Abstract
Background and Objective:
To describe the retinal vasculature and choriocapillaris, and transition zone between the diseased and healthy tissue, in eyes with inherited retinal degenerations using optical coherence tomography angiography (OCTA).
Patients and Methods:
Patients with inherited retinal degenerations were recruited for OCTA imaging. Retinal vasculature was assessed for increased intercapillary space and foveal avascular zone (FAZ) abnormalities. Choriocapillaris, retinal pigment epithelium (RPE) and photoreceptor disruption were noted and the borders were evaluated to speculate which layers become affected first.
Results:
Fourteen eyes of seven subjects with inherited retinal degenerations were included. All (100%) eyes demonstrated retinal thinning and increased intercapillary spaces overlying focal outer retinal changes. In all eyes the region of choriocapillaris changes was smaller than the region of overlying RPE and photoreceptor alteration, suggesting the vascular loss was secondary.
Conclusion:
OCTA is able to provide highly detailed vascular information in eyes with inherited retinal degenerations and may be useful to better understand the pathogenesis of these diseases.
Introduction
Inherited retinal degenerations are a broad group of hereditary disorders affecting the retina. Typically, these diseases affect the cone and rod photoreceptors and the retinal pigment epithelium (RPE), progress slowly and lead to an irreversible loss of vision.1 Retinitis pigmentosa (RP) is the most prevalent inherited retinal degeneration, affecting approximately 1 in 3000-4000 people.1-3 It primarily affects the rod photoreceptors in the mid-periphery and slowly progresses to the macula, leading to retinal hyperpigmentation called bone spicules in the periphery.1,4 Stargardt disease, in contrast, affects the central macula first and may be characterized by yellow pisciform flecks in the posterior pole. It is the most common macular degeneration related to mutations in the ABCA4 gene and is often referred to as fundus flavimaculatus.3,5 Disruption of the photoreceptors, RPE, and outer retina as seen on fundus autofluorescence and optical coherence tomography (OCT) in eyes with Stargardt disease are associated with a progressive decline in vision.6-7 Posterior polar annular choroidal dystrophy is a rare disease that is characterized by a peculiar pattern of atrophy around the optic nerve and arcades.3
Various studies have evaluated choroidal blood flow in inherited retinal degenerations. Studies using doppler flowmetry and magnetic resonance imaging have demonstrated a marked reduction in choroidal blood flow in eyes with RP that correlates with cone dysfunction and reduced a-wave on standard combined ERG.8-9 Furthermore, choroidal thickness measurements using enhanced depth imaging OCT have shown a statistically significant decrease in choroidal thickness in patients with RP.10 Choroidal changes in Stargardt disease were evaluated by Giani et al using indocyanine green angiography (ICGA), fluorescein angiography (FA), and OCT.11 Hypocyanescence was seen on ICGA at the areas of atrophy in 92% of Stargardt eyes. However, in these eyes the choroid was morphologically intact on SD-OCT leading the authors to postulate if there was selective loss of the choriocapillaris layer. Notably, changes in the retinal blood flow are poorly documented in inherited retinal degenerations.
In vivo imaging of the choroid has been historically challenging due to its location beneath the RPE and the fenestrated capillaries of the choriocapillaris causing dye leakage on FA and ICGA.12 In December 2013 Choi et al demonstrated the utility of OCT angiography (OCTA) in visualizing the choriocapillaris and choroidal vasculature in fine detail in healthy eyes.13 OCTA is a fast non-invasive imaging modality that provides high-resolution images of the retinal and choroidal vasculature without the use of exogenous dye. Assuming that the only things rapidly moving/changing in the back of the eye are erythrocytes in the retinal vasculature, the decorrelation signal between repeated OCT b-scans at the same retinal location is used by the OCTA software to create a vascular map (OCT angiogram). 14-15 En-face OCT angiograms are co-registered with corresponding OCT b-scans allowing for blood flow and structural information to be viewed simultaneously. The OCT angiograms can be scrolled through from internal limiting membrane to the deep choroid to visualize the different vascular plexuses.16 The present study aims to analyze the retinal vasculature and choriocapillaris and the transition zone between the diseased and healthy tissue in eyes with inherited retinal degenerations using OCTA. To our knowledge, this is the first study using OCTA to evaluate inherited retinal degenerations.
Patients and Methods
This study was approved by the institutional review board of Tufts Medical Center. Informed consent was obtained from patients in accordance with the Tufts Medical Center Institutional Review Board prior to examination. The research adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act of 1996. Patients with inherited retinal degenerations were recruited in a prospective manner from the retina service of New England Eye Center for imaging on the prototype AngioVue OCTA software of the commercially available RTVue XR Avanti spectral domain (SD)-OCT device (Optovue, Inc, Fremont, CA) between August 2014 and March 2015. The OCTA system operates at 70,000 A-scans per second to acquire 3 × 3 mm and 6 × 6 mm OCTA data sets each consisting of 304 × 304 A-scans in approximately 2.6 seconds. OCT angiograms are intrinsically co-registered with the corresponding OCT b-scans that were use to create the OCT angiograms and an en-face retinal thickness map. The exclusion criteria included eyes with retinal vascular diseases such as diabetic retinopathy, vascular occlusions and age-related macular degeneration (AMD).
The OCTA software was used to delineate the retinal vasculature by segmenting the volumetric data set to project blood flow between the internal limiting membrane and Bruch’s membrane and the choriocapillaris by segmenting a 10um layer directly below Bruch’s membrane. The OCT angiograms of the retinal vasculature were evaluated for increased intercapillary space and for enlargement or irregularity of the foveal avascular zone (FAZ). Increased intercapillary space was defined as an enlargement of the area between capillaries that appeared to be more than twice the normal size. The location of the increased intercapillary space was noted (ie foveal, central, peripheral) within the posterior pole. The co-registered OCT retinal thickness map was used to identify any retinal thinning. Capillary density loss, as defined by flow voids in an otherwise normally homogenous choriocapillaris layer, was evaluated on the OCT angiograms of the choriocapillaris, and location was noted (ie foveal, central, peripheral). A particular attention was paid to the transition zone between diseased and healthy tissue to evaluate the order of loss of the following layers: the RPE, photoreceptors, and choriocapillaris. The OCT angiograms were scrolled through while looking at the co-registered OCT b-scans in order to see the edges of RPE and photoreceptor loss simultaneously with the edge of choriocapillaris alteration.
Results
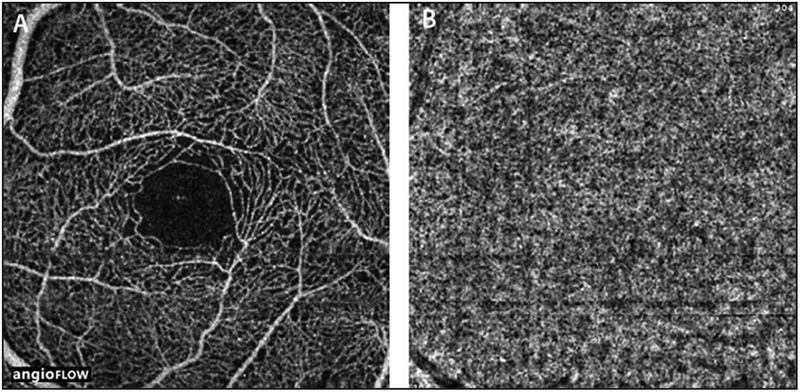
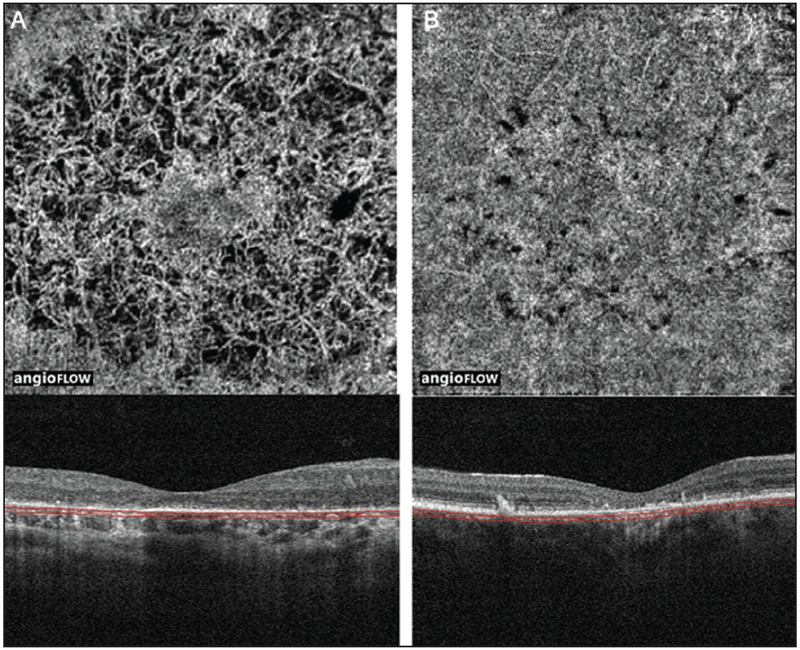
There were 14 eyes of seven subjects with inherited retinal degenerations included in this study. The average age was 53 years old with a range of 35-72 years. There were four women and three men. Five patients were Caucasian, one was Asian, and one was Hispanic. There were four eyes with RP, eight with Stargardt disease, and two with posterior polar annular choroidal dystrophy. For comparison, OCTA images of a normal eye of a 45 year old woman is shown in Figure 1.
Figure 1:
(A) 3 × 3 mm OCT angiogram of the retinal vasculature of the left eye of a normal eye. Note the dense, regular, spider-web-like appearance of the vasculature. (B) 3 × 3 mm OCT angiogram of the choriocapillaris of the same eye. Note the generally homogenous appearance.
Inner Retina
All 14/14 (100%) eyes with inherited retinal degenerations demonstrated retinal thinning and increased intercapillary spaces. However, the location of these changes varied by disease type. All four eyes (100%) affected by RP and all two eyes (100%) with posterior polar annular choroidal dystrophy showed these changes peripherally within the posterior pole (Figure 2). Of the eight eyes with Stargardt disease, all eyes (100%) had retinal thinning and increased intercapillary space centrally (Figure 3). Two of these eyes also demonstrated these changes temporally, and two of them showed these changes throughout the entire posterior pole. FAZ enlargement or gross irregularity was seen in seven of 14 eyes (50%), which included two eyes of one RP patient and five eyes of three Stargardt patients.
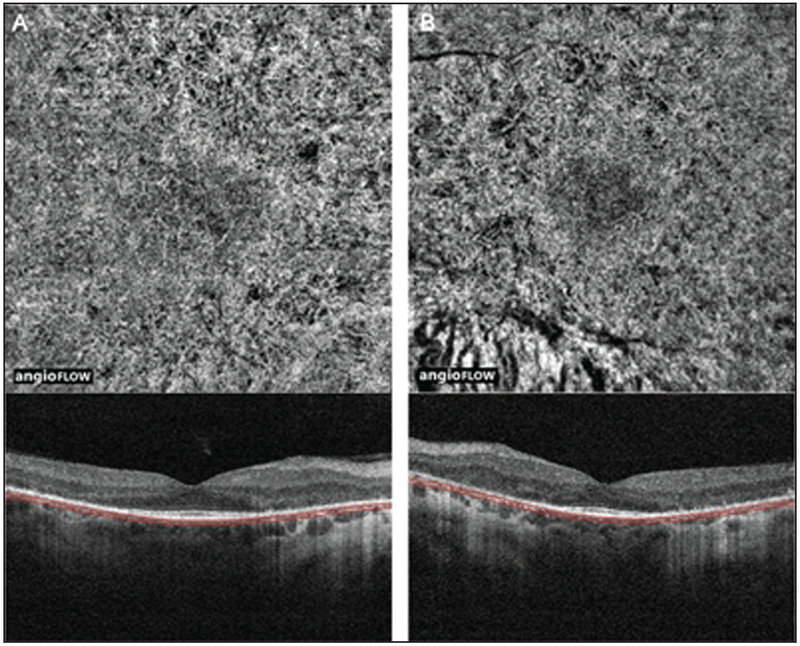
Figure 2:
(A) 6 × 6 mm OCT angiogram and corresponding OCT b-scan of the left eye of a patient with retinitis pigmentosa showing peripheral retinal thinning (inset: retinal thickness map), increased intercapillary spaces that are more prominent peripherally, and FAZ remodeling. Note the presence of CME. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. (B) 6 × 6 mm OCT angiogram and corresponding OCT b-scan of the left eye of a patient with posterior polar annular choroidal dystrophy showing peripheral retinal thinning (inset: retinal thickness map) and an increase in intercapillary spaces. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. OCT, optical coherence tomography; FAZ, foveal avascular zone; CME, cystoid macular edema.
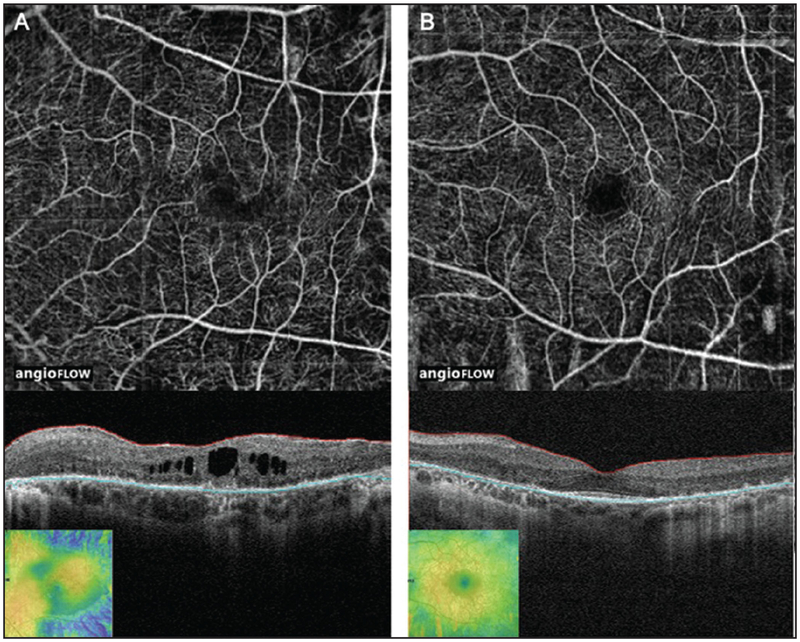
Figure 3:
(A) 3 × 3 mm OCT angiogram and corresponding OCT b-scan of the left eye of a patient with Stargardt disease showing central retinal thinning (inset: retinal thickness map), increased intercapillary spaces that are more prominent centrally, and FAZ remodeling. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. (B) 3 × 3 mm OCT angiogram and corresponding OCT b-scan of the right eye of a patient with Stargardt disease showing central retinal thinning, increased intercapillary spaces that are more prominent centrally, and FAZ remodeling. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram.OCT, optical coherence tomography; FAZ, foveal avascular zone.
Choriocapillaris/Photoreceptors/RPE
A region of choriocapillaris density loss was noted directly below the atrophied RPE and photoreceptor layers in 10/14 eyes (71%). Loss of the RPE and photoreceptors were seen in all 14 eyes (100%). Just as with the inner retinal changes, the location of these changes varied by disease type. Two eyes of one patient with RP (50%) demonstrated peripheral and foveal loss of these three layers (choriocapillaris, RPE, photoreceptors; Figure 4). The other two eyes with RP (50%) had an intact choriocapillaris within the posterior pole but peripheral loss of the photoreceptors and RPE. Both eyes (100%) with posterior polar annular choroidal dystrophy also showed these changes peripherally with a more subtle decrease in density of the choriocapillaris layer (Figure 5) than with the eyes of RP and Stargardt disease (Figures 4 and 6). Six of the eight eyes (75%) with Stargardt disease showed central loss of all three layers and two of the eight eyes (25%) from one patient had an intact choriocapillaris but loss of the RPE and photoreceptors centrally (Figure 6). Interestingly, three of the six eyes (50%) with central choriocapillaris loss demonstrated foveal sparing (Figure 6A).
Figure 4:
(A) 6 × 6 mm OCT angiogram and corresponding OCT b-scan of the right eye of a patient with retinitis pigmentosa showing peripheral and foveal loss of the choriocapillaris, RPE, photoreceptors. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. (B) 6 × 6 mm OCT angiogram and corresponding OCT b-scan of the left eye of the same patient showing peripheral and foveal loss of the choriocapillaris, RPE, photoreceptors. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. OCT, optical coherence tomography; RPE, retinal pigment epithelium.
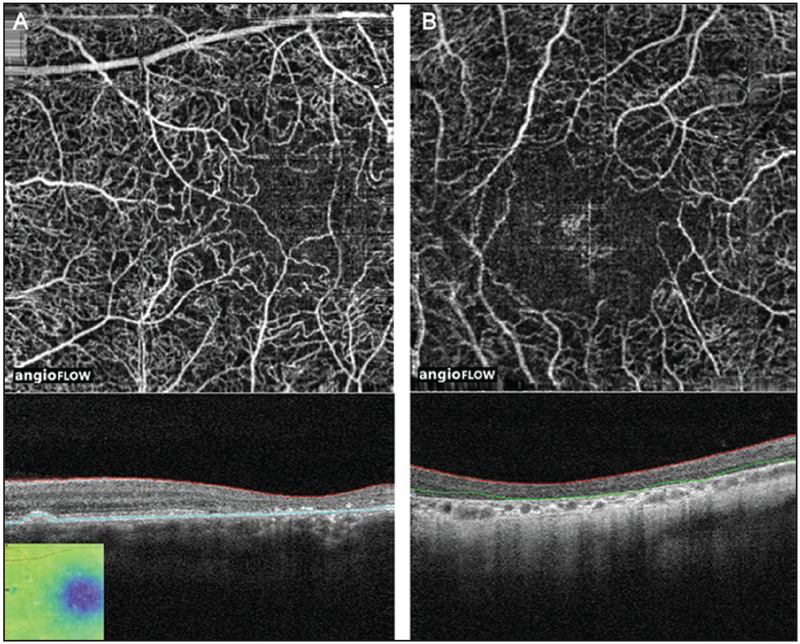
Figure 5:
(A) 6 × 6 mm OCT angiogram and corresponding OCT b-scan of the right eye of a patient with posterior polar annular choroidal dystrophy showing subtle peripheral loss of the RPE, photoreceptors and the choriocapillaris. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. (B) 6 × 6 mm OCT angiogram and corresponding OCT b-scan of the left eye of the same patient showing subtle peripheral loss of the RPE, photoreceptors and the choriocapillaris. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. OCT, optical coherence tomography; RPE, retinal pigment epithelium.
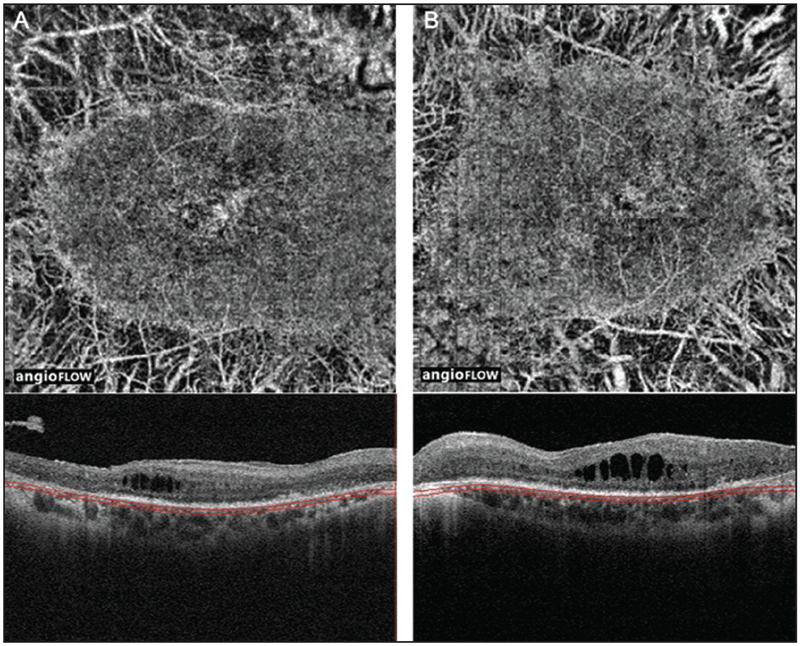
Figure 6:
(A) 3 × 3 mm OCT angiogram and corresponding OCT b-scan of the right eye of a patient with Stargardt disease showing central loss of RPE, photoreceptors and the choriocapillaris. Note that there is sparing of the choriocapillaris in the fovea. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. (B) 6 × 6 mm OCT angiogram and corresponding OCT b-scan of the right eye of a patient with Stargardt disease showing central loss of RPE and photoreceptors but an intact choriocapillaris. The dark areas of “loss” on the OCT angiogram are due to signal attenuation, not true choriocapillaris loss. The horizontal lines on the corresponding OCT B-scans show the region represented by OCT angiogram. OCT, optical coherence tomography; RPE, retinal pigment epithelium.
The transition zone between diseased and unaffected tissue was evaluated in detail. Two eyes of one patient with Stargardt disease did not have sufficient quality of the structural OCT b-scans to evaluate the RPE and photoreceptor loss in detail. Of the 12 eyes in which the transition zone was evaluated, all 12 (100%) clearly showed that the region of choriocapillaris changes was smaller than the region of RPE and photoreceptor loss suggesting that the choriocapillaris changes occurred after the RPE and photoreceptor changes. Furthermore, in four eyes of two patients, no choriocapillaris changes were noted but the RPE and photoreceptors were affected in these eyes (Figure 6B). In contrast, the borders of the RPE and photoreceptor loss were very close in all 12 eyes. In eight of the 12 eyes (67%) it appeared that the region of RPE loss was larger, suggesting that this change preceded the loss of the photoreceptors. In one eye (8%), the area of photoreceptor loss was more advanced. In the remaining three eyes (25%), there was no discernible difference between the regions of RPE and photoreceptor loss.
Discussion
This study used OCTA to evaluate the retinal vasculature and choriocapillaris in eyes with inherited retinal degenerations. The OCT angiograms of the inner retina consistently demonstrated total retinal thinning and increased intercapillary space in all eyes. The microvascular changes were in the same general distribution as that of the changes to the choriocapillaris, RPE, and photoreceptor loss. Patients with RP and posterior polar annular choroidal dystrophy showed increased intercapillary space peripherally within the posterior pole while eyes with Stargardt disease demonstrated these changes centrally in the macula. This suggests that the retinal vascular and choroidal changes in these common inherited retinal degenerations may be linked. The retinal vasculature in this patient population has yet to be well described in the literature and little is known about why cystoid macular edema sometimes occurs in these eyes. Longitudinal studies using OCTA may help elucidate if the retinal changes are related to formation of fluid.
Multiple studies have evaluated blood flow changes in eyes with inherited retinal degenerations suggesting that the circulation in these disease may be grossly altered.8-11 However, prior to OCTA, the choroidal circulation has been difficult to visualize in detail in vivo.12 This study utilizes the detailed angiographic information from OCTA and structural information from the co-registered OCT B-scans to evaluate the choriocapillaris. Choriocapillaris loss/atrophy was noted directly below the disrupted RPE and photoreceptor layers. However, by evaluating the transition zone between diseased and healthy tissue, it was noted that the region of choriocapillaris changes was smaller than the overlying RPE and photoreceptor loss. Meanwhile, the borders of RPE and photoreceptor loss coincided well. Notably, this finding was particularly marked in the four eyes (two eyes with RP and two eyes with Stargardt disease) that had an intact choriocapillaris layer but a clear disruption of the RPE and photoreceptors. This suggests that changes in these two layers may precede the choriocapillaris disruption. Longitudinal studies are warranted to confirm these findings and are expected to be useful to better understand the pathogenesis of these diseases to direct targeted therapies in future.
This study uses OCTA, a novel non-invasive imaging technique, to describe the retinal and choroidal vasculature in detail and evaluate the transition zone between healthy and diseased retina. The major limitation is the small sample size and cross-sectional nature. In future, prospective longitudinal OCTA studies involving a larger cohort of patients with a variety of inherited retinal degenerations may be useful to understand the natural history of the disease. Due to its ability to provide detailed angiographic information, OCTA is able to provide new insights into the effects of inherited retinal degenerations on the retinal and choroidal vasculature.
Acknowledgments
Conflicts of Interest: Thank you to the Massachusetts Lions Clubs, which supported this work. Jay S. Duker is a consultant for and receives research support from Carl Zeiss Meditec, OptoVue, and Topcon Medical Systems Inc. Nadia K. Waheed was a consultant for Iconic therapeutics, served the speaker’s bureau for Thrombogenics, and receives research support from Carl Zeiss Meditec.
Footnotes
This manuscript has not been presented at a meeting.
References
- 1.Witkin AJ, Ko TH, Fujimoto JG, et al. Ultra-high resolution optical coherence tomography assessment of photoreceptors in retinitis pigmentosa and related diseases. Am J Ophthalmol.2006;142(6):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis PJ. Genetics of inherited retinal disease. J R Soc Med 2006;99:189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hereditary Chorioretinal Dystrophies In: Yannuzzi LA, ed. The Retinal Atlas. Philadelphia: Elsevier Saunders; 2010:7–182. [Google Scholar]
- 4.Aizawa S, Mitamura Y, Baba T, Hagiwara A, Ogata K, Yamamoto S. Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye. 2009;23(2):304–308. [DOI] [PubMed] [Google Scholar]
- 5.McBain VA, Townend J, Lois N. Progression of retinal pigment epithelial atrophy in stardardt disease. Am J Ophthalmol. 2012;154(1):146–154. [DOI] [PubMed] [Google Scholar]
- 6.Salvatore S, Fishman GA, McAnany JJ, Genead MA. Association of dark-adapted visual function with retinal structural changes in patients with Stargardt disease. Retina. 2014;34(5):989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang WC, Cideciyan AV, Roman AJ, et al. Inner and outer retinal changes in retinal degenerations associated with ABCA4 mutations. Invest Ophthalmol Vis Sci. 2014;55:1810–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falsini B, Anselmi GM, Marangoni D, et al. Subfoveal choroidal blood flow and central retinal function in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011; 52:1064–1069. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Harrison JM, Nateras OSE, Chalfin S, Duong TQ. Decreased retinal–choroidal blood flow in retinitis pigmentosa as measured by MRI. Doc Ophthalmol. 2013;126:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhoot DS, Huo S, Yuan A, et al. Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol. 2013; 97:66–69. [DOI] [PubMed] [Google Scholar]
- 11.Giani A, Pellegrini M, Carini E, et al. The dark atrophy with indocyanine green angiography in stargardt disease. Invest Ophthalmol Vis Sci. 2012;53(7):3999–4004. [DOI] [PubMed] [Google Scholar]
- 12.Von Sallmann L The structure of the eye. Arch Ophthalmol. 1961;66:920–921. [Google Scholar]
- 13.Choi W, Mohler KJ, Potsaid B, et al. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS ONE 2013;8(12):e81499. doi: 10.1371/journal.pone.0081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsunaga D, Yi J, Puliafito CA, Kashani AH. OCT Angiography in healthy human subjects. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):510–515. [DOI] [PubMed] [Google Scholar]
- 15.de Carlo TE, Romano A, Waheed NK, et al. A review of optical coherence tomography angiography (OCTA). International Journal of Retina and Vitreous. 2015;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Carlo TE, Bonini Filho MA, Chin AT, et al. Spectral domain optical coherence tomography angiography (OCTA) of choroidal neovascularization. Ophthalmology. 2015;122(6):1228–1238. [DOI] [PubMed] [Google Scholar]