Abstract
The development of drug-loading technology will bring new and rapid development to the treatment of diseases. At present, drug delivery by nanoparticles, erythrocyte, and platelet have been studied extensively. Compared with traditional anticancer drugs, nano-drugs have shown many obvious advantages, disease treatment based on nanotechnology will bring a revolution in cancer treatment. Due to its inherent biocompatibility, large drug load and long half-life in the blood circulation, erythrocyte-inspired antibiotics, and some anticancer drugs delivery systems have also entered the clinical trial stage. At present, there are relatively few studies on drug delivery by platelets as carriers. It is necessary to overcome the shortcomings of platelets, such as easy activation, deformation, thrombosis, and difficult preservation. There are many ways to combine drugs with these carriers, and each has its own advantages and disadvantages. It is necessary to seek the best combination scheme to increase drug loading and reduce the damage to therapeutic components to the carriers, so as to bring more mature and reliable methods for the clinical application of drug delivery technology. Several drug-loading technologies and their development were described according to various categories. The combination of drugs and carriers is summarized for better understanding of its practical application.
Keywords: drug delivery technology, carrier, combination, tumor
Introduction
Short drug cycle time and difficulty in local treatment of tumor sites are two major challenges faced by traditional cancer treatment methods. Single anticancer drugs usually lead to cancer recurrence and acquire drug resistance. Combined therapy with multiple agents or different therapies improves the therapeutic effect, however,side effects are greater. Scientists are making great efforts to apply drug carrier technology to the anticancer war for solving these problems and further ensure the safety and effectiveness of cancer treatment. In the development of nanoparticle technology, nanoparticle-based chemical-siRNA combination therapy as an innovative strategy for malignant tumors treatment, which utilizes siRNA-mediated specific gene silencing to compensate for the incomplete anticancer effect of conventional chemotherapy. In addition, the combination of nanoparticles (NP) with peptide drugs, prodrugs, immunotherapy, and anti-infective drugs is also actively being studied and striving to be applied in clinical trials as soon as possible.1 Banerjee et al, showed that rod-like nanoparticles had higher cell absorption and trans-intestinal cell transport than spherical nanoparticles, which laid the foundation for the rational design of oral administration of nanodrugs.2 In many proposed drug delivery systems, red blood cells have become one of the better natural carrier systems in the history of drug delivery because of their long life, carrying breathing gas, and inherent ability to maintain their structural integrity.3 Drug carrier systems such as platelets and proteins have also been studied. Overall, the development of drug delivery systems can overcome many obstacles to traditional disease treatment.
NP as drug delivery systems
Nanotechnology is developing rapidly and contributing to clinical medicine. Nanoscale Drug Carriers is a kind of sub-micro particle drug delivery system, which belongs to nanoscale microscope. Drugs encapsulated in sub-particles can adjust the speed of drug release, increase the permeability of biofilm, change the distribution in vivo, and improve the bioavailability. NP are solid colloidal particles ranging in size from 10 to 100 nm used as a core in functionalization systems. They are generally composed of natural or synthetic macromolecule substances and can be used as carriers for conducting or transporting drugs. At present, NP used in medical treatment are generally accepted in size less than 100 nm.4 Due to the difference of materials and preparation technology, nanospheres and nanocapsules can be formed, which are collectively called NP. Solid lipid nanoparticles (SLN) are a new nanoparticle delivery system, which is being developed in recent years. SLN are a new nanoparticle delivery system, which is developing in recent years. It uses solid lipid (natural or synthetic) as a carrier and wraps drugs in lipid nuclei to prepare solid colloidal particles with a particle size of 50–1000 nm. The chemical materials of nanomaterials are chitosan, gelatin, branched polymers, carbon-based carriers, etc.4 Nanometer carrier used in medical applications must be biocompatible without causing an immune response or any negative effects. Drug-controlled release carriers can be achieved by changes in the physiological environment such as temperature, pH, osmotic pressure, or by enzymatic activity when drug nanometer carrier reach diseased tissues.5 In order to avoid injury to the patient’s body, smaller NP are easily removed by tissue exosmosis and kidney, while larger NP are quickly conditioned and cleared from the bloodstream by macrophages of the reticuloendothelial system (RES).6 Carbon nanomaterials and other drug carriers have been studied. Liposomes are the earliest drug carriers. Liposomes, NP based on solid lipids, dendrimer nanometer carrier, carbon nanomaterials are drug carrier has been studied, such as the drug carrier of Liposomes was among the first to be studied.7 In recent research, drug delivery system based on polymer micelles is a new kind of nano-carriers, forms micelles in water which possess hydrophilic and hydrophobic groups at the same time and can solubilize and encapsulate drugs.8
Advantages and disadvantages
Nano-drug delivery vectors show the advantage of enhanced permeability and retention effects in cancer tissues, which are caused by leakage of blood vessels and inefficient lymphatic drainage.9 Nanodrug delivery systems can release encapsulated molecules from nanometer carrier in a precise manner over time to maintain drug concentration in the therapeutic window, or they can be triggered by specific stimuli at a required release site. The nanometer carrier can improve the solubility and stability of encapsulated drugs and provide an opportunity to re-evaluate drug candidates previously neglected due to poor pharmacokinetics. Site-specific drug delivery can be achieved by using NP administered through different routes. Enhancing the solubility of highly hydrophobic drugs, providing sustained and controlled encapsulated drugs, and improving targeted therapies and specific ligands are also the advantages of nano-drug delivery technology.10,11 The research of inorganic layered nanomaterials in biomedical applications has also been progressing, because it has the advantages of adjustable particle size, crystal structure, aspheric shape, and high ion exchange capacity.9 Polymer micelle drug carrier is a kind of potential drug delivery system with good stability, strong drug-loading ability, and small particle size. It extends the application of the colloidal system in drug-controlled release and targeting.12 However, the environmental pollution caused by NP, the possible adverse effects of residual substances after drug release, and the toxicity of drugs to non-target organs need to be further studied. Limited lifetime of biodegradable NP may be conducive to solving these problems. Due to the many factors affecting the toxicity of NP and the difficulty in predicting these toxicities, it is necessary to study the toxicology of each new drug delivery systems preparation.13
Application and development
With the development of nano-drug-loading technology, drug production will achieve the goal of low cost, high efficiency, and automation. The role of drugs to achieve organ targeting will also be the focus of cancer treatment research in the 21st century. Nano-drug delivery technology overcomes many shortcomings and insurmountable problems of traditional drug, provides a new way for the research of new drug delivery systems (Table 1).14–19 For example, the research of polymer micelles as drug carriers mainly focuses on the delivery of two kinds of drugs. The first type is highly effective, toxic, and insoluble drugs, mainly including anticancer drugs, such as paclitaxel and doxorubicin. The second category is the drugs with the unstable physiological environment and low cell uptake rate, mainly genetic drugs, such as DNA (Figure 1).
Table 1.
Examples of therapeutic moieties loaded by nanoparticles/erythrocytes/platelet
| Drug carrier | Drug | Approaches | Application | First researcher |
|---|---|---|---|---|
| Nanoparticles | Paclitaxel | Cyclodextrin nanoparticles | MCF-7 human breast cancer cell line | Varan G14 |
| Doxorubicin | Injectable nanoparticle generator (iNPG) | Metastatic breast cancer | Rong X15 | |
| Dexamethasone | Lipid nanoparticles | Ocular drug delivery system | Junfeng B16 | |
| HIV integrase | Gold nanoparticle | HIV infection | Garrido C17 | |
| Vincristine sulfate | Liposome | Non-Hodgkin lymphoma | Rodriguez MA18 | |
| Daunorubicin | Liposome | Acute myeloid leukemia | Fassas A19 | |
| Erythrocyte | Enzyme | Hypotonic dialysis, Fusion with liposomes | Enzyme deficiency related diseases | Favrett ME25 |
| Dexamethasone | Hypotonic dialysis | Ulcerative Colitis | Bossa F26 | |
| Fasudil | Hypotonic dialysis | Pulmonary arterial hypertension | Gupta N27 | |
| L-asparaginase | Conjugation to cell-penetrating peptides | Acute lymphoblastic leukemia | Kwon YM28 | |
| Pravastatin | Endocytosis | Hypercholesterolemi-a | Harisa GE29 | |
| Platelet | Epidoxorubicin | Encapsulation | Myeloma | Lu D32 |
| Doxorubicin | Encapsulation | Lymphoma | Peipei X33 | |
| Factor viii | Encapsulation | Hemophilia A | Shi Q34 | |
| Factor iX | Encapsulation | Hemophilia B | Shi QZ35 |
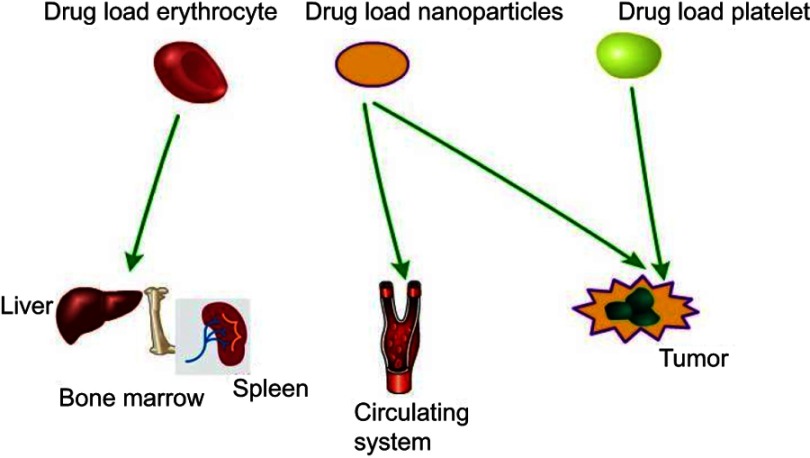
Figure 1.
Schematic of possible therapeutic applications of nanoparticles/erythrocyte/platelet drug carriers. Erythrocytes mainly target reticuloendothelial system (RES)-related organs (liver, bone marrow, spleen, etc.), while nanoparticles mainly target tumors and circulating system. Platelets mainly target tumors at present.
Erythrocyte-inspired delivery systems
Erythrocyte is double-sided concave round cakes with thicker edges and thinner middle shapes that allow them to maximize oxygen uptake from their surroundings. It also has flexibility, which allows it to pass through capillaries and release oxygen molecules, usually 6–8 microns in diameter. The function of red blood cells is to transport oxygen, carbon dioxide, electrolytes, glucose, and amino acids, which are essential substances for human metabolism. In addition, they also play a buffer role in acid–base balance and enhance phagocytosis, immune adherence, defense and so on. The half-life of human erythrocytes ranges from 100 days to 120 days. In adults, there are about 2–3×1013 order red blood cells. The function of the erythrocyte is to transport oxygen, carbon dioxide, electrolytes, glucose, and amino acids, which are essential substances for human metabolism. In addition, it plays a buffer role in acid–base balance, and also enhances the role of phagocytosis, immune adherence, and defense against infection. These characteristics lay the foundation for red blood cells as drug carriers.
Receptors, proteins, and functional groups on erythrocyte membranes provide binding sites for antibodies and drugs. Red blood cells also have good biocompatibility and biodegradability, so that they do not cause the immune reaction and produce toxic by-products. Importantly, the properties of erythrocyte membranes allow for higher drug loading and slower molecular release.20 Presently, the ability of scientists to manipulate erythrocyte membranes is increasing. The combination of polymer NP with erythrocyte membranes not only gives it the same long cycle life as natural red blood cells, but also optimizes the retention and release of controlled drugs.21 Erythrocyte as drug carriers can form different drug delivery systems, encapsulating drugs into non-diffusive prodrugs and releasing them in circulation, or combining drugs with protein or protein domains and encapsulating them into red blood cells are common ways.22
Advantages and disadvantages
Erythrocyte drug delivery system can deliver many kinds of materials, such as small molecule drugs, bioactive agents, liposomes and so on. Through membrane modification, these carriers can achieve drug delivery to many specific targets and promote the realization of targeted drug delivery. Because of the limitation of blood cell membranes, drugs will be released in a controlled manner, resulting in a significant reduction in steady-state concentration fluctuations.23 Changes in osmotic pressure during encapsulation may result in damage to erythrocyte membranes and changes in mechanical stability, plasticity, and permeability of erythrocytes. However, the preparation strategy of erythrocyte vectors has not been standardized and so the storage risk of erythrocyte vectors and blood contamination during drug loading is high.20
Application and development
Drug delivery system based on erythrocyte has attracted great attention because of the characteristics of high safety, long circulation, high drug loading, and low immunogenicity. It is a promising drug delivery system.24 Since red blood cells are engulfed by macrophages in the liver and spleen, they can introduce drugs into the RES of cells, thus contributing to the treatment of macrophage-related liver diseases (Figure 1). Erythrocyte-based delivery vectors have been developed to deliver a variety of therapeutic agents, including proteins, nucleic acids, and small molecule drugs, and some of them have entered the clinical trial stage and are used to treat various diseases, such as cancer-targeted therapy (Table 1).21,25–29 It can be seen that the clinical application of this delivery method has strong momentum for both solid tumors and hematological tumors, with the implementation of the combination of asparaginase and RBC vectors which can be used to treat acute lymphoblastic leukemia.25 Commonly used chemotherapeutic drugs such as vincristine and methotrexate, which are encapsulated in red blood cells, can improve their efficacy in vivo and prolong the effective time of treatment.30
Platelet-inspired delivery systems
Platelets are small pieces of cytoplasm released from the cytoplasm of mature megakaryocytes in bone marrow. Normal platelets in circulating blood are concave, oval or disc-shaped on both sides. The average diameter of human platelets is about 2–4 microns, the thickness is 0.5–1.5 microns, the average volume is 7 cubic microns, the normal value in vivo is about 100–300×109/L, and the average life span is 7–10 days. Since platelets can move and deform, they are multiform when observed by general methods. The main functions of platelets are coagulation and hemostasis, repairing damaged blood vessels. In the past decades, many studies have identified receptor–ligand interactions between activated platelets and cancer cells, including P-selectin ligands (cancer cells) – P-selectin (platelets), αIIbβ3 integrins (cancer cells and platelets) – fibrinogen (plasma), andα2β1 integrins (cancer cells and platelets) – Collagen (extracellular matrix) and so on.21 Tumor cells can induce platelet aggregation, while activated platelets protect or shield circulating tumor cells from physical factors such as intravascular shear stress, and help them escape epidemic surveillance. Importantly, platelets can also cause tumor cells to migrate to secondary sites on the vascular wall. Proteinases and cytokines secreted by platelets promote the formation of new blood vessels, which are essential for tumor-related angiogenesis and growth. These results laid a foundation for the application of platelet drug delivery technology in the treatment of cancer diseases.14 In platelet drug delivery systems, drugs are artificially exposed to agonists to facilitate their release. However, heterogeneity and complexity of cancer biology indicate that platelet-cancer cell adhesion using the same receptor–ligand interaction may lead to deviation of therapeutic objectives.12
Advantages and disadvantages
The advantages of using platelets as drug delivery carriers are as follows: a) platelets are readily available blood cells, b) through technical controlling, platelets can be exposed to agonists to release drugs. However, platelets are easily activated, deformed, and aggregated to form a thrombus, and their cytoplasmic components are complex. In the preparation of platelet carriers in vitro, centrifugation, washing, blowing, and temperature changes cause platelet activation and thrombosis, making the preparation of platelet carriers more difficult, with low yields and short periods of preservation. Kabiramide C is mainly used to inhibit platelet aggregation in platelet-mediated drug delivery technology, but it is harmful to human body, so finding a more suitable platelet inhibitor is also a challenge. Similar to the limitations on proper storage and contamination of red blood cells, platelets have similar problems. In genetic therapy, it is not clear whether platelet-specific gene expression affects the expression of endogenous proteins.20
Application and development
Platelet cytoplasmic components are more complex than red blood cells, and the characteristics of easily deformed and thrombosis make the preparation of platelet vectors more difficult. These factors make the related research of platelet vectors less. At present, more studies are focused on the use of platelets as carriers to transport DOX. Sarkar et al, showed that platelet-mediated DOX release can be effectively used in the clinical treatment of lymphoma.31 At the same time, many studies have suggested platelets can inspire a variety of therapeutic and targeted antibodies to achieve tumor immunotherapy (Figure 1), and can also be used in cardiovascular and neurological diseases (Table 1).32–35
Combination of drugs with carriers
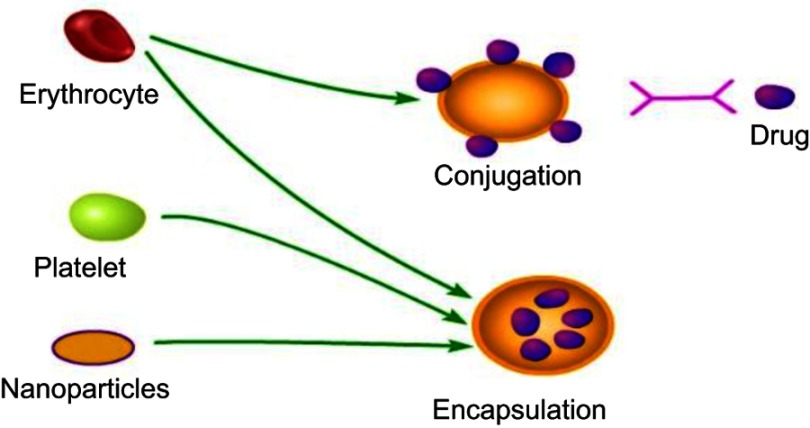
There are two main ways of drug carrier binding: drugs are encapsulated in cells or coupled onto a cells surface (Figure 2). In a drug delivery system with red blood cells as carriers, in order to achieve a better drug delivery effect, the two binding modes imitate the natural transport function of red blood cells as much as possible. Encapsulation helps to separate drugs from the body, but problems such as damage to carrier cells, storage of cells in later stages, and quality control need to be solved. These problems are particularly evident in drug delivery systems mediated by red blood cells, because there is no vesicle pathway to internalize external compounds on the surface of red blood cells.
Figure 2.
Encapsulation and conjugation are two main ways that drug attached to the carriers. Drugs can be encapsulated in erythrocyte or bound to cell membranes when erythrocyte is used as carriers. Drugs are mainly encapsulated in carriers, if nanoparticles or platelets are used as drug delivery carriers.
Drugs are encapsulated in cells
Hypotonic method
The hypotonic method developed in the 1970s is considered to be the standard method for encapsulating drugs. In a low osmotic solution, red blood cells can be used as osmometers and can expand reversibly to 25% of their original volume. Pores of 200–500 size are temporarily formed in the membrane, so substances can enter red blood cells through these holes. Under the condition of carefully controlling and restoring the isotonicity of the solution, these pores can be re-encapsulated and most of the biophysical and immunological characteristics of red blood cells can be preserved.21 Hypotonic dilution is considered to be the simplest and fastest encapsulation method. It is the first process of loading therapeutic fractions into red blood cells.20 However, the damage caused to erythrocytes by this technique is great. Hemolysis, exposure to phosphatidylserine, changes in cell morphology and membrane structure makes it necessary to monitor the loading process closely. More seriously, impaired biocompatibility of resealed red blood cells may be the biggest problem. Studies have shown that premature withdrawal from circulation may result in changes in membrane stiffness, further affecting cell shape, and reducing the deformation capacity required by cells through microcirculation.25,36 According to the same osmotic dissolution principle, but with different procedures, low osmotic hemolysis, low osmotic dilution, and low osmotic dialysis technologies have been developed successfully. The maturity of these technologies has also led to the development of red blood cell loaders, which allow drugs to be automatically packaged into red blood cells, providing a way to capture goods within RBCs.
Cell-penetrating peptides
Membrane-penetrating peptide is a new method used in erythrocyte drug delivery system in recent years. In the 1990s, it was found that a polypeptide consisting of 60 amino acid residues in the antennal homologous region of amphibian Drosophila could penetrate the cell membrane and drive the internalization of intracellular bioactive substances. Generally, the binding of CPP to membranes requires electrostatic interaction or amphiphilicity of peptides. Its two main families are arginine-rich and proline-rich. Many amphiphilic characteristics of CPP allow it to fold into alpha-helical and beta-sheet structures, some of which fold only in the presence of membranes, while others tend to present secondary structures in solution before interacting with membranes.37 Compared with the low osmotic method, the damage to cell membranes is more serious. Polypeptides can enter the cytoplasm or even the nucleus through the cell membranes, but the cell membranes are intact. Membrane-penetrating peptide consists of 16 amino acids, including seven positive residues and two very important Trp residues (Trp48 and Trp56). Membrane penetration may be caused by the interaction of Trp48 and Trp56 residues and other charged residues with cell membrane lipid matrix. Different from electroporation, microinjection, and other methods, there are some shortcomings, such as low molecule introduction rate, easy to cause cell damage and even death. Perforating peptide has many advantages, such as high affinity of cell membrane, fast penetration speed, and rapid degradation.38 In clinical research, the use of CPP to introduce insulin into cells for the treatment of diabetes mellitus, delivery of cytotoxic drugs for cancer, and delivery of low-concentration toxic drugs to the heart provide a very promising way.39 A large number of experiments have shown that CPPs combined with some anticancer drugs can increase the membrane permeability, drug release, and drug accumulation of cancer cells, thereby improving the therapeutic effect of cancer.40
Electroporation
Electroporation, also known as electrical transfection, improves the permeability of cell membranes instantaneously through the high-intensity electric field, thus absorbing foreign molecules in the surrounding media. This technology can transfer nucleotides, DNA and RNA, proteins, carbohydrates, dyes, and virus particles into prokaryotic and eukaryotic cells. Electroporation is a valuable and effective alternative to other physical and chemical conversion methods. At present, it is generally accepted that the description of the results of electroporation is the formation of water holes in lipid bilayers.41 Electroporation increases the permeability of cell membranes through non-thermal and non-chemical pathways. Kinosita and Tsong believe that ideal membrane permeability can be obtained by transient electrolysis when red blood cells are exposed to a strong external electric field.13 The development of standard electroporation and the integration of integrated therapy should bring more accurate and less side effects to the treatment of tumors and infectious diseases.40 Electroporation is essentially a physical method, so it does little damage to cells, and has the advantages of low toxicity and high efficiency.42 Spugnini et al, suggested that electroporation could improve the efficacy of bleomycin in the treatment of periocular and advanced head squamous cell carcinoma in cats with periocular cancer.43
Drug coupling with the cell membrane
In the traditional strategies, reversible or irreversible coupling between the therapeutic part and erythrocyte is the most widely used strategy. In addition to biotin-avidin, various drugs, such as viral antigen, immunoglobulin, and daunorubicin may also be bound to the surface of red blood cells through nonspecific chemical crosslinkers. Reagents are attached to the RBC surface through physical interaction. For example, polystyrene particles can adhere to RBC surface through nonspecific van der Waals and hydrogen bonds, which work through electrostatic force and hydrophobic force between particles and erythrocytes, respectively.20 Covalent ligation can accurately control the number of drug molecules connected to nanometer carrier, so it has advantages over other ligation methods.5 Biotin-avidin is also a commonly used chemical conjugate because of its high cell tolerance. Another way to attach drugs to red blood cells is to anchor affinity ligands such as peptides, antibodies, and antibody fragments onto the surface proteins of red blood cells, and then attach drugs to these ligands.
Conclusion
Up to now, the development of drug-loading technology is not perfect, and the drug delivery method based on NP is only used in the treatment of leukemia. In the preclinical model of leukemia, Dr. AK Rajasekaran and his team improved survival and quality of life through nanotechnology. The capsule of the drug, which was given a standard dose of 1/3, had good results and had no significant side effects. In addition, mice treated with NP had longer survival times than those who were given drugs in the traditional way. In the past decade or so, with the development of technology, the carriers of drug delivery have varied, but most of the drug delivery technologies have focused on tumor-targeted drugs, and the kinds of drugs transported and treated diseases are limited, drugs are absorbed into the body. “Dose-time” changes in distribution, metabolism, and excretion, or changes in “concentration and time” in blood, also need to be further studied, and the goal of drug delivery systems is to develop practical clinical treatments for patients. It is not enough to build a new distribution system.44,45 Searching for high-quality materials to deliver drugs to target cells, using cell-specific gene expression and disease physiology to increase drug targeting are challenges that will continue to be faced in the future and will have a significant impact on human health. Moreover, drug delivery technology with NP as a carrier can also participate in the completion of gene editing. The new gene editing drugs have more advantages than traditional drugs.46 In recent years, biomimetic nanopharmaceutical systems have become a hot topic in the field of nano-drug delivery. These carrier systems are mainly based on a variety of cells (red blood cells, immune cells, stem cells, etc.) or extracted cell membranes. It is the basic condition for these carriers to be used as a drug carrier system to ensure their approximate physiological state. At the same time, the drug encapsulation rate of cells is generally low, for example, when RBCs are used, the drug encapsulation rate is less than 20%. Traditional methods, such as dialysis and electroporation, have many disadvantages. The membrane-penetrating peptide and the newly developed chemical binder have been greatly improved. Therefore, it is very important to use different encapsulation methods to achieve the targeting and safety of drug delivery more efficient. Animal and clinical experiments must be done to test the efficacy and safety of cell-drug binding. Although there are still many problems to be solved in the drug carrier system and encapsulation technology at present, its unique superiority is bound to present a better prospect with the development of science and technology.47,48
Acknowledgments
This work was supported by the National Nature Science Foundation of China (number: 81170492), the National Nature Science Foundation of China (number: 81370673), the PLA Major Project (number: ANJ13J001), the Key Medical Projects of Jiangsu Province (BL2014078), the Key Department of Jiangsu Province (2016-2020), Jiangsu Social Development Project (Project No.: BE2018711).
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–185. doi: 10.1016/j.jconrel.2016.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzounakas VL, Karadimas DG, Papassideri IS, Seghatchian J, Antonelou MH. Erythrocyte-based drug delivery in transfusion medicine: wandering questions seeking answers. Transfus Apher Sci. 2017;56(4):626–634. doi: 10.1016/j.transci.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 3.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applicationsand hazards. Int J Nanomedicine. 2008;3(2):133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–1037. [DOI] [PubMed] [Google Scholar]
- 5.Cole AJ, Yang VC, David AE. Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29:323–332. doi: 10.1016/j.tibtech.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley S. Biological nanoparticles and their influence on organisms. Curr Opin Biotechnol. 2014;28:69–74. doi: 10.1016/j.copbio.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Luo Q, Gao H, et al. Cell membrane-inspired polymeric micelles as carriers for drug delivery. Biomater Sci. 2015;3(3):490–499. doi: 10.1039/c4bm00385c [DOI] [PubMed] [Google Scholar]
- 8.Saxena V, Diaz A, Clearfield A, Batteas JD, Hussain MD. Zirconium phosphate nanoplatelets: a biocompatible nanomaterial for drug delivery to cancer. Nanoscale. 2013;5(6):2328–2336. doi: 10.1039/c3nr34242e [DOI] [PubMed] [Google Scholar]
- 9.Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014;2014:180549. doi: 10.1155/2014/180549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal R, Macri LK, Kaplan HM, Kohn J. Nanoparticles and nanofibers for topical drug delivery. J Control Release. 2016;240:77–92. doi: 10.1016/j.jconrel.2015.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Ai Y, Wang L, et al. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials. 2016;76:52–65. doi: 10.1016/j.biomaterials.2015.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai J, Biazar E, Montazeri M, et al. Nanotoxicology and nanoparticle safety in biomedical designs. Int J Nanomedicine. 2011;6:1117–1127. doi: 10.2147/IJN.S16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu P, Wang R, Wang X, Ouyang J. Recent advancements in erythrocytes, platelets, and albumin as delivery systems. Onco Targets Ther. 2016;9:2873–2884. doi: 10.2147/OTT.S104691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varan G, Benito JM, Mellet CO, Bilensoy E. Development of polycationic amphiphilic cyclodextrin nanoparticles for anticancer drug delivery. Beilstein J Nanotechnol. 2017;8:1457–1468. doi: 10.3762/bjnano.8.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rong X, Guodong Z, Junhua M, et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol. 2016;34(4):414–418. doi: 10.1038/nbt.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junfeng B, Yan Z, Xin H, et al. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int J Nanomedicine. 2017;12:1329–1339. doi: 10.2147/IJN.S126199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrido C, Simpson CA, Dahl NP, et al. Gold nanoparticles to improve HIV drug delivery. Future Med Chem. 2015;7(9):1097–1107. doi: 10.4155/fmc.15.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez MA, Pytlik R, Kozak T, et al. Vincristine sulfate liposomes injection (Marqibo) in heavily pre-treated patients with refractory aggressive non-Hodgkin lymphoma. report of the pivotal phase. 2 study. Cancer. 2009;115(15):3475–3482. doi: 10.1002/cncr.24359 [DOI] [PubMed] [Google Scholar]
- 19.Fassas A, Anagnostopoulos A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk Lymphoma. 2005;46(6):795–802. doi: 10.1080/10428190400011625 [DOI] [PubMed] [Google Scholar]
- 20.Hu CM, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv Healthc Mater. 2012;1(5):537–547. doi: 10.1002/adhm.201200138 [DOI] [PubMed] [Google Scholar]
- 21.Magnani M, Rossi L. Approaches to erythrocyte-mediated drug delivery. Expert Opin Drug Deliv. 2014;11(5):677–687. doi: 10.1517/17425247.2014.889679 [DOI] [PubMed] [Google Scholar]
- 22.Piao J-G, Wang L, Gao F, You Y-Z, Xiong Y, Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8:10414–10425. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Su J, Liu G, et al. Advances of blood cell-based drug delivery systems. Eur J Pharm Sci. 2017;96:115–128. doi: 10.1016/j.ejps.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 24.Luk BT, Fang RH, Che-Ming J, et al. Safe and immunocompatible nanocarriers cloaked in rbc membranes for drug delivery to treat solid tumors. Theranostics. 2016;6(7):1004–1011. doi: 10.7150/thno.14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favretto ME, Cluitmans JCA, Bosman GJCGM,Brock R. Human erythrocytes as drug carriers: loading efficiency and side effects of hypotonic dialysis, chlorpromazine treatment and fusion with liposomes. J Control Release. 2013;170(3):343–351. doi: 10.1016/j.jconrel.2013.05.032 [DOI] [PubMed] [Google Scholar]
- 26.Bossa F, Latiano A, Rossi L, et al. Erythrocyte-mediated delivery of dexamethasone in patients with mild-to-moderate ulcerative colitis, refractory to mesalamine: a randomized, controlled study. Am J Gastroenterol. 2008;103:2509–2516. doi: 10.1111/j.1572-0241.2008.02103.x [DOI] [PubMed] [Google Scholar]
- 27.Gupta N, Patel B, Ahsan F, et al. Nano-engineered erythrocyte ghosts as inhalational carriers for delivery of fasudil: preparation and characterization. Pharm Res. 2014;31(6):1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon YM, Chung HS, Moon C, et al. L-Asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia. J Control Release. 2009;139(3):182–189. doi: 10.1016/j.jconrel.2009.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harisa GE, Ibrahim MF, Alanazi FK, et al. Characterization of human erythrocytes as potential carrier for pravastatin: an in vitro study. Int J Med Sci. 2011;8(3):222–230. doi: 10.7150/ijms.8.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427–3436. doi: 10.1182/blood-2009-10-247296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar S, Alam MA, Shaw J, Dasgupta AK. Drug delivery using platelet cancer cell interaction. Pharm Res. 2013;30(11):2785–2794. doi: 10.1007/s11095-013-1097-1 [DOI] [PubMed] [Google Scholar]
- 32.Lu D, Ning G, Baoan C, et al. Human platelets repurposed as vehicles for in vivo imaging of myeloma xenotransplants. Oncotarget. 2016;7(16):21076–21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peipei X, Huaqin Z, Bing C, et al. Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Sci Rep. 2017;7:42632. doi: 10.1038/srep42632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Q, Fahs SA, Wilcox DA, et al. Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunity. Blood. 2008;112(7):2713–2721. doi: 10.1182/blood-2008-03-140830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi QZ, Montgomery RR. Platelets as delivery systems for disease treatments. Adv Drug Deliv Rev. 2010;62(12):1196–1203. doi: 10.1016/j.addr.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villa CH, Seghatchian J, Muzykantov V. Drug delivery by erythrocytes: “Primum non nocere”. Transfus Apher Sci. 2016;55(3):275–280. doi: 10.1016/j.transci.2016.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalafatovic D, Giralt E. Cell-penetrating peptides: design strategies beyond primary structure and amphipathicity. Molecules. 2017;22(11). doi: 10.3390/molecules22111929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiang W, Xiang X, Huaping L, Ming Z. Structural characteristics and transmembrane mechanism of transmembrane peptides. Chemistry of Life. 2005;25(4):304–306. [Google Scholar]
- 39.Copolovici DM, Langel K, Eriste E, Langel Ü. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano. 2014;8(3):1972–1994. doi: 10.1021/nn4057269 [DOI] [PubMed] [Google Scholar]
- 40.Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622 [DOI] [PubMed] [Google Scholar]
- 41.Antonella B, Anna Lucia T, Maria Lina T, et al. Cell penetrating peptides as molecular carriers for anti-cancer agents. Molecules. 2018;23(2):295. doi: 10.3390/molecules23020295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Junfeng S, Yifan M, Jing Z, et al. A review on electroporation-based intracellular delivery. Molecules. 2018;23(11):3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spugnini EP, Pizzuto M, Filipponi M, et al. Electroporation enhances bleomycin efficacy in cats with periocular carcinoma and advanced squamous cell carcinoma of the head. J Vet Intern Med. 2015;29(5):1368–1375. doi: 10.1111/jvim.13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park K. Controlled drug delivery systems: past forward and future back. J Control Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yun YH, Lee BK, Park K. Controlled drug delivery: historical perspective for the next generation. J Control Release. 2015;219:2–7. doi: 10.1016/j.jconrel.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tibbitt MW, Dahlman JE, Langer R. Emerging frontiers in drug delivery. J Am Chem Soc. 2016;138(3):704–717. doi: 10.1021/jacs.5b09974 [DOI] [PubMed] [Google Scholar]
- 47.Yanshu L, Weihong G, Jianxin Z. Research progress of erythrocyte drug carrier. Chin Pharm J. 2004;39(5):324–327. [Google Scholar]
- 48.Bowei C, Shurui S, Guoyun W, et al. Preparation of multifunetional nanoscaled red blood cells drug delivery system and its photothermal and photodynanfic effects. Int J Biomed Eng. 2018;41(1):32–37. [Google Scholar]