Abstract
Background
Hantavirus pulmonary syndrome (HPS) is caused by Andes virus (ANDV) and related hantaviruses in the Americas. Despite a fatality rate of 40%, the pathogenesis of HPS is poorly understood and factors associated with severity, fatality, and survival remain elusive.
Methods
Ninety-three ANDV-infected HPS patients, of whom 34 had a fatal outcome, were retrospectively studied. Serum levels of cytokines and other inflammation-associated markers were analyzed using multiplex immunoassay and enzyme-linked immunosorbent assay. Associations with disease severity, fatal outcome, and survival were identified using logistic regression.
Results
HPS patients exhibited increased serum levels of markers associated with inflammation, intestinal damage, and microbial translocation compared to controls. Patients with fatal outcome displayed higher levels of interleukin (IL) 6, IL-10, interferon-γ, soluble tumor necrosis factor-related apoptosis-inducing ligand, and intestinal fatty acid–binding protein (I-FABP) than survivors. Levels of complement factor 5/5a were higher in survivors compared with fatal cases. IL-6 and I-FABP, the latter a marker for intestinal damage, were by multivariate analyses identified as independent markers associated with disease severity (odds ratio [OR], 2.25; 95% confidence interval [CI], 1.01–5.01) and fatal outcome (OR, 1.64; 95% CI, 1.01–2.64), respectively.
Conclusions
HPS patients displayed a multifaceted, systemic inflammatory response, with IL-6 and I-FABP as independent markers of disease severity and fatality, respectively.
Keywords: hantavirus pulmonary syndrome, cytokines, microbial translocation, intestinal fatty acid–binding protein, IL-6
Patients with hantavirus pulmonary syndrome exhibited strong inflammatory responses and increased serum levels of markers associated with microbial translocation and intestinal damage. Interleukin 6 and intestinal fatty acid–binding protein were identified as markers independently associated with severe disease and fatal outcome.
Hantaviruses comprise a family of negative-sense, single-stranded RNA viruses belonging to the Bunyavirales order. Certain members of the Hantaviridae family cause severe disease in humans: hantavirus pulmonary syndrome (HPS) in the Americas, and hemorrhagic fever with renal syndrome (HFRS) in Europe and Asia [1]. HPS is primarily caused by Andes virus (ANDV) in Argentina and Chile, and Sin Nombre virus in North America [1]. The first recognized HPS outbreak occurred in the Four Corners region of the United States in 1993 [2], and soon after, HPS was discovered also in South America [3]. Hantaviruses are continuing to cause sporadic outbreaks throughout the Americas. For example, the 2012 outbreak in Yosemite National Park, California, received worldwide attention [4]. With a fatality rate of 35%–40%, HPS represents one of the deadliest infectious human diseases [1, 5]. Yet, no US Food and Drug Administration (FDA)–approved vaccine or specific treatment exists.
Hantaviruses are normally transmitted to humans through inhalation of virus-contaminated rodent excreta [1], making HPS and HFRS zoonotic infectious diseases. While hantaviruses are in general not transmitted between humans, several cases of interhuman ANDV transmission have been reported [1, 6]. HPS patients initially present with flulike symptoms, such as fever and headache, as well as gastrointestinal symptoms including abdominal pain and diarrhea [7–9]. These symptoms are soon followed by a rapidly progressing pulmonary edema, often putting patients in life-threatening respiratory failure, and in the most severe cases cardiogenic shock leading to death [1, 8].
While the mechanisms behind HPS pathogenesis are poorly understood, hantavirus-induced immune responses have been suggested to drive immunopathology [1]. Immune responses upon hantavirus infection include strong inflammatory responses displayed by increased levels of proinflammatory cytokines and vigorous natural killer (NK) cell, CD8 T-cell, and B-cell responses [10–15]. However, studies providing more in-depth knowledge on how immune responses during hantavirus infection might affect the disease outcome are lacking, hampering the development of HPS interventions and treatments.
Here, we retrospectively characterized the systemic inflammatory response in the largest cohort of HPS patients analyzed to date, including 93 HPS patients, among whom 34 had a fatal outcome. With the aim of identifying correlates of fatal outcome and survival, we further identified serum markers associated with the respective outcomes, using logistic regression. Our findings strengthen previous observations [9, 14], showing that HPS patients display high levels of proinflammatory cytokines in general. We here present findings that delineate inflammatory responses in HPS patients with fatal vs nonfatal outcome. Most importantly, we show that markers of intestinal damage and microbial translocation are increased during HPS, and that multiple serum markers, including interleukin (IL) 6 and intestinal fatty acid–binding protein (I-FABP), are associated with severity and fatal outcome.
MATERIALS AND METHODS
Study Design and Patients
Serum samples from 93 HPS patients with confirmed ANDV infection were included in the study. Samples were collected for diagnostic purposes at the Hantavirus National Reference Laboratory in Buenos Aires, Argentina, during 2010–2016, and stored at –80ºC until analysis. Ethical approval was obtained from the institutional ethical committee at Administración Nacional de Laboratorios e Institutos de Salud “Dr Carlos G. Malbrán” in Buenos Aires, Argentina. HPS was confirmed by enzyme-linked immunosorbent assay (ELISA) detecting ANDV-specific immunoglobulin M and immunoglobulin G antibodies, as previously described [16], or by quantitative reverse-transcription polymerase chain reaction detecting ANDV RNA. In addition, serum from 33 community-matched controls was collected under informed consent.
Multiplex Immunoassay
A custom 15-plex Magnetic Luminex Screening assay (R&D Systems) was used for measurement of IL-1β, IL-2, IL-6, IL-10, IL-12 (p70), IL-15, IL-18, tumor necrosis factor (TNF), interferon gamma (IFN-γ), B-cell activating factor (BAFF), complement factor 5 (C5)/complement factor 5a (C5a), vascular endothelial growth factor (VEGF), soluble TNF-related apoptosis-inducing ligand (sTRAIL), granzyme A, and granzyme B according to the manufacturer’s instructions. Serum was diluted 1:2 prior to analysis.
Enzyme-Linked Immunosorbent Assay
Ferritin levels were measured in serum diluted 1:1000, using a commercially available human ferritin ELISA kit according to the manufacturer’s instructions (Abcam). Serum levels of lipopolysaccharide-binding protein (LBP), soluble CD14 (sCD14), I-FABP, and soluble CD25 (sCD25) were measured using DuoSet ELISA development kits (R&D Systems) according to the manufacturer’s guidelines. Serum was diluted 1:4000 for LBP, 1:2000 for sCD14, 1:5 for I-FABP, and 1:10 for sCD25 in ready-to-use ELISA diluent (Mabtech).
Statistical Analyses
Statistical analyses were performed using Graph Pad Prism version 7 and Stata version 13 software. Mann–Whitney U test was used for comparisons between 2 groups and Kruskal–Wallis test was used for comparisons of >2 groups. Spearman rank correlation coefficient was used for examining associations between serum markers. Principal component analysis (PCA) was performed for visualization of possible clusters, using the R package “scatterplot3d.” Serum marker concentrations used in the heatmap were standardized and clustered within each group.
Logistic regression analyses, adjusted for sex, age, and day of sampling, were performed using disease severity or fatal outcome as outcome and serum markers as predictors. The univariate analyses were carried out using standard logistic regression. The multivariate analyses were performed using penalized logistic regression (“ridge”). Ordinal logistic regression was used both for univariate and multivariate associations. Concentrations of IL-1β, IL-2, IL-6, IL-12, TNF, IFN-γ, VEGF, granzyme A, granzyme B, and I-FABP were log-transformed before analysis with PCA and logistic regression. Samples with concentrations below the detection limit were assigned a positive value smaller than the lowest detectable limit for that serum marker.
RESULTS
Study Cohort
Diagnostic serum samples from 93 HPS patients were collected at median of 6 days after reported symptom debut (range, 1–23 days). Two of the patients were diagnosed upon discharge and were thus excluded from logistic regression analyses. The HPS patients consisted of 27 females and 66 males at a mean age of 36 years (range, 7–85 years). Of the 93 patients, 34 had a fatal outcome. Patient characteristics are summarized in Table 1. Clinical data including white blood cell (WBC) count, platelet count, and hematocrit were available for a subset of the cohort (Table 1). In general, the HPS patients had increased WBC count and decreased platelet count, while the mean hematocrit was within normal range (Table 1). Patients were divided into 4 different severity groups, as previously described [13]: I, prodromal symptoms without respiratory involvement (n = 3); II, mild to moderate respiratory compromise without hemodynamic compromise (n = 37); III, severe respiratory insufficiency with hemodynamic compromise (n = 19); IV, severe respiratory insufficiency with refractory-to-treatment hemodynamic compromise and a final fatal outcome (n = 34). The control group consisted of 21 women and 12 men, with a mean age of 37 years (range, 24–69).
Table 1.
Patient Characteristics
| Patient Group | ||||
|---|---|---|---|---|
| Characteristic | HPS | HPS (Fatal) | HPS (Nonfatal) | Controls |
| No. of patients | 93 | 34 | 59 | 33 |
| Sex, female/male, No. | 27/66 | 12/22 | 15/44 | 21/12 |
| Age, y, mean ± SD | 36 ± 16 | 39 ± 18 | 34 ± 14 | 37 ± 11 |
| Days post symptom debut, median (range) | 6 (1–23) | 4 (1–13) | 7 (1–23) | NA |
| No. of patients per severity group, I/II/III/IV | 3/37/19/34 | 0/0/0/34 | 3/37/19/0 | NA |
| WBC count × 109/L, mean ± SDa | 12.2 ± 8.82b | 12.1 ± 9.72c | 12.3 ± 8.37d | ND |
| Platelet count × 109/L, mean ± SDe | 71.5 ± 49.6f | 66.8 ± 41.4g | 74.0 ± 53.6h | ND |
| Hematocrit, %, mean ± SDi | 44 ± 7.7j | 45 ± 9.0k | 44 ± 6.9l | ND |
Abbreviations: HPS, hantavirus pulmonary syndrome; NA, not applicable; ND, not done; SD, standard deviation; WBC, white blood cell.
aWBC: normal range, 4.5–10.5× 109/L.
bn = 74.
cn = 27.
dn = 47.
ePlatelet count: normal range, 150–400 × 109/L.
fn = 76.
gn = 26.
hn = 50.
iHematocrit: normal range, 37%–47% for females, 42%–52% for men.
jn = 71.
kn = 26.
ln = 45.
HPS Is Characterized by a Multifaceted Inflammatory Response
To characterize the systemic inflammatory response during HPS, levels of inflammation-associated serum markers were measured in HPS patients and controls, using multiplex immunoassay and ELISA. For initial statistical analyses, HPS patients were divided into 3 groups, depending on the day post symptom debut at which they had been sampled (days 1–4, n = 38; days 5–10, n = 38; days 11–23, n = 17). Serum levels of IL-1β, IL-6, IL-10, IL-18, TNF, IFN-γ, BAFF, C5/C5a, sCD25, granzyme A, and granzyme B were all significantly increased in all HPS groups, compared to controls (Figure 1; Supplementary Table 1). Levels of IL-12, IL-15, and ferritin were significantly higher in HPS patients sampled on days 1–4 and days 5–10, compared with controls, whereas IL-2 levels were significantly elevated only in HPS patients sampled at days 1–4 post symptom debut (Figure 1; Supplementary Table 1). Levels of sTRAIL and VEGF did not differ between HPS patients and controls (Figure 1; Supplementary Table 1). Together, these findings confirm previous studies showing that HPS patients display high levels of proinflammatory cytokines and IL-10 [9, 14]. In addition, these data show that HPS patients have increased systemic levels of BAFF, C5/C5a, granzyme A, granzyme B, and ferritin.
Figure 1.
Cytokines and inflammatory markers are increased in serum of patients with hantavirus pulmonary syndrome (HPS). Levels of serum markers in HPS patients, sampled either at days 1–4 (n = 38), days 5–10 (n = 38), or days 11–23 (n = 17) post symptom debut, and controls (n = 29–33). Kruskal–Wallis test; median values. *P < .05; **P < .01; ***P < .001; ****P < .0001. Abbreviations: BAFF, B-cell activating factor; C5, complement factor 5; C5a, complement factor 5a; IFN-γ, interferon gamma; IL, interleukin; sCD25, soluble CD25; sTRAIL, soluble TNF-related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor.
Markers Associated With Intestinal Damage and Microbial Translocation Are Increased During HPS
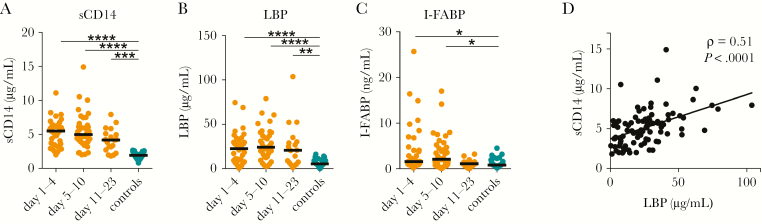
A potential driver of inflammation is microbial translocation (MT), referring to the leakage of bacteria or bacterial products from the gastrointestinal tract into the systemic circulation [17]. Given the systemic inflammation and gastrointestinal symptoms of HPS patients [7, 8], we were interested to investigate if markers of MT would be increased during HPS. LBP and sCD14 have been widely used as surrogate markers of MT [17]. Moreover, I-FABP, often measured in the context of MT, is a systemic marker for intestinal epithelial cell injury [18]. Serum levels of sCD14, LBP, and I-FABP were measured in HPS patients and controls. Levels of sCD14 and LBP were elevated in all groups of HPS patients compared to controls (Figure 2A and 2B; Supplementary Table 1). I-FABP levels were increased in HPS patients sampled at days 1–4 and days 5–10 post symptom debut (Figure 2C; Supplementary Table 1). Levels of sCD14 and LBP in HPS patients were positively correlated (ρ = .54, P < .0001) (Supplementary Figure 1). Furthermore, levels of sCD14 and I-FABP were positively correlated to levels of the inflammation-associated cytokines IL-6, IL-10, IL-12, IL-15, TNF, and IFN-γ (Supplementary Figure 1). Pairwise correlations between all serum markers are summarized in Supplementary Figure 1. Taken together, these data indicate that intestinal damage and MT may occur during HPS.
Figure 2.
Increased serum levels of soluble CD14 (sCD14), lipopolysaccharide-binding protein (LBP), and intestinal fatty acid–binding protein (I-FABP) indicate microbial translocation and intestinal epithelial cell damage during hantavirus pulmonary syndrome (HPS). Serum levels of sCD14 (A), LBP (B), and I-FABP (C) in HPS patients at days 1–4 (n = 38), days 5–10 (n = 38), and days 11–23 (n = 17) post symptom debut, and controls (n = 33). D, Spearman rank correlation coefficient between serum sCD14 and LBP, in HPS patients (n = 93). Kruskal–Wallis test; median values. *P < .05; **P < .01; ***P < .001; ****P < .0001. Abbreviations: I-FABP, intestinal fatty acid–binding protein; LBP, lipopolysaccharide-binding protein; sCD14, soluble CD14.
Levels of Multiple Serum Markers Differ Between Fatal and Nonfatal HPS
To get an overview of the differences in responses between fatal and nonfatal HPS cases and controls, data were summarized using PCA and heatmap analysis (Figure 3A and 3B). PCA separated fatal HPS cases from nonfatal cases, albeit not as marked as total HPS cases from controls (Figure 3A).
Figure 3.
Multiple serum markers distinguish fatal from nonfatal hantavirus pulmonary syndrome. A, Principal component (PC) analysis of patient groups and controls plotted against PC1–PC3. B, Heatmap displaying the standardized serum concentration of each marker in each patient. Colors depict high (red) or low (black) concentration. C, Levels of serum markers in patients with fatal and nonfatal HPS sampled on days 1–4 (fatal, n = 19; nonfatal, n = 19) and days 5–10 (fatal, n = 14; nonfatal, n = 23) post symptom debut. Mann–Whitney U test; median values. *P < .05; **P < .01; ***P < .001. Abbreviations: BAFF, B-cell activating factor; C5, complement factor 5; C5a, complement factor 5a; I-FABP, intestinal fatty acid–binding protein; IFN-γ, interferon gamma; IL, interleukin; LBP, lipopolysaccharide-binding protein; ns, not significant; PC, principal component; sCD14, soluble CD14; sCD25, soluble CD25; sTRAIL, soluble TNF-related apoptosis-inducing ligand; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Next, we sought to identify the specific serum markers discriminating patients with fatal vs nonfatal outcome. As only 1 of the patients who died was sampled later than day 10 (sampled at day 13) post symptom debut, groupwise comparisons were performed on patients sampled at days 1–4 (fatal, n = 19; nonfatal, n = 19) and days 5–10 (fatal, n = 14; nonfatal, n = 23) post symptom debut. Serum levels of IL-6 and I-FABP were significantly higher in patients with fatal compared to nonfatal outcome, both in patients sampled on days 1–4 and those sampled on days 5–10 post symptom debut (Figure 3C; Supplementary Table 2). In patients sampled on days 1–4, levels of C5/C5a were lower in patients with fatal outcome (Figure 3C; Supplementary Table 2). In patients sampled on days 5–10, higher levels of IL-10, IFN-γ, and sTRAIL were observed in fatal compared with nonfatal cases (Figure 3C; Supplementary Table 2). No significant differences were found for IL-1β, IL-2, IL-15, IL-18, TNF, BAFF, VEGF, sCD25, ferritin, granzyme A, granzyme B, sCD14, or LBP (Figure 3C; Supplementary Table 2). Together, these findings show that levels of several serum markers differ between HPS patients with fatal compared with nonfatal outcome, suggesting that certain responses may be associated with HPS severity and fatality.
IL-6 and I-FABP Are Associated With Severe Disease and Fatal Outcome
The observed differences in serum marker levels between fatal and nonfatal HPS prompted us to further investigate for possible associations with disease severity and outcome. Univariate and multivariate regression analyses adjusting for sex, age, and day of sampling were performed, using severity and fatal outcome as dependent variables.
We first explored associations to the disease severity. Univariate analyses showed that increased levels of IL-1β, IL-6, IL-15, ferritin, granzyme B, and I-FABP were associated with increased odds of severe disease, whereas C5/C5a was associated with decreased odds of severe disease (Figure 4A; Supplementary Table 3). Next, to identify serum markers that were independently associated with severe disease, multivariate analyses were performed. Interestingly, these analyses showed that IL-6 was the only marker independently associated with increased odds of more severe disease (Figure 4B; Supplementary Table 3). The multivariate analyses further showed that increased levels of C5/C5a and BAFF were significantly associated with decreased odds of more severe disease (Figure 4B; Supplementary Table 3).
Figure 4.
Elevated levels of interleukin 6 and intestinal fatty acid–binding protein are associated with increased risk for severe disease and fatal outcome. Forest plot displaying the standardized odds ratio (OR) for severe disease, calculated by univariate (A) and multivariate (B) analysis, comparing hantavirus pulmonary syndrome (HPS) severity groups I (n = 3), II (n = 35), III (n = 19), and IV (n = 34). Forest plot displaying the standardized OR for fatal outcome, calculated by univariate (C) and multivariate (D) analysis, comparing fatal (n = 34) and nonfatal (n = 57) HPS. Dots depict the estimated OR and whiskers depict the confidence interval. Colors depict significantly increased (red) or decreased (blue) OR (with 95% confidence interval), n = 91. Abbreviations: BAFF, B-cell activating factor; C5, complement factor 5; C5a, complement factor 5a; I-FABP, intestinal fatty acid–binding protein; IFN-γ, interferon gamma; IL, interleukin; LBP, lipopolysaccharide-binding protein; OR, odds ratio; sCD14, soluble CD14; sCD25, soluble CD25; sTRAIL, soluble TNF-related apoptosis-inducing ligand; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
The patient outcome data allowed for additional logistic regression analyses, using fatal outcome as dependent variable. In this univariate regression model, IL-6, IL-15, IFN-γ, ferritin, granzyme B, and I-FABP were identified as markers associated with increased odds of fatal outcome (Figure 4C; Supplementary Table 3). In contrast, C5/C5a was associated with decreased odds of fatal outcome (Figure 4C; Supplementary Table 3). Interestingly, multivariate analyses identified I-FABP as the only marker that was independently associated with increased odds of fatal outcome (Figure 4D; Supplementary Table 3). In conclusion, univariate analyses revealed associations between multiple serum markers and HPS severity and fatality, while the multivariate analyses highlighted IL-6 and I-FABP as independent factors associated with increased odds of severe disease and fatal outcome, respectively.
DISCUSSION
While an adequate immune response is essential for proper clearance of pathogens, a deregulated, exaggerated immune response can give rise to immunopathology. HPS is an acute, severe disease with a high fatality rate associated with an excessive immune response [1]. Still, correlates of fatality and survival are poorly known, impeding the development of treatments. Better understanding of pathological immune responses could aid in the development of treatments for hantavirus infection and other viral hemorrhagic fevers. Here, we characterized the inflammatory response in a unique cohort of HPS patients and identified correlates of fatality and survival.
Our findings, demonstrating a broad and strong inflammatory response in HPS patients (Figure 1), extend previous observations [9, 14]. Moreover, we demonstrate increased levels of BAFF, granzyme A, granzyme B, C5/C5a, and ferritin during HPS. We also observed increased levels of IL-1β, which is in contrast to 2 previous studies, in which no differences were found compared to healthy controls [9, 14]. While we did not observe elevated levels of VEGF and sTRAIL, increased levels of these markers have been shown in previous studies of HPS patients [14, 19].
The higher levels of C5/C5a observed in patients with nonfatal outcome (Figure 3), together with the associations found between increased C5/C5a levels and decreased odds of severe disease and fatal outcome (Figure 4B and 4C), suggest a possible role for the complement system in HPS pathogenesis. Likewise, our data showing an association between the B-cell–stimulating cytokine BAFF and decreased odds of severe HPS (Figure 4B) indicate a potential protective effect of BAFF. Further characterization of serum markers associated with survival during HPS could provide important leads on HPS pathogenesis and be useful in the development of therapies.
Strong cytotoxic cell responses have been repeatedly reported during human hantavirus infection [10–12] and in vitro, hantavirus-infected endothelial cells have been shown to activate NK cells via IL-15 [20]. The observed increases in serum levels of IL-2, IL-12, IL-15, and IL-18 in HPS patients (Figure 1) [14] suggest that hantavirus infection triggers secretion of a broad repertoire of cytokines that may activate cytotoxic lymphocytes. Moreover, the finding of increased systemic granzyme A and granzyme B levels (Figure 1) likely reflects high cytotoxic activity of these cells. The increased odds of severe disease and fatal outcome associated with increased levels of IL-15 and granzyme B (Figure 4A and 4C) may suggest that severely ill HPS patients are predisposed to mount stronger cytotoxic cell responses. Taken together, our data strengthen the view of HPS as a disease characterized by highly activated immune responses and inflammation [1].
Microbial translocation has been suggested as a driver of immune activation and inflammation during human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and dengue virus infection [21–23]. Here, we demonstrate increased serum levels of the MT surrogate markers sCD14 and LBP during HPS (Figure 2A and 2B). These data indicate that MT may occur during HPS. We also observed elevated levels of I-FABP (Figure 2C), suggesting injury of the intestinal epithelial cells of HPS patients. This raises the question as to whether intestinal damage could be associated with the gastrointestinal symptoms frequently observed in HPS patients [7, 8]. During Ebola virus disease, gastrointestinal symptoms are also common and have been associated with a fatal outcome [24]. Interestingly, I-FABP levels were higher in HPS patients with fatal compared with nonfatal outcome (Figure 3C) and in multivariate analysis, I-FABP was the only marker (out of 20) that was independently associated with increased odds of fatal outcome (Figure 4D). This suggests that intestinal damage marks a life-threatening stage of HPS. Possible causes of intestinal cell injury during HPS, causing subsequent leakage of I-FABP to the circulation, remain elusive. Several lines of evidence indicate that cells of the gastrointestinal tract could be infected during hantavirus infection. For instance, viral antigen can be detected in the small intestine and feces of bank voles, the natural host of HFRS-causing Puumala virus (PUUV) [25]. Furthermore, PUUV antigen has been detected in stomach and colon of HFRS patients [26]. As hantaviruses inhibit apoptosis [27], it is unlikely that systemic I-FABP levels would arise as a consequence of virus-induced cell death of infected intestinal epithelial cells. In support of this view, it was recently shown that human intestinal epithelial cells are susceptible to infection with PUUV in vitro and that infection does not cause cell death [28]. Possible causes of intestinal damage during HPS could be intestinal ischemia or bystander killing by cytotoxic cells or via soluble factors such as TNF [29, 30]. Unraveling the factors responsible for causing intestinal injury during HPS has the potential to reveal important and novel aspects of hantavirus-induced pathology.
The proinflammatory cytokines IL-1β, IL-6, and IL-15 as well as ferritin and granzyme B were all associated with a more severe disease in univariate analyses (Figure 4A). This suggests that certain inflammatory pathways might be more active in patients with severe HPS. In multivariate analyses, controlling for intermarker relationships, IL-6 was identified as the only marker that was independently associated with the disease severity of HPS (Figure 4B). These findings extend previous studies, in which increased IL-6 levels were suggested to be associated with the severity of HPS and HFRS [9, 31]. In addition, during Ebola virus disease, dengue virus infection, and Crimean–Congo hemorrhagic fever, elevated levels of IL-6 have been linked to disease severity [32–34], suggesting that this is a common characteristic of viral hemorrhagic fevers. Contemplating these findings, an interesting parallel can be drawn to cytokine release syndrome (CRS), a toxic immune reaction upon cancer treatment with chimeric antigen receptor–modified T cells, during which IL-6, IL-10, and IFN-γ are the main cytokines systemically increased [35]. Remarkably, CRS symptoms highly resemble those of hantavirus infection: flulike symptoms in the mild cases and, in the more severe cases, hypotension, vascular leakage, coagulopathy, and pulmonary edema [35]. The fact that blockage of the IL-6 receptor, by tocilizumab, has been successful in reversing CRS [36] raises the intriguing question as to whether hantavirus-infected patients could benefit from similar treatment.
In conclusion, this study provides novel insights into the inflammatory response during fatal and nonfatal HPS. Our results identify distinct inflammation-associated serum markers associated with severity and fatality in HPS. Importantly, multivariate analyses identified IL-6 as a marker associated with disease severity whereas I-FABP, a marker of intestinal damage, was shown to be associated with fatality. It is tempting to speculate that these markers may contribute toward prediction of disease severity in HPS.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support.This work was supported by grants from the Swedish Foundation for Strategic Research (grant number SB12-003 to H. G. L. and J. K.); the Swedish Research Council (grant numbers 521-2013-8623 to H. G. L. and J. K. and 321-2014-2818 to J. K.); Fondo para la Investigación Científica y Tecnológica; Ministerio de Ciencia (grant number PICTO 0112-2011 to V. P. M.); Administración Nacional de Laboratorios e Institutos de Salud “Dr Carlos G. Malbrán”; Ministerio de Salud (grant number FOCANLIS 2015 1532 to V. P. M.); and Consejo Nacional de Investigaciones Científicas y Técnicas (to P. S.).
Potential conflicts of interest.All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Vaheri A, Strandin T, Hepojoki J, et al. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol 2013; 11:539–50. [DOI] [PubMed] [Google Scholar]
- 2. Nichol ST, Spiropoulou CF, Morzunov S, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 1993; 262:914–7. [DOI] [PubMed] [Google Scholar]
- 3. López N, Padula P, Rossi C, Lázaro ME, Franze-Fernández MT. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology 1996; 220:223–6. [DOI] [PubMed] [Google Scholar]
- 4. Roehr B. US officials warn 39 countries about risk of hantavirus among travellers to Yosemite. BMJ 2012; 345:e6054. [DOI] [PubMed] [Google Scholar]
- 5. MacNeil A, Ksiazek TG, Rollin PE. Hantavirus pulmonary syndrome, United States, 1993–2009. Emerg Infect Dis 2011; 17:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez VP, Bellomo C, San Juan J, et al. Person-to-person transmission of Andes virus. Emerg Infect Dis 2005; 11:1848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oliveira RC, Sant’ana MM, Guterres A, et al. Hantavirus pulmonary syndrome in a highly endemic area of Brazil. Epidemiol Infect 2016; 144:1096–106. [DOI] [PubMed] [Google Scholar]
- 8. Duchin JS, Koster FT, Peters CJ, et al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N Engl J Med 1994; 330:949–55. [DOI] [PubMed] [Google Scholar]
- 9. Angulo J, Martínez-Valdebenito C, Marco C, et al. Serum levels of interleukin-6 are linked to the severity of the disease caused by Andes virus. PLoS Negl Trop Dis 2017; 11:e0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Björkström NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med 2011; 208:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kilpatrick ED, Terajima M, Koster FT, Catalina MD, Cruz J, Ennis FA. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J Immunol 1950 2004; 172:3297–304. [DOI] [PubMed] [Google Scholar]
- 12. Lindgren T, Ahlm C, Mohamed N, Evander M, Ljunggren HG, Björkström NK. Longitudinal analysis of the human T cell response during acute hantavirus infection. J Virol 2011; 85:10252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. García M, Iglesias A, Landoni VI, et al. Massive plasmablast response elicited in the acute phase of hantavirus pulmonary syndrome. Immunology 2017; 151:122–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morzunov SP, Khaiboullina SF, St Jeor S, Rizvanov AA, Lombardi VC. Multiplex analysis of serum cytokines in humans with hantavirus pulmonary syndrome. Front Immunol 2015; 6:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mori M, Rothman AL, Kurane I, et al. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J Infect Dis 1999; 179:295–302. [DOI] [PubMed] [Google Scholar]
- 16. Padula PJ, Rossi CM, Della Valle MO, et al. Development and evaluation of a solid-phase enzyme immunoassay based on Andes hantavirus recombinant nucleoprotein. J Med Microbiol 2000; 49:149–55. [DOI] [PubMed] [Google Scholar]
- 17. Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol 2012; 10:655–66. [DOI] [PubMed] [Google Scholar]
- 18. Pelsers MM, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 2003; 36:529–35. [DOI] [PubMed] [Google Scholar]
- 19. Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J Virol 2010; 84:11227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braun M, Björkström NK, Gupta S, et al. NK cell activation in human hantavirus infection explained by virus-induced IL-15/IL15Rα expression. PLoS Pathog 2014; 10:e1004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Weg CA, Pannuti CS, de Araújo ESA, et al. Microbial translocation is associated with extensive immune activation in dengue virus infected patients with severe disease. PLoS Negl Trop Dis 2013; 7:e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141:1220–30, 1230.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 24. Schieffelin JS, Shaffer JG, Goba A, et al. KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014; 371:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yanagihara R, Amyx HL, Gajdusek DC. Experimental infection with Puumala virus, the etiologic agent of nephropathia epidemica, in bank voles (Clethrionomys glareolus). J Virol 1985; 55:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Latus J, Tenner-Racz K, Racz P, et al. Detection of Puumala hantavirus antigen in human intestine during acute hantavirus infection. PLoS One 2014; 9:e98397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta S, Braun M, Tischler ND, et al. Hantavirus-infection confers resistance to cytotoxic lymphocyte-mediated apoptosis. PLoS Pathog 2013; 9:e1003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Witkowski PT, Perley CC, Brocato RL, et al. Gastrointestinal tract as entry route for hantavirus infection. Front Microbiol 2017; 8:1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grootjans J, Lenaerts K, Derikx JP, et al. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol 2010; 176:2283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Delgado ME, Grabinger T, Brunner T. Cell death at the intestinal epithelial front line. FEBS J 2016; 283:2701–19. [DOI] [PubMed] [Google Scholar]
- 31. Outinen TK, Mäkelä SM, Ala-Houhala IO, et al. The severity of Puumala hantavirus induced nephropathia epidemica can be better evaluated using plasma interleukin-6 than C-reactive protein determinations. BMC Infect Dis 2010; 10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vernet MA, Reynard S, Fizet A, et al. Clinical, virological, and biological parameters associated with outcomes of Ebola virus infection in Macenta, Guinea. JCI Insight 2017; 2:e88864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ergonul O, Tuncbilek S, Baykam N, Celikbas A, Dokuzoguz B. Evaluation of serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-alpha in patients with Crimean-Congo hemorrhagic fever. J Infect Dis 2006; 193:941–4. [DOI] [PubMed] [Google Scholar]
- 34. Juffrie M, Meer GM, Hack CE, et al. Inflammatory mediators in dengue virus infection in children: interleukin-6 and its relation to C-reactive protein and secretory phospholipase A2. Am J Trop Med Hyg 2001; 65:70–5. [DOI] [PubMed] [Google Scholar]
- 35. Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014; 20:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016; 6:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.