Abstract
Introduction:
Annual influenza vaccine safety monitoring is an important component of the influenza vaccination program in the United States to ensure that vaccines are safe, which is important for maintaining public trust in the national vaccination program. This is specially the case for influenza vaccines since the antigen composition of the viruses of which the vaccine is made often change from one season to the next, based on the circulating strain of influenza virus.
Areas covered:
This review describes the two surveillance systems used by the Centers for Disease Control and Prevention (CDC) to monitor the safety of influenza vaccines: 1) the Vaccine Adverse Event Reporting System (VAERS); and 2) the Vaccine Safety datalink (VSD).
Expert opinion:
VAERS and VSD are used routinely to monitor the safety of influenza vaccines in the United States, and over the years they have demonstrated their value in monitoring vaccine safety since their implementation in 1990. Both systems, although different, complemented each other well to study febrile seizures in young children following influenza vaccination during the 2010-2011 influenza season. Other examples of potential safety concerns after influenza vaccines are also presented and discussed.
Keywords: epidemiology, influenza vaccine, surveillance, vaccine safety, VAERS, VSD
Background
Influenza accounts for significant morbidity and mortality in the United States [1, 2]. Annual influenza vaccination is the primary way of preventing influenza and its complications. Annual influenza vaccine safety monitoring is an important component of the influenza vaccination program in the United States to ensure that vaccines are safe, which is crucial to maintain public trust in a national vaccination program. This is specially the case for influenza vaccines since the antigen composition of the viruses of which the vaccine is made often change from one season to the next, based on the circulating strain of influenza virus. The Centers for Disease Control and Prevention (CDC) uses two systems to monitor the safety of influenza vaccines: 1) the Vaccine Adverse Event Reporting System (VAERS) which is a front-line, national, spontaneous surveillance system that receives reports of adverse events (AEs) following vaccination in the United States; and 2) the Vaccine Safety datalink (VSD) which is a large linked database system used for active surveillance and research. In this review, we describe how these systems are used routinely to monitor the safety of influenza vaccines in the United States, including the types of analysis used to detect potential safety signals and their verification; and provide recent examples of safety concerns detected in both system and how VAERS and VSD complement each other.
Vaccine Adverse Events Reporting System (VAERS)
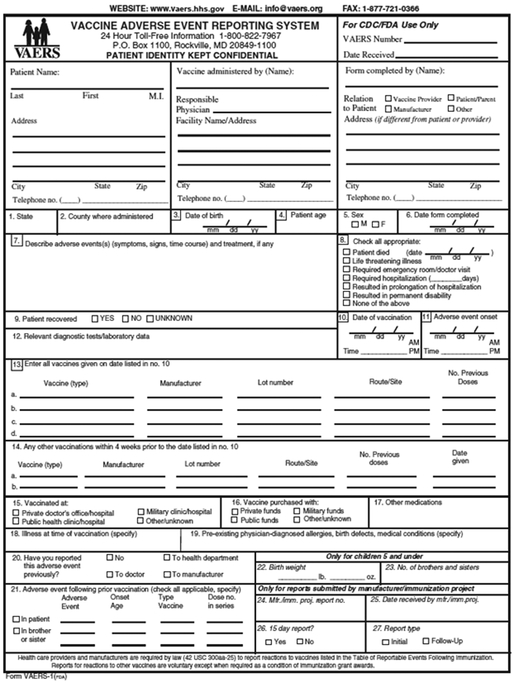
VAERS is a national vaccine safety surveillance system, co-administered by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) that receives spontaneous reports of AEs following vaccination [3]. VAERS, which was authorized by the National Childhood Vaccine Injury Act of 1986 and implemented in 1990, serves as the US early warning system to detect potential problems that may be related to vaccines. VAERS data are monitored to detect new, unusual, or rare vaccine AEs, increases in known AEs, and possible safety signals which may be evaluated in other studies. Monitoring of AEs is especially important whenever a new vaccine is licensed and recommended for use in the US population and this is often the case for the annual variation in antigenic composition of influenza vaccines. VAERS accepts reports from healthcare providers, vaccine recipients, manufacturers, and other reporters. The VAERS report form collects information on age, sex, vaccines administered, the AE experienced, medical conditions at the time of vaccination and medical history (Figure 1). Strengths of VAERS include its broad national scope and its ability to detect rare AEs, which makes it a good system for hypothesis generation. VAERS is also available to the public as a searchable database [4]. Limitations include over- or under-reporting, biased reporting, and inconsistency in quality and completeness of reports [3]. Publicity around adverse events may lead to over-reporting. For example, during the 2009 H1N1 pandemic a notable increase in pregnancy reports was observed [5] which was related to efforts to promote vaccination in pregnant women. In subsequent years, the number of pregnancy reports after influenza vaccines has decreased [6]. VAERS generally cannot assess causality between an AE and receipt of a vaccine and does not collect data on the number of vaccines administered. Therefore, it is neither possible to calculate the incidence or prevalence of an AE nor estimate an increase in risk of the AE. The VAERS form does not have a field for pregnancy reports; therefore, a special strategy is needed to search for these reports [5].
Figure 1.
Hard copy version of the VAERS form. An online version can also be accessed at: https://vaers.hhs.gov/esub/step1
Signs and symptoms of AEs are coded by trained personnel and entered into a database using the Medical Dictionary for Regulatory Activities (MedDRA), a clinically validated, internationally standardized medical terminology [7]. A VAERS report may be assigned one or more MedDRA preferred terms (PT). A PT is a distinct descriptor for a symptom, sign, disease, diagnosis, therapeutic indication, investigation, surgical, or medical procedure, and medical, social, or family history characteristic [8]. Reports are classified as serious based on the Code of Federal Regulations if one of the following is reported: death, life-threatening illness, hospitalization or prolongation of hospitalization, permanent disability, or a congenital anomaly [9]. For non-manufacturer serious reports, trained nurses will contact the person who filed VAERS reports to request additional information, including hospital records and autopsy reports when appropriate. Medical records are also routinely requested for certain pre-specified conditions such as Guillain-Barré Syndrome (GBS), and anaphylaxis. Medical records may also be requested for certain conditions (e.g., spontaneous abortion, stillbirth, birth defects) in some special populations (e.g., pregnant women). Reports with no AE (e.g., drug administered to patient of inappropriate age) may also be reported and are assigned MedDRA PTs.
Types of analysis for monitoring the safety of influenza vaccines in VAERS
There are four types of analysis used routinely to monitor the safety of influenza vaccines in the VAERS system; these include: 1) automated analysis of VAERS data; 2) clinical reviews; 3) crude reporting rates; and 4) data mining.
Automated analyses.
These types of analyses focus on numbers of reports and proportions, serious and non-serious reports, pre-specified outcomes, trends and historical comparisons (across years or influenza seasons), and specific vaccine products (e.g., new vaccines such as recombinant and cell culture-based influenza vaccines). Through these analyses, CDC looks for unusual or unexpected patterns of AEs, increases in known AEs, new AEs, and rare and/or serious AEs. For example, CDC automatically generates weekly tables and line lists for selected medical conditions after the inactivated influenza vaccines (IIV) and the quadrivalent live attenuated influenza vaccines (LAIV4) which are routinely monitored on a weekly basis each influenza season. One such example is shown in Table 1. Tables may also be generated for pre-specified medical conditions (e.g., anaphylaxis), and serious cases after newly licensed vaccines (e.g., cell culture-based influenza vaccines, IIV4) [Table 1b]. As an example, Table 2 shows reporting patterns for seizures after inactivated influenza vaccines during several influenza seasons. An increase in reporting was noted for seizures in the age group 6–23 months during the 2011–2012 season (30.80%) compared to the previous 2010–2011 (24.83%) season.
Table 1.
Sample tables automatically generated for surveillance of adverse events following administration of influenza vaccines in VAERS.
| (a) Total number of reports of inactivated and live influenza vaccines during the 2014-2015 and 2013-2104 influenza seasons as of 3/27/2015 | |||||||
|---|---|---|---|---|---|---|---|
| ALL REPORTS Received Following 2014-15 Seasonal Influenza Vaccines Compared to 2013-14 Seasonal Influenza Vaccines | |||||||
| Initial domestic reports only, VAERS reports as of 03/27/2015a | |||||||
| Influenza vaccine received | Total reports N |
Seriousb Fatal N(%) |
Seriousb Non-Fatal N(%) |
Non-Serious Reports N(%) |
Age in years Median (Range) |
Male N(%) |
Onset interval in daysc Median (Range) |
| Seasonal total (2014–2015) | 8,497 | 37 (0.4) | 435 (5.1) | 8,025 (94.4) | 51 (0,99) | 2,494 (29.4) | 0 (0,162) |
| Seasonal Inactivated | 8,028 | 33 (0.4) | 408 (5.1) | 7,587 (94.5) | 53 (0,99) | 2.276 (28.4) | 0 (0,162) |
| Seasonal Live, attenuated | 469 | 4 (0.9) | 27 (5.8) | 438 (93.4) | 10 (0.81) | 218 (46.5) | 1 (0,36) |
| Seasonal total (2013-2014) | 8,570 | 29 (0.3) | 477 (5 6) | 8,064 (94.1) | 48 (0,102) | 2.555 (29.8) | 0 (0,153) |
| Seasonal Inactivated | 7.915 | 28 (0.4) | 443 (5.6) | 7,444 (94.0) | 51 (0,102) | 2,254 (28.5) | 0 (0,153) |
| Seasonal Live, attenuated | 655 | 1 (0.2) | 34 (5.2) | 620 (94.7) | 11 (0,76) | 301 (46.0) | 0 (0,107) |
| (b) Reports of newly licensed influenza vaccines by severity and pre-specified conditions during the 2014-2015 influenza season | |||||||
| Reports of NEWLY LICENSED 2014-15 Influenza Vaccines by Severity and Pre-specified Outcomes | |||||||
| Initial domestic reports only, VAERS reports as of 03/27/2015a | |||||||
| Vaccines | All reports N | Seriousb Fatal N(%) |
Seriousb Non-Fatal N(%) |
GBS N(%) | Anaphylaxis N(%) | ||
| IIV4 | 1,218 | 1 (0.6) | 71 (5.8) | 14 (1.1) | 8 (0.7) | ||
| LAIV4 | 469 | 4 (0.9) | 27 (5.8) | 3 (0.6) | 2 (0.4) | ||
| RIV3 | 15 | 1 (6.7) | |||||
| ccIV3 | 222 | 1 (0.5) | 8 (3.6) | 4 (1.8) | 1 (0.5) | ||
IIV4: Quadrivalent inactivated influenza vaccine; LAIV4: quadrivalent live attenuated influenza vaccine; ccIV3: Flucelvax; RIV3: FluBlok
Table 2.
Reporting trends for seizure reports after 2014-2015 inactivated influenza vaccines (IIV) compared to 2013-2104 through 2004-2005 influenza seasons. Note the higher proportion of seizure reports among children aged 6-23 months during 2010-2011 (24.83%) and 2011-2012 (30.8%) influenza seasons compared to other seasons. 2010 – 2011 is the season during which a febrile seizure signal first appeared in VAERS and it continued for one more season.
| Reporting trends of SEIZURE Reports with Onset Interval 0-1 Day Following 2014-15 Inactivated Seasonal Influenza Vaccines (IIV)a Compared to 2013-14 Inactivated Seasonal Influenza Vaccines (IIV) through 2004-05 (IIV) (by season) By age-group and all ages, initial domestic reports only, VAERS reports as of 03/27/2015b | ||||||
|---|---|---|---|---|---|---|
| 6-23 mos | 2-4 yrs | All ages | ||||
| Season | Total Reports |
Seizure N (%) |
Total | Seizure N (%) |
Total Reports |
Seizure N (%) |
| 2014-15 IIV | 174 | 27 (15.52%) | 151 | 4 (2.65%) | 6000 | 75 (1.25%) |
| 2013-14 IIV | 175 | 30 (17.14%) | 162 | 13 (8.02%) | 5960 | 93 (1.56%) |
| 2012-13 IIV | 155 | 16 (10.32%) | 156 | 11 (7.05%) | 5513 | 69 (1.25%) |
| 2011-12 IIV | 276 | 85 (30.80%) | 147 | 12 (8.16%) | 5088 | 140 (2.75%) |
| 2010-11 IIV | 298 | 74 (24.83%) | 223 | 16 (7.17%) | 5930 | 140 (2.36%) |
| 2009-10 IIV | 253 | 36 (14.23%) | 216 | 13 (6.02%) | 4342 | 94 (2.16%) |
| 2008-09 IIV | 183 | 30 (16.39%) | 148 | 6 (4.05%) | 2960 | 63 (2.13%) |
| 2007-08 IIV | 224 | 50 (22.32%) | 152 | 12 (7.89%) | 2336 | 78 (3.34%) |
| 2006-07 IIV | 169 | 31 (18.34%) | 134 | 2 (1.49%) | 1836 | 46 (2.51%) |
| 2005-06 IIV | 159 | 23 (14.47%) | 112 | 5 (4.46%) | 1847 | 47 (2.54%) |
| 2004-05 IIV | 153 | 26 (16.99%) | 70 | 3 (4.29%) | 978 | 33 (3.37%) |
Seizure: MedDRA Codes Convulsion, Grand Mal Convulsion, Status Epilepticus, Convulsions Local, Febrile Convulsion
Reports with missing age and age 0 -5 months old were excluded
Vaccines type includes Inactivated only
IIV Includes all inactivated influenza vaccines (IIV3, IIV4, RIV3, ccIIV3)
Vaccination and Receive Date falls between 07/01 - 03/27 Per Season
Clinical review.
Clinical review of reports and medical records (if available) after influenza vaccines in VAERS may be performed to: 1) evaluate unusual or unexpected reporting of AEs following influenza vaccines; 2) evaluate new influenza vaccines or when new recommendations are made for existing vaccines; 3) monitor high-priority conditions (e.g., anaphylaxis, spontaneous abortion); and 4) evaluate data mining signals (signal assessment). Through this review, it may be possible to verify the diagnosis in the VAERS report or assign a specific diagnostic category to certain medical conditions (e.g., Brighton level criteria for anaphylaxis reports [10]). Through review of medical records, clinicians may characterize the completeness and quality of reports, the clinical and laboratory features, and assess for potential risk factors (e.g., co-administration of vaccines, underlying health conditions).
Crude reporting rates.
Incidence or prevalence of AEs would be an important measure to calculate as this could be compared to background incidence of the AEs and assess a safety concern. However, these measures cannot be calculated in VAERS because no data on the number of vaccines administered is collected. However, it may be possible to calculate crude reporting rates of AEs by using as proxy denominator data the number of doses of vaccine distributed or an estimate of the number of doses of vaccine administered. Because underreporting of AEs is an important limitation in VAERS, these rates are expected to be below the background rates for the conditions studied.
Data mining.
Data mining is the process of collecting, searching, and analyzing a large amount of data in a database in order to discover patterns or relationships [11]. Since AE incidence or prevalence rates cannot be calculated from VAERS data, data mining techniques have been developed to assess for disproportional reporting in the VAERS database [12]. Two techniques used for signal detection during routine safety monitoring of influenza vaccines include 1) proportional reporting ratio (PRR) and 2) Empirical Bayesian (EB) data mining [13–15].
In the setting of influenza vaccine safety monitoring, the PRR is a statistic used to compare the proportions of AEs for a specific influenza vaccine (e.g., Fluzone High-Dose) or influenza vaccine type (IIV4/LAIV4) with proportions of AEs for the same vaccine types in the previous influenza season [13]. For example, for the current 2015–2016 influenza season, the PRR is calculated for AEs after the quadrivalent inactivated influenza vaccine (IIV4) during the 2015–2016 influenza season and are compared to AEs after IIV4 during the 2014–2015 influenza season. Several PTs may exceed the threshold; however, the decision to conduct further assessment and clinical review of reports will depend on several factors (e.g., unexpected AE not seen before, AE in an unexpected age group, or an unexpected increase in an AE).
Empirical Bayesian (EB) data mining [14–15] is used to identify AEs reported more frequently than expected following a specific type (IIV/LAIV) or brand (Flucelvax®) of influenza vaccine in the VAERS database. Reports after a particular brand or type of influenza vaccine are compared with all other vaccines in the VAERS database. A vaccine-adverse event pairing “signals” when a statistical threshold is reached, i.e., when the vaccine-event pairs is reported at least twice as frequently as would be expected: lower bound of the 95% confidence interval surrounding the EB geometric mean [EB05] >2 [14–15]. Reports containing PTs that exceed the data mining threshold may be reviewed to characterize and verify the signal.
Two examples of disproportionate reporting or signals detected using EB data mining involved quadrivalent live attenuated influenza vaccine (LAIV4) and the cell-cultured trivalent subunit inactivated influenza vaccine, Flucelvax®. During 2013–2014 influenza season, a signal was detected for the PT “Influenza” associated with LAIV4 [16]. Review of the reports containing this PT revealed these were cases of vaccination failure. Studies conducted in other systems noted low LAIV4 vaccine effectiveness against the influenza A H1N1pdm09 (pandemic) strain, the predominant strain that season [17, 18]. Although it was not possible to conclude that this data mining signal was directly related to low LAIV4 vaccine effectiveness against the influenza A H1N1pdm09 virus, the finding was consistent with the epidemiologic data for the 2013–2014 influenza season [17, 18]. As another example, during 2013–2015, disproportional reporting was identified for the MedDRA PT ‘drug administered to patient of inappropriate age’ after the cell-cultured trivalent subunit inactivated influenza vaccine, Flucelvax® [19]. This vaccine is only recommended for adults but review of these reports revealed that in a substantial number of reports children received this vaccine; however, only 3% described an adverse health event associated with the misadministered vaccines (Table 3).
Table 3.
Disproportional reporting for preferred term ‘drug exposure to patient of inappropriate age’ and ‘incorrect route of drug administration’ * after Flucelvax® using Empirical Bayesian data mining during the 2014-2015 influenza season
| PREFERED TERM NAME | N | E | OE | EBGM | EB05 |
|---|---|---|---|---|---|
| INFLUENZA (SEASONAL) (FLUCELVAX 14-15) | |||||
| Drug administered to patient of inappropriate age | 34 | 2.58 | 13.2 | 12.47 | 9.35 |
| Incorrect route of drug administration | 7 | 0.41 | 17.17 | 12.18 | 5.89 |
n = Observed number of reports containing both the vaccine and symptom
E = Expected number of reports containing both the vaccine and symptom
OE = Ratio of n over E
EBGM = Empirical Bayes Geometric Mean estimate of OE (using the DuMouchel model) [ref 14]
EB05 = 90% lower confidence bound on EBGM (5th percentile of posterior)
‘Drug administered to patient of inappropriate age’ is a vaccination error PT which in this case denotes administration of Flucelvax® to persons less than 18 years in whom the vaccine is not recommended. This PT was disproportionally reported during the 2013-2014 season as well. ‘Incorrect route of drug administration’ is a vaccination error which refers to the incorrect administration of another vaccine, Tdap, given concomitantly with Flucelvax® on the same visit
Surveillance of adverse events after vaccination in special populations: pregnant women
The safety of influenza vaccines given to pregnant women is an important priority for CDC. Surveillance of AEs after vaccination with influenza vaccines in pregnant women is part of the routine monitoring of the safety of influenza vaccines which was initiated during the 2009 H1N1 influenza pandemic. The search for pregnancy reports is conducted using a special search strategy which includes: i) identifying PTs under two system organ classes (“Pregnancy, Puerperium, and Perinatal Conditions” and “Congenital, Familial, and Genetic Disorders”); ii) identify the PTs “drug exposure during pregnancy”, “maternal exposure during pregnancy” and “exposure during pregnancy” ; and iii) conducting a text string search for the term ‘preg’ in the symptom, pre-existing condition and medical history fields of the VAERS form [5]. For pregnancy reports, medical records are routinely requested and reviewed for certain conditions such as spontaneous abortion, stillbirth, and birth defects. No concerning patterns of pregnancy or infant AEs after the 2009 H1N1 influenza vaccines were observed [5]. A study on the safety of seasonal influenza vaccines in pregnancy from 2010 through 2015 did not find any safety concern [6].
Vaccine Safety Datalink (VSD)
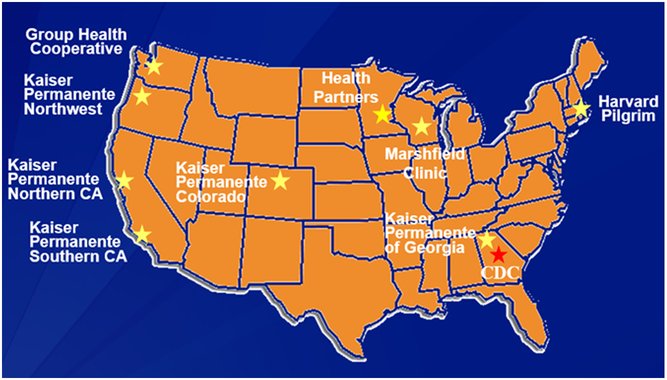
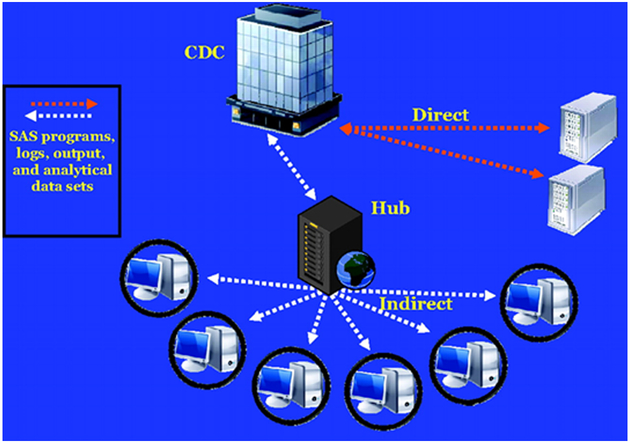
The Vaccine Safety Datalink (VSD) is a collaborative project between CDC and nine integrated health care organizations [Figure 2] [20, 21]. The nine sites are: Kaiser Permanente of Northern California, Oakland; Kaiser Permanente of Colorado, Denver; Marshfield Clinic Research Foundation, Marshfield, Wisconsin); Northwest Kaiser Permanente, Portland, Oregon; Group Health Cooperative, Seattle, Washington; Kaiser Permanente of Southern California, Pasadena; Health Partners, Bloomington, Minnesota; Kaiser Permanente of Georgia; and Harvard Pilgrim, Boston, Massachusetts. VSD, established in 1990, is a vital resource informing policy makers and the public about the safety of vaccines used in the United States. Large linked databases are used to identify and evaluate vaccine safety and conduct studies about potential AEs following immunization. VSD captures comprehensive medical and immunization histories for over 9 million people annually, approximately 3% of the US population. It uses electronic medical records and other administrative sources [Figure 3] to gather data on enrollees, including demographic and medical information, such as age and sex, health plan enrollment, vaccinations, hospitalizations, outpatient clinic visits, emergency or urgent care visits, and mortality data, as well as birth and pregnancy information [20]. Diagnosis codes from ambulatory, urgent care, emergency department and inpatient encounters had been based on the International Classification of Diseases, Ninth Revision (ICD-9). Beginning in October 2015, all VSD sites transitioned from ICD-9 diagnosis codes to ICD-10 codes. Cycle files are created annually to store information based on a standardized data dictionary. Since 2001, VSD has utilized a distributed data model (DDM), which, unlike the previous centralized data model, allows each site to maintain its data files on a secure server at the site rather than transferring data to CDC, thus greatly increasing the confidentiality and security of healthcare plan members’ data [20]. In addition to annually updated cycle files, dynamic data files (DDFs) have also been created since 2005, which use the same standardized data dictionary but are updated weekly. The combination of the DDM and DDFs enable VSD to gather data quickly and securely, and to have the capability to conduct near real-time post-licensure vaccine surveillance, such as Rapid Cycle Analysis (RCA).
Figure 2.
Vaccine Safety Datalink Sites in 2015
Figure 3.
Representation of the VSD distributed data model (DDM) (cited from Baggs et al, 2011).
Types of analysis for monitoring the safety of influenza vaccines in VSD
There are two types of analysis used to monitor the safety of influenza vaccines in VSD: 1) annual RCA; and 2) traditional retrospective studies on new influenza vaccines or particular AEs of interest.
Annual RCA
Annual near real-time vaccine safety surveillance using RCA is essential for influenza vaccine safety surveillance. VSD researchers developed RCA using weekly data collected from VSD sites. Each week, vaccination records and pre-specified medical outcomes of healthcare plan members from each site are collected. A historically-controlled design and/or a self-controlled design are used to sequentially monitor the occurrence of pre-specified outcomes following influenza vaccines, including trivalent inactivated influenza vaccine (IIV3), quadrivalent inactivated influenza vaccine (IIV4) and live attenuated influenza vaccine (LAIV). Other influenza vaccines with small numbers of vaccine recipients, such as Fluzone high dose IIV3, recombinant hemagglutinin vaccine (Flublok®), and intradermal IIV3 (Fluzone® Intradermal), are also closely monitored. For the historically-controlled design, the observed number of pre-specified outcomes of interest are compared with the expected number of events calculated from historical data as a baseline incidence rate. In a self-controlled design, the observed number of AEs for each individual in a pre-selected post-vaccination risk window are compared with the observed number of AEs for the same person in a pre-selected, pre-vaccination or post-vaccination control window. A signal is generated if the test statistic (log-likelihood ratio) exceeds the specified sequential boundary. Once a signal is generated, further analyses are conducted to determine if a signal is a result of an increased risk of an AE following vaccination. A variety of statistical methods were developed to implement RCA, including Poisson and binomial based Maximized Sequential Probability Ratio Test (MaxSPRT) [22] and conditional MaxSPRT [23]. Pre-specified medical outcomes that were monitored in prior influenza seasons include Guillain-Barré syndrome (GBS), Bell’s palsy, febrile seizure, anaphylaxis, and encephalitis. During the 2009 H1N1 influenza pandemic, VSD initiated RCA as soon as the H1N1 influenza vaccine became available in October 2009. The initial findings [24], as well as later end-of-season analysis [25], showed no major safety problems following H1N1 vaccination in 2009–2010, providing the public reassurance and confidence in the safety of this new vaccine.
Traditional retrospective epidemiological studies
VSD conducts vaccine safety studies based on questions or concerns raised from the medical literature and reports to VAERS. When there are new vaccines that have been recommended for use in the United States or changes in vaccine recommendations, VSD monitors the safety of these vaccines. For example, when monovalent H1N1 vaccine was available in 2009, assessment of the safety of the vaccine was carried out in multiple studies [25–27]. VSD large-linked longitudinal databases allow researchers to perform traditional retrospective epidemiological studies that employ cohort and case-control study designs. Novel methods have also been developed, such as case-centered approach [28]. In the past 5 years, as part of efforts to monitor the safety of vaccines in pregnant women, VSD has developed an algorithm to identify pregnant vaccine recipients who can be further evaluated for adverse pregnancy outcomes [29]. VSD investigators have published important studies on vaccine safety in pregnant women, including spontaneous abortion following trivalent inactivated influenza vaccine [30]; preterm or small for gestational age birth following maternal influenza vaccine [31]; fever, seizures, and neurologic disorders following 2009 H1N1 vaccine [32]; and others [33, 34]. A comprehensive review of safety of influenza vaccination during pregnancy can be found in Naleway et al. [35]. Recent VSD studies are evaluating the safety of simultaneous administration of multiple vaccines, such as co-administration of Tdap and influenza vaccines in pregnancy [36].
Limitations of VSD
When new influenza vaccines are licensed, their uptake may be slow and the small number of specific types of influenza vaccines administered may adversely impact the study power to study rare AEs. The number of AEs studied during a typical influenza season is limited to 7–10 priority outcomes using RCA. However for the 2009 H1N1 pandemic year, the number of outcomes was expanded and for certain conditions, multiple risk windows were assessed. Any medical condition that is a vaccine safety concern which is not included among the pre-specified conditions may go undetected, although signals from VAERS or other sources can be added to the conditions evaluated in VSD, which can quickly add new outcomes to be monitored under its current RCA methods. However, not all outcomes are well suited for real time analyses because of long risk windows or by being very nonspecific, and therefore may need to be studied using a more traditional retrospective methodology that can take longer to complete. Vaccines administered outside of the healthcare system of the VSD (e.g. pharmacies) may not be captured. However, use of risk interval methods in vaccinated individuals can help address this limitation.
Case example of influenza vaccine surveillance in VSD: RCA for the 2014–2015 influenza vaccines
For the 2014– 2015 influenza season, VSD investigators conducted weekly RCA to monitor seven medical outcomes which included acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, GBS, febrile seizures, and transverse myelitis following IIV3, IIV4, and LAIV in different age groups [37]. On October 1, 2014 after 23,271 doses of IIV4 were administered, a significantly increased risk of Bell’s palsy was detected following IIV4 for the age group ≥50 years with a relative risk (RR) of 11.3 (Table 4). On December 3, 2014, a signal was generated for an elevated risk of encephalitis following 2,691,270 doses of IIV3 among individuals ≥6 months old, with an estimated RR=2.18. However, after medical record review of Bell’s palsy and encephalitis cases, both signals were determined not to be true signals.
Table 4.
Preliminary signal information for three identified adverse events during the 2014-2015 influenza season in the Vaccine Safety Datalink.
| AE | Age group | Vaccine | Signal date | Signal dose | RR | Final assessment |
|---|---|---|---|---|---|---|
| Bell’s palsy | ≥50 years | IIV4 | 1-Oct-14 | 23,271 | 11.3 | Not verified |
| Encephalitis | ≥ 6 months | IIV3 | 3-Dec-14 | 2,691,270 | 2.18 | Not verified |
| Febrile seizure | 6-23 months | IIV3 | 10-Dec-14 | 43,641 | 17.5 | Verified |
IIV4: Quadrivalent inactivated influenza vaccine; IIV3: Trivalent inactivated influenza vaccine
Febrile seizures after the 2010–2011 influenza vaccine: Case example of a vaccine safety signal in both VAERS and the VSD
In April 2010, Australia halted its pediatric immunization program in children aged <5 years following an increased number of reports of fever and febrile seizures following 2010 CSL IIV3 vaccination [38]. Further studies in Australia and New Zealand verified this finding. In light of the findings in the Southern hemisphere, CDC and FDA implemented enhanced surveillance for seizures in children following all 2010–2011 influenza vaccines in the United States. This enhanced surveillance involved clinical review of all VAERS reports and medical records available for possible febrile seizure cases in children aged <5 years. Empirical Bayesian data mining was conducted biweekly during 2010–2011 to identify AEs reported more frequently than expected following IIV3 [39]. In data mining analysis for reports received by November 23, 2011, disproportionally higher reporting for ‘febrile convulsion’ following 2010–2011 Fluzone® was observed [40]. Review of medical records verified that these reports coded with the ‘febrile convulsion’ term were febrile seizures. No disproportionate reporting was seen with other influenza vaccines [41]. Concomitantly with the VAERS surveillance, VSD investigators conducted weekly rapid cycle analysis for febrile seizures after influenza vaccination. On a weekly basis, surveillance was conducted with the primary approach of a self-controlled risk interval design and the secondary approach of a current vs. historical vaccinee design [42]. Signals for seizures based on computerized data were identified in mid-November 2010 using a current vs. historical design and in late December 2010 using a self-controlled risk interval design. Further signal evaluation was conducted with chart-confirmed febrile seizure cases using only data from the self-controlled risk interval design. An increased risk for febrile seizures was observed predominately in Fluzone® vaccine recipients who received concomitant PCV13 [42]. The febrile seizure signal is an example of two different systems, VAERS and VSD, working and complementing each other to detect and evaluate a signal.
Conclusion
Two surveillance systems are used by CDC to routinely monitor the safety of influenza vaccine: VAERS and VSD. VAERS is the frontline, national surveillance system used to monitor influenza vaccine safety. VAERS’s national scope and its ability to detect rare adverse events make it a good system for hypothesis generation. As a national system, VAERS serves an important role in monitoring the safety of influenza vaccines. The VSD system, through participation of nine integrated health care organizations with large-linked databases, captures comprehensive medical and immunization histories for approximately 3% of the US population. VSD can be used to verify signals detected in VAERS and focused epidemiological studies may also be conducted to study specific vaccine safety issues that cannot be performed in VAERS. Routine annual influenza RCA conducted in VSD may provide timely and efficient post-licensure surveillance results on seasonal influenza vaccines.
Expert Opinion
Influenza can cause moderate to severe illness in all age groups particularly in the very young and the elderly. The most effective way to prevent disease and/or severe outcomes is through vaccination. Safe and effective influenza vaccines have been used during the last 60 years in the United States [2, 43]. Although the safety profile of influenza vaccines during pre-licensure studies has been re-assuring, the small sample size of these clinical trials limits their ability to detect rare AEs. Therefore, post-marketing surveillance of influenza vaccines is warranted and more so since the composition of influenza vaccines may change from year to year if the strains of circulating influenza virus change. Post-marketing surveillance of AEs after influenza vaccines is conducted by FDA and CDC through surveillance systems that include the Post-Licensure Rapid Immunization Safety Monitoring (PRISM), managed by FDA [44], VAERS, managed by both FDA and CDC, and the VSD, managed by CDC. Several other systems were used to monitor the safety of H1N1 influenza vaccines during the 2009 H1N1 pandemic [45]. As this review illustrates, VAERS and VSD have shown their value in monitoring vaccine safety since their implementation in 1990. Both systems, although different, complemented each other well to study febrile seizures in young children following influenza vaccination during the 2010–2011 influenza season [40–42]. However, both systems face a number of challenges for monitoring influenza vaccine safety. One such challenge involves the different types and brands of influenza vaccines available which need to be monitored and analyzed [46]. During the 2015–2016 season, there are both trivalent and quadrivalent influenza vaccines [2] and some brands have different routes of administration including intramuscular, intradermal, needle-free jet injection and nasal spray. Moreover, some influenza vaccines are given at higher antigen concentrations (Fluzone High-Dose) and some others at lower antigen concentration (e.g., Fluzone Intradermal Quadrivalent) [47,48]. These many different vaccines with unknown uptake for each particular age group make it challenging to evaluate them on an annual basis and emphasizes the importance of advance methods development for evaluation of influenza vaccine safety within VSD. In addition, the majority of influenza vaccine is administered in 2–3 months, which makes it more difficult to monitor outcomes that have long risk windows without acute onset
As a spontaneous system, VAERS has important limitations which can make it difficult to assess the safety of influenza vaccine in general as well as in specific groups (e.g., pregnant women). However, CDC and FDA are conducting updates to the VAERS form to improve VAERS reporting [49]. The VAERS form is being revised and new fields will be incorporated which will facilitate the search of reports, such as the pregnancy status of the vaccinee. CDC and FDA are seeking to improve and facilitate online reporting and to transition vaccine manufacturers to reporting using standardized messages through electronic data interchange. The incorporation of AE reminders and VAERS reporting capability directly into the software of electronic medical records has been shown in pilot programs to be useful [50]. Enhancements to both VAERS and VSD will strengthen their roles in the post-marketing surveillance of the safety of influenza vaccines.
Article highlights.
Annual influenza vaccine safety monitoring is an important component of the influenza vaccination program in the United States.
Two surveillance systems are used by the Centers for Disease Control and Prevention (CDC) to monitor the safety of influenza vaccines in the United States, the Vaccine Adverse Event Reporting System (VAERS), and the Vaccine Safety Datalink (VSD).
VAERS is a national vaccine safety surveillance system, co-administered by the CDC and the Food and Drug Administration (FDA) that receives spontaneous reports of adverse events (AEs) following vaccination.
VAERS data are monitored to detect new, unusual, or rare vaccine AEs, increases in known AEs, and possible safety signals which may be evaluated in other studies.
The Vaccine Safety Datalink (VSD) is a collaborative project between the CDC and nine healthcare organizations, which encompasses large linked databases of medical and immunization histories for over 9 million people annually. The VSD can perform epidemiological studies of specific vaccine safety issues that cannot be performed in VAERS
An example of how VAERS and VSD worked and complemented each other to detect and assess a ‘signal’ or safety concern is provided by a ‘signal’ for febrile seizures in children aged <5 years during the 2010-2011 influenza season.
Acknowlegments
We would like to thank Drs. Frank DeStefano for his valuable comments and advice. We thank CDC’s Immunization Safety Office staff whose work allowed this activity to be conducted.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC)
Footnotes
Conflict of interest: None of the authors have a conflict of interest.
References
- 1.Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza---United States, 1976–2007.MMWR Morb Mortal Wkly Rep. 2010. August 27;59(33):1057–62. [PubMed] [Google Scholar]
- 2.Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015. ;64(30):818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015. Vaccine. 2015;33(36):4398–405** An excellent review of the VAERS system, explaining its strengths and limitations and its importance in the US immunization program
- 4.Department of Health and Human Services. Centers for Disease Control and Prevention. http://wonder.cdc.gov/wonder/help/vaers.html.
- 5.Moro PL, Broder K, Zheteyeva Y, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2011;205(5):473.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moro PL, Lewis P, Cragan J, Tepper N. Safety of Seasonal Influenza Vaccines in Pregnancy in the Vaccine Adverse Event Reporting System, 2010 – 2014. 31st International Conference on Pharmacoepidemiology, August 23–26, 2015, Boston, MA. [Google Scholar]
- 7.Medical Dictionary for Regulatory Activities. Available at: http://www.meddramsso.com/ Accessed November 1, 2015.
- 8.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals. http://www.ich.org/products/meddra.html. [DOI] [PMC free article] [PubMed]
- 9.Food and Drug Administration. 21 CFR Part 600.80. Postmarketing reporting of adverse experiences. Vol 62: Federal Register, 1997:52252–3. [Google Scholar]
- 10.Rüggeberg JU, Gold MS, Bayas JM, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2007;25:5675–84. [DOI] [PubMed] [Google Scholar]
- 11.Banks D, Woo EJ, Burwen DR, et al. Comparing data mining methods on the VAERS database. Pharmacoepidemiol Drug Saf. 2005;14(9):601–9. [DOI] [PubMed] [Google Scholar]
- 12.Iskander J, Pool V, Zhou W, English-Bullard R, VAERS Team. Data mining in the US using the Vaccine Adverse Event Reporting System. Drug Saf. 2006;29(5):375–84. [DOI] [PubMed] [Google Scholar]
- 13.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483–6. [DOI] [PubMed] [Google Scholar]
- 14.DuMouchel W Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat. 1999;53:177–90. [Google Scholar]
- 15.Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25:381–92. [DOI] [PubMed] [Google Scholar]
- 16.Haber P, Moro PL, Cano M, et al. Post-licensure surveillance of quadrivalent live attenuated influenza vaccine United States, Vaccine Adverse Event Reporting System (VAERS), July 2013-June 2014. Vaccine. 2015;33(16):1987–92. [DOI] [PubMed] [Google Scholar]
- 17.Brendan F Update on Effectiveness of Live-Attenuated Versus Inactivated Influenza Vaccines in Children and Adolescents Aged 2–18 Years – US Flu VE Network Advisory Committee on Immunization Practice (ACIP) Meeting Presentation October 29, 2014. Available at http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/flu-03-flannery. [Google Scholar]
- 18.Ambrose C Effectiveness study: Preliminary results from 2013–14. MedImmune. Advisory Committee on Immunization Practice (ACIP) meeting presentation October 29, 2014. Available at http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/flu-03-flannery. [Google Scholar]
- 19.Moro PL, Winiecki S, Lewis P, et al. Surveillance of adverse events after the first trivalent inactivated influenza vaccine produced in mammalian cell culture (Flucelvax®) reported to the Vaccine Adverse Event Reporting System (VAERS), United States, 2013–2015. Vaccine. 2015;33(48):6684–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011; 127 (Suppl. 1): S45–53. [DOI] [PubMed] [Google Scholar]
- 21.McNeil MM, Gee J, Weintraub ES, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine 2014;32:5390–8.*Important review article describing the vaccine safety datalink
- 22.Kulldorff M, Davis RL, Kolczak M, et al. A maximized sequential probability ratio test for drug and vaccine safety surveillance. Sequential Anal. 2011;30:58–78. [Google Scholar]
- 23.Li L, Kulldorff M. A conditional maximized sequential probability ratio test for pharmacovigilance. Stat Med. 2010; 29: 284–95. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Safety of influenza A (H1N1)2009 monovalent vaccines—United States. MMWR Morb Mortal Wkly Rep. 2009. October 1–Nov 24;58:1351–6. [PubMed] [Google Scholar]
- 25.Lee GM, Greene SK, Weintraub ES, et al. H1N1and seasonal influenza vaccine safety in the Vaccine Safety Datalink Project. Am J Prev Med. 2011; 41:121–8. [DOI] [PubMed] [Google Scholar]
- 26.Dodd CN, Romio SA, Black S, et al. International collaboration to assess the risk of Guillain Barre Syndrome following influenza A (H1N1) 2009 monovalent vaccines. Vaccine 2013; 31: 4448–58. [DOI] [PubMed] [Google Scholar]
- 27.Duffy J, Weintraub E, Vellozzi C, DeStefano F. Narcolepsy and influenza A (H1N1) pandemic 2009 vaccination in the United States. Neurology. 2014; 83: 1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fireman B, Lee J, Lewis N, et al. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009; 170:650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naleway AL, Gold R, Kurosky S, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013;31(27):2898–903. [DOI] [PubMed] [Google Scholar]
- 30.Irving SA, Kieke BA, Donahue JG, et al. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstet Gynecol. 2013; 159–65. [DOI] [PubMed] [Google Scholar]
- 31.Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr. 2014;164:1051–7. [DOI] [PubMed] [Google Scholar]
- 32.Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al. Monovalent H1N1 influenza vaccine safety in preganant women, risks for acute adverse events. Vaccine. 2014; 32: 4985–92. [DOI] [PubMed] [Google Scholar]
- 33.Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659–67. [DOI] [PubMed] [Google Scholar]
- 34.Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol. 2013;121:519–25. [DOI] [PubMed] [Google Scholar]
- 35.Naleway AL, Irving SA, Henninger ML, et al. Safety of influenza vaccination during pregnancy: a review of subsequent maternal obstetric events and findings from two recent cohort studies. Vaccine. 2014;32:3122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakshmi S, Natalie M, Elyse K, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R, Stewart B, McNeil MM, et al. Post licensure surveillance of influenza vaccines in the vaccine safety datalink in the 2013–2014 and 2014–2015 seasons. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDC Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States During 2010–11. MMWR Morb Mortal Wkly Rep. 2010;59(31):989–92. [PubMed] [Google Scholar]
- 39.Leroy Z, Broder K, Menschik D, et al. Febrile seizures after 2010–2011 influenza vaccine in young children, United States: a vaccine safety signal from the vaccine adverse event reporting system. Vaccine. 2012;30(11):2020–3. [DOI] [PubMed] [Google Scholar]
- 40.Martin D, Menschik D, Bryant-Genevier M, Ball R. Data Mining for Prospective Early Detection of Safety Signals in the Vaccine Adverse Event Reporting System (VAERS): A Case Study of Febrile Seizures after a 2010–2011 Seasonal Influenza Virus Vaccine. Drug Saf. 2013. ;36(7):547–56 [DOI] [PubMed] [Google Scholar]
- 41.Broder KR, Martin DB, Vellozzi C. In the heat of a signal: responding to a vaccine safety signal for febrile seizures after 2010–11 influenza vaccine in young children, United States. Vaccine. 2012;30(11):2032–4.* This article provides an excellent summary of the febrile seizure signal detected in VAERS and the VSD
- 42.Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM; VSD Rapid Cycle Analysis Influenza Working Group. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010–2011. Vaccine. 2012;30(11):2024–31.* Important study describing how the seizure signal was detected and analyzed in the VSD
- 43.Hannoun C The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines. 2013;12(9):1085–94. [DOI] [PubMed] [Google Scholar]
- 44.Salmon D, Yih WK, Lee G, et al. PRISM Program Group. Success of program linking data sources to monitor H1N1 vaccine safety points to potential for even broader safety surveillance. Health Aff (Millwood). 2012;31(11):2518–27 [DOI] [PubMed] [Google Scholar]
- 45.Salmon DA, Akhtar A, Mergler MJ, et al. H1N1 Working Group of Federal Immunization Safety Task Force. Immunization-safety monitoring systems for the 2009 H1N1 monovalent influenza vaccination program. Pediatrics. 2011;127 Suppl 1:S78–86. [DOI] [PubMed] [Google Scholar]
- 46.Greene SK, Kulldorff M, Lewis EM, et al. Near real-time surveillance for influenza vaccine safety: proof-of-concept in the Vaccine Safety Datalink Project. Am J Epidemiol. 2010;171(2):177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Food and Drug Administration. Fluzone High-Dose vaccine insert. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305079.pdf
- 48.Food and Drug Administration. Fluzone Intradermal vaccine insert. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf.
- 49.Shimabukuro T Proposed changes to the Vaccine Adverse Event Reporting System (VAERS) reporting form. Advisory Committee on Immunization Practices, October 22, 2014 [Google Scholar]
- 50.Baker MA, Kaelber DC, Bar-Shain DS, et al. Advanced Clinical Decision Support for Vaccine Adverse Event Detection and Reporting. Clin Infect Dis. 2015;61(6):864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]