Abstract
The initial discovery that ob/ob mice become obese because of a recessive mutation of the leptin gene has been crucial to discover the melanocortin pathway to control appetite. In the melanocortin pathway, the fed state is signaled by abundance of circulating hormones such as leptin and insulin, which bind to receptors expressed at the surface of pro-opiomelanocortin (POMC) neurons to promote processing of POMC to the mature hormone α-melanocyte-stimulating hormone (α-MSH). α-MSH released by the POMC neurons then signals to decrease energy intake by binding to melanocortin-4 receptor (MC4R) expressed by MC4R neurons to the paraventricular nucleus (PVN). Conversely, in the “starved state” activity of agouti related neuropeptide (AgRP) and of neuropeptide Y (NPY)-expressing neurons is increased by decreased levels of circulating leptin and insulin and by the orexigenic hormone ghrelin to promote food intake. This initial understanding of the melanocortin pathway has recently been implemented by the description of the complex neuronal circuit that controls the activity of POMC, AgRP/NPY, and MC4R neurons and downstream signaling by these neurons. This review summarizes the progress done on the melanocortin pathway and describes how obesity alters this pathway to disrupt energy homeostasis. We also describe progress on how leptin and insulin receptors signal in POMC neurons, how MC4R signals, and how altered expression and traffic of MC4R change the acute signaling and desensitization properties of the receptor. We also describe how the discovery of the melanocortin pathway has led to the use of melanocortin agonists to treat obesity derived from genetic disorders.
Keywords: Melanocortin, Leptin, Hypothalamus, MC4R, AgRP, appetite
Introduction
In the melanocortin system, hormones of the “fed state” such as leptin and insulin, released in the bloodstream by adipocytes and by the β-cells of the pancreas, respectively, cross the blood-brain barrier to bind to leptin and insulin receptors on the surface of pro-opiomelanocortin (POMC) neurons to promote processing of POMC to the mature hormone α-melanocyte-stimulating hormone (α-MSH), which signals to decrease energy intake (Andermann and Lowell 2017; Cone 2006; Gautron, et al. 2015; Ghamari-Langroudi, et al. 2011; Morton, et al. 2014). In the fed state, leptin also binds to leptin receptors to inhibit secretion of agouti related neuropeptide (AgRP) and of neuropeptide Y (NPY) expressed by AgRP/NPY neurons. Conversely, in the “starved state” AgRP/NPY neuron activity is increased by decreased circulating leptin and insulin and by the orexigenic hormone ghrelin. Both POMC and AgRP/NPY neurons have their cell bodies in the arcuate nucleus of the hypothalamus and their axons project to the paraventricular nucleus (PVN). In the melanocortin pathway to regulate feeding, hypothalamic POMC neurons receive inhibitory signals from cholinergic neurons localized to the dorsomedial hypothalamus (DMH) that project to the arcuate nucleus (Jeong, et al. 2017), as well as from excitatory glutamatergic signals from steroidogenic factor (SF-1)-expressing neurons localized to the ventromedial hypothalamus (Konner and Bruning 2012). To control feeding, AGRP/NPY neurons project in addition to the PVN, to other brain regions such as the stria terminalis (BNST), the para-ventricular nucleus of the thalamus (PVT) and the lateral hypothalamus (LH) (Betley, et al. 2013). In the PVN, α-MSH released by POMC neurons interacts with melanocortin-4 receptor (MC4R) expressed by MC4R neurons to stabilize the receptor in an active conformation, with Gαq-mediated increase of intracellular calcium and decrease in food intake. AgRP antagonizes effects by α-MSH and also acts as inverse agonist to inhibit the constitutive activity of MC4R taking place in absence of agonist. MC4R neurons localized to the brainstem and to the spinal cord signals by Gs-dependent increase of intracellular cAMP to increase energy expenditure (Li, et al. 2016). MC4R also signals by inducing, in a G-protein independent manner, closure and opening, respectively, of the inwardly rectifying potassium channel, Kir7, thus modulating the firing activity of PVN neurons (Ghamari-Langroudi, et al. 2015). In this review, we will describe the role of POMC, AgRP and MC4R/MC3R neurons in the melanocortin system to control appetite and weight as well therapeutic implications.
POMC neurons localized in the hypothalamus and hindbrain are essential for energy homeostasis
POMC neurons localized to the hypothalamus and to the hindbrain are essential for energy homeostasis (Caron, et al. 2018; Dores, et al. 2016; Gautron et al. 2015; Mercer, et al. 2013; Toda, et al. 2017) (Fig.1). In POMC neurons, the POMC gene encodes a precursor polypeptide that undergoes cell-specific proteolytic cleavage to generate α-MSH (Toda et al. 2017). Mutations of POMC gene in both mice and humans lead to hyperphagia and obesity (Challis, et al. 2004; Muller, et al. 2016; Yaswen, et al. 1999). Injury to POMC neurons such as that derived by ablation of the mitochondrial protein mitofusin 2 or that by increased secretion of tumor necrosis factor alpha (TNFα) from microglia in obesity also leads to disrupted energy balance with increased food intake (Schneeberger, et al. 2013; Yi, et al. 2017). Consistent with a role for POMC neurons in energy balance, postnatal ablation of POMC neurons in mice induces an obese phenotype. However in mice with postnatal ablation of POMC neurons, obesity is unrelated to food intake and instead dependent on decreased energy expenditure (Greenman, et al. 2013). POMC neuron activity is regulated by other neurons. In this respect, under fasting conditions, POMC neurons receive inhibitory inputs from AgRP/NPY neurons (Fig.1) (Cone 2006; Cowley, et al. 2001; Horvath, et al. 1992; Pinto, et al. 2004). Other inhibitory inputs originate from noradrenergic neurons localized to the locus coeruleus and the hindbrain that send projections to the hypothalamus (Bouret and Richmond 2015; Varazzani, et al. 2015). Hypothalamic POMC neurons also receive inhibitory signals from cholinergic neurons localized to the dorsomedial hypothalamus (DMH) that project to the arcuate nucleus (Jeong, et al. 2017). The activity of hypothalamic POMC neurons is also modulated by sex hormones. In this respect, POMC neurons in the arcuate nucleus are responsive to estradiol administration to reduce food intake and body weight (Steyn, et al. 2018).
Fig. 1. POMC neurons in the melanocortin system.
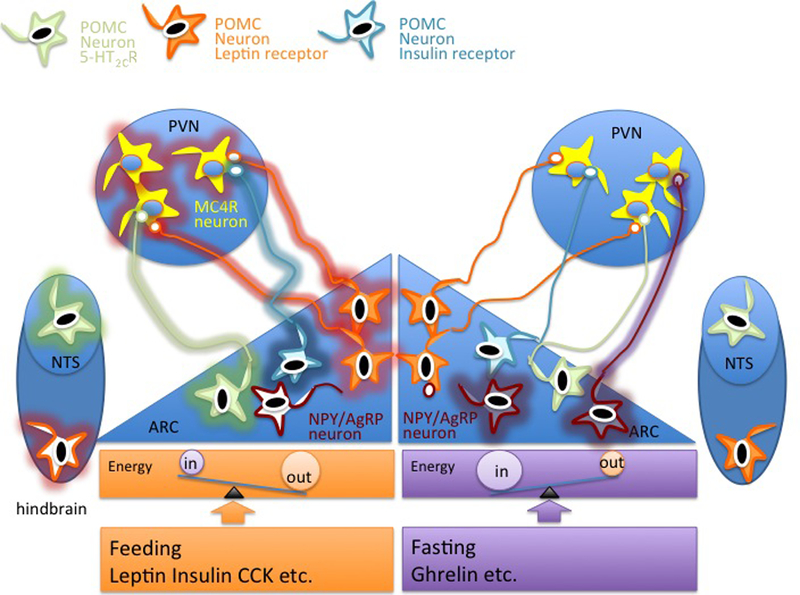
In the fed state, the signal to stop eating and to increase energy expenditure is conveyed by leptin and insulin released in the bloodstream by adipocytes and by the β-cells of the pancreas,respectively. These hormones cross the blood-brain barrier to reach the arcuate nucleus (ARC) of the hypothalamus and promote firing (indicated by glow around the cell perimeter) of distinct populations of POMC neurons expressing the LepR and insulin receptor. Other populations of POMC neurons in the arcuate nucleus and in the nucleus of the solitary tract (NTS) express the serotonin receptor 5-HT2CR. POMC neurons project to the paraventricular nucleus (PVN) to increase activity of MC4R neurons to decrease food intake and to increase energy expenditure. In the fasted state, POMC neurons in the arcuate nucleus are inhibited by decreased circulating leptin and insulin and by increased activation of AgRP/NPY neurons, which send inhibitory signals to reduce firing of POMC neurons and of MC4R neurons. References are in the main text.
POMC neurons and leptin signaling
POMC neurons are activated by leptin, a peptide hormone secreted by adipocytes, and more efficiently so by subcutaneous fat rather than by the omental fat and in a manner proportional to adipocyte size (Masuzaki, et al. 1995; Van Harmelen, et al. 1998). A pioneering discovery in the control of energy balance was the finding that mutations in the ob/ob mice and db/db mice induce obesity (Coleman 1973, 1978). It was later found that the ob/ob mice become obese because of a recessive mutation of the leptin gene (Zhang, et al. 1994). Also patients with leptin deficiency due to mutations of the leptin gene are obese. Although such leptin deficiency is rare in the population, it provides evidence that the hormone is essential for energy homeostasis in humans (Clement, et al. 1998; Montague, et al. 1997). Additional evidence that leptin is essential for energy homeostasis is the finding that delivery of leptin to mice and to humans with leptin deficiency corrects obesity (Farooqi, et al. 1999; Halaas, et al. 1995; Pelleymounter, et al. 1995). The identification of the leptin receptor (LepR) stemmed from the observation that iodinated leptin binds to the brain choroid plexus (Devos, et al. 1996). The LepR was cloned from a cDNA expression library derived from the murine choroid plexus and found to belong to the IL-6 receptor family (Tartaglia, et al. 1995). However, both obese db/db mice and fatty Zucker rats, which have elevated circulating leptin, have normal binding of leptin in the choroid plexus, comparable to that of lean rodents (Devos et al. 1996; Halaas et al. 1995). It was later discovered that the LepR has multiple splice forms and that the db/db mice and fatty Zucker rats become obese because of mutations which affects the intracellular domain of the long form of receptor, LepR, which is expressed in the hypothalamus including the POMC neurons (Chen, et al. 1996; Cheung, et al. 1997; Chua, et al. 1996; Iida, et al. 1996; Lee, et al. 1996; Phillips, et al. 1996; Takaya, et al. 1996). Conversely, the short form of LepR, expressed in the choroid plexus, can bind to leptin, but cannot signal (Bjorbaek, et al. 1997; Ghilardi and Skoda 1997).
Administration of leptin to brain slices induces depolarization of POMC neurons and reduces inhibition of POMC neurons by the AgRP/NPY neurons (Cowley et al. 2001). Deletion of LepR specifically in POMC neurons by using the Cre/Lox system finds that expression of LepR in these neurons is essential to body weight homeostasis (Balthasar, et al. 2004). Interestingly, it has been recently found that POMC neurons expressing LepR are required for the fasting-induced fall in leptin levels (Caron et al. 2018). LepR signals through multiple pathways (Fig. 2A and B)(Flak and Myers 2016; Toda et al. 2017; Wauman, et al. 2017). During leptin signaling, LepR, expressed at the plasma membrane as a dimer, activates receptor-associated Janus kinase 2 (JAK2) to phosphorylate LepR at Tyr1138, which then binds to signal transducer and activator of transcription 3 (Stat3). Stat3 is then phosphorylated by JAK2 to function as transcription factor (Bahrenberg, et al. 2002; Banks, et al. 2000; Bjorbaek et al. 1997; Ghilardi and Skoda 1997; Li and Friedman 1999). Activation of Stat3 by LepR, is essential to control food intake (Bates, et al. 2003; Buettner, et al. 2006; Cui, et al. 2004; Gao, et al. 2004; Zhang and Scarpace 2009). Binding of leptin to LepR also leads to downstream activation of Rho-kinase 1 (ROCK1), which phosphorylates and activates JAK2 in a pathway that is essential for leptin signaling to maintain energy homeostasis (Huang, et al. 2012). Binding of leptin to LepR also leads to JAK2 interaction with SH2-Bβ, which in turn promotes Insulin Receptor Substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase (PI3K) pathway (Anderwald, et al. 2002; Carvalheira, et al. 2003; Duan, et al. 2004; Kellerer, et al. 1997; Kim, et al. 2000; Li, et al. 2007; Wauman et al. 2017). The PI3K signaling pathway is essential for leptin-induced depolarization and firing of POMC neurons (Hill, et al. 2008; Kwon, et al. 2016). PI3K pathway also promotes phosphorylation and translocation of forkhead box protein O1 (FOXO1) from the nucleus to the cytosol, an effect that promotes transcription of POMC and increased expression of carboxypeptidase with increased processing of POMC to α-MSH, and suppression of food intake (Kim, et al. 2006; Kwon et al. 2016; Plum, et al. 2009)
Fig. 2. POMC neurons express leptin, insulin and serotonin receptors.
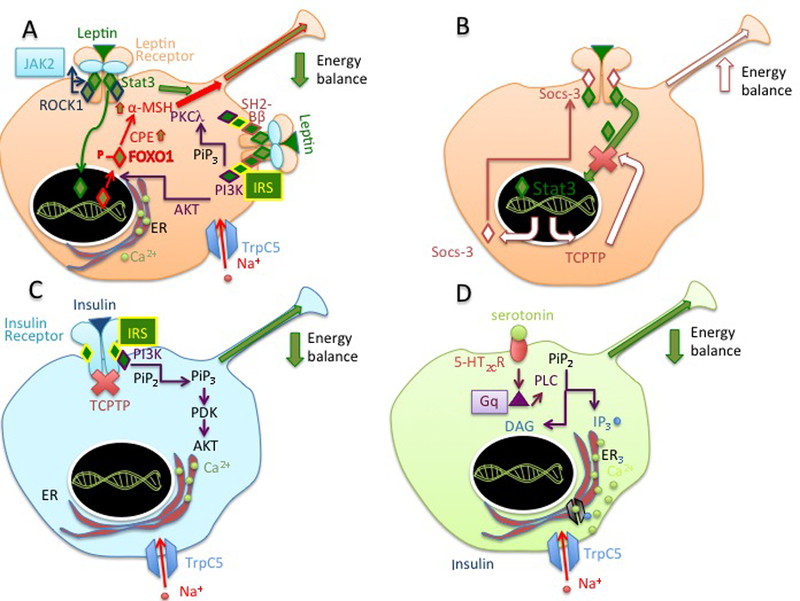
A) In the fed state, leptin bound to LepR expressed by POMC neurons in the arcuate nucleus and release of α-MSH hormone by multiple pathways initiated by activation of JAK2, a process that involves LepR-dependent activation of ROCK1. In one pathway initiated by JAK2, the kinase phosphorylates STAT3 to function as transcription factor. STAT3 promotes expression of the polypeptide POMC and processing of the pro-hormone to α-MSH. Binding of leptin to LepR induces another JAK2-dependent pathway where SH2-Bβ and IRS1 are recruited to activate the PI3K pathway. PI3K generates PIP3 from PIP2 at the plasma membrane. PIP3 recruits and activates of PKC including the atypical PKCλ. PI3K signaling leads to opening of TrpC5 to allow inward flux of Na+ and neuronal firing. PI3K pathway also promotes phosphorylation and translocation of FOXO1 from the nucleus to the cytosol to promote transcription of POMC, increased processing of POMC to α-MSH, and suppression of food intake B). Stat3 induces expression of factors involved in feed-back inhibitory pathways, such as that of Socs-3, which binds to LepR to inhibit receptor signaling. Stat 3 also induces expression of protein phosphatases such as TCPTP to terminate LepR to inhibit receptor signaling. C) In other populations of POMC neurons, the insulin receptor signals through PI3 kinase pathway to induce flux of Na+ into the cell through TrpC5 and neuronal firing. D) A population of POMC neurons expresses the GPCR 5-HT2CR. Binding of serotonin to HT2CR induces Gq-dependent activation of PLC, generation of increased intracellular IP3 and Ca2+, and opening of TrpC5 to allow Na+ into the cell and neuronal firing. Heterogeneity of POMC neurons expressing insulin, leptin and serotonin receptor is indicated by drawing cells expressing these receptors with different colors. References are in the main text.
The PI3K signaling pathway to control food intake and weight gain includes the atypical protein kinase C λ (aPKC λ) (Dorfman, et al. 2017b). An effect by leptin is to induce POMC neuron depolarization through a cation channel (Cowley et al. 2001). In this respect, leptin-dependent signaling through the PI3K pathway activates the transient receptor potential cation 5 (TrpC5) and this effect is essential to decrease food intake and increase energy expenditure (Gao, et al. 2017; Qiu, et al. 2014). The binding of leptin to LepR induces phosphorylation of the receptor also at Tyr985, which controls phosphorylation of the protein tyrosine phosphatase SHP-2, downstream activation of extracellular-regulated kinases-1/2 (ERK1/2), and increased expression of c-Fos (Banks et al. 2000; Bjorbak, et al. 2000; Li and Friedman 1999). An effect of STAT3 activation by the occupied LepR is to initiate the feed back inhibitory pathway to induce expression of suppressor of cytokine signaling-3 (Socs3) mRNA (Fig. 2B). Socs3 inhibits LepR signaling by binding to LepR Tyr985 (Bjorbak et al. 2000; Eyckerman, et al. 2000). Socs3 expression may contribute to increased food intake during pregnancy (Zampieri, et al. 2016). Other mechanisms to inhibit leptin signaling in feed back inhibitory pathway take place by dephosphorylation of LepR by the protein-tyrosine phosphatases 1B (PTP1B) and the STAT-1 and STAT-3 phosphatase T cell protein tyrosine phosphatase (TCPTP) (Loh, et al. 2011; Tsou, et al. 2012; White, et al. 2009; Zabolotny, et al. 2002). It has been recently proposed that leptin and insulin act on hypothalamic POMC neurons to increase energy expenditure by a pathway that involves PTP1B, and TCPTP and leads to increased browning of white adipose tissue (Dodd, et al. 2015; Zhang, et al. 2015). While deletion of LepR in POMC neurons has mild effects on body weight (Balthasar et al. 2004), deletion of LepR in large populations of hypothalamic regions produces profound obesity and metabolic dysfunction (Rupp, et al. 2018). It is likely that LepR expressed in other neuronal populations outside of the hypothalamus contribute to energy homeostasis. LepR is expressed by neurons localized to the hindbrain and to the brainstem (Barnes, et al. 2010). LepR expressed in these brain regions contributes to energy homeostasis by controlling meal size (Kanoski, et al. 2012) and mediates counter-regulatory responses to hypoglycemia during starvation (Flak and Myers 2016; Flak, et al. 2014). POMC neurons in the lateral hypothalamus, brainstem, and hindbrain express glucose transporter type 2 (Glut2). In these POMC neurons outside of the arcuate nucleus, Glut2-dependent glucose sensing functions to control thermoregulation by increasing leptin sensitivity (Mounien, et al. 2010). LepR expressed by non-neuronal cells such as the microglia is also implicated in control of energy homeostasis (Gao, et al. 2018).
POMC neuron and signaling in response to feeding, insulin, and serotonin.
Insulin functions to regulate energy homeostasis in POMC neurons by signaling through receptors expressed in a different population of hypothalamic POMC neurons than that expressing LepR (Williams, et al. 2010) (Fig. 2A and C). In the hypothalamus, insulin induces the association of PI3K with IRS-2, to promote Ser473 phosphorylation of Akt (Dodd and Tiganis 2017; Haeusler, et al. 2018; Torsoni, et al. 2003). Brain IRS-2 signaling functions in energy homeostasis (Taguchi, et al. 2007) and IRS-2 signaling in POMC neurons controls blood pressure and heart rate (do Carmo, et al. 2016). Insulin signal in POMC neurons suppresses appetite by a pathway that is similar to that of leptin because it also involves activation of PI3K (Xu, et al. 2005b) (Hill et al. 2008) (Al-Qassab, et al. 2009) and opening of TrpC5 with inward flow of Na+ (Qiu, et al. 2018b).
Feeding modulates termination of insulin signaling in POMC neurons. The protein phosphatase TCPTP dephosphorylates the insulin receptor and attenuates insulin signaling (Tiganis 2013). Feeding, by decreasing abundance of TCPTP, suppresses termination of insulin signal in POMC neurons. Conversely, fasting, by increasing TCPTP, promotes the termination of insulin signaling in in POMC neurons (Dodd, et al. 2018b). Thus, nutritional status modulates insulin responsiveness in POMC neurons. With this respect, hypothalamic POMC neurons are glucose sensitive and increase their firing rate in response to increased extracellular glucose concentration by a mechanism that involves increased ATP/ADP ratio, closure of K+ ATP channels, and cell depolarization (Ibrahim, et al. 2003; Parton, et al. 2007). Feeding is associated with firing of POMC neurons, an effect paralleled by increased generation of reactive oxygen species (ROS) in these neurons (Andrews, et al. 2008; Diano, et al. 2011; Horvath, et al. 2009). Moreover, central delivery of reactive oxygen species (ROS) promotes firing by POMC neurons and suppression of ROS formation in POMC neurons inhibits their activity (Diano et al. 2011). Therefore, nutrient sensing in POMC neurons appears to be mediated by changes in ROS generation. Generation of ROS takes place prevalently in mitochondria, which undergo fission/fusion cycles in POMC neurons, depending on availability of nutrients (Dietrich, et al. 2013; Schneeberger et al. 2013; Toda, et al. 2016). Dynamin-related protein (Drp1) functions in mitochondrial fission (Nasrallah and Horvath 2014). In response to feeding, the activation of Drp1 is decreased, resulting in increased mitochondrial size, increased generation of ROS, and neuronal activation (Santoro, et al. 2017). These data indicate that changes in mitochondrial size in POMC neurons modulate neuronal activation by altering generation of ROS. Another population of hypothalamic POMC neurons that regulates both energy and glucose homeostasis has been found to express the serotonin (5-HT) receptor 2C receptor (5-HT2CR), which signals to induce activation of TrpC5 and of the mammalian target of rapamycin (mTOR) pathway (Fig. 2D) (Barone, et al. 2018; Berglund, et al. 2013; Churruca, et al. 2008; Gao et al. 2017; Lam, et al., 2010; Lam, et al. 2011; Lam, et al. 2008; Sohn and Williams 2012; Sohn, et al. 2011). Consistent with the concept that different POMC neuron populations express LepR, insulin receptor and 5-HT2CR, single cell RNA sequencing analysis indicates that the population of POMC neurons residing in the arcuate nucleus is highly heterogeneous (Lam, et al. 2017). Interestingly, estradiol increases the excitability of POMC neurons by increasing the efficacy by which insulin activates canonical TrpC5 channels (Qiu, et al. 2018a). In the face of insulin and leptin affecting only a subpopulation of POMC neurons, noradrenaline instead decreases the activity of the POMC neurons through signaling by the α2A adrenergic receptor in a large population of POMC neurons, perhaps to promote food intake in response to challenges that require energy (Paeger, et al. 2017a).. In addition to the POMC neurons of the hypothalamus, other POMC neurons expressing 5-HT2CR may contribute to regulate appetite. In this respect, POMC neurons responsive to 5-HT2CR agonist localized to the Nucleus of the Solitary Tract (NTS) control food intake (D’Agostino, et al. 2018).
AgRP/NPY neurons promote feeding and function in adapted behavior under starvation
AgRP was originally identified as a peptide expressed by neurons in the mediobasal hypothalamus, which acts as antagonist of MC4R and of another member of the melanocortin receptor family expressed in brain, MC3R (Ellacott and Cone 2004). Ubiquitous expression of AgRP in transgenic mice induces obesity (Ollmann, et al. 1997). In the hypothalamus, AgRP-expressing neurons co-express NPY and respond to orexigenic and anorexigenic signals from the periphery to regulate feeding (Broberger, et al. 1998; Cowley, et al. 1999; Hahn, et al. 1998; van den Top, et al. 2004). Central delivery of AgRP induces increased feeding (Joppa, et al. 2007). Food deprivation induces increased expression of NPY and AgRP mRNA in the AgRP/NPY neurons, while re-feeding restores levels of these peptides (Swart, et al. 2002). In humans, abundance of AgRP and NPY correlates with body mass index (Alkemade, et al. 2012). Food deprivation increases activity of AgRP/NPY neurons (Takahashi and Cone 2005). Nutrients are necessary and sufficient for the reduction of AgRP/NPY neuron activity and this effect is proportional to the amount of calories being obtained (Betley et al. 2013; Chen, et al. 2015b; Mandelblat-Cerf, et al. 2015; Su, et al. 2017). AgRP/NPY neurons can also control feeding under conditions of appetite suppression (Padilla, et al. 2016). The activity of AgRP/NPY neurons is modulated within the brain by excitatory neurons that originate from the PVN and express thyrotropin-releasing hormone (TRH)(Krashes, et al. 2014), by excitatory glutamatergic inputs (Liu, et al. 2012), and by post-synaptic AMPK-dependent synaptogenesis and spinogenesis (Kong, et al. 2016).
Projections from the AgRP/NPY neurons converge with those from POMC neurons to MC4R neurons in the PVN to integrate control food intake and energy expenditure (Fig. 3) (Aponte, et al. 2011; Atasoy, et al. 2014; Atasoy, et al. 2012; Cowley et al. 1999; Cowley et al. 2001). AgRP/NPY neurons project also to POMC neurons of the arcuate nucleus and to neurons localized to the dorsomedial nucleus of the hypothalamus and to the rostral telencephalon and to the pons to control feeding (Bagnol, et al. 1999; Broberger et al. 1998; Haskell-Luevano, et al. 1999; Legradi and Lechan 1999; Singru, et al. 2006, 2007). AgRP/NPY neurons also project to BNST and to the lateral hypothalamic area to control, in addition to feeding, also insulin sensitivity in brown adipose tissue (Steculorum, et al. 2016) (Fig.3). Under starvation, projections of AgRP/NPY neuron to the BNST, to the medial nucleus of the amygdala (MeA) control adapted behavior such as modulation of aggression, fear and exploration to find food by releasing NPY (Betley et al. 2013; Burnett, et al. 2016; Dietrich, et al. 2015; Padilla et al. 2016). During hunger, AgRP neuron projections to the PBN inhibit inflammatory pain by signaling through NPY (Alhadeff, et al. 2018). During satiety, activation of AgRP neurons mimics hunger by a pathway to the insular cortex via the paraventricular thalamus (PVT) and basolateral amygdala (Livneh, et al. 2017).
Fig. 3. AgRP/NPY neurons drive food intake.
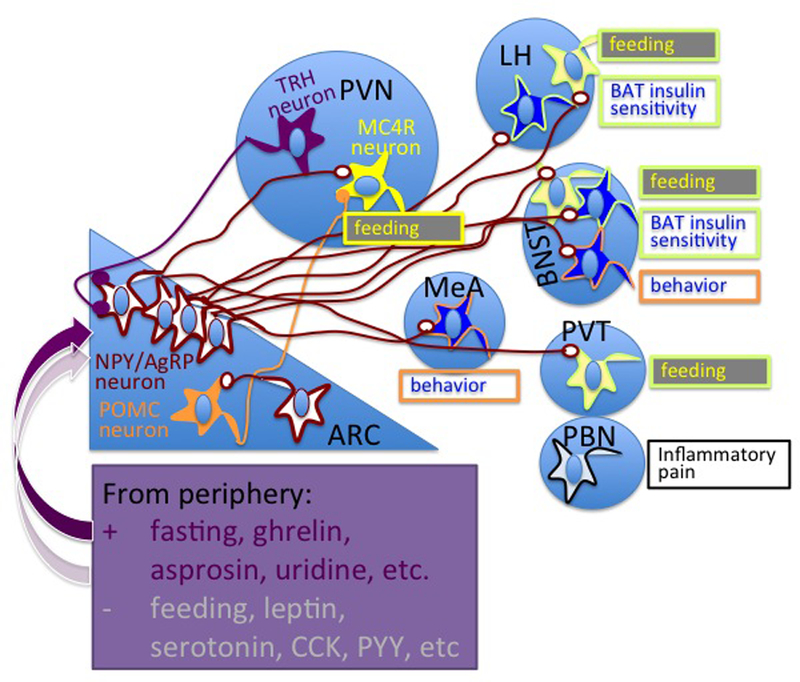
Fasting and circulating hormones released by the stomach induce activity of AgRP/NPY neurons localized to the arcuate nucleus (ARC) of the hypothalamus. To promote feeding, subpopulations of AgRP/NPY neurons send projections to: the paraventricular nucleus of hypothalamus (PVN), to synapse with MC4R neurons; and to neurons in the lateral hypothalamus (LH), bed nucleus of the stria terminalis (BNST) and the para-ventricular nucleus of the thalamus (PVT). Other projections to neurons in LH, medial amygdala (MeA), LH, and parabrachial nuclei control insulin sensitivity in brown adipose tissue (BAT) and suppress inflammatory pain in hunger condition. “Behavior” refers to behavior induced by the nutritional status of the organism such as modulation of aggression, fear and exploration to find food. adapted behavior such as modulation of aggression, fear and exploration to find food References are in the main text.
Fast and slow signals to induce feeding originate from subpopulations of arcuate nucleus AgRP/NPY neurons and by GABAergic neurons of the lateral hypothalamus
Specific activation of AgRP/NPY neurons by photostimulation of channelrhodopsin-2 evokes a voracious feeding response within minutes (Aponte et al. 2011). Subpopulations of AgRP/NPY neurons appear to be sufficient to promote food intake and do so by redundant pathways (Betley et al. 2013). In addition to AgRP and NPY, AgRP/NPY neurons release GABA, which is essential to energy homeostasis (Krashes, et al. 2013; Tong, et al. 2008; Wu and Palmiter 2011). Feeding responses evoked by designer receptors exclusively activated by designer drugs (DREADD) technology activation indicate that NPY and GABA released by AgRP/NPY neurons, convey fast signals to induce feeding, while AgRP instead induces feeding with effects that are delayed and more prolonged as compared to those by NPY and GABA (Krashes, et al. 2011; Krashes, et al. 2016; Krashes et al. 2013). Ablation of AgRP neurons results in starvation and activation of neurons in brain regions innervated by AgRP neurons (Wu, et al. 2008). Conversely, experiments where the AgRP/NPY neurons were ablated by making them selectively sensitive to diphtheria toxin or by expression of a neurotoxic form of ataxin-3, indicate that AgRP/NPY neurons are essential to control feeding in the adult mice, but not in neonatal mice, and thus suggesting compensatory pathways to promote food intake (Bewick, et al. 2005; Gropp, et al. 2005; Luquet, et al. 2005; Tan, et al. 2014). Similarly, mice where a Cre-lox strategy was used to induce progressive degeneration of hypothalamic neurons that express AgRP are normal, again suggesting existence of compensatory pathways (Xu, et al. 2005a). AgRP-null mice instead have normal food intake, body weight, and energy expenditure with reduced body weight after 6 months of age (Wortley, et al. 2005). Also transgenic mice with AgRP and NPY double-knockout have normal body weight and feeding response to starvation (Qian, et al. 2002), suggesting the existence of compensatory mechanisms to regulate energy homeostasis. In the hypothalamus other GABAergic neurons, besides the AgRP/NPY neurons contribute to food intake. In this respect, feeding can also be induced by the GABAergic neurons localized to the lateral hypothalamus and projecting to the PVN (Mangieri, et al. 2018)
Different roles of AgRP/NPY neurons in homeostatic and hedonic feeding
In the brain, multiple pathways function to control food intake, of which one is homeostatic feeding and the other is non-homeostatic, hedonic feeding (Pandit, et al. 2013; Sternson 2016). The brain reward circuit includes many brain areas, such as the ventral tegmental area (VTA), the nucleus accumbens, the lateral hypothalamus, the amygdala, the striatum and the prefrontal cortex (Pandit, et al. 2011; Pandit et al. 2013). In mice, AgRP/NPY neurons transmit negative valence signals (Betley, et al. 2015). When mice are fed normal chow, food caloric intake is the main parameter to decrease the activity of AgRP/NPY neuron activity and limit food intake (Su et al. 2017). Conversely, when mice are instead fed a highly palatable diet, AgRP/NPY neurons become dispensable for feeding and other neural circuits sensitive to emotion and stress take place to control food intake (Denis, et al. 2015).
Signaling in AgRP/NPY neurons
It is well established that AgRP/NPY neurons are activated by fasting and by ghrelin and are inhibited by leptin and by feeding (Hashiguchi, et al. 2017; Mani, et al. 2018; Pinto et al. 2004; van den Top et al. 2004; Yang, et al. 2011). However, the mechanism by which fasting promotes AgRP/NPY activation is yet to be completely understood. Ghrelin is a peptide hormone secreted by the stomach under conditions of food deprivation that stimulates food intake and adiposity (Kim, et al. 2003; Nakazato, et al. 2001; Shaw, et al. 2005; Toshinai, et al. 2001; Tschop, et al. 2000; Wren, et al. 2001; Wren, et al. 2000; Yang et al. 2011). AgRP neurons express the ghrelin receptor, also named growth hormone secretagogue receptor (GHsr), to mediate orexigenic as well as glucoregulatory actions of ghrelin (Mani, et al. 2017; Wang, et al. 2014; Zigman, et al. 2006). Experiments using brain slices indicate that GHsr is essential for ghrelin response to increase firing activity of AgRP/NPY neurons (Chen, et al. 2017b). Ghrelin induces activation of AgRP neurons by a mechanism that is dependent on mitochondrial uncoupling protein 2 (UCP2) and involves proliferation of mitochondria and increased mitochondrial respiration (Andrews et al. 2008; Diano 2013; Horvath et al. 2009). Such ghrelin dependent activation of AgRP/NPY neurons is driven by increased β-oxidation of fatty acids, which takes place by a mechanism that involves activation of 5’ AMP-activated protein kinase (AMPK). Active AMPK inhibits of Acetyl-CoA carboxylase (ACC) activity resulting in decreased production of malonyl CoA and increased fatty acid transport into the mitochondrial matrix by brain-specific carnitine palmitoyltransferase-1c (CPT-1c)(Horvath et al. 2009; Obici, et al. 2003; Obici and Rossetti 2003; Price, et al. 2002). Increased fatty acid oxidation is paralleled by increased mitochondrial respiration, with increased generation of reactive oxygen species (ROS) and ROS quenching by UCP2 (Andrews et al. 2008). These data indicate that mitochondrial function is essential for ghrelin signaling to reduce appetite. However, the function mitochondrial CPT-1c appears more complex than to promote food intake by ghrelin signaling in the AgRP/NPY neurons. This is because CPT-1c KO mice, while leaner than control mice, gain more weight upon exposure to high fat diet than control mice, without increasing their food intake (Wolfgang, et al. 2008; Wolfgang, et al. 2006). The role of ghrelin to control energy homeostasis is not yet completely understood because mice with loss of function mutations of the ghrelin system do not have altered body weight but, instead, altered glucose metabolism and insulin sensitivity (De Smet, et al. 2006; Mani and Zigman 2017; McFarlane, et al. 2014; Sun, et al. 2003; Sun, et al. 2008). Other hormones, such as leptin and insulin, may control activity of AgRP/NPY neurons in feeding and metabolism. In this respect, leptin signals to reduce food intake by inducing exclusion of Foxo1 from the nucleus of AgRP/NPY neurons and downstream expression of a purinergic GPCR, Gpr17 (Kitamura, et al. 2006; Ren, et al. 2015; Ren, et al. 2012). Insulin signaling also takes place in AgRP/NPY neurons to decrease their activity by inducing neuronal hyperpolarization. These effects control hepatic glucose production (Konner, et al. 2007). Insulin signaling in AgRP/NPY neurons is suppressed by TCPTP. Fasting induces TCPTP in AgRP/NPY neurons (Dodd, et al. 2018a). Feeding instead promotes the degradation of TCPTP. Reduced TCPTP enhances AgRP/NPY neuron insulin sensitivity and promotes downstream effects such as repression of hepatic gluconeogenesis (Dodd et al. 2018a). In addition to ghrelin, leptin and insulin, other factors such as serotonin, cholecystokinin (CCK), and peptide YY (PYY), released by the gastrointestinal tract in the general circulation, mediate effects of feeding to inhibit AgRP/NPY neurons (Beutler, et al. 2017). It has been found that asprosin, a hormone released by adipocytes under fasting conditions, in addition to stimulating glucose output from the liver (Romere, et al. 2016), also crosses the blood-brain-barrier to activate the AgRP/NPY neurons via a cAMP-dependent pathway and to inhibit POMC neuron activity by a pathway that involves the release of GABA by the AgRP/NPY neurons (Duerrschmid, et al. 2017). It has been recently discovered that, during fasting, plasma levels of the pyrimidine nucleoside uridine is increased, and that such elevated plasma uridine is required for the changes in thermoregulation and glucose metabolism that occur during fasting (Deng, et al. 2017). In the brain, extracellular uridine is converted to UTP (Ipata, et al. 2010). Interestingly, UDP activates AgRP/ NPY neurons by a pathway that is initiated by purinergic receptor 6 (P2Y6) signaling (Steculorum, et al. 2015). Thus the uridine and uridine nucleotides may be novel regulators of body metabolism and function of AgRP/NPY neurons. In addition to AgRP/NpY neurons, ghrelin promotes activation of somatostatin neurons in the hypothalamic tuberal nucleus to promote feeding by inhibiting downstream neurons in the PVN and to the BNST (Luo, et al. 2018).
MC4R neurons
MC4R is a member of a family of melanocortin G-protein-coupled receptors (GPCRs) cloned in 1992 that also include MC1R, MC2R, MC3R and MC5R (Chhajlani and Wikberg 1992; Cone 2005; Gantz, et al. 1993a; Gantz, et al. 1993b; Mountjoy, et al. 1992). MC4R is predominantly expressed in the brain, where it localizes to many areas (Cui, et al. 2012; Gantz et al. 1993b; Gautron, et al. 2010; Liu, et al. 2003; Mountjoy, et al. 1994; Rossi, et al. 2011; Shah, et al. 2014; Sohn, et al. 2013; Williams, et al. 2000) (Fig. 4) and in neuroendocrine cells of the intestine (Panaro, et al. 2014). MC4R is essential for appetite control as knock-out mice lacking MC4R have hyperphagia and obesity (Fan, et al. 1997; Huszar, et al. 1997). MC4R mutations are associated with human obesity (Farooqi and O’Rahilly 2008; Farooqi, et al. 2000; Lubrano-Berthelier, et al. 2006; Stutzmann, et al. 2008; Vaisse, et al. 2000; Vaisse, et al. 1998; Yeo, et al. 1998) this association is controversial in the case of MC3R mutations (Tao 2010b). The MC4R expressed in single-minded 1 (Sim1) and in MC4R glutamatergic neurons of the paraventricular nucleus (PVN) of hypothalamus and the amygdala functions to control food intake (Balthasar, et al. 2005; Garfield, et al. 2015; Shah et al. 2014) (Fig. 4). In the pathway to control appetite, populations of POMC neurons residing in the arcuate nucleus activate MC4R neurons in the PVN (Caron et al. 2018; Dores et al. 2016; Gautron et al. 2015; Mercer et al. 2013; Toda et al. 2017). Also activation of glutamate-releasing neurons that reside in the arcuate nucleus and co-express oxytocin receptor rapidly cause satiety (Fenselau, et al. 2017). These oxytocin receptor-expressing neurons engage MC4R neurons in the PVN through a fast glutamatergic transmission that is potentiated by α-MSH released from the POMC neurons. Other neurons, localized to the NTS and expressing CCK directly stimulate the activity of MC4R neurons in the PVN to signal (D’Agostino, et al. 2016). It has been recently proposed that the bone specific hormone lipocalin-2 (LCN2) suppresses appetite by crossing the blood-brain barrier and binding to MC4R in the PVN and ventromedial neurons of the hypothalamus (Mera, et al. 2018; Mosialou, et al. 2017). MC4R neurons are inhibited by the AgRP/NPY neurons residing in the arcuate nucleus (Aponte et al. 2011; Atasoy et al. 2014; Atasoy et al. 2012; Bagnol et al. 1999; Cowley et al. 1999; Cowley et al. 2001; Legradi and Lechan 1999). In genetic rodent models of obesity with elevated leptin levels abundance of melanocortin receptors is reduced in the NA and in VTA, which are areas of the brain involved in the reward circuit (Lindblom, et al. 2000) (Fig. 4). In the VTA, dopaminergic neurons that regulate palatable feeding are responsive to α-MSH and project to neurons of the NA (Lindblom, et al. 2002; Lindblom et al. 2000; Panaro et al. 2014; Pandit, et al. 2015; Roseberry 2013; Roseberry, et al. 2015; Szczypka, et al. 2000; Yen and Roseberry 2015). MC4R regulates other functions in addition to appetite. Administration of the MC4R and MC3R agonist MTII to the CNS of rats activates the hypothalamic melanocortin system and increases sympathoexcitation in the kidney and brown adipose tissue (Haynes, et al. 1999). Specifically, MC4R expressed in neurons of the dorsomedial nucleus of the hypothalamus (DMH) and MC4R expressed in pre-ganglionic cholinergic sympathetic neurons of the CNS localized in the intermediolateral nucleus (IML) of the spinal cord control both sympathetic outflow to adipose tissue and energy expenditure (Andermann and Lowell 2017; Berglund, et al. 2014; Chen, et al. 2004; Enriori, et al. 2011; Haynes et al. 1999; Rezai-Zadeh, et al. 2014; Rossi et al. 2011; Shrestha, et al. 2010) (Fig. 4). MC4R neurons are also implicated in glucose homeostasis. In this respect, pre-ganglionic cholinergic sympathetic neurons of the intermediolateral nucleus of the spinal cord (IML) that express MC4R control glucose output from liver (Berglund et al. 2014; Rossi et al. 2011). Lorcaserin, a 5-HT2CR agonist used as anti-obesity drug, decreases glycemia by acting on MC4R cholinergic neurons (Burke, et al. 2017). Interestingly, an association has been found between MC4R genotype and postpartum glycemic changes in humans, thus highlighting the relevance of MC4R in glucose metabolism in humans (de Carvalho, et al. 2017). Cholinergic parasympathetic MC4R neurons, such as those localized in the dorsal motor nucleus of the vagus (DMV) control insulin levels and gastric motility (Richardson, et al. 2013; Rossi et al. 2011; Sohn et al. 2013). In the PVN of the hypothalamus, in addition to food intake, melanocortin receptors modulate sympathetic outflow, blood pressure and heart rate (Kuo, et al. 2003; Li, et al. 2013; Li, et al. 2006; Skibicka and Grill 2009; Tallam, et al. 2005). In the PVN, MC4R also appears essential for hypertension in the offspring of obese rats (Samuelsson, et al. 2016). MC4R agonists increase blood pressure by targeting cholinergic neurons, including the sympathetic preganglionic neurons of the IML(Sohn et al. 2013). In the IML, MC4R neurons also control heart rate (Iwasa, et al. 2013) (Fig. 4). In the hindbrain, MC4R neurons control heart rate, but not blood pressure (do Carmo, et al. 2015) (do Carmo, et al. 2017).
Fig. 4. Localization and function of MC4R and MC3R in the central nervous system.
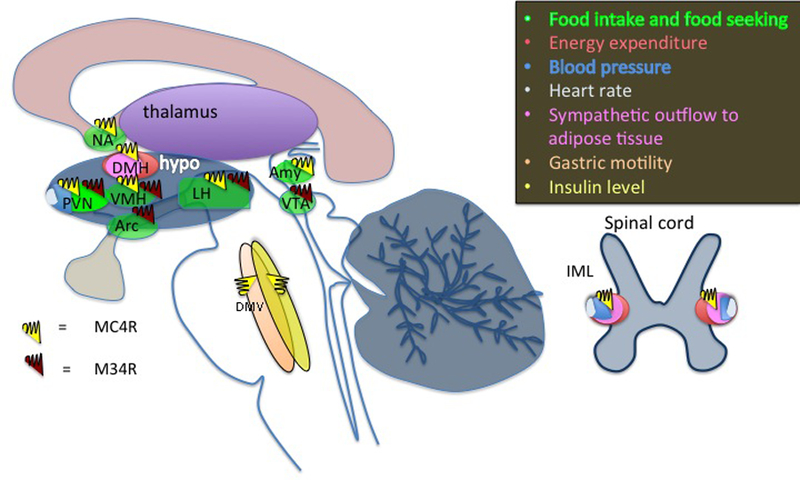
Amy, amygdala; DMH, dorsomedial nucleus of the hypothalamus; DMV, dorsal motor nucleus of the vagus IML, intermediolateral nucleus of the spinal cord; LH, lateral hypothalamus; NA, nucleus accumbens; PVN, paraventricular nucleus of hypothalamus; VMH, ventromedial nucleus of the hypothalamus; VTA, Ventral Tegmental Area. References are in the main text.
MC3R neurons
In the brain, MC3R is abundantly expressed in the arcuate nucleus, ventromedial nucleus of the hypothalamus (VMH), central linear nucleus of raphe, and dopaminergic neurons of the VTA; MC3R is instead moderately expressed in the lateral hypothalamic area and in the PVN (Gantz et al. 1993b; Jegou, et al. 2000; Lippert, et al. 2014; Roselli-Rehfuss, et al. 1993; Xia and Wikberg 1997). In the arcuate nucleus, MC3R is expressed in subpopulations of both POMC and AgRP/NPY neurons (Bagnol et al. 1999; Jegou et al. 2000). The obesity induced by MC3R knock out in mice is less profound than that by knock out of MC4R and mice lacking both MC3R and MC4R are heavier than mice lacking only MC4R, suggesting distinct roles to control food intake (Butler and Cone 2003; Butler, et al. 2017; Butler, et al. 2000; Chen, et al. 2000). Moreover, MC3R knock out mice do not develop fatty liver disease or severe insulin resistance like the MC4R mice (Ellacott, et al. 2007; You, et al. 2016). MC3R regulates normal fasting response (Marks, et al. 2003; Renquist, et al. 2012), adaptation to restricted feeding (Girardet, et al. 2017; Sutton, et al. 2010), food anticipatory activity (Girardet, et al. 2014; Vaanholt, et al. 2015), and enhanced motivation to acquire food during nutrient scarcity (Mavrikaki, et al. 2016). MC3R also functions to regulate nutrient partitioning in fat and liver tissues under conditions of fasting (Renquist et al. 2012). MC3R expressed in AgRP/NPY neurons functions to regulate inhibitory GABA release onto MC4R neurons in a pathway to tune energy balance from one set point to another (Bagnol et al. 1999; Cowley et al. 2001; Ghamari-Langroudi, et al. 2018).
MC4R and MC3R signaling
MC4R and MC3R are G-protein coupled receptors (GPCR) that, in the presence of the natural agonist α-MSH, couple to Gs and AgRP antagonizes this effect (Tao 2010a). In cells expressing exogenous MC4R, exposure to the natural agonist, α-MSH, induces activation of adenylate cyclase and increased production of cAMP (Gantz et al. 1993a) (Fig. 5). The increased concentration of cAMP induced by MC4R is thought to activate exchange protein directly activated by cAMP (EPAC), leading to ERK1/2- dependent phosphorylation of the transcription factor cAMP response element (CRE) binding protein (CREB), increased transcription of cFos and reduced phosphorylation and activity of AMP-activated protein kinase (AMPK) (Glas, et al. 2016; Yang and Tao 2016). In this respect, intracerebroventricular delivery of the MC3R/MC4R synthetic agonists induces anorexia as well as activation of transcription by CREB and expression of cFos in the PVN (Thiele, et al. 1998) (Benoit, et al. 2000) (Harris, et al. 2001; Lee, et al. 2001; Lu, et al. 2003; Rowland, et al. 2010; Sarkar, et al. 2002). On the other hand, other factors in addition to MC4R may modulate CREB-dependent control of energy homeostasis in the PVN. In this respect, it has been reported that, while lack of CREB in the Sim1 neurons of the PVN causes murine obesity, such effect can also take place in the absence of MC4R signaling (Chiappini, et al. 2011).
Fig. 5. MC4R signaling.
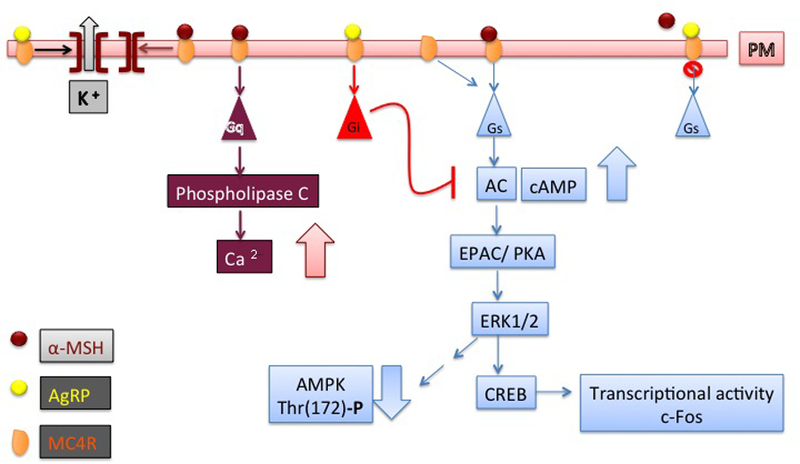
Binding of α-MSH to MC4R promotes receptor signal through Gs with activation of adenylate cyclase (AC) and increased generation of intracellular cAMP, followed by activation of PKA, EPAC, ERK1/2, CREB, and increased transcription of c-Fos as well as decreased AMPK activity. AgRP antagonizes these effects. The Gs signal induced by MC4R likely takes place in in the dorsomedial hypothalamus (DMH) to control energy expenditure. AgRP can also act a biased agonist to promote MC4R signal by Gi. MC4R can couple constitutively to both Gs and Gi, and AgRP blocks such signal, acting as an inverse agonist. MC4R in a complex with α-MSH also couples to Gq and induces activation of phospholipase C and increased intracellular cytosolic calcium. The Gq signal likely takes place in the paraventricular nucleus (PVN) of the hypothalamus to control food intake. MC4R in a complex with α-MSH opens the Kir7.1 channel to induce depolarization of MC4R neurons in a G-protein independent manner. AgRP acts as a biased agonist by opening the Kir7.1 channel to induce hyperpolarization of MC4R neurons. References are in the main text.
In addition to agonist-dependent coupling to Gs and generation of cAMP, MC4R can couple constitutively to both Gs and Gi. Constitutive coupling of MC4R to Gs in cells can be detected by exposure to the natural antagonist AgRP, thus indicating that the hormone is also an inverse agonist (Nijenhuis, et al. 2001; Oosterom, et al. 2001). Importantly, mutations that modulate the constitutive activity of MC4R to increase Gs signaling are linked with obesity in humans, thus suggesting that MC4R constitutive signaling is physiologically relevant (Proneth, et al. 2006; Srinivasan, et al. 2004; Vaisse et al. 2000). Interestingly, for some MC4R mutants with altered constitutive activity, AgRP can act as a biased agonist to promote ERK1/2 activation (Mo and Tao 2013; Wang and Tao 2011). It has been found that, in hypothalamic cells, MC4R can also couple to Gi and that MC4R constitutive activity through Gs and Gi/o can inhibit L-type voltage-gated calcium channels in neurons (Agosti, et al. 2017; Buch, et al. 2009). Moreover, MC4R activation by the synthetic agonist MTII inhibits presynaptic N-type calcium channels in amygdaloid complex neurons (Agosti, et al. 2014). Thus, signaling of MC4R by Gs and Gi controls calcium channel activity in neurons. In addition to Gs and Gi, MC4R couples to Gq (Peters and Scott 2009) and induces increased intracellular cytosolic calcium (Li and Lytton 2014; Newman, et al. 2006; Nickolls, et al. 2005). In this respect, it appears that peptide MC4R agonists induce both cAMP accumulation and calcium mobilization, while non-peptide agonists have blunted ability to induce calcium mobilization, thus indicating biased agonism (Nickolls et al. 2005). The ability of MC4R to signal through a specific pathway likely depends on the cell type where the receptor is being expressed. In this respect, it appears that Gs alpha signaling in the dorsomedial nucleus of the hypothalamus (DMH) and, to a lesser degree, in the PVN is important for regulation of energy expenditure (Chen, et al. 2012; Chen, et al. 2017a; Chen, et al. 2009). It also appears that, in mice, Gs expressed in MC4R cells regulates in addition to energy expenditure, also food intake, insulin sensitivity and cold-induced thermogenesis. Such effect may take place by mechanisms that include release of PYY from enteroendocrine cells of the intestine expressing MC4R (Panaro et al. 2014; Podyma, et al. 2018). Mice with PVN-specific loss of Gqα and G11α have hyperphagia and obesity and are relatively insensitive to delivery of MC4R agonist in the PVN, which would normally reduce food intake (Li et al. 2016). These findings indicate that signaling by MC4R to regulate appetite in the PVN is dependent upon Gqα and G11α. Mutations of adult type 3 adenylyl cyclase (Adcy3), a member of the adenylyl cyclase family that mediates Gs signaling, leads to obesity in mice (Wang, et al. 2009). Humans with variants of Adcy3 are also obese (Stergiakouli, et al. 2014; Wu, et al. 2016). Interestingly, tagged MC4R-GFP co-localizes with Adcy3 at the primary cilia of PVN neurons, while obesity-associated MC4R variants impair localization of the receptor to cilia (Siljee, et al. 2018; Tian, et al. 2018). These observations suggest that MC4R signaling though Adcy3 at cilia of hypothalamic neurons is essential for energy homeostasis (Varela and Horvath 2018). It also appears that α-MSH increases firing in MC4R neurons of the PVN in a G-protein independent manner by inducing depolarization through closure of the inwardly rectifying potassium channel, Kir7.1. Moreover, AgRP can act as a biased agonist by opening the Kir7.1 channel to induce hyperpolarization of MC4R neurons (Ghamari-Langroudi et al. 2015; Litt, et al. 2018). These data indicate that different MC4R signals may control food intake and energy expenditure at specific brain locations and that MC4R-dependent control of ion channel activity may contribute to receptor signal. MC4R activity can also be modulated by accessory factors. MRAP2 is a MC4R-interacting factor that is co-expressed with the receptor in the hypothalamus, localizes to the plasma membrane and endoplasmic reticulum (ER) and potentiates MC4R function. Deficiency of MRAP2 leads to obesity (Agulleiro, et al. 2013; Asai, et al. 2013; Chan, et al. 2009; Sebag, et al. 2013). Experiments where MRAP2 was overexpressed postnatally in MC4R neurons also indicate that MRAP2 functions to potentiate MC4R neurons (Bruschetta, et al. 2018). It has recently been found that MRAP2 affects other hypothalamic functions in addition to MC4R signaling, thus implicating multiple pathways to obesity by MRAP2 deficiency (Chaly, et al. 2016; Novoselova, et al. 2016). Nevertheless, the data offer evidence that interactions of MC4R with accessory factors along the secretory pathway modulate receptor function.
Intracellular traffic of MC4R and response to melanocortin receptor agonists
Studies based on undifferentiated cells, neuronal cells, and immortalized hypothalamic cells indicate that desensitization of MC4R takes place by a process where, upon prolonged agonist exposure, the receptor routes to the lysosomes, instead of being made available at the cell membrane (Gao, et al. 2003; Granell, et al. 2013; Mohammad, et al. 2007; Shinyama, et al. 2003). MC4R is internalized at the same rate in the presence or absence of α-MSH agonist (Mohammad et al. 2007). This constitutive endocytosis of MC4R is fast, by taking place with a t1/2 of approximately 3 min, is dependent on clathrin and membrane cholesterol, and is necessary to maintain receptor function (McDaniel, et al. 2012; Molden, et al. 2015). The rapid constitutive internalization and cycling back to the cell surface of MC4R is a specific feature of MC4R, as most other GPCRs, including the archetypal β2-adrenergic receptor, are endocytosed more efficiently in the presence of agonist (McDaniel et al. 2012). Agonist exposure induces binding of β-arrestin to the β2-adrenergic receptor, receptor internalization and desensitization (Drake, et al. 2006). In the case of MC4R, desensitization instead takes place because, in the presence of agonist, a population of constitutively recycling receptor is retained in the intracellular localization and routes to lysosomes (Granell et al. 2013; McDaniel et al. 2012). A factor implicated in MC4R intracellular traffic to lysosomes is Mahogunin Ring Finger-1, a RING domain-containing ubiquitin ligase, which also competes with Gsα to bind to MC4R (Overton and Leibel 2011; Perez-Oliva, et al. 2009). Desensitization of MC4R may also take place in vivo and manifest itself as “tachyphylaxis” to chronic, continuous MC4R treatment with cessation of weight loss (Bluher, et al. 2004; Pierroz, et al. 2002). Such loss of receptor function upon chronic exposure to MC4R agonists could limit their effects to reduce food intake and body weight. The unselective MC4R agonist peptide Melanotan II (MTII), the selective MC4R agonist peptides BIM-22511, and LY2112688, as well as the selective MC4R agonist THIQ, a small molecule, regulate food intake, energy expenditure, weight loss, sexual function and cardiovascular function (Adan, et al. 1999; Greenfield, et al. 2009; Kievit, et al. 2013; Kumar, et al. 2009; Kuo, et al. 2002; Martin, et al. 2002; Van der Ploeg, et al. 2002). In response to exposure to all of these agonists, MC4R desensitizes to the same extent as in response to the natural agonist, α-MSH (Molden et al. 2015). Interestingly, by using intermittent, rather than constant, delivery of MTII in rodents, it is possible to prolong the effects of MC4R agonists to reduce food intake, even if this treatment does not prevent tachyphylaxis (Cote, et al. 2017; Zhang, et al. 2010). It is also becoming evident that, in the face of common desensitization properties by different melanocortin receptor agonists, temporal effects induced by such agonists vary. With respect to MC1R, it has been found that, unlike α-MSH, MTII and another non-selective agonist active towards MC1R, 4-norleucine, 7-D-phenylalanine-α-MSH, have prolonged biological activity to darken frog skin (Sawyer, et al. 1980). With respect to MC4R, chronic treatment of obese primates with the selective MC4R agonist setmelanotide (also called RM-493), but not with another selective agonist LY2112688, results in persistent weight loss in non human promates(Kievit et al. 2013). In neuronal cells and in immortalized hypothalamic neurons where fluctuation of intracellular cAMP are measured by a temporally resolved Forster resonance energy transfer assay, the synthetic peptides MTII, a selective MC4R agonist, BIM-22511, and the non-peptide agonist THIQ can induce prolonged MC4R cAMP signaling after agonist withdrawal, while the other MC4R agonists such as α-MSH and LY2112688, do not have such property (Molden et al. 2015). It is possible that more persistent effects induced by some MC4R agonists to modulate energy homeostasis in vivo are linked to their biological property to induce prolonged receptor signal, rather than differences in the extent by which MC4R would desensitize.
Pharmacological chaperones and folding of MC4R
Recently, it has been discovered that it is possible to change the conformation of GPCRs by intracellular delivery of agonists and antagonists (Conn and Ulloa-Aguirre 2011). Many MC4R variants linked to obesity in humans have defective function because they are retained intracellularly (Ho and MacKenzie 1999; Ju, et al. 2018; Lubrano-Berthelier, et al. 2003; Nijenhuis, et al. 2003; Tao and Segaloff 2003). Intracellular retention of obesity-linked MC4R variants is dependent on their localization to the endoplasmic reticulum as misfolded, ubiquitinated proteins (Granell, et al. 2010; Rene, et al. 2010). Such misfolded MC4R mutants can be rescued by: a) pharmacological chaperones, namely lipophilic compounds that can enter cells and serve as a molecular scaffold to assist proper folding of misfolded proteins; b) by chemical chaperones, which likely modulate the folding capacity of the endoplasmic reticulum; c) by inhibitors of ubiquitination, which inhibit protein degradation by the proteasome (Granell, et al. 2012; Huang and Tao 2014; Meimaridou, et al. 2011; Rene et al. 2010; Tao and Conn 2014, 2018; Tao and Huang 2014). Interestingly, setmelanotide promotes weight loss in obese individuals expressing MC4R variants (Collet, et al. 2017) (Table I). Setmelanotide, when tested in cells, appears also acts as a pharmacological chaperone to promote receptor expression and function at the cell surface (Collet et al. 2017) (Table I). Changing conformation of newly synthesized MC4R along the secretory pathway may also affect the receptor properties not only to signal, but also to desensitize. In this respect, co-expressing α-MSH together with MC4R in the endoplasmic reticulum can rescue an obesity-linked variant retained in the endoplasmic reticulum and stabilize the wild-type receptor in an active conformation that does not route to lysosomes nor desensitizes (Granell et al. 2013). These observations indicate that MC4R conformation and ability to signal can be modulated by interactions with agonist in the endoplasmic reticulum.
Table I:
Natural and synthetic agonists of MC4R.
| hMC4R EC50 in vitro (nM) |
MC4R desensitizes (MC4R†) MC4R does not desensitize (MC4R‡) After agonist withdrawal : MC4R able to signal (MC4R*) MC4R unable to signal (MC4R**) Increased expression of MC4R at cell surface (MC4R⦿) |
Decreased body weight (↓BW) Decreased food intake (↓FI) Increased blood pressure (↑BP) Increased blood glucose (↑BG) Improved glucose metabolism (GM*) Increased heart rate (↑HR) |
Increased energy expenditure (↑EE ) Increased insulin sensitivity (↑IS) Increased lipolysis in white adipose tissue (↑WATL) Decreased fat (↓Fat) |
Increased neurogenesis (↑Neuro) Anti-inflammatory effects (↓Inf) |
|
|---|---|---|---|---|---|
| α-MSH | 4.69 (Kievit, et al. 2013) |
MC4R†; MC4R** (In vitro)
(Molden, et al. 2015) |
↓BW (h, only normal weight humans) intranasal delivery (Hallschmid, et al. 2008) ↓BW (r) ICV delivery (McMinn, et al. 2000) |
↑WATL (h) intranasal delivery (Wellhoner, et al. 2012) |
|
| NDP-α-MSH MTI |
0.0752 (Kievit et al. 2013) |
NT |
↓FI ICV delivery (Koegler, et al. 2001) |
↑IS (r) in hypothalamus ICV delivery (Chai, et al. 2009) |
↓Inf (In vitro) (Carniglia, et al. 2016) ↑Neuro (r) (Giuliani, et al. 2015) |
| MTII | 0.0542 (Kievit et al. 2013) |
MC4R†; MC4R* (In vitro)
(Molden et al. 2015) |
↓BW; ↓FI (r) ICV and IP delivery (Fan, et al. 1997) (Bluher, et al. 2004; Cote, et al. 2018) |
↑EE (r) IP delivery (Podyma, et al. 2018) |
|
| LY2112688 | 0.0857 (Kievit et al. 2013) |
MC4R†; MC4R** (In vitro) (Molden et al. 2015) |
↑BP; ↑HR (h)
(Greenfield, et al. 2009) ↑BP; ↑HR (nhp) (Kievit et al. 2013) |
↑WATL
In vitro human explants (Moller, et al. 2015) |
|
| Setmelanotide (BIM-22493) |
0.27 (Kievit et al. 2013) (Kumar, et al. 2009) |
MC4R⦿(In vitro) (Collet, et al. 2017) |
↓BW; no ↓BP (h) s.c. delivery, obese humans with LepR deficiency (Clement, et al. 2018) s.c. delivery obese humans with MC4R deficiency(Collet, et al. 2017) ↓BW; ↓FI; no ↑BP; GM* (nhp) (Kievit et al. 2013) |
↑EE (h) s.c. delivery, obese humans (Chen, et al. 2015) ↑EE ↓Fat (nhp) (Kievit et al. 2013) |
|
| Setmelanotide and glibencamide |
↓BW; ↓FI; GM* (r) (Clemmensen, et al. 2015) |
↑EE; ↓Fat (r) (Clemmensen et al. 2015) |
|||
| α-MSH-ER (intracellular α-MSH) |
NT |
MC4R‡; MC4R* (In vitro) (Granell, et al. 2013) |
|||
| MC4-NN2–0453 (peptide 19) MC4-NN1–0182 (peptide 11) |
88 (Conde-Frieboes, et al. 2012) 62 (Conde-Frieboes et al. 2012) |
NT NT |
No ↓BW; no ↑BP (h) s.c. delivery, obese humans {Royalty, 2014 #7286} ↓BW; GM*(r) (Fosgerau, et al. 2014) |
↑IS (r) (Fosgerau et al. 2014) |
|
| RO27–3225 | NT |
↓FI (r) ICV and IP delivery (Benoit, et al. 2000) |
↓Inf (r) IP delivery (Chen, et al. 2018) |
Humans (h); non-human primates (nhp); rodents (r)
Endoplasmic reticulum (ER); intracerebroventricular (ICV); intraperitoneal (IP); Leptin receptor (LepR); subcutaneous (s.c.) white adipose tissue (WAT)
The influence of diet-induced obesity on the melanocortin signaling.
Obesity by HF feeding induces damage to several regions of the hypothalamus implicated in energy homeostasis.
In male mice, exposure to high fat diet induces hypothalamic injury with inflammation, gliosis, and neuronal loss in the arcuate nucleus, medial eminence, and lateral hypothalamus (De Souza, et al. 2005; Dorfman, et al. 2017a; Dorfman and Thaler 2015; Moraes, et al. 2009; Thaler, et al. 2012; Velloso, et al. 2009; Yi et al. 2017). Female rodents exposed to HF diet, while having less severe hypothalamic injury and adverse metabolic consequences than male mice, nevertheless develop obesity (Atamni, et al. 2016; Chowen, et al. 2018; Dorfman et al. 2017a; Hong, et al. 2009; Qiu et al. 2018a). In the paraventricular nucleus (PVN) of the hypothalamus, Sim1 neurons are essential to control energy homeostasis and include the population of MC4R neurons that regulate appetite (Balthasar et al. 2005). In mice exposed to HF diet, while injury to POMC neurons and microgliosis in arcuate nucleus is specific to male mice, injury to Sim1 neurons of the PVN is a shared feature, taking place both in male and female mice (Nyamugenda, et al. 2019). Differently than in other regions of the hypothalamus, damage to Sim1 neurons by exposure to high fat diet is not paralleled by microgliosis (Nyamugenda et al. 2019). These contributions indicate, within the hypothalamus, there are region-specific mechanisms of neuronal damage by exposure to high fat diet.
Obesity by high fat feeding induces local activation and expansion of resident glia in the mediobasal hypothalamus, as well as recruitment of bone marrow-derived myeloid cells.
Obesity induces activation of the innate immune system and inflammation, which impacts many organs including adipose tissue, pancreas, liver, skeletal muscle and the brain (Saltiel and Olefsky 2017). Feeding rodents a high-fat diet induces, in hypothalamus, increased activation of c-Jun N-terminal kinase (JNK) and of nuclear factor-kappaB (NFkB) and expression of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) (De Souza et al. 2005). Already within the first week of high fat diet exposure, reactive gliosis and neuronal injury is detectable in the arcuate nucleus of the hypothalamus (Morari, et al. 2014; Thaler et al. 2012). Obesity induced by exposure to high-fat diet is associated with increased entry into the central nervous system of monocytes with the phenotype of activated microglia/macrophage (Buckman, et al. 2014). The mediobasal hypothalamus includes the arcuate nucleus, the medial eminence and the anterior part of the PVN (Timper and Bruning 2017). In diet-induced obesity (DIO), the recruitment of bone-marrow-derived myeloid cells in the mediobasal hypothalamus follows the earlier stage of hypothalamic inflammation, when hypothalamus-resident microglia are activated (Valdearcos, et al. 2017). In mice exposed to high fat diet, depletion of resident microglia from the mediobasal hypothalamus abolishes inflammation, reduces neuronal stress, suppresses food intake, and decreases weight gain (Valdearcos, et al. 2014). Moreover, inhibition of microglia expansion in mice exposed to high fat diet by central delivery of the antimitotic drug arabinofuranosyl cytidine inhibits hypothalamic and systemic inflammation and decreases weight gain (Andre, et al. 2016). Inhibition of NFkB-dependent signaling in microglia reduces both local microgliosis and recruitment of bone-marrow-derived myeloid cells in the mediobasal hypothalamus (Valdearcos et al. 2017). Moreover, even in mice that are not exposed to high fat diet, deletion of a negative regulator of NFkB, A20, induces hypothalamic microgliosis and increases food intake and weight gain (Valdearcos et al. 2017). Thus, in the mediobasal hypothalamus of mice treated with high fat diet, expansion and activation of resident microglia by the NFkB pathway promotes recruitment of myeloid cells from the circulation and neuronal injury. In DIO, upregulation of astrocytic IKKbeta/NF-kappaB pathway in astrocytes impairs astrocytic plasticity and promotes systemic adverse effects such as glucose intolerance, increased blood pressure, increased body weight and adiposity (Zhang, et al. 2017). Conversely, knockout of IKKbeta in astrocytes protects mice on a high fat diet from further weight gain by decreasing of food intake and increasing energy expenditure (Douglass, et al. 2017). It appears that male and female astrocytes respond differently to exposure to high fat diets (Chowen et al. 2018). In addition to microglia and astrocytes, the hypothalamus contains, especially the in median eminence, other resident glial cells such as monocyte-derived macrophages, which undergo expansion in mice exposed to high fat diet (Gao, et al. 2014; Kalin, et al. 2015; Lee, et al. 2018). Thus, under chronic exposure to high fat diet, activation of astrocytes, microglia, and macrophages residing in the hypothalamus and recruitment of activated bone-marrow-derived myeloid cells from the periphery disrupts hypothalamic control of energy homeostasis. Importantly, increased gliosis appears also in the hypothalamus of obese humans (Dorfman and Thaler 2015; Thaler et al. 2012).
Role of increased saturated fatty acids and lipoproteins to induce microglia and astrocyte activation.
In the arcuate nucleus, Toll-like receptor-4 (TLR4) is predominantly expressed in microglia. In DIO, increased levels of circulating saturated fatty acids, such as palmitic acid, activate TLR4 and modulate activity of neurons in the arcuate nucleus and feeding behavior (Milanski, et al. 2009; Reis, et al. 2015). Palmitic acid-induced activation of TLR4 in microglia has been found to promote phosphorylation and nuclear translocation NF-κB, increased secretion of pro-inflammatory cytokines, generation of nitric oxide (NO) and increased formation of reactive oxygen species (ROS) (Wang, et al. 2012). Conversely, knockdown of TLR4 in the arcuate nucleus of obese rats fed high fat diet, improves glucose homeostasis, attenuates insulin resistance, and reduces hepatic steatosis and adipocyte hypertrophy (Zhao, et al. 2017). Moreover, TAK-242, a TLR4 signaling inhibitor, decreases microglial activation in mice fed fat diet fat diet (Moser, et al. 2018). These data indicate that, in the hypothalamus of obese mice, activation of TLR4 in microglia by excess circulating saturated fatty acids plays a major role to regulate hypothalamic inflammation by initiating the NF-κB pathway to alter glucose metabolism, to induce insulin resistance, and to promote feeding. In obese mice, activation of GFAP-expressing glia promotes firing from orexigenic AgRP/NPY, but not from anorexigenic POMC neurons, and evokes feeding (Chen, et al. 2016). Thus activation of astrocytes mediated by IKKβ/NF-κB signaling has effects to alter activity of arcuate nucleus neurons involved in appetite control. Changes in lipoprotein abundance in DIO may also promote astrocytosis and disrupt glucose metabolism and energy homeostasis. With this respect, decreased circulating HDL, a risk factor for cardiovascular disease, correlates with obesity (Woudberg, et al. 2016). When loss of HDL is simulated in mice by knock-out of Apolipoprotein A1 (apoA-I), there is hypothalamic astrogliosis paralleled by disrupted hypothalamic mitochondrial function as well as by increased hepatic triglyceride content and glucose output (Gotz, et al. 2018). These data indicate that, in obesity, dyslipidemia with increased circulating saturated fatty acids and loss of HDL promotes hypothalamic inflammation and altered mitochondrial function in the hypothalamus.
Glial activation in DIO changes the architecture of the hypothalamus.
Glia is involved in the regulation of neuronal differentiation, proliferation, and synaptogenesis during development (Argente-Arizon, et al. 2015; Clarke and Barres 2013; Nedergaard, et al. 2003) (Argente-Arizon, et al. 2017). In adult rodents exposed to high fat diet, reactive gliosis modifies the architecture of the hypothalamus in the vicinity of POMC and AgRP/NPY neurons. In rats that are obese by being exposed to high fat diet, but not in rats that are diet-resistant, reactive gliosis in the arcuate nucleus reduces the accessibility of POMC and AgRP/NPY neurons to blood vessels and decreases synapses to the POMC neurons (Horvath, et al. 2010). High fat feeding induces activation of hypothalamic macrophages, which promotes nitric oxide synthase (iNOS)-dependent changes in blood-brain-barrier permeability, as well as altered glucose metabolism (Lee et al. 2018). Therefore in obesity, inflammation-induced changes in the properties of blood-brain-barrier may contribute to neuronal injury.
In DIO, changes in the cyto-architecture of the hypothalamus appear to include reduced adult neurogenesis. With this respect, it has been reported that murine adult astrocytes instruct stem cells to adopt a neuronal fate (Song, et al. 2002) and astrocytes themselves are, at least in some regions of the brain such as the ventricular-subventricular zone, precursors to neural stem cells (Doetsch, et al. 1999; Paul, et al. 2017). Neurogenesis has been found to take place in multiple brain regions, including the adult hypothalamus and hippocampus (Lindqvist, et al. 2006; Migaud, et al. 2010). In the hypothalamus, tancytes residing in the median eminence function to generate neurons (Lee, et al. 2012). In obesity, impaired neurogenesis affects multiple neuronal populations including of POMC and AgRP neurons (Lee et al. 2012; Li, et al. 2012; McNay, et al. 2012; Park, et al. 2010). Thus, in DIO, hypothalamic inflammation and neuronal injury is paralleled by decreased renewal of neurons relevant to energy homeostasis (Argente-Arizon et al. 2015).
Disrupted mitochondrial function in DIO impairs activation of POMC neurons in response to feeding.
Feeding controls mitochondrial function in hypothalamic neurons (Jin and Diano 2018). Increased glucose, by promoting increased ATP production in POMC neurons, enhances neuronal activation. Conversely, the activity of mitochondrial uncoupling protein 2 (UCP2) impairs glucose-stimulated ATP production in POMC neurons by promoting proton leak (Ma, et al. 2012; Parton et al. 2007). In POMC neurons, glucose-dependent generation of ROS is essential for neuronal firing (Andrews et al. 2008; Diano et al. 2011; Horvath et al. 2009). However, in DIO, glucose sensing by POMC neurons is impaired (Parton et al. 2007). With this respect, in hypothalamic POMC neurons of mice exposed to high fat diet, expression of peroxisome proliferator–activated receptor γ (PPAR-γ) is increased and cell peroxisome population is also increased, leading to decreased levels of ROS and suppressed neuronal firing (Diano et al. 2011). Thus, it appears that, in DIO, a mechanism of impaired glucose-dependent activation of POMC neurons is reduced levels of ROS. This is different than in AgRP/NPY neurons where suppression of ROS instead promotes the activity of AgRP/NPY neurons and feeding (Diano et al. 2011). Recently, it has been found that, when mitochondrial respiration is mildly impaired in POMC neurons of obese mice exposed to high fat diet, mitochondrial fatty acid utilization is improved, ROS generation is maintained, and neuronal activation continues to take place (Timper, et al. 2018).
Mitochondrial architecture regulates mitochondrial function in POMC neurons. With this respect, lack of MFN1 in POMC neurons impairs mitochondrial elongation induced by feeding and alters mitochondrial respiration with excessive generation of reactive oxygen species (Ramirez, et al. 2017). Exposure to high fat diet disrupts mitochondrial network with changes in mitochondrial morphology and decreases contacts sites between mitochondria and endoplasmic reticulum in POMC neurons (Diano 2011; Schneeberger et al. 2013). In mice exposed to high fat diet, hypersecretion of tumor necrosis factor (TNF)-α by activated glia changes mitochondrial morphology and induces mitochondrial stress in POMC neurons (Yi et al. 2017). Moreover, exposure to high fat diet impairs, in POMC neurons, calcium uptake in mitochondria, resulting in an increased level of cytosolic calcium and decreased excitability of these neurons (Paeger, et al. 2017b). These data converge to indicate that, in DIO, disrupted mitochondrial function with altered generation of ROS and increased cytosolic calcium suppresses nutrient-dependent excitability of POMC neurons.
Disrupted endoplasmic reticulum function in DIO impairs processing of POMC to α-MSH.
In the arcuate nucleus of the hypothalamus of mice exposed to high fat diet secretion of α-MSH from POMC neurons is impaired (Enriori, et al. 2007). Moreover, in mice exposed to high-fat diet, POMC neurons there is endoplasmic reticulum (ER) stress with defective processing of POMC precursor protein to generate α-MSH (Cakir, et al. 2013) as well as decreased mitochondria-ER contact sites (Schneeberger et al. 2013). Loss of α-MSH in POMC neurons of mice exposed to high fat diet impairs whole body glucose homeostasis by inducing, even before the onset of weight gain increased hepatic gluconeogenesis (Schneeberger, et al. 2015). When ER stress takes place, induction of the unfolded protein response with activation of the transcription factor spliced X-box binding protein 1 (Xbp1s) branch of the unfolded protein response (UPR) promotes restoration of ER homeostasis (Fu, et al. 2012). In mice that are obese by high fat diet feeding, induction of the unfolded protein response by overexpression of transcription factor spliced X-box binding protein 1 (Xbp1s) in POMC neurons protects against weight gain and suppresses hyperinsulinemia, hyperleptinemia, and glucose production (Williams, et al. 2014). These studies indicate that, in DIO, resolution of ER stress in POMC neurons is a target to reverse obesity and altered metabolism.
The onset of selective neuronal resistance to insulin and leptin in obesity
In DIO, systemic inflammation with increased levels of the inflammatory cytokines TNF-α and IL-6 parallels the onset of insulin and leptin resistance (Fu et al. 2012). With this respect, signaling by insulin and leptin is inhibited by components of inflammatory pathways such as inhibitor of nuclear factor kappa-B kinase β (IKKβ, also called IKK2) and c-Jun N-terminal kinase (JNK) (Haeusler et al. 2018). Expression of constitutively active IKKβ in hypothalamic neurons induces forced activation of the IKKβ /NF-kB pathway, increased expression of SOCS3, and, thereby, resistance to insulin and leptin signaling (Zhang, et al. 2008). Conversely, inactivation of SOCS3 in cells expressing the LepR restores leptin and insulin sensitivity in mice exposed to high fat diet (Pedroso, et al. 2014). Therefore, in DIO, systemic and local inflammation induces increased expression of SOCS3 in POMC neurons, resulting in leptin and insulin resistance. Leptin signaling by STAT3 is required for leptin regulation of energy balance (Bates et al. 2003). When mice are fed long-term a high fat diet, there is region-specific onset of leptin resistance in the arcuate nucleus with defective STAT3 signaling and increased expression of SOCS3, which functions in physiological termination of the leptin signal (Munzberg, et al. 2004) (Fig. 2). Interestingly, also chronic activation of STAT3 by expression of the constitutively active transcription factor in murine POMC neurons promotes, even when mice are fed chow diet, inhibition of both leptin and insulin signaling, up-regulation of SOCS3 expression, and increased weight gain (Ernst, et al. 2009). Together, these findings indicate that, in obesity, disrupted insulin signaling by inflammation disrupts the STAT3/SOCS3 signaling in POMC neurons, thereby resulting in insulin and leptin resistance. In AgRP neurons, the activation of the IKKb pathway increases action potential firing, without causing leptin resistance or obesity. Differently, inflammatory cytokines also activate another pathway, initiated by c-Jun N-terminal kinase 1 (JNK1). Activation of the JNK1 pathway results in increased firing by AgRP neurons and results in leptin and insulin resistance in these neurons as well as in peripheral tissues (Tsaousidou, et al. 2014). Thus, leptin resistance in AgRP neurons by activation of JNK1 may contribute to the onset of systemic insulin resistance in obesity.
In DIO, changes in other factors than increased SOCS3 levels may contribute to attenuation of the leptin signal and, thereby, leptin resistance. With this respect, the protein phosphatase PTP1B dephosphorylates JAK2 to terminate leptin signaling in POMC neurons, thereby regulating energy homeostasis (Banno, et al. 2010) (Bence, et al. 2006). Also TCPTP attenuates leptin signaling by dephosphorylating STAT3 (Loh et al. 2011) (Fig. 2B). In DIO, levels of both PTP1B and TCPTP are elevated (Dodd et al. 2015). Conversely, combined deficiency of PTP1B and TCPTP in POMC neurons enhances insulin and leptin signaling and prevents weight gain in mice fed high fat diet. Under these conditions, prevention of weight gain takes place because browning of white fat is increased and energy expenditure is enhanced (Dodd et al. 2015). Thus, in DIO, alteration of mechanisms to terminate leptin signaling results in hypothalamic resistance to anorexigenic hormones. Atypical PKCλ functions in the PI3K signaling pathway induced by leptin (Fig. 2). Male mice exposed to high fat diet have increases propensity to develop obesity, glucose intolerance, and insulin resistance when lacking PKCλ in POMC neurons (Dorfman et al. 2017b). Together these data indicate that alterations of leptin and insulin signaling at multiple steps including both factors implicated in the onset of the signal and signal termination may contribute to hypothalamic resistance to these hormones in obesity.
Neurons in the ventromedial hypothalamus (VMH) project excitatory synaptic inputs to POMC neurons in the arcuate nucleus (Sternson, et al. 2005). Steroidogenic factor 1 (SF-1)-expressing neurons in the VMH regulate feeding and anxiety (Klockener, et al. 2011; Viskaitis, et al. 2017). In the SF-1 neurons, insulin activates the PI3K signaling pathway with downstream activation of K+ATP channel, resulting in neuronal hyperpolarization and neuronal silencing (Klockener et al. 2011). In mice fed high fat diet, but not in mice fed control low fat diet, lack of insulin receptor signaling in SF-1 neurons protects against weight gain and impaired glucose homeostasis. In addition, these mice have also increased activation of POMC neurons. These data indicate that, in DIO, increased insulin sensitivity of SF-1 neurons may contribute to loss of excitatory glutamatergic inputs to POMC neuron with loss of POMC neuron activity (Klockener et al. 2011) (Timper and Bruning 2017). These studies indicate that onset of insulin resistance in populations of hypothalamic neurons outside of the arcuate nucleus contribute to energy homeostasis and glucose metabolism
Diet-induced obese mice (DIO) are resistant to the anorectic actions of leptin in POMC and AgRP nerons. Leptin increases sympathetic nerve activity resulting in increased thermogenesis taking place in interscapular brown adipose tissue (Enriori et al. 2011). This leptin-dependent increase of sympathetic outflow to the brown adipose tissue originates from neurons in dorsomedial hypothalamus and is increased in DIO (Enriori et al. 2011). Thus, in obesity, selective leptin sensitivity, taking place in neurons of the dorsomedial hypothalamus may underlie other systemic features associated with excessive weight gain, such as increased sympathetic outflow, blood pressure and heart rate.
Lipid stress and MC4R desensitization
MC4R constitutive traffic makes the receptor particularly sensitive to changes in cell environment. For example, depletion of membrane cholesterol, a condition that takes place in the diabetic hypothalamus (Suzuki, et al. 2010), appears to inhibit constitutive traffic of MC4R when modeled in cultured neuronal cells and immortalized hypothalamic neurons. Under such conditions, MC4R rapidly loses its ability to signal (McDaniel et al. 2012). Moreover, lipid stress by obesity and high-fat feeding induce injury to hypothalamic neurons and inflammation (Moraes et al. 2009) (De Souza et al. 2005; Thaler et al. 2012). Lipid stress modeled in cultured neuronal cells and immortalized hypothalamic neurons by increasing the concentration of extracellular palmitate induces loss of MC4R protein abundance and function (Cragle and Baldini 2014). However, in diet induced obese mice, the melanocortin system is instead over-responsive to MC4R agonists (Bluher et al. 2004; Enriori et al. 2007). An unexpected consequence of lipid stress is disruption of endocytosis following MC4R localization to clathrin-coated sites and inhibition of receptor desensitization. Such effects may underlie increased effectiveness of MC4R agonists in obesity (Cooney, et al. 2017).
Melanocortin agonists and in vivo studies
MC4R is considered as a target for anti-obesity therapy. However the receptor controls multiple pathways that include, in addition to energy homeostasis, also glucose metabolism, blood pressure and heart rate. In this respect, the first round of peptides being synthesized were 4-Norleucine, 7-d-phenylalanine-alpha-melanocyte-stimulating hormone (NDP-α-MSH, also called Melanotan I) and MTII (Hadley and Dorr 2006). NDP-α-MSH and MTII have superpotent activity as compared to that of α-MSH, are resistant to proteases and have prolonged activity (Al-Obeidi, et al. 1989a; Al-Obeidi, et al. 1989b; Hadley and Dorr 2006; Sawyer et al. 1980) (Table I). However, α-MSH and the relatively non-selective synthetic agonists NDP-α-MSH and MTII as well as the more selective agonist LY2112688, have side effects of increasing blood pressure and heart rate in rodents, non-human primates, and humans (Dunbar and Lu 2000; Greenfield 2011; Greenfield et al. 2009; Hill and Dunbar 2002; Kievit et al. 2013; Ni, et al. 2006; Rinne, et al. 2012). These effects are likely to be the result of activation of the sympathetic nervous system by the melanocortin receptor agonists (Fig. 4 and related text) and therefore preclude their use to treat obesity (Greenfield et al. 2009). These compounds have been patented and clinical trials have been carried out to study their effects to promote skin tanning and to improve sexual function in male and females (Hadley and Dorr 2006). However, potential risks to develop melanoma related to the use of unlicensed MT-II have been reported (Hjuler and Lorentzen 2014). Nevertheless, the first round of melanocortin receptor agonists have been the starting point to synthesize many other agonists and antagonists with elevated selectivity towards MC4R and MC3R (Ericson, et al. 2017; Hruby 2016). In this respect, chronic treatment of obese non-human primates with the selective MC4R agonist setmelanotide, but not with LY2112688, results in persistent weight loss without concomitant effects to increase blood pressure and heart rate and, instead, improvement of cardiovascular function (Kievit et al. 2013) (TableI). Importantly, setmelanotide, delivered to severely obese individuals with leptin deficiency, induces durable decrease in hyperphagia and body weight loss (Clement, et al. 2018). Moreover, obese patients with POMC deficiency have also durable decrease of hyperphagia and substantial weight loss upon treatment with setmelanotide (Collet et al. 2017; Kuhnen, et al. 2016; Muller et al. 2016). In this respect, a phase 2 clinical trial is undergoing to study the use of setmelanotide to treat patients with rare genetic disorders of obesity (ClinicalTrials.gov Identifier: NCT03013543). A phase 3 clinical trial is undergoing to study the use of setmelanotide to treat patients with LepR deficiency (ClinicalTrials.gov Identifier: NCT03287960). In obese individuals, short-term administration of setmelanotide increases resting energy expenditure and promotes fat oxidation (Chen, et al. 2015a). In mice with diet-induced obesity, simultaneous agonism at the GLP-1 receptor and MC4R induces body weight loss and improves glucose tolerance and cholesterol metabolism (Clemmensen, et al. 2015). Interestingly, some melanocortin agonists such as NDP-α-MSH and RO27–3225 have effects to reduce inflammation and promote neurogenesis, which may prove relevant to counteract injury to the hypothalamus in obesity (Carniglia, et al. 2016; Chen, et al. 2018; Giuliani, et al. 2015). These studies suggest that, in addition to obesity due to POMC or leptin deficiency, setmelanotide, in combination with GLP-1 agonists, may also be beneficial for treatment of obesity and diabetes. In conclusion, discoveries on the melanocortin system in the last two decades have revealed an unexpected complexity by which appetite control takes place and indicate possible therapeutic targets to reduce obesity.
Funding
This work was supported by National Institutes of Health Grant R01-DK102206 (to G.B.)
Footnotes
Declaration of interest
Authors have no conflicts of interest to disclose.
REFERENCES
- Adan RAH, Szklarczyk AW, Oosterom J, Brakkee JH, Nijenhuis WAJ, Schaaper WMM, Meloen RH & Gispen WH 1999. Characterization of melanocortin receptor ligands on cloned brain melanocortin receptors and on grooming behavior in the rat. European Journal of Pharmacology 378 249–258. [DOI] [PubMed] [Google Scholar]
- Agosti F, Cordisco Gonzalez S, Martinez Damonte V, Tolosa MJ, Di Siervi N, Schioth HB, Davio C, Perello M & Raingo J 2017. Melanocortin 4 receptor constitutive activity inhibits L-type voltage-gated calcium channels in neurons. Neuroscience 346 102–112. [DOI] [PubMed] [Google Scholar]
- Agosti F, Soto EJL, Cabral A, Castrogiovanni D, Schioth HB, Perello M & Raingo J 2014. Melanocortin 4 receptor activation inhibits presynaptic N-type calcium channels in amygdaloid complex neurons. European Journal of Neuroscience 40 2755–2765. [DOI] [PubMed] [Google Scholar]
- Agulleiro MJ, Cortes R, Fernandez-Duran B, Navarro S, Guillot R, Meimaridou E, Clark AJL & Cerda-Reverter JM 2013. Melanocortin 4 Receptor Becomes an ACTH Receptor by Coexpression of Melanocortin Receptor Accessory Protein 2. Molecular Endocrinology 27 1934–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Obeidi F, Castrucci AM, Hadley ME & Hruby VJ 1989a. Potent and prolonged acting cyclic lactam analogues of alpha-melanotropin: design based on molecular dynamics. Journal of Medicinal Chemistry 32 2555–2561. [DOI] [PubMed] [Google Scholar]
- Al-Obeidi F, Hruby VJ, Castrucci AM & Hadley ME 1989b. Design of potent linear alpha-melanotropin 4–10 analogues modified in positions 5 and 10. Journal of Medicinal Chemistry 32 174–179. [DOI] [PubMed] [Google Scholar]
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, et al. 2009. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metabolism 10 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC & Betley JN 2018. A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell 173 140–152 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkemade A, Yi CX, Pei L, Harakalova M, Swaab DF, la Fleur SE, Fliers E & Kalsbeek A 2012. AgRP and NPY expression in the human hypothalamic infundibular nucleus correlate with body mass index, whereas changes in alphaMSH are related to type 2 diabetes. J Clin Endocrinol Metab 97 E925–933. [DOI] [PubMed] [Google Scholar]
- Andermann ML & Lowell BB 2017. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 95 757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderwald C, Muller G, Koca G, Furnsinn C, Waldhausl W & Roden M 2002. Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2. Molecular Endocrinology 16 1612–1628. [DOI] [PubMed] [Google Scholar]
- Andre C, Guzman-Quevedo O, Rey C, Remus-Borel J, Clark S, Castellanos-Jankiewicz A, Ladeveze E, Leste-Lasserre T, Nadjar A, Abrous DN, et al. 2016. Inhibiting Microglia Expansion Prevents Diet-induced Hypothalamic and Peripheral Inflammation. Diabetes [DOI] [PubMed]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, et al. 2008. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D & Sternson SM 2011. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neuroscience 14 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente-Arizon P, Freire-Regatillo A, Argente J & Chowen JA 2015. Role of non-neuronal cells in body weight and appetite control. Front Endocrinol (Lausanne) 6 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente-Arizon P, Guerra-Cantera S, Garcia-Segura LM, Argente J & Chowen JA 2017. Glial cells and energy balance. Journal of Molecular Endocrinology 58 R59–R71. [DOI] [PubMed] [Google Scholar]
- Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, et al. 2013. Loss of Function of the Melanocortin 2 Receptor Accessory Protein 2 Is Associated with Mammalian Obesity. Science 341 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamni HJ, Mott R, Soller M & Iraqi FA 2016. High-fat-diet induced development of increased fasting glucose levels and impaired response to intraperitoneal glucose challenge in the collaborative cross mouse genetic reference population. Bmc Genetics 17 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Li WP, Su HH, Sertel SM, Scheffer LK, Simpson JH, Fetter RD & Sternson SM 2014. A genetically specified connectomics approach applied to long-range feeding regulatory circuits. Nature Neuroscience 17 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH & Sternson SM 2012. Deconstruction of a neural circuit for hunger. Nature 488 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS & Watson SJ 1999. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. Journal of Neuroscience 19 RC26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrenberg G, Behrmann I, Barthel A, Hekerman P, Heinrich PC, Joost HG & Becker W 2002. Identification of the critical sequence elements in the cytoplasmic domain of leptin receptor isoforms required for Janus kinase/signal transducer and activator of transcription activation by receptor heterodimers. Molecular Endocrinology 16 859–872. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr., Elmquist JK, et al. 2004. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42 983–991. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. 2005. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123 493–505. [DOI] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH & Myers MG Jr. 2000. Activation of downstream signals by the long form of the leptin receptor. Journal of Biological Chemistry 275 14563–14572. [DOI] [PubMed] [Google Scholar]
- Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W & Bence KK 2010. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. Journal of Clinical Investigation 120 720–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, Rogers RC, Van Meter MJ & Hermann GE 2010. Co-localization of TRHR1 and LepRb receptors on neurons in the hindbrain of the rat. Brain Research 1355 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone I, Melani R, Mainardi M, Scabia G, Scali M, Dattilo A, Ceccarini G, Vitti P, Santini F, Maffei L, et al. 2018. Fluoxetine Modulates the Activity of Hypothalamic POMC Neurons via mTOR Signaling. Molecular Neurobiology [DOI] [PubMed]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. 2003. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421 856–859. [DOI] [PubMed] [Google Scholar]
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG & Kahn BB 2006. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nature Medicine 12 917–924. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Schwartz MW, Lachey JL, Hagan MM, Rushing PA, Blake KA, Yagaloff KA, Kurylko G, Franco L, Danhoo W, et al. 2000. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. Journal of Neuroscience 20 3442–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, Vianna CR, Williams KW, Xu Y & Elmquist JK 2013. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. Journal of Clinical Investigation 123 5061–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Liu TM, Kong XX, Sohn JW, Vong L, Deng Z, Lee CE, Lee S, Williams KW, Olson DP, et al. 2014. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nature Neuroscience 17 911–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD & Sternson SM 2013. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y & Sternson SM 2015. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler LR, Chen Y, Ahn JS, Lin YC, Essner RA & Knight ZA 2017. Dynamics of Gut-Brain Communication Underlying Hunger. Neuron 96 461–475 e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA & Bloom SR 2005. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. Faseb Journal 19 1680–1682. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Uotani S, da Silva B & Flier JS 1997. Divergent signaling capacities of the long and short isoforms of the leptin receptor. Journal of Biological Chemistry 272 32686–32695. [DOI] [PubMed] [Google Scholar]
- Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS & Myers MG Jr. 2000. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. Journal of Biological Chemistry 275 40649–40657. [DOI] [PubMed] [Google Scholar]
- Bluher S, Ziotopoulou M, Bullen JW Jr., Moschos SJ, Ungsunan L, Kokkotou E, Maratos-Flier E & Mantzoros CS 2004. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes 53 82–90. [DOI] [PubMed] [Google Scholar]
- Bouret S & Richmond BJ 2015. Sensitivity of locus ceruleus neurons to reward value for goal-directed actions. Journal of Neuroscience 35 4005–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M & Hokfelt T 1998. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A 95 15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschetta G, Kim JD, Diano S & Chan LF 2018. Overexpression of melanocortin 2 receptor accessory protein 2 (MRAP2) in adult paraventricular MC4R neurons regulates energy intake and expenditure. Molecular Metabolism [DOI] [PMC free article] [PubMed]
- Buch TR, Heling D, Damm E, Gudermann T & Breit A 2009. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1–7 cells defines agouti-related protein as a biased agonist. Journal of Biological Chemistry 284 26411–26420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K & Ellacott KL 2014. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behavior and Immunity 35 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG Jr. & Rossetti L 2006. Critical role of STAT3 in leptin’s metabolic actions. Cell Metabolism 4 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LK, Ogunnowo-Bada E, Georgescu T, Cristiano C, de Morentin PBM, Valencia Torres L, D’Agostino G, Riches C, Heeley N, Ruan Y, et al. 2017. Lorcaserin improves glycemic control via a melanocortin neurocircuit. Molecular Metabolism 6 1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Bruning JC & Krashes MJ 2016. Hunger-Driven Motivational State Competition. Neuron 92 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA & Cone RD 2003. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Melanocortin System 994 240–245. [DOI] [PubMed] [Google Scholar]
- Butler AA, Girardet C, Mavrikaki M, Trevaskis JL, Macarthur H, Marks DL & Farr SA 2017. A Life without Hunger: The Ups (and Downs) to Modulating Melanocortin-3 Receptor Signaling. Frontiers in Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M & Cone RD 2000. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141 3518–3521. [DOI] [PubMed] [Google Scholar]
- Cakir I, Cyr NE, Perello M, Litvinov BP, Romero A, Stuart RC & Nillni EA 2013. Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. Journal of Biological Chemistry 288 17675–17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniglia L, Ramirez D, Durand D, Saba J, Caruso C & Lasaga M 2016. [Nle4, D-Phe7]-alpha-MSH Inhibits Toll-Like Receptor (TLR)2- and TLR4-Induced Microglial Activation and Promotes a M2-Like Phenotype. Plos One 11 e0158564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron A, Dungan Lemko HM, Castorena CM, Fujikawa T, Lee S, Lord CC, Ahmed N, Lee CE, Holland WL, Liu C, et al. 2018. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalheira JB, Ribeiro EB, Folli F, Velloso LA & Saad MJ 2003. Interaction between leptin and insulin signaling pathways differentially affects JAK-STAT and PI 3-kinase-mediated signaling in rat liver. Biol Chem 384 151–159. [DOI] [PubMed] [Google Scholar]
- Chai B, Li JY, Zhang W, Wang H & Mulholland MW 2009. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides 30 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, et al. 2004. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3–36). Proc Natl Acad Sci U S A 101 4695–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaly AL, Srisai D, Gardner EE & Sebag JA 2016. The Melanocortin Receptor Accessory Protein 2 promotes food intake through inhibition of the Prokineticin Receptor-1. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertova M, Elphick MR, Cheetham ME, et al. 2009. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci U S A 106 6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao LH, et al. 2000. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genetics 26 97–102. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et al. 1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84 491–495. [DOI] [PubMed] [Google Scholar]
- Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, Zhao X, Ring M, Psota TL, Cone RD, et al. 2015a. RM-493, a Melanocortin-4 Receptor (MC4R) Agonist Increases Resting Energy Expenditure in Obese Individuals. J Clin Endocrinol Metab jc20144024. [DOI] [PMC free article] [PubMed]
- Chen M, Berger A, Kablan A, Zhang J, Gavrilova O & Weinstein LS 2012. Gsalpha deficiency in the paraventricular nucleus of the hypothalamus partially contributes to obesity associated with Gsalpha mutations. Endocrinology 153 4256–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Shrestha YB, Podyma B, Cui Z, Naglieri B, Sun H, Ho T, Wilson EA, Li YQ, Gavrilova O, et al. 2017a. Gsalpha deficiency in the dorsomedial hypothalamus underlies obesity associated with Gsalpha mutations. Journal of Clinical Investigation 127 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wang J, Dickerson KE, Kelleher J, Xie T, Gupta D, Lai EW, Pacak K, Gavrilova O & Weinstein LS 2009. Central nervous system imprinting of the G protein G(s)alpha and its role in metabolic regulation. Cell Metabolism 9 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G & Han W 2016. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Williams SM, Grove KL & Smith MS 2004. Melanocortin 4 receptor-mediated hyperphagia and activation of neuropeptide Y expression in the dorsomedial hypothalamus during lactation. Journal of Neuroscience 24 5091–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhao L, Sherchan P, Ding Y, Yu J, Nowrangi D, Tang J, Xia Y & Zhang JH 2018. Activation of melanocortin receptor 4 with RO27–3225 attenuates neuroinflammation through AMPK/JNK/p38 MAPK pathway after intracerebral hemorrhage in mice. J Neuroinflammation 15 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Chen H, Zhou JJ, Pradhan G, Sun Y, Pan HL & Li DP 2017b. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. Journal of Neurochemistry 142 512–520. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW & Knight ZA 2015b. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK & Steiner RA 1997. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138 4489–4492. [DOI] [PubMed] [Google Scholar]
- Chhajlani V & Wikberg JE 1992. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. Febs Letters 309 417–420. [DOI] [PubMed] [Google Scholar]
- Chiappini F, Cunha LL, Harris JC & Hollenberg AN 2011. Lack of cAMP-response element-binding protein 1 in the hypothalamus causes obesity. Journal of Biological Chemistry 286 8094–8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowen JA, Argente-Arizon P, Freire-Regatillo A & Argente J 2018. Sex differences in the neuroendocrine control of metabolism and the implication of astrocytes. Front Neuroendocrinol 48 3–12. [DOI] [PubMed] [Google Scholar]
- Chua SC Jr., Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L & Leibel RL 1996. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271 994–996. [DOI] [PubMed] [Google Scholar]
- Churruca I, Portillo MP, Casis L, Gutierrez A, Macarulla MT & Echevarria E 2008. Effects of fluoxetine administration on hypothalamic melanocortin system in obese Zucker rats. Neuropeptides 42 293–299. [DOI] [PubMed] [Google Scholar]
- Clarke LE & Barres BA 2013. Emerging roles of astrocytes in neural circuit development. Nature Reviews Neuroscience 14 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, Biebermann H, Farooqi IS, Van der Ploeg L, Wolters B, Poitou C, Puder L, Fiedorek F, Gottesdiener K, Kleinau G, et al. 2018. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nature Medicine 24 551–555. [DOI] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, et al. 1998. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392 398–401. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Finan B, Fischer K, Tom RZ, Legutko B, Sehrer L, Heine D, Grassl N, Meyer CW, Henderson B, et al. 2015. Dual melanocortin-4 receptor and GLP-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice. Embo Molecular Medicine 7 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DL 1973. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia 9 294–298. [DOI] [PubMed] [Google Scholar]
- Coleman DL 1978. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14 141–148. [DOI] [PubMed] [Google Scholar]
- Collet TH, Dubern B, Mokrosinski J, Connors H, Keogh JM, Mendes de Oliveira E, Henning E, Poitou-Bernert C, Oppert JM, Tounian P, et al. 2017. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Molecular Metabolism 6 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Frieboes K, Thogersen H, Lau JF, Sensfuss U, Hansen TK, Christensen L, Spetzler J, Olsen HB, Nilsson C, Raun K, et al. 2012. Identification and in Vivo and in Vitro Characterization of Long Acting and Melanocortin 4 Receptor (MC4-R) Selective alpha-Melanocyte-Stimulating Hormone (alpha-MSH) Analogues. Journal of Medicinal Chemistry 55 1969–1977. [DOI] [PubMed] [Google Scholar]
- Cone RD 2005. Anatomy and regulation of the central melanocortin system. Nature Neuroscience 8 571–578. [DOI] [PubMed] [Google Scholar]
- Cone RD 2006. Studies on the physiological functions of the melanocortin system. Endocrine Reviews 27 736–749. [DOI] [PubMed] [Google Scholar]
- Conn PM & Ulloa-Aguirre A 2011. Pharmacological chaperones for misfolded gonadotropin-releasing hormone receptors. Adv Pharmacol 62 109–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney KA, Molden BM, Kowalczyk NS, Russell S & Baldini G 2017. Lipid stress inhibits endocytosis of Melanocortin-4 Receptor from modified clathrin-enriched sites and impairs receptor desensitization. Journal of Biological Chemistry [DOI] [PMC free article] [PubMed]
- Cote I, Green SM, Morgan D, Carter CS, Tumer N & Scarpace PJ 2018. Activation of the central melanocortin system in rats persistently reduces body and fat mass independently of caloric reduction. Can J Physiol Pharmacol 96 308–312. [DOI] [PubMed] [Google Scholar]
- Cote I, Sakarya Y, Kirichenko N, Morgan D, Carter CS, Tumer N & Scarpace PJ 2017. Activation of the central melanocortin system chronically reduces body mass without the necessity of long-term caloric restriction. Canadian Journal of Physiology and Pharmacology 95 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF & Cone RD 1999. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: Evidence of a cellular basis for the adipostat. Neuron 24 155–163. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD & Low MJ 2001. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411 480–484. [DOI] [PubMed] [Google Scholar]
- Cragle FK & Baldini G 2014. Mild lipid stress induces profound loss of MC4R protein abundance and function. Molecular Endocrinology 28 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Mason BL, Lee C, Nishi A, Elmquist JK & Lutter M 2012. Melanocortin 4 receptor signaling in dopamine 1 receptor neurons is required for procedural memory learning. Physiology & Behavior 106 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, et al. 2004. Essential role of STAT3 in body weight and glucose homeostasis. Molecular and Cellular Biology 24 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G, Lyons D, Cristiano C, Lettieri M, Olarte-Sanchez C, Burke LK, Greenwald-Yarnell M, Cansell C, Doslikova B, Georgescu T, et al. 2018. Nucleus of the Solitary Tract Serotonin 5-HT2C Receptors Modulate Food Intake. Cell Metabolism 28 619–630 e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G, Lyons DJ, Cristiano C, Burke LK, Madara JC, Campbell JN, Garcia AP, Land BB, Lowell BB, Dileone RJ, et al. 2016. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho AM, Shao P, Liu H, Cheng HL, Zheng Y, Leng J, Li W, Huang T, Wang T, Wang L, et al. 2017. The MC4R genotype is associated with postpartum weight reduction and glycemic changes among women with prior gestational diabetes: longitudinal analysis. Sci Rep 7 9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet B, Depoortere I, Moechars D, Swennen Q, Moreaux B, Cryns K, Tack J, Buyse J, Coulie B & Peeters TL 2006. Energy homeostasis and gastric emptying in ghrelin knockout mice. Journal of Pharmacology and Experimental Therapeutics 316 431–439. [DOI] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ & Velloso LA 2005. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146 4192–4199. [DOI] [PubMed] [Google Scholar]
- Deng YF, Wang ZV, Gordillo R, An Y, Zhang C, Liang QR, Yoshino J, Cautivo KM, De Brabander J, Elmquist JK, et al. 2017. An adipo-biliary-uridine axis that regulates energy homeostasis. Science 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis RG, Joly-Amado A, Webber E, Langlet F, Schaeffer M, Padilla SL, Cansell C, Dehouck B, Castel J, Delbes AS, et al. 2015. Palatability Can Drive Feeding Independent of AgRP Neurons. Cell Metabolism 22 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R, Richards JG, Campfield LA, Tartaglia LA, Guisez Y, van der Heyden J, Travernier J, Plaetinck G & Burn P 1996. OB protein binds specifically to the choroid plexus of mice and rats. Proc Natl Acad Sci U S A 93 5668–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S 2011. New aspects of melanocortin signaling: A role for PRCP in alpha-MSH degradation. Frontiers in Neuroendocrinology 32 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S 2013. Role of reactive oxygen species in hypothalamic regulation of energy metabolism. Endocrinol Metab (Seoul) 28 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E, Suyama S, Kelly K, Gyengesi E, Arbiser JL, et al. 2011. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nature Medicine 17 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Liu ZW & Horvath TL 2013. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Zimmer MR, Bober J & Horvath TL 2015. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160 1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA & Hall JE 2015. Role of hindbrain melanocortin-4 receptor activity in controlling cardiovascular and metabolic functions in spontaneously hypertensive rats. Journal of Hypertension 33 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, Perez de Lara CE & Hall JE 2017. Role of the brain melanocortins in blood pressure regulation. Biochim Biophys Acta [DOI] [PMC free article] [PubMed]
- do Carmo JM, da Silva AA, Wang Z, Freeman NJ, Alsheik AJ, Adi A & Hall JE 2016. Regulation of Blood Pressure, Appetite, and Glucose by Leptin After Inactivation of Insulin Receptor Substrate 2 Signaling in the Entire Brain or in Proopiomelanocortin Neurons. Hypertension 67 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, et al. 2015. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160 88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT, Lee-Young RS, Bruning JC & Tiganis T 2018a. TCPTP Regulates Insulin Signaling in AgRP Neurons to Coordinate Glucose Metabolism With Feeding. Diabetes 67 1246–1257. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Michael NJ, Lee-Young RS, Mangiafico SP, Pryor JT, Munder AC, Simonds SE, Bruning JC, Zhang ZY, Cowley MA, et al. 2018b. Insulin regulates POMC neuronal plasticity to control glucose metabolism. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT & Tiganis T 2017. Insulin action in the brain: Roles in energy and glucose homeostasis. Journal of Neuroendocrinology 29. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM & Alvarez-Buylla A 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97 703–716. [DOI] [PubMed] [Google Scholar]
- Dores RM, Liang L, Davis P, Thomas AL & Petko B 2016. 60 YEARS OF POMC: Melanocortin receptors: evolution of ligand selectivity for melanocortin peptides. Journal of Molecular Endocrinology 56 T119–133. [DOI] [PubMed] [Google Scholar]
- Dorfman MD, Krull JE, Douglass JD, Fasnacht R, Lara-Lince F, Meek TH, Shi X, Damian V, Nguyen HT, Matsen ME, et al. 2017a. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nature Communications 8 14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman MD, Krull JE, Scarlett JM, Guyenet SJ, Sajan MP, Damian V, Nguyen HT, Leitges M, Morton GJ, Farese RV, et al. 2017b. Deletion of Protein Kinase C lambda in POMC Neurons Predisposes to Diet-Induced Obesity. Diabetes 66 920–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman MD & Thaler JP 2015. Hypothalamic inflammation and gliosis in obesity. Curr Opin Endocrinol Diabetes Obes 22 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD & Thaler JP 2017. Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Molecular Metabolism 6 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Shenoy SK & Lefkowitz RJ 2006. Trafficking of G protein-coupled receptors. Circ Res 99 570–582. [DOI] [PubMed] [Google Scholar]
- Duan C, Li M & Rui L 2004. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. Journal of Biological Chemistry 279 43684–43691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C, Saha PK, Lee ME, Phillips KJ, Jain M, et al. 2017. Asprosin is a centrally acting orexigenic hormone. Nature Medicine 23 1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar JC & Lu HQ 2000. Proopiomelanocortin (POMC) products in the central regulation of sympathetic and cardiovascular dynamics: studies on melanocortin and opioid interactions. Peptides 21 211–217. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Murphy JG, Marks DL & Cone RD 2007. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology 148 6186–6194. [DOI] [PubMed] [Google Scholar]
- Ellacott KLJ & Cone RD 2004. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Progress in Hormone Research, Vol 59 59 395–408. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, et al. 2007. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metabolism 5 181–194. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C & Cowley MA 2011. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. Journal of Neuroscience 31 12189–12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson MD, Freeman KT, Schnell SM, Fleming KA & Haskell-Luevano C 2017. Structure-Activity Relationship Studies on a Macrocyclic Agouti-Related Protein (AGRP) Scaffold Reveal Agouti Signaling Protein (ASP) Residue Substitutions Maintain Melanocortin-4 Receptor Antagonist Potency and Result in Inverse Agonist Pharmacology at the Melanocortin-5 Receptor. Journal of Medicinal Chemistry [DOI] [PMC free article] [PubMed]
- Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Munzberg H, Hampel B, et al. 2009. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. Journal of Neuroscience 29 11582–11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J & Tavernier J 2000. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. Febs Letters 486 33–37. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ & Cone RD 1997. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385 165–168. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA & O’Rahilly S 1999. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341 879–884. [DOI] [PubMed] [Google Scholar]
- Farooqi IS & O’Rahilly S 2008. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nature Clinical Practice Endocrinology & Metabolism 4 569–577. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T & O’Rahilly S 2000. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. Journal of Clinical Investigation 106 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenselau H, Campbell JN, Verstegen AM, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, Mandelblat-Cerf Y, Livneh Y, et al. 2017. A rapidly acting glutamatergic ARC-->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nature Neuroscience 20 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN & Myers MG Jr. 2016. Minireview: CNS Mechanisms of Leptin Action. Molecular Endocrinology 30 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Patterson CM, Garfield AS, D’Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JT, Germani M, Jones JC, et al. 2014. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nature Neuroscience 17 1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosgerau K, Raun K, Nilsson C, Dahl K & Wulff BS 2014. Novel alpha-MSH analog causes weight loss in obese rats and minipigs and improves insulin sensitivity. Journal of Endocrinology 220 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Watkins SM & Hotamisligil GS 2012. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metabolism 15 623–634. [DOI] [PubMed] [Google Scholar]
- Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, Delvalle J & Yamada T 1993a. Molecular-Cloning of a Novel Melanocortin Receptor. Journal of Biological Chemistry 268 8246–8250. [PubMed] [Google Scholar]
- Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, Delvalle J & Yamada T 1993b. Molecular-Cloning, Expression, and Gene Localization of a 4th Melanocortin Receptor. Journal of Biological Chemistry 268 15174–15179. [PubMed] [Google Scholar]
- Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI & Fu XY 2004. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A 101 4661–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Ottaway N, Schriever SC, Legutko B, Garcia-Caceres C, de la Fuente E, Mergen C, Bour S, Thaler JP, Seeley RJ, et al. 2014. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vidal-Itriago A, Milanova I, Korpel NL, Kalsbeek MJ, Tom RZ, Kalsbeek A, Hofmann SM & Yi CX 2018. Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Molecular Metabolism 7 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Yao T, Deng Z, Sohn JW, Sun J, Huang Y, Kong X, Yu KJ, Wang RT, Chen H, et al. 2017. TrpC5 Mediates Acute Leptin and Serotonin Effects via Pomc Neurons. Cell Reports 18 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Lei D, Welch J, Le K, Lin J, Leng S & Duhl D 2003. Agonist-dependent internalization of the human melanocortin-4 receptors in human embryonic kidney 293 cells. Journal of Pharmacology and Experimental Therapeutics 307 870–877. [DOI] [PubMed] [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. 2015. A neural basis for melanocortin-4 receptor-regulated appetite. Nature Neuroscience 18 863–U299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron L, Elmquist JK & Williams KW 2015. Neural control of energy balance: translating circuits to therapies. Cell 161 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron L, Lee C, Funahashi H, Friedman J, Lee S & Elmquist J 2010. Melanocortin-4 Receptor Expression in a Vago-vagal Circuitry Involved in Postprandial Functions. Journal of Comparative Neurology 518 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Cakir I, Lippert RN, Sweeney P, Litt MJ, Ellacott KLJ & Cone RD 2018. Regulation of energy rheostasis by the melanocortin-3 receptor. Sci Adv 4 eaat0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, et al. 2015. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature 520 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Srisai D & Cone RD 2011. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc Natl Acad Sci U S A 108 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi N & Skoda RC 1997. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Molecular Endocrinology 11 393–399. [DOI] [PubMed] [Google Scholar]
- Girardet C, Mavrikaki M, Southern MR, Smith RG & Butler AA 2014. Assessing interactions between Ghsr and Mc3r reveals a role for AgRP in the expression of food anticipatory activity in male mice. Endocrinology 155 4843–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardet C, Mavrikaki MM, Stevens JR, Miller CA, Marks DL & Butler AA 2017. Melanocortin-3 receptors expressed in Nkx2.1(+ve) neurons are sufficient for controlling appetitive responses to hypocaloric conditioning. Scientific Reports 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani D, Neri L, Canalini F, Calevro A, Ottani A, Vandini E, Sena P, Zaffe D & Guarini S 2015. NDP-alpha-MSH induces intense neurogenesis and cognitive recovery in Alzheimer transgenic mice through activation of melanocortin MC4 receptors. Molecular and Cellular Neuroscience 67 13–21. [DOI] [PubMed] [Google Scholar]
- Glas E, Muckter H, Gudermann T & Breit A 2016. Exchange factors directly activated by cAMP mediate melanocortin 4 receptor-induced gene expression. Scientific Reports 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz A, Lehti M, Donelan E, Striese C, Cucuruz S, Sachs S, Yi CX, Woods SC, Wright SD, Muller TD, et al. 2018. Circulating HDL levels control hypothalamic astrogliosis via apoA-I. Journal of Lipid Research 59 1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granell S, Mohammad S, Ramanagoudr-Bhojappa R & Baldini G 2010. Obesity-linked variants of melanocortin-4 receptor are misfolded in the endoplasmic reticulum and can be rescued to the cell surface by a chemical chaperone. Molecular Endocrinology 24 1805–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granell S, Molden BM & Baldini G 2013. Exposure of MC4R to agonist in the endoplasmic reticulum stabilizes an active conformation of the receptor that does not desensitize. Proceedings of the National Academy of Sciences of the United States of America 110 E4733–E4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granell S, Serra-Juhe C, Martos-Moreno GA, Diaz F, Perez-Jurado LA, Baldini G & Argente J 2012. A novel melanocortin-4 receptor mutation MC4R-P272L associated with severe obesity has increased propensity to be ubiquitinated in the ER in the face of correct folding. Plos One 7 e50894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JR 2011. Melanocortin Signalling and the Regulation of Blood Pressure in Human Obesity. Journal of Neuroendocrinology 23 186–193. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, et al. 2009. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 360 44–52. [DOI] [PubMed] [Google Scholar]
- Greenman Y, Kuperman Y, Drori Y, Asa SL, Navon I, Forkosh O, Gil S, Stern N & Chen A 2013. Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Molecular Endocrinology 27 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. 2005. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neuroscience 8 1289–1291. [DOI] [PubMed] [Google Scholar]
- Hadley ME & Dorr RT 2006. Melanocortin peptide therapeutics: Historical milestones, clinical studies and commercialization. Peptides 27 921–930. [DOI] [PubMed] [Google Scholar]
- Haeusler RA, McGraw TE & Accili D 2018. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol 19 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG & Schwartz MW 1998. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neuroscience 1 271–272. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK & Friedman JM 1995. Weight-Reducing Effects of the Plasma-Protein Encoded by the Obese Gene. Science 269 543–546. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Perras B, Fehm HL, Kern W & Born J 2008. Towards the therapeutic use of intranasal neuropeptide administration in metabolic and cognitive disorders. Regul Pept 149 79–83. [DOI] [PubMed] [Google Scholar]
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C, Elmquist JK, Flier JS & Hollenberg AN 2001. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. Journal of Clinical Investigation 107 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi H, Sheng Z, Routh V, Gerzanich V, Simard JM & Bryan J 2017. Direct versus indirect actions of ghrelin on hypothalamic NPY neurons. Plos One 12 e0184261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL & Cone RD 1999. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology 140 1408–1415. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Djalali A, Sivitz WI & Mark AL 1999. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33 542–547. [DOI] [PubMed] [Google Scholar]
- Hill C & Dunbar JC 2002. The effects of acute and chronic alpha melanocyte stimulating hormone (alphaMSH) on cardiovascular dynamics in conscious rats. Peptides 23 1625–1630. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB & Elmquist JK 2008. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. Journal of Clinical Investigation 118 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjuler KF & Lorentzen HF 2014. Melanoma associated with the use of melanotan-II. Dermatology 228 34–36. [DOI] [PubMed] [Google Scholar]
- Ho GY & MacKenzie RG 1999. Functional characterization of mutations in melanocortin-4 receptor associated with human obesity. Journal of Biological Chemistry 274 35816–35822. [DOI] [PubMed] [Google Scholar]
- Hong J, Stubbins RE, Smith RR, Harvey AE & Nunez NP 2009. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J 8 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Andrews ZB & Diano S 2009. Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab 20 78–87. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Naftolin F, Kalra SP & Leranth C 1992. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology 131 2461–2467. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, et al. 2010. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proceedings of the National Academy of Sciences of the United States of America 107 14875–14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby VJ 2016. Design of cyclic peptides with biological activities from biologically active peptides: the case of peptide modulators of melanocortin receptors. Biopolymers 106 884–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Kong D, Byun KH, Ye C, Koda S, Lee DH, Oh BC, Lee SW, Lee B, Zabolotny JM, et al. 2012. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nature Neuroscience 15 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H & Tao Y-X 2014. A Small Molecule Agonist THIQ as a Novel Pharmacoperone for Intracellularly Retained Melanocortin-4 Receptor Mutants. International Journal of Biological Sciences 10 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88 131–141. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ & Kelly MJ 2003. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144 1331–1340. [DOI] [PubMed] [Google Scholar]
- Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M & Shima K 1996. Substitution at codon 269 (glutamine --> proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun 224 597–604. [DOI] [PubMed] [Google Scholar]
- Ipata PL, Barsotti C, Tozzi MG, Camici M & Balestri F 2010. Metabolic interplay between intra- and extra-cellular uridine metabolism via an ATP driven uridine-UTP cycle in brain. International Journal of Biochemistry & Cell Biology 42 932–937. [DOI] [PubMed] [Google Scholar]
- Iwasa M, Kawabe K & Sapru HN 2013. Activation of melanocortin receptors in the intermediolateral cell column of the upper thoracic cord elicits tachycardia in the rat. Am J Physiol Heart Circ Physiol 305 H885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegou S, Boutelet I & Vaudry H 2000. Melanocortin-3 receptor mRNA expression in pro-opiomelanocortin neurones of the rat arcuate nucleus. Journal of Neuroendocrinology 12 501–505. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Lee DK & Jo YH 2017. Cholinergic neurons in the dorsomedial hypothalamus regulate food intake. Molecular Metabolism 6 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S & Diano S 2018. Mitochondrial Dynamics and Hypothalamic Regulation of Metabolism. Endocrinology 159 3596–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joppa MA, Gogas KR, Foster AC & Markison S 2007. Central infusion of the melanocortin receptor antagonist agouti-related peptide (AgRP(83–132)) prevents cachexia-related symptoms induced by radiation and colon-26 tumors in mice. Peptides 28 636–642. [DOI] [PubMed] [Google Scholar]
- Ju SH, Cho GB & Sohn JW 2018. Understanding melanocortin-4 receptor control of neuronal circuits: Toward novel therapeutics for obesity syndrome. Pharmacological Research 129 10–19. [DOI] [PubMed] [Google Scholar]
- Kalin S, Heppner FL, Bechmann I, Prinz M, Tschop MH & Yi CX 2015. Hypothalamic innate immune reaction in obesity. Nat Rev Endocrinol 11 339–351. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, Bence KK, Hayes MR & Grill HJ 2012. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab 303 E496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerer M, Koch M, Metzinger E, Mushack J, Capp E & Haring HU 1997. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia 40 1358–1362. [DOI] [PubMed] [Google Scholar]
- Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL & Culler MD 2013. Chronic Treatment With a Melanocortin-4 Receptor Agonist Causes Weight Loss, Reduces Insulin Resistance, and Improves Cardiovascular Function in Diet-Induced Obese Rhesus Macaques. Diabetes 62 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, et al. 2006. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nature Neuroscience 9 901–906. [DOI] [PubMed] [Google Scholar]
- Kim MS, Yoon CY, Park KH, Shin CS, Park KS, Kim SY, Cho BY & Lee HK 2003. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport 14 1317–1320. [DOI] [PubMed] [Google Scholar]
- Kim YB, Uotani S, Pierroz DD, Flier JS & Kahn BB 2000. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology 141 2328–2339. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC Jr., Xu AW, Barsh GS, Rossetti L & Accili D 2006. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nature Medicine 12 534–540. [DOI] [PubMed] [Google Scholar]
- Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, et al. 2011. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nature Neuroscience 14 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegler FH, Grove KL, Schiffmacher A, Smith MS & Cameron JL 2001. Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology 142 2586–2592. [DOI] [PubMed] [Google Scholar]
- Kong D, Dagon Y, Campbell JN, Guo Y, Yang Z, Yi X, Aryal P, Wellenstein K, Kahn BB, Sabatini BL, et al. 2016. A Postsynaptic AMPK-->p21-Activated Kinase Pathway Drives Fasting-Induced Synaptic Plasticity in AgRP Neurons. Neuron 91 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner AC & Bruning JC 2012. Selective insulin and leptin resistance in metabolic disorders. Cell Metabolism 16 144–152. [DOI] [PubMed] [Google Scholar]
- Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al. 2007. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metabolism 5 438–449. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL & Lowell BB 2011. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. Journal of Clinical Investigation 121 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Lowell BB & Garfield AS 2016. Melanocortin-4 receptor-regulated energy homeostasis. Nature Neuroscience 19 206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S & Lowell BB 2013. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metabolism 18 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. 2014. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen P, Clement K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, Mai K, Blume-Peytavi U, Gruters A & Krude H 2016. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. New England Journal of Medicine 375 240–246. [DOI] [PubMed] [Google Scholar]
- Kumar KG, Sutton GM, Dong JZ, Roubert P, Plas P, Halem HA, Culler MD, Yang H, Dixit VD & Butler AA 2009. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R-and MC4R-deficient C57BL/6J mice. Peptides 30 1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Silva AA & Hall JE 2002. Chronic cardiovascular and renal responses to melanocortin-4 receptor activation or inhibition. Hypertension 40 386–386. [Google Scholar]
- Kuo JJ, Silva AA & Hall JE 2003. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 41 768–774. [DOI] [PubMed] [Google Scholar]
- Kwon O, Kim KW & Kim MS 2016. Leptin signalling pathways in hypothalamic neurons. Cellular and Molecular Life Sciences 73 1457–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BYH, Cimino I, Polex-Wolf J, Nicole Kohnke S, Rimmington D, Iyemere V, Heeley N, Cossetti C, Schulte R, Saraiva LR, et al. 2017. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Molecular Metabolism 6 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DD, Garfield AS, Marston OJ, Shaw J & Heisler LK Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav 97 84–91. [DOI] [PubMed] [Google Scholar]
- Lam DD, Garfield AS, Marston OJ, Shaw J & Heisler LK 2010. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav 97 84–91. [DOI] [PubMed] [Google Scholar]
- Lam DD, Leinninger GM, Louis GW, Garfield AS, Marston OJ, Leshan RL, Scheller EL, Christensen L, Donato J Jr., Xia J, et al. 2011. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metabolism 13 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O’Rahilly S & Heisler LK 2008. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Kim HJ, Lee YS, Kang GM, Lim HS, Lee SH, Song DK, Kwon O, Hwang I, Son M, et al. 2018. Hypothalamic Macrophage Inducible Nitric Oxide Synthase Mediates Obesity-Associated Hypothalamic Inflammation. Cell Reports 25 934–946 e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, et al. 2012. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nature Neuroscience 15 700–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Lee SH, Jung JW, Lee WT, Kim BJ, Park KW, Lim SK, Yoon CJ & Baik JH 2001. Differential regulation of cAMP-mediated gene transcription and ligand selectivity by MC3R and MC4R melanocortin receptors. European Journal of Biochemistry 268 582–591. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI & Friedman JM 1996. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379 632–635. [DOI] [PubMed] [Google Scholar]
- Legradi G & Lechan RM 1999. Agouti-related protein containing nerve terminals innervate thyrotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology 140 3643–3652. [DOI] [PubMed] [Google Scholar]
- Li C & Friedman JM 1999. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc Natl Acad Sci U S A 96 9677–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang Y & Cai D 2012. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nature cell biology 14 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Cui BP, Zhang LL, Sun HJ, Liu TY & Zhu GQ 2013. Melanocortin ¾ receptors in paraventricular nucleus modulate sympathetic outflow and blood pressure. Experimental Physiology 98 435–443. [DOI] [PubMed] [Google Scholar]
- Li XF & Lytton J 2014. An Essential Role for the K+-dependent Na+/Ca2+-exchanger, NCKX4, in Melanocortin-4-receptor-dependent Satiety. Journal of Biological Chemistry 289 25445–25459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Jackson KL, Stern JE, Rabeler B & Patel KP 2006. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 291 H2847–2856. [DOI] [PubMed] [Google Scholar]
- Li YQ, Shrestha Y, Pandey M, Chen M, Kablan A, Gavrilova O, Offermanns S & Weinstein LS 2016. G(q/11)alpha and G(s)alpha mediate distinct physiological responses to central melanocortins. Journal of Clinical Investigation 126 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhou Y, Carter-Su C, Myers MG Jr. & Rui L 2007. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Molecular Endocrinology 21 2270–2281. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Kask A, Hagg E, Harmark L, Bergstrom L & Wikberg J 2002. Chronic infusion of a melanocortin receptor agonist modulates dopamine receptor binding in the rat brain. Pharmacological Research 45 119–124. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Schioth HB, Watanobe H, Suda T, Wikberg JE & Bergstrom L 2000. Downregulation of melanocortin receptors in brain areas involved in food intake and reward mechanisms in obese (OLETF) rats. Brain Research 852 180–185. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P & Erlanson-Albertsson C 2006. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 13 1385–1388. [DOI] [PubMed] [Google Scholar]
- Lippert RN, Ellacott KL & Cone RD 2014. Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology 155 1718–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MJ, Cone RD & Ghamari-Langroudi M 2018. Characterization of MC4R Regulation of the Kir7.1 Channel Using the Tl(+) Flux Assay. Methods Mol Biol 1684 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM & Elmquist JK 2003. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. Journal of Neuroscience 23 7143–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL & Lowell BB 2012. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 73 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, et al. 2017. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K, Fukushima A, Zhang X, Galic S, Briggs D, Enriori PJ, Simonds S, Wiede F, Reichenbach A, Hauser C, et al. 2011. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metabolism 14 684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H & Watson SJ 2003. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. Journal of Neuroscience 23 7863–7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang SM, Bertrais S, Hercberg S, Basdevant A, Clement K, et al. 2006. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: Prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. Journal of Clinical Endocrinology & Metabolism 91 1811–1818. [DOI] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Durand E, Dubern B, Shapiro A, Dazin P, Weill J, Ferron C, Froguel P & Vaisse C 2003. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Human Molecular Genetics 12 145–153. [DOI] [PubMed] [Google Scholar]
- Luo SX, Huang J, Li Q, Mohammad H, Lee CY, Krishna K, Kok AM, Tan YL, Lim JY, Li H, et al. 2018. Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361 76–81. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS & Palmiter RD 2005. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310 683–685. [DOI] [PubMed] [Google Scholar]
- Ma ZA, Zhao Z & Turk J 2012. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Experimental diabetes research 2012 703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB & Andermann ML 2015. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri LR, Lu Y, Xu Y, Cassidy RM, Xu Y, Arenkiel BR & Tong Q 2018. A neural basis for antagonistic control of feeding and compulsive behaviors. Nature Communications 9 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Castorena CM, Osborne-Lawrence S, Vijayaraghavan P, Metzger NP, Elmquist JK & Zigman JM 2018. Ghrelin mediates exercise endurance and the feeding response post-exercise. Molecular Metabolism 9 114–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Osborne-Lawrence S, Mequinion M, Lawrence S, Gautron L, Andrews ZB & Zigman JM 2017. The role of ghrelin-responsive mediobasal hypothalamic neurons in mediating feeding responses to fasting. Molecular Metabolism 6 882–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK & Zigman JM 2017. Ghrelin as a Survival Hormone. Trends Endocrinol Metab 28 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DL, Butler AA, Turner R, Brookhart G & Cone RD 2003. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology 144 1513–1523. [DOI] [PubMed] [Google Scholar]
- Martin WJ, McGowan E, Cashen DE, Gantert LT, Drisko JE, Hom GJ, Nargund R, Sebhat I, Howard AD, Van der Ploeg LHT, et al. 2002. Activation of melanocortin MC4 receptors increases erectile activity in rats ex copula. European Journal of Pharmacology 454 71–79. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Isse N, Satoh N, Okazaki T, Shigemoto M, Mori K, Tamura N, Hosoda K, Yoshimasa Y, et al. 1995. Human obese gene expression. Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes 44 855–858. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Girardet C, Kern A, Brantley AF, Miller CA, Macarthur H, Marks DL & Butler AA 2016. Melanocortin-3 receptors in the limbic system mediate feeding-related motivational responses during weight loss. Molecular Metabolism 5 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel FK, Molden BM, Mohammad S, Baldini G, McPike L, Narducci P, Granell S & Baldini G 2012. Constitutive Cholesterol-dependent Endocytosis of Melanocortin-4 Receptor (MC4R) Is Essential to Maintain Receptor Responsiveness to alpha-Melanocyte-stimulating Hormone (alpha-MSH). Journal of Biological Chemistry 287 21873–21890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane MR, Brown MS, Goldstein JL & Zhao TJ 2014. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metabolism 20 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn JE, Wilkinson CW, Havel PJ, Woods SC & Schwartz MW 2000. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. American journal of physiology Regulatory, integrative and comparative physiology 279 R695–703. [DOI] [PubMed] [Google Scholar]
- McNay DEG, Briancon N, Kokoeva MV, Maratos-Flier E & Flier JS 2012. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. The Journal of clinical investigation 122 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimaridou E, Gooljar SB, Ramnarace N, Anthonypillai L, Clark AJL & Chapple JP 2011. The Cytosolic Chaperone Hsc70 Promotes Traffic to the Cell Surface of Intracellular Retained Melanocortin-4 Receptor Mutants. Molecular Endocrinology 25 1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera P, Ferron M & Mosialou I 2018. Regulation of Energy Metabolism by Bone-Derived Hormones. Cold Spring Harb Perspect Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Hentges ST, Meshul CK & Low MJ 2013. Unraveling the central proopiomelanocortin neural circuits. Front Neurosci 7 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I & Pillon D 2010. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. European Journal of Neuroscience 32 2042–2052. [DOI] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DML, Anhe G, Amaral ME, Takahashi HK, et al. 2009. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. The Journal of neuroscience : the official journal of the Society for Neuroscience 29 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo XL & Tao YX 2013. Activation of MAPK by inverse agonists in six naturally occurring constitutively active mutant human melanocortin-4 receptors. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1832 1939–1948. [DOI] [PubMed] [Google Scholar]
- Mohammad S, Baldini G, Granell S, Narducci P, Martelli AM & Baldini G 2007. Constitutive traffic of melanocortin-4 receptor in neuro2A cells and immortalized hypothalamic neurons. Journal of Biological Chemistry 282 4963–4974. [DOI] [PubMed] [Google Scholar]
- Molden BM, Cooney KA, West K, Van Der Ploeg LHT & Baldini G 2015. Temporal cAMP Signaling Selectivity by Natural and Synthetic MC4R Agonists. Molecular Endocrinology 29 1619–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller CL, Pedersen SB, Richelsen B, Conde-Frieboes KW, Raun K, Grove KL & Wulff BS 2015. Melanocortin agonists stimulate lipolysis in human adipose tissue explants but not in adipocytes. BMC Res Notes 8 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, et al. 1997. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387 903–908. [DOI] [PubMed] [Google Scholar]
- Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira ALR, Saad MJ, et al. 2009. High-fat diet induces apoptosis of hypothalamic neurons. Plos One 4 e5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morari J, Anhe GF, Nascimento LF, de Moura RF, Razolli D, Solon C, Guadagnini D, Souza G, Mattos AH, Tobar N, et al. 2014. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes 63 3770–3784. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Meek TH & Schwartz MW 2014. Neurobiology of food intake in health and disease. Nature Reviews Neuroscience 15 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VA, Uchoa MF & Pike CJ 2018. TLR4 inhibitor TAK-242 attenuates the adverse neural effects of diet-induced obesity. J Neuroinflammation 15 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J, et al. 2017. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 543 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounien L, Marty N, Tarussio D, Metref S, Genoux D, Preitner F, Foretz M & Thorens B 2010. Glut2-dependent glucose-sensing controls thermoregulation by enhancing the leptin sensitivity of NPY and POMC neurons. Faseb Journal 24 1747–1758. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB & Cone RD 1994. Localization of the Melanocortin-4 Receptor (Mc4-R) in Neuroendocrine and Autonomic Control-Circuits in the Brain. Molecular Endocrinology 8 1298–1308. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT & Cone RD 1992. The Cloning of a Family of Genes That Encode the Melanocortin Receptors. Science 257 1248–1251. [DOI] [PubMed] [Google Scholar]
- Muller TD, Tschop MH & O’Rahilly S 2016. Metabolic Precision Medicines: Curing POMC Deficiency. Cell Metabolism 24 194–195. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS & Bjorbaek C 2004. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145 4880–4889. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K & Matsukura S 2001. A role for ghrelin in the central regulation of feeding. Nature 409 194–198. [DOI] [PubMed] [Google Scholar]
- Nasrallah CM & Horvath TL 2014. Mitochondrial dynamics in the central regulation of metabolism. Nat Rev Endocrinol 10 650–658. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B & Goldman SA 2003. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26 523–530. [DOI] [PubMed] [Google Scholar]
- Newman EA, Chai BX, Zhang WZ, Li JY, Ammori JB & Mulholland MW 2006. Activation of the melanocortin-4 receptor mobilizes intracellular free calcium in immortalized hypothalamic neurons. Journal of Surgical Research 132 201–207. [DOI] [PubMed] [Google Scholar]
- Ni X-P, Butler AA, Cone RD & Humphreys MH 2006. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. Journal of Hypertension 24 2239–2246. [DOI] [PubMed] [Google Scholar]
- Nickolls SA, Fleck B, Hoare SR & Maki RA 2005. Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: evidence for ligand-specific conformational states. Journal of Pharmacology and Experimental Therapeutics 313 1281–1288. [DOI] [PubMed] [Google Scholar]
- Nijenhuis WAJ, Garner KM, van Rozen RJ & Adan RAH 2003. Poor cell surface expression of human melanocortin-4 receptor mutations associated with obesity. Journal of Biological Chemistry 278 22939–22945. [DOI] [PubMed] [Google Scholar]
- Nijenhuis WAJ, Oosterom J & Adan RAH 2001. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Molecular Endocrinology 15 164–171. [DOI] [PubMed] [Google Scholar]
- Novoselova TV, Larder R, Rimmington D, Lelliott C, Wynn EH, Gorrigan RJ, Tate PH, Guasti L, Sanger Mouse Genetics P, O’Rahilly S, et al. 2016. Loss of Mrap2 is associated with Sim1 deficiency and increased circulating cholesterol. Journal of Endocrinology 230 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamugenda E, Trentzsch M, Russell S, Miles T, Boysen G, Phelan KD & Baldini G 2019. Injury to hypothalamic Sim1 neurons is a common feature of obesity by exposure to high fat diet in male and female mice. Journal of Neurochemistry [DOI] [PMC free article] [PubMed]
- Obici S, Feng Z, Arduini A, Conti R & Rossetti L 2003. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nature Medicine 9 756–761. [DOI] [PubMed] [Google Scholar]
- Obici S & Rossetti L 2003. Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 144 5172–5178. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen YR, Gantz I & Barsh GS 1997. Antagonism of central melanocortin receptors in vitro and in vivo by Agouti-related protein. Science 278 135–138. [DOI] [PubMed] [Google Scholar]
- Oosterom J, Garner KM, den Dekker WK, Nijenhuiss WAJ, Gispen WH, Burbach JPH, Barsh GS & Adan RAH 2001. Common requirements for melanocortin-4 receptor selectivity of structurally unrelated melanocortin agonist and endogenous antagonist, Agouti protein. Journal of Biological Chemistry 276 931–936. [DOI] [PubMed] [Google Scholar]
- Overton JD & Leibel RL 2011. Mahoganoid and mahogany mutations rectify the obesity of the yellow mouse by effects on endosomal traffic of MC4R protein. Journal of Biological Chemistry 286 18914–18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, Quintana A, Zweifel LS, Ronnekleiv OK, Kelly MJ, et al. 2016. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nature Neuroscience 19 734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeger L, Karakasilioti I, Altmuller J, Frommolt P, Bruning J & Kloppenburg P 2017a. Antagonistic modulation of NPY/AgRP and POMC neurons in the arcuate nucleus by noradrenalin. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeger L, Pippow A, Hess S, Paehler M, Klein AC, Husch A, Pouzat C, Bruning JC & Kloppenburg P 2017b. Energy imbalance alters Ca(2+) handling and excitability of POMC neurons. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaro BL, Tough IR, Engelstoft MS, Matthews RT, Digby GJ, Moller CL, Svendsen B, Gribble F, Reimann F, Holst JJ, et al. 2014. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metabolism 20 1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, de Jong JW, Vanderschuren LJ & Adan RA 2011. Neurobiology of overeating and obesity: the role of melanocortins and beyond. European Journal of Pharmacology 660 28–42. [DOI] [PubMed] [Google Scholar]
- Pandit R, la Fleur SE & Adan RA 2013. The role of melanocortins and Neuropeptide Y in food reward. European Journal of Pharmacology 719 208–214. [DOI] [PubMed] [Google Scholar]
- Pandit R, van der Zwaal EM, Luijendijk MC, Brans MA, van Rozen AJ, Oude Ophuis RJ, Vanderschuren LJ, Adan RA & la Fleur SE 2015. Central melanocortins regulate the motivation for sucrose reward. Plos One 10 e0121768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Park M, Choi J, Park KY, Chung HY & Lee J 2010. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neuroscience Letters 482 235–239. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. 2007. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449 228–232. [DOI] [PubMed] [Google Scholar]
- Paul A, Chaker Z & Doetsch F 2017. Hypothalamic regulation of regionally distinct adult neural stem cells and neurogenesis. Science 356 1383–1386. [DOI] [PubMed] [Google Scholar]
- Pedroso JA, Buonfiglio DC, Cardinali LI, Furigo IC, Ramos-Lobo AM, Tirapegui J, Elias CF & Donato J Jr. 2014. Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Molecular Metabolism 3 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T & Collins F 1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269 540–543. [DOI] [PubMed] [Google Scholar]
- Perez-Oliva AB, Olivares C, Jimenez-Cervantes C & Garcia-Borron JC 2009. Mahogunin Ring Finger-1 (MGRN1) E3 Ubiquitin Ligase Inhibits Signaling from Melanocortin Receptor by Competition with G alpha(s). Journal of Biological Chemistry 284 31714–31725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MF & Scott CW 2009. Evaluating cellular impedance assays for detection of GPCR pleiotropic signaling and functional selectivity. Journal of Biomolecular Screening 14 246–255. [DOI] [PubMed] [Google Scholar]
- Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ & Hess JF 1996. Leptin receptor missense mutation in the fatty Zucker rat. Nature Genetics 13 18–19. [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS & Mantzoros CS 2002. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 51 1337–1345. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM & Horvath TL 2004. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304 110–115. [DOI] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, et al. 2009. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nature Medicine 15 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podyma B, Sun H, Wilson EA, Carlson B, Pritikin E, Gavrilova O, Weinstein LS & Chen M 2018. The stimulatory G protein Gsalpha is required in melanocortin 4 receptor-expressing cells for normal energy balance, thermogenesis, and glucose metabolism. Journal of Biological Chemistry 293 10993–11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N, van der Leij F, Jackson V, Corstorphine C, Thomson R, Sorensen A & Zammit V 2002. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics 80 433–442. [DOI] [PubMed] [Google Scholar]
- Proneth B, Xiang ZM, Pogozheva ID, Litherland SA, Gorbatyuk OS, Shaw AM, Millard WJ, Mosberg HI & Haskell-Luevano C 2006. Molecular mechanism of the constitutive activation of the L250Q human melanocortin-4 receptor polymorphism. Chemical Biology & Drug Design 67 215–229. [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, et al. 2002. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Molecular and Cellular Biology 22 5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Meza C, Navarro UV, Nestor CC, Wagner EJ, Ronnekleiv OK & Kelly MJ 2018a. Estradiol Protects Proopiomelanocortin Neurons Against Insulin Resistance. Endocrinology 159 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Wagner EJ, Ronnekleiv OK & Kelly MJ 2018b. Insulin and leptin excite anorexigenic pro-opiomelanocortin neurones via activation of TRPC5 channels. Journal of Neuroendocrinology 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Ronnekleiv OK & Kelly MJ 2014. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metabolism 19 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Gomez-Valades AG, Schneeberger M, Varela L, Haddad-Tovolli R, Altirriba J, Noguera E, Drougard A, Flores-Martinez A, Imbernon M, et al. 2017. Mitochondrial Dynamics Mediated by Mitofusin 1 Is Required for POMC Neuron Glucose-Sensing and Insulin Release Control. Cell Metabolism 25 1390–1399 e1396. [DOI] [PubMed] [Google Scholar]
- Reis WL, Yi CX, Gao Y, Tschop MH & Stern JE 2015. Brain innate immunity regulates hypothalamic arcuate neuronal activity and feeding behavior. Endocrinology 156 1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Cook JR, Kon N & Accili D 2015. Gpr17 in AgRP Neurons Regulates Feeding and Sensitivity to Insulin and Leptin. Diabetes 64 3670–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Orozco IJ, Su Y, Suyama S, Gutierrez-Juarez R, Horvath TL, Wardlaw SL, Plum L, Arancio O & Accili D 2012. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell 149 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rene P, Le Gouill C, Pogozheva ID, Lee G, Mosberg HI, Farooqi IS, Valenzano KJ & Bouvier M 2010. Pharmacological chaperones restore function to MC4R mutants responsible for severe early-onset obesity. Journal of Pharmacology and Experimental Therapeutics 335 520–532. [DOI] [PubMed] [Google Scholar]
- Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KLJ & Cone RD 2012. Melanocortin-3 receptor regulates the normal fasting response. Proceedings of the National Academy of Sciences of the United States of America 109 E1489–E1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Yu S, Jiang Y, Laque A, Schwartzenburg C, Morrison CD, Derbenev AV, Zsombok A & Munzberg H 2014. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Molecular Metabolism 3 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J, Cruz MT, Majumdar U, Lewin A, Kingsbury KA, Dezfuli G, Vicini S, Verbalis JG, Dretchen KL, Gillis RA, et al. 2013. Melanocortin signaling in the brainstem influences vagal outflow to the stomach. Journal of Neuroscience 33 13286–13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne P, Tikka S, Makela S, Streng T & Savontaus E 2012. Hemodynamic actions and mechanisms of systemically administered alpha-MSH analogs in mice. Peptides 38 150–158. [DOI] [PubMed] [Google Scholar]
- Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, Saha PK, Del Solar M, Zhu BK, York B, et al. 2016. Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell 165 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry AG 2013. Altered feeding and body weight following melanocortin administration to the ventral tegmental area in adult rats. Psychopharmacology (Berl) 226 25–34. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Stuhrman K & Dunigan AI 2015. Regulation of the mesocorticolimbic and mesostriatal dopamine systems by alpha-melanocyte stimulating hormone and agouti-related protein. Neurosci Biobehav Rev 56 15–25. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB & Cone RD 1993. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A 90 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB & Elmquist JK 2011. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metabolism 13 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE, Schaub JW, Robertson KL, Andreasen A & Haskell-Luevano C 2010. Effect of MTII on food intake and brain c-Fos in melanocortin-3, melanocortin-4, and double MC3 and MC4 receptor knockout mice. Peptides 31 2314–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp AC, Allison MB, Jones JC, Patterson CM, Faber CL, Bozadjieva N, Heisler LK, Seeley RJ, Olson DP & Myers MG Jr. 2018. Specific subpopulations of hypothalamic leptin receptor-expressing neurons mediate the effects of early developmental leptin receptor deletion on energy balance. Molecular Metabolism [DOI] [PMC free article] [PubMed]
- Saltiel AR & Olefsky JM 2017. Inflammatory mechanisms linking obesity and metabolic disease. Journal of Clinical Investigation 127 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AS, Mullier A, Maicas N, Oosterhuis NR, Eun Bae S, Novoselova TV, Chan LF, Pombo JM, Taylor PD, Joles JA, et al. 2016. Central role for melanocortin-4 receptors in offspring hypertension arising from maternal obesity. Proc Natl Acad Sci U S A 113 12298–12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A, Campolo M, Liu C, Sesaki H, Meli R, Liu ZW, Kim JD & Diano S 2017. DRP1 Suppresses Leptin and Glucose Sensing of POMC Neurons. Cell Metabolism 25 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Legradi G & Lechan RM 2002. Intracerebroventricular administration of alpha-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain Research 945 50–59. [DOI] [PubMed] [Google Scholar]
- Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB & Hadley ME 1980. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A 77 5754–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodriguez IC, Bortolozzi A, et al. 2013. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155 172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Gomez-Valades AG, Altirriba J, Sebastian D, Ramirez S, Garcia A, Esteban Y, Drougard A, Ferres-Coy A, Bortolozzi A, et al. 2015. Reduced alpha-MSH Underlies Hypothalamic ER-Stress-Induced Hepatic Gluconeogenesis. Cell Reports 12 361–370. [DOI] [PubMed] [Google Scholar]
- Sebag JA, Zhang C, Hinkle PM, Bradshaw AM & Cone RD 2013. Developmental Control of the Melanocortin-4 Receptor by MRAP2 Proteins in Zebrafish. Science 341 278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, Yang Z, Fuller PM, Elmquist JK & Lowell BB 2014. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci U S A 111 13193–13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AM, Irani BG, Moore MC, Haskell-Luevano C & Millard WJ 2005. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides 26 1720–1727. [DOI] [PubMed] [Google Scholar]
- Shinyama H, Masuzaki H, Fang H & Flier JS 2003. Regulation of melanocortin-4 receptor signaling: Agonist-mediated desensitization and internalization. Endocrinology 144 1301–1314. [DOI] [PubMed] [Google Scholar]
- Shrestha YB, Vaughan CH, Smith BJ, Song CK, Baro DJ & Bartness TJ 2010. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 299 R140–R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siljee JE, Wang Y, Bernard AA, Ersoy BA, Zhang S, Marley A, Von Zastrow M, Reiter JF & Vaisse C 2018. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nature Genetics 50 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singru PS, Sanchez E, Fekete C & Lechan RM 2006. Importance of Melanocortin Signaling in Refeeding-Induced Neuronal Activation and Satiety. Endocrinology [DOI] [PubMed]
- Singru PS, Sanchez E, Fekete C & Lechan RM 2007. Importance of melanocortin signaling in refeeding-induced neuronal activation and satiety. Endocrinology 148 638–646. [DOI] [PubMed] [Google Scholar]
- Skibicka KP & Grill HJ 2009. Hypothalamic and Hindbrain Melanocortin Receptors Contribute to the Feeding, Thermogenic, and Cardiovascular Action of Melanocortins. Endocrinology 150 5351–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Harris LE, Berglund ED, Liu TM, Vong L, Lowell BB, Balthasar N, Williams KW & Elmquist JK 2013. Melanocortin 4 Receptors Reciprocally Regulate Sympathetic and Parasympathetic Preganglionic Neurons. Cell 152 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW & Williams KW 2012. Functional Heterogeneity of Arcuate Nucleus Pro-Opiomelanocortin Neurons: Implications for Diverging Melanocortin Pathways. Molecular Neurobiology 45 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW & Elmquist JK 2011. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR & Vaisse C 2004. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. Journal of Clinical Investigation 114 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steculorum SM, Paeger L, Bremser S, Evers N, Hinze Y, Idzko M, Kloppenburg P & Bruning JC 2015. Hypothalamic UDP Increases in Obesity and Promotes Feeding via P2Y6-Dependent Activation of AgRP Neurons. Cell 162 1404–1417. [DOI] [PubMed] [Google Scholar]
- Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engstrom Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, et al. 2016. AgRP Neurons Control Systemic Insulin Sensitivity via Myostatin Expression in Brown Adipose Tissue. Cell 165 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiakouli E, Gaillard R, Tavare JM, Balthasar N, Loos RJ, Taal HR, Evans DM, Rivadeneira F, St Pourcain B, Uitterlinden AG, et al. 2014. Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity (Silver Spring) 22 2252–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM 2016. Hunger: The carrot and the stick. Molecular Metabolism 5 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM & Friedman JM 2005. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nature Neuroscience 8 1356–1363. [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Ngo ST, Chen VP, Bailey-Downs LC, Xie TY, Ghadami M, Brimijoin S, Freeman WM, Rubinstein M, Low MJ, et al. 2018. 17alpha-estradiol acts through hypothalamic pro-opiomelanocortin expressing neurons to reduce feeding behavior. Aging Cell 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O’Rahilly S, et al. 2008. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 57 2511–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Alhadeff AL & Betley JN 2017. Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Reports 21 2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ahmed S & Smith RG 2003. Deletion of ghrelin impairs neither growth nor appetite. Molecular and Cellular Biology 23 7973–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Butte NF, Garcia JM & Smith RG 2008. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 149 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Begriche K, Kumar KG, Gimble JM, Perez-Tilve D, Nogueiras R, McMillan RP, Hulver MW, Tschop MH & Butler AA 2010. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. Faseb Journal 24 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Lee K, Jing E, Biddinger SB, McDonald JG, Montine TJ, Craft S & Kahn CR 2010. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metabolism 12 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart I, Jahng JW, Overton JM & Houpt TA 2002. Hypothalamic NPY, AGRP, and POMC mRNA responses to leptin and refeeding in mice. Am J Physiol Regul Integr Comp Physiol 283 R1020–1026. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Rainey MA & Palmiter RD 2000. Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nature Genetics 25 102–104. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM & White MF 2007. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317 369–372. [DOI] [PubMed] [Google Scholar]
- Takahashi KA & Cone RD 2005. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146 1043–1047. [DOI] [PubMed] [Google Scholar]
- Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, Masuzaki H, Mori K, Tamura N, Hosoda K & Nakao K 1996. Molecular cloning of rat leptin receptor isoform complementary DNAs--identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun 225 75–83. [DOI] [PubMed] [Google Scholar]
- Tallam LS, Stec DE, Willis MA, da Silva AA & Hall JE 2005. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension 46 326–332. [DOI] [PubMed] [Google Scholar]
- Tan K, Knight ZA & Friedman JM 2014. Ablation of AgRP neurons impairs adaption to restricted feeding. Molecular Metabolism 3 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX 2010a. The Melanocortin-4 Receptor: Physiology, Pharmacology, and Pathophysiology. Endocrine Reviews 31 506–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX 2010b. Mutations in the melanocortin-3 receptor (MC3R) gene: Impact on human obesity or adiposity. Current Opinion in Investigational Drugs 11 1092–1096. [PubMed] [Google Scholar]
- Tao YX & Conn PM 2014. Chaperoning G protein-coupled receptors: from cell biology to therapeutics. Endocrine Reviews 35 602–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX & Conn PM 2018. Pharmacoperones as Novel Therapeutics for Diverse Protein Conformational Diseases. Physiol Rev 98 697–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX & Huang H 2014. Ipsen 5i is a novel potent pharmacoperone for intracellularly retained melanocortin-4 receptor mutants. Frontiers in Endocrinology 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX & Segaloff DL 2003. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology 144 4544–4551. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, et al. 1995. Identification and expression cloning of a leptin receptor, OB-R. Cell 83 1263–1271. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, et al. 2012. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation 122 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P & Seeley RJ 1998. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 274 R248–R254. [DOI] [PubMed] [Google Scholar]
- Tian Y, Peng B & Fu X 2018. New ADCY3 Variants Dance in Obesity Etiology. Trends Endocrinol Metab 29 361–363. [DOI] [PubMed] [Google Scholar]
- Tiganis T 2013. PTP1B and TCPTP--nonredundant phosphatases in insulin signaling and glucose homeostasis. Febs Journal 280 445–458. [DOI] [PubMed] [Google Scholar]
- Timper K & Bruning JC 2017. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Disease Models & Mechanisms 10 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timper K, Paeger L, Sanchez-Lasheras C, Varela L, Jais A, Nolte H, Vogt MC, Hausen AC, Heilinger C, Evers N, et al. 2018. Mild Impairment of Mitochondrial OXPHOS Promotes Fatty Acid Utilization in POMC Neurons and Improves Glucose Homeostasis in Obesity. Cell Reports 25 383–397 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda C, Kim JD, Impellizzeri D, Cuzzocrea S, Liu ZW & Diano S 2016. UCP2 Regulates Mitochondrial Fission and Ventromedial Nucleus Control of Glucose Responsiveness. Cell 164 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda C, Santoro A, Kim JD & Diano S 2017. POMC Neurons: From Birth to Death. Annu Rev Physiol 79 209–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK & Lowell BB 2008. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature Neuroscience 11 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsoni MA, Carvalheira JB, Pereira-Da-Silva M, de Carvalho-Filho MA, Saad MJ & Velloso LA 2003. Molecular and functional resistance to insulin in hypothalamus of rats exposed to cold. Am J Physiol Endocrinol Metab 285 E216–223. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K & Matsukura S 2001. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun 281 1220–1225. [DOI] [PubMed] [Google Scholar]
- Tsaousidou E, Paeger L, Belgardt BF, Pal M, Wunderlich CM, Bronneke H, Collienne U, Hampel B, Wunderlich FT, Schmidt-Supprian M, et al. 2014. Distinct Roles for JNK and IKK Activation in Agouti-Related Peptide Neurons in the Development of Obesity and Insulin Resistance. Cell Reports 9 1495–1506. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL & Heiman ML 2000. Ghrelin induces adiposity in rodents. Nature 407 908–913. [DOI] [PubMed] [Google Scholar]
- Tsou RC, Zimmer DJ, De Jonghe BC & Bence KK 2012. Deficiency of PTP1B in leptin receptor-expressing neurons leads to decreased body weight and adiposity in mice. Endocrinology 153 4227–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaanholt LM, Mitchell SE, Sinclair RE & Speakman JR 2015. Mice that are resistant to diet-induced weight loss have greater food anticipatory activity and altered melanocortin-3 receptor (MC3R) and dopamine receptor 2 (D2) gene expression. Hormones and Behavior 73 83–93. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B & Froguel P 2000. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. Journal of Clinical Investigation 106 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B & Froguel P 1998. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature Genetics 20 113–114. [DOI] [PubMed] [Google Scholar]
- Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres BA, Thaler JP, et al. 2017. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metabolism 26 185–197 e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW & Koliwad SK 2014. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Reports 9 2124–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM & Spanswick D 2004. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nature Neuroscience 7 493–494. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg LHT, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan XM, Drisko J, Cashen D, Sebhat I, Patchett AA, et al. 2002. A role for the melanocortin 4 receptor in sexual function. Proceedings of the National Academy of Sciences of the United States of America 99 11381–11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F & Arner P 1998. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 47 913–917. [DOI] [PubMed] [Google Scholar]
- Varazzani C, San-Galli A, Gilardeau S & Bouret S 2015. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. Journal of Neuroscience 35 7866–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela L & Horvath TL 2018. Neuronal Cilia: Another Player in the Melanocortin System. Trends Mol Med 24 333–334. [DOI] [PubMed] [Google Scholar]
- Velloso LA, Torsoni MA & Araujo EP 2009. Hypothalamic dysfunction in obesity. Reviews in the neurosciences 20 441–449. [DOI] [PubMed] [Google Scholar]
- Viskaitis P, Irvine EE, Smith MA, Choudhury AI, Alvarez-Curto E, Glegola JA, Hardy DG, Pedroni SMA, Paiva Pessoa MR, Fernando ABP, et al. 2017. Modulation of SF1 Neuron Activity Coordinately Regulates Both Feeding Behavior and Associated Emotional States. Cell Reports 21 3559–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, et al. 2014. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Molecular Metabolism 3 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li V, Chan GC, Phan T, Nudelman AS, Xia Z & Storm DR 2009. Adult type 3 adenylyl cyclase-deficient mice are obese. Plos One 4 e6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA & Hao A 2012. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signalling. Br J Nutr 107 229–241. [DOI] [PubMed] [Google Scholar]
- Wang ZQ & Tao YX 2011. Functional studies on twenty novel naturally occurring melanocortin-4 receptor mutations. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1812 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauman J, Zabeau L & Tavernier J 2017. The Leptin Receptor Complex: Heavier Than Expected? Front Endocrinol (Lausanne) 8 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellhoner P, Horster R, Jacobs F, Sayk F, Lehnert H & Dodt C 2012. Intranasal application of the melanocortin 4 receptor agonist MSH/ACTH(4–10) in humans causes lipolysis in white adipose tissue. International Journal of Obesity 36 703–708. [DOI] [PubMed] [Google Scholar]
- White CL, Whittington A, Barnes MJ, Wang Z, Bray GA & Morrison CD 2009. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. Am J Physiol Endocrinol Metab 296 E291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Kaplan JM & Grill HJ 2000. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-¾ receptor stimulation. Endocrinology 141 1332–1337. [DOI] [PubMed] [Google Scholar]
- Williams KW, Liu T, Kong X, Fukuda M, Deng Y, Berglund ED, Deng Z, Gao Y, Liu T, Sohn JW, et al. 2014. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metabolism 20 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF & Elmquist JK 2010. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. Journal of Neuroscience 30 2472–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang MJ, Cha SH, Millington DS, Cline G, Shulman GI, Suwa A, Asaumi M, Kurama T, Shimokawa T & Lane MD 2008. Brain-specific carnitine palmitoyl-transferase-1c: role in CNS fatty acid metabolism, food intake, and body weight. Journal of Neurochemistry 105 1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, Matsumoto S, Cha SH, Shimokawa T & Lane MD 2006. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci U S A 103 7282–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ & Sleeman MW 2005. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metabolism 2 421–427. [DOI] [PubMed] [Google Scholar]
- Woudberg NJ, Goedecke JH, Blackhurst D, Frias M, James R, Opie LH & Lecour S 2016. Association between ethnicity and obesity with high-density lipoprotein (HDL) function and subclass distribution. Lipids Health Dis 15 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, et al. 2001. Ghrelin causes hyperphagia and obesity in rats. Diabetes 50 2540–2547. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DGA, Ghatei MA, et al. 2000. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141 4325–4328. [DOI] [PubMed] [Google Scholar]
- Wu L, Shen C, Seed Ahmed M, Ostenson CG & Gu HF 2016. Adenylate cyclase 3: a new target for anti-obesity drug development. Obesity Reviews 17 907–914. [DOI] [PubMed] [Google Scholar]
- Wu Q, Howell MP & Palmiter RD 2008. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. Journal of Neuroscience 28 9218–9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q & Palmiter RD 2011. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. European Journal of Pharmacology 660 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y & Wikberg JE 1997. Postnatal expression of melanocortin-3 receptor in rat diencephalon and mesencephalon. Neuropharmacology 36 217–224. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW & Barsh GS 2005a. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol 3 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW & Barsh GS 2005b. PI3K integrates the action of insulin and leptin on hypothalamic neurons. Journal of Clinical Investigation 115 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH & Sternson SM 2011. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 146 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z & Tao YX 2016. Biased signaling initiated by agouti-related peptide through human melanocortin-3 and-4 receptors. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1862 1485–1494. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB & Hochgeschwender U 1999. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nature Medicine 5 1066–1070. [DOI] [PubMed] [Google Scholar]
- Yen HH & Roseberry AG 2015. Decreased consumption of rewarding sucrose solutions after injection of melanocortins into the ventral tegmental area of rats. Psychopharmacology (Berl) 232 285–294. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG & O’Rahilly S 1998. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature Genetics 20 111–112. [DOI] [PubMed] [Google Scholar]
- Yi CX, Walter M, Gao Y, Pitra S, Legutko B, Kalin S, Layritz C, Garcia-Caceres C, Bielohuby M, Bidlingmaier M, et al. 2017. TNFalpha drives mitochondrial stress in POMC neurons in obesity. Nature Communications 8 15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You PP, Hu HD, Chen YT, Zhao YL, Yang YQ, Wang TT, Xing RM, Shao YJ, Zhang W, Li DL, et al. 2016. Effects of Melanocortin 3 and 4 Receptor Deficiency on Energy Homeostasis in Rats. Scientific Reports 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, et al. 2002. PTP1B regulates leptin signal transduction in vivo. Dev Cell 2 489–495. [DOI] [PubMed] [Google Scholar]
- Zampieri TT, da Silva TE, de Paula Romeu D, Torrao AS & Donato J Jr. 2016. SOCS3 expression within leptin receptor-expressing cells regulates food intake and leptin sensitivity but does not affect weight gain in pregnant mice consuming a high-fat diet. Physiology & Behavior 157 109–115. [DOI] [PubMed] [Google Scholar]
- Zhang J & Scarpace PJ 2009. The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br J Pharmacol 158 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H & Cai D 2008. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Collazo R, Gao Y, Li G & Scarpace PJ 2010. Intermittent MTII application evokes repeated anorexia and robust fat and weight loss. Peptides 31 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L & Friedman JM 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372 425–432. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Reichel JM, Han C, Zuniga-Hertz JP & Cai D 2017. Astrocytic Process Plasticity and IKKbeta/NF-kappaB in Central Control of Blood Glucose, Blood Pressure, and Body Weight. Cell Metabolism 25 1091–1102 e1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Dodd GT & Tiganis T 2015. Protein Tyrosine Phosphatases in Hypothalamic Insulin and Leptin Signaling. Trends in Pharmacological Sciences 36 661–674. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li G, Li Y, Wang Y & Liu Z 2017. Knockdown of Tlr4 in the Arcuate Nucleus Improves Obesity Related Metabolic Disorders. Sci Rep 7 7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB & Elmquist JK 2006. Expression of ghrelin receptor mRNA in the rat and the mouse brain. Journal of Comparative Neurology 494 528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]


