Abstract
Background: The clinical efficacy and safety of Endostar combined with chemotherapy in the treatment of metastatic malignant melanoma (MM) were analyzed and the indicators capable of predicting the efficacy of the regimen were identified to guide clinical practice.
Patients and methods: The clinical data of 55 patients with metastatic MM without gene mutations who were treated with Endostar combined with dacarbazine and cisplatin were retrospectively analyzed. Efficacy was assessed using RECIST 1.1, and adverse events (AEs) were graded according to NCI-CTCAE 4.0. The log-rank test was used to compare the survival curves of patients in different subgroups, and stepwise multivariate Cox regression analysis was used to determine significant prognostic factors. Differences were considered statistically significant at P<0.05.
Results: Of the 55 patients, seven showed a partial response, 20 showed stable disease, and 28 showed progressive disease. The median progression-free survival was 17.9 months. AEs were controllable. Univariate analysis identified biotherapy, clinical stage, clinical classification, low baseline platelet count, platelet to albumin ratio (PAR), and platelet to globulin ratio (PGR) as factors affecting drug efficacy. Multivariate Cox regression analysis identified clinical stage and PAR as independent factors predicting the efficacy of the regimen.
Conclusions: Endostar combined with chemotherapy showed a curative effect on metastatic MM without gene mutations, and AEs were controllable. The baseline platelet count and derived PAR and PGR values were associated with the efficacy of the regimen. The potential value of efficacy prediction remains to be further verified by prospective random experiments.
Keywords: Endostar, melanoma, targeted therapy, inflammatory biomarkers, platelet to albumin ratio, platelet to globulin ratio
Introduction
Malignant melanoma (MM) is a highly aggressive malignant tumor that originates from neural crest melanocytes and is triggered by hyperplasia of abnormal melanocytes. Data released by the GLOBOCAN online database (gco.iarc.fr) in 2018 indicate that 287,723 new cases of MM of skin are diagnosed worldwide, and 60,712 of these patients die.1 The incidence of MM is increasing at a rate of 3% per year.2 MM is characterized by a high metastasis rate, high mortality, and strong drug resistance. The main cause of death is extensive metastasis, including to the lung, liver, bone, and brain.3 Clinically, MM is a highly invasive tumor that is mainly derived from the skin and mucosa and the tunica vasculosa. Lymphatic and hematogenous metastasis can occur in the early stage of tumorigenesis. Metastasis is detected in approximately 20% of patients with MM at first diagnosis, and in these cases the tumor has progressed to the advanced stage.4 The prognosis of metastasis to skin, subcutaneous tissues, or lymphoid tissues is relatively good; the prognosis of metastasis to lungs is moderate; the prognosis of metastasis to liver, bone, brain, or areas with high levels of lactate dehydrogenase is relatively poor. Patients with MM will potentially lose 20.4 years of their lifespan, which is significantly higher than the 16.6 years for all other malignant tumors.5 MM has become one of the malignant tumors that seriously threaten human health and identifying treatment strategies for MM is therefore important.
Angiogenesis provides abundant nutrients for the rapid growth of tumors, as well as a pathway for distant metastasis through blood circulation. The balance hypothesis of the angiogenic switch states that tumor angiogenesis is the result of the co-regulation of tumor angiogenesis inhibitors and tumor-promoting angiogenic factors. Inhibition of angiogenesis suppresses tumor growth. Rh-endostatin (Endostar) is an anti-tumor vascular targeted drug developed by experts in China through the addition of nine amino acids to endostatin. Endostar is a powerful drug targeting endogenous anti-angiogenic molecules and has shown excellent experimental results.6 The mechanisms of action of Endostar can be summarized as follows: 1. it inhibits the migration and proliferation of vascular endothelial cells, blocks neovascularization, and controls tumor growth; 2. it downregulates the expression of vascular endothelial growth factor (VEGF) by acting on the VEGF pathway, and decreases the activity of proteolytic enzymes, thereby blocking the G1 phase of endothelial cells, decreasing the level of Bcl-2 and directly inducing apoptosis of cancer cells; and 3. it reduces the probability of contact between vascular endothelial cells and interstitial or intercellular cells, which facilitates the effect of chemotherapeutics on attacking vascular endothelial cells and inducing apoptosis, thereby inhibiting the proliferation or metastasis of tumor cells.7–9 Since its arrival in the market, many clinical tests and studies have verified the efficacy, low drug resistance, and decreased adverse effects (AEs) of Endostar.10–13 Endostar has become the anti-tumor angiogenesis targeting drug with top priority for the first-line treatment of MM in China. Endostar is included in the basic strategies for the first-line treatment of metastatic MM, and is recommended by the Chinese Society of Clinical Oncology Diagnosis and Treatment Guide to Malignant Melanoma 2017.V1.
Recent studies show that inflammatory cells play an important role in tumorigenesis and can be used as an indicator of prognosis.14–20 However, there are no studies predicting the efficacy of Endostar combined with chemotherapy for the treatment of MM using inflammatory biomarkers. The present study analyzed the clinicopathological features of 55 patients with MM who were treated with Endostar combined with chemotherapy, and which identified relevant variables with prognostic value to explore the effectiveness and safety of this regimen. The efficacy of this regimen was also predicted from the perspective of clinicopathological features and pre-medication inflammatory biomarkers, providing theoretical support for the individualized treatment of patients.
Patients and methods
Patients
The present retrospective analysis included 55 stage unresectable III–IV patients with MM who had no KIT, BRAF, RAS (NRAS、KRAS、HRAS), MET or NF1 gene mutations and there was no evidence of MGMT methylation. All of the patients were treated with Endostar combined with dacarbazine (DTIC) and cisplatin (DDP) between August 2013 and June 2018 in the Department of Musculoskeletal Oncology, Fudan University Shanghai Cancer Center (Shangai, China). Of the 55 patients, 27 had unresectable stage III and 28 had stage IV MM based on the pathological examination performed in our hospital, with imaging examination showing lymphatic or distant metastasis. The medical information of all patients was available including primary and metastatic tumor sites, results of routine and imaging examinations, treatment records, survival follow-up data, and disease progression assessed by imaging. The study was approved by the Institutional Review Board of our hospital, and all patients and their guardians were informed and signed relevant documents.
Treatment
All patients agreed to use Endostar combined with DTIC and DDP regimens. The specific regimen was: DTIC (250 mg/m2) q.d. by intravenous drip for 5 consecutive days; DDP (25 mg/m2) q.d. for 3 consecutive days; and Endostar (7.5 mg/m2) q.d. for 14 consecutive days, with 2 weeks of rest, with one course of treatment consisting of 28 days. All patients received four to 15 courses of treatment, and a median of eight. Thirty-five (63.6%) patients were simultaneously treated with biotherapy. The specific regimen was interferon alfa-2b (IFN-α-2b) 3 MU/m2 and interleukins-2 (IL-2) 5 MU/m2, intravenous drip, once every other day and the duration of biotherapy was 1 year.
AEs were assessed, graded and recorded according to NCI-CTCAE 4.0. Patients with grade 1 or 2 AEs were advised to continue medication, with follow-up observations in the outpatient department. Patients with grade 3 or higher AEs were suggested to stop the medication and were hospitalized for symptomatic treatment until the grade of AEs was ≤2. The medication was continued if tolerated by the patient, and the medication was stopped if the patient’s safety was at risk or if serious sequelae were suspected.
Assessment
The follow-up ended on September 30, 2018. Baseline assessments included detailed medical history, physical examination, routine laboratory examination, pathological examination, and ultrasound, computer tomography (CT), or magnetic resonance imaging (MRI) of the primary or metastatic site. After four courses of treatment, RECIST 1.1 was used to assess efficacy, including complete response (CR), partial response (PR), stable disease (SD), and progression of disease (PD). The disease control rate (DCR) was the percentage of patients with CR + PR + SD; the objective response rate (ORR) was the percentage of patients with CR + PR. Progression-free survival (PFS) was defined as the time from the start of the combined regimen to PD or death. In cases of failure of the regimen, patients were advised to enter a clinical trial or to start treatment with other drugs such as apatinib. Pembrolizumab was approved in China in September 2018 for the treatment of MM. Therefore, it is not objective to assess the efficacy of this regimen based on the overall survival (OS) of patients, and no analysis or discussion were made.
Statistical analysis
All data were statistically processed using SPSS 25.0 software. The Kaplan-Meier method was used for survival analysis and the Log-Rank test was used to compare survival curves of patients in different subgroups. A stepwise multivariate Cox regression analysis was used to determine significant prognostic factors. Only prognostic factors with statistical significance in the univariate analysis were included in the multivariate analysis. Differences with a P-value <0.05 were considered statistically significant.
Results
Characteristics of patients at baseline
Of the 55 patients, 23 (41.8%) were men and 32 (58.2%) were women, and the median age was 58 years (range, 38–75 years). The clinical stage was based on the eighth edition of the American Joint Committee on Cancer (AJCC). The 27 patients with unresectable stage III disease were III N3c, while 28 patients (50.9%) with stage IV disease, six were M1a (10.9%), 12 were M1b (21.8%), 6 were M1c (10.9%), and four were M1d (7.3%). Of the 55 patients, 12 (21.8%) were diagnosed with mucosal type, 24 (43.6%) with acro-type, six (10.9%) with chronic sun-induced damage type, and 13 (23.7%) with non-chronic sun-induced damage type. Of 35 patients with melanoma of the limbs, 18 cases were at unresectable stage III, and 17 cases were at stage IV. Baseline data are shown in Table 1.
Table 1.
Patients’ characteristics and details of univariate analysis (N=55)
| Characteristics | No. of patients | % of total | Univariate P-value of progression free survival |
|---|---|---|---|
| Gender | |||
| Male | 23 | 41.8 | 0.3879 |
| Female | 32 | 58.2 | |
| Age (years) | |||
| Median, Range | 58 | 38~75 | |
| <60 | 34 | 61.8 | 0.1778 |
| ≥60 | 21 | 38.2 | |
| Baseline ECOG status | |||
| 0 | 13 | 23.6 | 0.7442 |
| 1 | 30 | 54.6 | |
| 2 | 12 | 21.8 | |
| AJCC stage | |||
| Unresectable III N3c | 27 | 49.1 | 0.0102* |
| IV | 28 | 50.9 | |
| M1a | 6 | 10.9 | 0.1729 |
| M1b | 12 | 21.8 | |
| M1c | 6 | 10.9 | |
| M1d | 4 | 7.3 | |
| Melanoma subtype | |||
| Mucosal | 12 | 21.8 | 0.0368* |
| Others | 43 | 78.2 | |
| Acra | 24 | 43.6 | |
| CSD | 6 | 10.9 | |
| NCSD | 13 | 23.7 | |
| Melanoma site | |||
| Head and neck | 12 | 21.8 | 0.7227 |
| Trunk | 8 | 14.6 | |
| Limbs | 35 | 63.6 | |
| Ulceration | |||
| Present | 13 | 23.6 | 0.8873 |
| Absent | 42 | 76.4 | |
| Clark level | |||
| III or IV | 22 | 40 | 0.4079 |
| V | 11 | 20 | |
| Breslow thickness (mm) | |||
| ≤1.0 | 2 | 3.6 | 0.3956 |
| 1.01~2.0 | 5 | 9.1 | |
| 2.01~4.0 | 15 | 27.3 | |
| >4.0 | 11 | 20 | |
| Biological Therapy | |||
| Yes | 35 | 63.6 | 0.0102* |
| No | 20 | 36.4 | |
| NLR | |||
| <1.37 | 8 | 14.5 | 0.4414 |
| ≥1.37 | 47 | 85.5 | |
| PLR | |||
| <206 | 46 | 83.6 | 0.0756 |
| ≥206 | 9 | 16.4 | |
| LMR | |||
| <4.83 | 36 | 65.5 | 0.1766 |
| ≥4.83 | 19 | 34.5 | |
| Platelet count (×109/L) | |||
| <168.5 | 18 | 32.7 | 0.0147* |
| ≥168.5 | 37 | 67.3 | |
| PAR | |||
| <4.251 | 18 | 32.7 | 0.0018** |
| ≥4.251 | 37 | 67.3 | |
| PGR | |||
| <4.694 | 11 | 20 | 0.0065** |
| ≥4.694 | 44 | 80 |
Note: *P <0.05 and **P <0.01.
Abbreviations: No, number; ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee on Cancer; CSD, melanomas on skin with chronic sun-induced damage; NCSD, melanomas on skin without chronic sun-induced damage; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PAR, platelet to albumin ratio; PGR, platelet to globulin ratio.
No main organ dysfunction was observed, and the Eastern Cooperative Oncology Group (ECOG) score was 0–2. The results of routine blood tests and liver function tests performed before drug administration were partly included in the statistical analysis, including lymphocytes, monocytes, neutrophils, platelets, albumin, globulin, and their ratios.
Efficacy and prognosis analysis
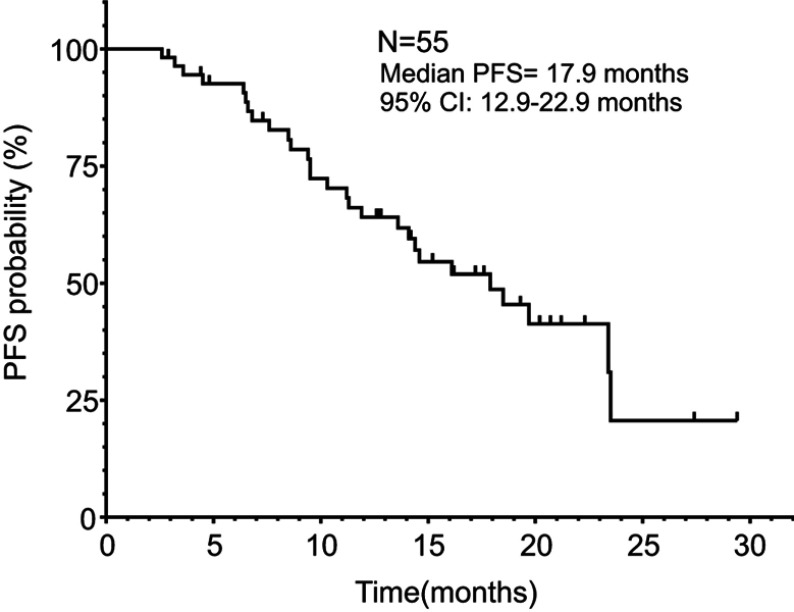
The 55 patients were followed-up for a median of 19.2 months (range, 2.9–37.1 months). Of the 55 patients, seven achieved PR, 20 had SD, and 28 had PD. The ORR and DCR were 12.7% and 49.1%, respectively. The median PFS (mPFS) was 17.9 months [95% confidence interval (CI), 12.9–22.9], and the PFS rates at 1 and 2 years were 64.1% and 20.7%, respectively. Details are shown in Figure 1. Due to 35 patients were simultaneously treated with biotherapy, and 22 non-responsive patients were subsequently treated with apatinib and 6 non-responsive patients were subsequently treated with pembrolizumab, 8 patients (4 patients in unresectable stage III and 4 patients in stage IV) died and the median OS (mOS) was not reached, respectively.
Figure 1.
Kaplan–Meier plot of PFS for all patients.
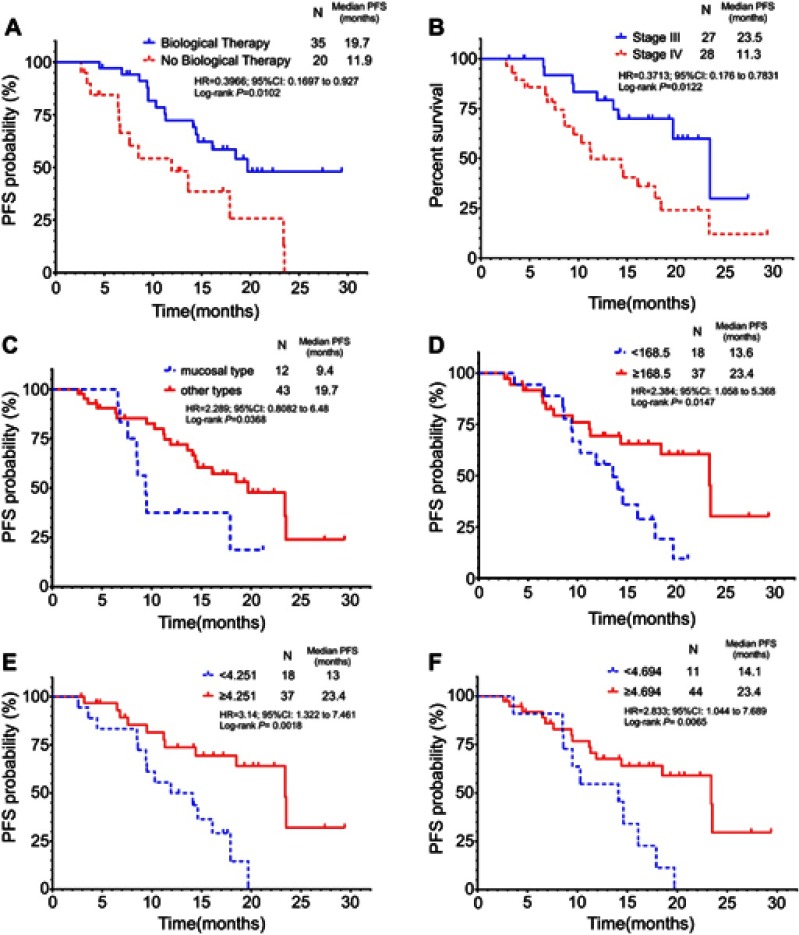
The results of univariate analysis showed that non-combined biotherapy (P=0.0102, Figure 2A), clinical stage IV (P=0.0102, Figure 2B), mucosal type (P=0.0368, Figure 2C), low pre-treatment platelet count (<168.5×109/L, P=0.0147, Figure 2D), low platelet to albumin ratio (PAR, <4.251, P=0.0018, Figure 2E), and low platelet to globulin ratio (PGR, <4.694, P=0.0065, Figure 2F) were poor prognostic factors affecting the efficacy of Endostar combined with DTIC and DDP regimens. Multivariate Cox regression analysis showed that the independent factors affecting the efficacy of the regimen were clinical stage [P=0.003, hazard ratio (HR) =3.926, 95% CI: 1.578–9.766] and PAR (P=0.005, HR =0.656, 95% CI: 0.489–0.880). Details are shown in Table 2.
Figure 2.
Subgroup analysis of progression-free survival by baseline clinical characteristics and inflammatory biomarkers, including (A) biological Therapy, (B) AJCC stage, (C) melanoma subtype, (D) platelet count, (E) platelet to albumin ratio,(F) platelet to globulin ratio.
Table 2.
Univariate and multivariate analysis for factors influencing progression free survival (PFS) of patients
| Variables | Progression free survival (PFS) | ||
|---|---|---|---|
| Univariate | Multivariate | ||
| P-value | HR (95%CI) | p-value | |
| Biological Therapy (Yes vs No) | 0.0102 | ||
| AJCC stage (stage III vs stage IV) | 0.0102 | 3.926 (1.578–9.766) | 0.003 |
| Melanoma subtype (Mucosal vs Others) | 0.0368 | ||
| Platelet count (<168.5 vs ≥168.5, ×109/L) | 0.0147 | ||
| PAR (<4.251 vs ≥4.251) | 0.0018 | 0.656 (0.489–0.880) | 0.005 |
| PGR (<4.694 vs ≥4.694) | 0.0065 | ||
Abbreviations: CI, confidence interval; AJCC, American Joint Committee on Cancer; PAR, platelet to albumin ratio; PGR, platelet to globulin ratio.
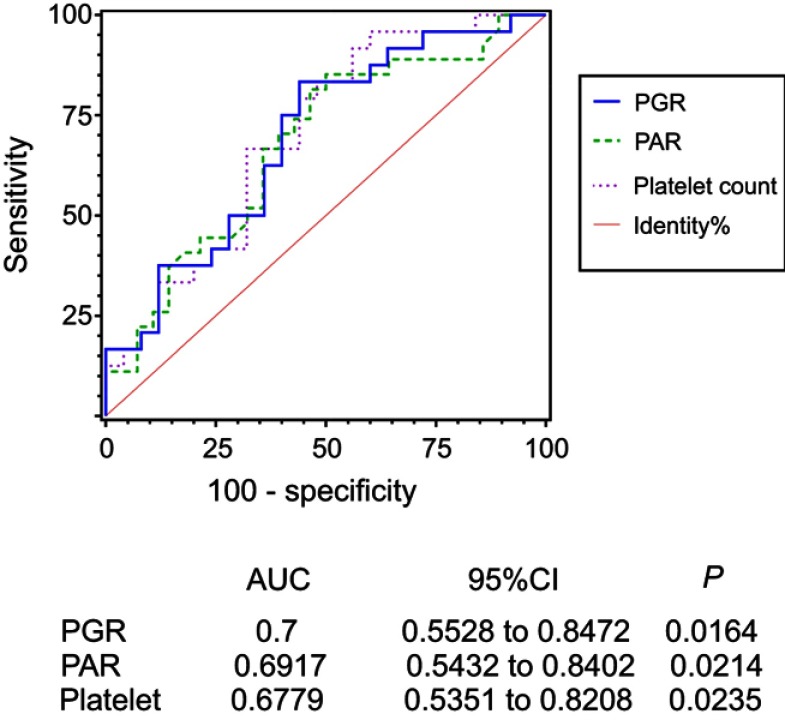
Based on the receiver operating characteristic (ROC) curves of platelet counts, PAR, and PGR, the area under curves (AUCs) were 0.6779 (95% CI =0.5351–0.8208, P=0.0235), 0.6917 (95% CI =0.5432–0.8402, P=0.0214), and 0.7 (95% CI =0.5528–0.8472, P=0.0164), respectively. Specifically, based on a PGR >4.694, the sensitivity for predicting the efficacy of Endostar combined with chemotherapy was 95.83% and the specificity was 40%; for PAR >4.251 as an independent risk factor, the sensitivity for predicting the efficacy of the regimen was 83.33% and the specificity was 56%. Details are shown in Figure 3.
Figure 3.
ROC curve analysis of the potential inflammatory biomarkers in patients with metastatic melanoma.
Abbreviations: AUC, area under curve; CI, confidence interval; PAR, platelet to albumin ratio; PGR, platelet to globulin ratio.
Adverse events
The combination of Endostar with DTIC and DDP was tolerated by all patients, and AEs ranged from Grade 1 to Grade 3, which are controllable. There were no Grade 4 or higher AEs. The most common AEs included myelosuppressive responses (leukocyte, platelet, or hemoglobin reduction), gastrointestinal reactions (nausea, vomiting, or loss of appetite) and impaired liver function (elevation of transaminase, bilirubin, or serum creatinine). Grade 3 AEs included decreased white blood cells or blood platelets and vomiting, which occurred in three patients, with an incidence of 5.5%. Details are provided in Table 3.
Table 3.
Adverse events experienced by the patients (N=55)
| Adverse events | Grade | Total | ||||
|---|---|---|---|---|---|---|
| 1 or 2 | 3 or 4 | |||||
| No. | % | No. | % | No. | % | |
| Gastrointestinal | ||||||
| Loss of appetite | 14 | 25.4 | 0 | 0 | 14 | 25.4 |
| Nausea | 18 | 32.7 | 0 | 0 | 18 | 32.7 |
| Vomiting | 14 | 25.4 | 1 | 1.8 | 15 | 27.2 |
| Hepatic | ||||||
| Increase of aminotransferase | 14 | 25.4 | 0 | 0 | 14 | 25.4 |
| Increase of bilirubin | 6 | 10.9 | 0 | 0 | 6 | 10.9 |
| Hematologic | ||||||
| Leucopenia | 13 | 23.6 | 1 | 1.8 | 14 | 25.4 |
| Thrombocytopenia | 14 | 25.4 | 1 | 1.8 | 15 | 27.2 |
| Decrease of hemoglobin | 12 | 21.8 | 0 | 0 | 12 | 21.8 |
| Others | ||||||
| Increase of LDH | 2 | 3.6 | 0 | 0 | 2 | 3.6 |
| Increase of creatinase | 1 | 1.8 | 0 | 0 | 1 | 1.8 |
| Fever | 20 | 36.4 | 0 | 0 | 20 | 36.4 |
| Fatigue | 5 | 9.1 | 0 | 0 | 5 | 9.1 |
| Hypertension | 2 | 3.6 | 0 | 0 | 2 | 3.6 |
| Trichomadesis | 4 | 7.3 | 0 | 0 | 4 | 7.3 |
Abbreviations: No, number; LDH, lactate dehydrogenase.
Discussion
Endostar is a novel antitumor drug that shows a significant synergistic effect with traditional chemotherapy (usually including DTIC and DDP). The efficacy of Endostar was verified in clinical trials of non-small cell lung cancer (NSCLC) at stage III, and Endostar was approved by the Chinese FDA for the clinical treatment of advanced NSCLC.7,12 A phase II Clinical trial of Endostar in patients with metastatic melanoma reported that Endostar combined with DTIC improves PFS (4.5 months vs 1.5 months, HR =0.578, P=0.013) and OS (12.0 months vs 8.0 months; HR =0.522, P=0.005) in patients with advanced MM compared with placebo combined with DTIC.10 DTIC and Melphalan are alkylating drugs. Melphalan loco-regional chemotherapy has been reported as a safe and effective therapy for stage III BRAF wild type melanoma patients who fail current systemic therapies, particularly in subjects with MGMT methylation.21 However, there was no evidence of MGMT methylation in our group, so the patients were not receiving Melphalan.
In our study, 35 patients (63.6%) who received combination treatment with biotherapy (ie, IFN-α-2b+IL-2) showed a longer PFS than patients who did not receive combination treatment (19.7 months vs 8.0 months, HR =0.3966, P=0.0102; Figure 2A). There were 16 patients in stage III and 19 patients in stage IV. There was no significant difference between the two cohorts (P=0.5076). A Phase III Trial showed that the relapse-free survival (RFS: 7.2 years vs 4.0 years, HR =0.75, P=0.015) of high-risk MM patients (stage IIIA-N2a–IIIC-N3) is prolonged if chemotherapy is combined with biotherapy compared with the group receiving only biotherapy (using only a high dose of IFN-α-2b).22 In our group, 27 patients (49.1%) were in unresectable stage III. Therefore, patients showed a longer mPFS, ie, 17.9 months. In terms of safety, many patients experienced multiple AEs. However, AEs were Grade 1–2, which can be controlled by dose interruption or supportive care. Grade 3 and higher AEs are rare, and no death related to treatment was recorded. This is similar to the results of clinical trials related to Endostar. Overall, the combination of Endostar with DTIC and DPP for the treatment of metastatic MM without genetic mutations is safe and well tolerated.
Tumor-specific factors such as clinical stage, tumor location, type, Clark classification, Breslow thickness, and ulceration are predictors of prognosis in addition to patient-specific factors such as age and gender.23–28 The present study analyzed the influence of the above factors on the PFS of patients and the efficacy of Endostar combined with chemotherapy. Univariate analysis showed that clinical stage (unresectable stage III vs stage IV, 23.5 months vs 11.3 months, HR =0.3713, P=0.0102; Figure 2B) and tumor type (mucosal type vs other types, 9.4 months vs 19.7 months, HR =2.289, P=0.0368; Figure 2C) can be used as a predictors of the efficacy of the regimen. The impact of age (P=0.3883), gender (P=0.1852), tumor location (P=0.7227), Clark grade (P=0.4079), Breslow thickness (P=0.3956), and ulcers (P=0.8873) on efficacy was not statistically significant. In particular, clinical stage can be used as an independent predictor of efficacy. In this group, 3 patients in stage III got PR, 15 patients got SD, while 4 patients in stage IV got PR and 5 patients got SD. ORR of the two cohorts were 11.1% in stage III and 14.3% in stage IV, respectively, with no statistical difference (chi-square test P=0.7204), while DCR was 66.7% in stage III and 32.1% in stage IV, respectively, with statistical difference (chi-square test P=0.0105). This indicates that patients with MM of non-mucosal type and unresectable stage III are more likely to benefit from Endostar combined with chemotherapy.
Under physiological conditions, the main functions of platelets include blood coagulation, hemostasis, and repair of damaged blood vessels. Platelets release platelet derived growth factor (PDGF), platelet factor, transforming growth factor-β, and VEGF, which can promote tumor neovascularization and tumor growth.29 High platelet counts are therefore associated with poor prognosis in patients with MM, as verified in many studies.30–32 However, the present study showed that the efficacy of Endostar combined with chemotherapy is greater in patients with high platelet counts. Based on a platelet count ≥168.5×109/L, the sensitivity for predicting a good efficacy of this regimen was 85.19% and the specificity was 50% (AUC =0.6779, P=0.0235; Figure 3). Endostar is an anti-angiogenic drug that can block the response to multiple growth factors,6 so it may inhabit the effect of platelets on promoting tumor angiogenesis and tumor cell proliferation, thereby improving the cell killing effect of chemotherapeutics. However, this does not explain the high efficacy of the regimen in patients with high platelet values. In addition, Yin et al.33 reported that PDGF receptor-β inhibitor slowed tumor growth but increased metastasis in combined radiotherapy and Endostar therapy. We hypothesized that the mechanism of action of Endostar included the reversal of the tumor promoting effect of platelets, or regulation by the level of PDGF released by platelets. However, no relevant studies have been identified, and this mechanism of action remains to be further studied.
Hanahan et al 34 proposed that one of the ten characteristics of tumors was the tumor-promoting inflammatory response, and the balance between inflammatory responses in tumors might play a role in predicting disease outcomes. Subsequent studies showed that inflammatory cell counts, albumin, globulin counts, and their ratios were predictors of prognosis in patients with MM, especially the neutrophil to lymphocyte ratio,14–17 platelet to lymphocyte ratio,17 and lymphocyte to monocyte ratio.20 We therefore included the inflammatory cell count from routine blood examination, the amount of albumin and immunoglobulins from the liver function examination, and the ratio of the above test resulted in the statistical analysis to identify factors predicting the efficacy of Endostar combined with chemotherapy. Preoperative PAR was identified as a predictor of prognosis in patients with cholangiocarcinoma and pancreatic ductal adenocarcinoma;18,19 however, there were no reports on the role of PGR in the prognosis of cancer patients. In the present study, PAR and PGR were used as predictors of efficacy. Specifically, PAR was an independent predictor of efficacy, and PGR had a high AUC (AUC =0.7, P=0.0164), indicating a certain predictive accuracy regarding efficacy. Since blood tests and liver function tests are routine examination items in patients undergoing chemotherapy, the results of these examinations should be used to recommend medications, and inflammatory biomarkers such as PAR and PGR should be determined as they had clinical application value.
The present study was a retrospective cohort study and it had several limitations. First, the use of single-center data and 55 cases as the subject of research was associated with bias. The study included patients that showed good compliance and who underwent more than four courses of treatment (4–15 courses of treatment, with a median of 8). Second, the OS of patients was not statistically analyzed and discussed. For those patients who failed Endostar combined with chemotherapy failed, they agreed to enter clinical trials or use other drugs such as apatinib or pembrolizumab. Apatinib is effective in the treatment of metastatic MM35,36 Pembrolizumab was approved by the US FDA on September 4, 2014 for the treatment of unresectable or metastatic MM.37,38 It has become a first-line drug for metastatic MM in Western countries and came into the market in China in September of this year. Thus, assessing the efficacy of this regimen based on the OS of patients was not objective. Finally, because metastatic MM patients treated with chemotherapy alone were not used as the control group, it was difficult to determine whether inflammatory biomarkers (platelet count, PAR, PGR) were predictors of chemotherapy efficacy or predictors of response to Endostar. In the other words, these biomarkers should be used in the treatment regimen of Endostar combined with chemotherapy. Prospective random experiments are necessary to analyze the significance of these indicators in the treatment of metastatic MM by Endostar and to determine precise cutoff values to guide clinical applications.
Conclusion
Endostar combined with DTIC and DDP showed efficacy in the treatment of metastatic MM without gene mutations, and the overall patient tolerance was good. Further clinical trials of this regimen are warranted. The development and clinical application of targeted drugs have diversified the therapy methods and choices for patients with metastatic MM. Patients who are more likely to benefit from a particular therapeutic regimen are commonly identified before the start of therapy to provide patients with the most appropriate therapy, protect them from unnecessary treatments or drug-related toxicities, improve the therapeutic response rate, and enhance the quality of life of patients. In patients treated with Endostar combined with chemotherapy, the baseline platelet counts and the platelet-derived PAR and PGR values were associated with the efficacy of this regimen based on this study and may have potential value for use in clinical practice and subsequent clinical trials. This remains to be further verified by prospective random experiments.
Acknowledgments
We thank the patients who shared their experiences with our oncologists and we thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. This article was supported by Simcere Pharmaceutical Group (Z-2014-06-18386).
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center and all aspects of the study comply with the Declaration of Helsinki. All patients and their guardians were informed and signed relevant documents.
Disclosure
All authors declare that they have no competing interests in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Tripp MK, Watson M, Balk SJ, Swetter SM, Gershenwald JE. State of the science on prevention and screening to reduce melanoma incidence and mortality: the time is now. CA Cancer J Clin. 2016;66(6):460–480. doi: 10.3322/caac.21352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Liu W, Gotlieb V. The rapidly evolving therapies for advanced melanoma–towards immunotherapy, molecular targeted therapy, and beyond. Crit Rev Oncol Hematol. 2016;99:91–99. doi: 10.1016/j.critrevonc.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 4.Lindsay CR, Spiliopoulou P, Waterston A. Blinded by the light: why the treatment of metastatic melanoma has created a new paradigm for the management of cancer. Ther Adv Med Oncol. 2015;7(2):107–121. doi: 10.1177/1758834014566619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekwueme DU, Guy GP, Li C, Rim SH, Parelkar P, Chen SC. The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity–United States, 2000 to 2006. J Am Acad Dermatol. 2011;65(5):S131–S133. doi: 10.1016/j.jaad.2011.04.036 [DOI] [PubMed] [Google Scholar]
- 6.Limaverde-Sousa G, Sternberg C, Ferreira CG. Antiangiogenesis beyond VEGF inhibition: a journey from antiangiogenic single-target to broad-spectrum agents. Cancer Treat Rev. 2014;40(4):548–557. doi: 10.1016/j.ctrv.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Yang X, Ding Y, et al. Recombinant human endostatin enhances the radioresponse in esophageal squamous cell carcinoma by normalizing tumor vasculature and reducing hypoxia. Sci Rep. 2015;5:14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Zheng D, Wei X, Jin Z, Zhang C, Li K. Effects of recombinant human endostatin and its synergy with cisplatin on circulating endothelial cells and tumor vascular normalization in A549 xenograft murine model. J Cancer Res Clin Oncol. 2012;138(7):1131–1144. doi: 10.1007/s00432-012-1189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li XQ, Shang BY, Wang DC, Zhang SH, Wu SY, Zhen YS. Endostar, a modified recombinant human endostatin, exhibits synergistic effects with dexamethasone on angiogenesis and hepatoma growth. Cancer Lett. 2011;301(2):212–220. doi: 10.1016/j.canlet.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 10.Cui C, Mao L, Chi Z, et al. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21(7):1456–1463. doi: 10.1038/mt.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Yao Q, Huang M, et al. A randomized Phase III trial of neoadjuvant recombinant human endostatin, docetaxel and epirubicin as first-line therapy for patients with breast cancer (CBCRT01). Int J Cancer. 2018;142(10):2130–2138. doi: 10.1002/ijc.31217 [DOI] [PubMed] [Google Scholar]
- 12.An J, Lv W. Endostar (rh-endostatin) versus placebo in combination with vinorelbine plus cisplatin chemotherapy regimen in treatment of advanced non-small cell lung cancer: a meta-analysis. Thorac Cancer. 2018;9(5):606–612. doi: 10.1111/1759-7714.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin T, Jiang F, Jin Q, Piao Y, Chen X. Endostar combined with gemcitabine and cisplatin chemotherapy for patients with metastatic nasopharyngeal carcinoma: an update. Transl Oncol. 2018;11(2):286–291. doi: 10.1016/j.tranon.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732–738. doi: 10.1093/annonc/mdw016 [DOI] [PubMed] [Google Scholar]
- 15.Teterycz P, Jagodzinska-Mucha P, Cybulska-Stopa B, et al. High baseline neutrophil-to-lymphocyte ratio predicts worse outcome in patients with metastatic BRAF-positive melanoma treated with BRAF and MEK inhibitors. Melanoma Res. 2018;28(5):435–441. doi: 10.1097/CMR.0000000000000461 [DOI] [PubMed] [Google Scholar]
- 16.Zhan H, Ma J, Jian Q. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in melanoma patients: a meta-analysis. Clin Chim Acta. 2018;484:136–140. doi: 10.1016/j.cca.2018.05.055 [DOI] [PubMed] [Google Scholar]
- 17.Wade RG, Robinson AV, Lo MCI, et al. Baseline neutrophil–lymphocyte and platelet–lymphocyte ratios as biomarkers of survival in cutaneous melanoma: a multicenter cohort study. Ann Surg Oncol. 2018;25(11):3341–3349. doi: 10.1245/s10434-018-6660-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirai Y, Shiba H, Haruki K, et al. Preoperative platelet–to–albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37(2):787–794. doi: 10.21873/anticanres.11378 [DOI] [PubMed] [Google Scholar]
- 19.Saito N, Shirai Y, Horiuchi T, et al. Preoperative platelet to albumin ratio predicts outcome of patients with cholangiocarcinoma. Anticancer Res. 2018;38(2):987–992. doi: 10.21873/anticanres.12313 [DOI] [PubMed] [Google Scholar]
- 20.Leontovich AA, Dronca RS, Nevala WK, et al. Effect of the lymphocyte-to-monocyte ratio on the clinical outcome of chemotherapy administration in advanced melanoma patients. Melanoma Res. 2017;27(1):32–42. doi: 10.1097/CMR.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 21.Guadagni S, Fiorentini G, Clementi M, et al. MGMT methylation correlates with melphalan pelvic perfusion survival in stage III melanoma patients: a pilot study. Melanoma Res. 2017;27(5):439–447. doi: 10.1097/CMR.0000000000000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flaherty LE, Othus M, Atkins MB, et al. Southwest oncology group S0008: a phase III trial of high-dose interferon alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma—an intergroup study of cancer and leukemia group B, children‘s oncology group, eastern cooperative oncology group, and southwest oncology group. J Clin Oncol. 2014;32(33):3771–3778. doi: 10.1200/JCO.2013.53.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksson H, Frohm-Nilsson M, Jaras J, et al. Prognostic factors in localized invasive primary cutaneous malignant melanoma: results of a large population-based study. Br J Dermatol. 2015;172(1):175–186. doi: 10.1111/bjd.13171 [DOI] [PubMed] [Google Scholar]
- 24.Maurichi A, Miceli R, Camerini T, et al. Prediction of survival in patients with thin melanoma: results from a multi-institution study. J Clin Oncol. 2014;32(23):2479–2485. doi: 10.1200/JCO.2013.54.2340 [DOI] [PubMed] [Google Scholar]
- 25.Lyth J, Hansson J, Ingvar C, et al. Prognostic subclassifications of T1 cutaneous melanomas based on ulceration, tumour thickness and Clark‘s level of invasion: results of a population-based study from the Swedish melanoma register. Br J Dermatol. 2013;168(4):779–786. doi: 10.1111/bjd.12095 [DOI] [PubMed] [Google Scholar]
- 26.Balch CM, Soong S, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013;20(12):3961–3968. doi: 10.1245/s10434-013-3100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.t Hout FEM, Haydu LE, Murali R, Bonenkamp JJ, Thompson JF, Scolyer RA. Prognostic importance of the extent of ulceration in patients with clinically localized cutaneous melanoma. Ann Surg. 2012;255(6):1165–1170. doi: 10.1097/SLA.0b013e31824c4b0b [DOI] [PubMed] [Google Scholar]
- 28.Balch CM, Gershenwald JE, Soong S, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–2459. doi: 10.1200/JCO.2009.27.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tesfamariam B. Involvement of platelets in tumor cell metastasis. Pharmacol Therapeut. 2016;157:112–119. [DOI] [PubMed] [Google Scholar]
- 30.Moreau J, Pelletier F, Biichle S, et al. Increased levels of circulating platelet-derived microparticles are associated with metastatic cutaneous melanoma. Exp Dermatol. 2017;26(10):961–963. doi: 10.1111/exd.13339 [DOI] [PubMed] [Google Scholar]
- 31.Bauer AT, Suckau J, Frank K, et al. von Willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood. 2015;125(20):3153–3163. doi: 10.1182/blood-2014-08-595686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker KA, Beckmann N, Adams C, et al. Melanoma cell metastasis via P-selectin-mediated activation of acid sphingomyelinase in platelets. Clin Exp Metastasis. 2017;34(1):25–35. doi: 10.1007/s10585-016-9826-6 [DOI] [PubMed] [Google Scholar]
- 33.Yin L, He J, Xue J, et al. PDGFR-β inhibitor slows tumor growth but increases metastasis in combined radiotherapy and Endostar therapy. Biomed Pharmacother. 2018;99:615–621. doi: 10.1016/j.biopha.2018.01.095 [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 35.Cui C, Zhou L, Lian B, et al. Safety and efficacy of apatinib combined with temozolomide in advanced melanoma patients after conventional treatment failure. Transl Oncol. 2018;11(5):1155–1159. doi: 10.1016/j.tranon.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Zhu H, Luo P, Chen S, Xu Y, Wang C. Apatinib mesylate tablet in the treatment of advanced malignant melanoma. Onco Targets Ther. 2018;11:5333–5338. doi: 10.2147/OTT.S175507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–1609. doi: 10.1001/jama.2016.4059 [DOI] [PubMed] [Google Scholar]
- 38.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.