Abstract
Background
Schistosomiasis increases the risk of human immunodeficiency virus (HIV) acquisition in women by mechanisms that are incompletely defined. Our objective was to determine how the cervical environment is impacted by Schistosoma haematobium or Schistosoma mansoni infection by quantifying gene expression in the cervical mucosa and cytokine levels in cervicovaginal lavage fluid.
Methods
We recruited women with and those without S. haematobium infection and women with and those without S. mansoni infection from separate villages in rural Tanzania with high prevalences of S. haematobium and S. mansoni, respectively. Infection status was determined by urine and stool microscopy and testing for serum circulating anodic antigen. RNA was extracted from cervical cytobrush samples for transcriptome analysis. Cytokine levels were measured by magnetic bead immunoassay.
Results
In the village where S. haematobium was prevalent, 110 genes were differentially expressed in the cervical mucosa of 18 women with versus 39 without S. haematobium infection. Among the 27 cytokines analyzed in cervicovaginal lavage fluid from women in this village, the level of interleukin 15 was lower in the S. haematobium–infected group (62.8 vs 102.9 pg/mL; adjusted P = .0013). Differences were not observed in the S. mansoni–prevalent villages between 11 women with and 29 without S. mansoni infection.
Conclusions
We demonstrate altered cervical mucosal gene expression and lower interleukin 15 levels in women with S. haematobium infection as compared to those with S. mansoni infection, which may influence HIV acquisition and cancer risks. Studies to determine the effects of antischistosome treatment on these mucosal alterations are needed.
Keywords: Schistosoma haematobium, Schistosoma mansoni, schistosomiasis, RNA-Seq, interleukin 15, cervicovaginal lavage
This case-control study found altered cervical mucosal gene expression and lower cervicovaginal interleukin 15 levels in women with Schistosoma haematobium infection but not in those with Schistosoma mansoni infection, which may influence human immunodeficiency virus acquisition and cancer risks.
Studies from Tanzania, Zimbabwe, and Mozambique support an increased risk of human immunodeficiency virus (HIV) acquisition in women with schistosome infection [1–5]. These studies report increased prevalence and incidence of HIV infection in women with schistosomiasis, including a longitudinal study that documented schistosome infection and a subsequent 2.8-fold increased odds of becoming HIV infected [5]. The World Health Organization and the Joint United Nations Programme on HIV/AIDS have declared schistosome infection to be a potentially modifiable risk factor for HIV acquisition [6, 7].
The mechanisms by which schistosomes modify a woman’s risk of HIV acquisition are unknown. The increase in HIV risk in schistosome-infected women but not men [5, 8] suggests that there are differential genital mucosal responses to schistosome infections. Although systemic effects may also play a role [9], most studies looking for causality have focused on the mucosa. Macroscopically, genital S. haematobium infection causes friability, neovascularization, and sandy patches on the cervix [10]. One study in Malawian women reported an increased density of CD4+ T lymphocytes and macrophages in cervical biopsy specimens with Schistosoma haematobium ova visualized [11]. Women with S. haematobium infection also have a greater proportion of monocytes in cervical cytobrush samples that express the HIV receptor CCR5 [12]. Although Schistosoma mansoni primarily influences the gastrointestinal tract, its eggs are widely distributed throughout genital tissues but at lower densities than in S. haematobium infection [13, 14]. A case series of women with S. mansoni eggs detected in genital tissue had visible cervical abnormalities that were similar to those described in women with S. haematobium infection [15]. To our knowledge, no studies have reported on the genital mucosal gene expression or cytokine levels in S. mansoni infection.
Our objective was to determine how the cervical environment is impacted by S. haematobium or S. mansoni infection by quantifying gene expression in the cervical mucosa and cytokine levels in cervicovaginal lavage fluid. To investigate the differential effects of schistosome species, we compared women with and those without S. haematobium infection and women with and those without S. mansoni infection. The goal of these efforts was to identify gene expression and cytokine differences in the genital mucosa of women with schistosome infections, to improve schistosomiasis treatment programs and guide HIV prevention strategies in schistosome-endemic areas.
MATERIALS AND METHODS
Study Sites and Population Screening
We invited a community-based sample of women of reproductive age living in rural villages in Tanzania with high prevalence of either S. haematobium or S. mansoni infection [2, 16] to participate in a study with free screening for schistosomiasis and HIV. The villages with S. mansoni infection were located closer to Lake Victoria, while the villages with S. haematobium were further inland, as previously described [2].
All women received individual HIV counseling and underwent rapid testing with same-day results. In accordance with Tanzanian national guidelines, whole blood collected by finger stick was tested with the Determine HIV-1/2 test (Alere, Waltham, MA), with confirmation of positive results with the Uni-Gold HIV 1/2 test (Trinity Biotech, Wicklow, Ireland). Women with a first-time diagnosis of HIV infection were referred to the nearest HIV care and treatment center for follow-up. Blood specimens were collected by phlebotomy for quantitation of serum circulating anodic antigen (CAA) at the Tanzanian National Institute for Medical Research laboratory in Mwanza. A 10-mL urine sample was filtered and examined microscopically for schistosome ova. The Kato-Katz technique was used to screen 5 slides per stool specimen collected for S. mansoni ova [17]. Our team returned to the study village 2 weeks later to provide results to all women and to offer eligible women the opportunity to participate in an additional portion of the study. All women found to have schistosome infections received free praziquantel treatment.
Study Design
Women eligible for enrollment were those with confirmed S. haematobium infection, with S. haematobium eggs visible on urine microscopy and a CAA-positive serum specimen, and women who were confirmed to be negative for schistosome infection but living in S. haematobium–endemic villages. We also enrolled women with confirmed S. mansoni infection, with S. mansoni eggs visible on stool microscopy and CAA-positive serum, and women who were confirmed to be negative for schistosome infection and living in S. mansoni–endemic villages. Infected and uninfected women were therefore recruited from the same schistosome-endemic villages. For each enrolled woman with schistosome infection, we invited the subsequent 2 uninfected women (from S. haematobium–endemic villages) or the subsequent 3 uninfected women (from S. mansoni–endemic villages) who had returned to obtain their screening results to enroll, as well. Among women in the schistosome-negative groups, schistosome eggs were not detected in urine or stool specimens by microscopy, and CAA was not detected in serum specimens.
Women who were pregnant were included in screening and treated for schistosomiasis if indicated, but they were excluded from further study owing to the need to perform endocervical brushing. Women provided written informed consent and underwent a structured interview in a private setting with a nurse fluent in the local language. At this visit, a peripheral blood specimen was collected into Tempus RNA isolation tubes. Women underwent a gynecologic examination by the study physician (J. A. D.). Cervical swab specimens were collected for sexually transmitted infection testing. Two endocervical cytobrush specimens were collected into RNAlater (Life Technologies, Carlsbad, CA). Cervicovaginal lavage was performed using a Pasteur pipette to wash 3 mL of 0.9% normal saline over the face of the cervix 3 times and then collect the fluid into a cryotube on ice, as previously described [18]. Acetic acid was used to perform cervical cancer screening in accordance with Tanzanian national guidelines [19].
Sample Processing and Testing
Rapid point-of-care Trichomonas testing was performed on site at the time of gynecologic examination (Osom Rapid Test, Sekisui Diagnostics, San Diego, CA). DNA was extracted from cervicovaginal swab specimens for chlamydia and gonorrhea quantitative polymerase chain reaction (qPCR) analysis, using the QIAamp DNA mini kit, and was quantified using the Artus CT/NG QS-RGQ kit (Qiagen, Hilden, Germany). Serum specimens for CAA testing were separated and stored at −20ºC within 10 hours of collection. Schistosome CAA testing was performed using up-converting phosphor technology as previously described, with CAA values >30 pg/mL considered positive [3, 20, 21].
RNA Isolation From Cervical Cytobrush Specimens
Cytobrush samples were disrupted using a Bead Mill 4 bead beater (Thermo-Fisher Scientific, Waltham, MA) in nuclease-free tubes with metal beads (Thermo-Fisher Scientific) for 1 minute at medium speed (3 m/second or 90 ×g). The RNAlater was removed from the bead tube and centrifuged for 6 minutes at 15 000 rpm. The supernatant was removed from the centrifuge tube (leaving the cell pellet intact) and placed back into the bead tube with the cervical cytobrush and was disrupted a second time at a speed of 4 m/second (120 ×g) for 1 minute. The supernatant was removed and RNA extracted with the RNeasy Mini Kit with on-column DNase digestion, according to product instructions (Qiagen).
RNA integrity was determined with a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA), and the RNA concentration was measured using the NanoDrop 8000 (ThermoFisher Scientific). Samples with RNA integrity numbers >6.4 and 28S to 18S ribosomal RNA ratios >1.2 were submitted for RNA sequencing. RNA sample preparation and next-generation sequencing (RNA-Seq) were performed in 2 batches for S. haematobium groups and in a single batch for S. mansoni groups.
RNA Library Preparation and RNA-Seq
RNA library preparation and RNA-Seq were performed at the Weill Cornell Genomics Core laboratory. Messenger RNA (mRNA) was prepared using the TruSeq RNA Sample Prep Kit v2 (Illumina, San Diego, CA) according to the manufacturer’s instructions. Prior to the sequencing run, samples were hybridized onto a patterned flow cell and amplified using a cBot fluidics device (Illumina). Patterned flow cells were sequenced on a HiSeq 4000 (Illumina) as single-end 50-bp reads. Illumina bcl2fastq2 Conversion Software was used to demultiplex samples and to convert per-cycle BCL base call files into FASTQ files for downstream data analysis. FastQC (Babraham Bioinformatics, Babraham, UK) was used to determine sequence quality. Reads were aligned to the human hg19 reference genome by using Tophat2 [22] and were counted with the HTSeq package [23].
Cytokine Measurement
Specimens obtained by cervicovaginal lavage were thawed and then centrifuged for 10 minutes at 10 000 ×g at 4oC. The supernatant was diluted 4-fold in Bio-Plex sample diluent (Bio-Rad Laboratories, Hercules, CA). Twenty-seven cytokines were labeled using the Bio-Plex Pro Human Cytokine Standard 27-Plex Group I magnetic bead immunoassay (Bio-Rad Laboratories). The following cytokines were measured: interleukin 1β, interleukin 1ra, interleukin 2, interleukin 4, interleukin 5, interleukin 6, interleukin 7, interleukin 8, interleukin 9, interleukin 10, interleukin 12p70, interleukin 13, interleukin 15 (IL-15), interleukin 17A, eotaxin, basic fibroblast growth factor, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interferon γ (IFN-γ), IFN-γ–induced protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein1α and 1β, plant-derived growth factor BB, RANTES, tumor necrosis factor α, and vascular endothelial growth factor. Quantitation was performed using a Luminex 100/200 System with Luminex xPONENT software (build 31.871.0; Luminex, Austin, TX). To control for interplate variability, 5 interplate specimen controls were included in duplicate on each of the plates.
Statistical Analysis
Demographic and clinical characteristics were expressed as numbers and percentages or as median values and interquartile ranges and were compared using the Fisher exact test, for discrete variables, and the Wilcoxon rank sum test, for continuous variables. We used principal component analysis to visualize sources of variation in the data.
For RNA-Seq data from cervical cytobrush specimens, we compared genes for differential expression in people with and those without schistosome infection, using DESeq2 in R (version 3.4.1) [24]. To adjust for batch effect, we included “transcriptome preparation batch” as an additional covariate for the differential expression analysis. In both RNA-Seq and cytokine analyses, P values were adjusted for multiple comparisons, using the procedure of Benjamini and Hochberg [25]. Genes that were differentially expressed with a false-detection rate (ie, adjusted P value [Padj]) of ≤.05 were included in subsequent analyses. All genes identified as being differentially expressed with Padj ≤ .05 in those with versus without schistosome infections were input into Ingenuity Pathway Analysis (Qiagen) and Webgestalt Kyto Encyclopedia of Genes and Genomes (KEGG) pathways analysis using overrepresentation enrichment analysis [26] to generate representative pathways.
Cytokine comparisons were done with the Wilcoxon rank sum test. Because our case definition required positive results of both CAA testing and microscopy, a large number of CAA-positive, egg-negative women with HIV infection were excluded. Therefore, we examined the effects of HIV infection in a subanalysis for both gene expression and cytokines. We assessed the influence of both S. haematobium infection and HIV infection, using 2-way analysis of variance to confirm that the effect of S. haematobium was significant while controlling for HIV.
Ethics
This study was approved by Bugando Medical Centre and the National Institute for Medical Research (both in Tanzania) and by the Weill Cornell Medical College. Study participants provided written informed consent. Those found to have sexually transmitted infections received free treatment for themselves and their sex partners in accordance with Tanzanian national guidelines. Those with HIV infection were referred for ongoing free care at the nearest HIV care and treatment center. Women with abnormalities during the acetic acid–based cervical cancer screening examination were given a referral appointment for repeat screening and possible cryotherapy at Bugando Medical Centre and money for transport to the appointment. All women who had schistosome infection as evidenced by urine, stool, or serum screening received free praziquantel treatment when they returned for their results. Women who consented to the next phase of the study received praziquantel the same day after completing all study procedures.
RESULTS
Characteristics of the Study Population
We screened 67 women in S. haematobium–endemic villages from April to November 2015 and 53 women from July to December 2016 (Supplementary Figure 1). Twenty-one women (16.7%) had confirmed S. haematobium infection, with both positive results of serum CAA testing and S. haematobium eggs visualized on urine microscopy. One of these women was pregnant and excluded from further study. Fourteen women had positive results of CAA testing but no schistosome eggs visualized in urine and stool specimens, of whom 3 (21%) were HIV infected; all were excluded from the study because of discordant test results. The remaining 20 women consented to additional testing. We also invited 44 women with serum and urine specimens confirmed to be negative for schistosome infection to participate in further testing. Following RNA extraction, 7 did not meet the threshold to continue to RNA-Seq, owing to either insufficient RNA quality or quantity. Ultimately, we analyzed data on 57 women from S. haematobium–endemic villages, 18 of whom were infected with S. haematobium.
We screened 80 women in S. mansoni–endemic villages during May 2015 for inclusion into the study. Of these, 1 woman had S. mansoni and S. haematobium coinfection and was treated and not studied further. There were also 18 women who had positive results of CAA testing but no schistosome eggs visualized in urine or stool specimens, of whom 1 (6%) was HIV infected. Fourteen women who were positive for CAA and had S. mansoni detected in stool specimens consented to participate; samples from 11 passed the RNA quality threshold to continue to RNA-Seq. We also enrolled 43 schistosome-uninfected women, and samples from 29 passed the threshold for RNA quality and quantity. Ultimately, we analyzed data from 40 women from S. mansoni–endemic villages, of whom 11 were and 29 were not infected with S. mansoni.
Between the groups of women from the same villages, those with S. haematobium infection were significantly younger than those without S. haematobium infection (Table 1). There were no other statistically significant demographic or clinical differences between S. haematobium– or S. mansoni–infected women and corresponding uninfected controls. Among a subset of cervical swab specimens available for testing, there were no significant differences in the prevalence of chlamydia, gonorrhea, or trichomoniasis between those with and those without schistosome infection. Two women (11.1%) with and 13 (33.3%) without S. haematobium infection were HIV infected (P = .11). The frequency of HIV infection among women with and those without S. mansoni infection was similar (18.2% and 17.2%, respectively).
Table 1.
Demographic and Clinical Characteristics of Study Participants, by Schistosoma haematobium and Schistosoma mansoni Infection Status
| Characteristic | S. haematobium Status | S. mansoni Status | ||||
|---|---|---|---|---|---|---|
| Infected(n = 18) | Uninfected(n = 39) | P difference | Infected(n = 11) | Uninfected(n = 29) | P difference | |
| Age, y | 21.5 (20–31) | 30 (26–35) | .0043 | 29 (27–35) | 32 (29–38) | .387 |
| Currently breastfeeding | 4 (23.5) | 13 (37.1) | .37 | 2 (18.2) | 7 (24.1) | 1.00 |
| Marital status | ||||||
| Married | 14 (77.8) | 27 (69.2) | .75 | 8 (72.7) | 22 (75.9) | 1.00 |
| Single/widowed/divorced | 4 (22.2) | 12 (30.8) | 3 (27.3) | 7 (24.1) | ||
| Years of school completed | 7 (0–7) | 7 (7–7) | .056 | 7 (5–7) | 7 (2–7) | .754 |
| Ever treated for schistosomiasisa | 3 (16.7) | 5 (12.8) | .70 | 2 (18.2) | 9 (31.0) | .693 |
| HIV positive | 2 (11.1) | 13 (33.3) | .11 | 2 (18.2) | 5 (17.2) | 1.00 |
| S. haematobium ova, no. per 10 mL of urine | 2 (2–12) | 0 (0–0) | <.001 | 0 | 0 | |
| S. mansoni ova, no. per g of stool | 0 | 0 | 48 (4.8–100.8) | 0 (0.0) | <.001 | |
| Serum schistosome CAA level, pg/mL | 218.6 (107.7–1320) | 0 (0–5) | <.001 | 2428 (950–6861) | 2.5 (0–4.7) | <.001 |
Data are no. (%) of participants or median value (interquartile range).
Abbreviations: CAA, circulating anodic antigen; HIV, human immunodeficiency virus.
aIncluding mass drug administration.
The factors listed in Table 1 were compared between the 2 groups. A comparison using principal component analysis revealed that the year of specimen collection was an important source of variation that needed to be included in the model for S. haematobium infection, while the other factors had no significant effect and were therefore not included as covariates. When the year of collection was included as a covariate, we identified 110 genes that were differentially expressed in the cervical brushing samples from women with and those without S. haematobium infection. Additional analysis of these 110 genes confirmed that their expression was not driven by other factors listed in Table 1, such as HIV infection status.
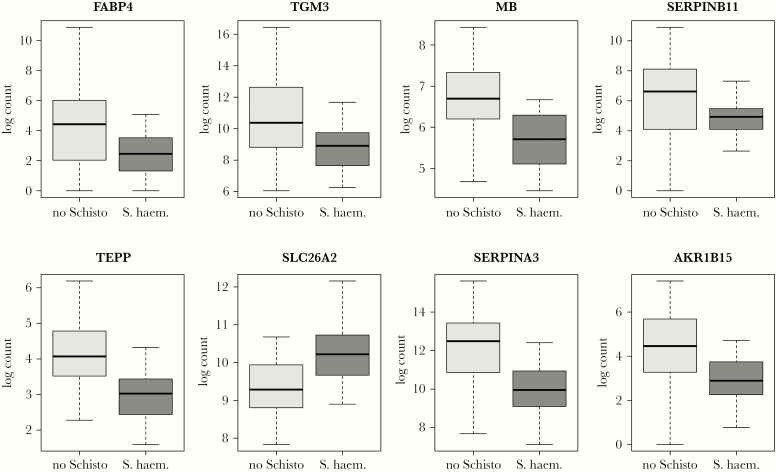
Box plots of the transcript levels of 8 genes for which comparisons between women with and those without S. haematobium infection yielded the lowest adjusted P values are shown in Figure 1. The genes were FABP4 (encoding fatty acid binding protein 4; log2 fold change in expression in infected women, −3.7), TGM3 (transglutaminase 3; fold change, −2.9), MB (myoglobin; fold change, −1.2), SERPINB11 (serpin family B member 11; fold change, −2.7), TEPP (testis, prostate and placenta expressed, FC = −1.4), SLC26A2 (solute carrier family 26 member 2; fold change, 1.0), SERPINA3 (serpin family A member 3; fold change, −2.1), and AKR1B15 (aldo-keto reductase family 1 member B15; fold change, −1.9). Of these genes, all but SLC26A2 were expressed at significantly lower levels in women with S. haematobium infection.
Figure 1.
Differences in transcript count in cervical cells for 8 representative genes found to be differentially expressed in women with and those without Schistosoma haematobium infection. The plots display median values (dark horizontal bar) and interquartile ranges (boxes), with error bars representing 1.5 times the interquartile range or the minimum/maximum values. The P value for differences was <.05 after adjustment for multiple comparisons for all genes shown. FABP4 encodes fatty acid binding protein 4; TGM3, transglutaminase 3; MB, myoglobin; SERPINB11, serpin family B member 11; TEPP, testis, prostate and placenta expressed; SLC26A2, solute carrier family 26 member 2; SERPINA3, serpin family A member 3; and AKR1B15, aldo-keto reductase family 1 member B15.
Ingenuity Pathways Analysis identified the top canonical pathway as “inhibition of matrix metalloproteinases” (P = 4.14 × 10–5). The second, third, and fourth canonical pathways, specified as follows, were related to cancer: “colorectal cancer metastasis signaling” (P = 2.42 × 10–4), “glioma invasiveness signaling” (P = 4.10 × 10–4), and “bladder cancer signaling” (P = 9.74 × 10–4), respectively. There were no statistically significantly enriched KEGG pathways, using a false-discovery rate cutoff of 0.05.
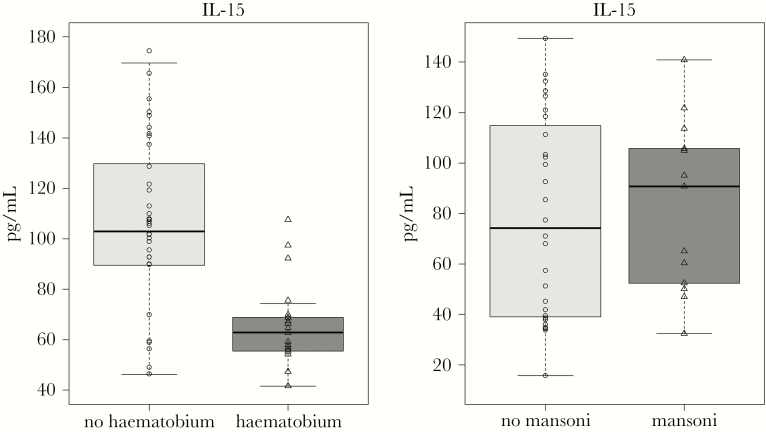
In cervicovaginal lavage fluid, IL-15 levels were lower in women with as compared to those without S. haematobium infection (68.2 vs 102.9 pg/mL; Padj = .0013; Figure 2). After adjustment for multiple comparisons, there were no statistically significant differences in levels of any of the other measured cytokines between women with and those without S. haematobium infection (Supplementary Table 1). An additional analysis that included HIV and S. haematobium status as factors by 2-way analysis of variance confirmed that S. haematobium status was significant (P = .0001) but HIV status was not (P = .2941) for the differences in IL-15 levels. Gene expression of IL-15 in cervical brushing samples was lower in the S. haematobium group (fold change, −0.3; Punadjusted = .065), but this did not reach statistical significance.
Figure 2.
Difference in interleukin 15 (IL-15) concentration in cervicovaginal lavage fluid from women with and those without Schistosoma haematobium infection (A) and women with and those without Schistosoma mansoni infection (B). The median IL-15 value was 62.8 pg/mL in women with S. haematobium infection, compared with 102.9 pg/mL in uninfected women (adjusted P = .0013). There was no significant difference in IL-15 concentrations between women with S. mansoni infection and schistosome- uninfected control women from the same village. The box plot displays median values (dark horizontal bars) and interquartile ranges (boxes), with error bars representing 1.5 times the interquartile range or the minimum/maximum values. Each triangle and square represents the IL-15 level for 1 unique patient in the specified group.
We found no genes that were differentially expressed in cervical brushing specimens from women with and those without S. mansoni infection on analysis with DESeq2. We also detected no statistically significant differences in levels of the 27 cytokines tested between the 2 groups (Supplementary Table 2)
DISCUSSION
Our investigation of human gene expression in the cervical mucosa and cytokine levels in cervical lavage fluid revealed important differences between women with and those without S. haematobium infection but not between women with and those without S. mansoni infection. In women with S. haematobium infection, we identified 110 genes that are differentially expressed in the cervix of women, which likely reflect effects of S. haematobium on the inflammatory response and tissue fibrosis. These genes were overrepresented in pathways related to cancer metastasis, reproductive system disease, and organismal injury. The decreased IL-15 level supports a hypothesis of immune exhaustion and impaired antiviral immune response in the cervix in women with S. haematobium and has not been previously reported. These differences suggest mechanisms by which S. haematobium could influence susceptibility to HIV acquisition in women. To our knowledge, this is the first study to demonstrate the effects of S. haematobium on global gene expression in human genital mucosal cells.
The analysis of cytokines in cervicovaginal lavage fluid showed lower levels of IL-15 in women infected with S. haematobium, independent of HIV status. IL-15 is an immune modulator that influences both innate and adaptive immune cells. A decreased IL-15 level leads to decreased natural killer cell activation, decreased survival, decreased proliferation of mononuclear cells, and decreased proliferation of memory CD8+ T cells [27–29]. This has been associated with decreased cytotoxic activity against herpes simplex virus [30] and increased susceptibility to mycobacterial infections in mice [31]. In human studies, there are lower levels of IL-15 in cervical tissue specimens from human papillomavirus type 16/18 (HPV16/18)–positive individuals with cervical cancer, compared with HPV16/18-negative individuals with cervical cancer [32], which is additional support for cervicovaginal cytokine levels being impacted by local infections.
IL-15 also plays an important role in the immune response to HIV by increasing cytotoxic and antiviral effects of natural killer cells and HIV-specific CD8+ T cells [33–35]. Further, stimulation by an IL-15 superagonist has been shown to prevent acquisition of HIV infection and to inhibit HIV replication [36]. Therefore, the decrease in the cervicovaginal IL-15 level that we observed in women infected with S. haematobium supports the hypothesis that an altered immune environment induced by schistosome infection contributes to susceptibility to HIV infection.
Ingenuity Pathway Analysis identified “inhibition of matrix metalloproteinases (MMPs)” as the top canonical pathway altered in the cervical mucosa during S. haematobium infection. Specifically, MMP-2 and MMP-16 expression was increased and expression of tissue inhibitor of MMP-3 (TIMP-3) was decreased in the cervix of women infected with S. haematobium. MMPs regulate extracellular matrix deposition by breaking down collagen and other extracellular matrix proteins. MMPs are also important regulators of inflammation involving cellular entry into tissues and activation of proinflammatory molecules [37, 38]. The ratio of MMP to TIMP gene expression correlates with levels of fibrosis in murine models of schistosomiasis, with an increased ratio corresponding to a greater degree of fibrosis [39, 40]. MMP-2 is also important in angiogenesis [41, 42], and its upregulation is consistent with the neovascularization of the cervix, which can occur in women with urogenital schistosomiasis [43]. Decreased expression of TIMP-3, which we found in S. haematobium–infected women, allows for increased protease activity and has been shown to increase angiogenesis, tumor invasion, and metastasis [44]. Alterations in expression of MMP and TIMP genes due to schistosome infection have been characterized in mice, but our study is the first to demonstrate these changes in human cervical cells.
Our analysis found no significant differences in gene expression or cytokine concentrations in the cervix of women infected with S. mansoni. Although S. mansoni is primarily an intestinal pathogen, autopsy studies have demonstrated the presence of S. mansoni ova in the urogenital tract, primarily the vagina and cervix [13, 14]. A case series in Tanzania found visible cervical lesions in up to half of women with biopsy-proven S. mansoni infection [15]. Furthermore, previous research in S. mansoni–endemic areas has found an association between S. mansoni infection and the risk of HIV acquisition in women [5]. It is possible that we did not identify effects because of the smaller sample size from S. mansoni–endemic villages or because of the lower density of eggs deposited in the cervix during S. mansoni infection as compared to S. haematobium infection. Further investigation of pathogenesis in S. mansoni and HIV coinfection is needed.
The sample size precluded control for potential confounders such as duration of schistosome infection, prior praziquantel treatment, and coinfections in a multivariate model. The study did not include preinfection samples, limiting conclusions as to whether changes in gene expression or cytokine levels are causally related to schistosome infection. Some of these obstacles are addressed in ongoing longitudinal studies that will analyze the cervical cells of schistosome-infected women after praziquantel treatment.
In conclusion, our study has, in the genital mucosa of women with S. haematobium infection, demonstrated molecular changes related to immune exhaustion and impaired antiviral immune response, potentially increasing susceptibility to HIV infection. These changes influence both the physical integrity of cervical tissue and the immune environment. Future studies are underway to elucidate gene expression changes over the course of schistosome infection, whether they reverse after praziquantel treatment, and their impact on susceptibility to HIV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Jo-ann Passmore, Smritee Dabee, and Shameem Jaumdally, for their helpful suggestions for optimizing our cytokine testing; Juana Gonzalez of the Kreuger Laboratory of Rockefeller University, for generously allowing us to use their Luminex instrument to read the plates and for guidance in setting up and analyzing the results; Claire Healy of the Ehrt Lab of Weill Cornell Medicine, for use of their magnetic 96-well plate separator; and the study participants, for their willingness to be a part of this project.
Disclaimer. The funders did not have a role in the decision to publish this article.
Financial support. This independent research was supported by the National Institutes of Health (grant K23 AI 110238), the Doris Duke Charitable Foundation (grant 2017067), and the Gilead Sciences Research Scholars Program in HIV from Gilead Sciences.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kjetland EF, Ndhlovu PD, Gomo E, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 2006; 20:593–600. [DOI] [PubMed] [Google Scholar]
- 2. Downs JA, Mguta C, Kaatano GM, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am J Trop Med Hyg 2011; 84:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Downs JA, van Dam GJ, Changalucha JM, et al. Association of schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg 2012; 87:868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brodish PH, Singh K. Association between schistosoma haematobium exposure and human immunodeficiency virus infection among females in Mozambique. Am J Trop Med Hyg 2016; 94:1040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Downs JA, Dupnik KM, van Dam GJ, et al. Effects of schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: a nested case-control study. PLoS Negl Trop Dis 2017; 11:e0005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Schistosomiasis. Geneva, Switzerland: World Health Organization, 2018. http://www.who.int/news-room/fact-sheets/detail/schistosomiasis Accessed 8 June 2018. [Google Scholar]
- 7. UNAIDS. The need for a holistic approach to women and HIV.2018. http://www.unaids.org/en/resources/presscentre/featurestories/2018/march/20180316_holistic-approach-to-women-and-hiv Accessed 8 June 2018.
- 8. Downs JA, de Dood CJ, Dee HE, et al. Schistosomiasis and human immunodeficiency virus in men in Tanzania. Am J Trop Med Hyg 2017; 96:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dupnik K, Reust M, Vick K, et al. Gene expression differences in host response to Schistosoma haematobium infection. Infect Immun 2018; 87. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kjetland EF, Ndhlovu PD, Mduluza T, et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 2005; 72:311–9. [PubMed] [Google Scholar]
- 11. Jourdan PM, Holmen SD, Gundersen SG, Roald B, Kjetland EF. HIV target cells in Schistosoma haematobium-infected female genital mucosa. Am J Trop Med Hyg 2011; 85:1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kleppa E, Ramsuran V, Zulu S, et al. Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PLoS One 2014; 9:e98593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gelfand M, Ross WF. II. The distribution of schistosome ova in the genito-urinary tract in subjects of bilharziasis. Trans R Soc Trop Med Hyg 1953; 47:218–20. [DOI] [PubMed] [Google Scholar]
- 14. Cheever AW, Kamel IA, Elwi AM, Mosimann JE, Danner R. Schistosoma mansoni and S. haematobium infections in Egypt. II. Quantitative parasitological findings at necropsy. Am J Trop Med Hyg 1977; 26:702–16. [DOI] [PubMed] [Google Scholar]
- 15. Poggensee G, Krantz I, Kiwelu I, Diedrich T, Feldmeier H. Presence of Schistosoma mansoni eggs in the cervix uteri of women in Mwanga District, Tanzania. Trans R Soc Trop Med Hyg 2001; 95:299–300. [DOI] [PubMed] [Google Scholar]
- 16. Downs JA, de Dood CJ, Dee HE, et al. Schistosomiasis and human immunodeficiency virus in men in Tanzania. Am J Trop Med Hyg 2017; 96:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berhe N, Medhin G, Erko B, et al. Variations in helminth faecal egg counts in Kato-Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop 2004; 92:205–12. [DOI] [PubMed] [Google Scholar]
- 18. Downs JA, Kabangila R, Verweij JJ, et al. Detectable urogenital schistosome DNA and cervical abnormalities 6 months after single-dose praziquantel in women with Schistosoma haematobium infection. Trop Med Int Health 2013; 18:1090–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of Health and Social Welfare. The national road map strategic plan to accelerate reduction of maternal, newborn, and child deaths in Tanzania (2016–2020). Dar es Salaam 2015. https://www.prb.org/wp-content/uploads/2018/05/National-Road-Map-Strategic-Plan-to-Accelerate-Reduction-of-Maternal-Newborn-and-Child-Deaths-in-Tanzania-2016-2020-One-Plan-II.pdf. Accessed 11 January 2019.
- 20. Corstjens PL, van Lieshout L, Zuiderwijk M, et al. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J Clin Microbiol 2008; 46:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corstjens PLAM, De Dood CJ, Kornelis D, et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology 2014; 141:1841–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 2013; 14:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015; 31:166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995; 57:289–300. [Google Scholar]
- 26. Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res 2013; 41:W77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol 2011; 11:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chehimi J, Marshall JD, Salvucci O, et al. IL-15 enhances immune functions during HIV infection. J Immunol 1997; 158:5978–87. [PubMed] [Google Scholar]
- 29. Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 2000; 191:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmad R, Sindhu STA, Toma E, Morisset R, Ahmad A. Studies on the production of IL-15 in HIV-infected/AIDS patients. J Clin Immunol 2003; 23:81–90. [DOI] [PubMed] [Google Scholar]
- 31. Umemura M, Hirose K, Wajjwaiku W, et al. Impaired IL-15 production associated with susceptibility of murine AIDS to mycobacterial infection. J Leukoc Biol 2001; 69:138–48. [PubMed] [Google Scholar]
- 32. Vidal AC, Skaar D, Maguire R, et al. IL-10, IL-15, IL-17, and GMCSF levels in cervical cancer tissue of Tanzanian women infected with HPV16/18 vs. non-HPV16/18 genotypes. Infect Agent Cancer 2015; 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mastroianni CM, d’Ettorre G, Forcina G, Vullo V. Teaching tired T cells to fight HIV: time to test IL-15 for immunotherapy? Trends Immunol 2004; 25:121–5. [DOI] [PubMed] [Google Scholar]
- 34. Oliva A, Kinter AL, Vaccarezza M, et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest 1998; 102:223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garrido C, Abad-Fernandez M, Tuyishime M, et al. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol 2018; 92:e00235–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seay K, Church C, Zheng JH, et al. In vivo activation of human NK cells by treatment with an Interleukin-15 superagonist potently inhibits acute in vivo hiv-1 infection in humanized mice. J Virol 2015; 89:6264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Detry B, Erpicum C, Paupert J, et al. Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood 2012; 119:5048–56. [DOI] [PubMed] [Google Scholar]
- 38. Song J, Wu C, Zhang X, Sorokin LM. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1β-induced peritonitis. J Immunol 2013; 190:401–10. [DOI] [PubMed] [Google Scholar]
- 39. Singh KP, Gerard HC, Hudson AP, Boros DL. Dynamics of collagen, MMP and TIMP gene expression during the granulomatous, fibrotic process induced by Schistosoma mansoni eggs. Ann Trop Med Parasitol 2004; 98:581–93. [DOI] [PubMed] [Google Scholar]
- 40. Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol 2001; 167:7017–26. [DOI] [PubMed] [Google Scholar]
- 41. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 2004; 16:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol 2015; 44-46:94–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jourdan PM, Roald B, Poggensee G, Gundersen SG, Kjetland EF. Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl Trop Dis 2011; 5:e1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adissu HA, McKerlie C, Di Grappa M, et al. Timp3 loss accelerates tumour invasion and increases prostate inflammation in a mouse model of prostate cancer. Prostate 2015; 75:1831–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.