Abstract
Methotrexate (MTX) efficacy in autoimmune arthritis is variable and unpredictable resulting in the need for the identification of biomarkers to guide drug therapy. This study utilizes the collagen-induced arthritis mouse model to investigate erythrocyte MTX disposition and anti-folate activity as biochemical markers of efficacy in autoimmune arthritis. Following induction of arthritis, DBA/1J mice were treated with once-weekly subcutaneous MTX at varying doses over a period of 40 days. At the completion of the study tissue samples were analyzed for MTX and folate content and assessed for their relationship with MTX efficacy. MTX treatment resulted in a reduction in disease activity that was variable and dose-dependent. Erythrocyte accumulation of MTX and its polyglutamate metabolites were dose proportionate, however, polyglutamate metabolites represented a mean±S.E.M. of 8.9±0.4% of total erythrocyte MTX, which is markedly lower than previously observed in humans and failed to display any significant association with MTX efficacy. MTX treatment resulted in reductions in erythrocyte 5-methyl-tetrahydrofolate (5mTHF) levels that were similar to those previously observed in human studies. Disease induction was associated with a decrease in liver 5mTHF and increased formyl-tetrahydrofolate (fTHF) that was normalized in MTX treated mice. MTX efficacy was associated with reductions in erythrocyte 5mTHF (P=0.04) and increases in liver 5mTHF (P=0.0001). Together, these findings demonstrate a relationship between alterations in tissue folate levels and MTX efficacy, and supports erythrocyte levels of 5mTHF as a marker of MTX efficacy in autoimmune arthritis.
Keywords: Methotrexate, Arthritis, Pharmacokinetics, Pharmacodynamics, Folates, Biomarkers
GRAPHICAL ABSTRACT
1. INTRODUCTION
Methotrexate (MTX) is the cornerstone of therapy for autoimmune arthritis, including rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA).(Giannini et al., 1992; Weinblatt, 2013; Weinblatt et al., 1985; Williams et al., 1985) However, response to MTX is variable with one-in-three patients failing to adequately respond and is characterized by a delayed onset-of-action requiring up to six-months of therapy for maximum response.(Giannini et al., 1992; Lambert et al., 2004; Ruperto et al., 2004; Sergeant et al., 2018) At this time there are no established biomarkers that allow clinicians to stratify patients based on their likelihood of response to MTX.(Funk and Becker, 2016) Therefore, with the current therapeutic goal of early and effective control of disease to improve long-term outcomes, there remains a critical need to identify clinical biomarkers associated with the efficacy of MTX to guide clinicians in the early selection and optimization of drug therapy.(Bluett and Barton, 2017; Smolewska, 2016) As a result, this work seeks to utilize an established autoimmune arthritis mouse model to define the relationship between MTX efficacy and proposed pharmacologic biomarkers, including erythrocyte levels of MTX polyglutamate metabolites and the major circulating form of folate, 5-methyl-tetrahydrofolate (5mTHF).
MTX is a potent inhibitor of dihydrofolate reductase (DHFR) and results in the depletion of the bioactive folate pool with inhibition of the various folate-dependent methylation reactions, including nucleotide and methionine biosynthesis.(Bailey and Gregory, 1999; Kremer, 2004; White and Goldman, 1976) MTX is also metabolized intracellularly through the addition of up to six glutamate residues.(Kim et al., 1993) The resulting polyglutamate metabolites become increasingly potent direct inhibitors of several folate-dependent enzymes.(Allegra et al., 1985a; Allegra et al., 1985b; Baram et al., 1988; Sant et al., 1992) As a result of the short circulating half-life of MTX and the relative abundance of MTX polyglutamate metabolites in tissues, it has been hypothesized that the pharmacological basis for MTX response in autoimmune arthritis is not through the inhibition of DHFR, but rather through the inhibition of the folate-dependent enzymes by the polyglutamate metabolites of MTX.(Chabner et al., 1985)
Efforts to identify clinical biomarkers of MTX efficacy have primarily focused on establishing the relationship between concentrations of the polyglutamate metabolites of MTX in patient erythrocytes and clinical response.(Danila et al., 2010) Some studies have demonstrated relationships between erythrocyte concentrations of these metabolites and efficacy, but overall findings have been conflicting.(de Rotte et al., 2015; Stamp et al., 2010) Recent work in our lab measuring folates in the erythrocytes of children with JIA have demonstrated that in addition to the accumulation of MTX polyglutamates in patient erythrocytes, MTX treatment is also associated with a marked depletion of circulating folate levels re.(Funk et al., 2014) In particular, JIA patients treated with MTX have an approximately 30% reduction in erythrocyte levels of the major circulating bioactive form of folate, 5mTHF. As a result, we hypothesize that erythrocyte 5mTHF levels represent an accessible pool of tissue folates that can be used to measure the pharmacologic response to MTX.
As a pharmacodynamic measurement of MTX activity we hypothesize that erythrocyte levels of 5mTHF represents a clinical biomarker of MTX efficacy that can be used to help guide drug therapy in the treatment of autoimmune arthritis. However, there remains limited data available evaluating the antifolate activity of MTX and its relationship with efficacy in autoimmune arthritis. Therefore, this work utilizes the established collagen-induced arthritis mouse model of autoimmune arthritis to evaluate the relationship of MTX efficacy with both MTX disposition and antifolate activity.
2. MATERIALS AND METHODS
2.1. Animals.
Male DBA/1J mice at 6 to 8 weeks of age were purchased from Jackson Laboratory (Bar Harbor, ME) and housed under pathogen-free conditions at an Association and Accreditation of Laboratory Animal Care approved Animal Care Unit at The University of Kansas. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health, and were conducted under a protocol approved by the Institutional Animal Care and Use Committee at The University of Kansas.
2.2. Disease Induction and Treatment.
Following a one-week acclimation period, mice at 7- to 9-weeks of age were administered intradermal tail injections of a commercially prepared collagen/CFA emulsion containing 1 mg/ml chicken type II collagen and 2 mg/ml killed mycobacterium tuberculosis H37Ra (Hooke Laboratories, Lawrence, MA) at experimental day 0. Mice undergoing the disease induction protocol received a subsequent intradermal tail booster injection on day 19 of collagen/IFA emulsion containing 1 mg/ml chicken type II collagen. The study included healthy control mice (n=5), and mice undergoing the disease induction protocol treated with vehicle alone that served as the positive disease control group (n=5), or mice undergoing the disease induction protocol treated with once weekly subcutaneous doses of MTX (n=20) at doses of 2, 10, 20, or 50 mg/kg with five mice in each dosing group. MTX dosing was initiated in all mice at the same time and was started following the first observable measure of disease activity as determined by disease scoring and resulted in a total of 6 weekly doses over the 54-day experimental period. Mice were fed with normal chow ad libitum. For mice developing clinical arthritis normal chow was substituted initially with moistened normal chow at day 37, and later with DietGel® Recovery (Clear H2O, Portland, ME) at day 47 due to concerns with animal weight loss and possible dehydration. Mice were closely monitored throughout the study for humane endpoints and resulted in the removal from the study of six mice due to extensive tail blistering secondary to the intradermal collagen emulsion injections. Two mice were found deceased following the second intradermal tail injection. Mice were monitored two to three times weekly for changes in weight, disease activity scoring, and paw volume measurements throughout the duration of the study. At experimental day 54, all mice were euthanized by CO2 asphyxiation and tissue samples were harvested for analysis. At the conclusion of the study there remained a total of 22 mice, including: 5 mice per group in the untreated healthy control group, 4 mice per group in the 0 and 50 mg/kg MTX treatment group, and 3 mice per group in the 2, 10, and 20 mg/kg MTX treatment group, with a total of 13 mice treated with MTX at varying doses.
2.3. Disease Activity Scoring.
Disease activity scores resulted from the assessment of clinical arthritis in all four limbs using an established scoring system.(Brand et al., 2007) Briefly, a disease severity score between 0 and 4 was determined for each limb by visual inspection and agreement by two investigators. A severity score of 0 was assigned if there was no evidence of erythema and swelling of the limb, a score of 1 was assigned for erythema and swelling isolated to a single digit, a score of 2 was assigned for erythema and swelling in > 1 digit or mild swelling of the entire paw, a score of 3 was assigned for erythema and swelling of the entire paw, and a score of 4 was assigned for severe swelling of the entire paw or limb ankyloses. The resulting disease activity score was the sum of the disease severity score for each limb with a maximum score of 16 and was individually tracked for each animal over the duration of the study.
2.4. Paw Volume Measurements.
Arthritis disease activity was also routinely monitored by measuring paw volume using limb water volume displacement.(Buyuktimkin et al., 2014) Briefly, paw volumes were determined by measuring the volume of water displaced by each paw by dipping each individual paw in a water bath and using a syringe to measure the volume of water displaced. Hind and front paws were submerged up to and including the ankle and elbow joints, respectively. Paw volume measurement were collected through the duration of the study and were reported as the sum of the total water volume displaced by all four paws for each animal.
2.5. Joint Histopathology Studies.
Disease activity was also assessed by histological examination of all four limbs of each animal at the completion of the study. Limbs were collected on day 54 of the study and fixed in 10% buffered formalin prior to embedding in paraffin and staining with hematoxylin and eosin. Arthritic disease activity was scored individually on a scale of 1 to 4 in each of five categories: acute inflammation, chronic inflammation, synovial membrane thickening, formation of pannus/granulation tissue, and articular damage. A score of 0.0 was assigned for no lesions or changes, a score of 0.5 to 1.0 was assigned for mild changes, a score of 1.5 to 2.0 was assigned for moderate changes, a score of 2.5 to 3.0 was assigned for marked changes, and a score of 3.5 to 4.0 was assigned for severe changes. The average composite joint histology score for the front and hind limbs of each mouse was determined individually with a maximum possible score of 20. Histopathology evaluations were conducted by Dr. Stanley Kosanke at the University of Oklahoma Health Sciences Center.
2.6. Methotrexate and Folate Analysis.
Blood samples were obtained by cardiac puncture following euthanization at experimental day 54 and were collected into potassium EDTA-containing tubes. Separation of blood into erythrocyte and plasma fractions was accomplished by centrifugation at 1600 g for 10 min at room temperature. The resulting erythrocyte pellet was washed once by re-suspension in PBS at room temperature and pelleted by centrifugation at 1600 g for 10 min at room temperature. Erythrocyte samples were stored at −80 °C prior to analysis. Fresh liver samples were also collected following euthanization and stored at −80 °C prior to analysis. Erythrocyte and liver samples were analyzed using an established UPLC-MS/MS method to quantify the tissue content of MTX and its polyglutamate metabolites containing up to a total of seven glutamic acid residues.(van Haandel et al., 2011) Similarly, erythrocyte content of 5mTHF and liver content of 5mTHF, fTHF, tetrahydrofolate (THF) and folic acid (FA) were determined using previously established UPLC-MS/MS assays.(Funk et al., 2014; van Haandel et al., 2012) Erythrocyte samples were normalized to packed erythrocyte volume and reported as nmole of analyte per liter of packed erythrocyte volume. Liver samples were normalized based on the weight of liver tissue from which the analytes were extracted and are reported as pmole per mg of tissue. Changes in tissue folate content were also expressed as a percentage change based on mean folate levels in animals with arthritis not treated with MTX. Comparisons with folate and MTX measurements from human subjects were derived from previously published work evaluating erythrocyte MTX and folate levels in patients with JIA.(Becker et al., 2010; Funk et al., 2014)
2.7. Statistical Analysis.
Data collection and statistical testing was conducted using the statistical package in Excel 2016 (Microsoft, Redmond, Washington) and JMP v11 (SAS Institute Inc, Cary, NC). Differences between the treatment groups and by dose stratification in paw volume measurements, changes in weight, and tissue folate levels were evaluated by unpaired two-tailed Student’s t-test analysis. Disease activity and histological scoring data were evaluated by non-parametric analysis using the Wilcoxon rank-sum test. Spearman’s rank correlation analyses were used for multivariable correlation testing, including evaluation of the relationship of tissue MTX and folate levels with measures of disease activity. The results of multivariable correlation analyses are presented as a Spearman’s correlation coefficient (ρ) along with corresponding P-values.
3. RESULTS
3.1. MTX efficacy in the collagen-induced arthritis (CIA) mouse model.
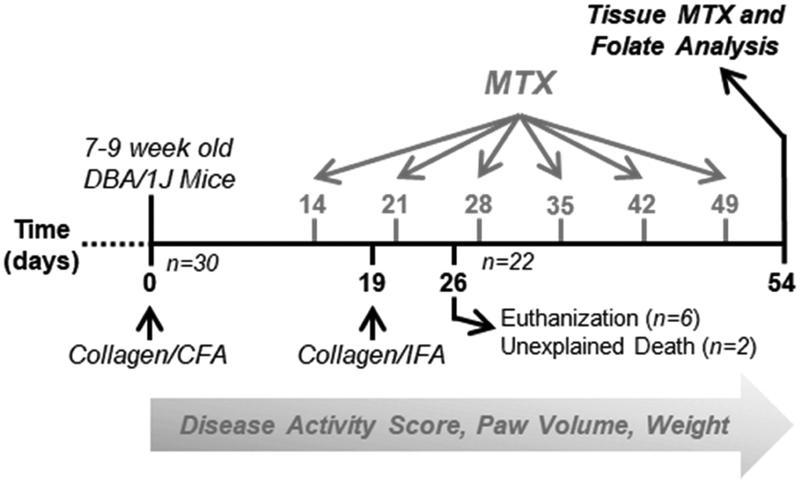
The efficacy of MTX in autoimmune arthritis was evaluated in the CIA mouse model using the experimental design illustrated in Fig. 1 and initially included two control groups with five mice per group and a MTX treatment group of twenty mice that was stratified into four subgroups of five mice per group based on the dose of MTX administered. Except for the healthy control mice, all mice underwent the CIA protocol with induction and booster injections at days 0 and 19, respectively. MTX treatment was initiated upon the first signs of disease activity and was marked by the observation of digit redness and swelling at day 14 in two mice. MTX was administered once weekly via the subcutaneous route at doses of 2, 10, 20 or 50 mg/kg and was initiated at the same time for all mice. Eight mice were removed during the study and were all mice undergoing the arthritis induction procedure. Two mice were found deceased shortly after their CIA booster injection given on day 19 and six mice were removed by the attending veterinarian and euthanized as a result of extensive tail blistering by day 26. At the completion of the study the healthy control group had five mice, the positive disease control group had four mice, and the MTX treatment group had 13 mice with three mice each receiving doses of 2, 10, and 20 mg/kg MTX and four mice receiving 50 mg/kg MTX.
Fig. 1. Diagram of disease induction and MTX treatment study design.
DBA/1J mice were split into 3 groups including a healthy control group, a CIA disease control group, and a MTX treatment group. The CIA disease control group of MTX treatment group underwent the CIA disease induction protocol including an initial injection of collagen/CFA at day 0 and a booster injection of collagen/IFA at day 19. The MTX treatment group were further stratified into 4 subgroups and administered subcutaneous MTX at doses of 2, 10, 20, or 50 mg/kg weekly starting on day 14 for a total of 6 doses. Disease activity scores, paw volume measurements, and animal weight were collected throughout the study. At day 54 mice were euthanized and tissues were collected for MTX and folate analysis. Eight mice were lost during the study and not included in the terminal analysis.
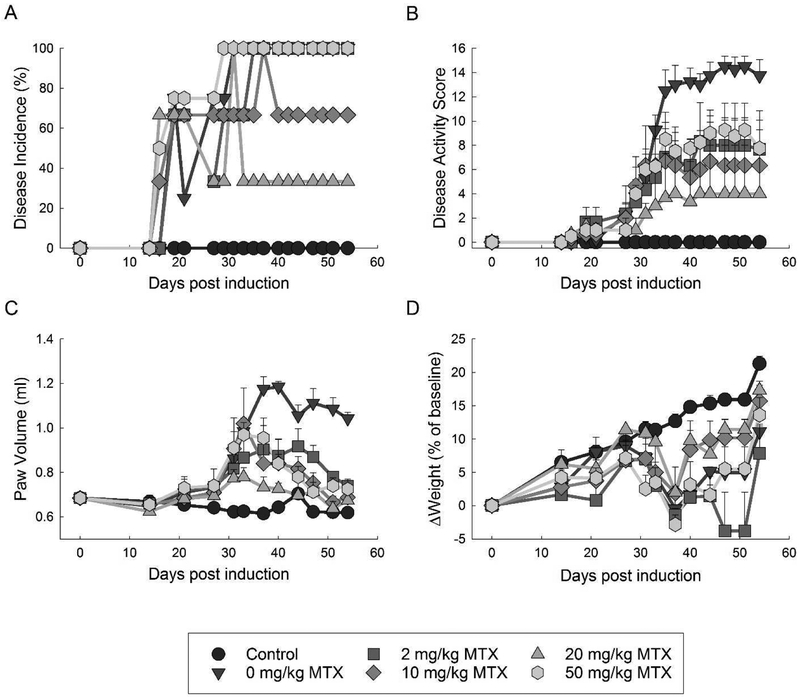
Over the duration of the study all mice undergoing the CIA regimen developed measurable disease activity, defined here as a disease activity score of at least one, at some point throughout the study and resulted in an overall induction incidence of 100% by day 37 (Fig. 2A). No measurable disease activity was noted in the healthy control group over the duration of the study. Several mice treated with 10 and 20 mg/kg MTX developed only transient digit inflammation resulting in a decreased overall incidence of disease throughout most of the study. At the completion of the study (i.e. day 54) 0% of the healthy mice, 100% of the CIA control mice, and 76% of the MTX treated mice had measurable disease activity. When stratified by MTX dose 100% of mice in the 2 and 50 mg/kg MTX groups had measurable disease activity, whereas 67% of mice in the 10 mg/kg MTX group and 33% in the 20 mg/kg MTX group had measurable disease activity.
Fig. 2. Effect of MTX on disease incidence and activity in the CIA mouse model.
Disease incidence and activity were compared amongst control mice and mice undergoing the CIA protocol treated with weekly subcutaneous MTX at doses of 0, 2, 10, 20, or 50 mg/kg. Groups were compared based on the incidence of arthritis (A), disease activity (B), paw volume (C), and change in weight from baseline (D). Parameters were measured over the 54-day experimental period and the resulting mean±S.E.M. for each experimental group is presented.
Measures of MTX efficacy in this study included disease activity scores, paw volume measurements, monitoring for changes in animal weight, and endpoint joint histology scoring. Disease activity scores appeared to peak around day 37 with minimal subsequent change throughout the duration of the study (Fig. 2B). Representative images of a normal paw from a control mouse and an active disease paw are provided in the supplemental data (Supplemental Fig. 1). Compared to the CIA control mice treated with vehicle alone (i.e. 0 mg/kg MTX), treatment with MTX at doses of 2, 10 and 20 mg/kg appeared to result in a dose dependent reduction in disease activity scores, whereas 50 mg/kg MTX appeared to display a paradoxical increase in disease activity scores to a level similar to those seen in the 2 mg/kg MTX group. Measurement of paw volume showed a continued increase in paw volume in the untreated CIA group up to day 37, with a trend towards decreasing paw volumes over the remainder of the study (Fig. 2C). Compared to the CIA control group, mice treated with MTX had markedly lower maximum paw volume measurements that approached those of untreated control mice by the end of the study. The most pronounced decrease in paw volume were observed with the 20 mg/kg MTX treatment group. Induction of arthritis corresponded to a marked suppression of weight gain that peaked at day 37 (Fig. 2D). However, by day 54 a strong rebound in weight was observed in all groups with a marked weight gain in the last week that likely reflected the switching of all animals to a nutritionally fortified soft water gel diet due to concerns of dehydration in some animals. Maximum suppression of weight gain was observed in the 2 mg/kg MTX treated mice, whereas mice treated with 0 and 50 mg/kg MTX displayed a similar level of suppression of weight gain, and the least suppression of weight gain was observed in the 10 and 20 mg/kg MTX treatment groups.
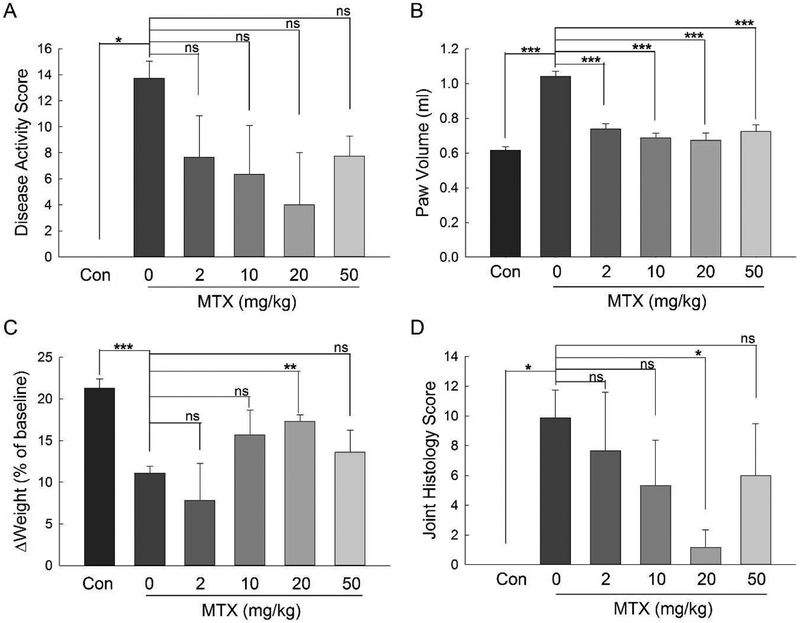
At the completion of the study on day 54, endpoint analysis of disease activity was completed and included evaluation of final disease activity scores, paw volume measurements, changes in weight from baseline, and post-euthanasia knee and elbow joint histological scoring by an independent pathologist. Based on disease activity scores, the CIA protocol resulted in a robust induction of arthritis that displayed minimal variability in the CIA control group (i.e. 0 mg/kg) with nearly maximal disease within the group based on the 16-point disease activity scale (Fig. 3A). Overall, mice treated with MTX as a group had reductions in mean±S.E.M. disease activity scores compared to the CIA control group (6.5±1.4 vs. 13.8±1.3, P=0.03). Evaluation of the dose-response relationship revealed a trend with 20 mg/kg MTX resulting in the greatest reduction in disease activity, however the dose stratified results did not reach statistical significance. Similarly, endpoint paw volumes as a measure of paw swelling and inflammation associated with disease activity showed that the CIA protocol resulted in a robust and reproducible increase in disease activity in the CIA disease control group (Fig. 3B). Similarly, mice treated with MTX as group had significant reductions in mean±S.E.M. paw volume measurements compared to the CIA control group (0.71±0.02 ml vs. 1.04±0.03 ml, P<0.0001) and stratification by dose showed that all of the MTX treatment regimens were associated with significant and similar reductions in paw swelling by the completion of the study (Fig. 3B). The CIA protocol also resulted in a marked and reproducible reduction in weight gain over the duration of the study that continued to be significant at the endpoint analysis (Fig. 3C). Overall, mice treated with MTX as a group had non-statistically significant increases in mean±S.E.M. percentage weight gain compared to the CIA control group (13.1±1.6% vs. 11.1±0.8%, P=0.19), however stratification by dose demonstrated a dose-dependent improvement in weight gain with a maximal and statistically significant effect observed with the 20 mg/kg MTX treatment group. Limb samples for each animal were submitted for histologic scoring and resulted in a composite score that further supported a robust and reproducible induction of disease in the CIA mice (Fig. 3D). As a group, mice treated with MTX had non-statistically significant lower mean±S.E.M. joint histology scores compared to the CIA disease control group (5.1±1.5 vs. 9.9±1.9, P=0.09), and consistent with the other measures of disease activity showed a dose-dependent and statistically significant reduction with MTX treatment at 20 mg/kg. Representative images from joint histologic scoring are provided in the supplemental data (Supplemental Fig. 2).
Fig. 3. Endpoint analysis of MTX efficacy in the CIA mouse model.
Endpoint measures of disease activity collected at day 54 of the study, including: disease activity scores (A), paw volume measurements (B), changes in weight from baseline (C), and joint histology scores (D) were compared in control mice and mice undergoing the CIA protocol treated with weekly subcutaneous MTX at doses of 0, 2, 10, 20, or 50 mg/kg. The groups were compared based on endpoint. The resulting mean±S.E.M. for each experimental group is presented and was compared by Wilcoxon rank-sum or Student’s t-test analysis, as appropriate.
Multivariable analysis by Spearman’s rank correlation analysis revealed a high level of correlation between the various independent measures of disease activity. Endpoint disease activity scores strongly correlated with endpoint paw volume measurements (ρ=0.86, P<0.0001), change in weight from baseline (ρ=−0.75, P<0.0001), and joint histology scores (ρ=0.85, P<0.0001). These endpoint measures of disease activity were used in all subsequent covariate analyses of potential biological markers of MTX efficacy in the CIA mice.
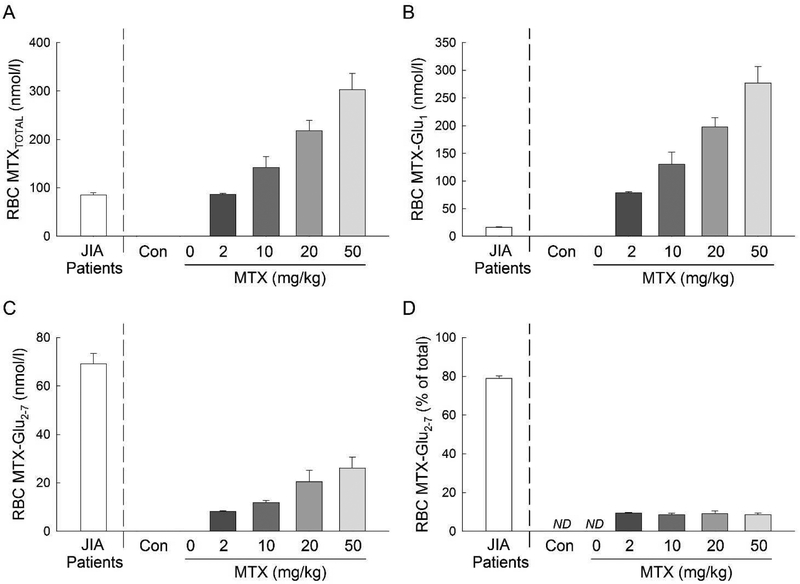
3.2. Tissue accumulation of MTX and its polyglutamate metabolites.
MTX uptake and formation of polyglutamate metabolites were measured at day 54 following six consecutive once weekly subcutaneous MTX injections (Fig. 4). For comparison, the observed erythrocyte concentrations of total MTX (MTXTOTAL), parent MTX (MTX-Glu1), and the polyglutamate metabolites of MTX (MTX-Glu2–7), and the percentage of erythrocyte MTX as a polyglutamate metabolite are plotted for each treatment condition for the CIA mice along with measured levels of these analytes from our previous work in patients with JIA for reference.(Becker et al., 2010) Erythrocyte concentrations of MTXTOTAL for mice treated with 2 mg/kg MTX were of a similar magnitude to those previously observed in patients with JIA receiving MTX therapy, and increased dose proportionately up to the highest tested dose of 50 mg/kg (Fig. 4A). Although the erythrocyte concentrations of MTX-Glu1 (Fig. 4B) and MTX-Glu2–7 (Fig. 4C) in mice also displayed a similar dose dependency, the majority of erythrocyte MTX existed in the parent form of the drug with relatively low levels of the polyglutamate metabolites. At the highest MTX dose tested, erythrocyte MTX-Glu1 levels were over 16-fold higher in mice than the reference JIA patient population, however erythrocyte MTX-Glu2–7 levels were 62% lower than those observed in the reference JIA population. The percentage of erythrocyte MTX existing as a polyglutamate (i.e. MTX-Glu2–7) did not change as a function of dose and represented a mean±S.E.M. of 8.9±0.4% of erythrocyte MTXTOTAL compared to 78.8±1.4% previously measured in patients with JIA (Fig. 4D).
Fig. 4. Erythrocyte MTX polyglutamate formation in the CIA mouse model.
Total erythrocyte MTX (A), erythrocyte concentrations of parent MTX (B), erythrocyte concentrations of polyglutamate metabolites of MTX (C), and the percent of MTXTOTAL existing as a polyglutamate (D) were compared amongst CIA mice treated with weekly subcutaneous MTX at doses of 0, 2, 10, 20, or 50 mg/kg. The resulting mean±S.E.M. for each experimental group is presented and was compared by Student’s t-test analysis.
Levels of MTX and its polyglutamate metabolites were also measured in liver samples collected at the completion of the study for each group of mice (Supplemental Fig. 3). Interestingly, Liver MTXTOTAL failed to demonstrate the clear dose-proportionality observed for erythrocyte MTXTOTAL, and included a lack of dose-proportionate changes in both levels of MTX-Glu1 and MTX-Glu2–7. In contrast, a higher percentage of MTX existing in its polyglutamated form was observed in the liver and was moderately proportional to dose with a mean±S.E.M. of 24.8±2.6% of liver MTXTOTAL in mice treated with 50 mg/kg MTX. No significant correlations were observed between liver and erythrocyte concentrations of MTX and resulted in poor correlations between measurements of liver and erythrocyte MTXTOTAL (ρ=0.46, P=0.11), as well as liver and erythrocyte MTX-Glu2–7 (ρ=0.48, P=0.09).
3.3. MTX levels fail to be associated with measures of efficacy.
Measured erythrocyte and liver levels of MTX were evaluated for their relationship with the various endpoint measures of MTX efficacy to interrogate the association of these potential biomarkers with the observed efficacy of MTX. By Spearman’s rank correlation analysis, liver and erythrocyte levels of MTXTOTAL, MTX-Glu1 and MTX-Glu2–7 were evaluated for their relationship with the endpoint measures of MTX efficacy, including: disease activity score, paw volume measurements, change in weight from baseline, or the joint histology score. Interestingly, no significant relationship between any of the measures of tissue MTX disposition and any of the endpoint measures of disease activity were observed (Supplemental Table 1).
3.4. Dysregulation of tissue folates following induction of arthritis and treatment with MTX.
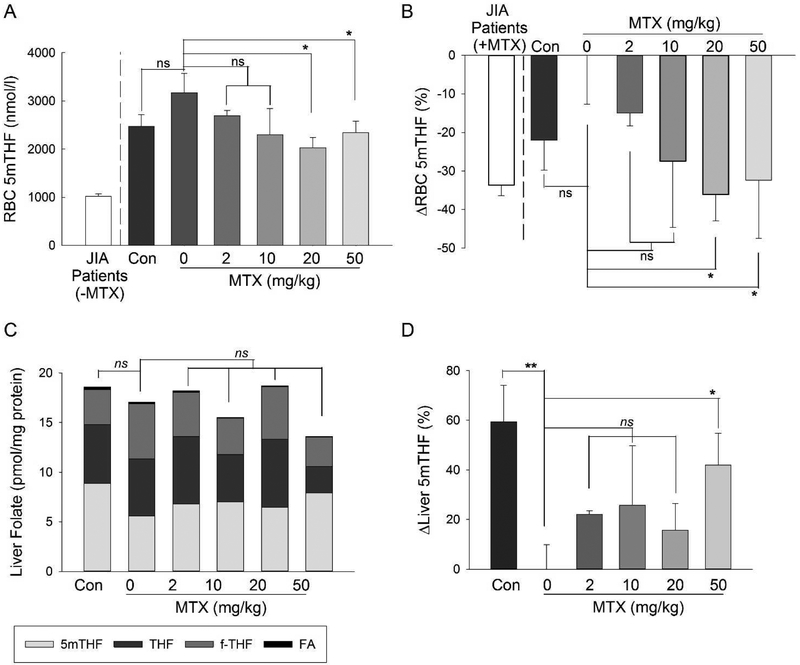
Mouse erythrocytes collected at the completion of the study were analyzed for 5mTHF content (Fig. 5A). Measured erythrocyte 5mTHF levels from our JIA patient population were plotted for reference and were less than half the concentration observed in mice and are consistent with the previously reported high folate levels in mice.(McKay et al., 2011) In the CIA control mice (i.e. 0 mg/kg), levels of 5mTHF were found to be slightly elevated compared to healthy control animals, however the difference did not reach statistical significance. Overall, the group of mice treated with MTX had significantly lower mean±S.E.M. erythrocyte 5mTHF levels compared to the CIA disease control group (2246±160 vs. 3170±400 nmol/l, P=0.02) and was associated with a dose-dependent reduction that peaked at 20 mg/kg MTX and was significant for both the 20 mg/kg and 50 mg/kg MTX treated mice. The percentage reduction in erythrocyte 5mTHF content using the untreated CIA mice (i.e. 0 mg/kg) as the reference group was evaluated and compared to our previous findings in the JIA patient population (Fig. 5B). Compared to the CIA disease control mice, treatment with 20 mg/kg and 50 mg/kg MTX weekly resulted in mean±S.E.M. reductions of erythrocyte 5mTHF of 36±7% and 32±8%, respectively, which is similar in magnitude to that previously observed in JIA patients treated with MTX.(Funk et al., 2014)
Fig. 5. Effect of MTX on erythrocyte and liver 5mTHF in the CIA mouse model.
Erythrocyte concentrations of 5mTHF (A), percentage change in erythrocyte 5mTHF (B), liver levels of 5mTHF (C), and percentage change in liver 5mTHF (D) were compared amongst control mice and mice undergoing the CIA protocol treated with weekly subcutaneous MTX at doses of 0, 2, 10, 20, or 50 mg/kg. The resulting mean±S.E.M. for each experimental group is presented and was compared by Student’s t-test analysis.
Liver folate content was also measured and included levels of FA, THF, f-THF and 5mTHF (Fig. 5C). Total liver folate, which is represented as the sum of the folates species measured, was not found to change significantly with the induction of arthritis. Total liver folate levels were not found to be different between the CIA disease control mice and the MTX treated mice, as a group or stratified by dose. However, comparisons of individual folate isoforms revealed that compared to healthy control mice, CIA control mice had significant reductions in mean±S.E.M. liver 5mTHF levels (8.9±0.8 vs. 5.6±0.6 nmol/l, P=0.003) as well as marked elevations in mean±S.E.M. liver f-THF levels (3.5±0.3 vs. 5.6±0.3 nmol/l, P=0.01) resulting in a shift in the mean±S.E.M. ratio for 5mTHF:f-THF in healthy control mice from 2.5±0.1 to 1.0±0.1 in the CIA control mice (P=0.005) that represented a shift in favor of the oxidized form of methylated folate in the disease mice. Mean±S.E.M. liver 5mTHF levels in the MTX treated mice were increased compared to the CIA disease control mice (7.1±0.4 vs. 5.6±0.6 nmol/l, P=0.08), but didn’t reach statistical significance. However, by MTX dose stratification the 50 mg/kg MTX treatment was associated with a significant increase in liver 5mTHF levels (Fig. 5D). Compared to the CIA control mice, mice receiving MTX treatment as a group had a significant overall reduction in mean±S.E.M. liver f-THF levels (5.6±0.3 vs. 4.0±0.4 nmol/l, P=0.02), and by dose stratification were statistically significant for the 10 mg/kg (P=0.02) and 50 mg/kg (P=0.0009) MTX treated mice. Treatment with 50 mg/kg MTX was also found to cause a marked reduction in mean±S.E.M. liver THF levels (5.7±0.6 vs. 2.6±0.5, P=0.007). No change in FA levels were observed either between disease and healthy groups, nor between untreated and MTX treated groups. As a result of MTX treatment causing an increase in liver 5mTHF and a decrease in liver f-THF, MTX treatment was associated with a significant increase in the mean±S.E.M. ratio of liver 5mTHF:f-THF from 1.0±0.1 in the CIA control mice to 2.0±0.2 in the MTX treated mice (P=0.02).
3.5. Association of tissue folate levels with MTX efficacy.
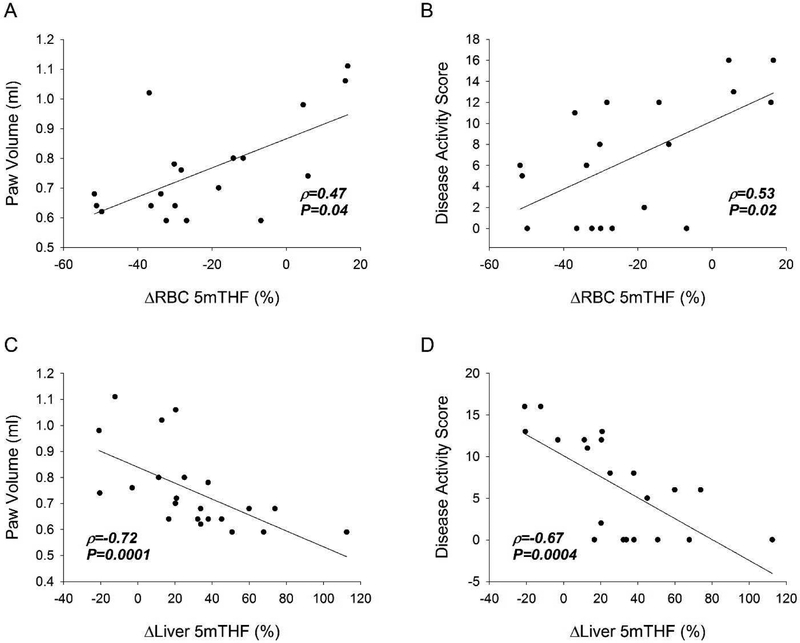
The association of measured folate levels in erythrocytes and the liver were evaluated for their association with the various endpoint measures of MTX efficacy. By Spearman’s rank correlation analysis, reductions in erythrocyte and liver 5mTHF were represented as a percentage change from the CIA control mice (Fig. 6). Reductions in erythrocyte 5mTHF levels were associated with reductions in both paw volume measurements (Fig. 6A) and disease activity scores (Fig. 6B). Similarly, increases in liver 5mTHF were associated with reductions in paw volume measurements (Fig. 6C) and reductions in disease activity scores (Fig. 6D). Increases in liver 5mTHF were also associated with increased MTX efficacy by both measured changes in weight from baseline (ρ=0.50, P=0.02) and by joint histology scores (ρ=−0.56, P=0.007). Measures of total liver folate levels, liver THF, f-THF and FA were not found to be significantly associated with any of the measures of MTX efficacy. However, increases in the ratio of liver 5mTHF:f-THF were associated with increased MTX efficacy by both paw volume measurements (ρ=−0.60, P=0.003) and by disease activity scores (ρ=−0.50, P=0.02).
Fig. 6. Association of tissue folates with MTX efficacy in the CIA mouse model.
The percentage change in erythrocyte 5mTHF in the CIA mouse model was evaluated by Spearman’s rank correlation analysis for their relationship with MTX efficacy by endpoint measures of paw volume (A) and disease activity (B). The percentage change in liver 5mTHF in the CIA mouse model was evaluated by Spearman’s rank correlation analysis for their relationship with MTX efficacy by endpoint measures of paw volume (C) and disease activity (D). The resulting Spearman’s correlation coefficient (ρ) and associated P-values are provided.
4. DISCUSSION
In this study we evaluated erythrocyte MTX polyglutamates and 5mTHF as putative pharmacologic markers of MTX response in autoimmune arthritis using the CIA mouse model. We found that our CIA protocol induced robust and reproducible arthritis that was responsive to MTX treatment. Although the response to MTX was variable, MTX treatment was found to be associated with the reduction in multiple independent measures of arthritis disease activity and displayed a dose-dependency. However, with our small sample sizes in each group ranging from three to five mice per group in our dose stratified analysis it is difficult to make definitive conclusions regarding the dose-dependence for the observed efficacy of MTX in this model. With a similar caution regarding our relatively small sample sizes in the dose-stratification analyses, we also found dose proportionate accumulation of MTX polyglutamates and a corresponding depletion of 5mTHF in circulating erythrocytes, along with corresponding measures of MTX polyglutamate accumulation and altered folate homeostasis in the liver. Interrogation of the association between the effect of MTX on disease activity (i.e. efficacy) and these molecular markers of MTX activity demonstrated that the efficacy of MTX was associated with the enhanced depletion of erythrocyte 5mTHF, but failed to demonstrate a similar relationship with the accumulation of MTX polyglutamates in erythrocytes. Therefore, these studies further support the role of erythrocyte 5mTHF as a molecular biomarker of MTX response in autoimmune arthritis.
Subcutaneous weekly MTX administration is common in clinical practice and was the basis for the dosing regimen chosen for this study.(Hazlewood et al., 2016; Vena et al., 2018) This regimen is based on the reduced risk of MTX-related toxicities associated with an extended dosing interval and the avoidance of variable bioavailability related to the saturable transporter mediated absorption across the gastrointestinal lumen when given orally.(Hoekstra et al., 2004; Radmanesh et al., 2011; Weinstein et al., 1973) However, based on previous data demonstrating reductions in erythrocyte 5mTHF in JIA patients receiving either oral or subcutaneous MTX, the observations made in this study are expected to similarly apply to the use of oral MTX.23 Interestingly, our data appeared to display a dose-dependent reduction in disease activity that peaked at 20 mg/kg weekly, but appeared to demonstrate reduced efficacy at 50 mg/kg. Although, unexpected, this apparent paradoxical dose-response relationship for MTX in autoimmune disease had been previously reported. Specifically, the MRL/lpr spontaneous autoimmune disease mouse model has previously demonstrated a dose-dependent reduction in arthritis disease activity at weekly MTX doses up to 25 mg/kg with an apparent reduction in efficacy at the 100 mg/kg weekly dose.(Baggott et al., 1992) Although the mechanistic basis for this paradoxical response remains unknown, the authors of the previous work associated these higher doses with MTX toxicity based on a measured decrease in mouse lifespan. Here, based on the known impact of MTX on the balance of effector and regulatory immune function, we speculate that MTX-mediated selective suppression of effector immunologic activity may be lost at higher MTX doses with increased activity towards immunoregulatory pathways resulting in an overall increased susceptibility to autoimmune disease induction and a reduction in MTX efficacy.(Charlton and Lafferty, 1995; Constantin et al., 1998; Herman et al., 2008; Yamaki et al., 2003)
Despite the widespread use of MTX as a positive control in drug efficacy studies utilizing the CIA mouse model, this is the first study to investigate the in vivo tissue formation of MTX polyglutamate metabolites and the antifolate effect of MTX in this model.(Lange et al., 2005; Luo et al., 2013; Neurath et al., 1999; Zhang et al., 2013) Previous work in a rat model of collagen-induced arthritis developed a mathematical pharmacokinetic-pharmacodynamic model for MTX polyglutamate formation and efficacy, however an exploration of the validity of MTX polyglutamates as a biochemical marker of efficacy was not undertaken.(Liu et al., 2013) The low level of MTX polyglutamate formation in the CIA mouse model was initially surprising based on the established efficacy of MTX in this model and the perceived importance of MTX polyglutamates as critical to the pharmacologic activity of MTX.(Chabner et al., 1985; Neurath et al., 1999) However, recent studies using dermal fibroblasts from the DBA/1J mouse strain have demonstrated that they are deficient in the formation of MTX polyglutamates, which might explain the high MTX dose requirements needed in these animals.(Inglis et al., 2007; You et al., 2013) In agreement with this previous report we found approximately 10 to 25% of tissue MTX to exist as a polyglutamate in the DBA/1J mice, which is far below the high level of polyglutamation that we typically see in patients (i.e. ~80%).(Becker et al., 2010) Despite the low level of MTX polyglutamate formation in our mice, we did observe efficacy with MTX, however, erythrocyte MTX polyglutamate levels failed to demonstrate any significant relationship with any of the measures of MTX efficacy. Therefore, this data supports MTX polyglutamates as an important marker of MTX exposure, as has been supported by studies showing the dose-dependent accumulation of these metabolites in erythrocytes with the potential use of these analytes to dose correct for pharmacokinetic variation and capture potential treatment non-adherence.(Dervieux et al., 2005; Hawwa et al., 2015; Stamp et al., 2009) However, the inability of our data to draw a correlation between the formation of these metabolites and efficacy suggests that non-pharmacokinetic variables may represent an important source for the observed variation in MTX efficacy.
Interestingly, we observe a slight but non-statistically significant elevation in erythrocyte levels of 5mTHF in the disease animals, and despite no change in total folate in the liver there was a shift in the ratio of liver 5mTHF to f-THF following induction of disease. Although previous work has shown that rheumatoid arthritis is associated with reduced circulating folate concentrations, unpublished work in our laboratory measuring erythrocyte levels of 5mTHF have demonstrated an association between increased erythrocyte 5mTHF content and reduced hematocrit in JIA patients with active disease.(Omer and Mowat, 1968) Therefore, we speculate that induction of disease likely results in an anemic response, similar to that observed in patients with autoimmune arthritis, and because the anemia is likely an iron-deficient anemia the existing erythrocytes are likely enriched in 5mTHF content resulting in an increase in the amount of 5mTHF per packed volume of erythrocytes.(Baer et al., 1990; Omer et al., 1970) The shift in liver folate homeostasis resulting in an increase in f-THF and decrease in 5mTHF is currently unexplained, but has been a documented phenomenon occurring in animals treated with methionine.(Brody et al., 1982) Whether the shift towards f-THF represents an enzymatic shift in the production of f-THF at the cost of 5mTHF production, or whether it represents the enzymatic or chemical oxidation of 5mTHF to f-THF is unknown, but we speculate that increased oxidative stress in the disease animal results in the chemical oxidation of 5mTHF resulting in the increased conversion to f-THF.(Choi, 2007; Lumb et al., 1988)
The observation that changes in tissue folate levels is directly associated with the efficacy of MTX appears to contradict previous clinical studies failing to measure changes in circulating folate levels in patients treated with MTX.(de Rotte et al., 2013; Morgan et al., 1994) In fact, these previous observations have served as the basis for the longstanding argument that the pharmacologic activity of MTX in autoimmune arthritis is independent of its antifolate activity.(Cronstein, 1992) However, in contrast to this prior work, recent work by our group has clearly demonstrated that initiation of MTX is associated with an approximately 30% reduction in erythrocyte 5mTHF levels in JIA patients despite the fact they were receiving daily folic acid supplementation.(Funk et al., 2014) We speculate the differing effects of MTX on folates in our studies and previous studies reflects differences in the analytical methodologies utilized to quantify folate levels, and believe the UPLC-MS/MS methods used in our studies are more selective and sensitive for the absolute quantification of folates.(van Haandel and Stobaugh, 2013) As a result, our data supports a clinically significant anti-folate effect of MTX that results in the reduction of erythrocyte concentrations of 5mTHF in both patients treated with MTX and in our CIA mouse model. Further, in the CIA mouse model we have now demonstrated that this anti-folate effect is directly associated with the efficacy of MTX and hypothesize that the same would be true in patients.
In conclusion, the key finding of this study is that MTX causes a reduction in arthritis disease activity in the CIA mouse model and fails to demonstrate a clear relationship with the tissue accumulation of MTX polyglutamates, but rather appears to be associated with MTX-mediated changes in tissue folate levels. Together, these results support tissue folate homeostasis as a key pharmacologic target of MTX therapy in autoimmune arthritis and indicates that erythrocyte 5mTHF levels may represent an accessible pharmacodynamic biomarker of MTX efficacy in the treatment of autoimmune arthritis.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the University of Kansas General Research Fund and a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# TL1TR002368).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no personal or financial conflicts of interest related to this work.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property. We further confirm that any aspect of the work covered in this manuscript that has involved experimental animals has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. We understand that the Corresponding Author is the sole contact for the Editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from ryanfunk@kumc.edu.
SUBMISSION DECLARATION
This work has not been previously published, nor is it under review for publication elsewhere. This manuscript has been reviewed and approved by all authors.
REFERENCES
- Allegra CJ, Chabner BA, Drake JC, Lutz R, Rodbard D, Jolivet J, 1985a. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. The Journal of biological chemistry 260, 9720–9726. [PubMed] [Google Scholar]
- Allegra CJ, Drake JC, Jolivet J, Chabner BA, 1985b. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proceedings of the National Academy of Sciences of the United States of America 82, 4881–4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer AN, Dessypris EN, Krantz SB, 1990. The pathogenesis of anemia in rheumatoid arthritis: a clinical and laboratory analysis. Seminars in arthritis and rheumatism 19, 209–223. [DOI] [PubMed] [Google Scholar]
- Baggott JE, Morgan SL, Freeberg LE, Hudson BB, Vaughn WH, Gopal Nair M, Krumdieck CL, Koopman WJ, Gay RE, Gay S, 1992. Long-term treatment of the MRL/lpr mouse with methotrexate and 10-deazaaminopterin. Agents Actions 35, 104–111. [DOI] [PubMed] [Google Scholar]
- Bailey LB, Gregory JF 3rd, 1999. Folate metabolism and requirements. The Journal of nutrition 129, 779–782. [DOI] [PubMed] [Google Scholar]
- Baram J, Chabner BA, Drake JC, Fitzhugh AL, Sholar PW, Allegra CJ, 1988. Identification and biochemical properties of 10-formyldihydrofolate, a novel folate found in methotrexate-treated cells. The Journal of biological chemistry 263, 7105–7111. [PubMed] [Google Scholar]
- Becker ML, van Haandel L, Gaedigk R, Lasky A, Hoeltzel M, Stobaugh J, Leeder JS, 2010. Analysis of intracellular methotrexate polyglutamates in patients with juvenile idiopathic arthritis: effect of route of administration on variability in intracellular methotrexate polyglutamate concentrations. Arthritis and rheumatism 62, 1803–1812. [DOI] [PubMed] [Google Scholar]
- Bluett J, Barton A, 2017. Precision Medicine in Rheumatoid Arthritis. Rheum Dis Clin North Am 43, 377–387. [DOI] [PubMed] [Google Scholar]
- Brand DD, Latham KA, Rosloniec EF, 2007. Collagen-induced arthritis. Nat Protoc 2, 1269–1275. [DOI] [PubMed] [Google Scholar]
- Brody T, Watson JE, Stokstad EL, 1982. Folate pentaglutamate and folate hexaglutamate mediated one-carbon metabolism. Biochemistry 21, 276–282. [DOI] [PubMed] [Google Scholar]
- Buyuktimkin B, Kiptoo P, Siahaan TJ, 2014. Bifunctional Peptide Inhibitors Suppress Interleukin-6 Proliferation and Ameliorates Murine Collagen-Induced Arthritis. J Clin Cell Immunol 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabner BA, Allegra CJ, Curt GA, Clendeninn NJ, Baram J, Koizumi S, Drake JC, Jolivet J, 1985. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest 76, 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton B, Lafferty KJ, 1995. The Th1/Th2 balance in autoimmunity. Curr Opin Immunol 7, 793–798. [DOI] [PubMed] [Google Scholar]
- Choi EM, 2007. Oxidative status of DBA/1J mice with type II collagen-induced arthritis. J Appl Toxicol 27, 472–481. [DOI] [PubMed] [Google Scholar]
- Constantin A, Loubet-Lescoulie P, Lambert N, Yassine-Diab B, Abbal M, Mazieres B, de Preval C, Cantagrel A, 1998. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis and rheumatism 41, 48–57. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, 1992. Molecular mechanism of methotrexate action in inflammation. Inflammation 16, 411–423. [DOI] [PubMed] [Google Scholar]
- Danila MI, Hughes LB, Brown EE, Morgan SL, Baggott JE, Arnett DK, Bridges SL Jr., 2010. Measurement of erythrocyte methotrexate polyglutamate levels: ready for clinical use in rheumatoid arthritis? Curr Rheumatol Rep 12, 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rotte MC, de Jong PH, Pluijm SM, Calasan MB, Barendregt PJ, van Zeben D, van der Lubbe PA, de Sonnaville PB, Lindemans J, Hazes JM, de Jonge R, 2013. Association of low baseline levels of erythrocyte folate with treatment nonresponse at three months in rheumatoid arthritis patients receiving methotrexate. Arthritis and rheumatism 65, 2803–2813. [DOI] [PubMed] [Google Scholar]
- de Rotte MC, den Boer E, de Jong PH, Pluijm SM, Calasan MB, Weel AE, Huisman AM, Gerards AH, van Schaeybroeck B, Wulffraat NM, Lindemans J, Hazes JM, de Jonge R, 2015. Methotrexate polyglutamates in erythrocytes are associated with lower disease activity in patients with rheumatoid arthritis. Annals of the rheumatic diseases 74, 408–414. [DOI] [PubMed] [Google Scholar]
- Dervieux T, Furst D, Lein DO, Capps R, Smith K, Caldwell J, Kremer J, 2005. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Annals of the rheumatic diseases 64, 1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk RS, Becker ML, 2016. Disease Modifying Anti-Rheumatic Drugs in Juvenile Idiopathic Arthritis: Striving for Individualized Therapy. Expert Rev Precision Med Drug Dev 1, 53–68. [Google Scholar]
- Funk RS, van Haandel L, Leeder JS, Becker ML, 2014. Folate depletion and increased glutamation in juvenile idiopathic arthritis patients treated with methotrexate. Arthritis & rheumatology 66, 3476–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, Fink CW, Newman AJ, Cassidy JT, Zemel LS, 1992. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children’s Study Group. The New England journal of medicine 326, 1043–1049. [DOI] [PubMed] [Google Scholar]
- Hawwa AF, AlBawab A, Rooney M, Wedderburn LR, Beresford MW, McElnay JC, 2015. Methotrexate polyglutamates as a potential marker of adherence to long-term therapy in children with juvenile idiopathic arthritis and juvenile dermatomyositis: an observational, cross-sectional study. Arthritis Res Ther 17, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood GS, Thorne JC, Pope JE, Lin D, Tin D, Boire G, Haraoui B, Hitchon CA, Keystone EC, Jamal S, Bykerk VP, Investigators C, 2016. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Annals of the rheumatic diseases 75, 1003–1008. [DOI] [PubMed] [Google Scholar]
- Herman S, Zurgil N, Langevitz P, Ehrenfeld M, Deutsch M, 2008. Methotrexate selectively modulates TH1/TH2 balance in active rheumatoid arthritis patients. Clin Exp Rheumatol 26, 317–323. [PubMed] [Google Scholar]
- Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, van de Laar M, 2004. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. The Journal of rheumatology 31, 645–648. [PubMed] [Google Scholar]
- Inglis JJ, Criado G, Medghalchi M, Andrews M, Sandison A, Feldmann M, Williams RO, 2007. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. Arthritis Res Ther 9, R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Lowe KE, Shane B, 1993. Regulation of folate and one-carbon metabolism in mammalian cells. IV. Role of folylpoly-gamma-glutamate synthetase in methotrexate metabolism and cytotoxicity. The Journal of biological chemistry 268, 21680–21685. [PubMed] [Google Scholar]
- Kremer JM, 2004. Toward a better understanding of methotrexate. Arthritis and rheumatism 50, 1370–1382. [DOI] [PubMed] [Google Scholar]
- Lambert CM, Sandhu S, Lochhead A, Hurst NP, McRorie E, Dhillon V, 2004. Dose escalation of parenteral methotrexate in active rheumatoid arthritis that has been unresponsive to conventional doses of methotrexate: a randomized, controlled trial. Arthritis and rheumatism 50, 364–371. [DOI] [PubMed] [Google Scholar]
- Lange F, Bajtner E, Rintisch C, Nandakumar KS, Sack U, Holmdahl R, 2005. Methotrexate ameliorates T cell dependent autoimmune arthritis and encephalomyelitis but not antibody induced or fibroblast induced arthritis. Annals of the rheumatic diseases 64, 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DY, Lon HK, Wang YL, DuBois DC, Almon RR, Jusko WJ, 2013. Pharmacokinetics, pharmacodynamics and toxicities of methotrexate in healthy and collagen-induced arthritic rats. Biopharm Drug Dispos 34, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb M, Chanarin I, Deacon R, Perry J, 1988. In vivo oxidation of the methyl group of hepatic 5-methyltetrahydrofolate. J Clin Pathol 41, 1158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Sun Y, Liu W, Qian C, Jin B, Tao F, Gu Y, Wu X, Shen Y, Xu Q, 2013. A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. Journal of immunology 191, 4969–4978. [DOI] [PubMed] [Google Scholar]
- McKay JA, Waltham KJ, Williams EA, Mathers JC, 2011. Folate depletion during pregnancy and lactation reduces genomic DNA methylation in murine adult offspring. Genes Nutr 6, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SL, Baggott JE, Vaughn WH, Austin JS, Veitch TA, Lee JY, Koopman WJ, Krumdieck CL, Alarcon GS, 1994. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann Intern Med 121, 833–841. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Hildner K, Becker C, Schlaak JF, Barbulescu K, Germann T, Schmitt E, Schirmacher P, Haralambous S, Pasparakis M, Meyer Zum Buschenfelde KH, Kollias G, Marker-Hermann E, 1999. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clinical and experimental immunology 115, 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer A, Finlayson ND, Shearman DJ, Samson RR, Girdwood RH, 1970. Plasma and erythrocyte folate in iron deficiency and folate deficiency. Blood 35, 821–828. [PubMed] [Google Scholar]
- Omer A, Mowat AG, 1968. Nature of anaemia in rheumatoid arthritis. IX. Folate metabolism in patients with rheumatoid arthritis. Annals of the rheumatic diseases 27, 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh M, Rafiei B, Moosavi ZB, Sina N, 2011. Weekly vs. daily administration of oral methotrexate (MTX) for generalized plaque psoriasis: a randomized controlled clinical trial. Int J Dermatol 50, 1291–1293. [DOI] [PubMed] [Google Scholar]
- Ruperto N, Murray KJ, Gerloni V, Wulffraat N, de Oliveira SK, Falcini F, Dolezalova P, Alessio M, Burgos-Vargas R, Corona F, Vesely R, Foster H, Davidson J, Zulian F, Asplin L, Baildam E, Consuegra JG, Ozdogan H, Saurenmann R, Joos R, Pistorio A, Woo P, Martini A, Pediatric Rheumatology International Trials, O., 2004. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis and rheumatism 50, 2191–2201. [DOI] [PubMed] [Google Scholar]
- Sant ME, Lyons SD, Phillips L, Christopherson RI, 1992. Antifolates induce inhibition of amido phosphoribosyltransferase in leukemia cells. The Journal of biological chemistry 267, 11038–11045. [PubMed] [Google Scholar]
- Sergeant JC, Hyrich KL, Anderson J, Kopec-Harding K, Hope HF, Symmons DPM, Co-Investigators R, Barton A, Verstappen SMM, 2018. Prediction of primary non-response to methotrexate therapy using demographic, clinical and psychosocial variables: results from the UK Rheumatoid Arthritis Medication Study (RAMS). Arthritis Res Ther 20, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolewska E, 2016. Are we closer to personalized therapy in juvenile idiopathic arthritis? Reumatologia 54, 151–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp LK, O’Donnell JL, Chapman PT, Zhang M, Frampton C, James J, Barclay ML, 2009. Determinants of red blood cell methotrexate polyglutamate concentrations in rheumatoid arthritis patients receiving long-term methotrexate treatment. Arthritis and rheumatism 60, 2248–2256. [DOI] [PubMed] [Google Scholar]
- Stamp LK, O’Donnell JL, Chapman PT, Zhang M, James J, Frampton C, Barclay ML, 2010. Methotrexate polyglutamate concentrations are not associated with disease control in rheumatoid arthritis patients receiving long-term methotrexate therapy. Arthritis and rheumatism 62, 359–368. [DOI] [PubMed] [Google Scholar]
- van Haandel L, Becker ML, Williams TD, Leeder JS, Stobaugh JF, 2011. Measurement of methotrexate polyglutamates in human erythrocytes by ion-pair UPLC-MS/MS. Bioanalysis 3, 2783–2796. [DOI] [PubMed] [Google Scholar]
- van Haandel L, Becker ML, Williams TD, Stobaugh JF, Leeder JS, 2012. Comprehensive quantitative measurement of folate polyglutamates in human erythrocytes by ion pairing ultra-performance liquid chromatography/tandem mass spectrometry. Rapid communications in mass spectrometry : RCM 26, 1617–1630. [DOI] [PubMed] [Google Scholar]
- van Haandel L, Stobaugh JF, 2013. Folate determination in human health: UPLC-MS/MS is the emerging methodology of choice. Bioanalysis 5, 3023–3031. [DOI] [PubMed] [Google Scholar]
- Vena GA, Cassano N, Iannone F, 2018. Update on subcutaneous methotrexate for inflammatory arthritis and psoriasis. Ther Clin Risk Manag 14, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt ME, 2013. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc 124, 16–25. [PMC free article] [PubMed] [Google Scholar]
- Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE, 1985. Efficacy of low-dose methotrexate in rheumatoid arthritis. The New England journal of medicine 312, 818–822. [DOI] [PubMed] [Google Scholar]
- Weinstein G, Roenigk HH Jr., Maibach H, 1973. Psoriasis-liver-methotrexate interactions. Arch Dermatol 108, 36–42. [PubMed] [Google Scholar]
- White JC, Goldman ID, 1976. Mechanism of action of methotrexate. IV. Free intracellular methotrexate required to suppress dihydrofolate reduction to tetrahydrofolate by Ehrlich ascites tumor cells in vitro. Mol Pharmacol 12, 711–719. [PubMed] [Google Scholar]
- Williams HJ, Willkens RF, Samuelson CO Jr., Alarcon GS, Guttadauria M, Yarboro C, Polisson RP, Weiner SR, Luggen ME, Billingsley LM, et al. , 1985. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis and rheumatism 28, 721–730. [DOI] [PubMed] [Google Scholar]
- Yamaki K, Uchida H, Harada Y, Li X, Yanagisawa R, Takano H, Hayashi H, Taneda S, Mori Y, Yoshino S, 2003. Effect of methotrexate on Th1 and Th2 immune responses in mice. J Pharm Pharmacol 55, 1661–1666. [DOI] [PubMed] [Google Scholar]
- You X, Williams A, Dervieux T, He W, Cronstein BN, 2013. Fibroblasts from methotrexate-sensitive mice accumulate methotrexate polyglutamates but those from methotrexate-resistant mice do not. Clin Exp Rheumatol 31, 433–435. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li P, Song S, Liu Y, Wang Q, Chang Y, Wu Y, Chen J, Zhao W, Zhang Y, Zhou A, Wei W, 2013. Comparative efficacy of TACI-Ig with TNF-alpha inhibitor and methotrexate in DBA/1 mice with collagen-induced arthritis. Eur J Pharmacol 708, 113–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.