Abstract
Objective:
To characterize progression of geographic atrophy (GA) associated with age-related macular degeneration in AREDS as measured by digitized fundus photographs.
Methods:
Fundus photographs from 181 of 4757 AREDS participants with a GA area of at least 0.5 disc areas at baseline or from participants who developed bilateral GA during follow-up were scanned, digitized, and evaluated longitudinally. Geographic atrophy area was determined using planimetry. Rates of progression from noncentral to central GA and of vision loss following development of central GA included the entire AREDS cohort.
Results:
Median initial lesion size was 4.3 mm2. Average change in digital area of GA from baseline was 2.03 mm2 (standard error of the mean, 0.24 mm2) at 1 year, 3.78 mm2 (0.24 mm2) at 2 years, 5.93 mm2 (0.34 mm2) at 3 years, and 1.78 mm2 (0.086 mm2) per year overall. Median time to developing central GA after any GA diagnosis was 2.5 years (95% confidence interval, 2.0-3.0). Average visual acuity decreased by 3.7 letters at first documentation of central GA, and by 22 letters at year 5.
Conclusions:
Growth of GA area can be reliably measured using standard fundus photographs that are digitized and subsequently graded at a reading center. Development of GA is associated with subsequent further growth of GA, development of central GA, and loss in central vision.
Geographic atrophy (GA) associated with age-related macular degeneration (AMD) is estimated to affect nearly 1% of the US population, with this prevalence expected to increase by 50% by the year 2020.1 The appearance of GA is characterized by 1 or more well-defined, usually more or less circular, patches of partial or complete depigmentation of the retinal pigment epithelium, typically with exposure of underlying large choroidal blood vessels.
To date, there are no available effective treatments to arrest the progression of GA, which often results in significant central vision loss and decline in activities of daily living.2 Design of studies to identify new treatment strategies for this form of AMD has been hampered by reliance on loss of visual acuity as the primary outcome variable. Although GA near the foveal center is associated with increased risk of vision loss, most eyes with GA will maintain normal vision for some time while the atrophy expands into the fovea, and when vision loss does occur it declines slowly over a period of years.3 It would therefore seem potentially advantageous to use sensitive and reliable measures of GA growth as an outcome measure for disease progression in clinical studies of GA.
Bilateral GA has been reported to be symmetric in initial appearance4 and growth.5 Sunness et al5 suggest that efficient trial designs for uniocular treatments are possible using growth of GA area as the primary outcome for patients with bilateral GA. They reported a high correlation in progression between eyes and observe that previous progression rates of GA are the strongest predictor of future progression, though the practicality of this approach in multicenter studies may represent a roadblock to implementation. Progression of GA area in cohorts from the United States and abroad using fundus photography and autofluorescence to evaluate area and growth over time suggests consistency in measurement across methods of evaluation and populations.6,7
The purpose of this study is to investigate the potential utility of progression of AMD-associated GA area as an outcome for future studies of treatment efficacy. This study includes participants enrolled in the Age-Related Eye Disease Study (AREDS), a long-term, multicenter, prospective study of the clinical course of AMD and age-related cataract as well as a clinical trial of high-dose antioxidants and zinc for the prevention of advanced AMD. We examine progression rates within the AREDS cohort, agreement in grading between 2 reading centers, impact of GA on visual acuity, the clinical course of GA, and correlation of growth for patients with bilateral GA and report on the test of treatment efficacy using change in area of GA as the outcome measure.
METHODS
A total of 3640 AREDS participants with signs of early AMD or a more advanced form of AMD were randomized to 1 of the 4 study treatments: placebo, antioxidants (500 mg of ascorbic acid; 400 IU of dl-alpha-tocopherol acetate; and 15 mg of beta carotene), zinc (80 mg as zinc oxide and copper; 2 mg as cupric oxide), and antioxidants plus zinc. The details of the study design and methods were published previously.8 Stereoscopic color fundus photographs were taken at baseline, the 2-year follow-up visit, and annually thereafter.
A subset of fundus photographs from AREDS participants were retrospectively digitized and graded for digital area of GA. All AREDS images for participants meeting either of the following criteria were selected:
1. Cumulative area of GA greater or equal to 0.5 disc areas (DAs) (1.33 mm2) within 1500 μm of the fovea in 1 or both eyes at baseline.
2. No GA, or cumulative area of GA less than 0.5 DAs in both eyes at baseline, with the participant developing a GA of 0.5 DAs or larger within 1500 μm of the fovea in both eyes at follow-up visits (not necessarily concurrently), with a minimum of 3 follow-up visits thereafter.
Once AREDS participants met either criterion, all subsequent images were included in the subset. Photographs from 294 eyes and 233 subjects, comprising a total of 1821 images, were graded. Fifty-two of the 233 participants had neovascular AMD at the time of GA development in at least 1 eye and are excluded from these analyses, as the GA may have been secondary to neovascular disease. The remaining 181 participants, contributing 251 eyes and 1341 images, are included in most of the analyses presented in this article. Analyses of right eyes and left eyes were similar. Summary statistics for change in area over time for all unilateral participants and a randomly selected eye for bilateral participants are included.
Analyses of progression from unilateral to bilateral GA, noncentral to central GA and vision loss following the development of central GA, included the entire AREDS cohort through final follow-up at December 31, 2005. Central GA was defined as definite GA involving the center point of the fovea. Development of GA was defined as any patch of definite GA regardless of size. Visual acuity was assessed by certified examiners using the Early Treatment for Diabetic Retinopathy Study (ETDRS) logMAR chart and a standardized refraction and visual acuity protocol (AREDS Manual of Operations, the EMMES Corporation, Rockville, Maryland).
GRADING METHODS
Photographs were digitized and evaluated longitudinally by 4 graders at the University of Wisconsin Fundus Reading Center, Madison. A 10% subset of photographs was regraded by at least 2 graders to determine reproducibility. Grading quality was assessed in a subsample of 87 photographs from 19 eyes and 10 participants that was independently graded by 2 reading centers, Doheny Image Reading Center, Los Angeles, California, and University of Wisconsin Fundus Reading Center. For both reading centers, the slides were scanned using a Nikon Coolscan 4000 (Nikon Corp, Tokyo, Japan) with standardized scanning parameters at a resolution of 500 pixels/inch. The images were then imported into an image viewer and grading software package (Topcon IMAGEnet for Wisconsin and Doheny Image Reading Center GRADOR for Doheny) for optimization and evaluation using a standardized procedure for image optimization that measures and adjusts for illumination and color balance.9
The area of GA was determined using planimetry, a computer-aided tool to draw around, quantify, and record affected areas. To use planimetry, the grader outlined the affected area and created a closed polygon. The grading software then calculated the area enclosed by the polygon based on the scale factor, which was determined by camera type. The proximity of GA to the macula was measured using a line tool. The grader drew a line from the closest edge of the polygon around the GA to the center of the macula. The grading software then calculated and displayed the distance.
For longitudinal grading, the images with the polygons and calculated areas displayed were saved (as copies) and then consulted during the grading of the subsequent visits. The entire series of visits was typically graded during the same session.
STATISTICAL ANALYSIS
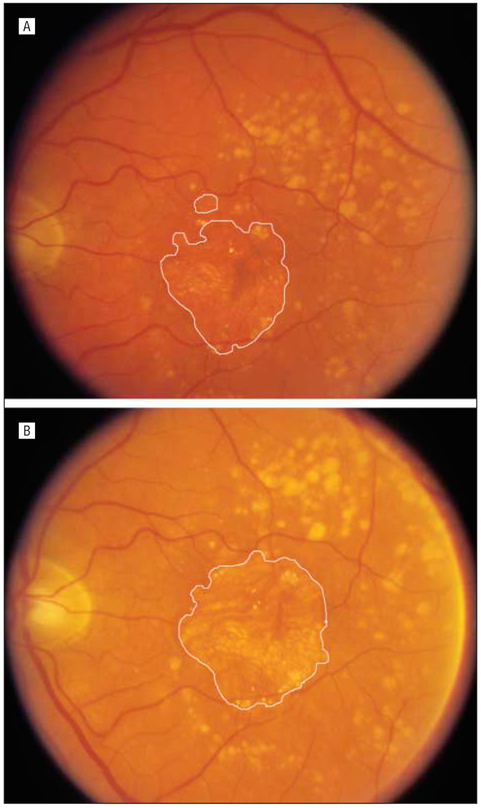
Demographic characteristics of the population are described with summary statistics, including frequency and percentage for categorical data and median, mean, and standard error of the mean (SEM) for continuous data. Baseline subgroups of DA for analysis were 0.5 DA to less than 0.75 DA, 0.75 DA to less than 4.0 dA, and 4.0 DA or greater. One DA is equal to approximately 2.66 mm2 Figure 1 provides examples of GA on fundus photographs and their corresponding GA area measurement from the digitized image.
Figure 1.
Example of geographic atrophy area. Geographic atrophy is defined by 3 criteria: presence of a circular shape; presence of a sharp well-demarcated edge; and diminished retinal pigment epithelium pigment (partial or complete depigmentation of the retinal pigment epithelium, typically with exposure of underlying choroidal blood vessels). A, Geographic atrophy area equals 5.33 mm2. B, Geographic atrophy area equals 8.23 mm2.
Digital area measurements, as assessed by 2 independent reading centers, were compared using the graphical presentation developed by Bland and Altman.10 The difference between the measurements is plotted against the mean of the measurements. The 95% confidence limits of agreement are calculated by components of variance estimation.
The MIXED procedure in SAS, version 8 (SAS Institute Inc, Cary, North Carolina), was used to model the change in digital area over the years of follow-up and test for treatment effect while adjusting for age, sex, smoking, and body mass index. The first-order autoregressive covariance structure was used to model the covariance structure of the repeated follow-up measures within participants. Models were fitted with and without quadratic terms. There was no adjustment for multiple comparisons. The second eye was not included in this analysis owing to the small gain in precision due to the high correlation between eyes.
RESULTS
GRADING QUALITY BETWEEN 2 READING CENTERS
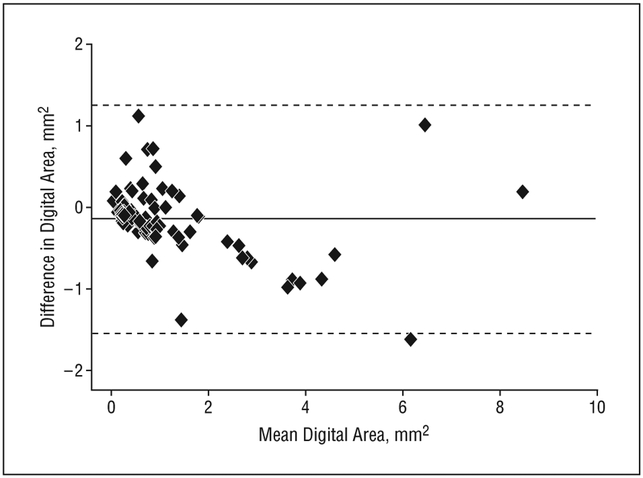
For this analysis, all images are included regardless of neovascular AMD status. Follow-up for these 10 participants varied (range, 1-11 years; mean, 4.78 years; median, 5.0 years). The Bland and Altman approach provides a graphical method for evaluating the agreement between the 2 reading centers over time, with the measurement for a given participant expected to change with time. The average difference, or mean bias, is estimated to be −0.14, indicating that measurements made by the first center are, on average, 0.14 mm2 of digital area less than those made by the second. The 95% limits of agreement, −1.54 to 1.25, suggest that a measurement of digital area from the first center is unlikely to exceed a measurement from the second center by more than 1.25 mm2 or to be less than 1.54 mm2. The Bland/Altman plot (Figure 2), in addition to the 95% limits of agreement, provides evidence that the GA area assessments from the 2 centers are similar for lesions measuring 4 mm2 or smaller. Data are too sparse to draw conclusions for lesions larger than this.
Figure 2.
Bland/Altman10 plot comparing 2 reading centers. The solid horizontal line is the overall average area. Dotted horizontal lines include 95% of the data. Difference is first center minus second center.
PATIENT DEMOGRAPHICS, GA SIZE, GROWTH RATE, AND RISK FACTORS
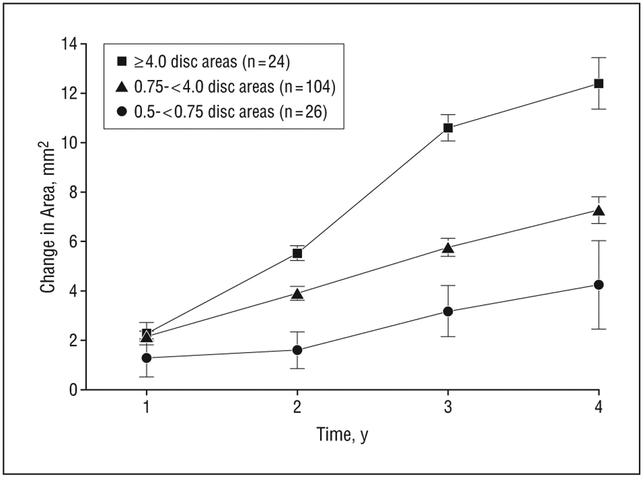
The demographics of the subset of participants without neovascular AMD accompanying GA in either eye at the time that their photographs were taken are presented in Table 1. The median length of follow-up for this subset was 6 years. Fifty-seven percent of participants were female and approximately 9% were current smokers. The mean age of this subset at the time of enrollment is 69.7 years (SD, 5.4 years; median, 71.0 years). Characteristics did not differ by length of follow-up. Participants contributed right-eye images only, left-eye images only, or a randomly selected eye from bilateral images. Median initial lesion size was 4.3 mm2, with a mean of 5.8 mm2 (SEM, 0.42 mm2). Lesions in the sample ranged from 1 mm2 to 45 mm2. Change in lesion size over time by baseline DA is presented in Table 2. The mean change (±standard error of the mean [SEM]) in digital area of GA from baseline was 2.03 mm2 (0.24 mm2) at 1 year, 3.78 mm2 (0.24 mm2) at 2 years, 5.93 mm2 (0.34 mm2) at 3 years, and 7.45 mm2 (0.51 mm2) at 4 years. A mixed-effects model resulted in age, smoking, sex, and body mass index not being predictive of GA growth (P > .2, data not shown). Baseline GA area was an important predictor of subsequent growth (estimate, 0.04; SEM, 0.017 mm2; P=.01) and the model yielded an estimate of linear growth of 1.78 mm2 per year (0.086 mm2 per year). A quadratic term was not significant (P>.9). Mean change over time for selected baseline area ranges illustrates the nearly linear growth of GA over time (Figure 3). An evaluation of lesion growth by the presence of large drusen was limited by the very few eyes that had GA lesions without evidence of large drusen in at least 1 eye (n=18). Three eyes with GA area smaller than 0.75 DAs (2.00 mm2) and without large drusen showed yearly growth rates of less than 0.25 mm2 (data not shown).
Table 1.
Demographics and Follow-up
| Characteristic | Value |
|---|---|
| Follow-up, No. of participants | |
| Any | 181 |
| >2y | 175 |
| >3y | 167 |
| Median follow-up, y | 6 |
| Age, mean (range), y | 69.7 (55-80) |
| Body mass index,a mean (range) | 28.3 (15-47) |
| Female sex, % | 57 |
| Smoking status, % | |
| Never | 37 |
| Former | 54 |
| Current | 9 |
| Initial digital area, mm2 | |
| Mean (SEM) | 5.8 (0.42) |
| Median (range) | 4.3(1-45) |
Calculated as weight in kilograms divided by height in meters squared.
Table 2.
Change in Digital DA by Year
| Baseline DAa in Randomly Selected Eye | ||||
|---|---|---|---|---|
| Characteristicb | 0.5-0.75 DA | 0.75-4.0 DAs | ≥4.0 DAs | Overall |
| Year 1 | ||||
| No. of eyes | 16 | 61 | 11 | 88 |
| Median (range) | 0.86 (0.00 to 7.10) | 1.50 (0.20 to 11.9) | 2.55 (−2.8 to 6.99) | 1.40 (−2.8 to 11.9) |
| Mean (SEM) | 1.29 (0.45) | 2.17 (0.30) | 2.27 (0.77) | 2.03 (0.24) |
| Year 2 | ||||
| No. of eyes | 25 | 104 | 24 | 153 |
| Median (range) | 0.90 (−0.06 to 4.92) | 3.30 (0.50 to 13.9) | 4.53 (−0.04 to 13.3) | 3.20 (−0.06 to 13.9) |
| Mean (SEM) | 1.60 (0.30) | 3.90 (0.28) | 5.53 (0.74) | 3.78 (0.24) |
| Year 3 | ||||
| No. of eyes | 26 | 103 | 19 | 148 |
| Median (range) | 2.63 (0.08 to 9.76) | 5.00 (0.50 to 19.1) | 10.6 (2.48 to 21.2) | 5.06 (0.08 to 21.2) |
| Mean (SEM) | 3.18 (0.53) | 5.76 (0.36) | 10.6 (1.03) | 5.93 (0.34) |
| Year 4 | ||||
| No. of eyes | 17 | 80 | 14 | 111 |
| Median (range) | 2.73 (0.23 to 15.9) | 6.52 (0.80 to 22.8) | 11.8 (1.31 to 24.1) | 6.53 (0.23 to 24.1) |
| Mean (SEM) | 4.24 (1.04) | 7.27 (0.54) | 12.4 (1.79) | 7.45 (0.51) |
Abbreviation: DA, disc area.
One DA is equal to 2.66 mm2.
Participants with geographic atrophy at Age-Related Eye Disease Study enrollment had annual photographs beginning at year 2.
Figure 3.
Mean geographic atrophy growth by baseline lesion size. Error bars indicate ±1 SEM.
EFFECT OF TREATMENT ASSIGNMENT ON GROWTH OF GA
To evaluate the effect of AREDS treatment on GA growth, data from all participants with digital gradings at baseline without evidence of neovascular disease were analyzed. Forty-eight participants with unilateral GA and 20 participants with bilateral GA greater or equal to 0.5 DAs (1.33 mm2) were available for evaluation of the effect of treatments (zinc vs no zinc, and antioxidants vs no antioxidants) on the change in digital area over time adjusting for age, sex, smoking history, and body mass index. The eye with highest baseline GA area was chosen as the study eye for participants with both eyes eligible. A repeated-measures analysis controlling for baseline GA area (assuming linear growth, a random slope, and intercept) and within-subject autoregressive covariance structure was used to test for treatment effect on GA growth. The results are provided in Table 3 and suggest no great benefit of AREDS-type supplements on the progression of GA. Tests for overall treatment effect, antioxidant main effect, and zinc main effect were not significant (P=.21, P=.06, and P=.43, respectively). The individual test of antioxidants plus zinc vs placebo was nominally significant at P=.05. Comparisons of antioxidants vs placebo (P=.19) and zinc vs placebo (P=.52) were not statistically significant.
Table 3.
GA Growth and AREDS Treatmenta
| Characteristic | Placebo (n=15) |
Antioxidants (n=13)b |
Zinc (n=14)c |
Antioxidants and Zinc (n=26)d |
|---|---|---|---|---|
| Baseline GA area, mm2 | 5.17 | 8.83 | 6.83 | 6.06 |
| 3-Year mean growth | 5.98 | 5.75 | 6.34 | 5.05 |
| Standard error | 1.1 | 1.5 | 1.6 | 0.7 |
| Estimated mean growth | 7.89 | 6.64 | 7.31 | 6.24 |
Abbreviations: AREDS, Age-Related Eye Disease Study; GA, geographicatrophy.
Overall significance, P=.21. Estimates adjusted for baseline age, sex, smoking, and body mass index.
Main effect, P=.06; vs placebo, P=.19.
Main effect, P=.43; vs placebo, P=.52.
vs Placebo, P=.05.
BILATERAL GA GROWTH RATES
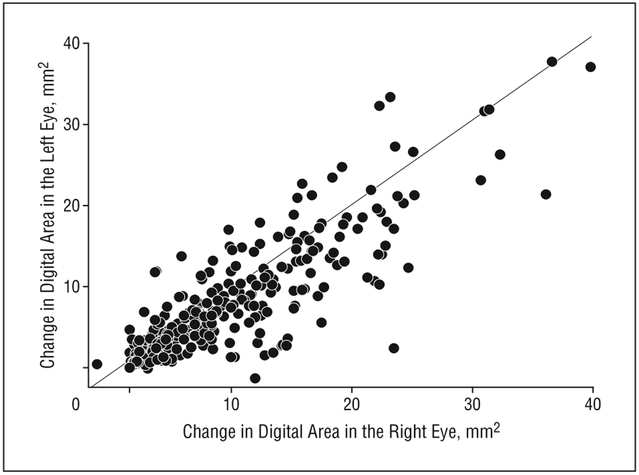
Bilateral GA was identified in 118 participants. However, 48 had been reported to have neovascular AMD in at least 1 eye prior to or concurrent with the photographic documentation of GA. The participants without any report of neovascular AMD are included in a scatterplot of the change in digital area from baseline between right and left eyes (Figure 4). The intraclass correlation coefficient is 0.88, suggesting that participants with bilateral GA experience similar changes in digital area in the right and left eyes.
Figure 4.
Bilateral geographic atrophy growth.
CENTRAL GA AND VISUAL ACUITY
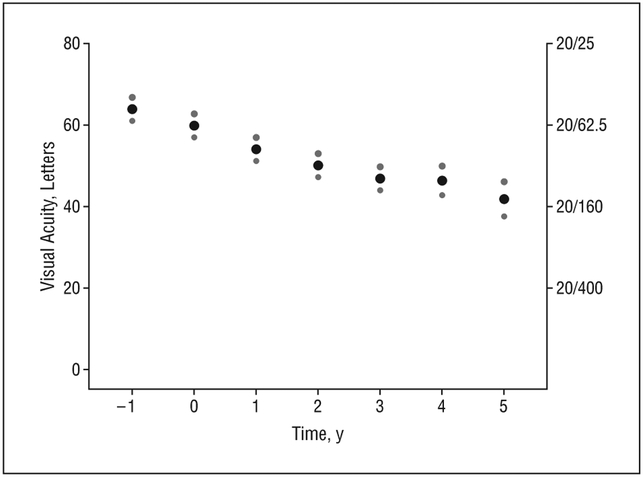
To evaluate the effect of central GA on visual acuity over time, participants who developed central GA during follow-up with no history of neovascularization were selected. Following central GA development, visual acuity is measured up until the last follow-up visit or the last visit for which the eye was free of neovascular disease. All AREDS participants began the study with at least 1 eye free of neovascularization or central GA. Subsequently, 271 right eyes and 295 left eyes (443 participants) developed central GA. Of these, 155 participants also have 5 years of follow-up after central GA development. Figure 5 displays the mean visual acuity 1 year prior to diagnosis of central GA to 5 years after the diagnosis of central GA with 95% confidence intervals. For the visit prior to central GA diagnosis, the mean visual acuity for eyes was 63.6 letters (approximately 20/50). Visual acuity decreased by an average of 3.7 letters at the time of first documentation of central GA. By year 5, the mean visual acuity was 41.9 letters (approximately 20/160), a 22-letter decrease.
Figure 5.
Mean visual acuity over time following development of central geographic atrophy. The small dots indicate 95% confidence intervals.
PROGRESSION FROM NONCENTRAL GA TO CENTRAL GA
The progression rate from noncentral GA to central GA in an eye was estimated from participants in whom GA was initially diagnosed during follow-up with no history of neovascularization. Follow-up data for each eye are included in this analysis unless neovascularization was detected. For participants who developed bilateral central GA, the eye that developed central GA faster is considered in this analysis. Four hundred forty-nine left eyes and 446 right eyes (686 participants) did not have neovascularization or any GA at baseline but later had any GA diagnosed during follow-up visits. Of these, 261 left eyes and 242 right eyes (397 participants) developed central GA. The Kaplan-Meier estimates of cumulative probability of progression over time are presented in Table 4. The median time to developing central GA after any GA diagnosis in at least 1 eye for the 397 participants was 2.5 years (95% confidence interval, 2.0-3.0). Thirty-five percent of participants had central GA at the same time that any GA was first identified.
Table 4.
Kaplan-Meier Estimates of Cumulative Probability of an Event
| Time | Estimate (95% Confidence Interval) |
|
|---|---|---|
| Central GA Development |
Bilateral GA Development |
|
| At initial GA developmenta | 0.35 (0.31-0.38) | 0.07 (0.05-0.09) |
| Year 2 | 0.50 (0.46-0.54) | 0.24 (0.21-0.28) |
| Year 4 | 0.66 (0.61-0.70) | 0.37 (0.32-.042) |
| Year 6 | 0.77 (0.72-0.82) | 0.47 (0.42-0.53) |
| Year 8 | 0.88 (0.82-0.94) | 0.54 (0.47-0.60) |
| Year 10 | 0.96 (0.89-1.00) | 0.62 (0.52-0.72) |
| Median time to event, y | 2.5 (2.0-3.0) | 7 (6.0-10.0) |
Abbreviation: GA, geographic atrophy.
Central GA or bilateral GA occurring at the same time that any GA was first identified.
PROGRESSION FROM UNILATERAL TO BILATERAL GA
To evaluate the rate of developing bilateral GA from the time that GA is diagnosed in 1 eye, participants with GA diagnosed during follow-up visits with no history of neovascularization were selected; follow-up data for each eye are included in this analysis unless neovascularization was diagnosed. Kaplan-Meier estimates of the cumulative probability of developing GA in the second eye from the time that the first eye was diagnosed with GA are provided in Table 4. Of the enrolled AREDS participants, 449 left eyes and 446 right eyes (686 participants) did not have neovascularization or GA at baseline in either eye but were later diagnosed with GA during the follow-up visits. Of these, 209 participants were diagnosed with bilateral GA during follow-up visits. The remaining 477 participants never had GA diagnosed in the second eye during follow-up. The median time to development of bilateral GA is 7 years from development of GA in the first eye (95% confidence interval, 6.0-10.0). Seven percent of participants had bilateral GA at the first visit that any GA was identified.
COMMENT
Geographic atrophy has a profound functional impact on patients. It has been shown to be associated with delayed dark adaptation for both rods and cones, reduced contrast sensitivity, and decreased reading ability.11-13 Data from AREDS support findings from others6,7 that GA area is as an important outcome measure for clinical trials. Our results show that about one-third of participants have central GA at the time we first identified GA; for the others there was a median time to progression from GA to central GA of 2 years. Visual acuity is often decreased prior to the development of central GA and for those who do not develop choroidal neovascularization vision is expected to decline an additional 22 letters on average during the next 5 years. Eyes that develop subsequent choroidal neovascularization have an even worse prognosis.14
Designing clinical trials will require identification of appropriate populations of patients with GA. Sunness et al7 showed that the strongest predictor of subsequent growth of GA is growth in the previous 2 years. However, relying on retrospective quantification of growth is often not practical. The pattern of increased fundus autofluorescence outside atrophic patches of GA may also be an important predictor of subsequent progression.6,15 These areas have shown variable degrees of decreased retinal sensitivity and decreased dark-adapted retinal sensitivity of the rod system. These decreases exceed the light-adapted sensitivity loss of the cone system in these patients.16,17 Although there are limitations in using fundus autofluorescence in the quantification of area of GA,18 it may have advantages over either fundus photography or fluorescein angiography in identifying high-risk patients. Our comparison of grading between 2 reading centers found good reliability of digitized fundus photographs, at least for lesions 4 mm2 or smaller. Data were sparse for lesions larger than 4 mm2.
In our analysis we found that a linear model of GA growth was superior to a quadratic model. Dreyhaupt et al19 reported on the findings from the fundus autofluorescence in an AMD study of 114 patients with GA in Germany followed up for a median of 1.9 years. They have shown that the linear model provides a better prediction of growth than nonlinear models, but the nonlinear model provides better agreement with assumptions. A quadratic growth rate might be expected if a GA lesion had a constant rate of centripetal expansion. However, the observation of an apparent linear relationship may be in part related to the overlapping areas of multiple lesions rather than the simple expanding growth of a single lesion and the possibility that growth rates may slow as the lesion expands out of the macular region. We found no impact on growth of GA for smoking history or body mass index. Similar findings have been previously reported.19
Holz et al6 reported on 195 eyes from 129 patients with GA with a median baseline GA area of 7.04 mm2. The median growth was 1.52 mm2 per year after a median of 1.8 years. Sunness et al7 reported a median growth rate of 2.2 mm2 per year after 2 years in their series of 212 eyes from 131 patients with a median baseline GA area of 7.9 mm2. Median area of baseline atrophy in our series is 4.3 mm2, which is somewhat smaller than that in the Holz and Sunness series, but yearly median growth was similar at 1.71 mm2 year, with a median follow-up of 6 years. Whether or not genetic variation could help predict GA growth rate or progression to central GA requires further study.
Sunness et al7 found a high concordance in baseline area of GA for patients with bilateral disease and a high correlation in 2-year enlargement rate (correlation coefficient, 0.76). A high correlation in growth was observed in bilateral GA within our series as well (correlation coefficient, 0.88). Prevalence of bilateral GA has been reported to range from 48% to 65% of GA cases.20-22 Interestingly, we found that the median time to development of bilateral GA was 7 years. This suggests that trials of uniocular treatments that confine the eligible population to those with bilateral GA, though more efficient in terms of sample size requirements, will omit a sizeable proportion of the potentially eligible population with GA. Possible delays in recruitment must be balanced against possible study design advantages resulting from the high correlation between eyes.
The AREDS has previously reported no statistically significant benefit of antioxidants or zinc on the progression of dry AMD or the development of any or central GA.23 Our analysis of progression of GA area also does not support a large benefit associated with AREDS-type supplements in delaying the progression of GA. The small sample size available does not permit detection of smaller but potentially clinically meaningful differences.
CONCLUSIONS
Geographic atrophy represents a progressive process. We have shown that development of GA is associated with further growth of GA, subsequent development of central GA, and subsequent loss in central vision. Finding new treatments to prevent GA or delay its progression has obvious public health significance. Our finding that GA area can be reliably measured using standard fundus photographs that are digitized and subsequently graded by a reading center and our characterization of the distribution of growth seen in this large population should be useful in the design of future studies.
Acknowledgments
Funding/Support: This report was supported by contracts from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Footnotes
Financial Disclosure: A US patent entitled “Nutritional supplement to treat macular degeneration” (patent No. 6 660 297) was issued on December 9, 2003; Dr Ferris is one of the inventors. The patent is owned by Bausch & Lomb. Dr Ferris has assigned his interest in the patent to the US Government and receives government compensation.
REFERENCES
- 1.Friedman DS, O’Colmain BJ, Muñoz B, et al. ; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. [DOI] [PubMed] [Google Scholar]
- 2.Lindblad AS, Clemons TE; Age-Related Eye Disease Study Research Group. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age-related macular degeneration, vision loss and lens opacity. AREDS Report No. 14. Arch Ophthalmol. 2005;123(9):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106(9):1768–1779. [DOI] [PubMed] [Google Scholar]
- 4.Bellmann C, Jorzik J, Spital G, Unnebrink K, Pauleikhoff D, Holz FG. Symmetry of bilateral lesions in geographic atrophy in patients with age-related macular degeneration. Arch Ophthalmol. 2002;120(5):579–584. [DOI] [PubMed] [Google Scholar]
- 5.Sunness JS, Applegate CA, Bressler NM, Hawkins BS Designing clinical trials for age-related geographic atrophy of the macula: enrollment data from the geographic atrophy natural history study. Retina. 2007;27(2):204–210. [DOI] [PubMed] [Google Scholar]
- 6.Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S; FAM-Study Group. Progression of geographic atrophy and impact on fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol 2007;143(3):463–472. [DOI] [PubMed] [Google Scholar]
- 7.Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007; 114(2):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS Report No 1. Control Clin Trials. 1999;20(6):573–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbard LD, Danis RP, Neider MW, et al. Brightness, contrast, and color balance of digital vs film retinal images in the Age-Related Eye Disease Study 2. Invest Ophthalmol Vis Sci 2008;49(8):3269–3282. [DOI] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. [DOI] [PubMed] [Google Scholar]
- 11.Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104(10):1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunness JS, Massof RW, Johnson MA, Finkelstein D, Fine SL. Peripheral retinal function in age-related macular degeneration. Arch Ophthalmol. 1985;103(6): 811–816. [DOI] [PubMed] [Google Scholar]
- 13.Sunness JS, Johnson MA, Massof RW, Marcus S. Retinal sensitivity over drusen and nondrusen areas: a study using fundus perimetry. Arch Ophthalmol. 1988; 106(8):1081–1084. [DOI] [PubMed] [Google Scholar]
- 14.Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis. 1999;5:25. [PubMed] [Google Scholar]
- 15.Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J, et al. ; Fundus Autofluorescence in Age-Related Macular Degeneration Study Group. Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMD. Invest Ophthalmol Vis Sci 2006;47(6):2648–2654. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz-Valckenberg S, Bültmann S, Dreyhaupt J, Bindewald A, Holz FG, Rohrschneider K Fundus autofluorescence and fundus perimetry in the junctional zone of geographic atrophy in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(12):4470–4476. [DOI] [PubMed] [Google Scholar]
- 17.Scholl HP, Bellmann C, Dandekar SS, Bird AC, Fitzke FW Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci 2004;45(2): 574–583. [DOI] [PubMed] [Google Scholar]
- 18.Sunness JS, Ziegler MD, Applegate CA. Issues in quantifying atrophic macular diseases using retinal autofluorescence. Retina. 2006;26(6):666–672. [DOI] [PubMed] [Google Scholar]
- 19.Dreyhaupt J, Mansmann U, Pritsch M, Dolar-Szczasny J, Bindewald A, Holz FG. Modeling the natural history of geographic atrophy in patients with age-related macular degeneration. Ophthalmic Epidemiol. 2005;12(6):353–362. [DOI] [PubMed] [Google Scholar]
- 20.Potter JW, Thallemer JM. Geographic atrophy of the retinal pigment epithelium: diagnosis and vision rehabilitation. J Am Optom Assoc. 1981;52(6):503–508. [PubMed] [Google Scholar]
- 21.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2(pt 5):552–577. [DOI] [PubMed] [Google Scholar]
- 22.Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92(5):615–627. [PubMed] [Google Scholar]
- 23.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta-carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch Ophthalmol. 2001;119(10):1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]