Abstract
Objectives:
Colorectal cancer (CRC) is the third most common cancer among Americans of South Asian (SA) descent and is a significant public health concern in SA communities. Rates of screening compliance among foreign-born SAs are very low. The goal of this study was to report on the development, acceptability, and preliminary impact of a culturally-targeted 1:1 intervention delivered in English, Hindi, and Urdu, called Desi-Sehat.
Design:
Ninety-three foreign-born SAs between the ages of 50 and 75 were recruited using community-based organization methods. Participants completed a baseline survey, participated in a 1:1 session with a community health educator, and a follow-up survey was administered four months after the baseline.
Results:
The acceptance rate was moderate (52.8%). Attendance at the intervention session was high. More than half of the population did not complete the follow-up survey (58.7%). Participant evaluations of the intervention were high. Intent-to-treat analyses indicate a 30% four month follow-up CRC screening uptake. There were significant increases in knowledge and significant reductions in perceived barriers to screening, worry about CRC screening tests, and worry about CRC. Effect sizes for significant changes were in the medium to large range.
Conclusions:
Desi Sehat was a well-evaluated and participation in the session was high, participant knowledge significantly increased, and screening barriers, worry about CRC, and worry about CRC screening tests declined significantly. Future studies should focus on enhancing recruitment and retention and include a randomized control design.
Keywords: Asian Americans, cancer, colorectal neoplasms, screening, minority groups
Introduction
At 3.4 million persons, South Asians (SA)—individuals originating from countries such as India and Pakistan—are the second fastest-growing ethnic community in the US (US Census Bureau 2010). Nearly 330,000 SA residents live in New Jersey, making the state home to the third largest SA population in the US and the most populous Asian group in the state (US Census Bureau 2010). Between the years of 2000 and 2010, the number of SA population grew by 78% in New Jersey (US Census Bureau 2010; Asian American Federation 2004). About 77% of SAs in this region are foreign-born, and the most common languages spoken at home among this population are Hindi, Gujarati, and Urdu.
As the SA population grows, the cancer burden in this population increases concomitantly. Literature prior to 2010 suggested that CRC incidence was lower among foreign-born SAs as compared with non-Hispanic (NH) whites (Ladabaum et al., 2014) However, emerging evidence suggests that CRC may be a public health concern among SAs. Although CRC incidence has declined among NH Whites, its incidence has not declined among SAs (Ladabaum et al., 2014). It is one of the top three most common cancers among SAs in the US (Jain, Mills, & Parikh-Patel, 2005). In addition, CRC incidence is increasing among female SAs (Jain et al., 2005; Giddings, Kwong, Parikh-Patel, Bates, & Snipe,s 2012; Liu, Wang, Sherman, Cockburn & Deapen, 2016). CRC incidence is higher among SAs living in the U.S. than those living in South Asia (Flood et al., 2000; Prehn et al., 1999). Compared with NH Whites, metastatic CRC is more common among foreign-born Asians than among NH Whites (Ladabaum et al., 2014)
Screening for CRC cancer reduces mortality by allowing for the detection of CRC at earlier stages, as well as to identify and remove adenomatous polyps. Many tests are available for screening, such as fecal occult blood tests (FOBTs), flexible sigmoidoscopy, and colonoscopy. Screening with FOBT has been shown to reduce colorectal cancer mortality (Mandel, Church, Ederer, & Bond, 1999; Hardcastle et al., 1996; Kronborg, Fenger, Olsen, Jorgensen, & Sondergaard, 1996). Screening with more sensitive FOBTs, flexible sigmoidoscopy, colonoscopy, or combinations of these tests may reduce the burden of colorectal cancer even more (Bevan & Rutter, 2018). Based on this data, the US Preventive Services Task Force (USP-STF) recommends routine CRC screening for average-risk persons between the ages of 50 and 75 (USP-STF, 2018).
Information about CRC screening adherence among SAs is limited. One key reason is that most studies aggregate SA’s data with other Asian groups (Kandula, Wen, Jacobs, & Lauderdale, 2006). The limited data suggests that CRC screening adherence is significantly lower among SAs than in the US population as well as compared with other Asian American subgroups. Rates of screening adherence among SAs range from 25% (Glenn, Chawla, Surani, & Bastini, 2009) to 59% (Homayoon, Shahidi, & Cheung, 2013) and are lower than national screening estimates of 65% (CDC 2013). Studies comparing CRC screening among subgroups of Asian Americans suggest that SAs have lower rates compared to Chinese, Japanese, and Vietnamese individuals (Lee, Lundquist, Ju, Luo, & Townsend, 2011). In our work examining CRC screening among foreign-born SAs living in New Jersey, 54% were adherent (Manne, Steinberg, Delnevo, Ulpe, & Sorice, 2015). In NYC, a study found that 36% of SAs had ever had a colonoscopy screening or an up-to-date screening, which was significantly lower than other ethnic groups. SAs were 31% less likely to have ever received a colonoscopy screening compared to NH White participants (Patel, under review).
Limited data elucidating reasons why foreign-born SAs have a lower adherence suggest that less awareness about CRC screening, fewer perceived benefits of screening, more perceived barriers to screening, lower perceived risk for CRC, and more medical mistrust are associated with lower screening adherence (Manne et al., 2015; Menon et al., 2014). Acculturation and access to health care are also known correlates of low screening adherence: Foreign-born SAs who have resided fewer years in the US, have low English fluency, and do not visit a primary care provider regularly are less likely to be screened (Manne et al.,2015; Menon et al., 2014). Given the disparity in CRC screening and data suggesting that acculturation and attitudes about CRC screening may contribute to non-adherence, effective approaches to improve CRC screening are needed for SAs. Unfortunately, there are no behavioral interventions developed and evaluated to improve uptake of CRC screening among foreign-born SAs in the US. Promising approaches in other minority populations have facilitated access to healthcare through personalized counseling (Christie et al. 2008) or provided linguistically-and culturally-matched education about CRC screening in community settings and facilitated healthcare access by providing the fecal occult blood test (FOBT) (Carney et al., 2014; Maxwell et al., 2010). In non-minority populations, evidence-based interventions include personalized barriers counseling, matching with a preferred screening test, and implementation planning (Daskalakis et al., 2014; Myers et al., 2013; Myers et al., 2008; Ritvo et al., 2015).
The current study reports on the development, acceptability, and preliminary impact of a culturally-targeted 1:1 intervention delivered in English, Hindi, and Urdu, called Desi Sehat (roughly translated as ‘South Asian Health’). The Desi Sehat intervention was developed by the study team to address the unique needs of the foreign-born SA population, and content was guided by the Cultural Explanatory Model (Lu et al. 2012). We offered factual information and addressed attitudinal and practical barriers to CRC screening that is not available to the foreign-born SA population in New Jersey. The intervention was attentive to cultural beliefs and values, socioeconomic factors, and unique barriers to medical care in immigrant communities. These unique cultural beliefs included valuing privacy and modesty (Lawton, Ahmad, Hanna, Douglas, & Hallowell, 2006), prioritizing family over individual health (Johnson et al. 1999; Palmer, Thomas, McGregor, von Wagner, & Raine, 2015; Patel, Phillips-Caesar, & Boutin-Foster 2012), and cultural beliefs about cancer (Lawton et al. 2006; Nelson, Geiger, & Mangione, 2002). Barriers to medical care included low English proficiency, low income, not having a regular primary care provider, and transportation. Desi Sehat was delivered in Hindi and Urdu by a member of the SA community, included printed material focused on the experiences of SA community members with CRC screening, contained information about CRC screening uptake in the New Jersey SA community, and incorporated culturally-relevant screening benefits (e.g., importance to family of maintaining health) and barriers (e.g., maintaining modesty). To address modesty, we provided referral to gender-matched health care providers.
Our study had two aims. The first aim was to evaluate Desi Sehat’s feasibility, measured by recruitment rates, participation in the intervention sessions, retention, and acceptability. The second aim was to examine the intervention’s preliminary impact on the primary outcome, screening uptake at follow-up, and on the secondary outcomes of screening intentions, CRC screening knowledge, and attitudes about screening (i.e., screening benefits and barriers, perceived cancer risk and worry, normative influences). Exploratory analyses were conducted to evaluate whether baseline demographic and health care access, screening intentions, knowledge, and attitudes were associated with intervention response (e.g., screening uptake). To this end, we conducted a pilot study among foreign-born SAs from New Jersey who were non-adherent to CRC screening.
Materials and Methods
Intervention Development
The intervention content was guided by a culturally and linguistically-adapted workbook developed in weekly meetings between study investigators over a six month period. Content was guided by the Preventive Health Model (PHM) (Myers et al. 2008) and the Cultural Explanatory Model (Lu et al. 2012). The PHM was selected because it is widely-used in behavioral CRC screening research, addresses key reasons why individuals do not have CRC screening, and has guided effective interventions. Key PHM constructs are salience and coherence of engaging in the behavior, CRC risk, CRC worry, screening response efficacy, and external support for screening (normative influences from family and friends). The PHM constructs have been associated with CRC uptake in a number of studies (Hawley et al. 2008; Shokar, Carlson, & Weller, 2010) and the PHM model has successfully guided interventions to improve CRC screening (Myers et al. 2013; Myers et al. 2008). The Desi Sehat workbook addressed knowledge about CRC and its risk factors, described screening guidelines and options, reviewed commonly-endorsed barriers to screening, possible screening benefits, planning a CRC screening test, and benefits and barriers counseling.
As noted previously, the Cultural Explanatory Model (Lu et al. 2012) was used to tailor the intervention for the SA community because it outlines unique factors that should be included in efforts to engage immigrant communites. The model suggests that sensitivity to cultural beliefs and values, socioeconomic factors, and unique barriers to care should guide intervention development for immigrant communities (Lu et al., 2012). Cultural and linguistic adaptations to the workbook included: 1) translation of written materials into Hindi and Urdu; 2) experiences and narratives regarding screening from SA community members; 3) facts about CRC screening uptake in the New Jersey SA community; 4) culturally-relevant screening benefits (e.g., importance to family of maintaining health) and barriers (e.g., modesty) that were illustrated by narratives by members of the SA community. Cultural and linguistic adaptations by the health educator were: 1) delivery in Hindi or Urdu, if desired; 2) delivery by a member of the SA community, and; 3) screening referral to gender-matched provider, if desired.
Drafts of the surveys and workbook were shown to community-based organization stakeholders, who provided feedback on its content, ease of understanding, and quality of the cultural adaptation. An iterative process was used to make changes to the workbook. The final workbook had 33 pages with six sections (see Table 1) and was translated into Hindi and Urdu. During the 1:1 session, the health educator: 1) clarified knowledge items answered incorrectly on the quiz; 2) discussed what participants had heard about screening; 3) facilitated selection of top benefits and barriers; 4) assisted the participants in understanding and addressing each barrier, 5) assisted participants with scheduling tests; and 6) conducted follow-up phone calls. During follow-up calls, the interventionist reviewed progress on plans made during the session, reminded participants to obtain screening, offered assistance in scheduling tests, and discussed barriers to screening [Table 1].
Table 1.
Description of Desi Sehat
| Section | Overview of content | # Pages |
|---|---|---|
| What is CRC and are you at risk? | Information about CRC Key terms Risk factors for CRC Quiz on CRC facts |
6 |
| How does screening save lives? | Description of how screening reduces risk Reviews low adherence in New Jersey |
1 |
| Is our South Asian community on-schedule for screening? | Physician picture and recommendation to be screened | 1 |
| What are the screening tests, what tests are recommended, and what does each test entail? | Reviews test procedures and preparation Comparison of test procedures |
5 |
| What do people from our community say about screening, and what have you heard about it? | Sample experiences of SAs who chose screening Discussion prompt to ask participant what he/she has heard about screening |
1 |
| Steps to making a decision | What test participant is considering What test participant prefers Understanding attitudes about screening Selection of benefits and barriers from a list Selection of top two benefits and top two barriers Narratives from South Asian men and women regarding barriers they have overcome Discussion of ways to address barriers Rating of confidence in plan to address barrier |
13 |
| Steps to scheduling a test | Pathways to screening test for those who have medical insurance and those who do not Information about Medicare coverage List of free or low-cost screening in NJ Planning documents for those who have health insurance and those who do not Assistance with scheduling a test |
11 |
Participants and Procedures
Eligibility criteria were 1) between 50 and 75 years of age; 2) born in Bangladesh, India, Pakistan, Nepal or Sri Lanka; 3) resides in New Jersey; 4) does not have a first degree relative with CRC or a personal history of CRC, and; 5) able to speak and read English, Hindi, or Urdu. The Institutional Review Board of the corresponding author’s institution approved this study. Informed consent was received from all participants.
Recruitment took place between February 2016 and July 2017. Participants were recruited by 1) distributing study information at local community, ethnic social groups, religious organizations, and professional organizations; 2) posting flyers at community ethnic grocery stores, restaurants, and places of worship; 3) distributing flyers at ethnic celebrations and health fairs, and; 4) collaborating with outreach staff at ten community-based organizations for in-person recruitment. Potential participants were approached by staff at these sites and eligibility was confirmed. Participants who saw study flyers in the community contacted the study staff by telephone and eligibility was determined.
All surveys and intervention materials were translated into Hindi and Urdu by CQ Fluency. Surveys were completed in person. Average time to complete the baseline survey was 45 minutes and 20 minutes for the follow-up. Participants received $25 for baseline,$25 for the 1:1 session, and $25 for the follow-up survey. After the baseline survey was completed, the 1:1 intervention session was scheduled. For 98% of participants, baseline surveys were completed at the time of recruitment. Most sessions were completed in the participant’s home. After the intervention, a call was scheduled to discuss progress and to troubleshoot remaining barriers. Participants were contacted to complete the follow-up survey four months after the baseline survey.
Intervention training
The intervention was delivered by a community health educator who spoke Hindi and Urdu. This educator was trained in the intervention by the authors (SM, VT). Sessions were audiotaped and reviewed for adherence to the intervention workbook. Regular supervision was provided. The interventionist completed a summary of the session, benefits and barriers counseling responses, screening test selected, and screening plans. Summaries were used in the follow-up phone call and analyzed descriptively to assess intervention feasibility.
Primary outcome measure: CRC screening
Two variables were calculated: 1) whether or not the participant has ever had CRC screening and; 2) whether the participant was currently on schedule for CRC screening (e.g., yearly for FOBT, every 5 years for sigmoidoscopy, or every 10 years for colonoscopy). Participants who never heard of any of the screening tests were categorized as never having screening and not on schedule for screening.
Secondary outcome measures
Screening intention
Four questions assessed likelihood of having a screening test of any kind, talking to your doctor about CRC screening tests, making an appointment for a test, and following through with the test if the doctor recommends it (Manne et al. 2015). Items were rated on a 10-point Likert scale (1= not at all likely, 10 = extremely likely). An average score was calculated. For analyses of this scale, we presented data on participants who did not have a screening test at follow-up (alpha = .91 at baseline, .95 at follow-up).
Screening benefits and barriers
A scale developed by Manne and colleagues (2002) consisted of 14 benefits and 13 barriers. Statements were rated on a 5-point Likert scale (5 = strongly agree, 1 = strongly disagree). One benefit item, ‘I think having colorectal cancer screening can protect my health,’ evidenced a low item-total correlation (−.45) and was excluded from the benefits scale. An average was computed. Reliability for benefits was .82 at baseline and .92 at follow up. Reliability for barriers was .80 at baseline and .84 at follow-up.
Cognitive and affective risk
Two items assessed cognitive risk: ‘I may get colorectal cancer if I don’t have colorectal cancer screening,’ and ‘If I don’t have colorectal cancer screening, I would feel very vulnerable to getting colorectal cancer in my lifetime. ‘Affective risk items were: ‘I am worried about having colorectal cancer screening tests,’ and ‘I worry about having colorectal cancer’ (1= strongly disagree, 5 =strongly agree). Reliability was .73 for cognitive risk at baseline and .87 at follow-up. Reliability was low for affective risk (.30 at baseline and .47 at follow-up), likely due to one item assessing worry about cancer and one item assessing worry about screening. Thus, the two items were analyzed separately.
Social norms
Social norms were perceived beliefs about family and friend support for screening and the desire to comply with family and friends’ support for CRC screening. Four items were included from Vernon and colleagues (1997) and were rated on a 5-point Likert-type scale (5 = strongly agree, 1 = strongly disagree). An average score was calculated. Higher scores indicate higher levels of perceived social influence. Reliability was .73 at baseline and .65 at follow-up.
Demographics and health care access
At baseline, participants completed measures of demographic characteristics (gender, age, marital status, education, income). They also completed measures of health care access including type of insurance and whether or not the participant visited a primary care provider yearly.
Acculturation
At baseline, participants completed Palmer and colleagues’ (2007) acculturation scale, a 17-item measure with three subscales: 1) behaviors suggesting greater acculturation in the host community (e.g., wearing Western style clothing); 2) attitudes indicative of greater or lesser acculturation (e.g., fears of discrimination), and; 3) behaviors associated with the society of origin (e.g., use of Asian media). All three subscales were transformed to a 0–100 scale and summed. A higher score indicated greater acculturation. Reliability was .85.
Evaluation
Six Likert-rated items assessed the acceptability of the program: was the information easy to understand, did it help to decide whether screening was right for you, was it helpful in increasing understanding of the advantages and disadvantages of screening, was there pressure to have a test, did it help overcome barriers to screening, and did it increase confidence about the steps to schedule a screening (1= strongly disagree, 7 = strongly agree). An average was computed. Reliability was .73. Open-ended questions solicited additional feedback.
Statistical Analysis Plan
To address the first aim, we calculated recruitment rates, participation in the intervention sessions, retention, and acceptability. We calculated descriptive statistics using SPSS using the treatment evaluation scale to measure acceptability. To address the second aim, we calculated a Chi-square test to evaluate the changes in the primary outcome of CRC screening uptake. We conducted an intent-to-treat analysis of all participants that used the self-reported outcomes and assumed participants without outcome data at follow-up had a “not screened” status. For the secondary outcomes of knowledge and attitudes, paired t-tests were calculated to evaluate changes in CRC knowledge, benefits, barriers, risk, norms and intentions from baseline to follow-up. For the exploratory analysis, the same approach of paired t-tests was adopted, with the intent-to-treat value for CRC screening uptake as the outcome. Because this is a pilot and feasibility study with a small sample size, we did not adopt a more sophisticated approach to the analyses (e.g., multivariate logistic regression)
Results
Feasibility
Enrollment
Of the 345 individuals approached, 171 were ineligible, 119 were ineligible due to age (less than 50 years, greater than 75 years), and 52 were ineligible for other reasons, including prior screening. Of the 174 eligible persons, 81 refused and 93 consented and completed the survey. Acceptance was 53.4%. Figure 1 shows the CONSORT diagram.
Figure 1.
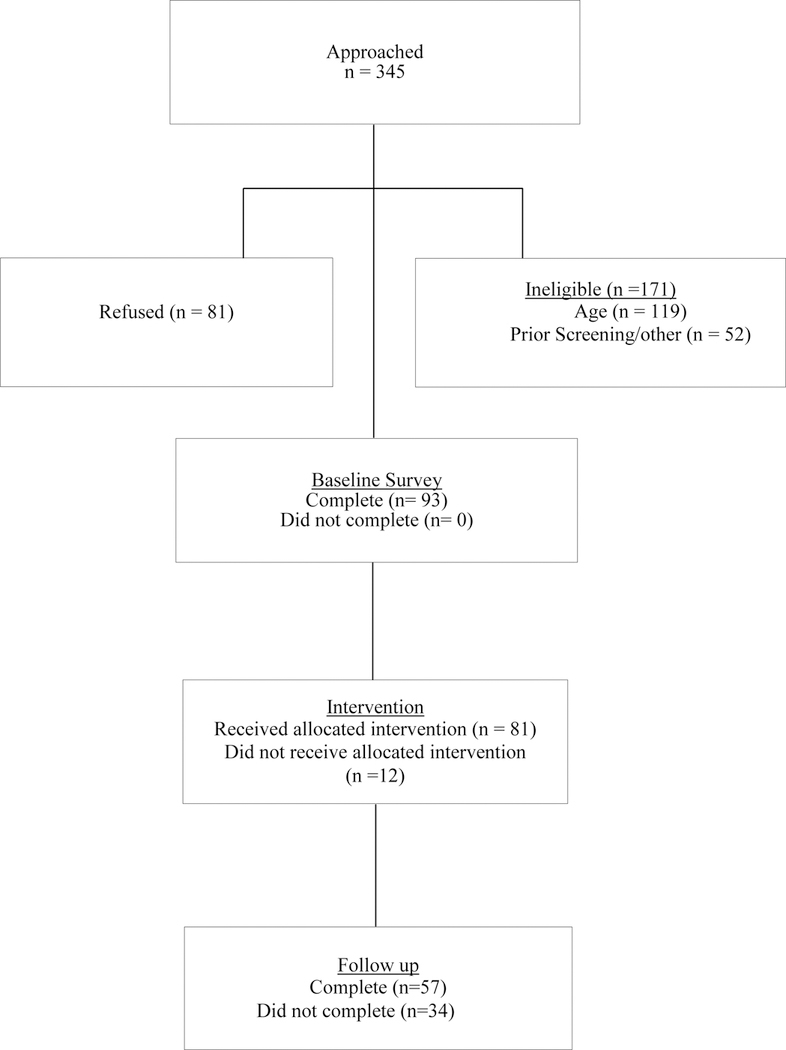
CONSORT diagram of the Desi Sehat pilot intervention
Session attendance and survey completion
Eighty-one participants (87.1%) completed the intervention session, and 57 participants (61.3%) completed follow-up surveys. Most common reasons for not completing follow-up surveys included dropping before the intervention session (n = 12) or not responding via telephone over several months (n = 18). We found no differences between those who completed follow-up surveys and those who did not with regard to age, income, education, sex, marital status, country of origin, acculturation, or whether or not a primary care provider was seen in the past year.
Descriptive information
Table 2 shows descriptive information on the study sample, which consisted of 48 men and 45 women. Seventy-six percent were born in India and 24% were born in Pakistan. Sixty-one percent resided in the US for more than 10 years. Primary languages were Hindi (64%) and Urdu (29%). Average age was 65 years (range = 50.7 – 75). Eighty percent carried health insurance, and 85% reported that they saw a primary care provider regularly. Approximately 35% did not complete a high school degree, and 80% reported a household income of less than $15,000/year.
Table 2.
Sample characteristics
| Variable | N | % |
|---|---|---|
| Sex | ||
| Male | 48 | 51.6 |
| Female | 45 | 48.4 |
| Country of birth | ||
| India | 71 | 76.3 |
| Pakistan | 22 | 23.7 |
| Primary Language | ||
| Hindi | 81 | 87.1 |
| Urdu | 12 | 12.9 |
| Years in US | ||
| < 1 | 9 | 9.7 |
| 1–3 | 3 | 3.2 |
| 4–5 | 7 | 7.5 |
| 6–10 | 17 | 18.3 |
| >10 | 57 | 61.3 |
| Education | ||
| ≤ 11th grade | 26 | 28.0 |
| High school, no diploma | 6 | 6.5 |
| High school degree | 13 | 14.0 |
| Some college | 13 | 14.0 |
| Associate degree | 4 | 4.3 |
| Bachelor’s degree | 28 | 29.0 |
| Master’s degree | 8 | 8.6 |
| Professional school degree | 2 | 2.2 |
| Employment status | ||
| Employed for wages | 18 | 19.4 |
| Self-employed | 2 | 2.2 |
| Homemaker | 21 | 22.6 |
| Retired | 39 | 41.9 |
| Unable to work | 13 | 14.0 |
| Marital Status | ||
| Married | 75 | 80.6 |
| Single | 2 | 2.2 |
| Divorced/widowed | 15 | 16.2 |
| Insurance | ||
| Private | 15 | 16.1 |
| Medicare | 25 | 26.1 |
| Medicaid | 34 | 36.6 |
| Uninsured | 18 | 19.4 |
| Missing | 1 | 1.1 |
| Income | ||
| < $10,000 | 59 | 63.4 |
| $10,000–$15,000 | 7 | 7.5 |
| $15,000–$20,000 | 8 | 8.6 |
| >$20,000 – $35,000 | 4 | 4.3 |
| $35,000–$50,000 | 4 | 4.3 |
| $50,000–$75,000 | 4 | 4.3 |
| Age (M and SD) | 65.3 | 6.9 |
| Primary care provider (yes) | 799 | 84.9 |
| Acculturation scale (M and SD) | 49.4 | 15.9 |
Acceptability
Overall, the intervention was evaluated positively (average of 5.45 on a 7-point scale), with participants reporting that: the material was easy to understand (M = 6.49, SD = .86), helped them decide about screening (M = 6.28, SD = 1.08), they felt little pressure to have a test (M = 6.22, SD = 1.10), it helped them overcome barriers to screening (M = 6.30, SD = 1.05), and it enhanced their confidence in understanding the steps to obtaining a test (M = 6.40, SD = 1.07). The highest-rated item indicated that participants’ understanding of the advantages and risks of screening increased (M = 6.51, SD = .81).
In terms of open-ended feedback, suggested changes included providing the FIT/FOBT test, using video technology rather than a paper workbook, and increasing the session length. Most qualitative feedback was positive, including the explanations about CRC and screening options were clear, the interaction with the health educator was positive, and the workbook was simple, user-friendly, and colorful.
Intervention response: Changes in CRC screening uptake
Of the 57 participants completing the follow-up survey, 28 (49%) completed screening and 29 (51%) did not. When participants who did not complete the follow-up survey were included in the analyses as ‘not screened,’ the percentage of screened participants was 30%.
Of the 28 completing CRC screening, 18 participants had an FIT/FOBT, nine participants had a colonoscopy, and one participant had an FIT and subsequent colonoscopy. Among the remaining 29 participants completing the follow-up survey, 13 (55%) reportedly scheduled a test.
Intervention response: Changes in knowledge and attitudes
Table 3 illustrates the pre-to post-test scores, the results of the paired t-tests, and effect sizes. Knowledge increased significantly, t (56) = −11.39, p < .001. Perceived screening barriers decreased significantly, t (55) = 4.69, p < .001. Worry about screening, t (56) = 4.42, p < .001 and worry about CRC, t (56) = 2.54, p < .05, also decreased significantly from pre-to post-test. Changes in screening benefits (t (57) = .42, p =.66), perceived cognitive risk (t (57) = .12, p = .90), and normative influence (t (56) = 1.52, p = .13) were not significant. Screening intentions declined, but this change was not significant (t (24) = 1.24, p = .23) [Table 3 near here]. Awareness of CRC screening tests increased from baseline (68.4% had heard of any of the tests) to follow-up (91.2% had heard of any of the tests), which is a 23% increase. This change was not significant (Chi-square = 2.05, p = .31).
Table 3.
Means, standard deviations, and t-tests for Pre-to Post-intervention
| Intervention outcomes | Baseline | Follow-up | t-test | Cohen’s d | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Screening intention | 8.18 | 1.38 | 7.41 | 2.66 | 1.34 | 0.36 |
| Knowledge (% correct) | 28.95 | 30.95 | 81.87 | 22.77 | 11.39*** | 1.95 |
| Benefits | 4.03 | 0.31 | 4.06 | 0.54 | −0.44 | 0.07 |
| Barriers | 3.02 | 0.52 | 2.59 | 0.55 | 4.69*** | 0.82 |
| Perceived cognitive risk | 3.49 | 0.70 | 3.47 | 0.90 | 0.12 | 0.02 |
| Worry about screening | 2.74 | 0.86 | 2.07 | 0.82 | 4.42*** | 0.79 |
| Worry about CRC | 3.25 | .85 | 2.86 | 1.08 | 2.54* | 0.40 |
| Normative influence | 4.09 | 0.69 | 3.92 | 0.61 | 1.52 | 0.28 |
Notes. CRC = colorectal cancer.
p < .05
p < .001.
The effect sizes are shown in Table 3. The significant change in worry about CRC represented a medium strength effect (Cohen’s d = 0.40), and large effects (i.e., Cohen’s d values of 0.80 and above) (Cohen, 1977) were observed for changes in knowledge, screening barriers, and worry about screening
Intervention Response: Exploratory Analysis of Predictors
Using t-tests for continuous variable and chi-square analyses for dichotomous variables, we compared demographic characteristics (age, gender, education, income), health care access (insurance status, regular primary care), acculturation, and baseline knowledge and attitudes of participants who completed CRC screening at follow-up with those who did not. With regard to demographics, there were no differences based on age, gender, education, income, or marital status. With regard to health care access, there were no differences with regard to insurance status or seeing a primary care annually. Acculturation was not significantly associated with screening uptake at follow-up. With regard to knowledge and attitudes, the only significant comparison was for baseline knowledge. Those who completed CRC screening reported significantly higher baseline knowledge than those who did not (t (55) = 2.71, p < .05). Screening uptake was not significantly associated with baseline intention, benefits, barriers, perceived risk, worry about screening, worry about CRC, and normative influence.
Discussion
Uptake of CRC screening among foreign-born SAs in New Jersey represents a health disparity, but no interventions have been developed to facilitate screening in this population. Our goal was to develop and pilot test a culturally-appropriate intervention to improve CRC screening uptake in this population. Our results indicate that Desi Sehat was positively evaluated by our participants and sessions were well-attended. Desi Sehat resulted in a CRC screening test uptake of 30.11% in intent-to-treat analyses. Importantly, its impact on knowledge and attitudes about CRC screening was promising in that participants reported increases in knowledge and awareness of screening tests and fewer barriers to screening.
The first aim was to evaluate the feasibility of Desi Sehat. Acceptance (53.4%) was lower than other community-based intervention trials focusing on immigrant Asian populations. Acceptance rates in prior studies have ranged from 78.3% to 91.5% (Tong et al., 2017; Tu et al., 2006). Comfort with focusing on CRC screening in the immigrant SA community may have contributed to lower uptake. However, intervention attendance was high (nearly 90%). This is comparable to other intervention studies with immigrant Asians (95% −100%) (Maxwell et al,. 2010; Nguyen et al., 2015; Tong et al., 2017). More than half of our participants did not complete follow-up surveys (58.7%). This rate is lower than other studies using community-based recruitment among Asians (77–98%) (Maxwell, et al., 2010; Jo et al., 2017; Tong et al., 2017; Cauresma et al., 2018). The lower follow-up rate shown in this study may be due to difficulty following up with participants who were recruited from community organizations who did not regularly attend events at the organizations. Our target population (primarily older immigrants) may pose follow-up challenges, including the inability to locate the participant by telephone. In future work, follow-up will include obtaining more complete contact information and enhancing engagement with participants over the follow-up period. Overall, participants positively evaluated the intervention as informative, easy to understand, and outlined steps to screening.
The second aim was to examine the impact of Desi Sehat on the primary outcome of CRC screening uptake and on the secondary outcomes of screening-related knowledge and attitudes. Intent-to-treat analyses indicated that Desi Sehat’s impact on self-reported CRC screening uptake was approximately 30%. This increase in screening uptake is similar to (25–30%; Maxwell et al., 2010) or higher than (9%; Cuaresma et al., 2018; 18%; 10.3%; Jo et al., 2017; Nguyen et al., 2017; 13%; Tong et al., 2017) studies focusing on other Asian populations. However, our CRC screening uptake was lower than other intervention studies that also targeted other Asian American groups (56%; Nguyen et al., 2015; 69.5%; 69.5%; Tu et al., 2006). There are several possible reasons that our screening uptake may not have been as high as these studies. Tu and colleagues’ study (2006) provided FOBT cards to participants and targeted patients seen in a primary care setting. Both of these additions would facilitate screening completion. Nguyen and colleagues (2015) implemented a more intensive intervention that included a second 60 to 90 minute in-person session for participants who did not have screening after three months, as well as additional calls and in-person visits to participants to remind them to have screening, assist with appointments, and to accompany to appointments. They also had a longer-term follow-up at six months, which may have resulted in higher uptake.
Desi Sehat’s impact on the secondary outcomes of knowledge and attitudes was promising: Desi Sehat resulted in increases in knowledge and reductions in perceived barriers to screening, worry about screening tests, and worry about CRC. The effect sizes were of a medium to large magnitude, which indicates that the intervention effectively increased knowledge and reduced concerns about cancer and screening. Prior CRC intervention studies among other Asian groups have found that interventions improve knowledge (e.g., Jo et al., 2017; Tong et al., 2017) and thus our findings are consistent with prior work. In addition, our finding that participants reported a decrease in worry about CRC is consistent with a prior study (Nguyen et al., 2015). If our findings are replicated in a larger randomized trial, our finding advance prior research by suggesting that CRC screening interventions among South Asian immigrants may reduce perceived screening barriers and worry about screening. The impact of behavioral interventions on barriers and screening worry has not been widely studied in the Asian immigrant population. The one intervention study among Filipino Americans that assessed the impact on perceived barriers did not illustrate a reduction in perceived barriers (Maxwell et al., 2011).
Finally, it is interesting to note that our preliminary analysis only resulted in one significant finding – that those who completed CRC screening reported significantly higher knowledge than those who did not complete screening. This finding has not been reported in other studies and suggests that future work using the Desi Sehat intervention may need to focus more intensively on persons who have less knowledge about CRC and screening. Prior studies have pointed to a range of moderators of screening uptake that we did not illustrate in the present study, including older age, living in the US for more than 10 years, and having a higher level of education (Cuaresma et al., 2018). Screening uptake was not significantly associated with gender, baseline intention, benefits, barriers, perceived risk, worry about screening, worry about CRC, and normative influence.
Limitations
The limitations of this study include the fact that it was not a randomized controlled clinical trial, the small sample size, and the fact that the intervention was delivered by one community health educator. Results may not be generalizable to all immigrant SAs, particularly those who speak other SA languages. We did not confirm self-reported screening tests. Finally, retention was biased towards participants who felt more positive about screening: Those who completed the follow-up reported significantly higher screening benefits (p < .05) and more worry about CRC (p < .01).
Future Directions and Conclusions
Future work should adopt a randomized control trial design and a larger sample so that treatment effects can be compared with usual care and treatment moderators can be better assessed. Our findings indicate that interventions targeting South Asian immigrants may achieve higher levels of recruitment and retention if they used targeted recruitment strategies (e.g., community partners who recruit study participants) and enhanced engagement strategies (e.g., more contact before the follow-up survey to facilitate follow-up survey return and anticipating travel to India during the follow-up period). Further, based on studies of other Asian American immigrant populations that have shown stronger effects, Desi Sehat’s efficacy might be bolstered if we provided easier access to screening (e.g., providing a FIT kit). Because persons with low knowledge were less likely to respond to Desi Sehat, targeting persons with very low levels of knowledge for more specialized or intensive navigation may enhance its impact. Finally, since knowledge, barriers to screening, and worry about screening and CRC were associated with screening uptake, Desi Sehat’s impact may be enhanced if there was a greater focus on these constructs.
In conclusion, as the older SA populations in the US continue to grow, both through migration and aging immigrant populations, addressing CRC screening disparities will continue to be important. Our study has demonstrated preliminary acceptability and efficacy, and warrants future study to examine its effectiveness in improving CRC screening for this population.
Acknowledgments
We would like to thank the community-based organizations who so generously worked with us on this study, as well as Victoria Taylor, Ph.D., who worked closely with us on the development of this intervention.
Funding details: This work was supported by the National Cancer Institute under Grant CA072720–18S4 (Community Health Education Supplement to P50) and funds from Rutgers Cancer Institute of New Jersey.
Footnotes
Declaration of interest statement
The authors declare that they have no potential conflicts of interest.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Contributor Information
Sharon L. Manne, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, sharon.manne@rutgers.edu.
Nadia Islam, New York University School of Medicine, New York, NY, Nadia.islam@nyumc.org.
Sara Frederick, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, sf503@cinj.rutgers.edu.
Usman Khan, Rutgers Robert Wood Johnson Medical School, usmanakhan@gmail.com.
Sunanda Gaur, Rutgers Robert Wood Johnson Medical School, gaursu@rwjms.rutgers.edu.
Anam Khan, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, ak866@cinj.rutgers.edu.
References
- Asian American Federation of New York Census Information Center. “Census Profile: New Jersey’s Asian American Population 2004.” http://www.aafny.org/cic/briefs/newjersey.pdf.
- Bevan R, & Rutter MD 2018. Colorectal Cancer Screening-Who, How, and When? Clin Endosc, 51(1), 37–49. 10.5946/ce.2017.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney PA, Lee-Lin F, Mongoue-Tchokote S, Mori M, Leung H, Lau C, Le TD, and Lieberman DA. 2014. “Improving colorectal cancer screening in Asian Americans: Results of a randomized intervention study.” Cancer 120 (11):1702–12. 10.1002/cncr.28640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuaresma CF, Sy AU, Nguyen TT, Ho RCS, Gildengorin GL, Tsoh JY,Stewart SL 2018. Results of a lay health education intervention to increase colorectal cancer screening among Filipino Americans: A cluster randomized controlled trial. Cancer, 124 Suppl 7, 1535–1542. 10.1002/cncr.31116 [DOI] [PubMed] [Google Scholar]
- Center for Disease Control (CDC). 2013. Vital signs: colorectal cancer screening test use — United States, 2012. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control 62:881–888. [PMC free article] [PubMed] [Google Scholar]
- Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, and Jandorf L. 2008. “A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities.” J Natl Med Assoc 100 (3):278–84. [DOI] [PubMed] [Google Scholar]
- Cohen J 1977. Statistical power analysis for the behavioral sciences Routledge. [Google Scholar]
- Daskalakis C, Vernon SW, Sifri R, DiCarlo M, Cocroft J, Sendecki JA, and Myers RE. 2014. “The effects of test preference, test access, and navigation on colorectal cancer screening.” Cancer Epidem Biomar 23 (8):1521–8. 10.1158/1055-9965.epi-13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood DM, Weiss NS, Cook LS, Emerson JC, Schwartz SM, & Potter JD 2000. Colorectal cancer incidence in Asian migrants to the United States and their descendants. Cancer Causes Control, 11(5), 403–411. [DOI] [PubMed] [Google Scholar]
- Giddings BH, Kwong SL, Parikh-Patel A, Bates JH, & Snipes KP. 2012. “Going against the tide: increasing incidence of colorectal cancer among Koreans, Filipinos, and South Asians in California, 1988–2007.” Cancer Cause Control 23 (5):691–702. 10.1007/s10552-012-9937-6. [DOI] [PubMed] [Google Scholar]
- Glenn BA, Chawla N, Surani Z, Bastani R 2009. Rates and sociodemographic correlates of cancer screening among South Asians. Journal of Community Health 34(2):113–121. [DOI] [PubMed] [Google Scholar]
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. 1996. Randomised controlled trial of faecal occult‐blood screening for colorectal cancer. Lancet 348: 1472–1477. [DOI] [PubMed] [Google Scholar]
- Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, and Kneuper S. 2008. “Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients.” Med Care 46 (9 Suppl 1):S10–6. 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- Homayoon B, Shahidi NC, & Cheung WY (2013). Impact of asian ethnicity on colorectal cancer screening: a population-based analysis. Am J Clin Oncol, 36(2), 167–173. 10.1097/COC.0b013e3182439068 [DOI] [PubMed] [Google Scholar]
- Jain RV, Mills PK, & Parikh-Patel A 2005. Cancer incidence in the south Asian population of California, 1988–2000. J Carcinog, 4, 21. 10.1186/1477-3163-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AM, Nguyen TT, Stewart S, Sung MJ, Gildengorin G, Tsoh JY, Kagawa-Singer M 2017. Lay health educators and print materials for the promotion of colorectal cancer screening among Korean Americans: A randomized comparative effectiveness study. Cancer, 123(14), 2705–2715. 10.1002/cncr.30568 [DOI] [PubMed] [Google Scholar]
- Johnson JL, Bottorff JL, Balneaves LG, Grewal S, Bhagat R, Hilton BA, and Clarke H. 1999. “South Asian womens’ views on the causes of breast cancer: images and explanations.” Patient Educ Couns 37 (3):243–54. [DOI] [PubMed] [Google Scholar]
- Kandula NR, Wen M, Jacobs EA, & Lauderdale DS 2006. Low rates of colorectal, cervical, and breast cancer screening in Asian Americans compared with non-Hispanic whites: Cultural influences or access to care? Cancer, 107(1), 184–192. 10.1002/cncr.21968 [DOI] [PubMed] [Google Scholar]
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O 1996. Randomised study of screening for colorectal cancer with faecal‐occult‐blood test. Lancet 348: 1467–1471. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Clarke Christina A., Press David J., Mannalithara Ajitha, Myer Parvathi A., Cheng Iona, and Gomez Scarlett Lin. 2014. “Colorectal Cancer Incidence in Asian Populations in California: Effect of Nativity and Neighborhood-Level Factors.” Am J Gastroenterol 109 (4):579–88. 10.1038/ajg.2013.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton J, Ahmad N, Hanna L, Douglas M, and Hallowell N. 2006. “‘I can’t do any serious exercise’: barriers to physical activity amongst people of Pakistani and Indian origin with Type 2 diabetes.” Health Educ Res 21 (1):43–54. 10.1093/her/cyh042. [DOI] [PubMed] [Google Scholar]
- Lee HY, Lundquist M, Ju E, Luo X, Townsend A 2011. Colorectal cancer screening disparities in Asian Americans and Pacific Islanders: which groups are most vulnerable? Ethnicity & Health 16(6):501–518. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang Y, Sherman R, Cockburn M LL, Deapen D 2016. Cancer in Los Angeles County: Trends by race/ethnicity, 1976–2006
- Lu M, Moritz S, Lorenzetti D, Sykes L, Straus S, and Quan H. 2012. “A systematic review of interventions to increase breast and cervical cancer screening uptake among Asian women.” BMC Public Health 12:413. 10.1186/1471-2458-12-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel JS, Church TR, Ederer F, Bond JH 1999. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 91: 434–437. [DOI] [PubMed] [Google Scholar]
- Manne S, Markowitz A, Winawer S, Meropol NJ, Haller D, Rakowski W, Babb J, and Jandorf L. 2002. “Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer.” Health Psychol 21 (1):3–15. [PubMed] [Google Scholar]
- Manne S, Steinberg MB, Delnevo C, Ulpe R, and Sorice K. 2015. “Colorectal Cancer Screening Among Foreign-born South Asians in the Metropolitan New York/New Jersey Region.” J Commun Health 40 (6):1075–83. 10.1007/s10900-015-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AE, Bastani R, Danao LL, Antonio C, Garcia GM, and Crespi CM. 2010. “Results of a community-based randomized trial to increase colorectal cancer screening among Filipino Americans.” Am J Public Health 100 (11):2228–34. 10.2105/ajph.2009.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AE, Crespi CM, Danao LL, Antonio C, Garcia GM, & Bastani R 2011. Alternative approaches to assessing intervention effectiveness in randomized trials: application in a colorectal cancer screening study. Cancer Causes Control, 22(9), 1233–1241. 10.1007/s10552-011-9793-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon U, Szolacha L,Prabhughate A, & Kue J 2014. Correlats of Colorectal cancer screening among South Asian immigrants in the United States. Cancer Nursing, 37, E19–27. [DOI] [PubMed] [Google Scholar]
- Myers RE, H. Bittner-Fagan C, Daskalakis R, Sifri SW, Vernon J, Cocroft M, Dicarlo N, Katurakes A, and Andrel J 2013. “A randomized controlled trial of a tailored navigation and a standard intervention in colorectal cancer screening.” Cancer Epidem Biomar 22 (1):109–17. 10.1158/1055-9965.epi-12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RE, Hyslop T, Sifri R, Bittner-Fagan H, Katurakes NC, Cocroft J, Dicarlo M, and Wolf T. 2008. Tailored navigation in colorectal cancer screening. Med Care 46 (9 Suppl 1):S123–31. 10.1097/MLR.0b013e31817fdf46. [DOI] [PubMed] [Google Scholar]
- Nelson K, Geiger AM, and Mangione CM. 2002. Effect of health beliefs on delays in care for abnormal cervical cytology in a multi-ethnic population. J Intern Med 17 (9):709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Bang H., Stewart Susan L., Nguyen Tung T., Ngoc Bui-Tong, and McPhee Stephen J.. 2015. Effectiveness of Lay Health Worker Outreach in Reducing Disparities in Colorectal Cancer Screening in Vietnamese Americans. Am J Public Health 105 (10):2083–9. 10.2105/AJPH.2015.302713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Tung T., Tsoh Janice Y., Woo Kent, Stewart Susan L., Le Gem M., Burke Adam, Gildengorin Ginny, et al. 2017. Colorectal Cancer Screening and Chinese Americans: Efficacy of Lay Health Worker Outreach and Print Materials. Am J Prev Med 52 (3):e67–e76. 10.1016/j.amepre.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer B, Macfarlane G, Afzal C, Esmail A, Silman A, and Lunt M. 2007. “Acculturation and the prevalence of pain amongst South Asian minority ethnic groups in the UK.” Rheumatology (Oxford, England) 46 (6):1009–14. 10.1093/rheumatology/kem037. [DOI] [PubMed] [Google Scholar]
- Palmer CK, Thomas MC, McGregor LM, von Wagner C, and Raine R. 2015. “Understanding low colorectal cancer screening uptake in South Asian faith communities in England--a qualitative study.” BMC Public Health 15:998. 10.1186/s12889-015-2334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Kranick J, Manne S, Shah K, Raveis V, Ravenell J, Yi S, Kwon S, Islam N Using a Population Health Equity Approach to Document Ongoing Disparities in Colorectal Cancer Screening in New York City South Asian Communities. Under review [DOI] [PMC free article] [PubMed]
- Patel M, Phillips-Caesar E, and Boutin-Foster C. 2012. “Barriers to lifestyle behavioral change in migrant South Asian populations.” J Immigr Minor Health 14 (5):774–85. 10.1007/s10903-011-9550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn A,LS, Clarke C, Packel L, Lum R, Lui S, Harper C, Lee M, Glaser S, West D 1999. Cancer incidence In Chinese, Japanese, and Filipinos In the US and Asia 1988—1992 [Google Scholar]
- Ritvo PG, Myers RE, Paszat LF, Tinmouth JM, McColeman J, Mitchell B, Serenity M, and Rabeneck L. 2015. “Personal navigation increases colorectal cancer screening uptake.” Cancer Epidem Biomar 24 (3):506–11. 10.1158/1055-9965.epi-14-0744. [DOI] [PubMed] [Google Scholar]
- Shokar NK, Carlson CA, and Weller SC. 2010. “Informed decision making changes test preferences for colorectal cancer screening in a diverse population.” Ann Fam Med 8 (2):141–50. 10.1370/afm.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Elisa K., Nguyen Tung T., Lo Penny, Stewart Susan L., Gildengorin Ginny L., Tsoh Janice Y., Jo Angela M., et al. 2017. “Lay health educators increase colorectal cancer screening among Hmong Americans: A cluster randomized controlled trial.” Cancer 123 (1):98–106. 10.1002/cncr.30265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu SP, Taylor V, Yasui Y, Chun A, Yip MP, Acorda E, Li L, and Bastani R. 2006. “Promoting culturally appropriate colorectal cancer screening through a health educator: a randomized controlled trial.” Cancer 107 (5):959–66. 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. “2010. Census Data” https://www.census.gov/2010census/data/.
- United States Preventative Services Task Force. Colorectal cancer screening guidelines 2015. Retreived May 31, 2018 https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening
- Vernon SW, Myers RE, and Tilley BC. 1997. “Development and validation of an instrument to measure factors related to colorectal cancer screening adherence.” Cancer Epidem Biomar 6 (10):825–32. [PubMed] [Google Scholar]


