Abstract
Background
Poorly differentiated thyroid carcinoma (PDTC) is an aggressive form of thyroid cancer that currently has limited effective treatment options. Immune checkpoint inhibitors (ICIs) have shown to be an effective treatment for a variety of carcinomas. In this study, we explore whether immune checkpoint pathways, such as Programmed cell death-ligand 1 (PD-L1) and Indoleamine 2,3-dioxygenase 1 (IDO1), are activated in a cohort of patients with PDTC to determine whether ICIs may be an effective therapy for these patients.
Methods
PDTC from 28 patients were stained for IDO1, PD-L1, and CD8 using immunohistochemistry. Staining was scored using an H-score, and PD-L1 and IDO1 expression was correlated with clinicopathologic characteristics. Positivity for PD-L1 and IDO1 was set at an H-score cutoff of 5.
Results
Twenty-five percent (n=7/28) of PDTC were positive for PD-L1 expression. Twenty-nine percent (n =2/7) of PD-L1 positive PDTCs also co-expressed IDO1. Expression of PD-L1 in PDTC was significantly associated with tumor size and multifocality, with a non-significant trend towards associations with older age, extra-thyroidal extension, presence of metastasis, higher stage, increased number of CD8+ T-cells, and decreased disease-free and overall survival.
Conclusions
PD-L1 expression occurs in a subset of PDTC, and is associated with a subset of clinical features of aggressive thyroid disease. Given the limited effective treatments for this patient population, consideration for ICIs as monotherapy or in combination with an IDO1 inhibitor should be explored as a novel treatment modality for patients with PDTC.
Keywords: Poorly-Differentiated Thyroid Carcinoma; PD-L1; IDO1; PD1; programmed cell death ligand 1; Indoleamine 2,3-Dioxygenase 1; immune checkpoint; immuno-oncology
Introduction
Poorly differentiated thyroid carcinoma (PDTC) is a follicular cell-derived thyroid neoplasm that has both histomorphologic features and clinical outcomes intermediate between well-differentiated thyroid cancers (WDTC, i.e. papillary or follicular carcinomas), and anaplastic (i.e. undifferentiated) thyroid carcinoma (ATC) [1]. The incidence of PDTC varies by country, ranging from 0.8% of thyroid cancers in Japan [2], to 2–3% in North America, and up to 15% in Northern Italy [3]. Unlike most WDTC, PDTC is an aggressive disease; extrathyroidal extension is present in up to 75% of patients, and distant metastasis is present in over half of patients [3][4]. The presence of PDTC, even as a minor tumor component (e.g. 10%) significantly reduces 10 year survival from 90% (for WDTC) to 50% [5][6].
Classification of a thyroid carcinoma as PDTC is based upon assessment of selected histomorphologic features included in the Turin criteria published by Volante et al. in 2007 [1]. These conventional diagnostic criteria consist of certain architectural patterns (such as an insular growth pattern), absence of papillary carcinoma nuclear features, and presence of certain other high grade histologic features [1]. Molecularly, as well as histologically, it appears that PDTC represents an intermediate step between WDTC (which often have BRAF, RAS, RET/PTC, or PAX8-PPARγ alterations), and ATC (which often harbor TP53, cyclin D1, TERT, and β-catenin abnormalities)[7–12]. PDTC often occurs in the setting of a prior or concurrent WDTC, where additional mutations may predispose to the transition to PDTC [7]. The histologic progression to PDTC often includes the accumulation of later-stage mutations such as TP53, TERT, and β-catenin, but without the full loss of differentiation seen in ATC [7–12].
Initial management of PDTC is similar to WDTC, with total thyroidectomy (if possible) and radioiodine being the mainstays of treatment. Unfortunately, these tumors are often poorly responsive, thus necessitating more aggressive therapy [3][4]. However, chemotherapy has not yet been shown to be effective, and is not currently recommended for routine management [13]. While multi-targeted tyrosine kinase inhibitors such as Lenvatinib improve progression-free survival in patients with advanced/metastatic WDTC, their role in PDTC is less clear [14, 15]. As such, enrollment in clinical trials is often recommended for patients with radioiodine-refractory metastatic disease, but outcomes are still generally poor. Immune checkpoint inhibitors (ICIs) are a promising treatment option in a number of malignancies, including head and neck cancers, and may be potentially useful in PDTC as well.
Programmed cell death-1 (PD-1) is an immune checkpoint molecule whose role is to suppress the CD8+ T-cell response upon interaction with its ligand, PD-L1 [16]. PD-1 is expressed on T-cells (as well as some other immune cells) in response to stimuli, such as interferon gamma, and upon interaction with PD-L1 (a membrane protein expressed on a variety of immune cells and which can be induced on cancer cells), results in T-cell anergy and apoptosis [17]. Although this response is physiologic in the context of preventing autoimmunity, it is pathologic in chronic infection and cancer, where it antagonizes the cytotoxic immune response and can allow for persistent infection and/or tumor escape from immune surveillance [18]. There has been extensive research on disrupting this axis (through inhibition of PD-1 or PD-L1) in order to reactivate the immune response for cytotoxic T cell killing. Three of these drugs have received FDA approval for treatment of several malignancies and are considered candidate first- or second-line therapies for a variety of cancers including melanoma [19], non-small cell lung carcinoma [20], head and neck squamous cell carcinoma [21], and merkel cell carcinoma [22].
The critical role of the PD-L1/PD-1 interaction has been demonstrated in both preclinical models and in clinical trials across many solid cancers. However, investigation into its use as a treatment option for thyroid cancer has been limited to date [23–27]. Cunha et. al. demonstrated higher levels of PD-L1 expression in papillary thyroid cancers compared to normal and benign lesions and showed a positive correlation between PDL-1 levels and infiltration of CD8+, CD4+, FoxP3+ lymphocytes, as well as tumor-associated macrophages [28]. French and colleagues found that PD-L1+ T-lymphocytes were enriched in lymph nodes with metastatic papillary thyroid carcinoma, and identified a strong association with extra-nodal invasion and recurrent disease [29]. In a preclinical study of ATC, PD-L1 blockade was shown to be effective only when combined with a BRAF inhibitor [30]. A recent case report also showed dramatic and durable response of an ATC patient to combined vemurafenib and PD-L1 inhibitor treatment [31]. The therapeutic effect of blocking either PD-1 or PD-L1 has not been previously tested in patients with thyroid cancer, but a multicenter clinical trial sponsored by International Thyroid Oncology Group (ITOG) has recently opened to examine the efficacy of PD-1 inhibitor therapy (NCT #02688608).
Many of the clinical trials using ICIs demonstrate that targeting multiple key immune regulators results in an improved clinical response over monotherapy based on resulting adaptive immune resistance mechanisms. One of these immune regulators that can contribute to adaptive immune resistance is Indoleamine 2,3-dioxygenase (IDO1), a cytoplasmic enzyme responsible for one of the rate-limiting steps of tryptophan metabolism [32]. There is some evidence that intrinsic IDO1 expression within a tumor may be linked with an altered tumor immune response leading to immune escape through local depletion of tryptophan, or the creation of immunosuppressive tryptophan metabolites [32]. Thus, IDO1 inhibitors are being actively investigated in clinical trials in combination with other ICIs [33].
While a limited number of publications have reported on PD-1/PD-L1 expression in WDTC and ATC, there is little published data on the expression levels of PD-L1 in PDTC. In this study, we examine the expression of the immune checkpoint molecules, PD-L1 and IDO1, in a cohort of patients with PDTC in order to determine whether there is a rationale for targeting the PD-1:PD-L1 axis and IDO1 in PDTC patients. A secondary goal of this study was to correlate PD-L1/IDO1 expression with clinical and pathologic features.
Materials and Methods
This study was performed with IRB approval from the Massachusetts General Hospital (MGH). A database of PDTC patients with surgical pathology was generated, reviewed and classified by an experienced thyroid pathologist (WCF) at MGH using the Turin criteria [1]. Specimens from 28 patients with sufficient in-house surgical material for immunohistochemical staining were selected. Five micron sections were cut from surgical blocks of tumor and stained with IDO1 immunohistochemical stain (Clone D5J4E, 1:40, Ph 6.0), and a combination PD-L1/CD8 double immunohistochemical stain (PD-L1: Cell Signaling Cat # 13684S @1:200 Rabbit Monoclonal Clone E1L3N; CD8: Leica RTU Cat # PA0183 Mouse Monoclonal Clone 4B11. Pre-treatment with Epitope Retrieval Solution 2 at Ph 9.0, for 20 minutes). Immunohistochemical stains were evaluated by two pathologists with experience in PD-L1 and IDO1 stain interpretation (WCF and MWR), both separately and at a double-headed scope.
Immunohistochemical stain interpretation
PD-L1 and IDO1 were both evaluated by H-Score calculation [34]. Briefly, the percentage of tumor cells staining for each antibody as negative, weak, moderate, and strong staining (0, 1+, 2+ and 3+), were enumerated as percentages of all tumor cells (e.g. 70% 0+, 10% 1+, and 20% 2+). These percentages were then multiplied by their intensities (0–3) to give a score ranging from 0–300 (from the previous example: 70×0+10×1+20×2=50). For PD-L1, only membranous tumor cell expression was scored as positive; for IDO1, cytoplasmic staining of tumor cells was scored as positive (membrane only staining was not observed) [35]. The cutoff for PD-L1 and IDO1 positivity in this cohort was set at an H-score threshold of 5 which included all cases with 5% or more positive staining. The latter cutoff has been used previously in establishing the efficacy of anti PD-L1 therapy, and is similar to that used for studies of head and neck squamous cell carcinoma [36, 37]. CD8+ tumor infiltrating lymphocytes (TILs) were semi-quantitatively graded on a scale from 0 to 3, with: 0 being absent or rare, 1+ being <5% of positive-staining lymphocytes overlapping tumor cells, 2+ being 5–25% of tumor cells with TILs present, and 3+ being >25% of tumor cells with TILs present. For purposes of analysis, scores of 2+ (i.e. 5% or more) were considered positive for abundant CD8+ TILs.
Statistical Analysis
Disease-free survival (DFS) was defined as time from date of diagnosis of the primary tumor to diagnosis of distant metastatic disease, or date of last follow up. Patients with distant metastases present at the time of diagnosis were considered to have a DFS of 0. Overall survival (OS) was defined as time from date of diagnosis of the primary tumor to death, or date of last follow up. Date of death was confirmed with the Social Security Death Index.
Demographic and clinical data analysis utilized STATA/IC 12.1 (Stata Corporation, College Station, TX). Group comparisons were made using the Student’s t-test, chi-square test, Fisher’s exact test, or Wilcoxon rank sum test, as appropriate. A p-value of <0.05 was considered significant. Kaplan-Meier survival analysis was performed. Cox regression analysis was performed to evaluate the association between PD-1 positivity on histologic analysis and survival outcomes.
Results
Slides from 28 patients were immunohistochemically stained for PD-L1/CD8 and IDO1. Of the 28 patients, 25% (n=7/28) were positive for PD-L1 at an H-score cutoff of 5 (median 32, range 7–119), and 75% (n=2½8) were negative for PD-L1. There were several distinct patterns of PD-L1 staining that were recognized, including diffuse membranous staining of tumor groups (Figure 1a), localized staining at the periphery of tumor groups (Figure 1b), and staining which co-localized with CD8+ TILs (Figure 1c).
Fig 1.
Patterns of immune checkpoint expression in PDTC. Common patterns included diffuse strong expression (a), peripheral edge expression (b), lymphocytic infiltrate co-expression (c). IDO1 expression was also present in similar patterns (lymphocytic infiltrate co-expression is shown here in d). In a-c: Brown=PD-L1, Red=CD8. In d: Brown=IDO1
The clinical and demographic characteristics between the PD-L1 positive and PD-L1 negative patients were comparable, except for tumor size, and presence of multifocality (Table 1). The mean age of the PDTC patient cohort was 64.9 years (range 13.6–86.2 years, SD 14.1 years), with PD-L1 positive patients being slightly, but non-significantly, older than the negative patients (67.1 versus 64.3 years respectively, p=0.67). The PDTC cohort was 46.3% male (n=13), with PD-L1 positive patients being 28.5% male (n=2), and PD-L1 negative patients being 52.4% male (n=11; p=0.396). Hashimoto thyroiditis was present in 14.3% of both the PD-L1 positive (n=1) and PD-L1 negative cohorts (n=3; p=0.281). Extrathyroidal extension was present in 83.3% (n=5) of PD-L1 positive cases compared with 52.4% (n=11) of the PD-L1 negative cases (p=0.484).
Table 1.
Clinical characteristics by PD-L1 status
| Characteristic | Study Cohort | PD-L1 Status | ||
|---|---|---|---|---|
| (n=28) | Positive (n=7) | Negative (n=21) | P-value | |
| N, median or mean | N, median or mean | N, median or mean | ||
| Mean age, years (SD) | 64.9 (14.1) | 67.1 (11.3) | 64.3 (15.0) | 0.666 |
| Male gender (%) | 13 (46.3) | 2 (28.5) | 11 (52.4) | 0.396 |
| Median tumor size, cm (IQR) | 6.5 (4.5,8.0) | 7.8 (7.0, 8.6) | 6.4 (4.0, 7.0) | 0.022 |
| Extrathyroidal extension (%) | 16 (59.3) | 5 (83.3) | 11 (52.4) | 0.484 |
| Multifocality (%) | 1 (4.2) | 1 (20.0) | 0 (0.0) | 0.046 |
| Hashimoto thyroiditis (%) | 4 (14.3) | 1 (14.3) | 3 (14.3) | 0.281 |
| Metastatic at diagnosis (%) | 9 (32.1) | 3 (42.9) | 6 (28.6) | 0.572 |
| Metastatic at any time (%) | 17 (60.7) | 5 (71.4) | 12 (57.1) | 0.831 |
| CD8 2+ staining (%) | 6 (21.4) | 3 (42.9) | 3 (14.3) | 0.144 |
| AJCC staging | 0.231 | |||
| Stage 3 (%) | 11 (39.3) | 1 (14.3) | 10 (47.6) | |
| Stage 4 (%) | 15 (53.6) | 5 (71.4) | 10 (47.6) | |
| Incomplete staging (%) | 2 (7.1) | 1 (14.3) | 1 (4.8) | |
SD – Standard deviation; IQR – Interquartile range; PD-L1- Programmed Cell Death Ligand 1; AJCC – American Joint Commission on Cancer
PD-L1 positive tumors were significantly larger in size than their PD-L1 negative counterparts (7.8 versus 6.4 cm; p=0.022, Table 1). Multifocality was present in only one case among the PD-L1 positive cohort and in none of the PD-L1 negative cohort (p=0.046).
Using the AJCC 7th Edition Staging Manual for thyroid carcinoma, the stage for cases of PDTC in our cohort was either stage 3 or 4 for all tumors examined (due to the small number of cases, we did not distinguish between 4A and 4B). For PD-L1 positive tumors, 71.4% (n=5) were stage 4, compared to 47.6% for PD-L1 negative tumors (n=10; p=.0231). 42.9% (n=3) of PD-L1 positive tumors and 28.6% (n=6) of PD-L1 negative tumors were metastatic at the time of diagnosis (p=0.572). 71.4% (n=5) of the PD-L1 positive tumors and 57.1% (n=12) of PD-L1 negative tumors developed metastatic disease during their clinical follow up (p=0.831). Histologically, all PDTC had either 1+ or 2+ CD8+ TILS. 42.9% (n=3/7) of PD-L1 positive tumors and 14.3% (n=3/21) of PD-L1 negative tumors had 2+ CD8 positive tumor infiltrating lymphocytes (p=.144).
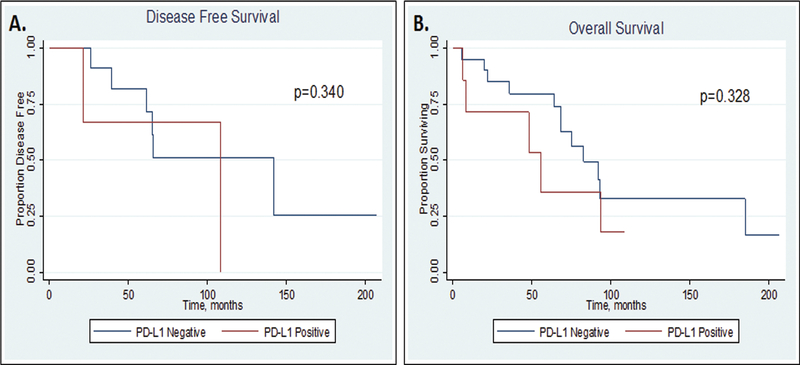
Median follow-up time for survival analysis among the PDTC cohort was 33 months in PD-L1 positive cases and 67 months in PD-L1 negative cases (p=0.103; Table 2, Figure 2). The 5-year disease-free and overall survival appeared shorter for patients with PD-L1 positive tumors compared to PD-L1 negative tumors, although this did not reach significance (DFS: 66.7% versus 81.8%, p=0.340, OS: 35.7% versus 79.7%, p=0.328, respectively, Table 2, Figure 2).
Table 2.
Follow up time, disease-free survival (DFS), and overall survival (OS)
| Study Cohort | PD-L1 Staining | |||||
|---|---|---|---|---|---|---|
| (n=28) | Positive (n=7) | Negative (n=21) | Hazard Ratio (for PD-L1 +) | 95% Confidence Interval | P-value | |
| Follow up time | -- | -- | 0.103 | |||
| Median (months) | 60.0 | 33.0 | 67 | -- | -- | -- |
| IQR (months) | 22.0, 81.0 | 6.0, 54.0 | 29.0, 82.5 | -- | -- | -- |
| Disease-free survival | 2.287 | 0.419–12.491 | 0.340 | |||
| Median (months) | 108.5 | 108.5 | 141.9 | -- | -- | -- |
| IQR (months) | 61.2, 141.9 | 21.3, 108.5 | 61.2, 141.9 | -- | -- | -- |
| 1-year DFS | 100% | 100% | 100% | -- | -- | -- |
| 5-year DFS | 78.8% | 66.7% | 81.8% | -- | -- | -- |
| Overall survival | 1.706 | 0.585–4.975 | 0.328 | |||
| Median (months) | 82.7 | 55.4 | 82.7 | -- | -- | -- |
| IQR (months) | 47.9, 185.0 | 8.2, 93.3 | 64.0, 185.0 | -- | -- | -- |
| 1-year OS | 88.9% | 71.4% | 95.0% | -- | -- | -- |
| 5-year OS | 69.3% | 35.7% | 79.7% | -- | -- | -- |
IQR – Interquartile range; PD-L1- Programmed Cell Death Ligand 1
Fig 2.
Kaplan Meier survival curves for disease-free survival (DFS; A) and overall survival (OS; B), by PD-L1 staining status.
Of the PD-L1 positive cases, 29% (n=2/7) of cases co-expressed IDO1 (Figure 1d). IDO positivity ranged from an H-score of 11 to 73 (median 70).
Discussion
New treatment strategies are urgently needed for patients with PDTC. Patients often rapidly acquire resistance to targeted therapies such as BRAF and multi-kinase inhibitors, and no other standard treatment options exist to meet this need. Combination strategies that target multiple aspects of the tumor microenvironment (immune cells, tumor blood vessel endothelium, and cancer cells themselves) could provide a more durable benefit for some patients. Recently, the development of neutralizing monoclonal antibodies to immune checkpoint molecules such as PD-1/PD-L1 has revolutionized our ability to overcome T-cell exhaustion, although resistance to this therapy has also been observed [38, 39]. Since cancer cells manipulate immune checkpoints to cause T-cell exhaustion and facilitate immune evasion (Figure 3), examining the role these checkpoints play in aggressive thyroid cancers such as PDTC and harnessing the power of new immune regulatory drugs are necessary and important steps forward in thyroid cancer management.
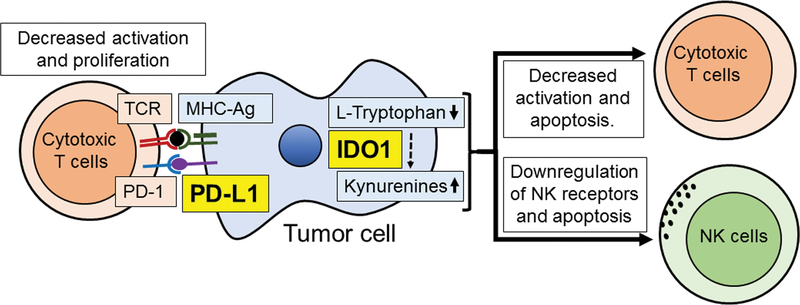
Fig 3.
Cartoon demonstrating the basic aspects of the PD-L1 and IDO1 immune checkpoint cascades. TCR: T-Cell Receptor; PD-1: Programmed Cell Death 1; PD-L1: Programmed Cell Death Ligand 1 MHC-Ag: Major Histocompatibility Complex protein-Antigen complex; IDO1:IIndoleamine 2 3-Dioxygenase 1; NK: Natural Killer.
A complex multifaceted immunosuppressive network in human tumors, including thyroid cancer, is just beginning to be better understood. This immunosuppressive network includes molecules on the surface of tumor cells that convey immunosuppressive activity (such as PD-L1), proteins that create an anti-inflammatory milieu (such as IDO1), and leukocyte infiltrates that contain regulatory T-cells (Treg), dendritic cells (DC), tumor-associated macrophages (TAMs), immature myeloid cells, and myeloid-derived suppressor cells (MDSCs), all of which work through various mechanisms to inhibit an effective immune response against the tumor. Greater understanding of this “immunosuppressive cellular network” will enable us to design rational combinatorial strategies that target both the immune cells and thyroid cancer cells [29][40][41]. Some thyroid cancers, especially those with the BRAFV600E mutation appear to show disturbed host-tumor immune surveillance, which may contribute to the poorer outcomes observed in this subset of patients [42]. To rationally design such combinations, it is critical to first determine the basic expression levels of the immune checkpoint inhibitor proteins in the tumor microenvironment. Subsequently, understanding how tumor cell signaling pathways alter the various infiltrating immune cells and their effector products (e.g. cytokines) will potentially allow for better manipulation of these pathways to kill tumor cells. The critical role of the PD-L1/PD-1 interaction has recently been investigated in thyroid cancer, but no large scale trials have yet analyzed the effects of combining therapies such as lenvatinib and immune checkpoint inhibitors [29, 30, 40].
The study presented here is one of the first examining both PD-L1 and IDO1 expression in PDTC. Several important aspects of immune checkpoint molecule expression are demonstrated in our cohort of PDTC. Most importantly, our series demonstrates that PD-L1 expression is common in PDTC, being present in 25% of these tumors, and IDO1 is co-expressed in a subset of the tumors. These findings raise the promising possibility that ICIs either as monotherapy or in combination may also have a role in the management of PDTC.
Although statistical significance was only identified linking PD-L1 with larger tumor size and with multifocality (which was present in only one case, limiting interpretation), it appears that for all clinical factors examined, PD-L1 positivity tended to be associated with poorer prognostic factors including: older patient age, larger tumor size, extra-thyroidal extension, higher AJCC stage, presence of metastatic disease at diagnosis, and shorter overall and disease-free survival. This data suggests that the presence of PD-L1 expression in tumor cells may be associated with more aggressive tumor behavior, as has been previously shown in papillary thyroid carcinoma (PTC), albeit using a different methodology and lower thresholds for calculating PD-L1 expression [43]. The statistical significance of this finding is limited by the size of our PDTC cohort, and larger series would be useful to further assess these observations. A further limitation of our study is the focality of PD-L1 expression in some cases, which we attempted to compensate for by staining whole slides with large areas of tumor rather than by using tissue microarrays. In view of the focal staining in some cases, studies that utilize tumor microarrays to assess the expression of immune biomarkers may underestimate the expression of immune checkpoint markers by PDTC. Of note, however, is that PDTC cases with positive PD-L1 expression tended to have a relatively high H-score, with only two positive cases below 10. Similar to its expression in PDTC, PD-L1 expression has also been associated with a worse prognosis in several other tumor types including breast [44], head and neck squamous cell carcinoma [45], renal cell carcinoma [46], non-small cell lung cancer [47], and others [44].
PD-L1 expression has previously been studied in several types of thyroid carcinoma, including WDTC and ATC, but its expression has not been thoroughly detailed in PDTC. PD-L1 expression in WDTC was reported as early as 2013 and was associated with aggressive features [48]. In papillary thyroid carcinoma, PD-L1 expression appears to occur in approximately 50% of tumors and is associated with decreased disease-free survival [43][49]. It is not clear what is responsible for the decreased rate of PD-L1 positivity in PDTC compared to PTC. There is the possibility that PD-L1 is less expressed by follicular carcinomas, and by extension, PDTC arising from FTC, but this hypothesis has yet to be proven. Assessing for this discrepancy is further complicated by the fact that underlying WDTC is unable to be determined in many cases of PDTC.
The expression of PD-L1 in tumor cells is reported to occur through two potential general mechanisms. The first mechanism is “intrinsic immune resistance,” where the up-regulation of PD-L1 occurs through the activation of an oncogene-associated pathway, such as KRAS (seen in lung cancer) [50] or alterations in the MAPK pathway (such as with BRAF mutations seen in thyroid cancer) [42]. In vitro studies have shown PD-L1 expression correlates with MAPK activation and is able to be altered with MEK and BRAF inhibitors [30, 51, 52]. This is important since both these agents are in clinical use for aggressive thyroid cancers, including PDTC. In ATC, which conceptually represents complete rather than partial loss of differentiation compared to PDTC, studies combining BRAF inhibitors with PD-L1 antibody treatment in a BRAFV600E mutant mouse model resulted in intense CD8+ lymphocyte infiltration and cytotoxicity, with more favorable CD8+/Treg ratios compared to each individual treatment, and ultimately led to a dramatic anti-tumor effect [30].
In the second pathway, termed “adaptive immune resistance,” tumoral expression of PD-L1 occurs in response to certain cytokines associated with T-cell mediated cytotoxic attack, such as interferon gamma, in order to dampen cytotoxic T-cell activity [53][54]. This T-cell activity is generally correlated to the immunogenicity of the tumor, which is increased in tumors with microsatellite instability (MSI-H) and in virus-associated carcinomas [55]. Thus, PD-L1 expression appears to be highly expressed on MSI-H colorectal carcinomas as well as virus-associated head and neck squamous cell carcinomas [56][57][58]. In response to the observation that these cancers also appear to demonstrate a greater response to PD-1 axis blockade [59], the FDA has recently approved pembrolizumab (an anti-PD-1 antibody) as a second-line treatment for advanced MSI-H cancers [60]. In our cohort, 2 of the 3 (67%) tumors with high CD8+ TILs and PD-L1 expression showed co-localization of PD-L1 (and IDO1) with CD8 positive T-lymphocytic infiltration, which may be explained by the adaptive response to immune checkpoint pathway activation.
Other patterns of PD-L1 expression in our PDTC cohort included positivity predominantly at the invasive edge of the tumor, likely also secondary to interaction with immune cells present within the stroma in these areas. It is interesting that a small number of PDTC in our cohort had diffuse strong expression of PD-L1 without robust CD8 positive infiltrates. As the majority of these tumors have not had genetic sequencing, it is unknown whether this staining pattern is secondary to an intrinsic driver mutation, but these patterns represent a potential for future study to better understand the mechanisms of immune checkpoint molecule expression in PDTC.
Our study of PDTC also identified a subset of cases that expressed IDO1, an immune regulatory molecule that creates an immunosuppressive environment, either through the local depletion of tryptophan, or through the creation of immunosuppressive tryptophan metabolites [32, 33]. Interferon gamma-associated IDO1 upregulation has been reported in some carcinomas, and its inhibition has sensitized tumors to interferon-alpha based treatment in animal models of kidney cancer [61]. Recently, IDO1 inhibitors have been developed and have entered clinical trials, often in combination with other ICIs. IDO1 overexpression has been reported in thyroid carcinomas where it has been shown to be associated with RET gene rearrangement [35][62]. In our study, IDO1 was infrequently expressed in isolation, but was more frequently co-expressed in PD-L1 positive cases, suggesting upregulation of both proteins secondary to interferon gamma expression, leading to evasion of host immune responses. In addition, this finding implies that the presence of IDO1 may have the potential to serve as an adaptive immune resistance mechanism to PD-1:PD-L1 inhibition, and that dual blockade of PD-L1 and IDO1 may be required to increase response rates to immune checkpoint blockade.
In summary, our study is among the first reporting the expression of PD-L1 and IDO1 in PDTC. PD-L1 expression was found to be relatively common in our series of PDTC, and a subset co-expressed IDO1. These findings provide a promising rationale to further explore ICIs as either monotherapy or in combination in PDTC patients who have limited effective, treatment options.
Acknowledgements and Disclosures
None of the authors have any conflicts of interest to disclose. This work is supported by NIH/NIDCR R01DE025340 (WCF and SIP); NIH 1R01CA149738-01 (SP); NIH 5T32DK00702842 (BJG); and Stand Up To Cancer-American Cancer Society Dream Team Translation Research Grant (MMK).
References
- 1.Volante M, Collini P, Nikiforov YE, et al. (2007) Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 31:1256–1264 [DOI] [PubMed] [Google Scholar]
- 2.Kakudo K, Bai Y, Katayama S, et al. (2009) Classification of follicular cell tumors of the thyroid gland: Analysis involving Japanese patients from one institute. Pathol Int 59:359–367 [DOI] [PubMed] [Google Scholar]
- 3.Sanders EM, LiVolsi VA, Brierley J, et al. (2007) An evidence-based review of poorly differentiated thyroid cancer. World J Surg 31:934–945 [DOI] [PubMed] [Google Scholar]
- 4.Lai HW, Lee CH, Chen JY, et al. (2006) Insular Thyroid Carcinoma: Collective Analysis of Clinicohistologic Prognostic Factors and Treatment Effect with Radioiodine or Radiation Therapy. J Am Coll Surg 203:715–722 [DOI] [PubMed] [Google Scholar]
- 5.Patel KN, Shaha AR (2014) Poorly differentiated thyroid cancer. Curr Opin Otolaryngol Head Neck Surg 22:121–6 [DOI] [PubMed] [Google Scholar]
- 6.Dettmer M, Schmitt A, Steinert H, et al. (2011) Poorly Differentiated Thyroid Carcinomas: How Much Poorly Differentiated is Needed? Am J Surg Pathol 35:1866–1872 [DOI] [PubMed] [Google Scholar]
- 7.Nikiforov YE (2004) Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr Pathol 15:319–327. doi: 10.1385/EP:15:4:319 [DOI] [PubMed] [Google Scholar]
- 8.Kunstman JW, Christofer Juhlin C, Goh G, et al. (2015) Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet 24:2318–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sykorova V, Dvorakova S, Vcelak J, et al. (2015) Search for new genetic biomarkers in poorly differentiated and anaplastic thyroid carcinomas using next generation sequencing. Anticancer Res 35:2029–2036 [PubMed] [Google Scholar]
- 10.Landa I, Ibrahimpasic T, Boucai L, et al. (2016) Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu B, Ghossein R (2016) Genomic Landscape of poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr Pathol 27:205–212 [DOI] [PubMed] [Google Scholar]
- 12.Liu R, Xing M (2016) TERT promoter mutations in thyroid cancer. Endocr Relat Cancer 23:R143–R155. doi: 10.1530/ERC-15-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacini F, Castagna MG, Brilli L, Pentheroudakis G (2012) Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23:110–9. doi: 10.1093/annonc/mds230 [DOI] [PubMed] [Google Scholar]
- 14.Cabanillas ME, Schlumberger M, Jarzab B, et al. (2015) A Phase 2 Trial of Lenvatinib (E7080) in Advanced, Progressive, Radioiodine-Refractory, Differentiated Thyroid Cancer: A Clinical Outcomes and Biomarker Assessment. Cancer 121:2749–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabanillas ME, Habra MA (2015) Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat Rev 42:47–55 [DOI] [PubMed] [Google Scholar]
- 16.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–64. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardhan K, Anagnostou T, Boussiotis VA (2016) The PD1:PD-L½ Pathway from Discovery to Clinical Implementation. Front Immunol 7:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyck L, Mills KHG (2017) Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol 47:765–779 [DOI] [PubMed] [Google Scholar]
- 19.Devji T, Levine O, Neupane B, et al. (2016) Systemic Therapy for Previously Untreated Advanced BRAF -Mutated Melanoma. JAMA Oncol 150:179–185 [DOI] [PubMed] [Google Scholar]
- 20.Ellis PM, Vella ET, Ung YC (2017) Immune Checkpoint Inhibitors for Patients With Advanced Non–Small-Cell Lung Cancer: A Systematic Review. Clin Lung Cancer [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Mirghani H, Amen F, Blanchard P, et al. (2015) Treatment de-escalation in HPV-positive oropharyngeal carcinoma: Ongoing trials, critical issues and perspectives. Int J Cancer 136:1494–1503 [DOI] [PubMed] [Google Scholar]
- 22.Kaufman HL, Russell J, Hamid O, et al. (2016) Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 17:1374–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha E, Wallin J, Kowanetz M (2015) PD-L1 Inhibition With MPDL3280A for Solid Tumors. Semin Oncol 42:484–487 [DOI] [PubMed] [Google Scholar]
- 24.Patnaik A, Kang SP, Rasco D, et al. (2015) Phase i study of pembrolizumab (MK-3475; Anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 21:4286–4293 [DOI] [PubMed] [Google Scholar]
- 25.Carter LL, Fouser LA, Jussif J, et al. (2002) PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur J Immunol 32:634–643 [DOI] [PubMed] [Google Scholar]
- 26.Brahmer JR, Tykodi SS, Chow LQM, et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen DS, Irving BA, Hodi FS (2012) Molecular pathways: Next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 18:6580–6587 [DOI] [PubMed] [Google Scholar]
- 28.Cunha LL, Marcello MA, Morari EC, et al. (2013) Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr Relat Cancer 20:103–110 [DOI] [PubMed] [Google Scholar]
- 29.French JD, Kotnis GR, Said S, et al. (2012) Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab 97:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brauner E, Gunda V, Vanden Borre P, et al. (2016) Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 7:17194–17211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kollipara R, Schneider B, Radovich M, et al. (2017) Exceptional Response with Immunotherapy in a Patient with Anaplastic Thyroid Cancer. Oncologist [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moffett JR, Namboodiri MA (2003) Tryptophan and the immune response. Immunol Cell Biol 81:247–265 [DOI] [PubMed] [Google Scholar]
- 33.Lob S, Konigsrainer A, Rammensee HG, et al. (2009) Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer 9:445–452 [DOI] [PubMed] [Google Scholar]
- 34.Azuma K, Ota K, Kawahara A, et al. (2014) Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 25:1935–1940 [DOI] [PubMed] [Google Scholar]
- 35.Moretti S, Menicali E, Voce P, et al. (2014) Indoleamine 2,3-Dioxygenase 1 (IDO1) Is Up-Regulated in Thyroid Carcinoma and Drives the Development of an Immunosuppressant Tumor Microenvironment. 99:832–840 [DOI] [PubMed] [Google Scholar]
- 36.Carbone DP, Reck M, Paz-Ares L, et al. (2017) First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 376:2415–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyford-Pike S, Peng S, Young GD, et al. (2013) Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q, Wu X (2017) Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol 46:210–219 [DOI] [PubMed] [Google Scholar]
- 39.Bellone M, Elia AR (2017) Constitutive and acquired mechanisms of resistance to immune checkpoint blockade in human cancer. Cytokine Growth Factor Rev 36:17–24 [DOI] [PubMed] [Google Scholar]
- 40.Severson JJ, Serracino HS, Mateescu V, et al. (2015) PD-1+Tim-3+ CD8+ T Lymphocytes Display Varied Degrees of Functional Exhaustion in Patients with Regionally Metastatic Differentiated Thyroid Cancer. Cancer Immunol Res 3:620–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn S, Kim TH, Kim SW, et al. (2017) Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer 24:97–106 [DOI] [PubMed] [Google Scholar]
- 42.Angell TE, Lechner MG, Jang JK, et al. (2014) BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 24:1385–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowdhury S, Veyhl J, Jessa F, et al. (2016) Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 7:32318–32328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Liu F, Liu L (2017) Prognostic significance of PD-L1 in solid tumors. Medicine (Baltimore) 96:e6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Wang P, Xu Y (2017) Prognostic value of programmed cell death ligand 1 expression in patients with head and neck cancer : A systematic review and meta- analysis. PLoS One 12:e0179536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu F, Xu L, Wang Q, et al. (2015) Clinicopathological and prognostic value of programmed death ligand-1 ( PD-L1 ) in renal cell carcinoma : a meta-analysis. 8:14595–14603 [PMC free article] [PubMed] [Google Scholar]
- 47.Pan Z, Ye F, Wu X, et al. (2015) Clinicopathological and prognostic significance of programmed cell death ligand1 ( PD-L1 ) expression in patients with non-small cell lung cancer : a meta-analysis. 1:462–470. doi: 10.3978/j.issn.2072-1439.2015.02.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunha LL, Marcello MA, Morari EC, et al. (2013) Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr Relat Cancer 20:103–110 [DOI] [PubMed] [Google Scholar]
- 49.Shi R, Qu N, Luo T, et al. (2017) Programmed Death-Ligand 1 Expression in Papillary Thyroid Cancer and Its Correlation with Clinicopathologic Factors and Recurrence. Thyroid 27:537–545 [DOI] [PubMed] [Google Scholar]
- 50.Chen N, Fang W, Lin Z, et al. (2017) KRAS mutation ‑ induced upregulation of PD ‑ L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang X, Zhou J, Giobbie-Hurder A, et al. (2013) The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 19:598–609. doi: 10.1158/1078-0432.CCR-12-2731 [DOI] [PubMed] [Google Scholar]
- 52.Ott PA, Bhardwaj N (2013) Impact of MAPK Pathway Activation in BRAFV600 Melanoma on T Cell and Dendritic Cell Function. Front Immunol 4:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S, Jang B, Lee S, et al. (2006) Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN- c -induced upregulation of B7-H1 ( CD274 ). FEBS Lett 580:755–762 [DOI] [PubMed] [Google Scholar]
- 54.Ribas A (2015) Adaptive immune resistance: How cancer protects from immune attack. Cancer Discov. 5:915–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rooney MS, Shukla SA, Wu CJ, et al. (2014) Article Molecular and Genetic Properties of Tumors Associated with Local Immune Cytolytic Activity. Cell 160:48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balermpas P, Rödel F, Krause M, et al. (2017) The PD-1/PD-L1 axis and human papilloma virus in patients with head and neck cancer after adjuvant chemoradiotherapy: A multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int J Cancer 141:594–603 [DOI] [PubMed] [Google Scholar]
- 57.Llosa NJ, Cruise M, Tam A, et al. (2014) The Vigorous Immune Microenvironment of Microsatellite Instable Colon Cancer Is Balanced by Multiple Counter-Inhibitory Checkpoints. 5:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenbaum MW, Bledsoe JR, Morales-oyarvide V, et al. (2016) PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol 29:1104–1112 [DOI] [PubMed] [Google Scholar]
- 59.Le DT, Le DT, Durham JN, et al. (2017) Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. 357:409–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.USFDA (2017) FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm?platform=hootsuite. Accessed 1 Jan 2017
- 61.Trott JF, Kim J, Aboud OA, et al. (2016) Inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget 7:66540–66557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moretti S, Menicali E, Nucci N, et al. (2017) Signal Transducer and Activator of Transcription 1 Plays a Pivotal Role in RET/PTC3 Oncogene-induced Expression of Indoleamine 2,3-Dioxygenase 1. J Biol Chem 292:1785–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]