Abstract
Background
Dual-task paradigms, in which an individual performs tasks separately and then concurrently, often demonstrate that people with neurodegenerative disorders experience more dual-task interference, defined as worse performance in the dual-task condition compared to the single-task condition.
Objective
To examine how gait-cognition dual-task performance differs between cognitively normal older adults with and without an APOE ε4 allele.
Methods
Twenty-nine individuals ages 60 to 72 with normal cognition completed a dual-task protocol in which walking and cognitive tasks (executive function, memory) were performed separately and concurrently. Fourteen participants carried APOE ε4 alleles (ε3/ε4 or ε2/ε4); fifteen had APOE genotypes (ε2/ε2, ε2/ε3, or ε3/ε3) associated with lower risk of Alzheimer’s disease (AD).
Results
The two risk groups did not differ by age, sex, race, education, or gait or cognitive measures under single-task conditions. Compared to low risk participants, APOE ε4 carriers tended to exhibit greater dual-task interference. Both the memory and executive function tasks resulted in dual-task interference on gait, but effect sizes for a group difference were larger when the cognitive task was executive function. In the dual-task protocol that combined walking and the executive function task, effect sizes for group difference in gait interference were larger (0.62–0.70) than for cognitive interference (0.45–0.47).
Discussion
Dual-task paradigms may reveal subtle changes in brain function in asymptomatic individuals at heightened risk of AD.
Keywords: Aging brain, cognitive performance, cognitive reserve, dementia, diagnosis, early detection, motor interference, phenotype, risk, stress test
INTRODUCTION
Alzheimer’s disease (AD) is among the top ten causes of death in the U.S. and is the only one among these with no proven prevention or cure [1, 2]. Because AD neuropathology develops in the brain years before the onset of obvious cognitive symptoms, a major barrier to the development and testing of therapies is the inability to reliably detect early disease. Although individuals with early AD may be difficult to distinguish from peers using traditional cognitive assessment, brain imaging studies suggest that early in the disease course, people with subclinical AD pathology may exhibit an altered metabolic response to certain task demands [3, 4]. It is plausible that the neurodegenerative process in the brains of these individuals reduces cognitive resources or limits the efficient recruitment and coordination of resources, which manifests as an impaired capacity to support performance in new or more challenging conditions.
One type of testing paradigm that directly assesses an individual’s response to increased task demand is a “dual-task” paradigm, in which the subject performs two discrete tasks, separately and then concurrently [5]. Dual-task interference refers to the decrement in performance of one or both tasks when they are performed concurrently, relative to when one task is performed alone [6]. Cognitive-motor dual-task paradigms, such as walking and talking, investigate the potential for interference in cognitive and/or motor function [7]. Cognition and walking are centrally integrated functions that are both affected by age and neurodegenerative disease [8, 9]. Gait-cognition dual-task paradigms have demonstrated that dual-task interference is more pronounced in older compared to younger adults [10] and in those with neurological disorders including stroke [11], Parkinson’s disease [12], and AD [13]. Gait interference during dual-tasking is associated with risk of falls [14, 15] and is increasingly incorporated as an outcome in rehabilitation research [16, 17].
Two recent studies suggest that dual-task tests, and specifically dual-task effects on gait speed, are indicative of risk of progression to dementia [18, 19]. The Gait and Brain Study included 112 communitydwelling participants with mild cognitive impairment who were followed for six years, with 27 individuals progressing to dementia. Single-task gait velocity was not predictive of progression to dementia, but greater reduction in a dual-task gait velocity while performing cognitive tasks (naming animals or counting backwards) was associated with progression to dementia [18]. The second study evaluated healthy older adults who were cognitively normal and free of mobility impairments, and the authors compared gait-cognition dual-task performance in 16 individuals with and 11 individuals without cerebral amyloid-β (Aβ), based on Pittsburgh Compound B (PiB) PET scans. While the two groups demonstrated similar dual-task cost on cognition, the group with cerebral Aβ exhibited greater dual-task cost to gait speed, suggesting that cerebral Aβ may be associated with gait slowing even in cognitively normal individuals [19]. Cerebral Aβ is a biomarker for AD pathology, although questions remain about the role of Aβ in disease progression and its specificity for identifying individuals in the early stages of AD [20].
The APOE ε4 allele, present in about 25% of the population, is the strongest genetic risk factor for AD [21]. Compared to individuals with an APOE ε3/ε3 genotype, those who are homozygous for ε4 (i.e., ε4/ε4) have a 12 to 15 times higher risk of developing AD, while those with the ε3/ε4 genotype are about three times more likely to develop AD [22]. Although allelic status of this single gene is not a perfect predictor of future AD, the APOE e4 allele is associated with earlier onset of AD [23]. A recent study in individuals with mild cognitive impairment suggests that there may also be a relationship between APOE ε4 carrier status and gait impairments [24]. In that study, individuals with mild cognitive impairment were more likely to exhibit worsening gait variability over one year of follow-up if they were also APOE ε4 carriers [24]. One purpose of our line of research is to better elucidate the role of APOE ε4 in the pre-symptomatic development of AD by examining dual-tasking interference patterns, according to APOE ε4 status.
The objective of the current study was to conduct a proof-of-concept study to investigate how performance on a gait-cognition dual-task protocol differs between cognitively normal older adults with and without an APOE ε4 allele. Our working hypothesis was that individuals with an APOE ε4 allele who are at higher risk of future AD—but currently asymptomatic—would experience greater dual-task interference compared to a lower risk group. In this preliminary study, we sought to characterize the magnitude and direction of effects. Consistent findings as predicted would then justify and aid in the design of a formal hypothesis-testing study with maximum conventionally accepted levels of Types I and II error.
METHODS
Study sample
The Duke Alzheimer’s Disease Prevention Registry is maintained by the Joseph and Kathleen Bryan Alzheimer’s Disease Research Center (Bryan ADRC) and includes about 4,300 volunteers between the ages 55–95 years who enlisted in the Registry because of their interest in being involved in studies that lead to preventive therapies for AD [25]. The PREPARE Cohort was drawn from the Registry and enrolled 1,399 cognitively healthy, community-dwelling registrants. To date, 1,294 PREPARE Cohort participants have provided blood/DNA samples. From this pool of participants, we sought individuals who were ≤72 years of age and were cognitively normal as defined by their performance on cognitive testing within the last 12 months, including: 1) ≥4 items correct on the delayed recall trial of the CERAD Word List [26, 27] and 2) a Montreal Cognitive Assessment (MoCA) score ≥25 [28]. We excluded individuals who endorsed subjective cognitive complaints. Participants were also required to be right-handed due to additional protocols, not presented here, that involved functional brain imaging. Screening interviews confirmed that all participants were willing and able to walk on a flat surface without using a mobility aid or assistance from another person and that they had not fallen in the last month.
Participants and investigators were masked to genotypes and genetic risk status of the study participants. One data manager at the Bryan ADRC maintained identifiable genetic data in conjunction with a remote coordinating laboratory. In order to recruit for the present study while ensuring that investigators, participants, and all study staff who interacted with participants remained masked to registrants’ genetic status, the data manager applied a recruitment rubric to identify individuals who met the above inclusion criteria and were either APOE ε4 carriers (ε3/ε4 or ε2/ε4) or lower risk genotypes (ε2/ε2, ε2/ε3, or ε3/ε3). Risk assignment was also supported by genotypic information at TOMM40, an allele that provides further AD risk information [29, 30]. Specifically, TOMM40 information was used to identify APOE ε3/ε3 individuals for the low risk group and to identify APOE ε3/ε4 individuals for the high risk group. The recruitment rubric called for up to 15 individuals in each risk group, with balancing of gender and race across risk groups. In total, 40 individuals were referred by the Bryan ADRC; six were excluded when more in-depth screening revealed that they did not meet inclusion criteria (e.g., participants or medical chart revealed subjective concerns about cognition) and four individuals declined. Prior to analysis, one participant was excluded when it was determined on data review that she did not meet genotype requirements for either group (she was APOE e3/e3 but with high risk TOMM40). The study protocol was approved by the Duke Institutional Review Board, and all participants provided informed consent.
Dual-task protocol
Dual-task cognition-gait paradigms include a gait task (e.g., walking) and a cognitive task (e.g., item recall), with each type of task performed separately, and then concurrently. This paradigm allows the experimenter to compare a participant’s performance on a given task in the single-task condition to performance on the same task in the dual-task condition [6, 31]. Dual-task interference occurs when the performance of a particular task in the dual-task condition is worse than performance on that task in the singletask condition. Because our goal was to compare performance differences between genetic risk groups (and study staff were masked to group status), all tasks were administered in the same order to each participant. First, each participant was instructed to ambulate at his or her usual pace on an electronic mat (gait task, single condition); next, the participant performed a memory task and then an executive function (EF) task in a seated position (cognitive tasks, single condition). Finally, the participant completed the cognitive tasks (memory task, then EF task) while ambulating on the mat (dual-task condition). The protocols for the gait task, the memory task, and the EF task are described below.
Gait measurements
The portable GAITRite® system (CIR Systems, Inc., Franklin, NJ) features an electronic, 2-foot × 20-foot mat [32]. When a person traverses the mat, the pressure-activated sensors measure the temporal (timing) and spatial (two-dimensional geometric position) parameters of each step. The software generates an automated report with calculated parameters. Participants were instructed to walk at a usual, comfortable pace. Participants began walking at a “starting line” which was two meters away from the mat, to allow participants to achieve steady state walking prior to stepping onto the mat. No practice trial occurred for the gait task. For the first 8 participants, gait parameters were calculated from three passes over the GAITRite® mat, obtained while the participants walked in a circular path that included the mat. Thereafter, due to a change in the system software, gait parameters could only be calculated from the first pass over the mat. However, in the dual-task condition participants continued walking in a circular route alongside the mat until the cognitive task was completed. The number of steps per pass across the mat ranged from 4 to 9, depending on the participant’s stride.
The gait parameters of interest in this protocol were gait speed and step length. Gait speed (m/s) is defined as the ambulation distance in meters divided by the ambulation time. Step length (cm) is defined as the distance from heel point of the current footfall to the heel point of the previous footfall on the opposite foot. We focused on these parameters for two reasons. First, prior studies suggest that individuals with AD exhibit dual-task cost to these metrics, with slowing of gait speed and shortening of step length while dual-tasking [33, 34]. Second, both gait speed and step length can be measured in an office setting without sophisticated technical equipment, which is ideal for widespread clinical utility, if dualtask protocols prove useful as early indicators of AD risk. Additionally, whereas some gait parameters require longer course lengths for reliable estimates, gait speed and cadence can be reliably estimated from shorter distances [35, 36], with gait speed commonly and reliably estimated in practice from courses 4 to 6 meters (~13 to 20 feet) in length [36].
Cognitive measurements
Memory task
The memory task includes an encoding portion, in which the participant is introduced to a set of words, and a recall portion, in which the participant recites the words from memory. In the encoding portion of the memory task, participants were shown words (one word at a time, displayed in large font in a notebook). The participant, seated in a chair, spoke each word out loud before being presented with the next word. The encoding portion of the memory task was performed in the same manner (with the participant seated) in both the single-task and dual-task condition. The task then requires the participant to recall all the words that he/she can remember. In the single-task condition, participants remained comfortably seated while recalling words; in the dual-task condition, participants recalled words while walking. In the dual-task condition, immediately after the last word cue was spoken out loud by the participant, the participant was asked to stand up and move to the start line for the gait task. The participant was instructed to walk and list recalled words while walking.
To standardize the 15-item word lists recalled by each participant, we used the word lists from the Rey Auditory Verbal Learning Test [37], with Form 1 used for the single-task condition and Form 2 used for dual-task condition. Before the single-task test was administered, the experimenter administered one practice trial to demonstrate the memory task procedure to participants. The practice trial included 5 unique words, and the participant remained seated for encoding and recall. The primary memory variable is accuracy, or percent of words correctly recalled. Response times were not collected for the memory task and a time limit was not imposed.
Executive function task
We administered the “Stop/Go” task, an executive control task from the Brief Test of Adult Cognition by Telephone (BTACT) [38]. This task tests attentional and inhibitory control and uses auditory cues, so it can be administered while the participant ambulates. The participant was cued with the word “red” or “green” and must respond with the word “stop” or “go.” Participants were first instructed to respond normally (red cues stop, green cues go) and then in reverse (red cues go, green cues stop). Participants were instructed to be as accurate as possible but respond as quickly as they can. First, instructions and practice trials were conducted while the participant was comfortably seated. For practice, participants responded to 5 to 7 trials in the EFnormal condition and were required to respond correctly to at least 4 trials in order to move on to practice the EFreverse condition. Participants practiced with 5 to 7 trials in the EFreverse condition and were required to respond correctly to at least four before moving on to the testing phase. The test included 20 trials in the normal condition (EFnormal) and 20 trials in the reverse (EFreverse) condition. The task was digitally recorded and each response time (RT) (the interval between the beginning of a word cue and the beginning of the correct verbal response) was measured in milliseconds (ms) by a single rater using Audacity® software version 2.1.3. In the single-task condition, the cues were administered while the participant was comfortably seated; in the dual-task condition, the cues were administered while the participant walked. From the starting line, the participant began walking when the first word cue was given. The experimenter walked behind the participant in a non-pacing position to administer word cues; participant and experimenter continued in a circular path alongside the GAITRite mat until each task (20 trials) was completed. Accuracy was >97% for this task in all conditions, so the variable of interest is average RT (after exclusion of any incorrect trials).
Statistical analysis
Descriptive statistics were used to characterize the cohort. To compare variables of interest in the high risk versus low risk group, t tests were employed for continuous variables and chi-squared tests were employed for dichotomous variables. Data were examined to ensure that assumptions of normality were met.
Our main objective was to examine group differences in dual-task interference. To assess interference when performing dual-tasks, we examined both the absolute difference (dual-task score − singletask score) and dual-task effect (DTE), expressed as a percentage (([dual-task score − single-task score]/single-task score) × 100%) [6]. When reporting DTE values for the RT variables (for which higher values indicate worse performance), the sign of the calculated DTE value was reversed, so that the meaning of positive and negative DTE values would be consistent across variables: positive DTE values always indicate that performance improved in the dual-task condition, whereas negative DTE values indicate performance worsened in the dualtask condition. We calculated absolute difference and DTE values for the following parameters: gait speed (for both EF and memory tasks), step length (for both EF and memory tasks), memory task accuracy, EF task response time in the normal condition, and EF task response time in the reverse condition.
We calculated the effect sizes (comparing high risk group to low risk group) for the absolute difference and the DTE of each variable of interest. Effect size is calculated as follows: (mean of group 1 − mean of group 2)/pooled standard deviation. We used these estimated effect sizes to calculate the sample size that would be needed to achieve >80% power to detect a statistically significant (p < 0.05, 2-sided) difference between groups. All statistical analyses were performed with SAS version 9.4.
Following recommendations for the presentation of patterns of dual-task interference [6, 39], we created a performance operating characteristic graph. Performance operating characteristic graphs plot each participant’s calculated DTEs to simultaneously illustrate DTE on task 1 (x axis) and DTE on task 2 (y axis). The center of each axis is set to DTE = zero, such that an observation at the center of the graph would indicate that performance on both tasks was equal in the single-task and dual-task condition. These representations are used to display resource allocation strategies of individual participants under dual-task conditions. For example, an observation of positive DTE on task 1 and negative DTE on task 2 would represent an individual whose performance on task 1 improved in the dual-task condition, while performance on task 2 declined, suggesting that the individual had prioritized task 1 over task 2 when the tasks were performed concurrently. On the performance operating characteristic graph we constructed, observations from the high risk group were red and observations from the low risk group were blue, in order to highlight potential group differences in allocation strategies.
RESULTS
Participant characteristics
The low risk genetic group included one individual with APOE ε2/ε2 genotype, nine individuals with APOE ε2/ε3 genotype, and five individuals with APOE ε3/ε3 genotype. The high risk genetic group (APOE ε4 carriers) included 11 individuals with APOE ε3/ε4 genotype and three individuals with APOE ε2/ε4 genotype. The ADPR is racially diverse, and 24% (7 of 29) participants in the current study identified as African-American. Almost 80% of participants were female and over 90% were college-educated. The two risk groups did not differ with respect to age, race, sex, proportion with a college degree, or MoCA score. Participant characteristics are summarized in Table 1.
Table 1:
Characteristics of the study population among low and high risk groups
| Characteristics | Low Risk Group N=15 |
High Risk Group N=14 |
p |
|---|---|---|---|
| Age in years, Mean (SD) | 66.7 (2.5) | 65.1 (4.0) | 0.22 |
| Sex (N, % male) | 3 (20.0) | 3 (21.4) | 0.99 |
| Race (N, % White) | 10 (66.7) | 12 (85.7) | 0.39 |
| Education (N, % completed college) | 13 (86.7) | 14 (100.0) | 0.48 |
| MoCA Score, Mean (SD) | 27.5 (1.6) | 28.3 (1.5) | 0.19 |
Risk group determined by genetic risk for Alzheimer’s disease; all individuals in the high risk group carry an APOE ε4 allele. MoCA, Montreal Cognitive Assessment. Central tendencies of age, education, and MoCA score were compared with t tests, and chi-squared tests were employed to compare sex and race.
Gait and cognitive measures in single-task and dual-task conditions
Mean gait and cognitive performance of each group, under single-task and dual-task conditions, are shown in Table 2. The two genetic risk groups performed similarly with respect to all measures of cognition and walking performed as single tasks. In both the high and low risk groups, mean performance worsened in the dual-task condition compared to the single-task condition; in other words, mean values of gait speed, step length, and accuracy are smaller in the dual-task condition, and mean response times are longer.
Table 2:
Gait and cognitive performance among genetic low and high risk (APOE ε4 carriers) groups under single-task and dual-task conditions
| Parameters | Low Risk Group Single-Task Mean (SD) N=15 |
Low Risk Group Dual-Task Mean (SD) N=15 |
High Risk Group Single-Task Mean (SD) N=14 |
High Risk Group Dual-Task Mean (SD) N=14 |
|---|---|---|---|---|
| Gait Parameters | ||||
| Gait Speed (m/s) | 1.10 (0.15) | 0.97 (0.21) | 1.17 (0.21) | 0.91 (0.18) |
| Step Length (cm) | 62.2 (7.3) | 58.4 (7.8) | 64.5 (10.2) | 56.2 (6.2) |
| Cognitive Parameters | ||||
| Memory (Accuracy) | 47.1 (12.2) | 40.0 (11.0) | 46.2 (10.6) | 42.9 (7.7) |
| EF Task-normal RT (ms) | 675.8 (83.3) | 729.4 (63.8) | 704.5 (90.9) | 727.1 (100.9) |
| EF Task-reverse RT (ms) | 755.7 (126.4) | 797.6 (128.8) | 784.1 (92.6) | 816.6 (123.1) |
SD, standard deviation; Accuracy, percent of words correctly recalled from the list of 15; EF, executive function task (Go/Stop Test), values reflect response time; RT, response time.
Group differences in cognitive interference
The mean reduction in cognitive scores seen with ambulation was relatively small. For example, in both high risk and low risk groups, the absolute difference between single-task and dual-task condition on the executive function test mean RTs was 50 ms or less (Table 3). The effect sizes for group differences in cognitive interference ranged from 0.09 to 0.47. Among the three cognitive variables, the largest effect sizes for cognitive interference were seen with the EFnormal task.
Table 3:
Dual-task interference on cognition among genetic low and high risk (APOE ε4 carriers) groups
| Cognitive Parameters | Absolute Difference |
Dual-Task Effect (DTEc) |
||||
|---|---|---|---|---|---|---|
| Low Risk Group N=15 Mean (SD) |
High Risk Group N=14 Mean (SD) |
Effect Size for Group Difference | Low Risk Group N=15 Mean (SD) |
High Risk Group N=14 Mean (SD) |
Effect Size for Group Difference | |
| Memory (Accuracy) | 7.1 (14.1) | 3.3 (9.3) | 0.31 | −11% (27%) | −4% (20%) | 0.30 |
| EF Task-normal RT (ms) | 50 (70) | 20 (70) | 0.45 | −9% (12%) | −4% (9%) | 0.47 |
| EF task-reverse RT (ms) | 40 (110) | 30 (100) | 0.09 | −7% (18%) | −4% (13%) | 0.16 |
Absolute difference, difference between parameter in single-task and dual-task condition; DTEc, Dual-Task Effect on cognition: [(dual-task − single-task)/single-task] × 100%. A negative sign precedes the formula when the variable of interest is response time because greater values of response time indicate worse performance; SD, standard deviation; Accuracy, percent of words correctly recalled from the list of 15; RT, response time; EF, executive function task (the go/stop test); values reflect response time.
Group differences in gait interference
The participants in the genetic high risk group exhibited greater mean dual-task interference on gait, compared to participants in the low risk group. Table 4 summarizes the degree of gait interference when walking is performed in conjunction with the three different cognitive tasks. The largest effect sizes for group difference in gait interference are seen with the EFnormal task, and these effect sizes range from 0.62 to 0.70. Although both risk groups exhibited slower mean gait speed in the dual-task compared to the single-task condition, larger mean reductions were observed among participants in the high risk group, compared to the low risk group. On average, the APOE ε4 carriers slowed their gate speed by 0.26 ± 0.20 m/s when performing the EFnormal task, as compared to the genetic low risk group change of 0.13 ± 0.14 m/s in gait speed (Fig. 1A). For reference, changes in gait speed of at least 0.1 to 0.2 m/s are considered clinically important across multiple patient groups [40]. In the high risk group (compared to low risk), average DTE values were more negative (indicating slower gait speed in the dual-task, compared to single-task, condition), and the range of dual-task effects on gait speed was wider (Fig. 1B). In other words, APOE ε4 carriers were more heterogeneous than non-carriers with regard to the degree of dualtask gait interference they exhibited.
Table 4:
Dual-task interference on gait among genetic low risk and high risk (APOE ε4 carriers) groups
| Gait Parameters | Absolute Difference |
Dual-Task Effect (DTEg) |
||||
|---|---|---|---|---|---|---|
| Low Risk Group N=15 Mean (SD) |
High Risk Group N=14 Mean (SD) |
Effect Size for Group Difference | Low Risk Group N=15 Mean (SD) |
High Risk Group N=14 Mean (SD) |
Effect Size for Group Difference | |
| Cognitive Task = Memory | ||||||
| Gait Speed (m/s) | 0.35 (0.24) | 0.40 (0.16) | 0.24 | −32% (22%) | −34% (13%) | 0.12 |
| Step Length (cm) | 8.9 (7.1) | 11.5 (5.2) | 0.40 | −14% (11%) | −18% (7%) | 0.36 |
| Cognitive Task = EFnormal | ||||||
| Gait Speed (m/s) | 0.13 (0.14) | 0.26 (0.20) | 0.70 | −12% (14%) | −21% (14%) | 0.62 |
| Step Length (cm) | 3.8 (4.7) | 8.3 (7.7) | 0.69 | −6% (8%) | −12% (10%) | 0.64 |
| Cognitive Task = EFreverse | ||||||
| Gait Speed (m/s) | 0.10 (0.17) | 0.19 (0.18) | 0.48 | −10% (16%) | −15% (13%) | 0.36 |
| Step Length (cm) | 3.2 (5.5) | 6.3 (6.3) | 0.52 | −5% (9%) | −9% (8%) | 0.45 |
Absolute difference, difference between parameter in single-task and dual-task condition; DTEg, Dual-Task Effect on gait: [(dual-task − single-task)/single-task] × 100%; SD, standard deviation.
Fig. 1.
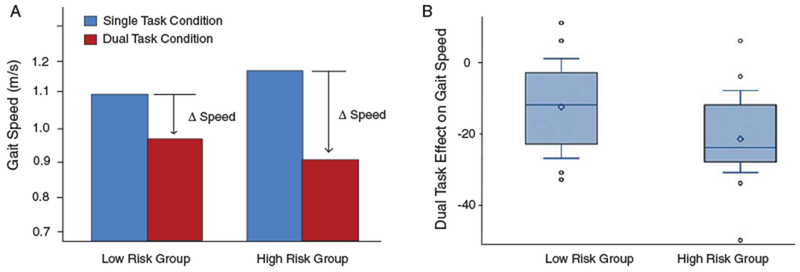
Dual-task interference on gait speed among cognitively normal older adults at low versus high genetic risk of AD. A) The bar graph illustrates mean gait speed of each group in the single-task (blue) and dual-task (red) condition. Both risk groups exhibited slower mean gait speed during the dual-task condition, but participants in the high risk group exhibited larger mean reductions in gait speed, compared to the low risk group. B) The dual-task effect (DTE) on gait speed is calculated as: [(dual-task gait speed − single-task gait speed)/single-task gait speed] × 100%. This box plot displays the range of DTE values calculated for all participants.
Sample size estimates to detect significant group differences in dual-task interference
Based on the effect sizes displayed in Tables 3 and 4, we estimated the sample size that would be required to achieve 80% power to detect significant differences (alpha error <0.05, two-sided) between high risk and low risk groups in dual-task interference. Assuming a conservative effect size of 0.6 for gait interference during the EFnormal task (Table 4), adequate power would be achieved with 45 individuals per genetic risk group. Assuming an effect size for cognitive interference of 0.4 (based on the EFnormal task data in Table 3), an adequately powered study would require 176 participants per group to detect such a difference. None of the group differences observed in the pilot study, which was under-powered, were statistically significant.
Patterns of cognitive versus gait dual-task interference: performance operating characteristic
Figure 2 presents the performance operating characteristic graph for the dual-task protocol that combined walking with the EFnormal task (the cognitive task that yielded the largest effect sizes for a group difference in dual-task interference). In this plot, each participant in the genetic high risk group (APOE ε4 carriers) is represented in red; each participant in the genetic low risk group is represented in blue. As shown in Fig. 2, most participants had negative DTE values for both the gait task and the cognitive task, such that 15 of 29 observations are in the lower left quadrant. This quadrant is termed “mutual interference,” meaning that these individuals had worse performance on both motor and cognitive tasks in the dual-task condition. Four observations appear in the lower right quadrant indicating four individuals (three low risk, one high risk) who exhibited “gait priority trade-off,” meaning that gait speed was better but cognitive performance was worse in the dual-task, compared to single-task, condition. In contrast, seven observations are in the upper left quadrant, indicating seven individuals (two low risk, five high risk) who exhibited “cognitive priority tradeoff,” meaning that cognitive performance was better while gait speed was worse in the dual-task condition. The improvements that these individuals exhibited in cognitive RT during the dual-task condition was minimal, as no DTE-cognitive values were larger than 10%. In other words, any improvements in cognition from single- to dual-task conditions represented changes of 10% or less. Three individuals (one low risk, two high risk) exhibited DTE-cognitive values very close to zero, whereas DTE-gait values are negative, meaning gait speed worsened in the dual-task while cognitive performance was stable. This pattern has been termed “cognitive-related gait interference [6].” No individuals in this study exhibited mutual facilitation, a situation in which both task performances improve under dual-task conditions.
Fig. 2.
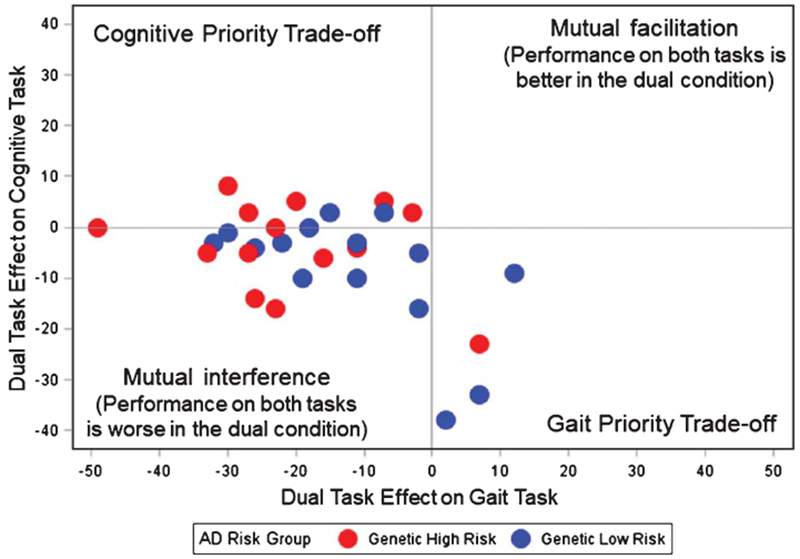
Patterns of dual-task interference on gait and cognition among participants. The scatter plot displays each individual’s dual-task effect (DTE) for the gait task (gait speed) and for the cognitive task (response time during the normal executive function [EFnormal] task). DTE, calculated as [(dual-task value − single-task value)/single-task value] × 100%, equals zero when an individual’s performance on a given task is the same in the single-task and dual-task conditions. Positive DTE values indicate performance was better in the dual-task condition; negative DTE values indicate performance was worse in the dual-task condition. Participants in the genetic high risk group (APOE ε4 carriers) are represented in red; participants in the genetic low risk group are represented in blue. Gait priority trade-offs (gait speed improves during dual-task, while cognitive performance declines) were more often seen among low risk participants, whereas cognitive priority trade-offs (cognitive performance improves, while gait speed declines) were more often seen among high risk participants.
DISCUSSION
In this study, cognitively normal individuals with an APOE ε4 allele tended to exhibit greater dualtask interference with walking and cognitive tasks, compared to a comparable group of individuals without an APOE ε4 allele. Dual-task interference was consistently observed in the expected direction: the genetic high risk group tended to exhibit greater declines in performance in the dual-task condition, as compared to the low risk group, across all the measures. The largest effect sizes were seen when walking was combined with an executive function task, and the effect sizes for gait interference were larger (0.62–0.70) than the observed effect sizes for cognitive interference (0.30 to 0.47). Our results are consistent with a recent study which reported dualtask cost to gait (but not cognition) among cognitively normal individuals with cerebral Aβ, compared to those without this AD biomarker [19]. Our study lends further support to the notion that gait-cognition dual-task paradigms have the potential to evoke a phenotype in individuals who are in early stages of AD yet are asymptomatic under more traditional testing conditions.
This proof-of-concept study was based on the premise that more individuals in the genetic high risk group, compared to the low risk group, are in preclinical stages of AD and that poor performance with dual-tasking is associated with underlying AD. However, an alternative possibility is that APOE ε4 status affects dual-task performance, independent of the development of AD pathology. Indeed, previous research suggests that APOE ε4 status may play a role in age-related changes in motor function. Two studies in community-dwelling older adults found that presence of an APOE ε4 allele predicted faster declines in gait speed [41] and motor function [42], even after adjusting for cognitive status and health factors. Another study reported that the relationship between cerebral Aβ burden and gait speed in non-demented individuals was rendered non-significant after accounting for APOE gene status [43]. Understanding how APOE genotype may influence the ability of dual-task paradigms to predict dementia merits additional study. Longitudinal research in participants with mild cognitive impairment suggests that dual-task gait interference is predictive of those who will go on to develop dementia [18] and that APOE ε4 status may also predict progression of their cognitive decline and gait impairments [24]. Longitudinal research in cognitively intact adults is needed to determine whether gait interference during cognition-motor dual-tasking may predict future AD diagnoses in this population, and whether the ability of dualtask protocols to predict future phenotypes differs by genotype. Both genotype and gait interference can be feasibly observed in asymptomatic individuals, and future studies should test whether these two markers yield interactive or additive prognostic value for AD.
Despite widespread interest in the use of dual-task methodology to examine the interplay between gait and cognition, the precise neural mechanisms that underlie dual-task interference are unknown, with two major theories in existence [6]. The serial bottleneck model proposes that only one information processing operation can occur at a time, giving rise to a decrement in function when two tasks are attempted simultaneously [44]. The capacity sharing model assumes that multiple tasks can be accomplished in parallel, but the neural capacity to accomplish any number of tasks is a limited resource [45]. In either model, when multiple tasks are conducted simultaneously, the individual may need to employ conscious or subconscious strategies to allocate resources—sequentially or in parallel—to the different tasks. One intriguing possibility is that preferred strategies for accomplishing dual-tasks may change over time and may be altered by disease states or rehabilitation. For example, one review noted that during inpatient rehabilitation after an acute stroke event, patients tend to exhibit mutual interference (or dualtask costs to gait speed and cognition), while patients examined several months post-stroke exhibited cognitive-related motor interference (preserved cognitive performance, worsened gait) [7]. Motor interference during cognitive tasks has also been described in individuals with Parkinson’s disease and AD [31].
Our findings are consistent with previous literature in that AD-related dual-task costs to gait speed are relatively larger than AD-related dual-task costs to cognition [18, 31]. It remains to be determined whether patterns of cognitive-motor interference may shift as the disease progresses from its subclinical to more advanced stages, and whether such patterns may have diagnostic value. Although our study is too small to be definitive, the results in Fig. 2 show that while most participants exhibited mutual interference, when trade-offs did occur, low risk participants were more likely to prioritize gait (at a cost to cognition), whereas high risk participants were more likely to prioritize cognition (at a cost to gait). Future research is needed to examine whether gait priority during dual-tasking may be a good prognostic indicator with respect to AD risk, while cognitive priority (preserved or improved cognitive performance, while gait speed suffers) may be a worrisome risk factor for AD.
Another unanswered question is the degree to which gait/cognition priority patterns are modifiable across the AD spectrum, and whether rehabilitation to modify dual-task trade-offs may benefit patients by improving clinically meaningful outcomes such as cognitive trajectories or falls. One study reported that older adults with mild dementia who participated in an 8-week physical exercise/cognitive stimulation rehabilitation program had similar single-task gait speed before and after the intervention, but significant improvement in dual-task cost to gait speed [16]. This effect was more pronounced among participants who also demonstrated objective improvements in cognitive assessment scores, leading the authors to conclude that the shift toward greater prioritization of gait during the dual-task condition may be driven by cognitive improvements and more strategic allocation of resources across the two tasks.
Another important question for future research is to identify which cognitive tasks are best suited to elicit group differences in dual-task interference while walking. The effect sizes reported here suggest that the EFnormal task was superior to the memory task or the more difficult EFreverse in its ability to distinguish between groups based on dual-task interference. A recent meta-analysis noted that the AD-related gait interference is most pronounced when the cognitive tasks involve internal interfering factors (e.g., mental tracking) rather than external interfering factors (e.g., reaction time to simple cue) [31]. The authors posit that cognitive tasks that rely on internal interfering factors may involve more complex neural networks, which are more likely to be inter-linked with neural networks involved in gait control. It is possible that such tasks are more likely to disturb gait because they draw on shared and limited resources to a greater extent than tasks that rely on external interfering factors, which entail more simple, bottom-up stimuli recognition. However, the meta-analysis demonstrated that tasks associated with internal interfering factors (mental tracking, verbal fluency, and working memory) elicited gait interference in both healthy participants and participants with neurodegenerative disorders [31]. It remains to be determined which type of cognitive tasks should be incorporated in dualtask protocols when the goal is to discern between healthy adults and those harboring early neurodegenerative disease. In the study that examined cognitively normal adults with and without cerebral Aβ, group differences were detected in gait speed across a variety of cognitive tasks: working memory, go/no-go response inhibition, motor sequencing with hand gestures, and dialing a phone [19].
Our study has several strengths. To our knowledge, no previous study has investigated the relationship between genetic risk status and dual-task performance in cognitively normal older adults. We enhanced the precision of risk group assignment by utilizing TOMM40 information to supplement group assignments based on the APOE gene. Participants and investigators were masked to genetic status. We used recent cognitive testing, medical record review, and subject interviews to confirm that individuals in both risk groups were cognitively intact and free of subjective cognitive symptoms. We utilized the GAITRite® equipment to obtain more accurate measurement of gait parameters, although specialized equipment is not necessary to measure gait speed and step length in the clinical setting.
Our study also has limitations that should be taken into account in the interpretation of findings. A main limitation is the small sample size of this pilot study, which means that we lack the statistical power to explicitly test our hypothesis. Second, aside from genetic status, we do not have information from participants about other biomarkers for AD (e.g., brain imaging markers, cerebrospinal fluid markers) or how the groups differed on the basis of other important health factors that may have confounded our results. For example, if our high risk group happened to be less mobile or have more comorbid conditions (such as stroke) compared to our low risk group, these factors could have driven the interference patterns we observed. Additionally, information about other biomarkers of subclinical AD may have helped to further risk stratify or understand the variability in dual-task interference observed in both groups. Third, due to an unexpected change in the software capabilities of the electronic mat used to collect our gait parameters, we were forced to adjust our gait task protocol during the study. From the first 8 participants, gait speed and step length were averaged from three passes across the 20-foot mat, whereas the subsequent 21 participants had these parameters estimated from a single pass across the mat. Prior research suggests that reliable estimates of usual gait speed can be obtained with courses shorter than 20 feet [35, 36]. Participants from both risk groups were run during the time periods before and after the protocol was adjusted, further decreasing the likelihood that main results were affected by the mid-study protocol change. Finally, our cross-sectional study does not allow us to make inferences about whether dualtask interference may be predictive of future AD risk.
Although this pilot study is too small to demonstrate conclusive differences in dual-task effects between individuals with and without an APOE ε4 allele, the findings justify future research to determine how dual-task protocols may predict future cognitive and motor trajectories in people without obvious motor or cognitive symptoms. The observed effect sizes are consistently in the hypothesized direction, with APOE ε4 carriers exhibiting more interference than non-carriers during dual-tasking. Furthermore, the APOE ε4 carriers demonstrated a tendency to prioritize cognitive performance over gait performance in the dual-task condition. Further study is needed to determine whether patterns of cognitive-motor interference in dual-tasking paradigms can be useful in early detection of AD, and which dual-task protocols are best able to discern individuals with early AD from their peers.
ACKNOWLEDGMENTS
This study was supported by the Duke Claude D. Pepper Older Americans Independence Center (NIA P30AG028716-11S1) and the Joseph and Kathleen Bryan Alzheimer’s Disease Research Center. Dr. Whitson’s effort and intellectual contributions to the study were further supported by R01AG043438 and UH2AG056925.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0016r2).
REFERENCES
- [1].Xu J, Murphy SL, Kochanek KD, Bastian BA (2016) Deaths: Final data for 2013. Natl Vital Stat Rep 64, 1–119. [PubMed] [Google Scholar]
- [2].James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA (2014) Contribution of Alzheimer disease to mortality in the United States. Neurology 82, 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Oh H, Jagust WJ (2013) Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta-amyloid. J Neurosci 33, 18425–18437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oh H, Steffener J, Razlighi QR, Habeck C, Stern Y (2016) beta-Amyloid deposition is associated with decreased right prefrontal activation during task switching among cognitively normal elderly. J Neurosci 36, 1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Della Sala S, Baddeley A, Papagno C, Spinnler H (1995) Dual-task paradigm: A means to examine the central executive. Ann N Y Acad Sci 769, 161–171. [DOI] [PubMed] [Google Scholar]
- [6].Plummer P, Eskes G (2015) Measuring treatment effects on dual-task performance: A framework for research and clinical practice. Front Hum Neurosci 9, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Plummer P, Eskes G, Wallace S, Giuffrida C, Fraas M, Campbell G, Clifton K, Skidmore ER, American Congress of Rehabilitation Medicine Stroke Networking Group Cognition Task Force (2013) Cognitive-motor interference during functional mobility after stroke: State of the science and implications for future research. Arch Phys Med Rehabil 94, 2565–2574 e2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, Black SE, Camicioli R, Carlson MC, Ferrucci L, Guralnik JM, Hausdorff JM, Kaye J, Launer LJ, Lipsitz LA, Verghese J, Rosano C (2013) Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci 68, 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA, Boxer AL, Buchman AS, Cruickshanks KJ, Devanand DP, Duffy CJ, Gall CM, Gates GA, Granholm AC, Hensch T, Holtzer R, Hyman BT, Lin FR, McKee AC, Morris JC, Petersen RC, Silbert LC, Struble RG, Trojanowski JQ, Verghese J, Wilson DA, Xu S, Zhang LI (2015) At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement 11, 70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Plummer-D’Amato P, Altmann LJ, Reilly K (2011) Dualtask effects of spontaneous speech and executive function on gait in aging: Exaggerated effects in slow walkers. Gait Posture 33, 233–237. [DOI] [PubMed] [Google Scholar]
- [11].Plummer-D’Amato P, Altmann LJ, Saracino D, Fox E, Behrman AL, Marsiske M (2008) Interactions between cognitive tasks and gait after stroke: A dual task study. Gait Posture 27, 683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kelly VE, Eusterbrock AJ, Shumway-Cook A (2012) A review of dual-task walking deficits in people with Parkinson’s disease: Motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis 2012, 918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Camicioli R, Howieson D, Lehman S, Kaye J (1997) Talking while walking: The effect of a dual task in aging and Alzheimer’s disease. Neurology 48, 955–958. [DOI] [PubMed] [Google Scholar]
- [14].Hofheinz M, Schusterschitz C (2010) Dual task interference in estimating the risk of falls and measuring change: A comparative, psychometric study of four measurements. Clin Rehabil 24, 831–842. [DOI] [PubMed] [Google Scholar]
- [15].Muir-Hunter SW, Wittwer JE (2016) Dual-task testing to predict falls in community-dwelling older adults: A systematic review. Physiotherapy 102, 29–40. [DOI] [PubMed] [Google Scholar]
- [16].Tay L, Lim WS, Chan M, Ali N, Chong MS (2016) A Combined Cognitive Stimulation and Physical Exercise Programme (MINDVital) in early dementia: Differential effects on single- and dual-task gait performance. Gerontology 62, 604–610. [DOI] [PubMed] [Google Scholar]
- [17].Liu YC, Yang YR, Tsai YA, Wang RY (2017) Cognitive and motor dual task gait training improve dual task gait performance after stroke - A randomized controlled pilot trial. Sci Rep 7, 4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, Borrie MJ, Hachinski VC, Wells J, Riccio PM, Schapira M, Sejdic E, Camicioli RM, Bartha R, McIlroy WE, Muir-Hunter S (2017) Association of dual-task gait with incident dementia in mild cognitive impairment: Results from the Gait and Brain Study. JAMA Neurol 74, 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nadkarni NK, Lopez OL, Perera S, Studenski SA, Snitz BE, Erickson KI, Mathis CA, Nebes RD, Redfern M, Klunk WE (2017) Cerebral amyloid deposition and dual-tasking in cognitively normal, mobility unimpaired older adults. J Gerontol A Biol Sci Med Sci 72, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marcus C, Mena E, Subramaniam RM (2014) Brain PET in the diagnosis of Alzheimer’s disease. Clin Nucl Med 39, e413–422; quiz e423-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–1356. [PubMed] [Google Scholar]
- [23].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- [24].Sakurai R, Montero-Odasso M (2017) Apolipoprotein E4 allele and gait performance in mild cognitive impairment: Results from the Gait and Brain Study. J Gerontol A Biol Sci Med Sci 72, 1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Romero HR, Welsh-Bohmer KA, Gwyther LP, Edmonds HL, Plassman BL, Germain CM, McCart M, Hayden KM, Pieper C, Roses AD (2014) Community engagement in diverse populations for Alzheimer disease prevention trials. Alzheimer Dis Assoc Disord 28, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fillenbaum GG, Heyman A, Huber MS, Ganguli M, Unverzagt FW (2001) Performance of elderly African American and White community residents on the CERAD Neuropsychological Battery. J Int Neuropsychol Soc 7, 502–509. [DOI] [PubMed] [Google Scholar]
- [27].Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A (1994) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 44, 609–614. [DOI] [PubMed] [Google Scholar]
- [28].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: Abrief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [29].Lutz MW, Crenshaw DG, Saunders AM, Roses AD (2010) Genetic variation at a single locus and age of onset for Alzheimer’s disease. Alzheimers Dement 6, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lutz MW, Crenshaw D, Welsh-Bohmer KA, Burns DK, Roses AD (2016) New genetic approaches to AD: Lessons from APOE-TOMM40 phylogenetics. Curr Neurol Neurosci Rep 16, 48. [DOI] [PubMed] [Google Scholar]
- [31].Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J (2011) Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci Biobehav Rev 35, 715–728. [DOI] [PubMed] [Google Scholar]
- [32].Bilney B, Morris M, Webster K (2003) Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 17, 68–74. [DOI] [PubMed] [Google Scholar]
- [33].Cedervall Y, Halvorsen K, Aberg AC (2014) A longitudinal study of gait function and characteristics of gait disturbance in individuals with Alzheimer’s disease. Gait Posture 39, 1022–1027. [DOI] [PubMed] [Google Scholar]
- [34].Maquet D, Lekeu F, Warzee E, Gillain S, Wojtasik V, Salmon E, Petermans J, Croisier JL (2010) Gait analysis in elderly adult patients with mild cognitive impairment and patients with mild Alzheimer’s disease: Simple versus dual task: A preliminary report. Clin Physiol Funct Imaging 30, 51–56. [DOI] [PubMed] [Google Scholar]
- [35].Hollman JH, Childs KB, McNeil ML, Mueller AC, Quilter CM, Youdas JW (2010) Number of strides required for reliable measurements of pace, rhythm and variability parameters of gait during normal and dual task walking in older individuals. Gait Posture 32, 23–28. [DOI] [PubMed] [Google Scholar]
- [36].Kim HJ, Park I, Lee HJ, Lee O (2016) The reliability and validity of gait speed with different walking pace and distances against general health, physical function, and chronic disease in aged adults. J Exerc Nutrition Biochem 20, 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hawkins KA, Dean D, Pearlson GD (2004) Alternative forms of the Rey Auditory Verbal Learning Test: A review. Behav Neurol 15, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tun PA, Lachman ME (2006) Telephone assessment of cognitive function in adulthood: The Brief Test of Adult Cognition by Telephone. Age Ageing 35, 629–632. [DOI] [PubMed] [Google Scholar]
- [39].Granholm E, Marder SR, Asarnow RF (1996) Dual-task performance operating characteristics, resource limitations, and automatic processing in schizophrenia. Neuropsychology 10, 11–21. [Google Scholar]
- [40].Bohannon RW, Glenney SS (2014) Minimal clinically important difference for change in comfortable gait speed of adults with pathology: A systematic review. J Eval Clin Pract 20, 295–300. [DOI] [PubMed] [Google Scholar]
- [41].Verghese J, Holtzer R, Wang C, Katz MJ, Barzilai N, Lipton RB (2013) Role of APOE genotype in gait decline and disability in aging. J Gerontol A Biol Sci Med Sci 68, 1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA (2009) Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord 23, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nadkarni NK, Perera S, Snitz BE, Mathis CA, Price J, Williamson JD, DeKosky ST, Klunk WE, Lopez OL (2017) Association of brain amyloid-beta with slow gait in elderly individuals without dementia: Influence of cognition and apolipoprotein E epsilon4 genotype. JAMA Neurol 74, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pashler H (1994) Dual-task interference in simple tasks: Data and theory. Psychol Bull 116, 220–244. [DOI] [PubMed] [Google Scholar]
- [45].Tombu M, Jolicoeur P (2005) Testing the predictions of the central capacity sharing model. J Exp Psychol Hum Percept Perform 31, 790–802. [DOI] [PubMed] [Google Scholar]


