a careful evaluation of risks and benefits and alternative options for treatment should be discussed with the parents.
Acknowledgments
This work was supported in part by grant U24 006964 from the National Institute on Minority Health and Health Disparities, National Institutes of Health, and the Pediatric Dentistry Department, College of Dentistry, New York University.
ABBREVIATION KEY
- EPA
Environmental Protection Agency
- ISA
Ionic strength adjustment
- ISE
Ion selective electrode
- SDF
Silver diamine fluoride
- si-ICP-MS
Simultaneous inductively coupled plasma–mass spectrometer
Footnotes
Disclosure. None of the authors reported any disclosures.
Contributor Information
Yasmi O. Crystal, College of Dentistry, New York University, New York, NY..
Sasan Rabieh, Department of Biomaterials, College of Dentistry, New York University, New York, NY..
Malvin N. Janal, Department of Epidemiology and Health Promotion, College of Dentistry, New York University, New York, NY..
Sarunphorn Rasamimari, College of Dentistry, New York University, New York, NY..
Timothy G. Bromage, Department of Biomaterials, College of Dentistry, New York University, New York, NY..
References
- 1.Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet.” J Dent Res 2009;88(2):116–125. [DOI] [PubMed] [Google Scholar]
- 2.Yamaga R, Nishino M, Yoshida S, Yokomizo I. Diammine silver fluoride and its clinical application. J Osaka Unic Dent Sch 1972;12(20):1–10. [PubMed] [Google Scholar]
- 3.Mei ML, Lo ECM, Chu CH. Arresting dentine caries with silver diamine fluoride: what’s behind it? J Dent Res 2018;97(7):751–758. [DOI] [PubMed] [Google Scholar]
- 4.Zhao IS, Gao SS, Hiraishi N, et al. Mechanisms of silver diamine fluoride on arresting caries: a literature review. Int Dent J 2018;68(2):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crystal YO, Niederman R. Silver diamine fluoride treatment considerations in children’s caries management. Pediatr Dent 2016;38(7):466–471. [PMC free article] [PubMed] [Google Scholar]
- 6.Advantage Arrest: SDF 38% [package insert] West Palm Beach, Florida: Elevate Oral Care; 2018. Available at: http://www.elevateoralcare.com/site/images/AA_PI_040715.pdf. Accessed November 18, 2018. [Google Scholar]
- 7.Elevate Oral Care Advantage Arrest SDF 38%-Bottle Elevate Oral Care website; Available at: http://www.elevateoralcare.com/dentist/AdvantageArrest/Advantage-Arrest-Silver-Diamine-Fluoride-38. Accessed November 18, 2018. [Google Scholar]
- 8.Elevate Oral Care. Safety data sheet: Advantage Arrest SDF 38% Available at: http://www.elevateoralcare.com/site/images/AASDS082415.pdf. Accessed November 18, 2018.
- 9.Gao SS, Zhao IS, Hiraishi N, et al. Clinical trials of silver diamine fluoride in arresting caries among children: a systematic review. JDR Clin Transl Res 2016; 1(3):201–210. [DOI] [PubMed] [Google Scholar]
- 10.Crystal YO, Marghalani AA, Ureles SD, et al. Use of silver diamine fluoride for dental caries management in children and adolescents, including those with special health care needs. Pediatr Dent 2017;39(5):135–145. [PubMed] [Google Scholar]
- 11.Phantumvanit P, Makino Y, Ogawa H, et al. WHO global consultation on public health intervention against early childhood caries. Community Dent Oral Epidemiol 2018;46(3):280–287. [DOI] [PubMed] [Google Scholar]
- 12.Horst JA, Ellenikiotis H, Milgrom PL. UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications and consent. J Calif Dent Assoc 2016;44(1):16–28. [PMC free article] [PubMed] [Google Scholar]
- 13.Mei ML, Chu CH, Lo EC, Samaranayake LP. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int J Paediatr Dent 2013;23(4): 279–285. [DOI] [PubMed] [Google Scholar]
- 14.Bäuchle M, Lüdecke T, Rabieh S, Calnek K, Bromage TG. Quantification of 71 detected elements from Li to U for aqueous samples by simultaneous-inductively coupled plasma-mass spectrometry. RSC Adv 2018;8: 37008–37020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Mier EA, Cury JA, Heilman JR, et al. Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res 2011; 45(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez I, Hardisson A, Paz S, et al. Fluoride intake from the consumption of refreshment drinks and natural juices. J Food Compost Anal 2018;72:97–103. [Google Scholar]
- 17.Bolann BJ, Rahil-Khazen R, Henriksen H, Isrenn R, Ulvik RJ. Evaluation of methods for trace-element determination with emphasis on their usability in the clinical routine laboratory. Scand J Clin Lab Invest 2007;67(4):353–366. [DOI] [PubMed] [Google Scholar]
- 18.German EJ, Hurst MA, Wood D. Reliability of drop size from multi-dose eye drop bottles: is it cause for concern? Eye (Lond) 1999;13(pt 1):93–100. [DOI] [PubMed] [Google Scholar]
- 19.Vasquez E, Zegarra G, Chirinos E, et al. Short term serum pharmacokinetics of diammine silver fluoride after oral application. BMC Oral Health 2012;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elevate Oral Care. The Silver Bulletin 1; October 25, 2017. Available at: http://www.elevateoralcare.com/Landing-Pages/silverbulletinv1. Accessed November 18, 2018.
- 21.Integrated Risk Information System (IRIS) Chemical Assessment Summary NCfEA, US Environmental Protection Agency; Silver: CASRN 7440–22-4. Last revised December 1, 1991. Available at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0099_summary.pdf. Accessed November 18, 2018. [Google Scholar]


