Abstract
Objective
To compare the long-term survival following transcatheter aortic valve implantation (TAVI) in an octogenarian population with that in a younger population.
Methods
This retrospective study included 274 patients that underwent TAVI for severe symptomatic aortic stenosis. The study group was divided into two age groups, as those with an age ≥ 80 years (octogenarians, n = 132), and age < 80 (younger patients, n = 142). The two groups were compared in terms of clinical outcomes and survival. In addition, significant predictors of survival were estimated.
Results
Non-cardiac mortality (during follow-up) (21.9% vs. 10.5%, P = 0.01) and in-hospital stroke (8.3% vs. 2.8%, P = 0.01) were more common among octogenarians. The two groups did not differ in terms of mean survival (41.0 ± 2.1 vs. 38.2 ± 2.2 months, respectively, P = 0.18). Multivariate analysis identified left ventricular ejection fraction < 35% (OR: 2.17, 95% CI: 1.17–4.03; P = 0.01), preoperative of moderate to severe mitral insufficiency (OR: 1.88, 95% CI: 1.15–3.06; P = 0.01), postoperative major and life-threating bleeding (OR: 2.49, 95% CI: 1.05–5.89; P = 0.03), and in-hospital stroke (OR: 2.29, 95% CI: 1.04–5.04; P = 0.03) as potential predictors of poor survival.
Conclusions
In this study, similarly favorable survival outcomes were achieved in the elderly population as in younger patients, despite the presence of comorbid conditions. A consideration should be given to non-surgical management of severe aortic stenosis with the TAVI procedure in elderly patients, in the absence of co-existent conditions associated with shortened life expectancy.
Keywords: Aortic stenosis, Clinical outcome, Non-surgical management, Octogenarian, The elderly
1. Introduction
The prevalence of aortic stenosis (AS) increases with age.[1] Of all cases with severe symptomatic aortic stenosis, approximately one third cannot be referred to surgical management due to advanced age and comorbid conditions.[2] In such patients, transcatheter aortic valve implantation (TAVI) can be undertaken with lower procedure-related risks.
TAVI is known to be superior to medical treatment in patients with high-risk for surgical replacement.[3] Currently, TAVI is also used in patients with low-risk for surgery. In patients with intermediate surgical risk, similar clinical outcomes with surgery can be obtained with TAVI procedure; and in low-risk groups better outcomes are attained with TAVI.[4],[5]
As suggested by previous studies, the mean age of patients undergoing a TAVI procedure is approximately 80 years, regardless of the risk status for surgery.[6] This elderly population is likely to have many comorbid conditions, which may increase the morbidity and mortality in the long-term. A number of factors may increase the mortality in this elderly population including frailty (a condition defined on the basis of the presence of symptoms such as fatigue, unexplained weight loss, frequent infections, falls, and delirium), increased risk of experiencing spontaneous cerebrovascular events, or malignancy.[7] In patients undergoing TAVI, not only the procedural success rates, but also the long-term survival rates should be taken into consideration. In this regard, there is a scarcity of data on the long-term survival following TAVI in different age groups.
This study aimed to compare the long-term survival following TAVI in an octogenarian population, who traditionally represents a high-risk group for cardiovascular procedures, with that in a younger population.
2. Methods
A total of 274 patients that underwent TAVI at the Cardiology Unit, Bezmialem Foundation University for aortic stenosis between 2012 and 2017 were retrospectively evaluated. For the purpose of analyses, the study group was divided into two age groups, as those with an age ≥ 80 years (octogenarians, n = 132), and age < 80 years (younger patients, n = 142).
In each patient, medical history was obtained and physical examination was performed. This was followed by trans-thoracic and trans-esophageal echocardiography, carotid ultrasound, coronary angiography, aortography, ilio-femoral arteriography, and computed tomography angiography examinations.
All patients had been diagnosed with severe symptomatic native aortic valve stenosis, and all represented high-risk patients. Indications for a TAVI procedure included the following: an aortic annulus diameter of 20–27 mm; an ascending aortic diameter < 45 mm; age ≥ 75 years with a logistic EuroSCORE > 15% or age ≥ 60 years and presence of an additional risk factor including cirrhosis, respiratory failure, pulmonary hypertension, previous cardiac surgery, right ventricular dysfunction, hostile thorax (radiotherapy, burns, previous thoracic pleurodesis or multiple thoracotomy), severe connective tissue disease, cachexia, or porcelain aorta. Patients with a life expectancy < 1 year were excluded.
Pre-operative demographic data was assessed using the European System for Cardiac Operative Risk Evaluation (EuroSCORE) definitions,[8] while post-operative outcomes and clinical end-points were reported in accordance with the recommendations of the updated Valve Academic Research Consortium (VARC)-2.[9] Thus, vascular complications were classified in two categories (minor or major), bleeding in three categories (minor, major, and life-threatening), and acute kidney injury in 3 stages.[9]
Furthermore, composite end-points defined in VARC-2 such as the device success, early safety (at 30 days), and 1-year clinical efficacy were also evaluated.[9] Device success was defined as the absence of procedural mortality in addition to correct positioning and achievement of intended performance. Early safety (at 30 days) was defined as the absence of all-cause mortality, stroke, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary artery obstruction requiring intervention, major vascular complication and valve-related dysfunction requiring repeat procedure (balloon aortic valvuloplasty, TAVI, or surgical aortic valve replacement). Clinical efficacy at one year was defined as the absence of the following: all-cause mortality, stroke, hospitalizations for valve-related symptoms or worsening congestive heart failure, New York Heart Association (NYHA) class III or IV, valve-related dysfunction.[9]
2.1. Procedure
All TAVI procedures were performed via the trans-femoral route under general anesthesia. Prophylactic antibiotics were administered 1 h before the procedure, and 100 IU/kg heparin was given during the procedure. A temporary pacemaker lead was inserted into the right ventricle through the femoral vein. An Amplatz Extra Stiff Guide-Wire was advanced into the apex of the left ventricle by placing a 16-F sheath through the femoral artery. The aortic valve was dilated once using a 20 × 40 mm valvuloplasty balloon, with a pacemaker rate of less than 200/min. Simultaneously, fluoroscopic balloon sizing measurements were performed to determine the valve size. The valve system was advanced to the aortic valve through the sheath. The prosthetic valve was placed at the aortic annulus, and optimal opening was achieved. An aortography was performed to check any significant failure. All patients received dual antiplatelet chemotherapy (100 mg of acetylsalicylic acid and 75 mg of clopidogrel) for the first 6 months following surgery.
2.2. Statistical Analysis
Statistical Package for Social Sciences (SPSS) for Windows (version 17.0) software (SPSS Inc., Chicago, IL, USA) was used for the analysis of data. Intergroup comparisons of continuous variables were done by Mann-Whitney U test and chi-square test or Fisher's exact test was used for the comparisons of categorical variables. Long-term survival analyses were done using Kaplan-Meier test. Univariate and multivariate analyses of potential predictors for long-term mortality were done using Log rank test and Cox regression analysis, respectively. A P value < 0.05 was considered indication of statistical significance.
3. Results
3.1. Baseline characteristics
Table 1 demonstrates baseline characteristics of the patients. Logistic EuroSCORE was higher (21.4% ± 3.3% vs. 20.3% ± 3.7%, P = 0.01) and aortic mean gradient was lower (46.5 ± 11 vs. 49.3 ± 12 mmHg, P = 0.04) in the octogenarian group when compared to younger patients. In addition, preoperative critical condition was more common among octogenarians (7.5% vs. 2.1%, P = 0.03). The two groups did not differ with regard to other baseline characteristics (P > 0.05 for all).
Table 1. Baseline characteristics of the patients.
| Octogenarians (≥ 80 yrs) (n = 132) | Younger patients (< 80 yrs) (n = 142) | P | |
| Age, yrs | 84.3 ± 3.1 | 72.6 ± 5.8 | 0.000 |
| Female gender | 81 (61.3%) | 91 (64%) | 0.64 |
| Diabetes | 34 (25.7%) | 46 (32.3%) | 0.22 |
| Hypertension | 82 (62.1%) | 87 (61.2%) | 0.88 |
| Dyslipidemia | 22 (16.6%) | 25 (17.6%) | 0.83 |
| History of smoking | 42 (31.8%) | 45 (31.6%) | 0.98 |
| COPD | 44 (33.3%) | 48 (33.8%) | 0.93 |
| Peripheral vascular disease | 28 (21.2%) | 31 (21.8%) | 0.90 |
| Coronary artery disease | 74 (56%) | 74 (52.1%) | 0.51 |
| Cerebrovascular disease | 9 (6.8%) | 13 (9.1%) | 0.47 |
| Serum creatinine >1.5 mg/dL | 20 (15.1%) | 19 (13.3%) | 0.67 |
| Atrial fibrillation | 49 (37.1%) | 38 (26.7%) | 0.06 |
| Previous MI (< 90 days) | 7 (5.3%) | 6 (4.2%) | 0.67 |
| Preoperative critical condition | 10 (7.5%) | 3 (2.1%) | 0.03 |
| Prior CABG | 12 (9%) | 21 (14.7%) | 0.14 |
| Aortic mean gradient, mmHg, | 46.5 ± 11 | 49.3 ± 12 | 0.04 |
| Aortic valve area, cm2 | 0.71 ± 0.1 | 0.69 ± 0.1 | 0.29 |
| LVEF | 50.6% ± 9.2% | 48.7% ± 11% | 0.12 |
| PAB > 40 mmHg | 24 (18.1%) | 29 (20.4%) | 0.63 |
| Mitral regurgitation | |||
| Mild | 14 (10.6%) | 16 (11.2%) | 0.70 |
| Moderate | 8 (6.0%) | 12 (8.4%) | |
| Severe | 10 (7.5%) | 9 (6.3%) | |
| NYHA class | |||
| I-II | 26 (19.6%) | 35 (24.6%) | 0.19 |
| III-IV | 106 (80.3%) | 107 (75.3%) | |
| Logistic EuroSCORE | 21.4% ± 3.3% | 20.3% ± 3.7% | 0.01 |
Data are presented as mean ± SD or n (%). CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; PAB: pulmonary arterial pressure.
3.2. Procedural and postoperative outcomes
Table 2 shows the distribution of implanted prosthetic valve types across groups. The two groups did not differ in the distribution of valve types (P =0.38). Table 3 compares the two groups in terms of clinical outcomes. The mean duration of follow-up was 25.1 ± 19.1 vs. 27.1 ± 20.0 months for octogenarians and younger patients, respectively (P > 0.05). Non-cardiac mortality (during follow-up) (21.9% vs. 10.5%, P = 0.01) and in-hospital stroke (8.3% vs. 2.8%, P = 0.01) were more common among octogenarians. On the other hand, the two groups were similar in terms of all other clinical outcomes (Table 3).
Table 2. Distribution of implanted prosthetic valve types across groups.
| Type of prosthesis | Octogenarians (≥ 80 yrs) (n = 132) | Younger patients (< 80 yrs) (n = 142) |
| Edwards Sapien XT | 59 (44.7%) | 78 (54.9%) |
| CoreValve | 46 (34.8%) | 45 (31.7%) |
| Direct Flow Medical | 13 (9.9%) | 11 (7.8%) |
| Symetis Acurate valve | 3 (2.3%) | 5 (3.5%) |
| Lotus Valve System | 7 (5.3%) | 1 (0.7%) |
| Portico Transcatheter Heart Valves | 4 (3.0%) | 2 (1.4%) |
Data presented as n (%). Prosthesis names are trademarks of their respective companies: Edwards Sapien XT, Edwards Lifesciences, Irvine, CA, USA; CoreValve prosthesis, Medtronic, Minneapolis, MN, USA; Direct Flow Medical, Direct Flow Medical Inc., Santa Rosa, CA, USA; Symetis Acurate, Symetis Inc., Ecublens, Switzerland; Lotus Valve System, Boston Scientific, Natick, Massachusetts; Portico Transcatheheter Heart Valves, St. Jude Medical, Saint Paul, Minnesota.
Table 3. Clinical outcomes.
| Octogenarians (≥ 80 yrs) (n = 132) | Younger patients (< 80 yrs) (n = 142) | P | |
| Device success (VARC-2) | 128 (96.9%) | 140 (98.5%) | 0.35 |
| Conversion to SAVR | 2 (1.5%) | 1 (0.7%) | 0.61 |
| Coronary obstruction | 0 | 1 (0.7%) | 1.0 |
| Paravalvular leak | |||
| None | 106 (80.3%) | 119 (83.8%) | 0.61 |
| Mild | 21 (15.9%) | 16 (11.2%) | |
| Moderate | 3 (2.2%) | 4 (2.8%) | |
| Severe | 2 (1.5%) | 3 (2.1%) | |
| Vascular complications | |||
| Major | 4 (3%) | 3 (2.1%) | 0.53 |
| Minor | 18 (13.6%) | 14 (9.8%) | |
| Bleeding | |||
| Minor | 23 (17.4%) | 22 (15.4%) | 0.68 |
| Major | 5 (3.7%) | 3 (2.1%) | |
| Life-threatening | 3 (2.2%) | 2 (1.4%) | |
| Acute kidney injury | |||
| Stage 1 | 10 (7.5%) | 16 (11.2%) | 0.75 |
| Stage 2 | 6 (4.5%) | 4 (2.8%) | |
| Stage 3 | 3 (2.2%) | 3 (2.1%) | |
| New-onset atrial fibrillation | 8 (6%) | 10 (7%) | 0.74 |
| Stroke | 11 (8.3%) | 4 (2.8%) | 0.04 |
| Permanent pacemaker implantation | 14 (10.6%) | 11 (7.7%) | 0.41 |
| All-cause mortality (30 days) | 12 (9%) | 14 (9.8%) | 0.82 |
| Early safety (30 days) (VARC-2) | 106 (80.3%) | 117 (82.3%) | 0.65 |
| Clinical efficacy (1 year) (VARC-2) | 89 (67.4%) | 100 (70.4%) | 0.59 |
| Cardiovascular mortality (follow-up) | 30 (22.7%) | 38(26.7%) | 0.44 |
| Noncardiac mortality (follow-up) | 29 (21.9%) | 15 (10.5%) | 0.01 |
Data are presented as n (%). VARC: Valve Academic Research Consortium; SAVR: surgical aortic valve replacement.
3.3. Survival
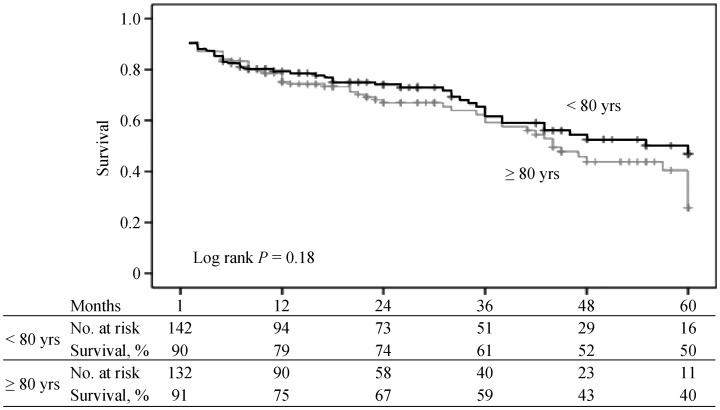
1-, 2-, 3-, 4-, and 5-year survival rates were 79%, 74%, 61%, 52%, and 50%, respectively, in the octogenarian group. Corresponding figures for younger patients were 75%, 67%, 59%, 43%, and 40%, respectively. The two groups did not differ in terms of mean survival (41.0 ± 2.1 vs. 38.2 ± 2.2 months, respectively, P = 0.18) (Figure 1). In univariate analysis, among preoperative variables, presence of chronic obstructive pulmonary disease (COPD); pulmonary hypertension, moderate to severe mitral insufficiency, preoperative chronic renal insufficiency, and an ejection fraction < 35% were associated with poor survival outcome. Among postoperative variables, major life-threatening bleeding, in-hospital stroke, moderate to severe paravalvular leak and acute kidney injury were associated with poor survival. Multivariate analysis identified left ventricular ejection fraction < 35% (OR: 2.17, 95% CI: 1.17–4.03, P = 0.01), preoperative of moderate to severe mitral insufficiency (OR: 1.88, 95% CI: 1.15–3.06, P = 0.01), postoperative major and life-threating bleeding (OR: 2.49, 95% CI: 1.05–5.89, P = 0.03), and in-hospital stroke (OR: 2.29, 95% CI: 1.04–5.04, P = 0.03) as potential predictors of poor survival (Table 4).
Figure 1. Kaplan Meier survival curves for patients < 80 vs. ≥ 80 years old.
Table 4. Univariate (log rank) and multivariate (cox regression) analysis of potential predictors for long-term mortality.
| Variables | Univariate |
Multivariate |
||
| Mean survival (months) | P | OR (95% CI) | P | |
| Age | ||||
| < 80 yrs | 41.0 ± 2.1 | 0.17 | ||
| ≥ 80 yrs | 38.2 ± 2.2 | |||
| Gender | ||||
| Male | 40.4 ± 2.4 | 0.77 | ||
| Female | 39.3 ± 1.9 | |||
| Diabetes mellitus | ||||
| Absent | 39.8 ± 1.8 | 0.94 | ||
| Present | 39.3 ± 2.7 | |||
| COPD | ||||
| Absent | 41.8 ± 1.8 | 0.04 | ||
| Present | 36.5 ± 1.4 | |||
| LVEF | ||||
| ≥ 35% | 40.8 ± 1.5 | 0.004 | 2.17 (1.17–4.03) | 0.01 |
| < 35% | 24.8 ± 5.6 | |||
| PHT | ||||
| Absent | 40.9 ± 1.6 | 0.05 | ||
| Present | 34.6 ± 3.4 | |||
| Coronary artery stenosis | ||||
| Absent | 41.1 ± 2.1 | 0.19 | ||
| Present | 38.4 ± 2.1 | |||
| Moderate to severe mitral insufficiency | ||||
| Absent | 41.9 ± 1.6 | 0.000 | 1.88 (1.15–3.06) | 0.01 |
| Present | 26.2 ± 3.8 | |||
| Peripheral vascular disease | ||||
| Absent | 40.5 ± 1.6 | 0.21 | ||
| Present | 35.3 ± 3.5 | |||
| Chronic renal insufficiency(preoperative) | ||||
| Absent | 40.9 ± 1.6 | |||
| Present | 31.7 ± 4.0 | |||
| Pacemaker implantation | ||||
| Absent | 40.1 ± 1.5 | 0.31 | ||
| Present | 29.6 ± 4.3 | |||
| New-onset atrial fibrillation | ||||
| Absent | 40.1 ± 1.5 | 0.29 | ||
| Present | 33.5 ± 6.7 | |||
| Vascular complication | ||||
| Absent | 40.2 ± 1.5 | 0.004 | ||
| Present | 14.9 ± 5.8 | |||
| Major and life threating bleeding | ||||
| Absent | 40.5 ± 1.5 | 0.001 | 2.49 (1.05–5.89) | 0.03 |
| Present | 22.6 ± 7.5 | |||
| In-hospital stroke | ||||
| Absent | 40.3 ± 1.5 | 0.04 | 2.29 (1.04–5.04) | 0.03 |
| Present | 26.0 ± 7.9 | |||
| Moderate to severe PVL | ||||
| Absent | 40.2 ± 1.5 | 0.05 | ||
| Present | 24.2 ± 7.4 | |||
| Acute kidney injury (postoperative) | ||||
| Absent | 40.5 ± 1.5 | 0.01 | ||
| Present | 27.3 ± 7.0 | |||
Data are presented as mean ± SD unless other indicated. COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; PHT: pulmonary hypertension; PVL: paravalvular leakage.
4. Discussion
In this study, we observed similar long-term survival rates in octogenarian and younger patients after a TAVI procedure. The potential predictors of poor long-term survival identified in our study were pre-operative left ventricular ejection fraction below 35%, presence of moderate to severe mitral insufficiency, major or life-threatening postoperative bleeding, and in-hospital stroke. The 30-day mortality was approximately 9% in both groups and the 5-year survival rates were 40% and 50% in octogenarians and younger subjects, respectively.
Due to the advanced age of the patient population, life expectancy and expected long-term survival after a TAVI procedure are important considerations. Until now, most comparisons involving the long-term survival rates generally utilized parameters such as the procedure-related factors (trans-femoral versus trans-apical), gender, presence of kidney failure, and ECG or echocardiography findings.[10]–[14] Again, studies examining the effect of age on survival rates mainly involved within-group comparisons among patients in their 80's, or comparisons with those over 90 years of age. However, comparison of survival between patients with advanced age, i.e., over 80 years of age, with that in a younger age group could provide more insights. In this regard, we believe that our study may represent the first of its kind.
Most studies of TAVI involved patients between 80 and 85 years of age.[6],[15] In a review of 13,857 patients with a mean age of 81.5 ± 7.0 years, the long term survivals with TAVI at 1, 2, 3, and 5 years were 83%, 75%, 65%, 48%, respectively.[6] The corresponding figures in our study among octogenarians (84.3 ± 3.1 years) and younger patients (72.6 ± 5.8 years) were 79%, 74%, 61%, 50% vs. 75%, 67%, 59%, 40%, respectively, in line with the published data.
In one previous study involving 276 TAVI patients with a mean age of 82 years, no significant differences were found in terms of peri-procedural and in-hospital complication rates as well as long term survival rates when compared with a group of patients ≥ 87 years of age.[16] Again, in another study, patients over 80 years of age were divided into three groups as those between 80 and 85 years of age, 85 and 90 years of age, and > 90 years of age, and a lower 1-year mortality rate was observed in those between 80 and 85 years of age as compared to other two groups.[17] In our study, a Kaplan-Meier analysis failed to identify significant long-term survival differences between patients ≥ 80 or < 80 years of age (log-rank P = 0.18).
Previously, potential predictors of long-term mortality have been examined in large patient series. In one such report by Zahn, et al.[18] with 1444 patients in the German Transcatheter Aortic Valve Implantation Registry, the following predictors of 5-year mortality were identified: female gender, renal failure, prior mitral regurgitation ≥ II°, residual aortic regurgitation ≥ II°, atrial fibrillation, low gradient aortic stenosis, prior decompensation, frailty, surgical TAVI, age, prior myocardial infarction, urgent TAVI, and diabetes mellitus. In the UK Transcatheter Aortic Valve Implantation Registry involving 870 patients, Duncan, et al.[19] found that renal dysfunction, atrial fibrillation, logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) ≥ 18.5, respiratory dysfunction, ventricular dysfunction (left ventricular ejection fraction < 30%), coronary artery disease, and age as independent predictors of mortality at 5 years. In the current study, while moderate to severe mitral failure (OR: 1.88, 95% CI: 1.15–3.06, P = 0.01), left ventricular ejection fraction < 35% (OR: 2.17, 95% CI: 1.17–4.03, P = 0.01), and chronic renal failure (OR: 1.59, CI 0.95–2.66, P = 0.07) emerged as predictors of mortality, age did not appear to play a role in this regard. In a single center retrospective experience from Italy with 338 patients, D'Onofrio, et al.[20] identified para-valvular leak, acute renal failure, and previous history of myocardial infarction as independent predictors of mortality. Again, long-term predictors of mortality reported from a single center study from the US by Escárcega, et al.[21] included vascular complications, more than mild aortic insufficiency, atrial fibrillation and in-hospital stroke. In our study in-hospital stroke (OR: 2.29, 95% CI: 1.04–5.04, P = 0.03), and major and life-threatening bleeding (OR: 2.49, 95% CI: 1.05–5.89, P = 0.03) were predictors of poor long term survival.
Our octogenarian patients had significantly increased non-cardiac mortality than younger patients, which is an unsurprising finding given the high incidence of comorbid conditions in this elderly individuals. Despite similar pre-operative echocardiographic and demographic characteristics in the two study groups, cardiovascular problems represented main cause of death in younger patients of our study, leading to similar outcomes in terms of survival when compared with the octogenarians. As the life expectancy in this younger patients is longer than that in octogenarians, it may be prudent to take preventive operative measures and to pay more attention to the treatments during the course of follow-up in an effort to reduce cardiovascular mortality.
Our study was performed in a single high-volume center with the inclusion of patients undergoing a TAVI procedure. A larger patient series may be achieved via a national registry study.
TAVI was found to positively affect the survival in the elderly patients, in whom surgery had not been performed.[22] Based on our results, similarly good rates of survival were achieved in this elderly population as in younger patients, despite the presence of comorbid conditions that could negatively impact the outcomes. A consideration should be given to non-surgical management of severe aortic stenosis with the TAVI procedure in elderly patients, in the absence of co-existent conditions associated with shortened life expectancy such as advanced malignancy.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Lindroos M, Kupari M, Heikkila J, et al. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 2.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 3.Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–2491. doi: 10.1016/S0140-6736(15)60290-2. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 5.Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol. 2018;72:2095–2105. doi: 10.1016/j.jacc.2018.08.1033. [DOI] [PubMed] [Google Scholar]
- 6.Chakos A, Wilson-Smith A, Arora S, et al. Long term outcomes of transcatheter aortic valve implantation (TAVI): a systematic review of 5-year survival and beyond. Ann Cardiothorac Surg. 2017;6:432–443. doi: 10.21037/acs.2017.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 9.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 10.Ferro CJ, Chue CD, de Belder MA, et al. Impact of renal function on survival after transcatheter aortic valve implantation (TAVI): an analysis of the UK TAVI registry. Heart. 2015;101:546–552. doi: 10.1136/heartjnl-2014-307041. [DOI] [PubMed] [Google Scholar]
- 11.Ewe SH, Ajmone Marsan N, Pepi M, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–1120. doi: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Kumar N, Khera R, Fonarow GC, et al. Comparison of outcomes of transfemoral versus transapical approach for transcatheter aortic valve implantation. Am J Cardiol. 2018;122:1520–1526. doi: 10.1016/j.amjcard.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Erez A, Segev A, Medvedofsky D, et al. Factors affecting survival in men versus women following transcatheter aortic valve implantation. Am J Cardiol. 2014;113:701–705. doi: 10.1016/j.amjcard.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 14.Robert R, Porot G, Vernay C, et al. Incidence, predictive factors, and prognostic impact of silent atrial fibrillation after transcatheter aortic valve implantation. Am J Cardiol. 2018;122:446–454. doi: 10.1016/j.amjcard.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Messori A, Trippoli S, Biancari F. Early and intermediate survival after transcatheter aortic valve implantation: systematic review and meta-analysis of 14 studies. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orvin K, Assali A, Vaknin-Assa H, et al. Efficacy and safety of transcatheter aortic valve implantation in aortic stenosis patients with extreme age. J Invasive Cardiol. 2015;27:475–480. [PubMed] [Google Scholar]
- 17.Yamamoto M, Mouillet G, Meguro K, et al. Clinical results of transcatheter aortic valve implantation in octogenarians and nonagenarians: insights from the FRANCE-2 registry. Ann Thorac Surg. 2014;97:29–36. doi: 10.1016/j.athoracsur.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 18.Zahn R, Werner N, Gerckens U, et al. Five-year follow-up after transcatheter aortic valve implantation for symptomatic aortic stenosis. Heart. 2017;103:1970–1976. doi: 10.1136/heartjnl-2016-311004. [DOI] [PubMed] [Google Scholar]
- 19.Duncan A, Ludman P, Banya W, et al. Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv. 2015;8:645–653. doi: 10.1016/j.jcin.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 20.D'Onofrio A, Facchin M, Besola L, et al. Intermediate clinical and hemodynamic outcomes after transcatheter aortic valve implantation. Ann Thorac Surg. 2016;101:881–888. doi: 10.1016/j.athoracsur.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Escarcega RO, Lipinski MJ, Baker NC, et al. Analysis of long-term survival following transcatheter aortic valve implantation from a single high-volume center. Am J Cardiol. 2015;116:256–263. doi: 10.1016/j.amjcard.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Perrin N, Frei A, Noble S. Transcatheter aortic valve implantation: Update in 2018. Eur J Intern Med. 2018;55:12–19. doi: 10.1016/j.ejim.2018.07.002. [DOI] [PubMed] [Google Scholar]