Abstract
Background
Obstructive uropathy (OU) is a common cause of end-stage renal disease (ESRD) in children. Children who escape the newborn period with mild-to-moderate chronic kidney disease (CKD) continue to be at increased risk. The predictive ability of clinically available markers throughout childhood is poorly defined.
Methods
Patients with OU were identified in the Chronic Kidney Disease in Children Study. The primary outcome of interest was renal replacement therapy (RRT) (cases). Controls were age matched and defined as patients within the OU cohort who did not require RRT during study follow-up.
Results
In total, 27 cases and 41 age-matched controls were identified. Median age at baseline and age at outcome measurement were 10 vs. 16 years, respectively. First available glomerular filtration rate (GFR) (36.9 vs. 53.5 mL/min per 1.73 m2), urine protein/creatinine (Cr) (0.40 vs. 0.22 mg/mg) and microalbumin/Cr (0.58 vs. 0.03 mg/mg), and serum CO2 (20 vs. 22 mmol/L) and hemoglobin (12.4 vs. 13.2 g/dL) differed significantly between cases and controls, respectively. GFR declined 3.07 mL/min per 1.73 m2/year faster in cases compared to that in controls (p < 0.0001). Urine protein/Cr and microalbumin/Cr increased by 0.16 and 0.11 per year more in cases compared to those in controls, respectively (p ≤ 0.001 for both). Serum phosphate increased by 0.11 mg/dL and serum albumin and hemoglobin decreased by 0.04 (g/dL) and 0.14 (g/dL) per year more for cases compared to those for controls, respectively (p < 0.05 for all).
Conclusions
Age-specific baseline and longitudinal measures of readily available clinical measures predict progression to ESRD in children with mild-to-moderate CKD from OU.
Keywords: Obstruction, Uropathy, Chronic kidney disease, End-stage renal disease, Prediction, CKiD
Background
The term obstructive uropathy (OU) encompasses a constellation of diagnoses affecting the urinary system. The common thread grouping these diagnoses together is the impedance of urine flow resulting in renal injury. Most causes of pediatric OU are congenital and impact nephrogenesis and kidney maturation, contributing significantly to pediatric chronic kidney disease (CKD) and end-stage renal disease (ESRD) [1]. Population studies estimate that OU accounts for 16.5% of children with kidney transplants and 13% of children receiving dialysis [2, 3]. Despite continued improvement in techniques for early diagnosis and management of OU in both the prenatal and postnatal periods, a significant number of these children still progress to ESRD [4–7].
Predicting CKD progression in children with OU is critically important in order to enable early intervention and allow targeted surveillance. The majority of research to date has focused on posterior urethral valves (PUV); however, less common forms of urinary obstruction also contribute to uropathy in children. In children with PUV, serum nadir creatinine (Cr) under 1 mg/dL by the first year of life is used as a prognostic indicator, with those whose Cr is above this nadir being more likely to develop renal failure than those below. This single cutoff of serum creatinine is suboptimal in risk stratifying many patients. In particular, those whose nadir is below 1 mg/dL appear to have a more variable course with some not progressing at all during follow-up [8].
Historically, this lower risk group of patients has not been as well studied, but is important to understand as it lends an opportunity for intervention and prevention given that their kidney prognosis appears less defined. Some of the difficulties with determining the progression of renal decline in this group is that renal injury is likely multifactorial and additive, including the initial injury and potentially modifiable insults throughout childhood. In children with PUV specifically, bladder dysfunction occurs in over half of patients, typically progressing from that of spastic high pressures to increased capacity myogenic failure [9, 10]. Also, the renin-angiotensin system (RAS) is activated in OU through tubule-interstitial damage which may play a role in the timing and progression of CKD [11, 12].
Further complicating the study of these children is that many demonstrate delayed signs of renal insufficiency that only surface during their teenage or adult years [13, 14]. This lag time of over a decade and potential influence of puberty makes studying this group of OU children difficult and highlights the importance of prospective longitudinal studies. Progressive renal damage in these children likely occurs due to a multitude of factors, including but not limited to bladder dysfunction, chronic vesicoureteral reflux, persistent/recurrent obstruction, polyuria, hyperfiltration, and infections, as well as acceleration of growth, increased body mass, and elevated blood pressure during pubertal years [15, 16]. It is thus essential to define the natural history of renal decline over many years in these children, as well as predictors and trends in clinical markers that may precede GFR decline. Also, by improving our understanding of the natural progression of these children, mechanistic knowledge gaps can be narrowed, allowing future studies to focus on targeted novel biomarkers and potential disease modifiers. This study utilized a prospective longitudinal cohort of children with mild-to-moderate CKD to better characterize renal decline in children with OU and identify potential trends in clinically available predictors specific to those who progress to ESRD and those who do not.
Materials and methods
Study population
The Chronic Kidney Disease in Children (CKiD) study is a prospective, observational study of children with mild-to-moderate CKD recruited from 48 North American Pediatric Nephrology Centers. Enrollment for CKiD initiated in 2005 (CKiD cohort 1) and was initially designed to capture children ages 1–16 with a glomerular filtration rate (GFR) of 30–90 mL/min per 1.73 m2. In 2011 (CKiD cohort 2), these criteria were further restricted to a GFR of 45–90 mL/min per 1.73 m2 to allow longer follow-up prior to CKD progression. Between cohorts 1 and 2, a total of 891 children have been enrolled in CKiD. All subjects undergo baseline evaluation and yearly follow-up visits of which details for the study design and methods have been previously published [17]. For this study, we nested a case-control design within the CKiD cohort by matching children who received renal replacement therapy (RRT), defined as renal transplant or dialysis, to children who did not receive RRT during follow-up.
Measurements and data collection
Patients with OU as their primary diagnosis code were identified in CKiD. No further granularity of data is available in CKiD as to the clinical diagnosis resulting in OU (i.e., PUV, prune belly syndrome vs. obstruction at the ureteropelvic or ureterovesical junction could not be distinguished). We reviewed patient sociodemographic characteristics, follow-up duration, urine markers (protein/Cr and microalbumin/ Cr), and serum testing (CO2 mmol/L, phosphate mg/dL, albumin g/dL, hemoglobin g/dL). These specific markers were chosen for analysis, as they are both available in CKiD and commonly measured in clinical settings, facilitating application to patient care. GFR (mL/min per 1.73 m2) was measured by either disappearance of iohexol (iGFR) or estimated as a function of sex, height, serum Cr, cystatin C, and BUN using the CKiD derived formula, which has been previously described [18]. The primary outcome of interest was RRT (defined in our study as “cases”). “Controls” were defined as patients within the OU cohort who did not receive RRT during the study period.
Cohort development
In total, 149 patients with OU were identified, with 33 meeting the case definition. Only patients with at least 3 study visits measuring GFR were included, resulting in 32 cases and 69 controls. Matching of cases and controls was performed based on age at baseline and time on study (i.e., control’s follow-up length had to be longer than or equal to the case’s length of time free of RRT), using a random computer-generated sequence. Cases were matched to controls without replacement so that each case had a unique set of controls. Cases for which an appropriate control match was unavailable (n = 5 cases) were excluded from the analysis. The final matched population allowed for up to a 3:1 ratio for controls per case.
Statistical analysis
Patient characteristics (age, sex, race), follow-up duration, and clinical predictor variables for RRT (GFR, urine protein/Cr and microalbumin/Cr, serum CO2 mmol/L, phosphate mg/ dL, albumin g/dL, and hemoglobin g/dL) were compared between cases and controls at baseline visit using chi-squared tests for categorical variables and the Wilcoxon rank-sum test for continuous variables. Clinical predictor variables were then compared longitudinally between cases and controls using linear mixed-effects models, whereby the variable was modeled as a function of time to RRT and case vs. control status. For all matched cases and controls, the slope for time to RRT was anchored at the time of RRT for the case. All models included a patient random intercept and random slopes for time to RRT, to allow for patient-specific progression to ESRD, and an interaction term between time to RRT and case status in order to test for slope differences between cases and controls. iGFR measurement was preferentially used when available, and eGFR was included for improved linear modeling for visits where only estimations were calculated.
Results
After exclusions and matching, we identified 27 cases and 41 controls. There was no difference noted between cases and controls for age at baseline, age at outcome, sex, and race. Follow-up duration was longer for controls than cases (6.8 vs. 4.9 years, respectively) due to CKiD study censoring once the patient progresses to ESRD. Median age at baseline and age at outcome measurement were 10 vs. 16 years, respectively. Baseline visit GFR, urine protein/Cr, serum CO2, and serum hemoglobin differed significantly between cases and controls. A significant difference was also found for urine microalbumin/Cr; however, this variable was not collected at all baseline visits, as it was added later and thus represents earliest measurement available. No difference was seen for baseline serum phosphate or albumin (Table 1). Evaluating changes in GFR over time between cases and controls (Fig. 1), cases declined 3.07 mL/min per 1.73 m2/year faster compared to controls, which was statistically significant (Table 2). Expanding this analysis to other clinical variables, significant differences in change over time for cases and controls were identified for urine protein/Cr, urine microalbumin/Cr (Table 2, Fig. 2), serum phosphate, serum albumin, and serum hemoglobin (Table 2, Fig. 3). Only serum CO2 failed to show statistical differences in this analysis.
Table 1.
Baseline patient characteristics of the obstructive uropathy cohort
| Cases (n = 27) | Controls (n = 41) | p | |
|---|---|---|---|
| Age at baseline (years) | 10.0 (9−13) | 10.0 (9−13) | 0.950 |
| Age at outcome (years) | 15.9 (12.7, 16.9) | 15.8 (12.7, 16.6) | 0.821 |
| Male sex | 23 (85.2) | 33 (80.5) | 0.751 |
| Nonwhite race | 7 (25.9) | 18 (43.9) | 0.133 |
| GFR (mL/min per 1.73 m2) | 36.9 (28.6, 42.2) | 53.5 (44.5, 64.6) | < 0.0001* |
| Urine protein/Cr (mg/mg) | 0.40 (0.23, 0.84) | 0.22 (0.11, 0.47) | 0.010* |
| Urine microalbumin/Cr** (mg/mg) | 0.58 (0.27, 1.68) | 0.03 (0.01, 0.14) | < 0.0001* |
| Serum CO2 (mmol/L) | 20 (18, 20) | 22 (20, 25) | 0.002* |
| Serum phosphate (mg/dL) | 4.6 (4.3, 5.1) | 4.5 (3.9, 4.8) | 0.125 |
| Serum albumin (g/dL) | 4.3 (4.2, 4.4) | 4.4 (4.3, 4.5) | 0.270 |
| Serum hemoglobin (g/dL) | 12.4 (11.4, 13.3) | 13.2 (12.3, 14.3) | 0.020* |
| Follow-up duration (years) | 4.9 (3.4, 5.9) | 6.8 (5.2, 7.7) | < 0.001* |
Controls matched to cases by age at study entry and time on study. Data reported as median (interquartile range) for continuous variables and n (%) for categorical variables
GFR glomerular filtration rate, Cr creatinine, n number
Clinical significance (p < 0.05)
Urine microalbumin/Cr estimated at 3–5 years before matched case
Fig. 1.
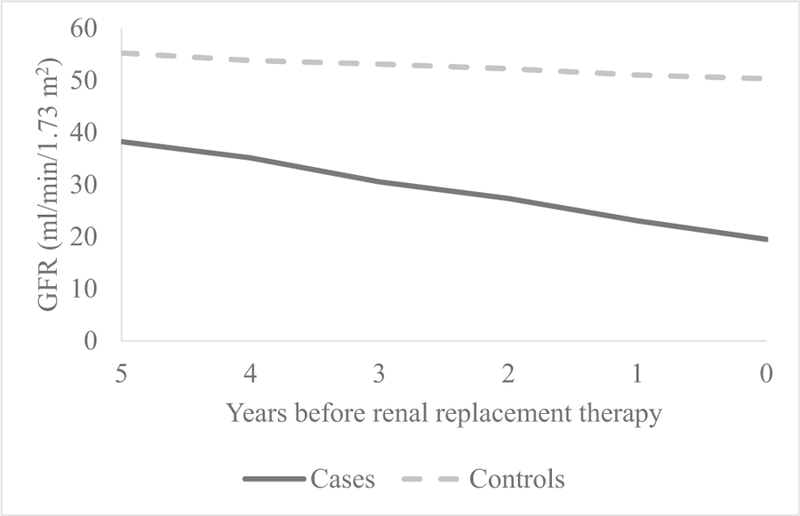
Estimated change in glomerular filtration rate (GFR) over time
Table 2.
Estimated change per year in clinical predictors over time in the obstructive uropathy cohort
| Cases |
Controls |
Cases-controls |
||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | |
| GFR (mL/min per 1.73 m2) | − 4.12 (− 5.05, − 3.18) | < 0.0001* | − 1.05 (− 1.83, − 0.27) | 0.009* | − 3.07 (− 4.28, − 1.86) | < 0.0001* |
| Urine protein/Cr (mg/mg) | 0.24 (0.17, 0.32) | < 0.0001* | 0.09 (0.03, 0.15) | 0.005* | 0.16 (0.06, 0.25) | 0.001* |
| Urine microalbumin/Cr (mg/mg) | 0.18 (0.11, 0.25) | < 0.0001* | 0.07 (0.02, 0.12) | 0.009* | 0.11 (0.02, 0.20) | < 0.0001* |
| Serum CO2 (mmol/L) | − 0.05 (− 0.36, 0.27) | 0.775 | 0.06 (− 0.20, 0.32) | 0.654 | − 0.10 (− 0.51, 0.30) | 0.613 |
| Serum phosphate (mg/dL) | 0.07 (0.02, 0.13) | 0.008* | − 0.04 (− 0.08, 0.01) | 0.094 | 0.11 (0.04, 0.18) | 0.002* |
| Serum albumin (g/dL) | − 0.02 (− 0.05, 0.00) | 0.077 | 0.02 (0.00, 0.04) | 0.120 | − 0.04 (− 0.08, − 0.01) | 0.019* |
| Serum hemoglobin (g/dL) | − 0.12 (− 0.23, − 0.01) | 0.026* | 0.02 (− 0.07, 0.11) | 0.609 | − 0.14 (− 0.28, − 0.01) | 0.041* |
All statistics estimated from linear mixed-effects models
GFR glomerular filtration rate, Cr creatinine, CI confidence interval
Clinical significance (p < 0.05)
Fig. 2.
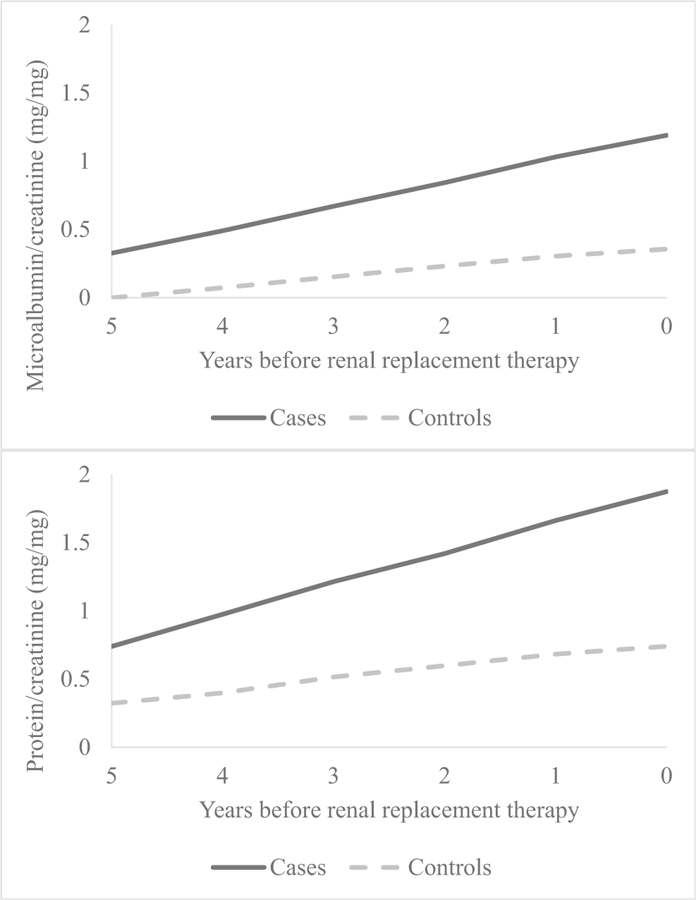
Estimated change in statistically significant urinary predictors over time
Fig. 3.
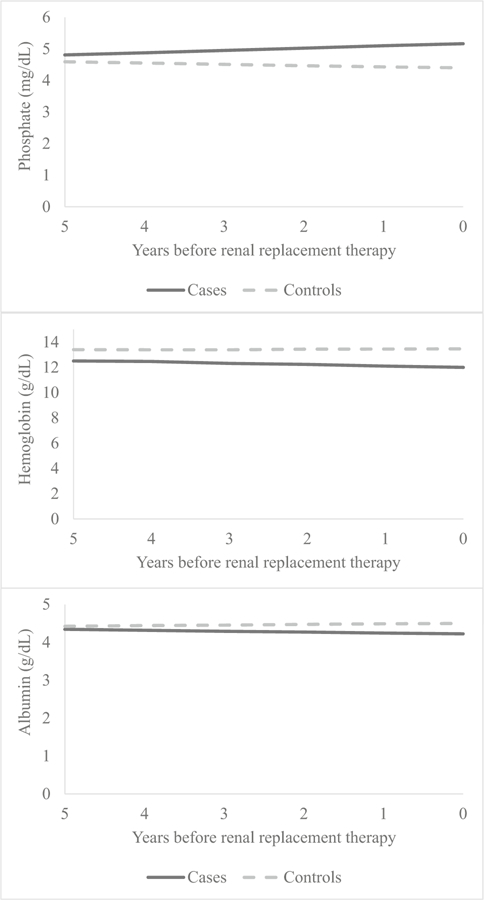
Estimated change in statistically significant serum predictors over time
Discussion
In the current study, we were able to identify patients with OU who were found to have opposing clinical courses and evaluate risk factors by outcome. The initial analysis focused on GFR, showing both a significant difference at study entry and change over time when comparing cases and controls. This suggests that in the years prior to progression, both a single GFR measurement and its trend over time provide predictive value. The analysis was then expanded to include urine protein/Cr, urine microalbumin/Cr, and serum (CO2, hemoglobin, phosphate) differences both at initial measurement and longitudinally. All markers evaluated showed some level of prediction on either difference in baseline measure, change over time, or both.
To our knowledge, this is the first study of its kind to evaluate predictive measures of renal progression specifically in OU from a prospectively collected longitudinal dataset such as CKiD. The advantage of this study design was the ability to study predictors at enrollment and over time in two cohorts of patients where the renal outcome at last follow-up was known. The ability of these measures to provide information on progression risk up to 5 years before the outcome has the potential for significant clinical impact. In a disease like PUV, where silent bladder dysfunction or recurrent obstruction may contribute to accelerated renal progression, sequential targeted blood or urine screening could provide an opportunity to intervene earlier and change the clinical course. Also in areas where specialty care is sparse, risk stratification using these measures could identify patients at greater need for urology and/or nephrology consultation and testing.
Merging data from CKiD and the ESCAPE trial, a recently published study also found that combining GFR, proteinuria, and CKD diagnosis allows improved prediction for disease progression compared to GFR alone [19]. This highlights the potential strength in combining clinical markers to improve risk stratification. Also, in adults with proteinuria, the association between strict blood pressure control and inhibition of the RAS is well documented in delaying progression of CKD [20]. In children, blood pressure maintained in the low range of normal using ramipril delayed progression to ESRD. After instituting ramipril therapy, a significant decrease in proteinuria was seen in most patients and was predictive of renal protection. This study also stratified the effect of ramipril by diagnosis, demonstrating that glomerular diseases showed the most significant response [21]. These insights into the association between the RAS and CKD progression in children highlight the need for both improved early risk stratification and refinement of intervention strategies to delay progression. It is also evident that all childhood CKD is not created equal, and patients would likely benefit more from diagnosis-specific risk stratification and intervention therapies.
Notwithstanding the advantages of using the CKiD dataset to study predictors of renal progression in OU, there are significant limitations to this study. Unfortunately, the level of granularity available for diagnosis is limited to OU with no further characterization. Thus, the OU cohort likely comprised a heterogeneous population including PUV, prune belly syndrome, urethral stricture, and ureteropelvic and ureterovesical junction obstructions that were either bilateral or with an abnormal or absent contralateral kidney. PUV and prune belly syndrome likely represent the majority of patients in our study; however, the precise distribution is unknown. This is significant given bladder-level dysfunction can impact clinical course, especially in patients with PUV and prune belly syndrome. Despite this limitation in the ability to differentiate between specific causes of OU and the unique clinical course associated with each, we were still able to identify overall trends in a heterogeneous group. Presumably, the ability to stratify by specific diagnosis would have improved prediction of each disease course, thus not undermining our current findings. Also of concern in our study is that the median age at outcome was 16 years and some controls may eventually become cases if followed longer. One would expect however that if some cases were misclassified as controls due to limited follow-up, this would bias the results towards the null and not diminish the study conclusions.
This study is also limited by its potential selection bias in that patients were recruited from pediatric nephrology centers. Not only are these patients likely to have access to more specialized care, enrollment in the CKiD demands close follow-up and screening. The combination of these factors likely results in optimization of renal outcomes compared to a general population of OU patients with comparable disease severity. Thus, the clinical course observed in this study may not accurately represent the general population of children with mild-to-moderate CKD from OU. This point however may represent an opportunity rather than a limitation, given the greater potential impact from risk screening in a patient population not receiving a comparable level of surveillance for disease progression. Lastly, given the small number of children studied between our two cohorts, further stratification by renal protective medication use and blood pressure control was not feasible. Given the known effects these factors have on renal progression in CKD, future studies will require incorporation of these variables.
In conclusion, we identified both baseline and longitudinal differences in commonly collected urine and serum measurements that were able to predict ESRD in the years preceding RRT. Optimal risk stratification would likely result from a combination of age-specific baseline and longitudinal measures for which ideal cutoffs still require investigation. Future studies will focus on defining optimal combinations and cutoff values of these clinical markers to hone risk models, as well as the addition of candidate CKD progression biomarkers which may add predictive value. Ideally, children identified at high risk would undergo closer surveillance and testing that may allow changes in clinical management to slow or halt progression.
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-082194, U01-DK-66116). The CKiD website is located at https://statepi.jhsph.edu/ckid.
Footnotes
Compliance with ethical standards
This study was assigned exempt status by the institutional review board due to the de-identification of data received from CKiD.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Benfield MR, McDonald RA, Bartosh S, Ho PL, Harmon W (2003) Changing trends in pediatric transplantation: 2001 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant 7:321–335 [DOI] [PubMed] [Google Scholar]
- 2.Roth KS, Koo HP, Spottswood SE, Chan JC (2002) Obstructive uropathy: an important cause of chronic renal failure in children. Clin Pediatr (Phila) 41:309–314. 10.1177/000992280204100503 [DOI] [PubMed] [Google Scholar]
- 3.Weaver DJ Jr., Somers MJG, Martz K, Mitsnefes MM (2017) Clinical outcomes and survival in pediatric patients initiating chronic dialysis: a report of the NAPRTCS registry. Pediatr Nephrol 32: 2319–2330. doi: 10.1007/s00467-017-3759-4 [DOI] [PubMed] [Google Scholar]
- 4.Parkhouse HF, Barratt TM, Dillon MJ, Duffy PG, Fay J, Ransley PG, Woodhouse CR, Williams DI (1988) Long-term outcome of boys with posterior urethral valves. Br J Urol 62:59–62 [DOI] [PubMed] [Google Scholar]
- 5.Reinberg Y, de Castano I, Gonzalez R (1992) Prognosis for patients with prenatally diagnosed posterior urethral valves. J Urol 148: 125–126 [DOI] [PubMed] [Google Scholar]
- 6.El-Ghoneimi A, Desgrippes A, Luton D, Macher MA, Guibourdenche J, Garel C, Muller F, Vuillard E, Lottmann H, Nessmann C, Oury JF, Aigrain Y (1999) Outcome of posterior urethral valves: to what extent is it improved by prenatal diagnosis? J Urol 162:849–853 [DOI] [PubMed] [Google Scholar]
- 7.Smith GH, Canning DA, Schulman SL, Snyder HM 3rd, Duckett JW (1996) The long-term outcome of posterior urethral valves treated with primary valve ablation and observation. J Urol 155:1730–1734 [PubMed] [Google Scholar]
- 8.Sarhan OM, El-Ghoneimi AA, Helmy TE, Dawaba MS, Ghali AM, Ibrahiemel HI (2011) Posterior urethral valves: multivariate analysis of factors affecting the final renal outcome. J Urol 185(6 Suppl):2491–2495. 10.1016/j.juro.2011.01.023 [DOI] [PubMed] [Google Scholar]
- 9.Hennus PM, van der Heijden GJ, Bosch JL, de Jong TP, de Kort LM (2012) A systematic review on renal and bladder dysfunction after endoscopic treatment of infravesical obstruction in boys. PLoS One 7:e44663 10.1371/journal.pone.0044663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmdahl G, Sillen U, Hanson E, Hermansson G, Hjalmas K (1996) Bladder dysfunction in boys with posterior urethral valves before and after puberty. J Urol 155:694–698 [PubMed] [Google Scholar]
- 11.Bajpai M, Chaturvedi PK, Bal CS, Sharma MC, Kalaivani M (2013) Posterior urethral valves: persistent renin angiotensin system activation after valve ablation and role of pre-emptive therapy with angiotensin converting enzyme-inhibitors on renal recovery. J Indian Assoc Pediatr Surg 18:74–78. 10.4103/0971-9261.109357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarger WE, Schocken DD, Harris RH (1980) Obstructive nephropathy in the rat: possible roles for the renin-angiotensin system, prostaglandins, and thromboxanes in postobstructive renal function. J Clin Invest 65:400–412. 10.1172/JCI109683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, Izzi C, Scolari F, Mattioli G, Allegri L, Ghiggeri GM (2009) Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76:528–533. 10.1038/ki.2009.220 [DOI] [PubMed] [Google Scholar]
- 14.Wuhl E, van Stralen KJ, Verrina E, Bjerre A, Wanner C, Heaf JG, Zurriaga O, Hoitsma A, Niaudet P, Palsson R, Ravani P, Jager KJ, Schaefer F (2013) Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol 8:67–74. 10.2215/CJN.03310412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez Pereira P, Espinosa L, Martinez Urrutina MJ, Lobato R, Navarro M, Jaureguizar E (2003) Posterior urethral valves: prognostic factors. BJU Int 91:687–690 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez Celedon C, Bitsori M, Tullus K (2007) Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol 22:1014–1020. 10.1007/s00467-007-0459-5 [DOI] [PubMed] [Google Scholar]
- 17.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA (2006) Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1:1006–1015. 10.2215/CJN.01941205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furth SL, Pierce C, Hui WF, White CA, Wong CS, Schaefer F, Wuhl E, Abraham AG, Warady BA, Chronic Kidney Disease in Children (CKiD); Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) Study Investigators (2018) Estimating time to ESRD in children with CKD. Am J Kidney Dis 71:783–792. 10.1053/j.ajkd.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggenenti P, Perna A, Gherardi G, Benini R, Remuzzi G (2000) Chronic proteinuric nephropathies: outcomes and response to treatment in a prospective cohort of 352 patients with different patterns of renal injury. Am J Kidney Dis 35:1155–1165 [DOI] [PubMed] [Google Scholar]
- 21.ESCAPE Trial Group, Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Moller K, Wigger M, Peruzzi L, Mehls O, Schaefer F (2009) Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361:1639–1650. 10.1056/NEJMoa0902066 [DOI] [PubMed] [Google Scholar]


