Abstract
Purpose: The study aimed to evaluate the analgesic efficiency of dexmedetomidine (DEX) when added to levobupivacaine in continuous ultrasound-guided serratus anterior plane block (SAPB) performed at the end of major thoracic surgery.
Methods: This randomized, double-blind trial included 50 adults undergoing thoracic surgery. Continuous SAPB was performed at the end of surgery. Patients were randomized into two groups. Group L (n=25) received levobupivacaine only while Group DL (n=25) received a mixture of levobupivacaine and DEX. All patients received intravenous (IV) paracetamol every 8 hrs. Morphine IV was given according to VAS score of pain as a 5 mg loading dose. The primary outcome measure was postoperative pain intensity. Secondary outcome measures were postoperative morphine consumption and adverse effects.
Results: Analgesia was satisfactory in the two groups up to 24 hrs. VAS score was significantly lower in group DL compared to group L between 6 and 24 hrs postoperatively. Total morphine consumption was significantly lower in group DL compared to group L (p<0.001). Up to 12 hrs postoperatively, sedation score was significantly lower in group DL compared to group L. Afterwards, all patients were fully alert. All values of mean arterial pressure and heart rate were within the clinically accepted ranges. There were no recorded cases of hypotension or bradycardia in the whole studied group.
Conclusions: Continuous SAPB with levobupivacaine plus DEX seems to be a promising analgesic alternative following thoracotomy. Combined with IV paracetamol, this approach provided adequate analgesia and proper sedation.
Trial registration: ISRCTN registry; study ID: ISRCTN35517318
Keywords: serratus anterior plane block, dexmedetomidine, thoracic surgery, post-thoracotomy
Introduction
Thoracotomy is usually followed by severe pain which may result in hypoxemia, and ventilation–perfusion imbalance.1 Proper management of acute post-thoracotomy pain is crucial not only to avoid early morbidity complications but also to prevent chronic post-thoracotomy pain syndrome.2 Regional anesthetic techniques have been effectively used for pain relief following thoracic surgical procedures. These include intercostal nerve block, thoracic epidural anesthesia (TEA), and thoracic paravertebral blocks.3
TEA may fail in near 30% of the cases owing to technical difficulties of catheter placement or due to catheter dislodgement.4 Also, high epidural catheterization is associated with a risk of spinal hematoma and epidural abscess.5 Paravertebral block can control acute pain and reduce the development of chronic pain, but it carries the risk of accidental dural puncture, hematoma, and pneumothorax.6
Serratus anterior plane block (SAPB) is another regional technique that can induce analgesia between T2 and T9 levels. This way, it can be appropriate for analgesia in thoracic surgery. Under ultrasonographic guidance, SAPB can be under or above the serratus muscle.7 A recent cadaveric study concluded that SAPB seems to provide its effect through blockade of the lateral cutaneous branches of the intercostal nerves.8 Previous reports showed that after superficial SAPB, the duration of sensory block was between 7 and 12 hrs.7,8
Dexmedetomidine, a selective α2-adrenergic agonist, possesses analgesic and sedative properties.9 It was successfully used to prolong the local anesthetic effect in epidural,10 intrathecal,11 and paravertebral block.12 Dexmedetomidine intravenous infusion was effectively used as adjuvant to general anesthesia in cases of laparoscopic surgery13 and craniotomy.14 In healthy volunteers, it demonstrated similarity with natural sleep.15
The aim of this study is to evaluate the analgesic efficiency of dexmedetomidine when added to levobupivacaine in continuous ultrasound-guided serratus anterior plane block performed at the end of major thoracic surgery.
Patients and methods
This randomized, double-blind trial included 50 adult patients undergoing thoracic surgery in the period from June 2017 to January 2018. The study was carried out at National Cancer Institute, Cairo University after approval of the local ethical committee, and all patients have provided written informed consents to participate. The study was approved by the Institutional Review Board of the National Cancer Institute (Approval no.: 201617011.2P). Patients were given a full and detailed explanation of the study protocol and were informed about the potential benefits of the development of a successful technique as well as the potential side-effects. The study implemented the principles of the Declaration of Helsinki (1964) and its following revisions. The study was registered on the ISRCTN registry with study ID ISRCTN35517318.
Inclusion criteria were age from 20 to 60 years, ASA physical status I-II class, and body mass index (BMI) <40 kg/m2. Exclusion criteria were refusal for SAPB, defective coagulation, neuropathy, pregnancy, clinically significant cardiovascular, pulmonary, renal or hepatic diseases, infection at the site of injection, history of opioid dependence and allergy to the study drugs.
General anesthesia
A uniform anesthetic technique was used in two groups, namely, after preoxygenation with 100% oxygen for 3 mins, induction of anesthesia was by IV propofol 2 mg/kg, fentanyl 200 μg and atracurium 0.5 mg/kg to facilitate endotracheal intubation. Anesthesia was been maintained with isoflurane 1–2% in 50% air in oxygen mixtures. Intermittent dose of atracurium and fentanyl was used for muscle relaxation and analgesia, respectively. All patients were mechanically ventilated to maintain end-tidal carbon dioxide tension around 35 mmHg.
At the end of surgery, ultrasound-guided SAPB was done using a serratus anterior catheter. Patients were randomized by simple randomization using computer-generated random numbers into two groups. Group L (n=25) received a bolus of 30 mL of 0.25% levobupivacaine followed by an infusion of 5 mL/hr of 0.125% levobupivacaine. Group DL (n=25) received a bolus of 30 mL of 0.25% levobupivacaine plus 1 μg/kg dexmedetomidine followed by an infusion of 0.125% levobupivacaine plus 0.2 μg/kg/hour dexmedetomidine (Precedex 200 µg vials, Pfizer, Egypt) at a rate of 5 mL/hr.
Technique of SAPB
Patients are placed in the lateral position with the diseased side up. A linear ultrasound transducer (10–12 MHz, M-Turbo Ultrasound, USA) is placed over the mid-clavicular region of the thoracic cage in a sagittal plane (Figure 1). The fifth rib is identified in the mid-axillary line. The following muscles are identified easily overlying the fifth rib: the latissimus dorsi (superficial and posterior), teres major (superior) and serratus muscle (deep and inferior). As an extra-reference point, the thoracodorsal artery is used to aid the identification of the plane superficial to the serratus muscle. The needle (22G, 50-mm Tuohy needle) is introduced in-plane with respect to the ultrasound probe targeting the plane superficial to the serratus muscle. Under continuous ultrasound guidance, local anesthetic solution is injected then a catheter is threaded.
Figure 1.
An ultrasound image of serratus anterior plane block.
Inhalation anesthetic was discontinued and neuromuscular blockade was reversed by IV injection of neostigmine 0.05 mg/kg with atropine 0.01 mg/kg after fulfilling the criteria for extubation. Patients were extubated and transferred to surgical ICU. All patients were monitored with 5 leads electrocardiogram (ECG), pulse oximetry, non-invasive blood pressure monitoring (NIBP), end-tidal CO2, and TOF monitoring.
For postoperative pain management, all patients received intravenous (IV) paracetamol 1 gm every 8 hrs (Injectemol, Pharco B International, Pharma-tech) then morphine IV (0.1 mg/kg) to keep VAS score less than 3. Morphine consumption during the first 24 postoperative hours as a rescue drug was calculated.
Patients were monitored for systemic blood pressure, heart rate, and oxygen saturation every 10 min for the initial 1 hr of the blockade; every 30 mins for the next 2 hrs and then 2 hourly for the next 12 hrs. As soon as the patient is alert enough, VAS score of pain (10 mm vertical scale from 0 to 10 where zero means no pain and 10 is the worst pain) was recorded every 2 hrs. Sedation was assessed using the observer’s assessment of alertness/sedation (OAA/S) scale.16 The scale was scored as a composite score ranging from 1 (deep sleep) to 5 (alert). Signs of morphine side effects (nausea, vomiting, dizziness, an unusual pleasant feeling, sweating, headache, anxiety, and constipation) were monitored. In case of nausea and/or vomiting, 10 mg metoclopramide was given intravenously.
The primary outcome measure was postoperative pain intensity while patients were breathing quietly. Secondary outcome measures were postoperative morphine consumption and adverse effects including nausea, vomiting, hypotension bradycardia, and cardiac arrhythmia.
Sample size
No previous studies tested the effect of adding dexmedetomidine to local anesthetics in serratus anterior plane block. Thus, we calculated the required sample based on a study using the same combination for paravertebral block.17 According to this study, based on our primary outcome measure (VAS score), 6 patients are required to have a 95% chance of detecting, as significant at the 5% level, a lower VAS score after 16 hrs in the experimental group (3.0) compared to 4.79 in the control group. In view of the difference in technique, disease type, and surgical procedure, we will include 25 patients in each group to test the effect of adding dexmedetomidine on pain control, opioid consumption, and hemodynamic stability. The sample size was estimated using the online power calculator for continuous outcome superiority trial under Sealed Envelope Ltd. 2012. Available from: https://www.sealedenvelope.com/power/continuoussuperiority/
Statistical analysis
Statistical analysis was done using IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY, USA). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. For quantitative data, comparison between the two groups was done using independent sample t-test or Mann–Whitney test. Comparison of repeated measures was done using ANOVA for repeated measures or Friedman test followed by a pairwise comparison. All tests were two-tailed. A p-value<0.05 was considered significant.
Results
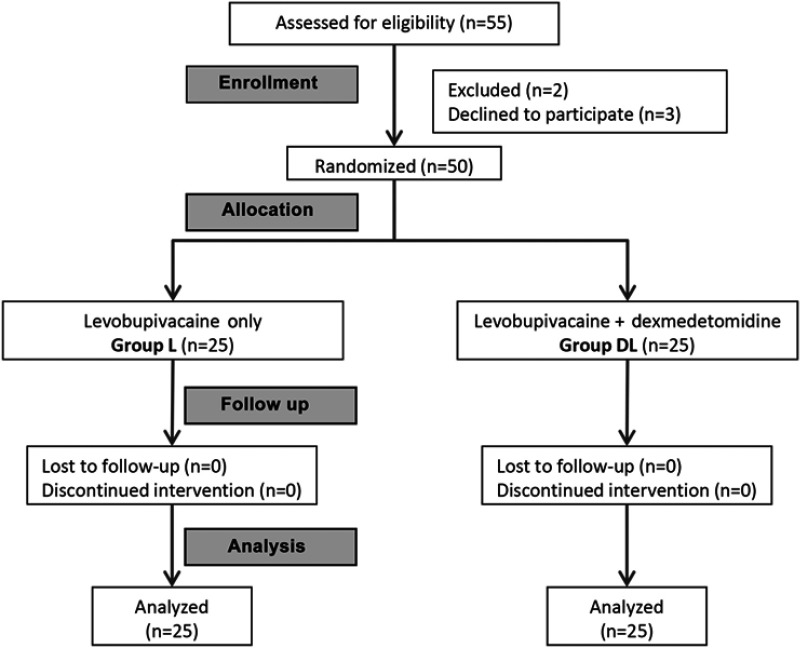
The flow of the patients is illustrated in CONSORT flow chart (Figure 2). The baseline characteristics of the two studied groups are shown in Table 1. The two groups were comparable in demographic and clinical characteristics. There was no significant difference between the two groups in the total dose of intraoperative fentanyl and time of last fentanyl dose (Table 1). Analgesia was satisfactory in the 2 groups up to 24 hrs after surgery. Pain intensity in terms of VAS score was significantly lower in group DL compared to group L between 6 and 24 hrs postoperatively (Table 2). Total Morphine consumption was significantly lower in group DL compared to group L (p<0.001). Up to 12 hrs postoperatively, OAA/S sedation score was significantly lower in group DL compared to group L (Table 2), ie, patients in the DL group were more sedated. Afterwards, all patients were fully alert with a score 5.
Figure 2.
CONSORT flow diagram.
Table 1.
Baseline characteristic of the two studied groups
| Group L (n=25) |
Group DL (n=25) |
p-value | |
|---|---|---|---|
| Age (years) | 37.0±5.2 | 38.0±4.0 | 0.575 |
| Sex (male/female) | 13/12 | 14/11 | 0.777 |
| Body mass index (kg/m2) | 27.4±1.5 | 28.3±1.6 | 0.061 |
| Baseline hemodynamics | |||
| Mean arterial pressure (mmHg) | 100±3 | 90±2 | <0.001 |
| Heart rate | 90±1 | 85±2 | <0.001 |
| SPO2 (%) | 95±1 | 94±1 | 0.012 |
| American Society of Anesthesiologists (ASA) class (I/II) | 14/11 | 15/10 | 0.774 |
| Duration of surgery (min) | 151.4±8.7 | 155.0±8.5 | 0.147 |
| Total dose of fentanyl (µg) | 280±16 | 279±20 | 0.758 |
| Time of last fentanyl dose (min) | 108±6 | 105±6 | 0.084 |
| Type of surgery | |||
| Lobectomy | 8 (32%) | 7 (28%) | |
| Pneumonectomy | 5 (20%) | 6 (24%) | 1.000 |
| Cyst excision | 7 (28%) | 8 (32%) | |
| Decortication | 5 (20%) | 4 (16%) |
Notes: Data are presented as mean±SD or number (%). Group L received levobupivacaine only. Group DL received a mixture of levobupivacaine and dexmedetomidine.
Table 2.
Clinical data in the postoperative period in the two studied groups
| Group L n=25 |
Group DL n=25 |
p-value | |
|---|---|---|---|
| Visual analog scale score | |||
| Baseline postoperative | 2 (1–3) | 1 (1–1) | <0.001 |
| After 2 hrs | 2 (1–3) | 2 (1–3) | 1.000 |
| After 6 hrs | 3 (2–4) | 2 (1–3) | <0.001 |
| After 12 hrs | 4 (3–5) | 3 (2–4) | <0.001 |
| After 18 hrs | 4 (3–5) | 3 (2–4) | <0.001 |
| After 24 hrs | 2 (1–3) | 1 (1–2) | <0.001 |
| Sedation score | |||
| Baseline postoperative | 4 (3–4) | 3 (2–4) | <0.001 |
| After 2 hrs | 4 (3–4) | 3 (3–4) | <0.001 |
| After 6 hrs | 5 (4–5) | 4 (4–5) | <0.001 |
| After 12 hrs | 5 (5–5) | 5 (4–5) | 0.020 |
| Total morphine consumption (mg) | 6.7±2.0 | 3.8±1.3 | <0.001 |
Notes: Data are presented as median (range) or mean±SD. Group L received levobupivacaine only. Group DL received a mixture of levobupivacaine and dexmedetomidine.
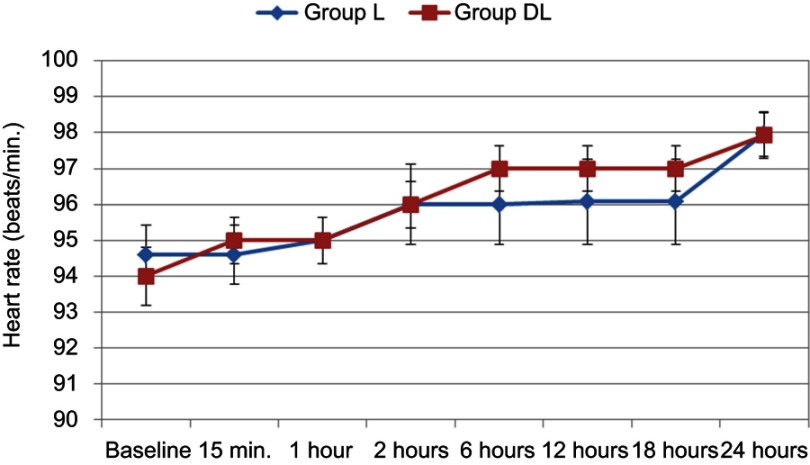
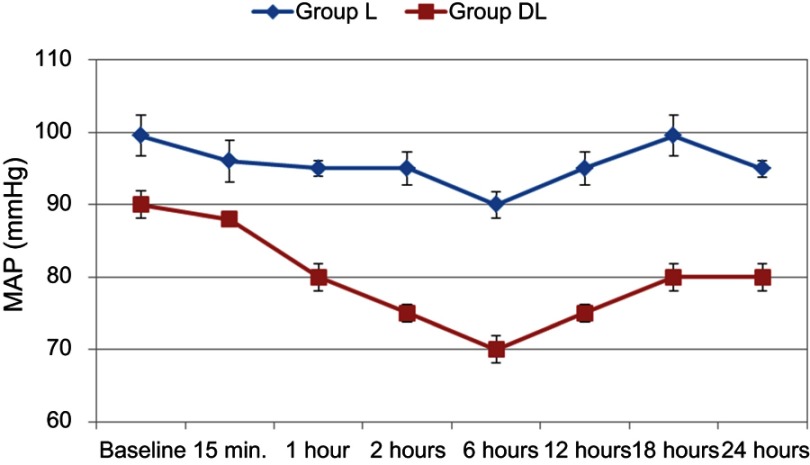
There are statistically significant differences between the two groups in hemodynamic parameters (Figures 3 and 4). Also, the heart rate and mean arterial pressure showed statistically significant change throughout the 24 postoperative hours in the 2 groups. Nevertheless, all values were within the clinically accepted ranges. There were no recorded cases of hypotension or bradycardia in the whole studied group.
Figure 3.
Changes of the heart rate during the first 48 postoperative hours in the 2 studied groups.
Notes: Group L received levobupivacaine only. Group DL received a mixture of levobupivacaine and dexmedetomidine.
Figure 4.
Changes of the mean arterial pressure (MAP) during the first 48 postoperative hours in the 2 studied groups.
Notes: Group L received levobupivacaine only. Group DL received a mixture of levobupivacaine and dexmedetomidine.
Discussion
The results of this study demonstrated that dexmedetomidine is a beneficial adjuvant to levobupivacaine for continuous serratus anterior plane block after thoracic oncologic surgeries. SAPB provided a good analgesic effect up to 12 hrs postoperatively. The addition of dexmedetomidine potentiated analgesia to cover the first 24 hrs of the postoperative period with significant reduction of morphine consumption and preservation of stable hemodynamics. Dexmedetomidine provided superior sedative effect throughout the 24 postoperative hours without causing respiratory depression.
In thoracic surgery, sources of pain involve nociceptive and neuropathic mechanisms originating from somatic and visceral afferents. These involve intercostal nerves at the sites of incision and thoracic drains, the vagus nerve and phrenic nerve in the pleura, and the brachial plexus in the ipsilateral shoulder.8 Owing to the multifactorial nature of acute thoracotomy pain a multimodal analgesic approach that targets multiple sites along the pain pathway, and combines intravenous opioids or non-steroidal anti-inflammatory drugs (NSAIDs) with regional anesthesia.10
In the current study, pain was managed by IV paracetamol injection every 8 hrs in addition to continuous SAPB. IV morphine was given as a rescue analgesic drug. Continuous dexmedetomidine local infusion combined with the local anesthetic was intended to add a third dimension of pain management to gain the benefit of its antinociceptive effect against both cutaneous and visceral pain.18,19
The current study demonstrated analgesic effectiveness of dexmedetomidine when administered locally in SAPB. Previous studies showed moderate analgesic benefit of systemic dexmedetomidine which was superior to paracetamol.20 However, this analgesic advantage is commonly associated with significant hypotension and bradycardia.21
In addition to pain relief, dexmedetomidine continuous infusion in SAPB was associated with better sedation during the early postoperative period (12 hrs). Intravenous continuous infusion injection of dexmedetomidine is used effectively to produce adequate levels of sedation during different types of surgical procedures.22–24 Dexmedetomidine has also extra benefits in perioperative care such as anxiolysis, control of postoperative shivering, and PONV.20
Despite the lack of α2-adrenoceptors on the axon of the normal peripheral nerve.25 Four mechanisms have been proposed for the action of α2-adrenoceptor agonist in peripheral nerve block. These include centrally mediated analgesia, α2B-adrenoceptor mediated vasoconstrictive effects, attenuation of the inflammatory response, and direct action on a peripheral nerve. However, the former three mechanisms cannot fully explain the efficacy of dexmedetomidine in peripheral nerve block.26
It was shown that α2-adrenoceptor agonist enhances activity-dependent hyperpolarization by inhibiting the hyperpolarization-activated cation (Ih) current which plays a key role in cell excitability, especially the firing frequency.27 The Ih current acts to reset a nerve for subsequent action potentials. When blocked, the Ih current enhances hyperpolarization and inhibits subsequent action potentials.28
In light of the results of the current study, levobupivacaine combined with dexmedetomidine through continuous SAPB seems to be a promising alternative for pain relief following major surgeries involving thoracotomy. We combined by IV paracetamol, this approach provided satisfactory pain amelioration and good sedation during the early postoperative period.
This approach seems to satisfy the requirement of a multimodal method as it considers the multiple nociceptive pathways, the pharmacologically distinct mechanisms, analgesic time with minimal side effects, especially respiratory depression.29 If we add the easy application compared to other methods in the management of thoracotomy pain, SAPB seems to be favorable. We suggest carrying out controlled studies to compare the effectiveness of the current approach versus other regional analgesic methods of thoracotomy pain relief.
Data availability
The authors declare that de-identified participants’ data will be available whenever requested. The corresponding author will be ready to send the data for any authority on request by an email message. The data will be available this way for up to 3 months after the paper is published.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Doan LV, Augustus J, Androphy R, Schechter D, Gharibo C. Mitigating the impact of acute and chronic post-thoracotomy pain. J Cardiothorac Vasc Anesth. 2014;28(4):1048–1056. doi: 10.1053/j.jvca.2014.02.021 [DOI] [PubMed] [Google Scholar]
- 2.Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2009;36(1):170–180. doi: 10.1016/j.ejcts.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 3.Kiss G, Castillo M. Non-intubated anesthesia in thoracic surgery-technical issues. Ann Transl Med. 2015;3(8):109. doi: 10.3978/j.issn.2305-5839.2015.05.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Br J Anaesth. 2012;109(2):144–154. doi: 10.1093/bja/aes214 [DOI] [PubMed] [Google Scholar]
- 5.Rosero EB, Joshi GP. Nationwide incidence of serious complications of epidural analgesia in the United States. Acta Anaesthesiol Scand. 2016;60(6):810–820. doi: 10.1111/aas.12702 [DOI] [PubMed] [Google Scholar]
- 6.Gärtner R, Jensen M-B, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985–1992. doi: 10.1001/jama.2009.1568 [DOI] [PubMed] [Google Scholar]
- 7.Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68(11):1107–1113. doi: 10.1111/anae.12344 [DOI] [PubMed] [Google Scholar]
- 8.Okmen K, Bm O, Uysal S. Serratus Anterior Plane (SAP) block used for thoracotomy analgesia: a case report. Korean J Pain. 2016;29(3):189–192. doi: 10.3344/kjp.2016.29.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7(4):221–226. [DOI] [PubMed] [Google Scholar]
- 10.Shaikh SI, Mahesh SB. The efficacy and safety of epidural dexmedetomidine and clonidine with bupivacaine in patients undergoing lower limb orthopedic surgeries. J Anaesthesiol Clin Pharmacol. 2016;32(2):203–209. doi: 10.4103/0970-9185.182104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27(3):339–343. doi: 10.4103/0970-9185.83678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohta M, Kalra B, Sethi AK, Kaur N. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. J Anesth. 2016;30(2):252–260. doi: 10.1007/s00540-015-2123-8 [DOI] [PubMed] [Google Scholar]
- 13.Salman N, Uzun S, Coskun F, Salman MA, Salman AE, Aypar U. Dexmedetomidine as a substitute for remifentanil in ambulatory gynecologic laparoscopic surgery. Saudi Med J. 2009;30(1):77–81. [PubMed] [Google Scholar]
- 14.Turgut N, Turkmen A, Ali A, Altan A. Remifentanil-propofol vs dexmedetomidine-propofol–anesthesia for supratentorial craniotomy. Middle East J Anaesthesiol. 2009;20(1):63–70. [PubMed] [Google Scholar]
- 15.Hsu Y-W, Cortinez LI, Robertson KM, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101(5):1066–1076. [DOI] [PubMed] [Google Scholar]
- 16.Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–251. [PubMed] [Google Scholar]
- 17.Sinha S, Mukherjee M, Chatterjee S, et al. Comparative study of analgesic efficacy of ropivacaine with ropivacaine plus dexmedetomidine for paravertebral block in unilateral renal surgery – anaesthesia, pain & intensive care. Anaesth Pain Intensive Care. 2012;16(1):38–42. [Google Scholar]
- 18.Sullivan AF, Kalso EA, McQuay HJ, Dickenson AH. The antinociceptive actions of dexmedetomidine on dorsal horn neuronal responses in the anaesthetized rat. Eur J Pharmacol. 1992;215(1):127–133. [DOI] [PubMed] [Google Scholar]
- 19.Ulger F, Bozkurt A, Bilge SS, et al. The antinociceptive effects of intravenous dexmedetomidine in colorectal distension-induced visceral pain in rats: the role of opioid receptors. Anesth Analg. 2009;109(2):616–622. doi: 10.1213/ane.0b013e3181a9fae2 [DOI] [PubMed] [Google Scholar]
- 20.Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116(6):1312–1322. doi: 10.1097/ALN.0b013e31825681cb [DOI] [PubMed] [Google Scholar]
- 21.Wijeysundera DN, Bender JS, Beattie WS. Alpha-2 adrenergic agonists for the prevention of cardiac complications among patients undergoing surgery. Cochrane Database Syst Rev. 2009;4:CD004126. doi: 10.1002/14651858.CD004126.pub2 [DOI] [PubMed] [Google Scholar]
- 22.Ko K-H, Jun I-J, Lee S, Lim Y, Yoo B, Kim K-M. Effective dose of dexmedetomidine to induce adequate sedation in elderly patients under spinal anesthesia. Korean J Anesthesiol. 2015;68(6):575–580. doi: 10.4097/kjae.2015.68.6.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Han Z, Zhou H, Wang N, Ma H. Effective loading dose of dexmedetomidine to induce adequate sedation in parturients undergoing caesarean section under spinal anaesthesia. Turk J Anaesthesiol Reanim. 2017;45(5):260–263. doi: 10.5152/TJAR.2017.04578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song J, Kim W-M, Lee S-H, Yoon MH. Dexmedetomidine for sedation of patients undergoing elective surgery under regional anesthesia. Korean J Anesthesiol. 2013;65(3):203–208. doi: 10.4097/kjae.2013.65.3.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pöpping DM, Elia N, Marret E, Wenk M, Tramèr MR. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: a meta-analysis of randomized trials. Anesthesiology. 2009;111(2):406–415. doi: 10.1097/ALN.0b013e3181aae897 [DOI] [PubMed] [Google Scholar]
- 26.Leem JW, Choi Y, Han SM, Yoon MJ, Sim JY, Leem SW. Conduction block by clonidine is not mediated by alpha2-adrenergic receptors in rat sciatic nerve fibers. Reg Anesth Pain Med. 2000;25(6):620–625. doi: 10.1053/rapm.2000.16160 [DOI] [PubMed] [Google Scholar]
- 27.Dalle C, Schneider M, Clergue F, Bretton C, Jirounek P. Inhibition of the I(h) current in isolated peripheral nerve: a novel mode of peripheral antinociception?. Muscle Nerve. 2001;24(2):254–261. [DOI] [PubMed] [Google Scholar]
- 28.Helal SM, Eskandr AM, Gaballah KM, Gaarour IS. Effects of perineural administration of dexmedetomidine in combination with bupivacaine in a femoral-sciatic nerve block. Saudi J Anaesth. 2016;10(1):18–24. doi: 10.4103/1658-354X.169469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and Treating Pain after Thoracic Surgery. Anesthesiol J Am Soc Anesthesiol. 2006;104(3):594–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that de-identified participants’ data will be available whenever requested. The corresponding author will be ready to send the data for any authority on request by an email message. The data will be available this way for up to 3 months after the paper is published.