Abstract
The classical theory of cortical systematic variation has been independently described in reptiles, monotremes, marsupials and placental mammals, including primates, suggesting a common bauplan in the evolution of the cortex. The Structural Model is based on the systematic variation of the cortex and is a platform for advancing testable hypotheses about cortical organization and function across species, including humans. The Structural Model captures the overall laminar structure of areas by dividing the cortical architectonic continuum into discrete categories (cortical types), which can be used to test hypotheses about cortical organization. By type, the phylogenetically ancient limbic cortices ─ which form a ring at the base of the cerebral hemisphere ─ are agranular if they lack layer IV, or dysgranular if they have an incipient granular layer IV. Beyond the dysgranular areas, eulaminate type cortices have six layers. The number and laminar elaboration of eulaminate areas differs depending on species or cortical system within a species. The construct of cortical type retains the topology of the systematic variation of the cortex and forms the basis for a predictive Structural Model, which has successfully linked cortical variation to the laminar pattern and strength of cortical connections, the continuum of plasticity and stability of areas, the regularities in the distribution of classical and novel markers, and the preferential vulnerability of limbic areas to neurodegenerative and psychiatric diseases. The origin of cortical types has been recently traced to cortical development, and helps explain the variability of diseases with an onset in ontogeny.
Keywords: limbic cortex, cortical hierarchies, homology, phylogeny, brain pathology, glia
1. Introduction
Neuroscience is a relative newcomer to the era of big data, preceded by genetics, molecular biology and cancer biology (Weinberg 2014). But in the era of ‘big data’ novel brain markers obtained from mice to humans have already yielded a bewildering amount of information. New cellular, molecular and gene expression patterns of the cerebral cortex have supplanted classical architectonic methods to stain neurons and glia (Campbell 1905; Brodmann 1909/1999; von Economo 1927/2009; Bailey and von Bonin 1951; Braak 1980). Novel markers have helped to sort out cortical areas with a higher degree of confidence (Ding et al. 2016; Glasser et al. 2016; Palomero-Gallagher and Zilles 2017; Zilles and Palomero-Gallagher 2017; Palomero-Gallagher and Zilles 2018).
The accumulation of big data in neuroscience, particularly in imaging studies [e.g., Glasser et al. (2016)], has largely proceeded outside a theoretical framework outpacing efforts to organize and interpret them. What do the novel brain data offer for understanding the organization of the cerebral cortex? We argue that recent findings obtained by mining big databases support a classical and hitherto largely overlooked theoretical framework. The fundamental principle of this framework is that the built-in architecture of the cortex varies systematically along the cortical landscape. New data support this classical principle and can be subsumed under it. Below we provide evidence that the built-in architecture of the cortex provides a theory that has led and continues to lead to several testable predictions about cortical microstructure, connections, plasticity and vulnerability to disease. The origin of the systematic variation of the cortex can be traced to development in ontogeny and evolution. In Box 1 we list the most relevant terms.
Box 1. Definitions of main concepts used in the text.
| Allocortex: Ancestral part of the cerebral cortex, which includes the hippocampal formation (archicortex) and the primary olfactory cortex (paleocortex). |
| Cortical area: A portion of the cerebral cortex with distinct cyto-, myelo-, and chemoarchitectonic features. |
| Cortical type: Describes a category of cortical areas with comparable laminar differentiation, regardless of placement within a cortical sensory, high order association or motor system. |
| Eulaminate cortex: Neocortical areas with well-developed layer IV. |
| Isocortex: A term that also defines neocortex with six layers (eulaminate). |
| Limbic cortex: Neocortical areas found on the edge (limit) of the hemisphere; they form the base or stem of the cortex. Limbic areas are either agranular (lack inner layer IV) or dysgranular (have an incipient layer IV). |
|
Periallocortex: Neocortical areas neighboring the allocortex (agranular cortex). Proisocortex: Neocortical areas between periallocortex (agranular) and isocortex (eulaminate) areas. Proisocortical areas are dysgranular. |
|
Neocortex: The cortex beyond the allocortex, which is the newest part of the cerebral cortex. Reptiles have a very small neocortex, whereas mammals have comparatively a large neocortex. The number of layers and their distinction increases in neocortical areas along gradients of laminar differentiation. |
|
Theories and Principles Dual Origin of the Neocortex: A hypothesis stating that the neocortex developed in evolution from two periallocortical (ancestral parahippocampal and paraolfactory) moieties (Dart 1934; Abbie 1940, 1942; Sanides 1962). |
|
Structural Model: A predictive model that relates the type of areas to their connections (Barbas 1986; Barbas and Rempel-Clower 1997), the plasticity/stability continuum, preferential vulnerability of areas to neurologic and psychiatric diseases, the timing of their development, and to the variety of neural progenitors. |
2. Many cortical areas but few cortical types
Neuroscientists rely on maps to interpret data, whether obtained using anatomic, genetic, physiologic or functional imaging methods. Classical architectonic maps of the cortex were constructed by examining how cellular features vary between neighboring areas. Working from paper-thin sections stained to see all neurons and glia, investigators placed borders where they detected changes in the appearance of neurons and their arrangement in cortical layers. This is how Brodmann produced a human cortical map, which is the most frequently used map to this day (Brodmann 1909/1999).
The mapping method used by Brodmann (1909/1999) and others is simple in technique but difficult for analysis because differences between neighboring areas are often subtle. The inevitable variations among such maps have engendered skepticism by critics. In recent times, mapping areas following Brodmann’s methodology has been reinforced by the use of new techniques and markers that help sharpen borders and layers of areas (Palomero-Gallagher et al. 2008; Palomero-Gallagher et al. 2009; Henssen et al. 2016; Malikovic et al. 2016; Palomero-Gallagher and Zilles 2017; Palomero-Gallagher et al. 2018).
A different approach to subdivide the cortex emerged with the studies of von Economo (1927/2009) and Sanides (1962). Working independently, these investigators observed that the laminar architecture of the cortex varies systematically. This important observation provided a road map to subdivide the cortex in a consistent way. To begin with, von Economo and Sanides observed that some neocortical areas in the human cortex do not have six layers from the get-go, and remain that way during the life span. Some areas lack an inner layer IV and belong to the agranular cortical type. Nearby areas have an incipient layer IV and belong to the dysgranular type (Fig. 1). Easily spotted at the base of the cortex, we refer to agranular and dysgranular areas collectively as ‘limbic’, a term that describes their position on the edge of the hemisphere (Broca 1878), though not their architecture. Areas with six layers ─ which are eulaminate in type ─ are found beyond dysgranular areas. The principle of systematic variation of the cortex provided the framework for von Economo and Sanides to parcellate the cortex from a theoretical perspective.
Figure 1.
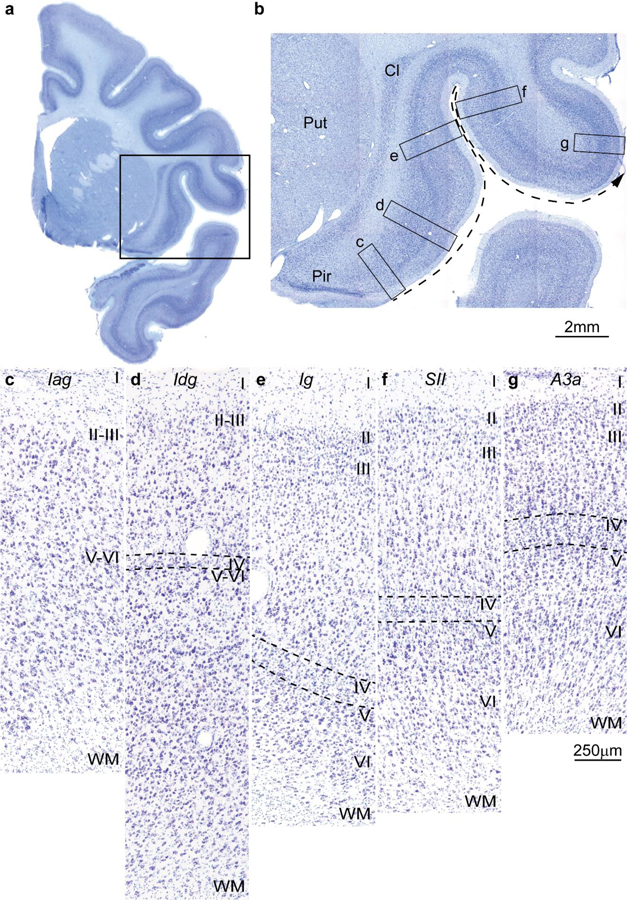
Cortical types and laminar gradients of differentiation in the insula and adjacent frontal cortex of the rhesus monkey (Nissl staining). a, Coronal section of the rhesus monkey brain at the level of the frontotemporal junction stained for Nissl. b, Closer view of the insula and the frontal operculum, shows the levels of photomicrographs in c-g; the dashed arrow shows the increasing trend of laminar differentiation. c, The agranular insula (Iag) is next to the primary olfactory cortex (Pir in b) and lacks layer IV. d, Dysgranular insula (Idg) has a rudimentary layer IV (dashed lines). e, Layer IV (dashed lines) is better developed in the adjacent granular insula (Ig). f, The secondary somatosensory area (SII) has a denser layer IV (dashed lines) and better differentiation of the other layers than the granular insula. g, Area 3a, which is part of the primary somatosensory cortex, has thicker and denser layer IV (dashed lines) and its superficial layers are denser than in SII. Abbreviations: A3a: area 3a, Cl: claustrum; Iag: insula agranular; Idg: insula dysgranular; Ig: insula granular; Pir: piriform cortex in the primary olfactory cortex; Put: putamen; SII: secondary somatosensory area; WM: white matter. Roman numerals indicate cortical layers. Calibration bar in g applies to c-g.
Variation in the cortex can be described by the term ‘cortical type’, an abstraction that refers to the overall laminar structure of areas and not their topography or membership to a cortical system. Several structural features combine to define cortical type: the presence or absence of layer IV; the thickness or density of layer IV, when present; the distinction between adjacent layers; the relative population of neurons in the upper compared to the deep layers (Fig. 1). Other features correlate with, but do not consistently capture cortical type. For example, myelin is a very useful marker because it increases in density from limbic to eulaminate areas (Fig. 2), but is not a universal proxy for cortical type [e.g., Barbas and Pandya (1989); Barbas and García-Cabezas (2015); Garcia-Cabezas et al. (2017); Barbas et al. (2018); Zikopoulos et al. (2018a)]. Another example is the non-phosphorylated intermediate neurofilament protein SMI-32 (Fig. 3) that in the primate cortex labels a subset of pyramidal projection neurons (Campbell and Morrison 1989) and can be used as a sensitive architectonic marker (Hof et al. 1995; Nimchinsky et al. 1996; Barbas and García-Cabezas 2015, 2016; García-Cabezas and Barbas 2017; Barbas et al. 2018; Joyce and Barbas 2018). Similarly, neuronal density is generally higher in sensory and association areas that have the clearest lamination (e.g., V1), but is not useful for the primary motor cortex, where neurons are large, yielding an overall lower density.
Figure 2.
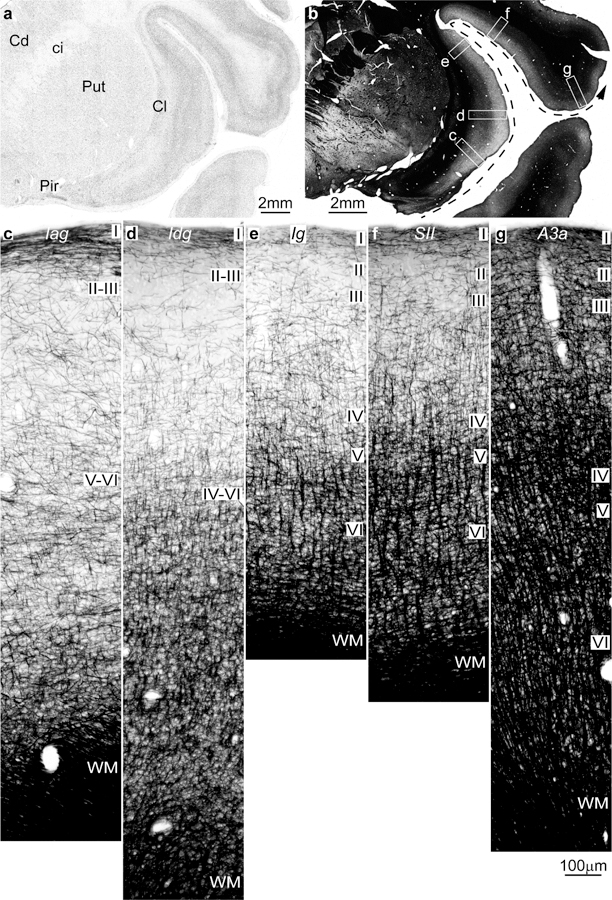
Intracortical myelin varies systematically in parallel with laminar differentiation in the insula and adjacent frontal cortex in the rhesus monkey (Gallyas staining). a, Coronal section of the rhesus monkey brain at the level of the frontotemporal junction stained for Nissl shows the insula and the frontal operculum. b, Adjacent section to a is stained for myelin (black) and shows the levels of photomicrographs c-g; the dashed arrow shows the trend of increasing laminar differentiation. c, The agranular insula (Iag) is next to the primary olfactory cortex (Pir in a) and has comparatively fewer myelinated axons than the adjacent area. d, Dysgranular insula (Idg) is slightly better myelinated. e, The granular insula (Ig) has more myelinated axons organized into vertical bundles than Iag and Idg. f, The secondary somatosensory area (SII) has more myelinated axons than the insular areas with well-developed vertical bundles in the deep layers and abundant small horizontal axons in the middle layers. g, Area 3a (A3a) in the primary somatosensory cortex has more myelinated axons than SII in all layers. Abbreviations: A3a: area 3a, Cd: caudate; ci: internal capsule; Cl: claustrum; Iag: insula agranular; Idg: insula dysgranular; Ig: insula granular; Pir: piriform cortex in the primary olfactory cortex; Put: putamen; SII: secondary somatosensory area; WM: white matter. Roman numerals indicate cortical layers. Calibration bar in g applies to c-g.
Figure 3.
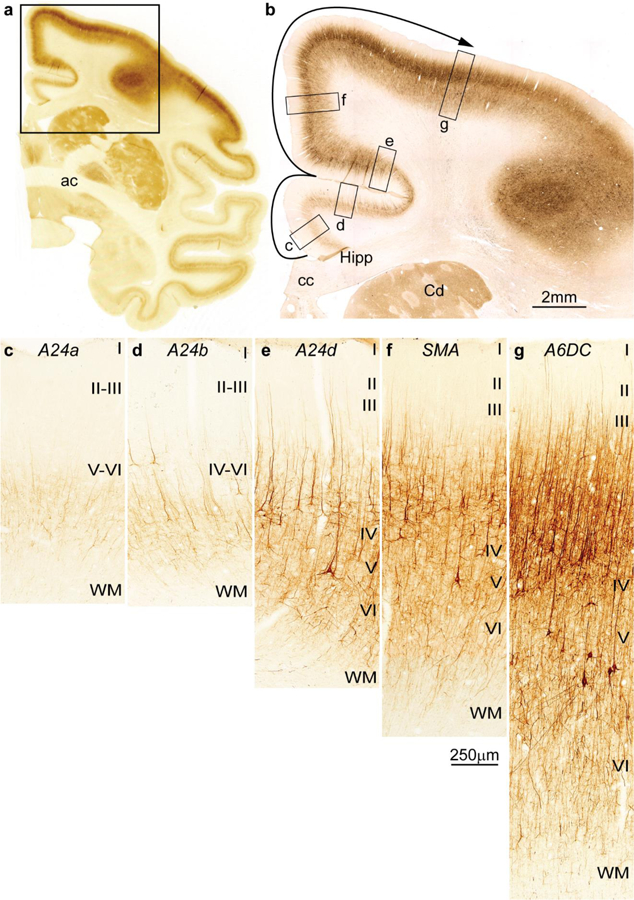
Cortical types and laminar gradients of differentiation in the cingulate and frontal cortex of the rhesus monkey (SMI-32 staining). a, Coronal section of the rhesus monkey brain at the level of the anterior commissure (ac) stained for the nonphosphorylated neurofilament protein SMI-32. b, Higher magnification shows the cingulate and dorsal frontal cortex and the levels of photomicrographs c-g; the solid arrow shows the trend of increasing laminar differentiation. c, Agranular area 24a (A24a) in the cingulate cortex is next to the anterior extension of the hippocampal formation (Hipp in b) and has few SMI-32 labeled neurons restricted to the deep layers. d, Area 24b (A24b) in the lower bank of the cingulate sulcus has few labeled SMI-32 neurons in the deep layers and some at the bottom of layer III. e, Area 24d (A24d) in the upper bank of the cingulate sulcus has more SMI-32 neurons than the other cingulate areas in the deep layers and in layer III, demarcating a band of unstained tissue that corresponds to layer IV. f, The supplementary motor area (SMA) and (g) premotor area 6 dorsocaudal (A6DC) have more neurons labeled for SMI-32 than the cingulate areas; in both SMA and A6DC layer IV stands out as a band of unlabeled tissue between the bottom of layer III and the upper part of layer V. Abbreviations: A24a: cingulate area 24a; A24b: cingulate area 24b; A24d: cingulate area 24d; A6DC: premotor area 6 dorsocaudal; ac: anterior commissure; cc: corpus callosum; Cd: caudate; Hipp: anterior extension of the hippocampal formation; SMA: supplementary motor area; WM: white matter. Roman numerals indicate cortical layers. Calibration bar in f applies to c-g.
2.1. How many types of cortex are there?
The limbic areas have the simplest architecture and form a ring at the base of the entire cortex, as in the stem of a mushroom (Fig. 4). Consequently, all cortical systems have a limbic component, whether they are visual, auditory, somatosensory or motor, as shown in previous studies (e.g., Sanides (1972); see also Barbas (1988); Pandya et al. (1988); Barbas (2015); Pandya et al. (2015)]. Eulaminate type cortices radiate from the basal stem to form the cap of the mushroom. In old world primates and humans, the cap, and to a lesser extent the dysgranular limbic areas, are curled into complex convolutions due to the great expansion of the cortex.
Figure 4.
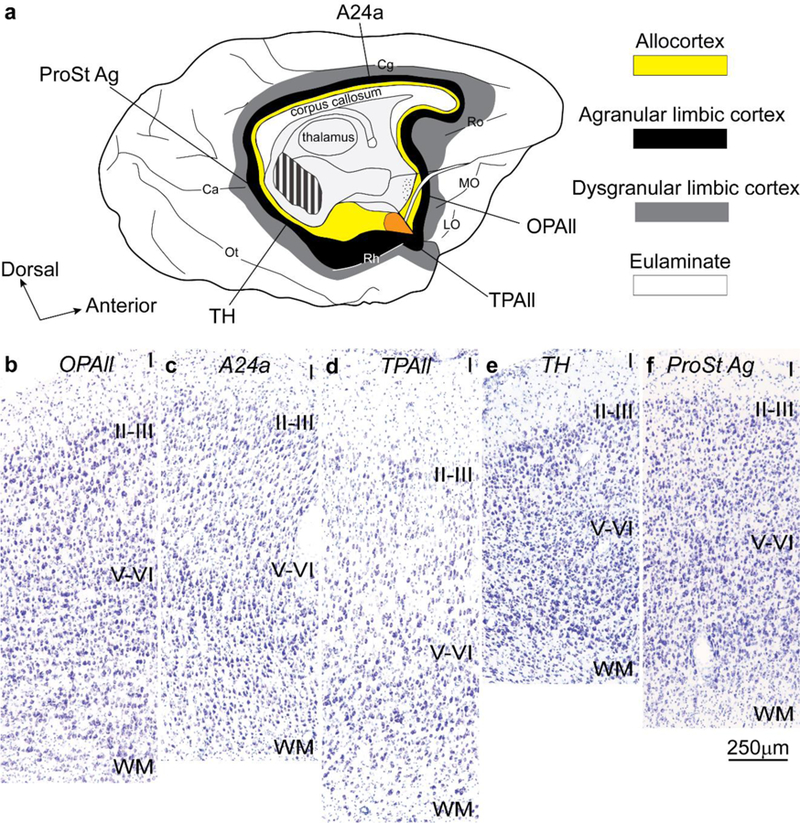
Neocortical areas with the simplest laminar structure form a ring at the edge (base) of the hemisphere. a, Tilted view of the macaque brain shows the medial and basal aspects of the left hemisphere. Allocortical areas (yellow) are at the edge (base) of the hemisphere; agranular limbic areas (black) are periallocortical; dysgranular limbic areas (grey) are between the periallocortex and eulaminate areas (white). Orange shows the place of the amygdala. b-f show agranular limbic areas without layer IV (Nissl staining). These areas are distributed along the periallocortical ring (black) of the neocortex at the foot of each cortical system; they have overall comparable laminar structure. Abbreviations: A24a: cingulate area 24a; Ca: calcarine fissure; Cg: cingulate sulcus; LO: lateral orbital sulcus; MO: medial orbital sulcus; OPAll: orbital periallocortex; Ot: occipitotemporal sulcus; ProSt Ag: area prostriata agranular; Rh: rhinal sulcus; Ro: rostral sulcus; TH: medial temporal area TH; TPAll: temporal periallocortex. Calibration bar in f applies to b-f.
The classification of types into agranular areas is straight forward. Dysgranular areas are interposed between agranular areas and eulaminate areas with a clearly identifiable layer IV. Dysgranular areas have a poorly developed layer IV, which is both thin and less dense than layer IV of eulaminate areas (Fig. 1). The rest of the cortex is composed of eulaminate areas, but the number of cortical types that can be distinguished reliably varies by species and system, in the range of about 3 to 8. The number of cortical types also varies by the degree of desired refinement. Some investigators lump areas with intermediate lamination together, whereas others make finer distinctions [e.g., Hilgetag et al. (2016); Zikopoulos et al. (2018a)].
Cortical expansion obeys the topologic rule of the systematic variation of the cortex. Among eulaminate areas, lamination is least differentiated in areas that are near dysgranular areas, and most distinct in areas that are the most distant from the limbic areas (Fig. 1 & 3). Based on the topological rule and gradual changes in type, areas that differ markedly from each other in laminar structure thus are not neighbors.
2.2. Cortical type versus cortical area
How does type differ from area? Area is a specific term, type is a general term. Area defines a small region that is unique in the cortex and can be identified by cellular and molecular markers, which distinguish it from neighboring areas. A recurrent theme in the literature is the discovery of new sensory or motor areas [e.g., Woolsey (1963); Kaas (2004)]. Using a variety of methods, investigators have subdivided large regions of sensory cortex, based on representation of the sensory periphery in the visual system or the body surface in the somatosensory system.
The areas along a cortical sensory system have different physiological properties, but also differ in their laminar architecture, and thus represent different types of cortex. Within a given cortical system, be it visual, somatosensory, or motor, type differs along a functional gradient in the respective system. In the cortical sensory systems, the areas with the best delineated layers are the primary areas. The primary visual cortex, in particular, has the most distinct laminar architecture in the primate cortex. By function, areas with highly delineated laminar structure, have specialized properties, such as small receptive fields in neurons of the primary visual (V1) or somatosensory cortex. Areas that are at an increasing distance from the primary areas have neurons with progressively larger receptive fields and more complex properties [reviewed in Hubel (1988); Rosa et al. (1997)].
Type is thus a general term because it applies across cortical sensory or motor systems. Type describes the general laminar structure of an area, regardless of modality. Below we examine why type but not area underlies the organization of the cortex.
3. Cortical type allows testable predictions for cortical organization
The philosopher Karl Popper proposed that a given scientific theory can be tested only indirectly by confirming or rejecting predictions derived from such a theory. First, a hypothesis is advanced inspired by observations. Second, predictions are deduced logically from the hypothesis. Third, the predictions are experimentally supported or rejected. If empirical data show that the predictions are wrong the hypothesis is falsified. If the data confirm the predictions, then the hypothesis is provisionally supported. According to Popper, scientific hypotheses can be accepted only provisionally, because a single instance of falsification with new data would be enough to reject them (Popper 1959).
The construct of cortical type ─ as embodied in the Structural Model ─ has been used to predict connections, synaptic plasticity, selective vulnerability, and the development of cortical areas. Cortical systematic variation also suggests the likely origin of the cortex in evolution. Below, we elaborate on the explanatory power of the Structural Model along multiple dimensions and its usefulness for guiding new research on the cerebral cortex. We propose that the systematic variation in cortical structure and the embedded construct of cortical type represent a theory that explains multiple levels of cortical organization that have been consistently verified by empirical data.
3.1. Cortical type is associated with connections
Studies based on the ablation-degeneration method to study anterograde pathways showed that sensory information flows sequentially from primary sensory to unimodal association areas, to multimodal association areas, and finally to limbic areas (Jones and Powell 1970; Mesulam 1998). The introduction of neural tracers in the early ‘70s provided a clear picture of the laminar origin and termination of cortical projection neurons. In the cortical sensory systems, pathways were called ‘feedforward’ if they originated from a cortical area that is closer to the sensory periphery than the area of termination, and ‘feedback’ if they proceeded in the opposite direction. These pathways have different laminar distributions [e.g., Rockland and Pandya (1979); Maunsell and Van Essen (1983)]. Feedforward connections originate in neurons in the upper layers (mostly layer III) and their axons terminate in the middle layers, which include layer IV. Feedback connections originate in the deep layers (V and VI) and their axons terminate in the upper layers, and especially layer I, to a lesser extent layer VI, but avoid the middle layers. Projections that originate in both upper and deep layers and terminate in all layers were previously lumped into one category called ‘lateral’ (Felleman and Van Essen 1991).
The interpretation of pathways by the direction of sensory signals (Felleman and Van Essen 1991) proved to be too restrictive, because non-sensory areas revealed similar patterns. Specifically, studies of the prefrontal cortex showed that connections do not depend on areas but depend on cortical type (Barbas 1986; Barbas and Rempel-Clower 1997). Areas of the simplest type, such as the limbic, project in a ‘feedback’ pattern to areas with more elaborate type. Connections in the reverse direction ─ from eulaminate to limbic ─ originate mostly in layer III and terminate in the middle (deep III to upper V) layers (see examples in Fig. 5).
Figure 5.
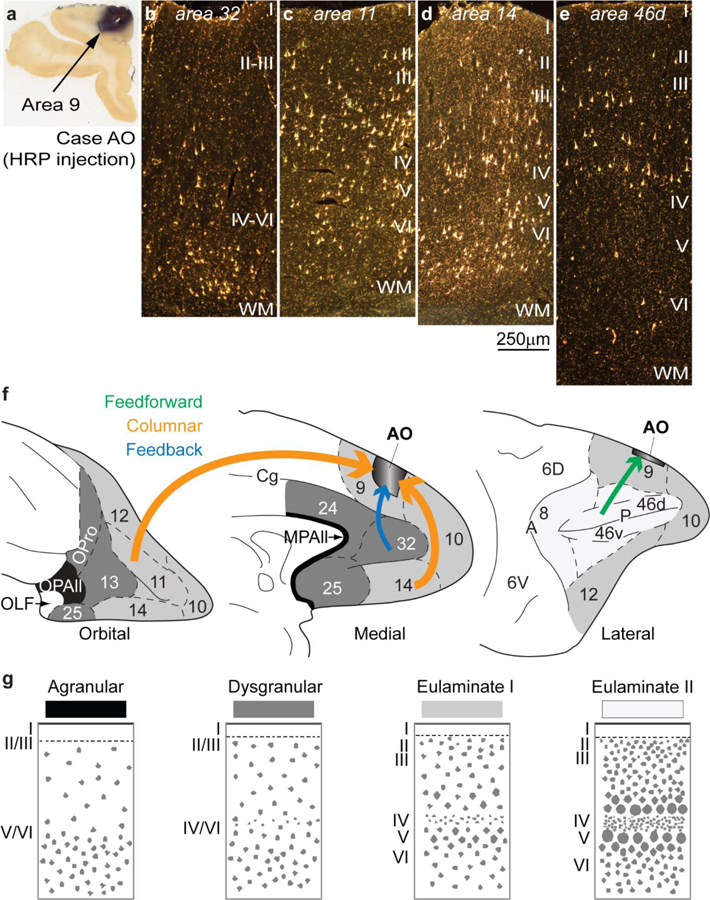
Examples of connections predicted by the relational Structural Model. a, Injection of the neural tracer horseradish peroxidase (HRP) in area 9 of the dorsolateral prefrontal cortex in a rhesus monkey brain. b, Retrogradely labeled neurons in anterior cingulate area 32 are concentrated in the deep layers V-VI, typical of a feedback pattern of connections originating from an area with a simpler laminar structure (area 32) and directed to an area with a more elaborate laminar structure (area 9). c-d, In area 11 (c) and in area 14 (d) there are labeled neurons in superficial layers II-III and in the deep layers V-VI, typical of a columnar pattern of connections between areas of a comparable type; note absence of labeled neurons in layer IV, which do not project out of the cortex. e, Retrogradely labeled neurons in the dorsal part of area 46d are concentrated in the superficial layers II-III, typical of a predominant feedforward pattern of connections, originating from an area with a more elaborate architecture (area 46d) to an area with comparatively simpler architecture (area 9). f, Sketches of the prefrontal cortex of the rhesus monkey show the orbital (left), medial (center), and lateral (right) surfaces; agranular areas are shown in black, dysgranular areas in dark grey, and eulaminate areas are depicted progressively with lighter grey. Arrow color indicates type of connection; arrow thickness indicates relative strength of pathways; thicker arrows suggest more robust connections. g, Cortical types (agranular, dysgranular, eulaminate I, and eulaminate II) are represented in four sketches. Abbreviations: HRP: horseradish peroxidase; MPAll: medial periallocortex; OLF: primary olfactory cortex; OPAll: orbital periallocortex; OPro: orbital proisocortex. Arabic numerals correspond to prefrontal cortical areas according to Barbas and Pandya (1989). Roman numerals indicate cortical layers. Calibration bar in d applies to b-e.
Connections of prefrontal areas with the rest of the cortex show a regularity that parallels closely the systematic variation within each cortical system: connections are distributed in a variety of laminar patterns. The regularity of laminar connections and cortical systematic variation can be distilled into a new principle: connections depend on the relationship of the type of connected areas (Barbas and Rempel-Clower 1997). We have called this relationship the Structural Model for connections [reviewed in Barbas (2015)]. When two areas differ markedly in laminar structure and they are connected, the pattern fits clearly within the ‘feedforward’ or ‘feedback’ categories. But because the cortex varies systematically, there are multiple permutations of type differences between linked areas and thus multiple patterns in the laminar distribution of connections. The closer the areas are in type, the more layers are involved in the connections. For example, when two areas belong to the same type, the connections between them are columnar: projection neurons originate in all layers (except for layers I and IV) (Barbas 1986), and their axons terminate in all layers (Barbas and Rempel-Clower 1997). Connections in sensory and non-sensory cortices can be subsumed under the general rules of the relational Structural Model.
The Structural Model has successfully predicted the pattern of connections in a variety of systems and species (Barbas et al. 1999; Barbas et al. 2005a; Grant and Hilgetag 2005; Medalla and Barbas 2006; Hilgetag and Grant 2010; Beul et al. 2015; Hilgetag et al. 2016; Beul et al. 2017; García-Cabezas and Barbas 2017; Goulas et al. 2017; Burt et al. 2018; Joyce and Barbas 2018). The model also predicts the strength of connections. Neighboring areas of a comparable type are strongly connected (Barbas and Pandya 1989), but so are areas that are at a considerable distance from each other, provided they belong to a similar cortical type (Pandya et al. 1988; Rempel-Clower and Barbas 2000; Medalla and Barbas 2006; Joyce and Barbas 2018; Zikopoulos et al. 2018a). Predictions of cortical connections based on the Structural Model have been verified without fail. On the other hand, models based on the anterior-posterior dimension of the cortex (Badre and D’Esposito 2007; Cahalane et al. 2012), the distance between areas (Ercsey-Ravasz et al. 2013) or cortical thickness (He et al. 2007) have met with contradictions, and thus are falsifiable (Goulas et al. 2014; Hilgetag et al. 2016; Beul et al. 2017; Burt et al. 2018).
The Structural Model can be applied to the cerebral cortex of humans to predict the laminar pattern and strength of connections in the absence of invasive tract tracing methods, as has been suggested (Barbas and Rempel-Clower 1997). These predictions can also be used to focus on specific networks to study typical development and disruption of circuit interactions in disease (Zikopoulos and Barbas 2010, 2013; Zikopoulos et al. 2018a; Zikopoulos et al. 2018b), and provide the pathway basis for models of consciousness (Chanes and Barrett 2016). Recent findings from functional studies in humans (Wei et al. 2019) are consistent with the Structural Model.
3.2. The Structural Model predicts the degree of synaptic plasticity or stability
The architectonic features of limbic areas suggest high plasticity. Limbic areas express some developmental markers into adulthood that cease to be expressed in other areas after ontogeny (Barbas 1995). Recent evidence has corroborated this hypothesis by providing direct evidence that cortical type is linked to the cortical plasticity-stability continuum of areas. Markers of synaptic plasticity are more concentrated in limbic than in eulaminate areas. In contrast, markers of stability have a higher concentration in eulaminate than in limbic areas. These features and functions are relative, and once again, consistent with the systematic variation of the cortex (Garcia-Cabezas et al. 2017).
We provide a few examples to illustrate this point. The growth associated protein GAP-43 is found at the edge of growing axons in development, when it is universally expressed across cortices (Benowitz and Routtenberg 1997). In the adult cortex, however, GAP-43 is expressed in measurable amounts only in limbic areas in primates. On the other hand, markers associated with stability show the opposite trend. One of these is myelin, which is highest in areas with the best architectonic differentiation, and decreases gradually towards the limbic cortices (Vogt and Vogt 1919; Braitenberg 1962; Sanides 1970; Nieuwenhuys and Broere 2017). Interestingly, GAP-43 and myelin associated proteins are mutually antagonistic (Kapfhammer and Schwab 1994). The high level of GAP-43 in the limbic anterior cingulate cortex, for example, helps explain the comparatively late myelination of this region (Zikopoulos and Barbas 2010), even though neuronal migration is completed early (Rakic 2002).
Limbic areas generally have a lower neuron density (Barbas and Pandya 1989; Dombrowski et al. 2001). Quantitative architectonic findings in the prefrontal cortex have shown that as a group the limbic areas have overall lower density in the upper layers than in the deep layers, when layer IV is considered separately (Dombrowski et al. 2001). This pattern is particularly prominent in subgenual area 25 in both rhesus monkeys and humans (Joyce and Barbas (2018); Zikopoulos et al. (2018a)]. Additional quantitative studies using unbiased methods are needed to test whether this pattern is found in other cortical systems as well. A lower density of neurons appears to be permissive for development of a rich dendritic tree with a high density of spines in limbic areas. Dendritic complexity decreases gradually through adjacent areas and is lowest in the well-laminated areas in all systems in primates [Elston (2003); Elston et al. (2005); reviewed in García-Cabezas et al. (2018)]. Markers of plasticity thus are expressed at higher levels in limbic than in eulaminate areas, whereas markers of stability show the opposite trend (Garcia-Cabezas et al. 2017).
3.3. The Structural Model predicts preferential vulnerability to disease
The high plasticity of limbic areas comes at a cost. Limbic areas are more vulnerable to neurodegenerative and psychiatric diseases. For example, pathological aggregation of aberrant proteins in Alzheimer’s and Parkinson’s diseases begins in limbic areas and spreads slowly to adjacent eulaminate areas (Arnold et al. 1991; Duyckaerts et al. 1998; Braak et al. 2006; Brettschneider et al. 2015; Braak and Del Tredici 2018). Limbic areas in the frontal and temporal lobes are also more vulnerable to epileptiform activity (Penfield and Jasper 1954; Gloor et al. 1982; Holmes et al. 2004; Tucker et al. 2007), which spreads to adjacent eulaminate areas with which they are connected. We have suggested that in prefrontal limbic areas, a lower density of inhibitory parvalbumin neurons ─ which exercise strong perisomatic inhibition of nearby pyramidal neurons ─ may contribute to vulnerability to epileptiform activity (Barbas 1995; Dombrowski et al. 2001; Barbas et al. 2005b; Zikopoulos and Barbas 2013). Limbic areas are also the preferred target of herpes virus infections (Damasio and Van Hoesen 1985).
Limbic areas are often implicated in psychiatric diseases as well, such as depression (Mayberg et al. 2005) and autism (Zikopoulos and Barbas 2010, 2013; Zikopoulos et al. 2018a; Zikopoulos et al. 2018b). There is evidence that the size of neurons and the underlying white matter pathways of limbic areas are altered in individuals with autism (van Kooten et al. 2008; Wegiel et al. 2010; Zikopoulos and Barbas 2010; Santos et al. 2011; Jacot-Descombes et al. 2012; Zikopoulos et al. 2018a). This evidence suggests that limbic areas and their cortico-cortical pathways are more vulnerable to developmental disruption than other areas (García-Cabezas et al. 2018). In combination, these observations support the hypothesis that cortical type predicts a series of features that predispose areas to preferential vulnerability to disease.
The idea that vulnerability to disease is linked to plasticity has been firmly established for other body tissues. Cancer, for example, most frequently affects the most plastic tissues which have high turnover throughout life, such as epithelial tissue, which accounts for over 80% of all cancers (Siegel et al. 2018). In the nervous system, most cancers are glia-related, which can proliferate in the adult nervous system (del Río-Hortega 1934/1962).
4. Laminar structure in the cerebral cortex is rooted in development
How do different types of cortex come about to underlie so many aspects of cortical organization? We hypothesized that differences in laminar structure among cortices emerge in development (Dombrowski et al. 2001). According to this hypothesis, limbic areas have a shorter ontogenetic period, and thus fewer neurons migrate to the cortex than in eulaminate areas. Moreover, because the mammalian cortex develops from inside out (Sidman and Rakic 1973), a shorter ontogenetic period would result in fewer neurons in the upper layers, which develop last. This hypothesis needs to be addressed with developmental data in future studies.
There are many missing pieces on the development of the nervous system. A developmental study in fetal rhesus monkeys, however, included a mix of areas that could test our developmental hypothesis. The study showed that completion of neurogenesis in area 24 ends at embryonic day 70 (E70), earlier than in area 11 (E80), which ends earlier than in area 46 (E90) (Rakic 2002). By cortical type, area 24 is limbic, area 11 is an intermediate eulaminate area, and area 46 has the best lamination within the prefrontal cortex. The highly laminated primary visual cortex in rhesus monkeys continues to develop until E100, more than any other visual area, including the adjacent area V2 (Rakic 1974, 1976a). The development of these prefrontal and occipital areas is thus consistent with the hypothesis that areas belonging to different types have a predicted ontogenetic sequence.
We recently showed that cortical type in humans is directly linked to variation of progenitor zones below prospective areas that differ in cortical type (Barbas and García-Cabezas 2016). In addition to the classical ventricular zone (VZ), in mammalian species there is a subventricular zone (SVZ), found above the VZ. The SVZ is a complex embryonic layer that is further subdivided into inner (ISVZ) and outer (OSVZ) sectors. These discoveries reveal a wealth in progenitor cells [reviewed in Noctor et al. (2007); Borrell and Reillo (2012); LaMonica et al. (2012)]. The classical bipolar radial glial cells reside in the VZ. In the SVZ there are translocated outer radial glial cells along with intermediate progenitor cells. The three progenitor classes differ by morphology, position, and expression of molecular factors [reviewed in Borrell and Reillo (2012); LaMonica et al. (2012); Dehay et al. (2015); Martinez-Cerdeno and Noctor (2016, 2018)].
In the developing human brain the specialized SVZ increases progressively in thickness and complexity in the direction from prospective limbic cortices to prospective eulaminate cortices. This evidence provides a developmental mechanism for the differences in neuronal density or other markers that are characteristic of different types of cortex. The difference in thickness is particularly striking in the OSVZ, which gives rise to the upper cortical layers, which we found to be denser with neurons in eulaminate than in limbic areas in adults, at least in the prefrontal cortex (Dombrowski et al. 2001; Joyce and Barbas 2018; Zikopoulos et al. 2018a). The OSVZ is very thin below prospective limbic areas and thickens progressively below prospective eulaminate areas in frontal (Barbas and García-Cabezas 2016), as well as in temporal areas, as shown in Figure 6. The systematic variation of the adult cortex thus is explained by systematic differences in the distribution of progenitors along zones that give rise to different types of cortex. In the rat embryo, VZ and SVZ show less variation across areas and the SVZ is less developed compared to the human (Fig. 7).
Figure 6.
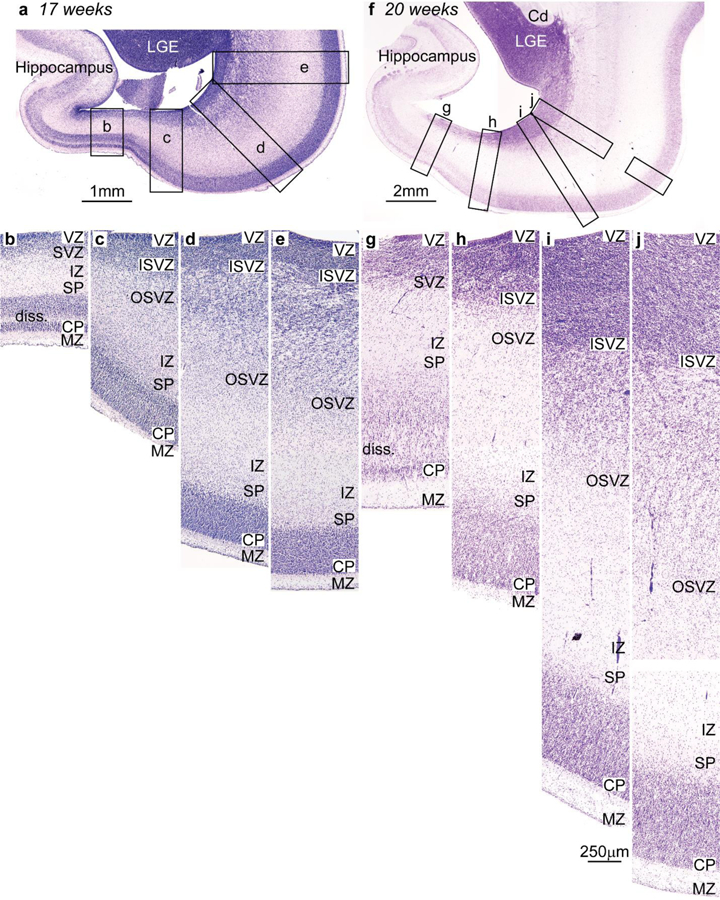
Systematic variation of embryonic layers in the prospective temporal lobe of the human embryo (Nissl staining). Germinal zones and prospective cortical layers are named according to Smart et al. (2002) and Bystron et al. (2008). a-e, Photomicrographs from a human fetus of 17 weeks gestational age stained for Nissl. a, Low power photomicrograph at the level of the temporal lobe shows the levels of photomicrographs b-e. b, The germinal zones of the prospective entorhinal cortex next to the hippocampal formation are composed of thin ventricular (VZ) and subventricular (SVZ) zones; the SVZ has no sublayers. c, The SVZ of the prospective perirhinal cortex is divided into inner (ISVZ) and outer (OSVZ) zones. d, The OSVZ is more prominent in prospective lateral (eulaminate) temporal areas. e, The cell density and the thickness of the OSVZ increases progressively in a lateral direction along a line of increasing laminar elaboration. f-j, Photomicrographs from a human fetus of 20 weeks gestational age stained for Nissl. f, Low power photomicrograph at the level of the temporal lobe shows the levels of photomicrographs g-j. g, The germinal zones of the prospective entorhinal cortex next to the hippocampal formation are thicker compared to b, but the SVZ still does not show two subzones. h, The prospective perirhinal cortex has thicker VZ and SVZ; the latter is subdivided into ISVZ and OSVZ. i, Prospective lateral eulaminate areas have thicker VZ and ISVZ than prospective entorhinal and perirhinal areas; the OSVZ predominates. j, The thickness of the OSVZ increases progressively in the lateral direction consistent with increased laminar definition in the adult. Abbreviations: Cd: caudate; CP, cortical plate; ISVZ, inner subventricular zone; IZ, intermediate zone; LGE, lateral ganglionic eminence; MZ, marginal zone; OSVZ, outer subventricular zone; SP, subplate; SVZ, subventricular zone; VZ, ventricular zone. Calibration bar in i applies to b-e and g-j. [Note: This figure is a re-examination of material from an earlier paper (Reillo et al. 2011)].
Figure 7.
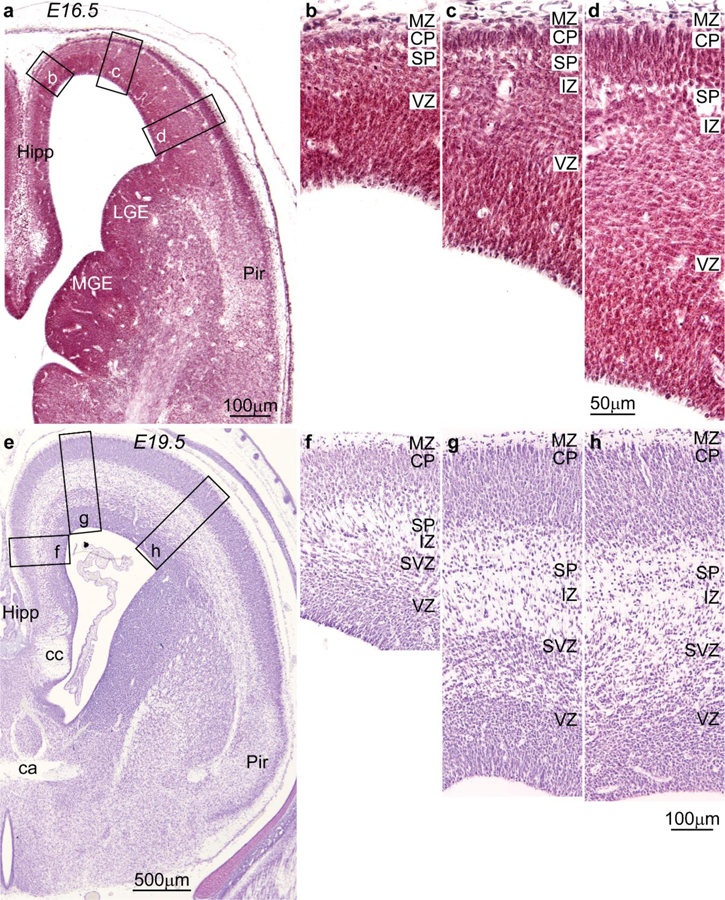
Systematic variation of embryonic layers in the prospective frontal cortex of the rat embryo (Nissl staining). Germinal zones and prospective cortical layers are named according to Bystron et al. (2008) and Reillo et al. (2011). a-d, Photomicrographs from a rat embryo of 16.5 days gestational age stained for Nissl. a, Low power photomicrograph at the level of the interventricular foramen shows the levels of photomicrographs b-d. b, The germinal zones of the prospective cingulate cortex next to the hippocampal formation (Hipp in a) are composed of the ventricular (VZ), the intermediate zone (IZ) which contains many cells migrating to the cortical plate, the subplate (SP), and the cortical plate (CP) which is thin. c, d, Prospective lateral areas have slightly thicker VZ with progressive thicker CP; the IZ contains many cells migrating to the CP. At this stage, a subventricular zone (SVZ) cannot be distinguished with Nissl stain. e-h, Photomicrographs from a rat embryo of 19.5 days gestational age stained for Nissl. e, Low power photomicrograph at the level of the anterior commissure (ca) shows the levels of photomicrographs f-h. f, The prospective cingulate area shows well differentiated VZ and SVZ. g, h, In a lateral direction, the VZ and the SVZ are slightly thicker, but a clear division of the SVZ into inner and outer zones is not apparent. Abbreviations: ca: anterior commissure; cc: corpus callosum; CP, cortical plate; Hipp: anterior extension of the hippocampal formation; IZ, intermediate zone; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; MZ, marginal zone; Pir: piriform cortex in the primary olfactory cortex; SP, subplate; SVZ, subventricular zone; VZ, ventricular zone. Calibration bar in d applies to b-d; calibration bar in h applies to f-h. [Note: This figure is an examination of material from a gift of Dr. Alan Peters].
The linkage of different cortices to progenitor status was previously unsuspected because most developmental studies in primates had focused on the primary sensory areas (Smart et al. 2002; Lukaszewicz et al. 2005; Lukaszewicz et al. 2006; Martinez-Cerdeno et al. 2012; Fame et al. 2017). A more inclusive study in the human quantitatively showed that prospective limbic cingulate and insular regions in the human embryo have fewer mitoses than prospective lateral eulaminate areas, which show higher proliferative activity in the OSVZ (Reillo et al. 2011).
5. Linkage of cortical type to cortical evolution
The developmental linkage of the systematic variation of the cortex suggests that it may also be traced to evolution. Ramón y Cajal described a process of simplification in the laminar structure of the cerebral cortex from humans to rodents. Mammals with comparatively simpler cortex do not generally have an inner granular layer IV and the other cortical layers are overall poorly differentiated (Ramón y Cajal 1904/2002), as seen in Figure 8.
Figure 8.
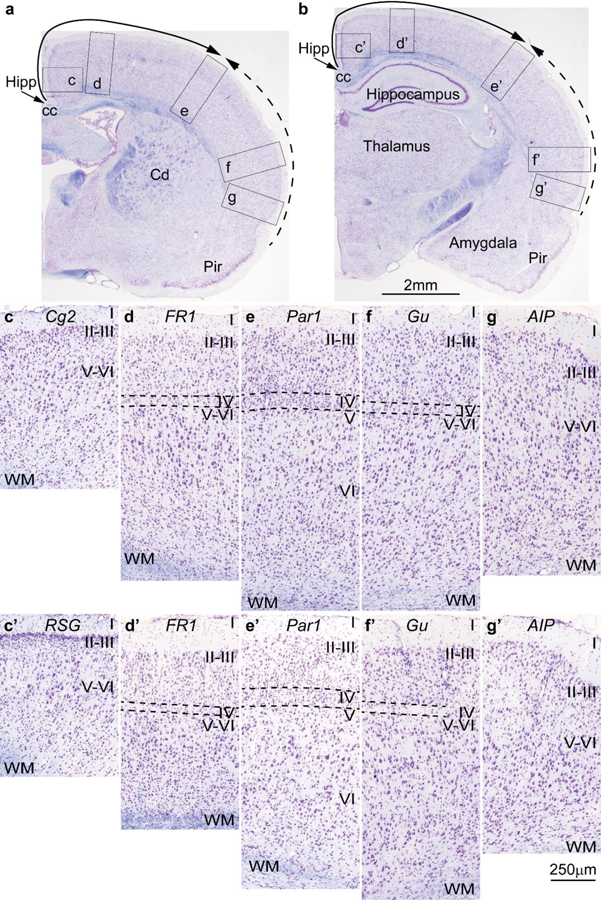
Cortical types and laminar gradients of differentiation in the cerebral cortex of the adult rat (stained for Nissl –purple- and myelin –blue-). Areas are named according to Zilles (1985). a, b, Photomicrographs of coronal sections in an adult rat brain stained for Nissl and myelin show the levels of photomicrographs c-g (from a) and c’-g’ (from b); the solid and dashed arrows show trends of increasing laminar differentiation. c-c’, Cingulate area 2 (Cg2) and the granular retrosplenial cortex (RSG) are found next to the anterior extension of the hippocampal formation (Hipp in a, b) and lack layer IV. d-d’, Frontal cortex, area 1 (FR1, primary motor cortex) has rudimentary layer IV (dashed lines). e-e’, Parietal cortex, area 1 (Par1, primary somesthetic cortex) has identifiable layer IV (dashed lines). f-f’, The gustatory cortex (Gu) has rudimentary layer IV (dashed lines). g-g’, The posterior part of the agranular insular cortex (AIP) is next to the piriform cortex (Pir in a, b) and lacks layer IV. Abbreviations: AIP: agranular insular cortex, posterior part; cc: corpus callosum; Cd: caudate; Cg2: Cingulate area 2; FR1: Frontal cortex, area 1 (primary motor cortex); Gu: gustatory cortex; Hipp: anterior extension of the hippocampal formation; Par1: Parietal cortex, area 1 (primary somesthetic cortex); Pir: piriform cortex in the primary olfactory cortex; RSG: granular retrosplenial cortex; WM: white matter. Roman numerals indicate cortical layers. Calibration bar in b applies to a-b. Calibration bar in g’ applies to c-g and c’-g’. [Note: This figure is an examination of material from a gift of Dr. Alan Peters].
Two investigators after Cajal realized that the laminar structure of the cerebral cortex undergoes progressive differentiation across species, manifested by the gradual emergence of an inner granular layer (Dart 1934; Abbie 1940, 1942). They also proposed that the neocortex has a Dual Origin, one traced to the ancient olfactory cortex (dashed arrows in Fig. 1 & 8), and the other to the ancestral hippocampal cortex (solid arrows in Fig. 3 & 8; see Table 1 for nomenclature). These two anlagen were identified in comparative studies in non-mammalian species and were later discovered independently across mammalian species [reviewed in Sanides (1970); Reep (1984)]. The neocortical components that emerge from the dual origin show parallel laminar gradations that expand in evolution through successive rings. An ancestral ring of agranular type neocortex is present in all mammals. The last ring includes the well-laminated primary visual, somesthetic and auditory areas in primates [reviewed in Sanides (1970); Reep (1984); Pandya et al. (1988); Pandya et al. (2015)]. The topological relation of the rings is hard to visualize in primate gyrencephalic brains because the neocortex grew through successive rings beyond the ancestral limbic stem, like the cap of an oversized curly mushroom. Interestingly, data on neurogenesis across cortical areas in non-human primates (Rakic 1976b, 2002) are compatible with the emergence of systematic cortical variation in evolution: areas of progressively more elaborate laminar architecture finalize their development later. Gradients of lamination across prospective cortical areas during development (Barbas and García-Cabezas 2016) also support this hypothesis. Future studies comparing the developmental features of limbic and eulaminate areas across mammalian species and systems are needed to test the hypothesis of the Dual Origin of the Neocortex.
Table 1.
Nomenclature for cortical types
| Brodmann (1909/1999) | Vogt and Vogt (1919) | Ariëns Kappers et al. (1936) | Filimonoff (1947) | Yakovlev (1959) | Sanides (1970) | Braak (1980) | This paper | Puelles (2017) |
|---|---|---|---|---|---|---|---|---|
| Heterogenetic | Allocortex | Archicortex (hippocampus) | Cortex incompletes (hippocampu & olfactory cortex) |
Entopallium (hippocampus & olfactory cortex) |
Allocortex (hippocampus & olfactory cortex) |
Allocortex sensu stricto (hippocampus & olfactory cortex) |
Allocortex (hippocampus & olfactory cortex) |
Medial Pallium |
| Paleocortex (olfactory cortex) | Ventral Pallium | |||||||
| Homogenetic | Isocortex | Neocortex | Cortex intermedius | Mesopallium | Periallocortex (agranular) | Mesocortex (Periallocortex) | Agranular limbic cortex | Lateral Pallium Medial Pallium |
| Proisocortex (dysgranular) | Mesocortex (Proisocortex) | Dysgranular limbic cortex | ||||||
| Cortex completus | Ectopallium | Isocortex | Isocortex sensu stricto | Eulaminate | Dorsal Pallium |
Further support for the hypothesis of the Dual Origin of the Neocortex emerged from modern genoarchitectonic studies in developing embryos of mice and non-mammalian species [reviewed in Puelles (2011); Nieuwenhuys and Puelles (2016); Puelles (2017)]. These investigators suggest that the neocortex appeared in evolution as an island surrounded by allocortex. Specification and patterning of the ancestral cortex is thought to be guided by two organizers: the cortical hem dorsally at the ancestral hippocampal anlage, and the antihem at the olfactory anlage ventrally (Subramanian et al. 2009). These organizers secrete specific patterning molecules that diffusely help form complementary gradients that may have directed neocortical expansion (Medina and Abellan 2009; Subramanian et al. 2009; Montiel and Aboitiz 2015) (Fig. 9). This evidence from the developmental perspective is consistent with the hypothesis of the Dual Origin of the Neocortex. The conclusion from these studies is that the cortical type of a given area suggests its antiquity in phylogeny: the more elaborate the laminar architecture of a cortical area, the newer in evolution. This hypothesis needs to be tested with further genoarchitectonic studies across mammalian species.
Figure 9.
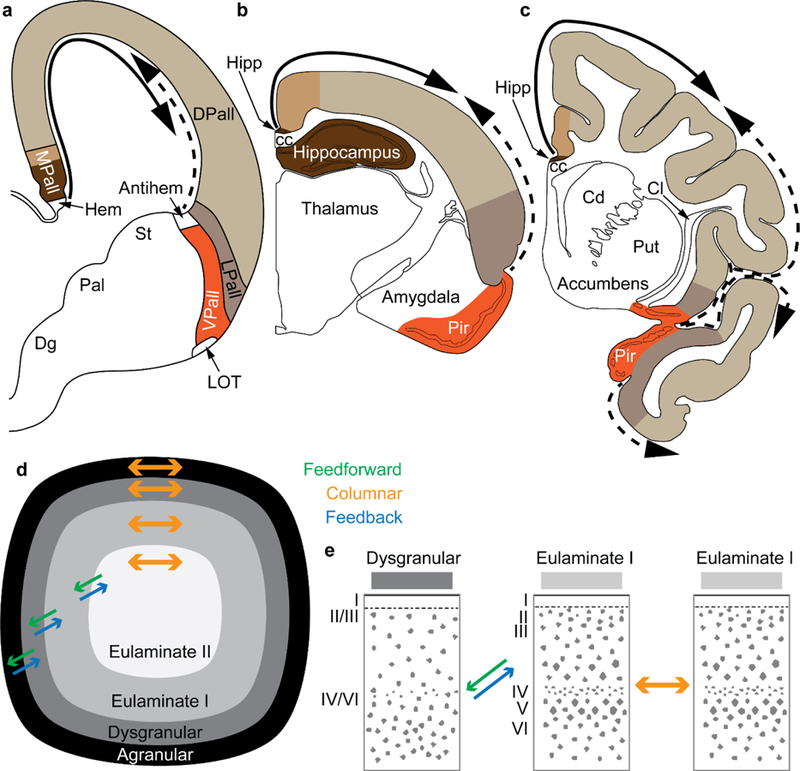
Two organizers for the expansion of the neocortex in development and evolution: the hem and antihem. a, Sketch of the mammalian telencephalon in development in coronal section shows the pallial (cortical) sectors [medial pallium (MPall), dorsal pallium (DPall), lateral pallium (LPall), and ventral pallium (VPall)], the hem, and the antihem according to the terminology of Subramanian et al. (2009), Montiel and Aboitiz (2015), and Puelles (2017) with the addition of the distinction of two parts on the MPall sector corresponding to allocortex (hippocampus) and periallocortex (agranular/dysgranular cingulate areas) in rats (b) and primates (c) based on architectonic analysis of these species in adults. The hem, found next to the roof plate, and the antihem, found in the corticostriatal junction, are secondary organizers that secrete morphogen proteins that form two overlapping gradients (solid and dashed arrows). b, Sketch of the rat adult brain in a coronal section; the adult derivatives of the developmental pallial sectors are colored as in a. The solid arrow shows the trend of laminar differentiation traced to the ancestral hippocampal cortex; the dashed arrow shows the trend of laminar differentiation traced to the ancestral olfactory cortex (Pir). c, Sketch of the adult rhesus monkey brain in coronal section; the adult derivatives of the developmental pallial sectors are tinted as in a. The solid arrow shows the dorsal trend of laminar differentiation traced to the ancestral hippocampal cortex; the dashed arrow shows the ventral trend of laminar differentiation traced to the ancestral olfactory cortex Pir). Note that DPall derivatives are more expansive than in the rat and extend to dorsal and ventral regions. d, Schematic of the primate cerebral cortex shows the arrangement of cortical types in rings. Laminar differentiation progresses from the outer or basal (black and dark grey) to the inner rings (lighter shades of grey). The edge of the cortex (black and dark grey) is actually thin compared to the greatly expanded eulaminate areas in the center. Cortical areas have stronger connections with other areas in the same ring and display columnar patterns of connections (orange arrows). Connections between areas in different rings (i.e., of different cortical type) are less strong than connections within the same ring and display feedback (blue arrows) and feedforward (green arrows) laminar patterns of connections. e, according to the Structural Model (Barbas and Rempel-Clower 1997), the laminar pattern of connections is related to the cortical type difference of the connected areas. Pathways form dysgranular to eulaminate areas are feedback (blue arrow); pathways from eulaminate to dysgranular are feedforward (green arrow); pathways between areas of comparable cortical type are columnar (orange arrow). Abbreviations: cc: corpus callosum; Cd: caudate; Cl: claustrum; Dg: Subpallial diagonal domain; DPall: dorsal pallium; Hipp: anterior extension of the hippocampal formation; LOT: lateral olfactory tract; LPall: lateral pallium; MPall: medial pallium; Pal: pallidum; Pir: piriform cortex in the primary olfactory cortex; Put: putamen; St: striatum; VPall: ventral pallium. Roman numerals indicate cortical layers.
The fact that the Dual Origin of the Neocortex was proposed independently in so many species (Table 2), first in reptiles by Dart (1934), then in monotremes and marsupials by Abbie (1940, 1942), then in the human brain by Sanides (1962), is not likely to be a random coincidence. The independent discovery of the Dual Origin of the Neocortex rather suggests a common pattern in the development of the cortex in evolution. These ideas were based on astute observations of cortical structure using simple stains for neurons and glia and are supported by modern genoarchitectonic studies, as summarized above.
Table 2.
Species examined for the Dual Origin of the Neocortex hypothesis
| Reptiles | Agama, Zonurus, Gecko, Testudo, Chameleon | Dart (1934) | ||
| Mammals | Monotremes | Tachyglossus | Abbie (1940) | |
| Marsupials | Perameles | Abbie (1942) | ||
| Placental | Rodents | Rat | Sanides (1970) and this paper | |
| Cetacean | Dolphin | Morgane et al. (1990) | ||
| Felidae | Cat | Sanides and Hoffmann (1969) | ||
| Primates | Slow loris | Sanides and Krishnamurti (1967) | ||
| Squirrel monkey | Sanides (1968) | |||
| Macaque |
Pandya and Sanides (1973); Mesulam and Mufson (1982); Pandya and Barbas (1985); Pandya et al. (1988); Barbas and Pandya (1989) and this paper |
|||
| Human | Sanides (1962, 1964); Galaburda and Sanides (1980) | |||
Note: Reep (1984) analyzed extensively data from the literature with new diagrams about the Dual Origin of the Neocortex in the following species: tachyglossus, opossum, hamster, mouse, rat, hedgegog, rabbit, sheep, cat, dog, tree shrew, galago, macaque, and human.
Current ideas about the specialization in cortical systems in adult primates are consistent with the hypothesis of the Dual Origin of the Neocortex. The dorsal trend in the visual system includes a series of areas that have a bias for processing the spatial aspects of the visual environment (Ungerleider and Mishkin 1982). According to the dual origin hypothesis, the dorsal trend may be traced to the ancestral hippocampal cortex. The ventral trend includes visual areas that process the features of visual stimuli, having origin in an ancestral olfactory cortex, the key sensory system in reptiles. Primates rely heavily on the visual modality, and visual cortices have expanded considerably. Allman and Kaas have suggested that the many visual cortices in primates may reflect duplications in the genome (Allman 2000). Specializations into dorsal and ventral systems have also been reported for other cortical sensory systems (Romanski et al. 1999), as well as for the motor-premotor cortical system (Pandya and Barbas 1985; Barbas and Pandya 1987).
6. Summary and conclusions: The multiple uses of the Structural Model to guide research
The Structural Model was the first to link the pattern and strength of connections to the classical theory of cortical systematic variation [(Barbas 1986; Barbas and Rempel-Clower 1997): reviewed in Barbas (2015)]. The relational Structural Model allows predictions about patterns and strength of connections, the degree of synaptic plasticity, and the vulnerability of cortical areas to psychiatric and neurologic diseases. Cortical type, as embodied in the Structural Model, is rooted in the systematic variation of the cortex, which has expanded in laminar complexity through many rings in humans. Most rings beyond the basal limbic are not present in mammals with simpler cortex. The prefrontal cortex of rodents is largely agranular (Krettek and Price 1977; Wise 2008). By contrast, the prefrontal cortex of primates has agranular and dysgranular as well as a predominant array of eulaminate cortices (Barbas and Pandya 1989; Petrides et al. 2012). This evidence suggests that limbic areas across mammalian species are homologous, defined as areas that have the same developmental origin (Wagner 1989; Nieuwenhuys and Puelles 2016), and likely share evolutionary origin. The construct of cortical type thus provides the basis to suggest homologies of cortical areas and facilitate comparisons across species. Based on this evidence, eulaminate areas with thick and dense layer IV in the primate brain (e.g., Fig. 1F, G) do not have homologous counterparts in the rodent cortex. Rodents have primary areas that lack the elaborate laminar definition of the primary areas in primates. This evidence suggests that primary areas in rodents and primates are analogous but not homologous.
Taking into account cortical type should help focus neurodevelopmental studies in primate embryos on the limbic areas that are understudied [e.g., Nowakowski et al. (2017)] in spite of their predilection to pathology. Spatiotemporal analysis of gene expression along the primate developing cortex may help identify factors and time windows of neurodevelopmental disruption. Integrative single-cell analysis [e.g., Woodworth et al. (2017)] applied to neurons of limbic areas in neurotypical humans and those with psychiatric diseases may help zero in on molecular factors that affect the structure and function in human diseases (García-Cabezas et al. 2018). Genetic and epigenetic studies of the human cortex [e.g., Lake et al. (2017); Roy et al. (2018)] would benefit from a focus on cortical type differences to compare areas with different molecular, laminar, cellular, and connectional profiles, as suggested (García-Cabezas et al. 2018). Predicting ontogenetic period by cortical type can provide information on windows of vulnerability and help explain the variability in diseases, such as autism (Zikopoulos and Barbas 2010, 2013; Zikopoulos et al. 2018a; Zikopoulos et al. 2018b).
Conceptualizing the construct of cortical type as employed in the Structural Model opens up avenues to understand seemingly puzzling phenomena, such as similar expression of markers in areas that are not neighbors (Roy et al. 2018), or connections between them. Distant areas can be connected because they are sisters in type by membership to the same or neighboring ring of cortical differentiation, and not by their Euclidean distance [reviewed in Hilgetag et al. (2016)]. Novel markers that reveal patterns of expression in the cortex (Burt et al. 2018) support the classical principle of cortical systematic variation, notwithstanding the proliferation of new names to describe them (see Table 3). Novel markers may add to the dimensions that describe cortical type and sharpen its definition. Big data alone, however, do not provide insights for understanding the brain, the same way that big data have not led to understanding cancer (Weinberg 2014). A theoretical framework is needed to provide context to organize new data and to provide insights on brain connections, function, and dysfunction in brain diseases.
Table 3.
Different nomenclature for the same regularities and principles
| Barbas (1986); Barbas and Rempel-Clower (1997) | Laminar structure (cortical type) | Laminar pattern of connections | Structural Model: Relation of laminar structure to cortical connections |
| van den Heuvel et al. (2015); van den Heuvel and Yeo (2017); Scholtens et al. (2018); Scholtens and van den Heuvel (2018); Wei et al. (2019) | Microscale pattern of cortical organization | Macroscale pattern of connectivity | Bridging/linking/relating the micro & macroscale organization of the cortex |
| Huntenburg et al. (2018) | Cortical microstructure | Macroscale connectivity | Sensorimotor-to-transmodal gradient of cortical organization* |
| Burt et al. (2018) | Microanatomy, microcircuit specialization, microscale properties | Hierarchical long range interactions | Hierarchical gradients of microscale properties contribute to the macroscale specialization of cortical function |
First proposed by Jones and Powell (1970) and then elaborated by Mesulam (1985).
Astute observations of animals, plants and rocks allowed Darwin to make a big leap and formulate the most enduring theory of evolution (Darwin 1859), as was the case for Cajal, who came up with the neuron doctrine after simple observations under a monocular microscope (Ramón y Cajal 1937). Neuroscience has a lot to learn from classical theories that are based on empirical observations to derive principles and test them rigorously with new data. A theoretical framework and new data can go hand-in-hand to inform and advance both.
Acknowledgements
This work was supported by the National Institutes of Health (NINDS R01 NS024760; NIMH R01 MH057414; R01 MH117785; and R01 MH101209). The sections of rat brain shown in Figures 7 and 8 were a generous gift from Dr. Alan Peters.
Footnotes
Disclosure of potential conflicts of interest: The authors declare that they have no conflict of interest.
References
- Abbie AA (1940) Cortical lamination in the monotremata. J Comp Neurol 72:429–467 [Google Scholar]
- Abbie AA (1942) Cortical lamination in a polyprotodont marsupial, perameles nasuta. J Comp Neurol 76:509–536 [Google Scholar]
- Allman J (2000) Evolving brains Scientific American Library, New York [Google Scholar]
- Ariëns Kappers CU, Huber GC, Crosby EC (1936) The comparative anatomy of the nervous system of vertebrates, including man Macmillan, New York, [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1:103–116 [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M (2007) Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. JCogn Neurosci 19 (12):2082–2099 [DOI] [PubMed] [Google Scholar]
- Bailey P, von Bonin G (1951) The Isocortex of Man University of Illinois Press, Urbana [Google Scholar]
- Barbas H (1986) Pattern in the laminar origin of corticocortical connections. J Comp Neurol 252:415–422 [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN (1987) Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 256:211–218 [DOI] [PubMed] [Google Scholar]
- Barbas H (1988) Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol 276:313–342 [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN (1989) Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 286 (3):353–375 [DOI] [PubMed] [Google Scholar]
- Barbas H (1995) Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev 19:499–510 [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N (1997) Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex 7:635–646 [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL (1999) Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol 410:343–367 [DOI] [PubMed] [Google Scholar]
- Barbas H, Hilgetag CC, Saha S, Dermon CR, Suski JL (2005a) Parallel organization of contralateral and ipsilateral prefrontal cortical projections in the rhesus monkey. BMC Neurosci 6 (1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P (2005b) Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex 15 (9):1356–1370 [DOI] [PubMed] [Google Scholar]
- Barbas H (2015) General Cortical and special Prefrontal Connections: Principles from Structure to Function. Annu Rev Neurosci 38:269–289 [DOI] [PubMed] [Google Scholar]
- Barbas H, García-Cabezas MA (2015) Motor cortex layer 4: less is more. Trends Neurosci 38 (5):259–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, García-Cabezas MA (2016) How the prefrontal executive got its stripes. Curr Opin Neurobiol 40:125–134. doi: 10.1016/j.conb.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Wang J, Joyce MKP, García-Cabezas MA (2018) Pathway mechanism for excitatory and inhibitory control in working memory. J Neurophysiol 120:2659–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20 (2):84–91 [DOI] [PubMed] [Google Scholar]
- Beul SF, Grant S, Hilgetag CC (2015) A predictive model of the cat cortical connectome based on cytoarchitecture and distance. Brain Struct Funct 220 (6):3167–3184. doi: 10.1007/s00429-014-0849-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beul SF, Barbas H, Hilgetag CC (2017) A Predictive Structural Model of the Primate Connectome. Scientific reports 7:43176. doi: 10.1038/srep43176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, Reillo I (2012) Emerging roles of neural stem cells in cerebral cortex development and evolution. Developmental neurobiology 72 (7):955–971. doi: 10.1002/dneu.22013 [DOI] [PubMed] [Google Scholar]
- Braak H (1980) Architectonics of the human telencephalic cortex. Studies of brain function, vol 4 Springer-Verlag, Berlin; New York [Google Scholar]
- Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Movement disorders : official journal of the Movement Disorder Society 21 (12):2042–2051. doi: 10.1002/mds.21065 [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K (2018) Spreading of Tau Pathology in Sporadic Alzheimer’s Disease Along Cortico-cortical Top-Down Connections. Cereb Cortex 28 (9):3372–3384. doi: 10.1093/cercor/bhy152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V (1962) A note on myeloarchitectonics. J Comp Neurol 118:141–156 [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ (2015) Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 16 (2):109–120. doi: 10.1038/nrn3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P (1878) Anatomie compaŕe des circonvolutions ćŕbrales: Le grand lobe limbique et la scissure limbique dans la śrie des mammif̀res. Revue D’anthropologie 1:385–498 [Google Scholar]
- Brodmann K (1909/1999) Brodmann’s Localisation in the Cerebral Cortex. Translated from German by Laurence J. Garey Imperial College Press, London [Google Scholar]
- Burt JB, Demirtas M, Eckner WJ, Navejar NM, Ji JL, Martin WJ, Bernacchia A, Anticevic A, Murray JD (2018) Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimagin topography. Nat Neurosci 9:1251–1259. doi: 10.1038/s41593-018-0195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: Boulder Committee revisited. NatRevNeurosci 9 (2):110–122 [DOI] [PubMed] [Google Scholar]
- Cahalane DJ, Charvet CJ, Finlay BL (2012) Systematic, balancing gradients in neuron density and number across the primate isocortex. Front Neuroanat 6:28. doi: 10.3389/fnana.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AW (1905) Histological studies on the localisation of cerebral function University Press, Cambridge, [Google Scholar]
- Campbell MJ, Morrison JH (1989) Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol 282:191–205 [DOI] [PubMed] [Google Scholar]
- Chanes L, Barrett LF (2016) Redefining the Role of Limbic Areas in Cortical Processing. Trends Cogn Sci 20 (2):96–106. doi: 10.1016/j.tics.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Van Hoesen GW (1985) The limbic system and the localisation of herpes simplex encephalitis. Journal of neurology, neurosurgery, and psychiatry 48 (4):297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RA (1934) The dual structure of the neopallium: Its history and significance. J Anat 69:3–19 [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1859) On the origin of species by means of natural selection: or, The preservation of favoured races in the struggle for life John Murray, Albemarle Street, London: [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Kennedy H, Kosik KS (2015) The outer subventricular zone and primate-specific cortical complexification. Neuron 85 (4):683–694. doi: 10.1016/j.neuron.2014.12.060 [DOI] [PubMed] [Google Scholar]
- del Río-Hortega P (1934/1962) The microscopic anatomy of tumors of the central and peripheral nervous system Thomas, Springfield, Ill., [Google Scholar]
- Ding SL, Royall JJ, Sunkin SM, Ng L, Facer BA, Lesnar P, Guillozet-Bongaarts A, McMurray B, Szafer A, Dolbeare TA, Stevens A, Tirrell L, Benner T, Caldejon S, Dalley RA, Dee N, Lau C, Nyhus J, Reding M, Riley ZL, Sandman D, Shen E, van der Kouwe A, Varjabedian A, Wright M, Zollei L, Dang C, Knowles JA, Koch C, Phillips JW, Sestan N, Wohnoutka P, Zielke HR, Hohmann JG, Jones AR, Bernard A, Hawrylycz MJ, Hof PR, Fischl B, Lein ES (2016) Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol 524 (16):3127–3481. doi: 10.1002/cne.24080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski SM, Hilgetag CC, Barbas H (2001) Quantitative architecture distinguishes prefrontal cortical systems in the rhesus monkey. Cereb Cortex 11:975–988 [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Colle MA, Dessi F, Piette F, Hauw JJ (1998) Progression of Alzheimer histopathological changes. Acta neurologica Belgica 98 (2):180–185 [PubMed] [Google Scholar]
- Elston GN (2003) Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex 13 (11):1124–1138 [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J (2005) A study of pyramidal cell structure in the cingulate cortex of the macaque monkey with comparative notes on inferotemporal and primary visual cortex. Cereb Cortex 15 (1):64–73 [DOI] [PubMed] [Google Scholar]
- Ercsey-Ravasz M, Markov NT, Lamy C, Van Essen DC, Knoblauch K, Toroczkai Z, Kennedy H (2013) A predictive network model of cerebral cortical connectivity based on a distance rule. Neuron 80 (1):184–197. doi: 10.1016/j.neuron.2013.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fame RM, Dehay C, Kennedy H, Macklis JD (2017) Subtype-Specific Genes that Characterize Subpopulations of Callosal Projection Neurons in Mouse Identify Molecularly Homologous Populations in Macaque Cortex. Cereb Cortex 27 (3):1817–1830. doi: 10.1093/cercor/bhw023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47 [DOI] [PubMed] [Google Scholar]
- Filimonoff IN (1947) A rational subdivision of the cerebral cortex. Arch Neurol Psychiat 58:296–311 [DOI] [PubMed] [Google Scholar]
- Galaburda A, Sanides F (1980) Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol 190:597–610 [DOI] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Joyce MP, John Y, Zikopoulos B, Barbas H (2017) Mirror trends of plasticity and stability indicators in primate prefrontal cortex. Eur J Neurosci 46 (8):2392–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabezas MA, Barbas H (2017) Anterior Cingulate Pathways May Affect Emotions Through Orbitofrontal Cortex. Cereb Cortex 27 (10):4891–4910. doi: 10.1093/cercor/bhw284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabezas MA, Barbas H, Zikopoulos B (2018) Parallel development of chromatin patterns, neuron morphology, and connections: Potential for disruption in autism. Frontiers in Neuroanatomy 12:70 10.3389/fnana.2018.00070. doi: 10.3389/fnana.2018.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC (2016) A multi-modal parcellation of human cerebral cortex. Nature 536 (7615):171–178. doi: 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S (1982) The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Annals of Neurology 12:129–144 [DOI] [PubMed] [Google Scholar]
- Goulas A, Uylings HB, Stiers P (2014) Mapping the hierarchical layout of the structural network of the macaque prefrontal cortex. Cereb Cortex 24 (5):1178–1194. doi: 10.1093/cercor/bhs399 [DOI] [PubMed] [Google Scholar]
- Goulas A, Uylings HB, Hilgetag CC (2017) Principles of ipsilateral and contralateral cortico-cortical connectivity in the mouse. Brain Struct Funct 222 (3):1281–1295. doi: 10.1007/s00429-016-1277-y [DOI] [PubMed] [Google Scholar]
- Grant S, Hilgetag CC (2005) Graded classes of cortical connections: quantitative analyses of laminar projections to motion areas of cat extrastriate cortex. Eur J Neurosci 22 (3):681–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC (2007) Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17 (10):2407–2419 [DOI] [PubMed] [Google Scholar]
- Henssen A, Zilles K, Palomero-Gallagher N, Schleicher A, Mohlberg H, Gerboga F, Eickhoff SB, Bludau S, Amunts K (2016) Cytoarchitecture and probability maps of the human medial orbitofrontal cortex. Cortex 75:87–112. doi: 10.1016/j.cortex.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Grant S (2010) Cytoarchitectural differences are a key determinant of laminar projection origins in the visual cortex. NeuroImage 51 (3):1006–1017. doi: 10.1016/j.neuroimage.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Medalla M, Beul S, Barbas H (2016) The primate connectome in context: principles of connections of the cortical visual system. NeuroImage 134:685–702. doi:doi: 10.1016/j.neuroimage.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Mufson EJ, Morrison JH (1995) Human orbitofrontal cortex: cytoarchitecture and quantitative immunohistochemical parcellation. J Comp Neurol 359:48–68 [DOI] [PubMed] [Google Scholar]
- Holmes MD, Brown M, Tucker DM (2004) Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia 45 (12):1568–1579. doi: 10.1111/j.0013-9580.2004.23204.x [DOI] [PubMed] [Google Scholar]
- Hubel DH (1988) Eye, brain, and vision. Scientific American Library series, vol no 22 Scientific American Library; : Distributed by Freeman WH, New York [Google Scholar]
- Huntenburg JM, Bazin PL, Margulies DS (2018) Large-Scale Gradients in Human Cortical Organization. Trends Cogn Sci 22 (1):21–31. doi: 10.1016/j.tics.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Jacot-Descombes S, Uppal N, Wicinski B, Santos M, Schmeidler J, Giannakopoulos P, Heinsen H, Schmitz C, Hof PR (2012) Decreased pyramidal neuron size in Brodmann areas 44 and 45 in patients with autism. Acta Neuropathol 124 (1):67–79. doi: 10.1007/s00401-012-0976-6 [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS (1970) An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain 93:793–820 [DOI] [PubMed] [Google Scholar]
- Joyce MP, Barbas H (2018) Cortical connections position primate area 25 as a keystone for interoception, emotion, and memory. J Neurosci 38 (7):1677–1698. doi: 10.1523/JNEUROSCI.2363-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH (2004) Evolution of somatosensory and motor cortex in primates. AnatRecA DiscovMolCell EvolBiol 281 (1):1148–1156 [DOI] [PubMed] [Google Scholar]
- Kapfhammer JP, Schwab ME (1994) Inverse patterns of myelination and GAP-43 expression in the adult CNS: neurite growth inhibitors as regulators of neuronal plasticity? J Comp Neurol 340 (2):194–206 [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171 (2):157–191 [DOI] [PubMed] [Google Scholar]
- Lake BB, Chen S, Sos BC, Fan J, Kaeser GE, Yung YC, Duong TE, Gao D, Chun J, Kharchenko PV, Zhang K (2017) Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nature biotechnology doi: 10.1038/nbt.4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonica BE, Lui JH, Wang X, Kriegstein AR (2012) OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease. Curr Opin Neurobiol 22 (5):747–753. doi: 10.1016/j.conb.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C (2005) G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron 47 (3):353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Cortay V, Giroud P, Berland M, Smart I, Kennedy H, Dehay C (2006) The concerted modulation of proliferation and migration contributes to the specification of the cytoarchitecture and dimensions of cortical areas. Cereb Cortex 16 Suppl 1:i26–i34 [DOI] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Kujovic M, Palomero-Gallagher N, Eickhoff SB, Zilles K (2016) Cytoarchitecture of the human lateral occipital cortex: mapping of two extrastriate areas hOc4la and hOc4lp. Brain Struct Funct 221 (4):1877–1897. doi: 10.1007/s00429-015-1009-8 [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Cunningham CL, Camacho J, Antczak JL, Prakash AN, Cziep ME, Walker AI, Noctor SC (2012) Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS One 7 (1):e30178. doi: 10.1371/journal.pone.0030178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC (2016) Cortical evolution 2015: Discussion of neural progenitor cell nomenclature. J Comp Neurol 524 (3):704–709. doi: 10.1002/cne.23909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC (2018) Neural Progenitor Cell Terminology. Front Neuroanat 12:104. doi: 10.3389/fnana.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JHR, Van Essen DC (1983) The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci 3:2563–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45 (5):651–660 [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H (2006) Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci 23 (1):161–179 [DOI] [PubMed] [Google Scholar]
- Medina L, Abellan A (2009) Development and evolution of the pallium. Seminars in cell & developmental biology 20 (6):698–711. doi: 10.1016/j.semcdb.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ (1982) Insula of the old world monkey. I: Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol 212:1–22 [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1985) Patterns in behavioral neuroanatomy: Association areas, the limbic system, and hemispheric specialization. In: Mesulam MM (ed) Principles of Behavioral Neurology F. A. Davis Company, Philadelphia, pp 1–70 [Google Scholar]
- Mesulam MM (1998) From sensation to cognition. Brain 121:1013–1052 [DOI] [PubMed] [Google Scholar]
- Montiel JF, Aboitiz F (2015) Pallial patterning and the origin of the isocortex. Front Neurosci 9:377. doi: 10.3389/fnins.2015.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ, Glezer II, Jacobs MS (1990) Comparative and evolutionary anatomy of the visual cortex of the dolphin. Cereb Cortex 8B:215–262 [Google Scholar]
- Nieuwenhuys R, Puelles L (2016) Towards a new neuromorphology Springer International Publishing, [Google Scholar]
- Nieuwenhuys R, Broere CA (2017) A map of the human neocortex showing the estimated overall myelin content of the individual architectonic areas based on the studies of Adolf Hopf. Brain Struct Funct 222 (1):465–480. doi: 10.1007/s00429-016-1228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Hof PR, Young WG, Morrison JH (1996) Neurochemical, morphologic, and laminar characterization of cortical projection neurons in the cingulate motor areas of the macaque monkey. J Comp Neurol 374:136–160 [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR (2007) Contribution of intermediate progenitor cells to cortical histogenesis. Arch Neurol 64 (5):639–642. doi: 10.1001/archneur.64.5.639 [DOI] [PubMed] [Google Scholar]
- Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, Di Lullo E, Haeussler M, Sandoval-Espinosa C, Liu SJ, Velmeshev D, Ounadjela JR, Shuga J, Wang X, Lim DA, West JA, Leyrat AA, Kent WJ, Kriegstein AR (2017) Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 358 (6368):1318–1323. doi: 10.1126/science.aap8809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B (2008) Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol 508 (6):906–926. doi: 10.1002/cne.21684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K (2009) Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp 30 (8):2336–2355. doi: 10.1002/hbm.20667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Zilles K (2017) Cortical layers: Cyto-, myelo-, receptor- and synaptic architecture in human cortical areas. Neuroimage doi: 10.1016/j.neuroimage.2017.08.035 [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Hoffstaedter F, Mohlberg H, Eickhoff SB, Amunts K, Zilles K (2018) Human Pregenual Anterior Cingulate Cortex: Structural, Functional, and Connectional Heterogeneity. Cereb Cortex doi: 10.1093/cercor/bhy124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Zilles K (2018) Cyto- and receptor architectonic mapping of the human brain. Handbook of clinical neurology 150:355–387. doi: 10.1016/B978-0-444-63639-3.00024-4 [DOI] [PubMed] [Google Scholar]
- Pandya D, Seltzer B, Petrides M, Cipolloni PB (2015) Cerebral Cortex: Architecture, Connections, and the Dual Origin Concept Oxford University Press, New York [Google Scholar]
- Pandya DN, Sanides F (1973) Architectonic parcellation of the temporal operculum in rhesus monkey and its projection pattern. ZAnatEntwickl-Gesch 139:127–161 [DOI] [PubMed] [Google Scholar]
- Pandya DN, Barbas H (1985) Architecture and connections of the premotor areas in the rhesus monkey [Commentary to Supplementary motor area structure and function: Review and hypotheses, Golberg G (1985); Behav Brain Sci, 8: 567–616]. The Behavioral and brain sciences 8 (4):595–596 [Google Scholar]
- Pandya DN, Seltzer B, Barbas H (1988) Input-output organization of the primate cerebral cortex. In: Steklis HD, Erwin J (eds) Comparative Primate Biology, Vol. 4: Neurosciences Liss Alan R., New York (NY), pp 39–80 [Google Scholar]
- Penfield W, Jasper H (1954) Epilepsy and the functional anatomy of the human brain Little, Brown and Company, Boston [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN (2012) The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex 48 (1):46–57. doi: 10.1016/j.cortex.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Popper KR (1959) The logic of scientific discovery Basic Books, New York, [Google Scholar]
- Puelles L (2011) Pallio-pallial tangential migrations and growth signaling: new scenario for cortical evolution? Brain, behavior and evolution 78 (1):108–127. doi: 10.1159/000327905 [DOI] [PubMed] [Google Scholar]
- Puelles L (2017) Comments on the Updated Tetrapartite Pallium Model in the Mouse and Chick, Featuring a Homologous Claustro-Insular Complex. Brain, behavior and evolution 90 (2):171–189. doi: 10.1159/000479782 [DOI] [PubMed] [Google Scholar]
- Rakic P (1974) Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 183:425–426 [DOI] [PubMed] [Google Scholar]
- Rakic P (1976a) Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature 261:467–471 [DOI] [PubMed] [Google Scholar]
- Rakic P (1976b) Differences in the time of origin and in eventual distribution of neurons in areas 17 and 18 of visual cortex in rhesus monkey. Exp Brain Res Suppl 1:244–248 [Google Scholar]
- Rakic P (2002) Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci 3 (1):65–71 [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S (1904/2002) Textura del sistema nervioso del hombre y de los vertebrados. Tomo II, segunda parte Aragón Gobierno de. Departamento de Cultura y Turismo, Zaragoza, Spain [Google Scholar]
- Ramón y Cajal S (1937) Recollections of my life. Memoirs of the American philosophical society, vol VIII, pt I-II, 1937 The American philosophical society, Philadelphia, [Google Scholar]
- Reep R (1984) Relationship between prefrontal and limbic cortex: A comparative and anatomical review. Brain Behavior and Evolution 25:1–80 [DOI] [PubMed] [Google Scholar]
- Reillo I, Romero CD, García-Cabezas MA, Borrell V (2011) A Role for Intermediate Radial Glia in the Tangential Expansion of the Mammalian Cerebral Cortex. Cereb Cortex 21 (7):1674–1694 [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H (2000) The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cereb Cortex 10 (9):851–865 [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN (1979) Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res 179:3–20 [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP (1999) Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci 2:1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MG, Casagrande VA, Preuss T, Kaas JH (1997) Visual field representation in striate and prestriate cortices of a prosimian primate (Galago garnetti). J Neurophysiol 77:3193–3217 [DOI] [PubMed] [Google Scholar]
- Roy M, Sorokina O, Skene N, Simonnet C, Mazzo F, Zwart R, Sher E, Smith C, Armstrong JD, Grant SGN (2018) Proteomic analysis of postsynaptic proteins in regions of the human neocortex. Nat Neurosci 21 (1):130–138. doi: 10.1038/s41593-017-0025-9 [DOI] [PubMed] [Google Scholar]
- Sanides F (1962) [Architectonics of the human frontal lobe of the brain. With a demonstration of the principles of its formation as a reflection of phylogenetic differentiation of the cerebral cortex]. Monographien aus dem Gesamtgebiete der Neurologie und Psychiatrie 98:1–201 [PubMed] [Google Scholar]
- Sanides F (1964) The Cyto-myeloarchitecture of the Human Frontal Lobe and its Relation to Phylogenetic Differentiation of the Cerebral Cortex. Journal fur Hirnforschung 7:269–282 [PubMed] [Google Scholar]
- Sanides F, Krishnamurti A (1967) Cytoarchitectonic subdivisions of sensorimotor and prefrontal regions and of bordering insular and limbic fields in slow loris (Nycticebus coucang coucang). J Hirnforsch 9:225–252 [PubMed] [Google Scholar]
- Sanides F (1968) The architecture of the cortical taste nerve areas in squirrel monkey (Saimiri sciureus) and their relationships to insular, sensorimotor and prefrontal regions. Brain Res 8:97–124 [DOI] [PubMed] [Google Scholar]
- Sanides F, Hoffmann J (1969) Cyto- and myeloarchitecture of the visual cortex of the cat and of the surrounding integration cortices. Journal fur Hirnforschung 11 (1):79–104 [PubMed] [Google Scholar]
- Sanides F (1970) Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: Noback CR, Montagna W (eds) The Primate Brain: Advances in Primatology Appleton-Century-Crofts Educational Division/Meredith Corporation, New York (NY), pp 137–208 [Google Scholar]
- Sanides F (1972) Representation in the cerebral cortex and its areal lamination pattern. In: Bourne GH (ed) The Structure and Function of Nervous Tissue, vol V Academic Press, New York & London, pp 329–453 [Google Scholar]
- Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, Heinsen H, Schmitz C, Hof PR (2011) Von Economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Res 1380:206–217. doi: 10.1016/j.brainres.2010.08.067 [DOI] [PubMed] [Google Scholar]
- Scholtens LH, Feldman Barrett L, van den Heuvel MP (2018) Cross-Species Evidence of Interplay Between Neural Connectivity at the Micro- and Macroscale of Connectome Organization in Human, Mouse, and Rat Brain. Brain connectivity 8 (10):595–603. doi: 10.1089/brain.2018.0622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtens LH, van den Heuvel MP (2018) Multimodal Connectomics in Psychiatry: Bridging Scales From Micro to Macro. Biological psychiatry Cognitive neuroscience and neuroimaging doi: 10.1016/j.bpsc.2018.03.017 [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P (1973) Neuronal migration, with special reference to developing human brain: a review. Brain Res 62:1–35 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA: a cancer journal for clinicians 68 (1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H (2002) Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex 12 (1):37–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian L, Remedios R, Shetty A, Tole S (2009) Signals from the edges: the cortical hem and antihem in telencephalic development. Seminars in cell & developmental biology 20 (6):712–718. doi: 10.1016/j.semcdb.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM, Brown M, Luu P, Holmes MD (2007) Discharges in ventromedial frontal cortex during absence spells. Epilepsy & behavior : E&B 11 (4):546–557 [DOI] [PubMed] [Google Scholar]
- Ungerleider L, Mishkin M (1982) Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW (eds) Analysis of Visual Behavior MIT Press, Cambridge, pp 549–586 [Google Scholar]
- van den Heuvel MP, Scholtens LH, Feldman Barrett L, Hilgetag CC, de Reus MA (2015) Bridging Cytoarchitectonics and Connectomics in Human Cerebral Cortex. J Neurosci 35 (41):13943–13948. doi: 10.1523/JNEUROSCI.2630-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Yeo BTT (2017) A Spotlight on Bridging Microscale and Macroscale Human Brain Architecture. Neuron 93 (6):1248–1251. doi: 10.1016/j.neuron.2017.02.048 [DOI] [PubMed] [Google Scholar]
- van Kooten IA, Palmen SJ, von Cappeln P, Steinbusch HW, Korr H, Heinsen H, Hof PR, van Engeland H, Schmitz C (2008) Neurons in the fusiform gyrus are fewer and smaller in autism. Brain 131 (Pt 4):987–999 [DOI] [PubMed] [Google Scholar]
- Vogt C, Vogt O (1919) Allgemeinere Ergebnisse unserer Hirnforschung. J Psychol Neurol 25:279–462 [Google Scholar]
- von Economo C (1927/2009) Cellular structure of the human cerebral cortex (Translated and edited by Triarhou Lazaros C.). Karger, Basel (Switzerland: ) [Google Scholar]
- Wagner GP (1989) The Origin of Morphological Characters and the Biological Basis of Homology. Evolution; international journal of organic evolution 43 (6):1157–1171. doi: 10.1111/j.1558-5646.1989.tb02566.x [DOI] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H, Marchi E, Ma SY, Chauhan A, Chauhan V, Bobrowicz TW, de Leon M, Louis LA, Cohen IL, London E, Brown WT, Wisniewski T (2010) The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathologica 119 (6):755–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Scholtens LH, Turk E, Van den Heuvel MP (2019) Multiscale examination of cytoarchitectonic similarity and human brain connectivity. Network Neuroscience 3 (1):124–137. doi: 10.1162/netn_a_00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA (2014) Coming full circle-from endless complexity to simplicity and back again. Cell 157 (1):267–271. doi: 10.1016/j.cell.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Wise SP (2008) Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 31 (12):599–608. doi: 10.1016/j.tins.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth MB, Girskis KM, Walsh CA (2017) Building a lineage from single cells: genetic techniques for cell lineage tracking. Nature reviews Genetics 18 (4):230–244. doi: 10.1038/nrg.2016.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey CN (1963) Comparative Studies on Localization in Precentral and Supplementary Motor Areas. International journal of neurology 4:13–20 [PubMed] [Google Scholar]
- Yakovlev PI (1959) Pathoarchitectonic studies of cerebral malformations. III. Arrhinencephalies (holotelencephalies). Journal of neuropathology and experimental neurology 18 (1):22–55 [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H (2010) Changes in prefrontal axons may disrupt the network in autism. J Neurosci 30 (44):14595–14609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H (2013) Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci 7:609. doi: 10.3389/fnhum.2013.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Garcia-Cabezas MA, Barbas H (2018a) Parallel trends in cortical grey and white matter architecture and connections in primates allow fine study of pathways in humans and reveal network disruptions in autism. PLoS Biol 16 (2). doi: 10.1371/journal.pbio.2004559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Liu X, Tepe J, Trutzer I, John YJ, Barbas H (2018b) Opposite development of short-and long-range anterior cingulate pathways in autism. Acta Neuropathol doi: 10.1007/s00401-018-1904-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N (2017) Multiple Transmitter Receptors in Regions and Layers of the Human Cerebral Cortex. Front Neuroanat 11:78. doi: 10.3389/fnana.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles KJ (1985) The cortex of the rat : a stereotaxic atlas Springer-Verlag, Berlin ; New York [Google Scholar]


