Abstract
While recently completing a study of the effects of stimulating the lateral preoptic area (LPO) and ventral pallidum (VP) on locomotion and other movements, we also noticed LPO and VP effects on motivational drive and threat tolerance. Here, we have investigated these latter effects by testing conditioned place preference (CPP), behavior on the elevated plus maze (EPM) and the willingness of sated rats to occupy a harshly lit open field center to acquire sweet pellets, a measure of threat tolerance, following infusions of vehicle or bicuculline (bic) into the LPO and VP. LPO bic infusions robustly increased total locomotion, and, in direct proportion, occupancy of both the harshly lit field center and open arms of the EPM. LPO bic also generated CPP, but did not increase sweet pellet ingestion. These effects were attenuated by dopamine D1 and D2 receptor antagonists, whether given individually or as a cocktail and systemically or infused bilaterally into the nucleus accumbens. VP bic infusions did not increase total locomotion, but preferentially increased field center occupancy. VP bic-infused rats compulsively ingested sweet pellets and did so even under the spotlight, whereas harsh illumination suppressed pellet ingestion in the control groups. VP bic produced CPP and increased open arm occupancy on the EPM. These effects were attenuated by pretreatment with dopamine receptor antagonists given systemically or as bilateral infusions into the VP, except for % distance in the field center (by D1 or D2 antagonists) and pellet ingestion (by D1 antagonist). Thus, boldness generated in association with LPO activation is tightly tied to locomotor activation and, as is locomotion itself, strongly DA-dependent, whereas that accompanying stimulation of the VP is independent of locomotor activation and, at least in part, DA signaling. Furthermore, respective emboldened behaviors elicited from neither LPO nor VP could clearly be attributed to goal pursuit. Rather, emboldening of behavior seems more to be a fixed action response not fundamentally different than previously for reported locomotion, pivoting, backing, gnawing and eating elicited by basal forebrain stimulation.
Keywords: basal forebrain, anxiety, locomotion, decision, dopamine
Introduction
Two distinct neural structures, the lateral preoptic area (LPO) and ventral pallidum (VP), sitting side by side in the subcommissural basal forebrain are well situated to modulate adaptive behavior (Zahm et al., 2014a; Root et al., 2015; Barker et al., 2017; Subramanian et al., 2018). Mainly via relays in the lateral septum and accumbens (Acb), both receive dense striatal-like inputs conveying information that in some way reflects representations and recollections of the organism’s internal and external environment and experiences assembled by manifold cortical and cortical-like association structures (Heimer and Wilson, 1975; Swanson, 1976; Heimer, 1978; Beckstead, 1979; Groenewegen et al, 1982; 1991; Kelley and Domesick, 1982; Kelley et al., 1982; Wayner et al., 1983; Alonso and Kohler, 1984; Russchen and Price, 1984; Phillipson and Griffiths, 1985; Caffe et al., 1987; Swanson et al.,1987a;b; McGeorge and Faull, 1989; Sesack et al., 1989; Zahm, 1989; Jakab and Leranth; 1990; Zahm and Heimer, 1990; King et al., 1993; Buchanan et al., 1994; Canteras et al., 1995; Haber et al., 1995; Chikama et al., 1997; Risold and Swanson, 1997; Leranth et al., 1999; Ferry et al., 2000; Swanson, 2000; Chiba et al., 2001; Vertes, 2004; Reynolds and Zahm, 2005; Zahm et al., 2013a; Root et al., 2015; Reichard et al., 2017). By projections to lateral hypothalamus and brainstem behavioral effectors, LPO and VP may directly influence behavioral synthesis and the autonomics (Swanson, 1976; Phillipson, 1979; Haber et al., 1985; Rye et al., 1987; Steininger et al., 1992; Groenewegen et al., 1993; Kalivas et al., 1993; Zahm et al., 2001; Geisler and Zahm, 2005; 2006; Yetinkoff et al., 2015). By reciprocal connections with the Acb and VTA (Wayner et al., 1983; Haber et al., 1985; Zahm, 1989; Groenwegen et al., 1993; Spooren et al., 1996; Zahm et al., 1996; Zahm et al., 2013a; Yetnikoff et al., 2015; Barker et al., 2017), LPO and VP may influence the release of dopamine in the forebrain (Yokel and Wise, 1975; Lyness at al., 1979; Roberts et al., 1980; Wise, 1984; Abercrombie et al., 1989; Wise and Rompre, 1989; Everitt, 1990; Mitchell and Gratton, 1994; Belujon and Grace, 2015) and, via projections to the lateral habenula, forebrain levels of dopamine, serotonin, norepinephrine, acetylcholine, GABA and other neuromodulators conveyed by long ascending neuromodulatory pathways (Sutherland, 1982; Geisler and Trimble, 2008; Zahm and Root, 2017). By reëntrant projections relayed in the thalamus, LPO and VP may contribute to modulation of cortical representations.
Recent work in this lab has shown that the LPO and VP, despite such general similarities in connectivity, contribute in quite distinct ways to locomotion and other movements (Zahm et al., 2014a; Subramanian et al., 2018). While doing that work, we encountered evidence suggesting that the locomotor effects of stimulating the LPO and VP are accompanied by altered motivational drive and threat tolerance (Zahm et al., 2013b; 2014b). Here, we report these data formally and provide assessments of conditioned place preference (CPP) and behavior on the elevated plus maze (EPM) made following infusions of bicuculline (bic) into the LPO and VP. Parts of the CPP and EPM data were presented at a recent conference (Reichard et al., 2016).
Methods and Materials
Subjects
Male Sprague Dawley rats (250–320g) were group and single housed prior to and following surgeries, respectively, on a 12-hr light-dark cycle in a temperature- and humidity-controlled vivarium with chow and water available ad libitum. All protocols affecting them were approved by the Saint Louis University Institutional Animal Care and Use Committee in accordance with guidelines provided in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
All chemicals and drugs, except as noted, were acquired from the Sigma Chemical Company (St. Louis, MO, USA). The gamma-aminobutyric acid A (GABAA) receptor antagonist, 9(R)-(−)-bicuculline methbromide (bic), and dopamine receptor antagonists, R(+)-SCH-23390 (SCH), S-(−)-eticlopride hydrobromide (etic) were dissolved in sterile 0.9% saline (Hospira Inc., Lake Forest, IL). Haloperidol (hal) was prepared in a mixture of 20% ethanol, 50% propylene glycol and 30% distilled water as a 2 mg/ml stock solution and diluted as required with sterile 0.9% saline. Hal and etic preferentially bind D2-D3 relative to D1 class dopamine receptors, whereas SCH preferentially binds the D1 receptor (Christensen et al., 1984; Hall et al., 1985; Köhler et al., 1986; Leslie et al., 1987 a; b; Sokoloff et al., 1990; Bourne, 2001).
Guide Cannulae Placement
The rats were deeply anesthetized by intraperitoneal injections of a mixture of ketamine (72 mg/kg) and xylazine (11.2 mg/kg) injected as a cocktail consisting of 45% ketamine (100 mg/ml), 35% xylazine (20 mg/ml) and 20% saline at a dose of 0.16 ml/100g of body weight. The anesthetized rats were placed into a Kopf stereotaxic instrument and incisions were made to expose the skulls, in which small burr holes were made over the LPO unilaterally and the VP, which in separate groups was targeted unilaterally (VP uni) and bilalerally (VP bi). Stainless steel guide cannulae (22 gauge) 13 mm in length were inserted to a depth 2 mm above the centers of the targets and fixed with dental cement anchored to stainless steel screws set in the skull. Stainless steel wire obturators were inserted into the guide cannulae to maintain patency. The incisions were closed with wound clips and the rats were given i.p. injections of Buprenorphine SR (1mg/kg) and kept warm until they awoke.
Consistent with the earlier results showing that unilateral LPO infusions of bic produce a behavioral response no different than bilateral LPO bic infusions (Zahm et al., 2014a; Subramanian et al., 2018), only unilateral LPO bic infusions were utilized here. In contrast, and consistent with our previous discovery of the necessity of infusing bilaterally into the VP in order to produce a behavioral response (see preceding refs.), the VP was infused mainly bilaterally (VP bi) in this study, except in the threat tolerance experiments, which also utilized some unilateral VP infusions (VP uni).
Behavioral Testing
Three to five days after cannulae were implanted, the rats were brought to the behavior testing room where they were habituated to handling on three consecutive days. On test days, the rats were removed from the home cages, the obturators were removed and stainless steel injector cannulae (28 gauge) 15 mm in length, connected by a short length of polyethylene tubing (PE 20 0.38/1.09, Stoelting, Wood Dale, IL, cat. # 51155) to the Luer needle of a 1.0 μl Hamilton syringe, were inserted to a depth 2 mm beyond the tips of the guide cannulae. Infusate was propelled through the injectors at a rate of 0.25 μl/min by a syringe pump (Harvard Apparatus, Holliston, MA, model ‘11’ plus) for a duration of one minute, resulting in infusions of 0.25 μl. For infusions into the LPO and VP, bic was prepared at a dilution of 1 mg/3 ml, which resulted in delivery of 67 ng/0.25 μl. This concentration and rate of delivery produced robust locomotor activation in previous studies (Reynolds et al., 2006; Zahm et al., 2014a; Subramanian et al., 2018). After the infusions the injectors were removed and the rats were placed in one of the various test devices described below.
Locomotion and threat tolerance were measured during the rats’ light period in standard activity monitors, each comprising a square, white, 43.2 × 43.2 cm floor and clear, 30.5 cm high plexiglass walls (Med Associates, St. Albans, VT). Fixtures containing horizontal rows of 16 point-source infrared illuminators equispaced at 2.54 cm were bracketed to the wall on one side of the chambers at heights of 2.54 and 12.7 cm from the monitor floor. Photodetectors were affixed to the opposing walls. The lower bank comprised two sets of illuminators and detectors mounted on adjacent walls at a right angle to each other so as to form a grid of 16 × 16 intersecting light beams for measurement of horizontal (forward) locomotion, whereas the upper consisted of a single illuminator/detector pair for detection of rearing. Beam breaks were evaluated with the aid of Activity Monitor software (Med Associates). The monitors were housed inside sound attenuating chambers with exhaust fans running (Med Associates).
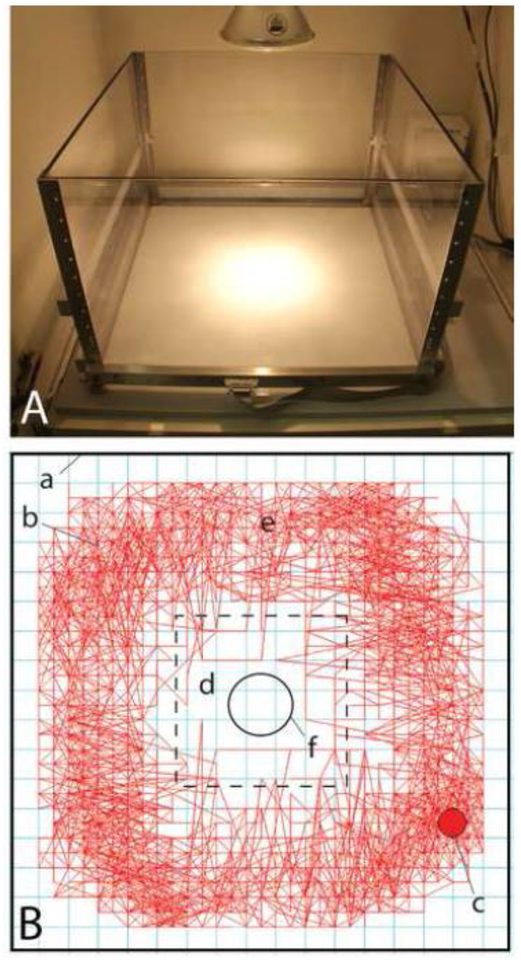
To assess threat tolerance, unconditioned anxiety produced in rats by strong light and their reluctance to occupy open space were exploited. Briefly, activity monitors were modified with a ceiling mounted 90W, 120V, 1260 lumens spotlight aimed at the center of the chamber floor (Fig. 1A) in addition to the two dim CML 182 28V 100mA bulbs provided by the manufacturer. To determine if activations of LPO and VP affect the unconditioned tendency of rats to avoid intense illumination and the center of an open field, total distance and distance in the field center (expressed raw or as percent of total distance) were measured. Three conditions were varied – illumination (house light vs. spotlight), infusate (vehicle vs. bic) and the infused brain structure (LPO vs. VP infused unilaterally (VP uni) and bilaterally (VP bi). Because preliminary experiments (Zahm et al., 2013b; 2014b) showed that rats rarely enter a dimly or harshly lit field center, unless enticed (Fig. 1B), field centers were baited with 20 45 mg sweet pellets (TestDiet, Richmond, IN, cat. #1811256), necessitating an additional accounting of numbers of ingested pellets. To assess dopamine receptor involvement in threat tolerance, groups of rats were given subcutaneous injections of hal (0.1 mg/kg) or SCH (0.01 mg/kg) 10 minutes prior to being placed in the apparatus for an additional 10 minutes for measurement of baseline activity, after which they were removed, given intracranial infusions of bic or vehicle and returned to the apparatus for testing.
Figure 1:
Apparatus in which locomotion and threat tolerance were recorded (A) and a diagram of the data generated (B). Locomotion monitors (A) comprised chambers with plexiglas walls lit either by two dim incandescent bulbs situated in the upper rear corners of the sound attenuating chambers in which the monitors were enclosed or by spotlights that produced intense illumination in the center of the monitor floor, as shown. Panel B provides additional detail, showing the monitor walls (a), a trace of a rat’s itinerary during a test session (b), the digitial representation of the rat’s center of mass (c), areas regarded as the field center (d, enclosed by the broken line) and periphery (e, outside of the broken line) and the digrammatic outline of the food receptacle that was baited with sweet pellets (f).
CPP was measured during the dark period using a standard CPP apparatus and dedicated software (MED-PC-IV), both acquired from Med Associates. The apparatus consists of two rectangular chambers with distinct flooring (bars vs grids) and wall colors (black with white horizontal lines vs white with black vertical lines) separated by a gray center compartment. Guillotine doors enable confinement of animals in any of the chambers. During three days of habituation (days 1–3), the rats were placed in the center compartment with all doors open and permitted to freely explore the apparatus for thirty minutes. On day 4, a pre-conditioning assessment was recorded of the time spent in each compartment followed by 3 days (days 5–7) of twice daily conditioning sessions separated by at least one hour. Just before one of the daily conditioning sessions, bic was infused into the LPO unilaterally or VP bilaterally, after which the rats were confined in one of the large compartments (thereafter designated ‘bic-paired’) for twenty minutes. The other daily conditioning session was preceded by a vehicle infusion into the same structure and the animals were confined in the other large chamber (thereafter ‘vehicle-paired’) for thirty minutes. To offset the expectation that bic infusions elicit CPP, chambers in which animals spent less time during the preconditioning assessment were paired with bic infusions. The order of bic and vehicle conditioning sessions was reversed on successive days. During a post-conditioning assessment on day 8, the animals were placed in the center compartment and time spent in each chamber was recorded as they freely explored the apparatus for thirty minutes. Times in the bic-paired chamber during the pre- and postconditioning assessments were statistically compared.
EPM behavior was measured during the rats’ dark period using an apparatus consisting of two 112 × 10 cm runways mutually bisecting at a right angle and situated 70 cm above the floor (Med Associates). One runway is open to the room and the other is enclosed. Relative time spent on the open and closed arms provides a measure of anxiety. At testing, rats received infusions of bic or vehicle into the LPO unilaterally or VP bilaterally, as described, five minutes prior to being placed at the hub where the open and closed runways cross. Ratios of open to total arm entries, time spent on the arms after each entry and the ratio of time spent on open and closed arms were recorded. Veh and bic groups were run in counterbalanced order in succesive trials to minimize repeated exposures to the apparatus.
Systemic administration of DA antagonists:
To determine if dopamine receptor signaling modulates threat tolerance or motivation states elicited by LPO or VP bic infusions, groups of animals received intraperitoneal injections of vehicle, SCH (0.01 mg/kg) or hal (0.1 mg/kg) thirty minutes prior to the start of all CPP conditioning sessions or 30 min before testing on the EPM.
Site-specific administration of DA antagonists – Acb:
To determine if dopamine receptor signaling in the Acb is required for the expression of LPO-bic elicited CPP, rats with implanted guide cannulae targeting the Acb bilaterally and LPO unilaterally were habituated, subjected to a preconditioning assessment and, just before conditioning sessions, given bilateral Acb infusions of a cocktail containing SCH (2 μg) and etic (10 μg) in 0.5 μl over 2 min and, immediately after, a unilateral infusion of bic into the LPO. Vehicle infusions into the LPO and Acb preceded confinement in the other chamber. After three days of such conditioning, the rats underwent post-conditioning assessment. For groups tested on the EPM, DA antagonist or veh infusions were made just prior to bic or veh infusions
Site-specific administration of DA antagonists – VP:
To determine if dopamine receptor signaling in the VP is required for VP-bic elicited CPP, rats with guide cannulae targeting the VP bilaterally were habituated and given a preconditioning assessment as previously described. The rats were then given bilateral VP infusions of a cocktail containing SCH (2 μg), eticl (10 μg) and bic (67 ng) in 0.25 μl over 1 min in one chamber (bic-paired) and an equivalent volume of vehicle prior to being confined in the other chamber. After three days of such conditioning, a post-conditioning assessment was done. For groups tested on the EPM, DA antagonist or veh infusions were made just prior to bic or veh infusions
Experimental groups and statistics
During the course of the study guide cannulae were implanted, mainly bilaterally, into the LPO and VP. After a week of recovery, measurements were done on each rat with different combinations of injection (vehicle, hal, SCH) and infusion (vehicle or bic infused unilaterally or bilaterally) on five consecutive days under vigilance for lethargy or other signs of illness, detection of which warranted immediate removal of a rat from the study. Duplicate measurements (same drug-infusion combination more than once in the same rat) were mostly avoided. Sample sizes are given in the legends of figures showing the data.
Threat tolerance:
Three factors described above (infused structure, infusion (bic vs veh) and drug (hal or SCH vs veh) were initally tested with a 3-way ANOVA and the Holm-Sidak post hoc test. In view of the profound effects of the spotlight, experiments run under dim house lighting and the spotlight were subsequently tested separately using a 2-way ANOVA to distinguish structure vs infusate (veh vs bic), also assessed post hoc with the Holm-Sidak test. The effects of hal and SCH on locomotion, % distance in the field center and pellet ingestion were analyzed with t-tests. Remaining comparisons from the threat tolerance antagonist experiments were made with an ANOVA followed by Student-Newman-Keuls post hoc testing.
CPP:
The amounts of time groups spent in the bic-paired chamber during preconditioning and postconditioning assessments were statistically compared using paired t-tests with the critical limit set at p < 0.05. Rats that spent >70% in one chamber during the preconditioning assessment were excluded from analysis.
To determine the effects of dopamine antagonists, a conditioning score was calculated by subtracting time in the bic-paired chamber during the preconditioning assessment from time in that chamber during the post-conditioning assessment. Treatment groups were statistically compared with a one-way ANOVA and a post hoc Holm-Sidak test with the critical limit set at p < 0.05.
EPM:
The ratios of time spent on the open to closed arms and open to total arm entries, and the time on arms per entry following LPO and VP bic infusions were tested statistically against the respective saline infusion values using paired t-tests with the critical limit set at p < 0.05. A one-way ANOVA and a post hoc Holm-Sidak test with the critical limit set at p <0.05 was used to compare the effects of dopamine antagonists and vehicle.
Localization of Injections
The rats were deeply anesthetized and perfused as described. The brains were removed and placed in the same fixative for four hours and then in a 30% sucrose solution overnight. Slabs of brain containing infusion sites were sectioned with a cryostat into three adjacent series of 50 μm thick coronal sections. The first series was thaw-mounted onto subbed glass slides and the rest were stored in glass vials containing 0.1 M SPB. The mounted sections were defatted overnight in a 1:1 solution of chloroform and methanol and, after rehydration in decreasing concentrations of methanol in water (100%, 95%, 90%, 80%, 70% and 50%), placed in a filtered solution of 0.1% cresyl violet acetate in 0.3% glacial acetic acid for 10 minutes. The stained sections were dehydrated in increasing concentrations of MeOH and then placed in xylene prior to coverslipping with Permount. Sections were viewed with brightfield optics with an Olympus BX51 microscope and the locations of the injector tips were mapped with the aid of the Neurolucida hardware/software platform (MBF Bioscience, version 4.70.3). The maps were exported to Adobe Illustrator CS2 for preparation of illustrations.
Results
Injection sites
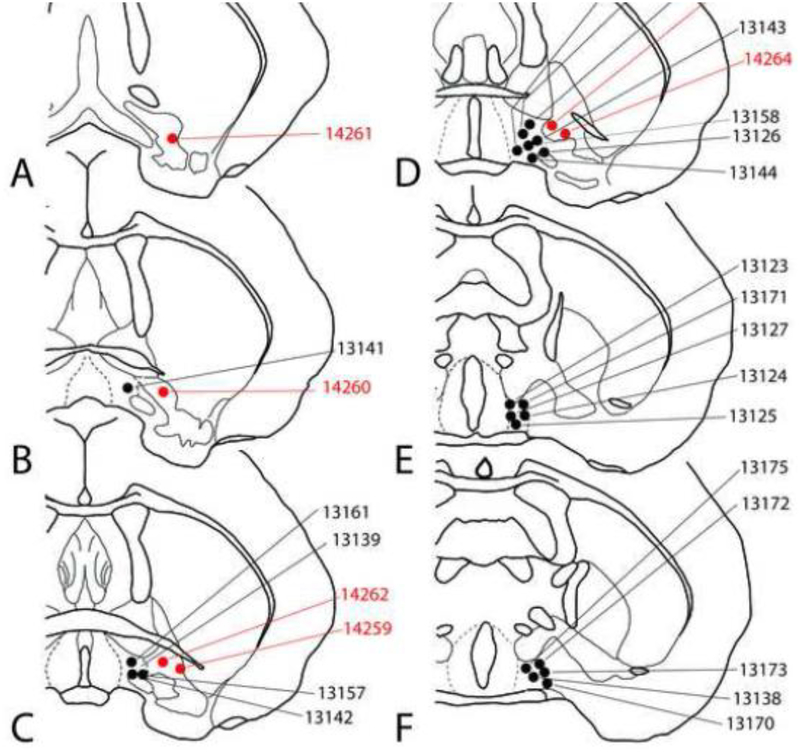
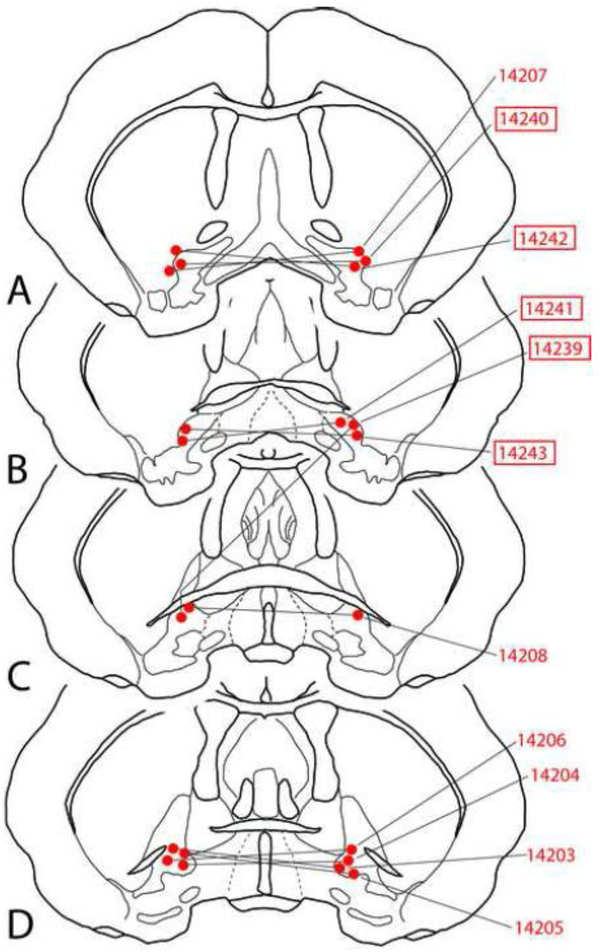
Infusion sites used in the threat tolerance experiments are shown in Figures 2 and 3. For the CPP and EPM experiments, injection sites are included in the figures with the respective data. The microscopic appearance of the injection sites is comparable to that illustrated in our recent papers (Zahm et al., 2014a; Subramanian et al., 2018).
Figure 2:
Diagrams showing representative unilateral infusion sites in the LPO (black dots) and VP (red dots). Additional unilateral VP infusion sites are shown in Figure 3.
Figure 3:
Diagrams showing representative bilateral infusion sites in the VP (red dots). Those with boxed case numbers were tested, in addition, with infusions on one side only to supplement the sample of unilateral VP infusions shown in Figure 2.
Threat tolerance
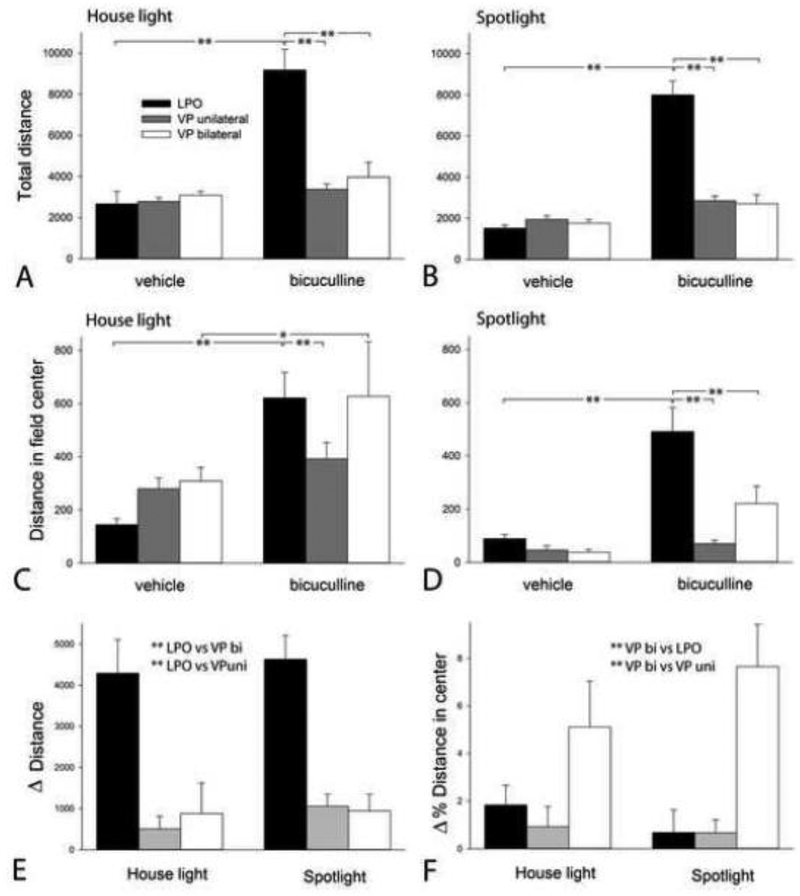
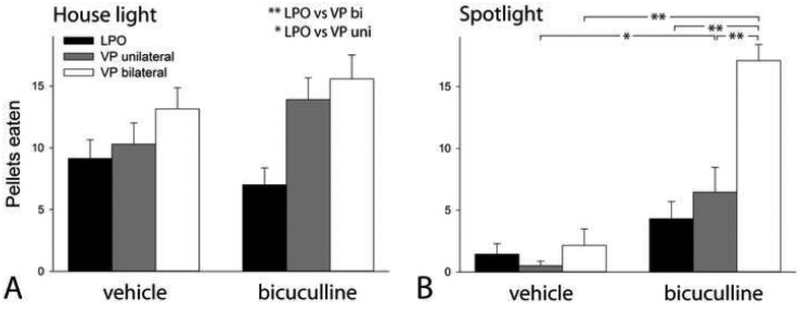
Significant main effects of illumination on total distance (3-way ANOVA, F1,264=10.321; P<0.002) and distance in the field center (3-way ANOVA, F1,264=23.698; P<0.001) indicated that spotlighting as compared to house lighting suppresses locomotion (note the generalized reduction of bar values across all groups in Figures 4B and D as compared to 4A and C, respectively). Significant main effects on total distance of infused structure (F1,264=30.681; P<0.001) and infusate (F2,264=69.121; P<0.001) were complicated by a significant structure X infusate interaction (F2,264=38.410; P<0.001), as were significant main effects on distance in the field center of infused structure (F1,264=10.677; P<0.001) and infusate (F2,264=45.397; P<0.001) by a structure X infusate interaction (F1,264=15.163; P<0.001). The Holm-Sidak post hoc test, in turn, detected multiple significant comparisons among various levels of the all of the outcomes, but no significant structure X illumination X infusate interactions. The potent effects of brilliant field center illumination on locomotion and field center occupancy suggested that separate testing of groups run under house lighting and spotlighting might better explicate the effects of bicuculline infusions. Accordingly, a description follows of the results of a 2-way ANOVA and Holm-Sidak post-hoc tests (shown on the graphs) applied to data acquired separately under dim house lighting (Fig. 4A, C and E) and spotlighting (Fig. 4B, D and F).
Figure 4:
Graphs showing total distance traversed (A and B) and distance traversed in the field center (C and D) after infusions of bicuculline (bic) or vehicle (veh) into the lateral preoptic area (LPO, black bars) and ventral pallidum (VP), infused either unilaterally (uni, gray bars) or bilaterally (bi, white bars), in locomotor monitors illuminated by dim house lights (A and C) or a bright spotlight aimed at the field center (B and D). Panels E and F, respectively, show change (Δ) in distance traversed (E) and Δ % of total distance traversed in the field center (F) due to bic infusion (calculated as bic values minus veh values). Data (n= 30 for LPO; 20 for VP uni and 19 fo VP bi) were tested with a 3-way ANOVA (results provided in the text) and, alternatively, for House light (A and C) and Spotlight (E and F), considered separately, and Δ Distance (E) and Δ % Distance in the center (F), by a 2-way ANOVA (results are given with graphs). p-values: ** 0.01<*< 0.01.
As we previously reported (Zahm et al., 2014a; Subramanian et al., 2018), locomotion was significantly activated by infusion of bic into the LPO (p<0.0001, Holm-Sidak), but not VP uni or VP bi, whether in dim house lighting (Fig 4A; F2,132=19.660, p<0.001) or under the spotlight (Fig 4B; F2,132=34.445, p<0.001). Expressed as relative change (Δ) after infusions (bic value minus veh value), total distance after LPO bic infusions far outstripped that after infusions of bic into VP uni and VP bi (Fig. 4E; F2,166=19.259, p<0.001). Pertinent to threat tolerance, infusions of bic into both the LPO and VP bi increased locomotion within the field center in both house lighting (Fig. 4C; F2, 132=6.521, p=0.002) and under the spotlight (Fig. 4D; F2, 132=9.248, p<0.001). However, Δ % distance in the center after LPO bic infusions was strictly proportional to the increase in overall locomotion, whereas, following VP bi bic infusions, Δ % distance in the field center was disproportionately increased, even (particularly) under the spot light (Fig. 4F; F2,168=11.154, p<0.001).
Suppression of sweet pellet ingestion by the spotlight was reversed by VP infusions of bic. Testing was done shortly after a full night with ad lib access to chow, which rendered most rats sated to chow. Consequently, control rats in dim house lighting typically ingested only half to three-quarters of the 20 sweet pellets provided (Fig. 5A). The spotlight produced profound suppression of sweet pellet ingestion (Fig. 5B, as compared to 5A; 3-way ANOVA - F1,264=52.271; P<0.001) that was partially (modestly, p<0.05) and completely (p<0.001) reversed, respectively, after infusions of bic into VP uni and VP bi, both in dim house lighting (2-way ANOVA – F2,132=8.320; P<0.001) and in the spotlight (2-way ANOVA – F2,132=15.894; P<0.001). Thus, spotlit VP bi-infused rats ingested numbers of sweet pellets similar to the house lit control groups and significantly greater than the other spotlit vehicle and bic groups (p<0.001).
Figure 5:
Graphs showing pellets eaten after infusions of bicuculline (bic) or vehicle (veh) into the lateral preoptic area (LPO, black bars) and ventral pallidum (VP), infused either unilaterally (uni, gray bars) or bilaterally (bi, white bars), in locomotor monitors illuminated by dim house lights (A) or a bright spotlight aimed at the field center (B). Data (n= 30 for LPO; 20 for VP uni and 19 fo VP bi) were tested with a 3-way ANOVA (results provided in the text) and, alternatively, for House light (A) and Spotlight (B), considered separately, by a 2-way ANOVA (results are given with graphs). ** p < 0.001; * p < 0.01.
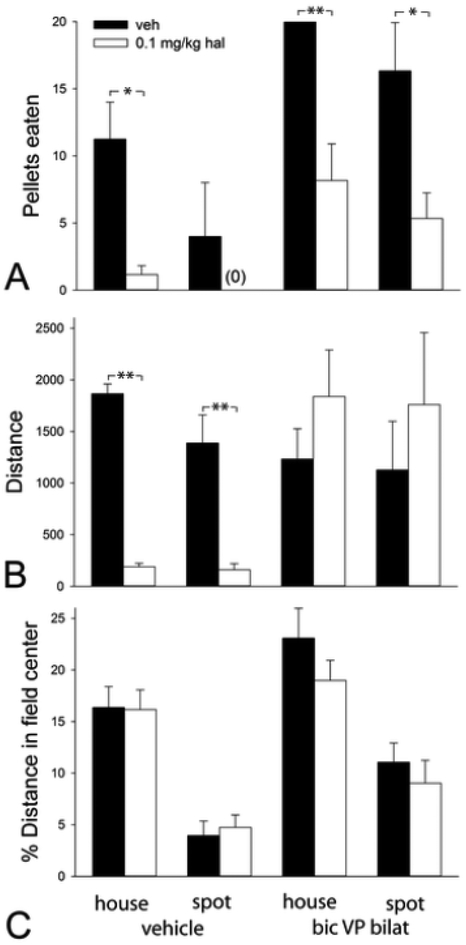
Following vehicle infusions into VP bi, hal given at 0.1 mg/kg profoundly suppressed ingestion of sweet pellets in house lighting and further suppressed pellet ingestion inhibited by the spotlight (Fig. 6A; p=0.018 [bic vehicle, house light]; ns [bic vehicle, spotlight]). VP bi bic infusions reversed the inhibition of sweet pellet ingestion due to spotlighting (Fig. 5B; 6A), but not that due to hal (Fig. 6A; p<0.001 [bic, house light]; p=0.014 [bic, spotlight]). The suppressive effect of hal on VP bi-stimulated pellet ingestion did not reflect impaired motor function, because hal did not attenuate and, indeed, may have slightly enhanced locomotion produced by VP bi bic in the spotlight (Fig 6B). Percent distance in the field center also was increased by VP bi infusions of bic (F3,18=15.378, p<0.001 [hal vehicle]; F3,19=13.262, p,0.001 [hal]), and, in contrast to sweet pellet ingestion, the increase was not attenuated by pretreatment with 0.1 mg/kg hal (Fig. 6C).
Figure 6:
Graphs showing the effects of administration of haloperidol (hal, 0.1 mg/kg) on numbers of pellets eaten (A), total distance traversed (B) and % distance of total distance traversed in the field center (C) following bilateral (bilat) infusions of bicuculline (bic) or vehicle (veh) into the ventral pallidum (VP) in activity monitors illuminated by dim house lights (house) or or a bright spotlight aimed at the field center (spot). Hal was compared to hal vehicle (n=6) with t- tests. Further comparisons were made separately for vehicle and bic groups with ANOVA followed by Student-Newman-Keuls post hoc tests. The critical limit set at p < 0.05. p-values: ***<0.001< **< 0.01< *< 0.05.
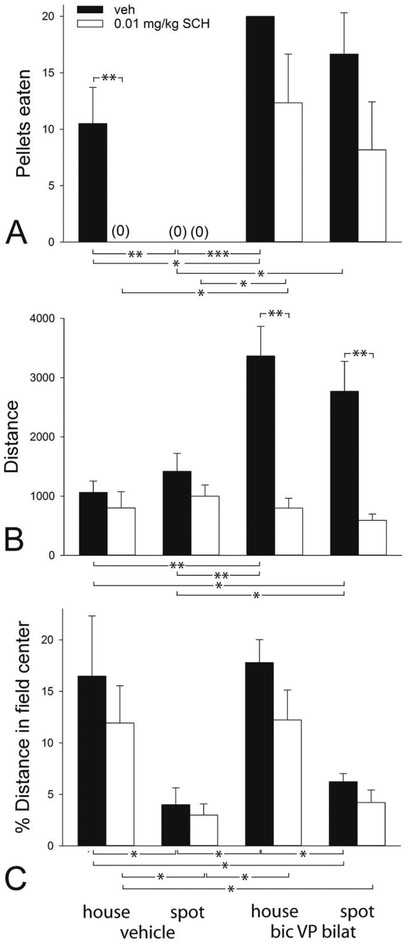
SCH given at 0.01 mg/kg also profoundly suppressed pellet taking by the vehicle-infused control group (p=0.005 [bic vehicle, house light]), but not so after VP bi bic infusions (Fig 7A). An apparent downward trend in pellet ingestion in the SCH pretreated groups following VP bi bic infusions seems likely related to the profound impairment of locomotion by SCH in the VP bi bic infused groups (Fig. 7B; p<0.001 [bic, house light]; p=0.002 [bic, spotlight]). However, % distance in the field center was unaffected by SCH (Fig. 7C).
Figure 7:
Graphs showing the effects of administration of SCH-23390 (SCH, 0.01 mg/kg) on numbers of pellets eaten (A), total distance traversed (B) and % distance of total distance traversed in the field center (C) following bilateral (bilat) infusions of bicuculline (bic) or vehicle (veh) into the ventral pallidum (VP) in activity monitors illuminated by dim house lights (house) or or a bright spotlight aimed at the field center (spot). Statistics are as in Fig. 6 legend.
Conditioned place preference
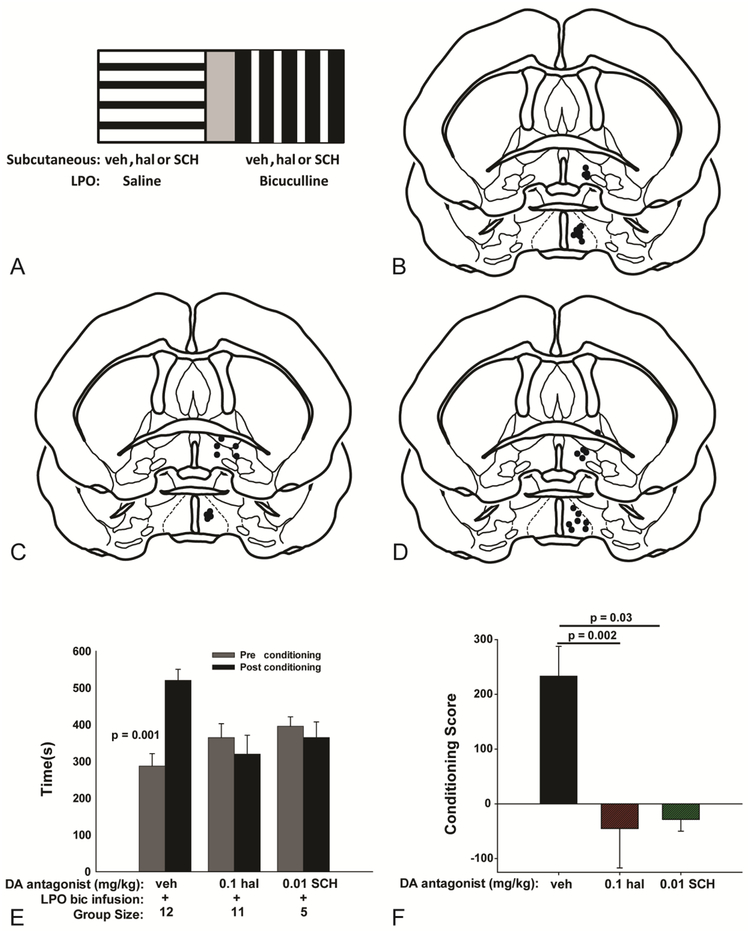
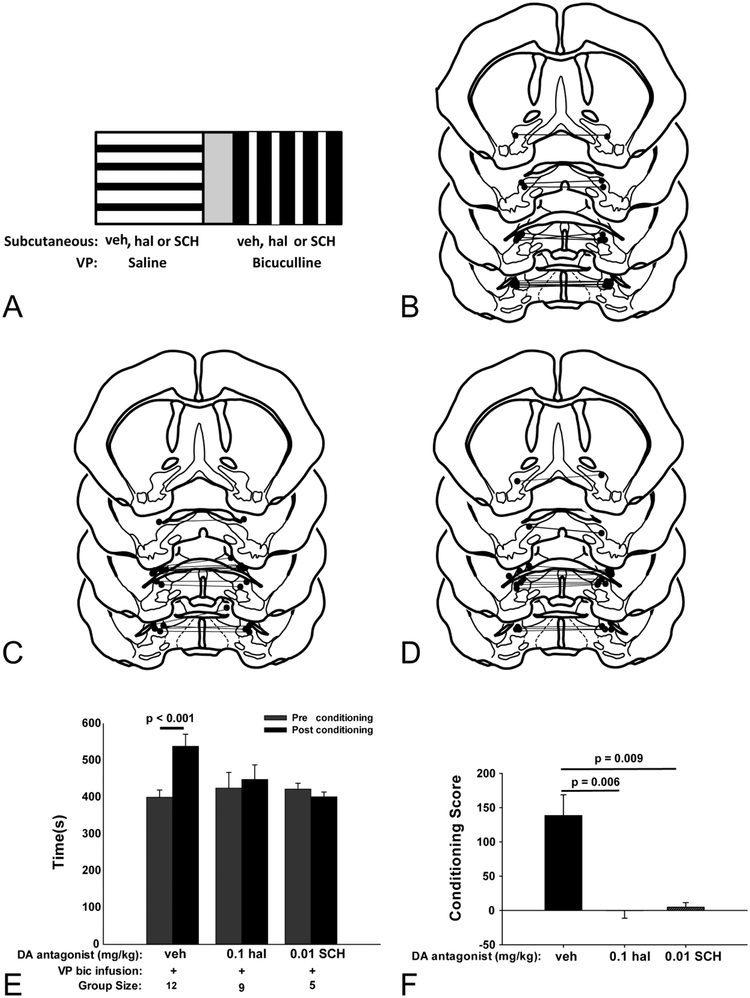
Bic infusions into the LPO during conditioning trials (Fig. 8A–D) significantly increased the time postconditioning spent in the bic-paired chamber (Fig. 8E) as compared to the preconditioning assessment (Means±SEM: 520 sec ± 30.3 vs 287sec ± 33.7 [t11= −4.262, p= 0.001]). This effect was blocked in rats injected before conditioning sessions with either 0.1 mg/kg hal (319 sec ± 51.3 vs 364 ± 37.8; t10= 0.625, p= 0.55) or 0.01 mg/kg SCH (365 sec ± 42.5 vs 395.2 ± 25.7; t4= 1.465, p = 0.217). Because rats display widely varying preferences during preconditioning assessments, the magnitude and valence of time spent in the bic-paired chamber was expressed alternatively with the aid of a conditioning score (see Methods). Comparison of the conditioning scores (Fig. 8F) revealed a significant main effect of drug treatment [F3,36= 7.1, p= 0.002], shown by Holm-Sidak post hoc analysis to reflect significantly lower scores after injections of 0.1 mg/kg hal (−45 ± 71.9) or 0.01 mg/kg SCH (−19.5 ± 21.2) compared to the group that received LPO bic infusions and vehicle injections (233 ± 54.6). Thus, a preferred behavioral state requiring both D1 and D2 receptor signaling accompanies LPO disinhibition. Locomotion during LPO bic conditioning sessions (data not shown) also exhibited a significant main effect of drug treatment [F3,25= 20.1, p< 0.001] revealed by Holm-Sidak post hoc analysis to reflect significantly less locomotion in animals that received LPO bic infusions and injections of 0.1mg/kg hal (1415 ± 137) or 0.01mg/kg SCH (1479 ± 96.7) as compared to controls (2429 ± 126.9). That said, locomotion was significantly greater in all treatment groups during bic as compared to vehicle conditioning sessions.
Figure 8:
Schematic illustrating LPO place preference conditioning sessions (A). Cannula placements for vehicle (B) 0.01mg/kg SCH and 0.1 mg/kg hal (D) treatment groups tested in place preference and EPM. Time spent in the LPO bic-paired chamber during the preconditioning (gray bars) and post conditioning (black bars) assessments (E). Time in the LPO-bic paired chamber significantly increased during the post compared to the pre conditioning assessment in only the control group. Statistical comparison of the conditioning scores from each treatment group shows that place preference acquired from LPO bic infusions requires signaling through both D1 and D2 receptors (F).
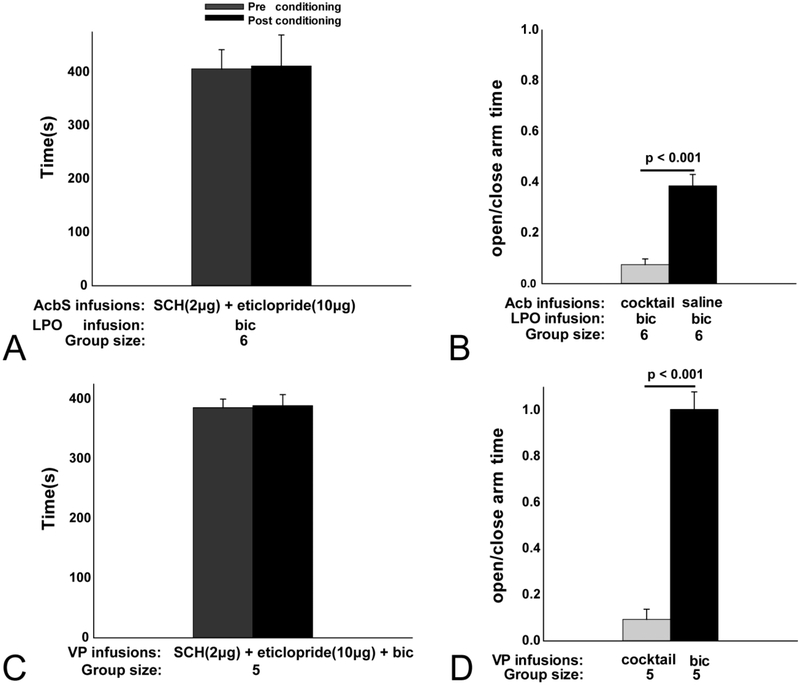
To test if the effects of dopamine receptor antagonists on LPO-elicited CPP are due to antagonist actions in the Acb, rats with guide cannulae targeting the LPO unilaterally and Acb bilaterally underwent CPP testing. Conditioning sessions during which a cocktail containing SCH (2.0 μg) and etic (10 μg) was infused bilaterally into the Acb and bic was infused into the LPO did not result in significantly (t5=0.646, p=0.547) more time spent in the LPO bic-paired chamber during the postconditioning (416 ± 10.9s) compared to the preconditioning (401.6 ± 12.6s) assessment (Fig. 9A), indicating that LPO bic-elicited CPP (Fig. 8E and F) requires dopamine receptor signaling in the Acb.
Figure 9:
Conditioning with bilateral infusions into the Acb of a cocktail containing SCH (2 μg) + eticlopride (10 μg) combined with LPO bic infusions did not significantly alter time in the LPO bic paired-chamber during the post- (black bar) compared to the pre- (gray bar) conditioning assessment (A). The ratio of open to closed arm time following LPO bic infusions paired with bilateral Acb saline infusions (black bar) was significantly decreased when the LPO bic infusions were paired instead with bilateral infusions into the Acb of antagonists cocktail (gray bar) (B). Conditioning with bilateral infusions into the VP of a cocktail containing SCH (2 μg), eticlopride (10 μg) and bic did not significantly alter time in the cocktail-paired chamber during the post- (black bar) compared to the pre- (gray) conditioning assessment (C). The ratio of open to closed arm time was significantly lower following bilateral infusions into the VP of the cocktail (gray bar) as compared to infusions into VP of only bic (black bar) (D).
Bilateral infusions of bic into the VP during conditioning (Fig. 10A–D) significantly increased time in the bic-paired chamber (Fig. 10E) during the postconditioning as compared to preconditioning assessment (538sec ± 32.8 vs 400sec ± 19.6 [t11= −4.565, p< 0.001]). This effect was blocked in rats injected prior to conditioning sessions with 0.1 mg/kg hal (448 sec ± 39.6 vs 424 sec ± 42.6 [t8 = 0.009, p= 0.99]) or 0.01 mg/kg SCH (401 sec ± 12.6 vs 422 sec ± 15.9 [t4= −0.673, p= 0.538]). Comparison of the conditioning scores (Fig. 10F) revealed a significant main effect of drug treatment (F2,25= 9.92, p= 0.003) shown by Holm-Sidak post hoc analysis to reflect significantly lower conditioning scores in animals that received bilateral VP bic infusions and injections of 0.1 mg/kg hal (−0.11 ± 11.2) or 0.01 mg/kg SCH (4.6 ± 6.8) during conditioning as compared to control groups that received bic infusions and vehicle injections (138 ± 30.3). Thus, a preferred behavioral state requiring both D1 and D2 receptor signaling accompanies VP disinhibition. Pivoting and gnawing that accompany VP disinhibition (Zahm et al., 2014a; Subramanian et al., 2018) were observed during all VP bic conditioning sessions.
Figure 10:
Schematic illustrating VP place preference conditioning sessions (A). Cannula placements for vehicle (B) 0.01mg/kg SCH and 0.1 mg/kg hal (D) treatment groups tested in CPP and EPM. Time spent in the VP bic-paired chamber during the preconditioning (gray bars) and post conditioning (black bars) assessments (E). Time in the VP-bic paired chamber significantly increased during the post compared to the pre conditioning assessment in only the control group. Statistical comparison of the conditioning scores from each treatment group showed that place preference acquired from VP bic infusions requires signaling through both D1 and D2 receptors (F).
Conditioning with bilateral VP infusions of a cocktail containing dopamine receptor antagonists and bicuculline did not significantly (t4=−0.539, p=0.618) alter time in the cocktail-paired chamber during the post (389.2 ± 18.4s) compared to the pre (385.8 ± 14.2s) conditioning assessment (Fig. 9C), indicating that CPP elicited by VP bic (Fig. 10E and F) requires dopamine receptor signaling in the VP.
Elevated plus maze
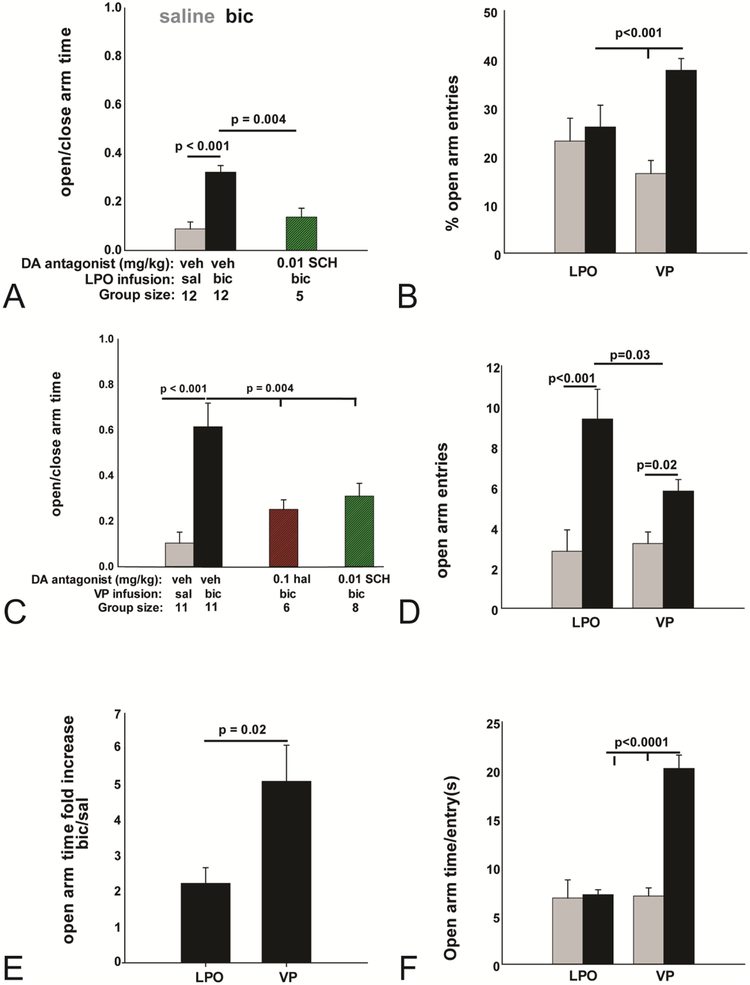
Bic as compared to vehicle infusions into the LPO (Fig. 11A) significantly increased the ratio of time spent on the open as compared to closed arms of the EPM (0.32 ± 0.03 vs 0.09 ± 0.02 [t10= 8.56, p< 0.001]). This ratio was significantly lowered [F10,26= 32.449, p < 0.001] in animals pre-injected with 0.01 mg/kg SCH as compared to vehicle (0.138 ± 0.04 [t16= 3.826, p=0.004]). Unaccountably, rats that received hal at 0.1 mg/kg prior to LPO bic infusions repeatedly fell or leapt (two subjects) from the open arms of the EPM and were not included in the statistical evaluation. Together, the results indicate that an EPM anxiolytic effect requiring signaling through D1 and D2 receptors accompanies disinhibition of the LPO.
Figure 11:
(A) The ratio of open to closed arm time significantly increased following LPO bic infusions (black bar) as compared to LPO saline infusions (gray bar). The bic-elicited increase was attenuated by pretreatment with SCH (green hashed bar). However, VP bic infusions elicited a higher percentage of open arm entries, expressed as percent of total, compared to VP saline or LPO bic infusions which were not significantly different than LPO saline (B). (C) The ratio of open to closed arm time significantly increased following VP bic infusions (black bar) compared to VP saline infusions (gray bar). The bic-elicited increase was significantly attenuated by pretreatment with haloperidol (red hashed bar) or SCH (green hashed bar). The number of open arm entries was significantly increased by bic infusions (black bars) into the LPO or VP as compared to the respective saline infusions (gray bars). (D) Open arm entries were significantly more numerous following bic infusions into the LPO compared to VP. (E) The fold increase in time spent on the plus maze open arms following bic as compared to saline was significantly greater for VP as compared to LPO infusions. (F) VP bic infusions increased the time spent each time the animal entered an open arm as compared to VP saline or LPO bic infusions. Open arm time spent per entry was no different following LPO bic as compared to LPO saline infusions.
To test if the effects of dopamine receptor antagonists on LPO-elicited EPM behavior are due to actions in the Acb, rats with guide cannulae targeting the LPO unilaterally and Acb bilaterally underwent EPM testing. Bilateral infusions of a cocktail containing etic (10 μg) and SCH (2 μg) into the Acb significantly (t5= 7.630, p < 0.001) reduced the LPO bic-elicited open/closed arm time ratio (Fig. 9B) compared to Acb vehicle infusions (Acb cocktail + LPO bic: 0.075 ± 0.02 vs Acb saline + LPO bic: 0.386 ± 0.05). In contrast, total arm entries in this condition were not different than controls (data not shown, but see Fig. 11B). The results indicate that emboldened EPM behavior that accompanies LPO stimulation requires dopamine receptor signaling in the Acb.
Bilateral infusions into the VP of bic as compared to vehicle (Fig. 11C) significantly increased the ratio of time spent on the open as compared to closed arms of the EPM (0.674 ± 0.09 vs 0.1 ± 0.03 [t9= 6.16, p= 0.0001]). This effect was restored to vehicle levels (0.1 mg/kg hal: t18=0.395, p=0.696; 0.01 SCH t14=0.788, p=0.788) in animals pretreated with 0.1 mg/kg hal (0.08 ± 0.02 [F10,26 = 32.449, p < 0.001]) or 0.01 mg/kg SCH (0.03 ± 0.006 [F3,33 = 37.9, p < 0.001]). Taken together, these results demonstrate that emboldened EPM behavior that accompanies bilateral disinhibition of the VP requires signaling through D1 and D2 receptors. Most rats displayed brief episodes of pivoting and gnawing in the enclosed arms and one rat was excluded from the analysis for this reason due to prolonged pivoting (180 seconds). Interestingly, rats never pivoted on the open arms.
Bilateral infusions into the VP of a cocktail containing bic, etic (0.1 mg/kg) and SCH (0.01 mg/kg) significantly (t4=10.192, p< 0.001) reduced the ratio of open to closed arm time (Fig. 9D) compared to bilateral VP bic infusions (VP cocktail: 0.09 ± 0.05 vs VP bic: 0.926 ± 0.08), indicating that emboldened EPM behavior that accompanies bilateral inhibition of the VP requires D1 and D2 dopamine receptor signaling in the VP.
Comparison of LPO and VP EPM data
EPM open arm entries (Fig. 11D) significantly increased following bic as compared to vehicle infusions into the LPO (8.5 ± 0.9 vs 2.2 ± 0.7 [t10= −5.33, p< 0.001]) and VP (5.8 ± 0.6 vs 3.2 ± 0.6; [t9= −2.82, p= 0.02]). Significantly more open arm entries were recorded after LPO-bic than VP-bic infusions (Fig.11D; [t19= 2.35, p=0.03]), suggesting that the emboldening effect is greater after disinhibiting the LPO than VP. However, open arm entries calculated as percent of total (Fig. 11B) were significantly more numerous after VP than LPO bic infusions (VP: 0.37 ± 0.02 vs LPO: 0.22 ± 0.03 [t19= −4.373, p= 0.02]). Furthermore, fold increase in time on the open arms (Fig. 11E) was significantly greater following VP (5.1 ± 1.0) than LPO (2.6 ± 1.0) bic infusions (t16 = 2.57, p=0.02). Accordingly, more time was spent per open arm entry (Fig. 11F) following VP bic (bic: 20.2 ± 1.4 vs sal: 7.0 ± 0.8; t9 = 6.96, p<0.0001) than LPO bic (bic: 7.1 ± 0.5 vs sal:6.8 ± 1.9; t19=9.18, p<0.0001) infusions as compared to vehicle, whereas time spent on the open arms per entry did not differ significantly between bic and vehicle infusions into the LPO. Taken together, the data indicate that, in contrast to the LPO, disinhibition of the VP produces a tolerance of EPM open arms not linked to an increase in locomotor activation.
Discussion
Based upon observed increases in % distance in the field center, sweet pellet ingestion and EPM open arm entries, summarized in Table 1, we can assert that stimulation of both the LPO and VP embolden behavior.
Table 1.
Summary of experiments and results
| Treatments | Effects* | ||||||
|---|---|---|---|---|---|---|---|
| threat tolerance experiments | |||||||
| Bicuculline | Dopamine antagonists | total distance | % distance in center | pellets eaten | CPP | EPM open arms | |
| Site of infusion | Specificity | Site of delivery | |||||
| LPO | ↑ | ↔ | ↔ | ↑ | ↑ | ||
| D2 | systemic | block | n/a | n/a | block | block** | |
| D1 | systemic | block | n/a | n/a | block | block | |
| D2+D1 | Acb bi | nd | n/a | n/a | block | block | |
| VP bi | ↔ | ↑ | ↑ | ↑ | ↑ | ||
| D2 | systemic | n/a | ↔ | block | block | block | |
| D1 | systemic | n/a | ↔ | ↔ | block | block | |
| D2+D1 | VP bi | n/a | nd | nd | block | block | |
Symbols show the effects of LPO and VP bi bicuculline infusions on the indicated experimental parameters and of dopamine antagonists on effects elicited by LPO- and VP bicuculline.
Rats jumped off the open arms (see Results).
Symbols: ↑- increased or produced; ↔ - no effect; block - effect reversal; n/a - not applicable; nd - not done
Abbreviations: Acb bi – accumbens, bilateral; CPP – conditioned place preference: D1 - dopamine D1 receptor; D2 – dopamine D2 receptor; EPM – elevated plus maze; LPO – lateral preoptic area; VP bi – ventral pallidum, bilateral
LPO bic infusions produced increases in total locomotion and field center occupancy, which were directly proportional to each other, but did not increase ingestion of sweet pellets. LPO bic also increased occupancy on the open arms of the EPM, also in a manner proportionate to the potentiation of locomotion. Accordingly, the reduction in LPO bic-elicited occupancy of the open field center and open arms of the EPM caused by SCH and hal was likely due to reductions in overall locomotion that accompanied the administration of the D1 and D2 dopamine antagonists. Indeed, the apparent emboldening effect of LPO bic was so linked to locomotion that it might alternatively be interpreted as an epiphenomenon of LPO-elicited locomotor activation. Nevertheless, LPO bic increased occupancy of space that, according to the control experiments, rats normally would prefer not to occupy – a sine qua non of emboldening. This effect might be explained, at least in part, by CPP, a reflection of increased sense of well-being that accompanied locomotion potentiated by LPO bic infusions, in the glow of which the open field center and EPM open arms may be less threatening. To be sure, the locomotor and CPP effects of stimulating the LPO resemble those elicited by drugs that activate the mesoaccumbens DA pathway or otherwise cause release of DA in the Acb (Kelly et al., 1975), so it is consistent that LPO bic-elicited CPP was reversed by DA antagonists infused directly into the Acb during the conditioning sessions (as well as by systemic administration of DA antagonists). Thus, a capacity of LPO stimulation to embolden behavior may occur in lock-step with DA release in the Acb.
VP bic infusions increased field center occupancy in the absence of a general increase in locomotor activation. When field center occupancy after VP bic infusions was calculated as % total locomotion, it represented a significantly greater increase than after LPO bic infusions. In addition, VP bic-infused rats boldly ingested sweet pellets under the spotlight, in contrast to subjects in all of the control groups, which ingested almost no pellets in harsh illumination. Presumably, pellets were ingested compulsively by the VP bic group as a fixed behavioral response to VP bic infusions (Cromwell and Berridge, 1993; Stratford et al., 1999; Shimura et al., 2006; Smith and Berridge, 2007; Smith et al., 2009; Stratford and Wirtshafter, 2012; 2013; Covelo et al., 2014; Subramanian et al., 2018), rather than from hunger or to satisfy a sweet appetite. Importantly, however, VP-infused rats discounted imputed threat and aversiveness of the spotlight to do so. Whether they were inclined to eat in the harsh light due to the strength of the compulsion, because discharging the compulsion is in itself rewarding, or because VP bic-induced boldness promotes disregard of the spotlight was not apparent from the open field data. However, VP bic also increased open arm occupancy on the EPM, where sweet pellets were unavailable, which argues against a goal-related basis for the emboldening effect of VP. On the other hand, VP bic-elicited pellet ingestion was attenuated by the DA D2 receptor antagonist hal, which renders reinforcers less reinforcing (Wise, 2004). In contrast to pellet ingestion, center field occupancy increased by VP bic infusions remained increased in the presence of hal. Inasmuch as infusions of bic into the VP also produced CPP, enhanced sense of well being may underlie the willingness of VP bic-infused rats to occupy (and ingest pellets in) the harshly lit field center. Were this the case, however, a treatment that prevents VP bic-associated development of CPP would be expected also to block the VP bic-elicited increase in field center occupancy. As it turned out, hal prevented VP bic-generated CPP, suggesting that the failure of hal to decrease VP bic-elicited field center occupancy may more reflect a sort of threat-blindness not associated with well-being. These discrepancies are at present unexplained, but it is pertinent that Floresco et al. (2003) reported that stimulation of VP does not activate ventral tegmental DA neurons or cause release of DA in the Acb, which suggests, in any event, that the behavioral effects of VP stimulation are independent of DA release in the Acb (see also below). However, our data suggest that VP bic-elicited pellet ingestion, at least, requires D2 receptor stimulation somewhere in the brain, possibly the VP itself, insofar as we also show that selective blockade of D2 + D1 receptors in the VP blocks VP bic-elicited CPP and EPM open arm occupancy. Obviously, these interactions require further investigation, but what is not in dispute is that rats subjected to VP bic infusions reliably and vigorously ingest sweet pellets in a harshly illuminated field center and boldly occupy the open arms of the EPM, whereas control rats do not.
We thus have abundant evidence linking of one sort of emboldened behavior to stimulation of the LPO and another to activation of the VP. Boldness generated in association with LPO activation is tightly tied to locomotor activation and, as is locomotion itself, strongly DA-dependent (Kelly et al., 1975), whereas that accompanying stimulation of the VP was independent of locomotor activation and, at least as regards field center occupancy in harsh illumination, DA signaling. Furthermore, in the case of neither LPO nor VP could the respective emboldened behavior be clearly attributed to goal pursuit – sweet pellet ingestion was not increased after LPO stimulation and, after VP activation, pellet consumption is a fixed action response unrelated to normal feeding cues. Rather, the data suggest that emboldening of behavior is itself an obligatory response linked to the downstream effects of stimulating the VP much akin to other fixed action motor responses, such as pivoting, backing, gnawing and eating elicited by VP stimulation (Subramanian et al., 2018).
Methodological considerations
This paper is the fourth in a series from our lab describing behavioral effects of bic infusions into the LPO and VP. Important methodological concerns, such as the localization and appearance of infusion sites, control infusions and mechanism of action of bic have been addressed at length in the earlier papers, which were concerned exclusively with motor responses (Reynolds et al., 2006; Zahm et al., 2014a; Subramanian et al., 2018).
CPP:
Notably, our assessment of VP bic-elicited CPP differs from Gong et al. (1997), who reported no CPP after VP bic. This discrepancy may reflect differences in cannula placements or numbers of VP-bic conditioning sessions (two in Gong et al., three here). Furthermore, the CPP and EPM experiments in this study were performed during the dark part of the diurnal cycle, when rats are active. Two groups of our rats (n=12) did not show CPP after conditioning during light hours and actually spent very little time exploring the apparatus during the postconditioning assessment (data not shown), consistent with the negative result of Gong et al. (1997).
Concentrations of Hal and SCH:
Relatively low doses of hal at 0.1 mg/kg and SCH at 0.01 mg/kg strongly suppressed basal locomotion and produced moderate attenuation of forebrain-stimulated locomotion, which raises the possibility that accompanying reductions of field center occupancy and pellet ingestion reflected impairments of performance rather than motivation. This was addressed by normalizing critical data, such as field center distance and open arm occupancy as % of total. In contrast, the effects of DA antagonists on pellet ingestion were less susceptible to motor impairments. First, insofar as VP bic rescues the hal-elicited motor deficit (Subramanian et al., 2018; present data), the accompanying hal-elicited reduction in VP bic-elicited pellet ingestion, CPP and EPM open arm occupancy cannot be attributed to motor impairment. Alternatively, pellet ingestion was not significantly reduced by SCH after VP bic infusions, despite a moderate to severe impairment in locomotion, which is consistent with a minimal effect of the D1 antagonist and, for that matter, motor impairment, on VP bic-elicited eating. Preliminary experiments with lower doses of hal and SCH that left locomotion mildly impaired, had no effects on the critical dependent variables of the study, i.e., % distance in the field center, pellet consumption, CPP and EPM. Alternatively, increasing the concentrations of hal and SCH beyond 0.1 and 0.01 mg/kg, respectively, produced pronounced suppression of locomotion, which prevented assessment of behavior, i.e., rats remained stationary in a corner of the apparatus throughout the trials.
Effects of systemic and site-specific DA antagonists
Our data showed that alterations in CPP and EPM behavior elicited by disinhibition of the LPO require DA receptor signaling in the Acb, but did not exclude the possibility that DA receptor stimulation is also required elsewhere, e.g., in the VP. Alternatively, by co-infusing bic and DA antagonists into the VP we showed that VP-elicited behavioral states require DA receptor signaling in the VP, but, again, this did not exclude a possibility that DA receptor stimulation is also required elsewhere, e.g., in the Acb. Despite our keen interest to explore these alternatives more fully, technical constraints prevented us from infusing bic into the LPO along with DA antagonists bilaterally into the VP or bic bilaterally into the VP along with DA antagonists bilaterally into the Acb. Fortunately, the literature contains much information useful to the interpretation of the experiments we were able to do.
Inasmuch as the LPO and VP both project similarly to brainstem behavior effectors and structures that influence them (refs. cited in the Introduction), both LPO and VP should be able to influence motivated behaviors independently of DA release in the Acb. However, this seems unlikely to be the case for LPO bic infusions because co-infusing DA antagonists into the Acb prevented the increases in CPP and EPM open arm tolerance. That is, if LPO disinhibition sends motivationally relevant signals downstream, independent of Acb DA release, CPP and emboldened EPM open arm behavior should not be affected by blockade of DA receptors upstream in the Acb. Alternatively, behavioral boldness elicited by VP bic infusions was disrupted by DA antagonists co-infused within the VP itself, which was not unexpected given that VP activation does not increase the activity of VTA DA neurons or Acb DA release (Floresco et al., 2003). In point of fact, stimulation and inhibition of VP or VP terminals in the VTA, respectively, suppressed and increased the firing rates of VTA dopamine neurons (Wu et al., 1996; Floresco et al., 2003; Hjelmstad et al., 2013; Mahler et al., 2014). Accordingly, it seems unlikely the CPP response to VP bic is contingent upon dopamine signaling in the Acb. Instead, CPP elicited by bic infusions into the VP functionally resembles CPP due to VP psychostimulant infusions – both act to increase extracellular DA concentration in the VP (Gong et al., 1996;1998) - consistent with reports that, e.g., stimulation of VP DA receptors elicits conditioned reward (Fletcher et al., 1998) and facilitates cocaine CPP (Gong et al., 1997) and cocaine self-administration (Sizemore et al., 1998). Furthermore, VP lesions and inactivation of VP block the rewarding effects of Acb DA (Swerdlow et al., 1983) and drugs that increase Acb DA concentrations (Hubner and Koob, 1990; Hiroi and White, 1993; Subramanian et al., 2018). Thus, motivational potency typically attributed to Acb dopamine release may actually be conveyed to behavioral effectors, at least in part, by outputs from the VP. If neural substrates for motoric (Sherrington; 1910; Brown, 1911; Woods, 1964; Chaurand et al., 1974; Micco, 1974; Waldbillig, 1975) and motivational (Bard, 1928;1934a;b; Wolfle et al., 1971; Glickman and Schiff, 1967; Venkatraman et al., 2017) responding are intrinsic to the brainstem and disinhibition of the VP elicits both motoric and motivational responding, it is reasonable to speculate that VP conveys a motivationally relevant signal directly to the brainstem and/or, via other ascending modulatory projections and the thalamus, to the telencephalon (Zahm, 2006; Heimer et al., 2008; Zahm and Root, 2017), independently of Acb DA release. However, just as VP bic infusions fail to generate the kind of (integrated, coordinated) locomotor activation that accompanies LPO stimulation or DA release in the Acb (Subramanian et al., 2018), the ‘emboldening’ response to VP bic also lacks a genuine, i.e., goal-linked, motivational component, and thus may be better regarded as another “fixed action” response along with pivoting dyskinesias, compulsive gnawing and so on, observed after activation of the VP (Subramanian et al., 2018).
Concluding remarks
A consistent theme that over the years has characterized conceptualizations of the neural substrates of adaptive behavior is that cognitive-affective states proceed from telencephalic integration of neural signaling reflecting genetically transmitted behavioral imperatives and the external and internal environments via pathways that descend to motor networks - behavioral programs - intrinsic to the brainstem and spinal cord (Sherrington, 1910; Brown; 1911; Bard, 1934a;b; Chaurand et al., 1974; Micco, 1974; Waldbillig, 1975; Woods, 1964; Nauta,1986; Alheid and Heimer, 1988; Swanson, 1987; 2000; Holestege, 1992; Nieuwenhuys,1996; Zahm, 2000; 2006; Pinker, 2003; Morgane et al., 2005; Heimer et al., 2008; Uddin et al., 2014; Barrett, 2017; Pessoa, 2017). A crucial advance in this progression owes to Lennart Heimer, George Alheid, Jose de Olmos and other of their colleagues in the conceptualization of basal forebrain functional-anatomical macrosystems (e.g., Heimer, 1972; Heimer and Wilson, 1975; Alheid and Heimer, 1988; Heimer and Alheid, 1991; Heimer et al., 1991; McDonald, 1991; McDonald et al., 1996; 1999; Shi and Cassell, 1998; Zahm, 1999; Shammah-Lagnado and Santiago, 1999; Zahm, 2000; 2006; Reynolds and Zahm, 2005; Heimer and Van Hoesen, 2006; Heimer et al., 2008; Reichard et al., 2017). It is implicit in the organization of macrosystems that competing and cooperating pathways descend to modulate adaptive movements and motivational tone by acting on influencers of brainstem behavioral effectors and long reëntrant ascending and thalamocortical pathways. The present study aligns with this model of brain organization by showing that two output structures, LPO and VP, serving adjacent basal forebrain macrosystems act by different mechanisms to embolden behavior.
Acknowledgments
Grant support: USPHS NIH grants NS-23805 (DSZ) and T32 GM008306 (RAR)
1) Funding: The work was supported by USPHS NIH grant NS-23805 to DSZ. RAR received support from USPHS NIH grant T32 GM008306.
List of Abbreviations
- Acb
accumbens
- bic
9(R)-(−)-bicuculline methbromide
- CPP
conditioned place preference
- D1
dopamine D1 receptor
- D2
dopamine D2 receptor
- EPM
elevated plus maze
- etic
S-(−)-eticlopride hydrobromide
- hal
haloperidol
- i.p.
Intraperitoneal
- LPO
lateral preoptic area
- SCH
R(+)-SCH-23390
- VP
ventral pallidum
- VP bi
VP, infused bilaterally
- VP uni
VP, infused unilaterally
- VTA
ventral tegmental area
Footnotes
2) Disclosure of potential conflicts of interest: The authors report no conflicts of interest
3) Research involving Human Participants and/or Animals: There were no human subjects. Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250–375 g were used in accordance with policy mandated in the Public Health Service Policy on Humane Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/references/phspol.htm) provided by the U.S. Department of Health and Human Services with oversight by the Saint Louis University Animal Care Committee. Veterinary care was provided by the Saint Louis University Department of Comparative Medicine.
4) Ethical approval: All authors read and approved the final submitted manuscript.
5) Informed consent: There were no human subjects.
Literature cited:
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ (1989) Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem 52, 1655–1658. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid and corticopetal components of substantia innominata. Neuroscience 27: 1–39. [DOI] [PubMed] [Google Scholar]
- Alonso A, Kohler C (1984) A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J. Comp. Neurol 225: 327–343. [DOI] [PubMed] [Google Scholar]
- Bard P (1928) A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. American Journal of Physiology, 84, 490–515. [Google Scholar]
- Bard P (1934a) On emotional expression after decortication with some remarks on certain theoretical views: Part I. Psychological Review, 41, 309–329. [Google Scholar]
- Bard P (1934b) On emotional expression after decortication with some remarks on certain theoretical views: Part II. Psychological Review, 41, 424–449. [Google Scholar]
- Barker DJ, Miranda-Barrientos J, Zhang S, Root DH, Wang HL, Liu B, Calipari ES, Morales M (2017) Lateral Preoptic Control of the Lateral Habenula through Convergent Glutamate and GABA Transmission. Cell reports 21:1757–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2017) The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci 12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM (1979) An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J. Comp. Neurol 184:43–62. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. (2015) Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc. R. Soc. B 282: 20142516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JA (2001) SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS drug reviews 7:399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG (1911) The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond Ser B 84:308–319. [Google Scholar]
- Buchanan SL, Thompson RH, Maxwell BL, Powell DA (1994) Efferent connections of the medial prefrontal cortex in the rabbit. Exp. Brain Res 100:469–483. [DOI] [PubMed] [Google Scholar]
- Caffe AR, van Leeuwen FW, Luiten PG (1987) Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J. Comp. Neurol 261:237–252. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW (1995) Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J. Comp. Neurol 360:213–24. [DOI] [PubMed] [Google Scholar]
- Chaurand JP, Vergnes M, Karli P (1974) [Elicitation of aggressive behavior by electrical stimulation of ventral mesencephalic tegmentum in the rat (author’s transl)]. Physiology & behavior 12:771–778. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K (2001) Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res 888:83–101. [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN (1997) Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J. Neurosci 17:9686–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AV, Arnt J, Hyttel J, Larsen JJ, Svendsen O (1984) Pharmacological effects of a specific dopamine D-1 antagonist SCH 23390 in comparison with neuroleptics. Life sciences 34:1529–1540. [DOI] [PubMed] [Google Scholar]
- Covelo IR, Patel ZI, Luviano JA, Stratford TR, Wirtshafter D (2014) Manipulation of GABA in the ventral pallidum, but not the nucleus accumbens, induces intense, preferential, fat consumption in rats. Behav Brain Res 270:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC (1993) Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res 624:1–10. [DOI] [PubMed] [Google Scholar]
- Everitt BJ (1990) Sexual motivation:a neural and behavioral analysis of the mechanisms underlying appetitive copulatory responses of malerats. Neurosci Biobehav Rev 14:217–32. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Öngür D, An X, Price JL (2000) Prefrontal cortical projections to the striatum in Macaque monkeys: Evidence for an organization related to prefrontal networks. J. Comp. Neurol 425:447–470. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Sabijan MS, DeSousa NJ (1998) Injections of D-amphetamine into the ventral pallidum increase locomotor activity and responding for conditioned reward: a comparison with injections into the nucleus accumbens. Brain Research 805(1–2):29–40. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6(9):968–73. [DOI] [PubMed] [Google Scholar]
- Geisler S, Trimble M (2008) The lateral habenula: no longer neglected. CNS Spectr 13, 484–489. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS (2005) Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J. Comp. Neurol 490: 270–294. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS (2006) Neurotensin afferents of the ventral tegmental area in the rat: [1] re-examination of their origins and [2] responses to acute psychostimulant and antipsychotic drug administration. Eur. J. Neurosci 24, 116–134. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Schiff BB (1967) A biological theory of reinforcement. Psychol Rev 74:81–109. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB Jr. (1996) Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Research 707:64–74. [DOI] [PubMed] [Google Scholar]
- Gong W, Justice JB Jr, Neill D (1997) Dissociation of locomotor and conditioned place preference responses following manipulation of GABA-A and AMPA receptors in ventral pallidum. Prog Neuropsychopharmacol Biol Psychiatry 21(5):839–52. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB Jr. (1998) GABAergic modulation of ventral pallidal dopamine release studied by in vivo microdialysis in the freely moving rat. Synapse 29(4):406–12. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Room P, Witter MP, Lohman AHM (1982) Cortical afferents of the nucleus accumbens in the cat, studies with anterograde and retrograde transport techniques. Neuroscience 7:977–95. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AHM (1991) The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization In: Uylings HBM, Van Eden CG, De Bruin JPC, Corner MA, Feenstra MGP, editors. Progress in Brain Research, 85 Amsterdam: Elsevier, p. 95–118. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN (1993) Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience 57:113–142. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ (1985) Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J. Comp. Neurol 235: 322–335. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E (1995) The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci 15:4851–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Kohler C, Gawell L (1985) Some in vitro receptor binding properties of [3H]eticlopride, a novel substituted benzamide, selective for dopamine-D2 receptors in the rat brain. European journal of pharmacology 111:191–199. [DOI] [PubMed] [Google Scholar]
- Heimer L (1972) The olfactory connections of the diencephalon in the rat: an experimental light- and electron-microscopic study with special emphasis on the problem of terminal degeneration. Brain Behav. Evol 6, 484–523. [DOI] [PubMed] [Google Scholar]
- Heimer L (1978) The Olfactory Cortex and the Ventral Striatum In: Livingston KE, Hornkiewicz O (Eds.), Limbic Mechanism Plenum Press, New York, pp. 95–187. [Google Scholar]
- Heimer L, Alheid GF (1991) Piecing together the puzzle of basal forebrain anatomy. Adv Exp Med Biol 295:1–42. [DOI] [PubMed] [Google Scholar]
- Heimer L, de Olmos J, Alheid GF, Zaborszky L (1991a) “Perestroika” in the basal forebrain: opening the border between neurology and psychiatry. Progress in brain research 87:109–165. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW (2006) The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev 30:126–147. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW, Trimble M, Zahm DS (2008) Anatomy of neuropsychiatry: The new anatomy of the basal forebrain and its implications for neuropsychiatric illness Amsterdam: Academic Press/Elsevier. [Google Scholar]
- Heimer L, Wilson RD (1975) The subcortical projections of allocortex. Similarities in the neural associations of the hippocampus, the piriform cortex and the neocortex In Golgi Centennial Symposium Proceedings Edited by Santini M: 177–196. Raven Press, New York. [Google Scholar]
- Hjelmstad GO, Xia Y, Margolis EB, Fields HL (2013) Opioid Modulation of Ventral Pallidal Afferents to Ventral Tegmental Area Neurons. The Journal of Neuroscience, 33(15): 6454–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirori N, White NM (1991) The amphetamine conditioned place preference: differential involvement of dopamine receptor subtypes and two dopaminergic terminals. Brain Research 552:141–152. [DOI] [PubMed] [Google Scholar]
- Holstege G (1992) The emotional motor system. Eur J Neurosci 30:67–79. [PubMed] [Google Scholar]
- Hubner CB, Koob GF (1990) The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Research 508(1):20–9. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Leranth C (1990) Catecholaminergic, GABAergic, and hippocamposeptal innervation of GABAergic “somatospiny” neurons in the rat lateral septal area. J. Comp. Neurol 302:305–321. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA (1993) GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience 57:1047–1060. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB (1982) The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience 7:2321–2335. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ (1982) The amygdalostriatal projection in the rat--an anatomical study by anterograde and retrograde tracing methods. Neuroscience 7:615–630. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD (1975) Amphetamine and apomorphine responses in the rat following 6-OHDA lesion of the nucleus accumbens septi and corpus striatum. BrainRes 94:507–22. [DOI] [PubMed] [Google Scholar]
- King BM, Cook JT, Rossiter KN, Rollins BL (2003) Obesity-inducing amygdala lesions: examination of anterograde degeneration and retrograde transport, Am. J. Physiol. Regul. Integr. Comp. Physiol 284:965–82. [DOI] [PubMed] [Google Scholar]
- Köhler C, Hall H, Gawell L (1986) Regional in vivo binding of the substituted benzamide [3H]eticlopride in the rat brain: evidence for selective labelling of dopamine receptors. European journal of pharmacology 120:217–226. [DOI] [PubMed] [Google Scholar]
- Leranth C, Carpi D, Buzsaki G, Kiss J (1999) The entorhino-septosupramammillary nucleus connection in the rat: morphological basis of a feedback mechanism regulating hippocampal theta rhythm. Neuroscience 88:701–718. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Bennett JP Jr (1987a) Striatal D1- and D2-dopamine receptor sites are separately detectable in vivo. Brain Res 415:90–97. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Bennett JP Jr (1987b) [3H]spiperone binds selectively to rat striatal D2 dopamine receptors in vivo: a kinetic and pharmacological analysis. Brain Res 407:253–262. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Friedle NM, More KE (1979) Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav 11:663–6. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G (2014) Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature Neuroscience 17:577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ (1991) Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 44:15–33. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L (1996) Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71:55–75. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M (1999) Cortical afferents to the extended amygdala. Ann N Y Acad Sci 877:309–338. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM (1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29:503–37. [DOI] [PubMed] [Google Scholar]
- Micco DJ (1974) Complex behaviors elicited by stimulation of the dorsal pontine tegmentum in rats. Brain Res 75: 172–176. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A (1996) Involvement of mesolimbic dopamine neurons in sexual behaviors: implications for the neurobiology of motivation. Rev Neurosci 5:317–29. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ (2005) A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol 75(2):143–60. [DOI] [PubMed] [Google Scholar]
- Nauta WJH (1986) Ciruitous connections linking cerebral cortex, limbic system and corpus striatum In: Doane BK, Livingston KE, editors. Limbic System: Functional Organization and Clinical Disorders New York: Raven Press, pp. 43–54. [Google Scholar]
- Nieuwenhuys R (1996) The greater limbic system, the emotional motor system and the brain. Prog Brain Res 107:551–80. [DOI] [PubMed] [Google Scholar]
- Pessoa L (2017) A network model of the Emotional Brain Trends in Cognitive Sciences 5:357–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT (1979) Afferent projections to the ventral tegmental area of tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J. Comp. Neurol 187:117–144. [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Griffiths AC (1985) The topographical order of inputs to nucleus accumbens in the rat. Neuroscience 16:175–96. [DOI] [PubMed] [Google Scholar]
- Pinker S (2003) The Blank Slate The Modern Denial of Human Nature Penguin Books, New York, NY. [Google Scholar]
- Reichard RA, Parsley KP, Subramanian S, Zahm DS (2018) Non-homeostatic ingestion elicited by disinhibition of the ventral pallidum bun not lateral preoptic area: dissoiative roles of D1 and D2 receptors, in review
- Reichard RA, Parsley KP, Zahm DS (2016) Comparison of stimulations of the lateral preoptic area and ventral pallidum using measures of reward, anxiety and ingestion. Soc Neurosci Abstr 453.23. [Google Scholar]
- Reichard RA, Subramanian S, Desta MT, Sura T, Becker ML, Ghobadi CW, Parsley KP, Zahm DS (2017) Abundant collateralization of temporal lobe projections to the accumbens, bed nucleus of stria terminalis, central amygdala and lateral septum. Brain Structure and Function 222:1971–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS (2005) Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci 25:11757–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Geisler S, Berod A, Zahm DS (2006) Neurotensin antagonist acutely and robustly attenuates locomotion that accompanies stimulation of a neurotensin-containing pathway from rostrobasal forebrain to the ventral tegmental area. Eur JNeurosci 24: 188–196. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW (1997) Connections of the rat lateral septal complex. Brain Research Reveiws 24: 115–195. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Koob GF, Klonoff P, Fibiger HC (1980) Extinction and recovery of cocaine self- administration following 6-hydroxydopamine lesions of the nucleus accumbens. J Pharmacol Exp Ther 224:662–73. [DOI] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC (2015) The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Progress in Neurobiology 130:29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT, Price JL (1984) Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-L as an anterograde tracer. Neurosci Lett 47:15–22. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH (1987) Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum, J. Comp. Neurol 259 483–528. [DOI] [PubMed] [Google Scholar]
- Sesack S, Deutch AY, Roth RH, Bunney BS (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin, J. Comp. Neurol 290:213–242. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Santiago AC (1999) Projections of the amygdalopiriform transition area (APir). A PHA-L study in the rat. Ann N York Acad Sci 877:655–660. [DOI] [PubMed] [Google Scholar]
- Sherrington CS (1910) Flexion-reflex of the limb, crossed extension reflex and the stepping reflex and standing. J Physiol 40:28–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD (1998) Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol 399:440–468. [DOI] [PubMed] [Google Scholar]
- Shimura T, Imaoka H, Yamamoto T (2006) Neurochemical modulation of ingestive behavior in the ventral pallidum. The European journal of neuroscience 23:1596–1604. [DOI] [PubMed] [Google Scholar]
- Sizemore GM, Co C, Hemby S, Koves TR, Smith JE (1998) The effects of 6-OHDA lesions of the ventral pallidum on cocaine self administration. College on Problems of Drug Dependence Abstracts, 134. [Google Scholar]
- Smith KS, Berridge KC (2007) Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27:1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009) Ventral pallidum roles in reward and motivation. Behav Brain Res 196:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Lynd-Balta E, Mitchell S, Haber SN (1996) Ventral pallidostriatal pathway in the monkey: evidence for modulation of basal ganglia circuits. J Comp Neurol 370: 295–312. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Rye DB, Wainer BH (1992) Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J. Comp. Neurol 321: 515–543. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE, Simansky KJ (1999) Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res 825:199–203. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D (2012) Evidence that the nucleus accumbens shell, ventral pallidum, and lateral hypothalamus are components of a lateralized feeding circuit. Behav Brain Res 226:548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D (2013) Lateral hypothalamic involvement in feeding elicited from the ventral pallidum. The European journal of neuroscience 37:648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Reichard RA, Stevenson HS, Schwartz ZM, Parsley KP, Zahm DS (2018) Lateral preoptic and ventral pallidal roles in locomotion and other movements, Brain structure & function, 223:2907–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ (1982) The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev 6, 1–13. [DOI] [PubMed] [Google Scholar]
- Swanson LW (1976) An autoradiographic study of the efferent connections of the preoptic region in the rat. J. Comp. Neurol 167: 227–256. [DOI] [PubMed] [Google Scholar]
- Swanson LW (1987) Limbic system In: Adelman G, Smith BH, editors. Encyclopedia of Neuroscience 2nd ed. Vol. 2, Amsterdam; Elsevier; pp. 1053–1055. [Google Scholar]
- Swanson LW (2000) Cerebral hemisphere regulation of motivated behavior. Brain Res 886:113–164. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kohler C, Bjorkland A (1987a) The limbic region. I. The septohippocampal system In: Bjorkland A, Hokfelt T, Swanson LW (Eds.), Integrated Systems of the CNS, Part I. Hypothalamus,Hippocampus, Amygdala, Retina Handbook of Chemical Neuroanatomy, vol. 5 Elsevier, Amsterdam, pp. 125–277. [Google Scholar]
- Swanson LW, Mogenson GJ, Simerly RB, Wu M (1987b) Anatomical and electrophysiological evidence for a projection from the medial preoptic area to the ‘mesencephalic and subthalamic locomotor regions’ in the rat, Brain research 405:108–122. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Swanson LW and Koob GF (1983) Electrolytic lesions of the substantia innominate and lateral preoptic area attenuate the ‘supersensitive’ locomotor response to apomorphine resulting from denervation of the nucleus accumbens. Brain Research 306:141–148. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kinnison J, Pessoa L, Anderson ML (2014) Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J. Cogn. Neurosci 26:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman A, Edlow BL, Immordino-Yang MH (2017) The Brainstem in Emotion: A Review. Frontiers in Neuroanatomy, 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. [DOI] [PubMed] [Google Scholar]
- Waldbillig RJ (1975) Attack, eating, drinking, and gnawing elicited by electrical stimulation of rat mesencephalon and pons. J. Comp. Physiol. and Psychol 89: 200–212. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Barone FC, Scharoun SL, Guevara-Aguilar R, Aguilar-Baturoni HU (1983) Limbic connections to the lateral preoptic area: A horseradish peroxidase study in the rat. Neuroscience & Biobehavioral Reveiws 7: 375–384. [DOI] [PubMed] [Google Scholar]
- Wise RA (1984) Neural mechanisms of the reinforcing action of cocaine. NIDA Res Monogr 50:15–33. [PubMed] [Google Scholar]
- Wise RA (2004) Dopamine, learning and motivation. Nature reviews. Neuroscience 5:483–494. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225. [DOI] [PubMed] [Google Scholar]
- Woods JW (1964) Behavior of chronic decerebrate rats. J Neurophysiol 27:635–44. [DOI] [PubMed] [Google Scholar]
- Wolfle TL, Mayer DJ, Carder B, Liebeskind JC (1971) Motivational effects of electrical stimulation in dorsal tegmentum of the rat. Physiol. Behav 7: 569–574. [DOI] [PubMed] [Google Scholar]
- Wu M, Hrycyshyn AW, Brudzynski SW (1996) Subpallidal outputs to the nucleus accumbens and the ventral tegmental area: anatomical and electrophysiological studies. Brain Research 740:151–61. [DOI] [PubMed] [Google Scholar]
- Yetnikoff L,Cheng AY, Lavezzi HN, Parsley KP, Zahm DS (2015) Sources of input to the rostromedial tegmental nucleus, ventral tegmental area, and lateral habenula compared: A study in rat. J. Comp. Neurol 523: 2426–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA, Wise RW (1975) Increased lever pressing for Amphetamine after Pimozide in Rats: Implications for a Dopamine Theory of Reward. Science 187:547–549. [DOI] [PubMed] [Google Scholar]
- Zahm DS (1989) The ventral striatopallidal parts of the basal ganglia in the rat--II. Compartmentation of ventral pallidal efferents. Neuroscience 30:33–50. [DOI] [PubMed] [Google Scholar]
- Zahm DS (1999) Functional-anatomical implications of the nucleus accumbens core and shell subterritories, Annals of the New York Academy of Sciences 877:13–128. [DOI] [PubMed] [Google Scholar]
- Zahm DS (2000) An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev 24: 85–105. [DOI] [PubMed] [Google Scholar]
- Zahm DS (2006) The evolving theory of basal forebrain functional-anatomical “macrosystems”. Neurosci Biobehav Rev, 30(2):148–172. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Williams EA, Qin S, Berod A (2001) Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined. Neuroscience 104:841–851. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L (1990) Two transpallidal pathways originating in the rat nucleus accumbens. J. Comp. Neurol 302:437–446. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Parsley KP, Schwartz ZM, Cheng AY (2013a) On lateral septum-Like characteristics of outputs from the accumbal hedonic “hotspot” of Peciña and Berridge with commentary on the transitional nature of basal forebrain “boundaries”. The Journal of comparative neurology 521(1):50–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Root DH (2017) Review of the cytology and connections of the lateral habenula, an avatar of adaptive behaving. Pharmacol Biochem Behav 162:3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Schwartz ZM, Lavezzi HN, Yetinkoff L, Parsley KP (2014a) Comparison of the locomotor-activating effects of bicuculline infusions into the preoptic area and ventral pallidum. Brain Struct Funct 219:511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Schwartz ZM, Parsley KP (2013b) Anxiolytic effects of lateral preoptic area (LPO) activation. Soc Neurosci Abstr 393.16. [Google Scholar]
- Zahm DS, Stevenson HS, Schwartz ZM, Parsley KP (2014b) Activation of ventral pallidum versus lateral preoptic area: double dissociation by threat discounting and locomotion. Soc Neurosci Abstr 267.11. [Google Scholar]
- Zahm DS, Williams E, Wohltmann C (1996) Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J. Comp. Neurol 364: 340–362. [DOI] [PubMed] [Google Scholar]