Abstract
The coxsackievirus and adenovirus receptor (CAR) mediates viral attachment and infection, but its physiologic functions have not been described. In nonpolarized cells, CAR localized to homotypic intercellular contacts, mediated homotypic cell aggregation, and recruited the tight junction protein ZO-1 to sites of cell–cell contact. In polarized epithelial cells, CAR and ZO-1 colocalized to tight junctions and could be coprecipitated from cell lysates. CAR expression led to reduced passage of macromolecules and ions across cell monolayers, and soluble CAR inhibited the formation of functional tight junctions. Virus entry into polarized epithelium required disruption of tight junctions. These results indicate that CAR is a component of the tight junction and of the functional barrier to paracellular solute movement. Sequestration of CAR in tight junctions may limit virus infection across epithelial surfaces.
Group B coxsackieviruses and a number of adenovirus serotypes initiate infection by binding to the coxsackievirus and adenovirus receptor (CAR) (1–3). CAR is a 46-kDa integral membrane protein with a typical transmembrane region, a long cytoplasmic domain, and an extracellular region composed of two Ig-like domains (1, 2). Both adenovirus (4) and coxsackievirus (5) interact with the N-terminal domain. Homologs of human CAR have been characterized in mice (2, 6), rats, pigs, dogs (7), and zebrafish (8). The murine and human proteins are very similar (91% amino acid identity within the extracellular domain, 77% within the transmembrane domain, and 95% identity within the cytoplasmic domain). Variant isoforms of CAR, which differ only at the C terminus and which most likely result from alternative splicing, have been identified in mice (6), humans, and rats (7). Despite evidence of its evolutionary conservation in mammals and nonmammalian vertebrates, the function of CAR is not known.
Tight junctions are continuous circumferential intercellular contacts at the apical poles of lateral cell membranes, appearing in electron micrographs as a series of discrete contacts between the plasma membranes of adjacent cells (9). Tight junctions form a barrier that regulates the paracellular transit of water, solutes, and immune cells across an epithelium (10), and are essential for establishing cell polarity by separating the apical and basolateral domains of polarized epithelial cells (11). ZO-1, the first tight junction protein identified (12, 13), is an intracellular peripheral membrane scaffolding protein important for tight junction structure and assembly. In the present study, we found that in polarized epithelial cells CAR is expressed at the tight junction, where it associates with ZO-1 and functions in the barrier to the movement of macromolecules and ions.
Materials and Methods
Cell Culture.
T-84, CALU-3, and 16HBE14o- cells were grown in 10% CO2 in a 1:1 mixture of DMEM and Ham's F-12 medium with 10% FCS. To establish polarized monolayers, 5 × 105 cells per well were plated on 12-mm diameter polyester filters with a pore size of 0.4 μm (Transwell Clears, Corning-Costar, Cambridge, MA). Cells were cultured for 1 week with medium changed every other day in both the upper and lower chambers of the Transwell. Madin–Darby canine kidney (MDCK) cells stably expressing human CAR (isoform 1 as originally reported in ref. 1) (MDCK-CAR) or transfected with vector alone (MDCK-pcDNA) were grown in DMEM with 10% FCS and 0.5 mg/ml G418 under 10% CO2/90% air as described (14). Chinese hamster ovary (CHO) cells stably transfected with cDNA constructs encoding human CAR (1) (CHO-CAR cells), CAR linked to a glycosylphosphatidylinositol (GPI) anchor (GPI-CAR), or transfected with vector alone (CHO-pcDNA) were cultured in nucleoside-free α-MEM + 10% dialyzed FCS as described (15).
Human well-differentiated tracheobronchial epithelial cells derived from airway specimens were obtained and cultured as described (16). Briefly, 3 × 105 human airway cells were plated onto Transwell-col inserts (diameter, 24 mm; pore size 0.4 μm) and confluent monolayers were maintained with an air–liquid interface (medium in the lower chamber only) for ≈30 days.
Immunofluorescence Microscopy.
For CAR staining, CHO cells were grown on glass coverslips for 2 days and fixed in 1% paraformaldehyde in PBS for 30 min at room temperature. Cells were incubated in 10% goat serum and 0.2% Triton X-100 in PBS with a polyclonal anti-CAR rabbit antibody that had been raised against recombinant CAR extracellular domain and purified by affinity to the extracellular domain immobilized on N-hydroxysuccinimide-Sepharose. After washing with PBS, cells were incubated with FITC-conjugated goat antibody to rabbit Ig (Sigma) in PBS with 10% goat serum and 0.2% Triton X-100. Specificity of the antibody was confirmed by Western blot and immunofluorescence of CAR-expressing and mock-transfected CHO cells (data not shown). For ZO-1 staining, fixed CHO cells were exposed to polyclonal rabbit anti-ZO-1 antibody (Zymed) in PBS with 10% goat serum and 0.2% Triton X-100 followed by a FITC-conjugated secondary antibody. Cells were examined with a Nikon Eclipse 800 fluorescence microscope.
For localization of CAR and ZO-1 in human well-differentiated airway epithelium, monolayers were fixed in 4% paraformaldehyde in PBS for 30 min. Cells were stained with affinity-purified rabbit anti-CAR antibody and mouse monoclonal anti-ZO-1 antibody (Zymed) in PBS with 10% goat serum and 0.2% Triton X-100 for 4 h. Cells were washed and exposed to Cy-3-conjugated goat anti-rabbit Ig and FITC-conjugated goat anti-mouse Ig for 2 h. Cells were examined with a Leica TCS 4D confocal microscope. Human airway epithelium showed no staining with the control monoclonal antibody MOPC 195 or with preimmune rabbit serum.
Confluent monolayers of T-84, CALU-3, or 16HBE14o- cells were fixed in 1% paraformaldehyde for 30 min and stained with affinity-purified rabbit anti-CAR antibody and mouse monoclonal anti-ZO-1 antibody (Zymed) for 3–4 h in PBS with 10% goat serum and 0.2% Triton X-100 followed by FITC-conjugated goat anti-mouse Ig and Cy-3-conjugated goat anti-rabbit Ig (Jackson ImmunoResearch Laboratories). Cells were examined by confocal microscopy. T-84 cells showed no staining with preimmune rabbit serum or the control monoclonal antibody MOPC 195. There was no bleed-through of fluorescence from either the FITC channel into the Cy-3 channel or the Cy-3 channel into the FITC channel when cells were stained only for CAR or ZO-1. There was no cross-species reactivity seen for the antirabbit or the antimouse secondary antibodies. For detection of endogenous CAR in MDCK cells, cells were fixed in cold methanol, rather than paraformaldehyde, and stained as above; no immunoreactivity with the affinity-purified rabbit anti-CAR antibody was detected in paraformaldehyde-fixed MDCK cells.
Electron Microscopy.
Polarized monolayers of T-84 cells were fixed in 4% paraformaldehyde and 0.2% glutaraldehyde in 100 mM sodium cacodylate buffer (pH 7.4) with 1 mM CaCl2 for 3 h at 4°C. The monolayers were dehydrated by using a graded series of ethanol concentrations and infiltrated in LR white. After sectioning, specimens were blocked in 10 mM sodium phosphate with 0.9% sodium chloride with 1% ovalbumin + 0.2% cold water fish skin gelatin for 1 h. Grids were incubated overnight at 4°C with rabbit polyclonal anti-CAR and mouse monoclonal anti-ZO-1 antibody. After washing, the grids were incubated for 1 h at room temperature with 8-nm gold-labeled anti-mouse antibody to detect ZO-1 and 18-nm gold-labeled anti-rabbit antibody to detect CAR. Grids were counterstained with 2% uranyl acetate and examined with a JEOL–JEM 1010 electron microscope. No staining was evident with preimmune rabbit serum or the control antibody MOPC 195. There was no cross-species reactivity seen for the gold-labeled anti-rabbit or anti-mouse secondary antibodies.
Immunoprecipitation.
Confluent monolayers of T-84 cells were washed twice with PBS and removed from the tissue culture flask with a cell scraper. Cells were lysed for 30 min at 4°C in PBS with 0.5% Triton X-100 and 0.02% SDS with 1 mM DTT, 2 mM PMSF, and 0.15 trypsin inhibitory units (TIU)/ml aprotinin. Insoluble material was pelleted at high speed in a microcentrifuge for 15 min in the cold. The supernatant was precleared for 1 h with protein G beads. The supernatant was incubated overnight at 4°C with the anti-CAR monoclonal antibody RmcB covalently linked to protein G beads, with a rabbit anti-ZO-1 antibody (Zymed), or with the control monoclonal antibody MOPC 195 linked to protein G beads. The beads were washed on ice with PBS containing 0.5% Triton X-100, then boiled for 5 min in 30 μl Laemmli sample buffer. Beads were separated by brief centrifugation, and supernatant was run on an SDS/7.5% polyacrylamide gel and transferred to a poly(vinylidene difluoride) (PVDF) membrane. The membrane was blocked overnight in PBS with 10% milk and probed with mouse anti-ZO-1 antibody (Zymed) or rabbit anti-CAR antiserum followed by horseradish peroxidase-conjugated antibody to rabbit or mouse Ig (Amersham Pharmacia) in PBS with milk, then developed with ECL reagents (Amersham Pharmacia), and exposed to film.
FITC-Dextran Flux.
Confluent monolayers of CHO-CAR or mock-transfected CHO cells were grown on transwell membranes and FITC-dextran (1 mg/ml; average molecular weight 38,200) was added to the upper chamber. After 2 h, aliquots were collected from the lower chamber and assayed by fluorimetry (excitation wavelength set at 492 nm and emission at 520 nm).
Transepithelial Resistance (TER) Measurement.
Confluent monolayers of mock-transfected (MDCK-pcDNA) or CAR-expressing MDCK cells (14) were grown on Transwell membranes at confluency for 5 days. The TER was measured with an epithelial voltohmmeter (World Precision Instruments, Sarasota, FL).
Inhibition of Tight Junction Formation with Soluble CAR.
To disrupt tight junctions, monolayers of T-84 cells grown on Transwell filters were exposed to 2.5 mM EDTA in PBS for 30 min at 37°C. This disruption resulted in a decrease in the TER of the monolayers from 4,767 ± 398 Ω⋅cm2 to 190 ± 29 Ω⋅cm2. EDTA was replaced with medium alone, medium containing 5 μg of the hCAR extracellular domain produced as an Ig–Fc fusion protein (soluble CAR) (17), or medium containing 5 μg of the avian sarcoma virus envelope protein produced as an Fc fusion protein [ASVenv Fc (control protein)]. The TER was measured as an indication of tight junction reassembly.
Cell Adhesion Assay.
Cells were trypsinized into a single cell suspension at a concentration of 1 × 106 cells per ml and mixed gently at room temperature for 5 h. The number of aggregated cells (>2 cells contacting each other) was determined by using a hemacytometer. In some experiments, CHO-CAR cells labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI; 20 μg/ml) and CHO-pcDNA cells labeled with 3,3′-dioctadecyloxacarbocyanine (DiO; 40 μg/ml) were mixed in equal numbers, incubated together for 5 h, and examined by fluorescence microscopy.
Infection of Polarized Monolayers with Coxsackievirus and Adenovirus.
Confluent monolayers of T-84 cells were exposed to 10 plaque-forming units per cell of coxsackievirus B3-Nancy (CBV3), which initiates infection by binding to CAR and not the alternative receptor decay accelerating factor (18), or adenovirus type 5 encoding green fluorescent protein (AdGFP) from either the apical or basolateral surface. Basolateral infection was performed by inverting transwell inserts and applying virus to the basolateral surface. For coxsackievirus infection, cells were exposed to virus for 1 h, washed, and incubated for 16 h at 37°C. Cells were fixed with a 50:50 methanol/acetone mix, stained for virus with a CBV3 mouse monoclonal antibody (Chemicon) followed by FITC-conjugated goat anti-mouse Ig, and examined by immunofluorescence microscopy. Staining for viral antigens was specific, as determined by absence of staining in noninfected cells, and absence of staining with a control antibody. For adenovirus infection, monolayers were exposed to virus for 2 h, washed, incubated for 48 h at 37°C, and examined for GFP expression by fluorescence microscopy. In some experiments, tight junctions were disrupted with EDTA before infection as described above. To determine whether CBV3 or AdGFP infection of T-84 cells was CAR-dependent, monolayers were incubated with a 1:100 dilution of anti-CAR serum or preimmune serum for 1 h after tight junctions were disrupted with EDTA and before infection by CBV3 or AdGFP.
Results
CAR Is Concentrated at Homotypic Cell–Cell Contacts.
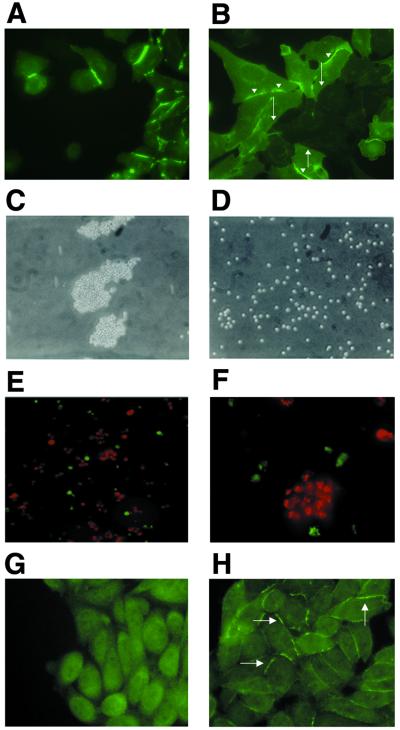
In CHO-CAR cells, CAR was highly concentrated at sites of cell–cell contact (Fig. 1A). Localization was seen only when CAR was present on both opposing cell membranes and not where CHO-CAR cells came into contact with mock-transfected cells (Fig. 1B). CAR linked to the cell membrane by means of a GPI anchor (GPI-CAR), and thus lacking cytoplasmic and transmembrane domains, also concentrated at sites of cell–cell contact in CHO cells (data not shown); this suggests that CAR localization to intercellular contacts was driven by homotypic interactions between the extracellular domains of CAR molecules on opposing cell membranes.
Figure 1.
CAR in transfected cells. (A and B) CAR is localized at homotypic cell junctions in CAR-expressing CHO cells. (A) CHO cells stably transfected with CAR were stained with anti-CAR antibody and examined by immunofluorescence microscopy. CAR staining is enhanced at sites of intercellular contact. (B) Mock-transfected (CAR-negative) and CAR-transfected CHO cells were plated together on coverslips and then stained for CAR 48 h later. CAR is concentrated at contacts between CAR-positive cells (arrowheads), but not at contacts between CAR-positive and CAR-negative cells (filled arrows). (C and D) CAR mediates aggregation of CAR-transfected CHO cells. Clustering of CAR-transfected (C) and mock-transfected (D) CHO cells viewed by light microscopy. (E and F) CAR-mediated aggregation is caused by homophilic interactions. CHO-CAR cells labeled with DiI (red) and mock-transfected CHO cells labeled with DiO (green) were mixed in equal numbers then incubated together for 5 h. (E, ×200; F, ×600.) (G and H) CAR-expressing and mock-transfected CHO cells were stained for ZO-1. (G) Mock-transfected CHO cells show diffuse cytoplasmic staining of ZO-1. (H) ZO-1 localized at intercellular contacts between CHO-CAR cells.
CAR Mediates Homotypic Cell Adhesion.
When incubated with gentle agitation, CHO-CAR cells formed large aggregates (Fig. 1C), whereas CHO-pcDNA cells remained primarily as single cells (Fig. 1D). Similar results were obtained with CAR-transfected murine L cells (data not shown). In separate experiments, 68 ± 6% of CHO-CAR cells formed aggregates after 5 h as compared with 14 ± 7% of mock-transfected cells. When CHO-CAR cells labeled with fluorescent DiI (red) were mixed with DiO labeled CHO-pcDNA cells (green), the CAR-expressing cells formed large aggregates that did not incorporate the mock-transfectants (Fig. 1 E and F). These results demonstrate that CAR-mediated aggregation resulted from homophilic interactions. While these studies were in progress, Honda et al. (19) demonstrated that murine CAR can mediate homophilic aggregation of C6 rat glioma cells.
CAR Recruits ZO-1 to Cell–Cell Contacts in CHO Cells.
ZO-1 was expressed diffusely throughout the cytoplasm of CHO-pcDNA cells (Fig. 1G). In contrast, in CHO-CAR cells, ZO-1 localized to sites of cell–cell contact (Fig. 1H), suggesting that ZO-1 associates with CAR and that CAR recruits ZO-1 to intercellular junctions in CHO cells. CHO-CAR cells and mock-transfected CHO cells expressed similar amounts of ZO-1 as determined by Western blotting of cell lysates (data not shown). In transfected CHO cells lacking the complete cytoplasmic domain (35), ZO-1 staining was diffuse and not concentrated at intercellular junctions, indicating that ZO-1 localization to cell–cell contacts depended on interactions with the CAR cytoplasmic domain (data not shown).
CAR and ZO-1 Associate in Epithelial Cell Tight Junctions.
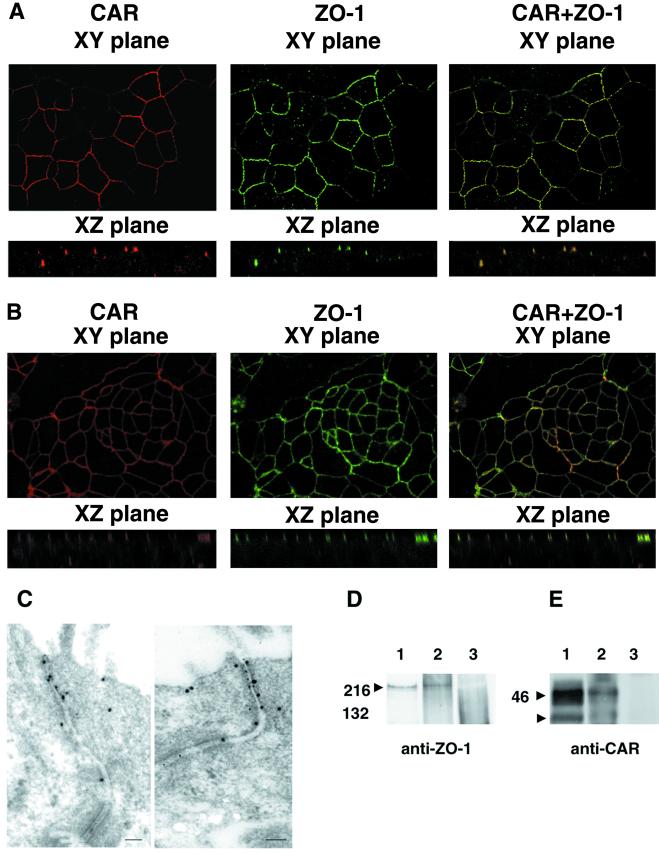
ZO-1 is localized to tight junctions of mature epithelial cells. We stained primary human tracheobronchial airway cells for CAR and ZO-1 after the cells had been cultured on semipermeable Transwell filters for 1 month—conditions that result in the formation of a well-differentiated epithelial monolayer. CAR and ZO-1 colocalized at the apical pole of the lateral cell membrane (Fig. 2A), indicating that CAR, like ZO-1, is concentrated in tight junctions. Similar colocalization of endogenous CAR and ZO-1 was seen in the human colonic cell line T-84 (Fig. 2B), in the human airway epithelial cell lines CALU-3 and 16HBE14o- (not shown), and in MDCK cells (not shown).
Figure 2.
Association between CAR and ZO-1 in epithelial cells. (A and B) CAR and ZO-1 colocalize when examined by confocal microscopy. (A) Human well-differentiated tracheobronchial epithelial cells were stained with anti-CAR polyclonal rabbit serum and Cy3-conjugated secondary antibody (red) or with monoclonal anti-ZO-1 antibody and FITC-conjugated secondary antibody (green). (B) Polarized T-84 colonic epithelial cells were examined as in A. (C) Colocalization of CAR and ZO-1 by immunoelectron microscopy. T-84 cell monolayers were fixed and stained with rabbit polyclonal anti-CAR and mouse monoclonal anti-ZO-1 antibody, then with 8-nm gold-labeled anti-mouse Ig to detect ZO-1 and 18-nm gold-labeled anti-rabbit Ig antibodies to detect CAR. Cells were examined by electron microscopy as described in Materials and Methods. (D and E) CAR and ZO-1 coprecipitate. T-84 cell lysates were immunoprecipitated with anti-ZO-1 antibody (D lane 1 and E lane 2), anti-CAR antibody (D lane 2 and E lane 1), or with a control antibody (D and E lane 3). Immunoprecipitates were electrophoresed in SDS/polyacrylamide gels, transferred to a poly(vinylidene difluoride) membrane, and probed with a monoclonal anti-ZO-1 antibody (D) or anti-CAR antiserum (E) as described in Materials and Methods. Arrowheads mark the location of ZO-1 (D) and CAR (E), which immunoprecipitates as a doublet (22).
Immunoelectron microscopy, which has been the definitive technique for localizing proteins to specialized cell junctions, demonstrated that CAR and ZO-1 localized together at the apical junction of T-84 cells in structures with morphologic characteristics of tight junctions (Fig. 2C). Although occasional CAR and ZO-1 molecules were seen at other locations, both molecules were heavily concentrated at tight junctions.
To determine whether there was a physical association between CAR and ZO-1, we immunoprecipitated endogenous CAR from detergent lysates of T-84 cells, then immunoblotted the precipitates with a monoclonal anti-ZO-1 antibody (Fig. 2D). ZO-1 was precipitated by the anti-CAR monoclonal antibody RmcB and by the anti-ZO-1 antibody. In a reciprocal experiment, we immunoprecipitated endogenous ZO-1 from T-84 cells and immunoblotted the precipitates with anti-CAR antiserum (Fig. 2E). CAR was precipitated by anti-ZO-1 antibody as well as by RmcB. Neither ZO-1 nor CAR was precipitated by control antibodies. Taken together, these results indicate that CAR and ZO-1 associate with each other, either through a direct interaction or through an interaction involving one or more other junctional proteins.
CAR Expression Limits Paracellular Solute Flow.
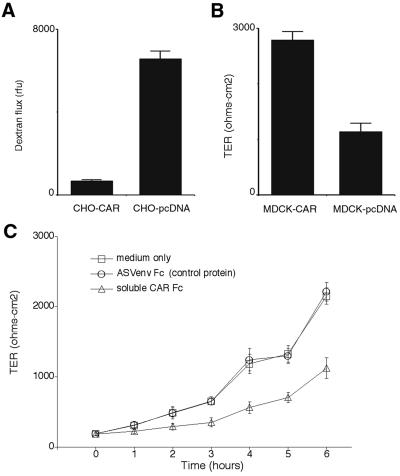
The tight junction functions as a barrier to movement of both large molecules and ions. We measured the passage of a macromolecule, FITC-dextran, across CHO cell monolayers grown on Transwell membranes. Significantly less dextran crossed monolayers of CHO-CAR cells than crossed CHO-pcDNA cell monolayers (Fig. 3A). The transepithelial resistance—a measure of ion flux—across polarized monolayers of MDCK cells transfected with human CAR was significantly greater than that measured across monolayers of mock-transfected MDCK cells (Fig. 3B). We used EDTA to disrupt the junctions between T-84 cells, then permitted tight junctions to regenerate in medium alone or in the presence of excess recombinant CAR extracellular domain (Fig. 3C). Electrical resistance of monolayers exposed to control protein did not differ from that of untreated monolayers. In contrast, transepithelial resistance was significantly reduced in monolayers exposed to soluble CAR. All these results demonstrate a role for CAR in junctional barrier function.
Figure 3.
CAR is a barrier to paracellular solute and ion movement. (A) FITC dextran flux was measured across CHO cell monolayers. Confluent monolayers of CHO-CAR and mock-transfected CHO cells were grown on transwell membranes. FITC-labeled dextran was added to the upper chamber and after 2 h, aliquots of fluid from the lower chamber were collected and assayed by fluorimetry. Graph shows mean and standard deviation for triplicate monolayers. Results are representative of three experiments. (B) TER of MDCK cell monolayers. Confluent monolayers of mock-transfected (MDCK-pcDNA) or CAR-expressing MDCK cells were grown on Transwell membranes at confluency for 5 days. TER was measured with an epithelial voltohmmeter. Graph shows mean and standard deviation for quadruplicate monolayers. Results are representative of three experiments. (C) Soluble CAR inhibits the formation of tight junctions. Monolayers of T-84 cells were exposed to EDTA to disrupt tight junctions. EDTA was replaced with medium alone, medium containing 5 μg of soluble CAR, or medium containing 5 μg of the avian sarcoma virus envelope protein [ASVenv Fc (control protein)]. The TER was measured as an indication of tight junction reassembly. Graph shows mean and standard deviation for triplicate monolayers. Results are representative of three experiments.
Virus Infection Is Limited by CAR Localization.
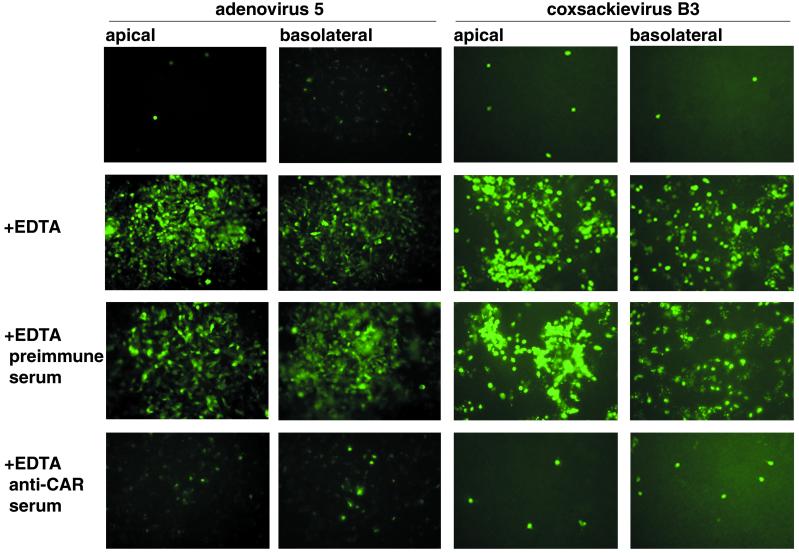
To determine whether virus could infect T-84 cells when CAR was localized to tight junctions, polarized monolayers were exposed, from either the apical or basolateral side, to coxsackievirus B3 or to adenovirus type 5 encoding GFP. Under either condition, cells were rarely infected with either virus, as determined by expression of coxsackievirus protein or expression of GFP (Fig. 4). Disruption of tight junctions with EDTA resulted in a significant increase in infection of epithelial cells. Infection after EDTA treatment was inhibited by polyclonal anti-CAR antiserum but not by preimmune serum. In control experiments, EDTA treatment of CHO cell monolayers grown on Transwell filters did not alter their susceptibility to infection (not shown). These results indicate that CAR sequestered within tight junctions is inaccessible to coxsackievirus and adenovirus, and that disruption of junctions facilitates infection by permitting virus to interact with CAR.
Figure 4.
CAR-mediated coxsackievirus and adenovirus infection of polarized T-84 cells requires disruption of tight junctions. Confluent monolayers of T-84 cells were exposed to coxsackievirus B3 or to adenovirus 5 encoding GFP from either the apical or basolateral surface. Cells were examined for coxsackievirus protein expression 16 h later or for GFP expression 48 h later. In some experiments, tight junctions were disrupted with EDTA before monolayers were exposed to virus. In some experiments, monolayers were incubated with rabbit anti-CAR antiserum or with preimmune rabbit serum after EDTA treatment.
Discussion
We and others have previously observed that CAR is absent from the apical surface of polarized epithelial cells (16, 20–22). In the experiments reported here we found that CAR is localized to the tight junction itself, where it associates, directly or indirectly, with the junctional scaffolding protein ZO-1.
A number of proteins are found at tight junctions. Most of them, like ZO-1, are peripheral membrane proteins associated with the cytoplasmic face of the junctional membrane (10). Only three tight-junction proteins are known to span the membrane. Occludin (23) and the claudin family of junction proteins (24, 25) have multiple membrane-spanning domains, whereas junctional adhesion molecule, a member of the Ig superfamily, has a single membrane-spanning domain (26). Based on the observations reported here, CAR is the fourth integral membrane component of the tight junction to be identified, and the second with a single transmembrane domain.
Because the transmembrane components of the tight junction extend into the paracellular space, they are likely to be important for junctional barrier function, whereas cytoplasmic peripheral membrane components may serve in assembly and regulation of junctions, tethering to intracellular structures, and transmission of signals. Occludin (27), claudins (28), and junctional adhesion molecule (26, 29) all have adhesive properties, which may contribute to a paracellular seal that prevents movement of solutes. We found that CAR promoted cell adhesion and restricted the movement of both solutes and ions between transfected cells. It is thus likely that CAR contributes to the barrier function of tight junctions.
In an earlier study, CAR was seen to be expressed diffusely on the basolateral membrane of cultured airway epithelial cells (16), rather than concentrated specifically at tight junctions. However, consistent with our present findings, we more recently observed that CAR was concentrated at the apex of the basolateral membrane of airway cells in culture and in tracheobronchial tissue specimens (14). This variation may reflect differences in the origin of the airway cells, in culture conditions, or in degree of maturation. A subpopulation of junctional adhesion molecule is present on the lateral membrane as well as in tight junctions of some polarized cells (30), and similar observations have been reported for occludin (31). In nonpolarized cells, CAR may be expressed at other cell contacts. We have observed that in cardiac myocytes, CAR colocalized with N-cadherin at intercalated discs (data not shown), a site where ZO-1 is also expressed (32).
The redistribution of ZO-1 in CAR-transfected cells and the coprecipitation of ZO-1 and CAR from cell lysates indicate that CAR associates with the tight-junction protein ZO-1. The CAR cytoplasmic domain contains potential recognition sites for protein–protein interaction domains: the C-terminal motif SIV may interact with PDZ domains, which recognize the C-terminal motif [(S/T)-X-(hydrophobic amino acid) (33)]; the peptide PTLPP resembles the SH3 domain recognition motif PXXPX (34). CAR's association with ZO-1 may depend on interactions with ZO-1's PDZ or SH3 domains. The CAR C terminus contains multiple serine, threonine, and tyrosine residues that may be sites of phosphorylation; phosphorylation regulates association of other integral membrane proteins with the tight junction (31). We found that CAR expression led to relocalization of ZO-1 in a nonpolarized cell; the association between CAR and ZO-1 may affect the subcellular localization of these proteins in polarized cells as well.
The CAR N-terminal Ig-like domain forms homodimers both in crystals and in solution (8); this dimerization may be responsible for CAR accumulation at cell–cell contacts as well as for CAR-mediated cell adhesion. We found that human CAR promoted homotypic cell aggregation, consistent with results recently reported for murine CAR (19). However, it is not certain that CAR's primary function involves adhesion per se as opposed to intercellular recognition. We recently observed that CAR expression leads to modulation of the cell cycle regulatory proteins p21 and Rb and inhibits the growth of bladder carcinoma cells, effects that are prevented by antibody that recognizes the CAR extracellular domain (35). Homotypic CAR interactions may thus initiate signals contributing to contact-dependent inhibition of cell growth.
A deficiency of viral receptors on the epithelial cell surface is recognized as a barrier to adenovirus infection from the apical side (16, 20, 21), and we now find that sequestration of CAR in tight junctions can be a barrier to adenovirus infection from the basolateral surface as well. In addition, localization of CAR in the tight junction is an impediment to coxsackievirus infection. Junctional adhesion molecule, another transmembrane component of the tight junction, was recently identified as a receptor for mammalian reoviruses (36). It thus appears that three unrelated viruses—all of which must traverse respiratory or gastrointestinal epithelium in the course of infection—have evolved to interact with receptors that may be inaccessible from the epithelial surface. Infection by these pathogens in vivo may require loss of epithelial integrity, transport across M cells of the intestine (37), or interactions with alternative receptors present on the apical surface.
Acknowledgments
We thank H. C. J. Ertl for the adenovirus type 5 encoding GFP, N. Shah for her electron microscopy work, S. Puhalla for assistance with confocal microscopy, A. Fanning for advice, and M. Korn for discussions of CAR localization in nonpolarized cells. We thank M. Schneider and S. Coffin for criticism of the manuscript, and T. Ohman, J. Gaetz, and J. Petrella for technical assistance. This research was supported by National Institutes of Health Grants HL54734, T32 AI07278, HL07443, and HL51818, the Cystic Fibrosis Foundation, and The Gillson Longenbaugh Foundation, a Pediatric Infectious Diseases Society Fellowship Award funded by Glaxo–SmithKline, and the American Heart Association.
Abbreviations
- MDCK
Madin–Darby canine kidney
- CAR
coxsackievirus and adenovirus receptor
- GPI
glycosylphosphatidylinositol
- CHO
Chinese hamster ovary cells
- TER
transepithelial resistance
- DiO
3,3′-dioctadecyloxacarbocyanine
- AdGFP
adenovirus type 5 encoding green fluorescent protein
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwit M S, Crowell R L, Finberg R W. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Tomko R P, Xu R, Philipson L. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roelvink P W, Lizonova A, Lee J G, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bewley M, Springer K, Zhang Y-B, Freimuth P, Flanagan J. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 5.He Y, Chipman P R, Howitt J, Bator C M, Whitt M A, Baker T S, Kuhn R J, Anderson C W, Freimuth P, Rossmann M G. Nat Struct Biol. 2001;8:874–878. doi: 10.1038/nsb1001-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, Veghel R, Houtsmuller A, Schultheiss H P, Lamers J, Poller W. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 8.van Raaij M J, Chouin E, van der Zandt H, Bergelson J M, Cusack S. Structure (London) 2000;8:1147–1155. doi: 10.1016/s0969-2126(00)00528-1. [DOI] [PubMed] [Google Scholar]
- 9.Farquahr M, Palade G. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanning A S, Mitic L L, Anderson J M. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson B R, Keon B H. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Anderson J M, Stevenson B R, Jesaitis L A, Goodenough D A, Mooseker M S. J Cell Biol. 1988;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevenson B R, Siliciano J D, Mooseker M S, Goodenough D A. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickles R J, Fahrner J A, Petrella J M, Boucher R C, Bergelson J M. J Virol. 2000;74:6050–6057. doi: 10.1128/jvi.74.13.6050-6057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Bergelson J M. J Virol. 1999;73:2559–2562. doi: 10.1128/jvi.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martino T A, Petric M, Weingartl H, Bergelson J M, Opavsky M A, Richardson C D, Modlin J F, Finberg R W, Kain K C, Willis N, et al. Virology. 2000;271:99–108. doi: 10.1006/viro.2000.0324. [DOI] [PubMed] [Google Scholar]
- 18.Bergelson J M, Mohanty J G, Crowell R L, St. John N F, Lublin D M, Finberg R W. J Virol. 1995;69:1903–1906. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda T, Saitoh H, Masuko M, Katagiri-Abe T, Tominaga K, Kozakai I, Kobayashi K, Kumanishi T, Watanabe Y G, Odani S, Kuwano R. Brain Res Mol Brain Res. 2000;77:19–28. doi: 10.1016/s0169-328x(00)00036-x. [DOI] [PubMed] [Google Scholar]
- 20.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. J Clin Invest. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 22.Cohen C J, Gaetz J, Ohman T, Bergelson J M. J Biol Chem. 2001;276:25392–25398. doi: 10.1074/jbc.M009531200. [DOI] [PubMed] [Google Scholar]
- 23.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morita K, Furuse M, Fujimoto K, Tsukita S. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Itallie C M, Anderson J M. J Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- 28.Kubota K, Furuse M, Sasaki H, Sonoda N, Fujita K, Nagafuchi A, Tsukita S. Curr Biol. 1999;9:1035–1038. doi: 10.1016/s0960-9822(99)80452-7. [DOI] [PubMed] [Google Scholar]
- 29.Liang T W, DeMarco R A, Mrsny R J, Gurney A, Gray A, Hooley J, Aaron H L, Huang A, Klassen T, Tumas D B, Fong S. Am J Physiol. 2000;279:C1733–C1743. doi: 10.1152/ajpcell.2000.279.6.C1733. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Estrada O M, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. J Biol Chem. 2001;276:9291–9296. doi: 10.1074/jbc.M006991200. [DOI] [PubMed] [Google Scholar]
- 31.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. J Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toyofuku T, Yabuki S, Otsu K, Kanzuya T, Hori M, Tada M. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 33.Songyang Z, Fanning A S, Fu C, Xu J, Marfatia S M, Chishti A H, Crompton A, Chan A C, Anderson J M, Cantley L C. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 34.Pawson T, Scott J D. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 35.Okegawa T, Pong R, Li Y, Bergelson J, Sagalowsky A, Hsieh J. Cancer Res. 2001;61:6592–6600. [PubMed] [Google Scholar]
- 36.Barton E, Forrest J, Connoly J, Chappell J, Liu Y, Schnell F, Nusrat A, Parkos C, Dermody T. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 37.Wolf J L, Rubin D H, Finberg R, Kauffman R S, Sharpe A H, Trier J S, Fields B N. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]