Abstract
Sensory photoreceptors underpin light-dependent adaptations of organismal physiology, development and behavior in nature. Adapted for optogenetics, sensory photoreceptors become genetically-encoded actuators and reporters to enable the non-invasive, spatiotemporally accurate and reversible control by light of cellular processes. Rooted in a mechanistic understanding of natural photoreceptors, artificial photoreceptors with customized light-gated function have been engineered that greatly expand the scope of optogenetics beyond the original application of light-controlled ion flow. As we survey presently, UV/blue-light-sensitive photoreceptors have particularly allowed optogenetics to transcend its initial neuroscience applications by unlocking numerous additional cellular processes and parameters for optogenetic intervention, including gene expression, DNA recombination, subcellular localization, cytoskeleton dynamics, intracellular protein stability, signal transduction cascades, apoptosis and enzyme activity. The engineering of novel photoreceptors benefits from powerful and reusable design strategies, most importantly light-dependent protein association and (un)folding reactions. Additionally, modified versions of these same sensory photoreceptors serve as fluorescent proteins and generators of singlet oxygen, thereby further enriching the optogenetic toolkit. The available and upcoming UV/blue-light-sensitive actuators and reporters enable the detailed and quantitative interrogation of cellular signal networks and processes in increasingly more precise and illuminating manners.
Graphical Abstract
1. Introduction
The ability to sense and respond to stimuli is a basic hallmark of life. Light within the near-UV to near-infrared region of the electromagnetic spectrum represents a crucial environmental stimulus that is processed by a multitude of both sessile and motile organisms across all kingdoms of life. Beyond its role as the primary energy source in photosynthesis, light carries vital spatial and temporal information; light sensitivity thus bestows an evolutionary advantage on organisms by endowing them with a sense of where and when. Important and widespread physiological adaptations to light absorption include developmental and behavioral responses, entrainment of the diurnal rhythm and phototaxis. To utilize the information content of incident light for the regulation of biological processes, nature has evolved a plethora of so-called sensory photoreceptor proteins.1,2 Notably, such sensory photoreceptors are distinct from the pigments in photosynthesis, e.g., light-harvesting complexes and photosynthetic reaction centers, and from photoenzymes,3 which primarily absorb light for its energy content to drive demanding chemical reactions, e.g., the oxidative splitting of water. Sensory photoreceptors generally harbor an organic chromophore that is sensitive to certain bands within the electromagnetic spectrum. Photon absorption by the dark-adapted state of the photoreceptor initiates a series of photochemical reactions (“photocycle”) that couple the chromophore to the surrounding protein scaffold. These changes culminate in shifting the photoreceptor from the dark-adapted to the light-adapted (or, “signaling”) states, which differ in their structures, dynamics and biological activity. These conformations are often simply referred to as the ‘dark state’ and ‘lit state’. (However, we discourage denoting these as “ground” and “excited” states as these terms also refer to the electronic configuration of molecular orbitals, and it is important to note that both the dark- and light-adapted states feature chromophores that are generally electronic ground states). Usually, the photocycle is fully reversible, with the metastable signaling state spontaneously decaying in a thermal reaction back to the dark-adapted state. Based on chromophore identity and photocycle, sensory photoreceptors divide into approximately ten different classes. Taken together, one can consider sensory photoreceptors as signal processors or transducers that convert one type of signal (light) into another (a biological response). Photoreceptors can be functionally dissected into a photosensor (“input”) module that harbors the chromophore and mediates light absorption, and an effector (“output”) module that elicits downstream physiological responses. Often, photosensor and effector moieties localize to distinct domains of the photoreceptor and can hence be physically separated into distinct parts.
The light-dependent adaptations in nature mediated by sensory photoreceptors display key desirable properties: genetic encoding, reversibility, and exquisite resolution in time and space. These benefits have made sensory photoreceptors versatile and powerful actuators for the targeted control of cellular processes and parameters. In an approach dubbed “optogenetics”,4 targeted cells (or, tissues, organs or organisms) are rendered light-sensitive via the heterologous expression of suitable sensory photoreceptors. Light can then be used as a perturbatory stimulus to trigger defined physiological responses. Compared to other stimuli, e.g., addition of chemical compounds, the optogenetic approach excels in its reversibility, genetic encodability, spatiotemporal acuity and non-invasiveness. Optogenetics originated in the neurosciences, as reviewed by Bamberg, and at first solely relied on microalgal and bacterial rhodopsin photoreceptors that function as light-driven ion pumps and light-gated ion channels.5–8 With these actuators in hand, ion flux across the plasma membrane either against or along the electrochemical gradient has been controlled by light, and action potentials have been elicited at will. While light-regulated ion pumps and channels continue to serve as extremely versatile and powerful actuators, the past several years have readily demonstrated the broader generality of optogenetics to many other kinds of light-regulated tools and applications.
Such advances have been enabled by protein engineering strategies that have been particularly successful for several classes of soluble photoreceptors sensitive to near-UV and blue light (BL). In this article, we chiefly consider pertinent approaches based on three types of flavin-binding, blue-light-sensitive photoreceptors that have proven most versatile for optogenetics: the Light-Oxygen-Voltage (LOV) domains,9,10 the Blue Light sensors Utilizing Flavin adenine dinucleotide (BLUF) domains11,12 and cryptochromes.13 We will also discuss applications derived from the BL-sensitive photoactive yellow protein14 (PYP, and the broader group of xanthopsins) and the UV-B-sensitive photoreceptor UVR8.15 By contrast, photoreceptors from other classes are treated in the accompanying reviews by Gärtner, Bamberg, Engelhard, and Kandori on phytochromes and rhodopsins. We begin by reviewing the photochemistry, the molecular architectures and the predominant signaling strategies used by the listed UV-light/BL receptors in section 2. The mechanistic elucidation of light-dependent allostery in natural photoreceptors and their constituent modules directly informs the rational engineering of novel photoreceptors that translate desired light stimuli into customized cellular output. Although the so-far implemented photoreceptor engineering studies are diverse, a small set of particularly successful design strategies emerge, as discussed in section 3. Using naturally occurring and engineered UV-light/BL-sensitive photoreceptors, many cellular activities and parameters have been subjected to optogenetic control including gene expression, cellular cytoskeleton and motility, and signal transduction (cf. section 4.). The applications of these photoreceptors can be further expanded by abrogating their normal photochemistry while retaining their ability to specifically incorporate their chromophores (cf. section 5.). By doing so, novel fluorescent proteins and blue-light-driven generators of singlet oxygen have been devised that further enrich the optogenetic toolkit as versatile reporters and actuators.
2. Blue-Light-Responsive Photoreceptors
2.1. Classes of Blue-light-responsive Photoreceptors
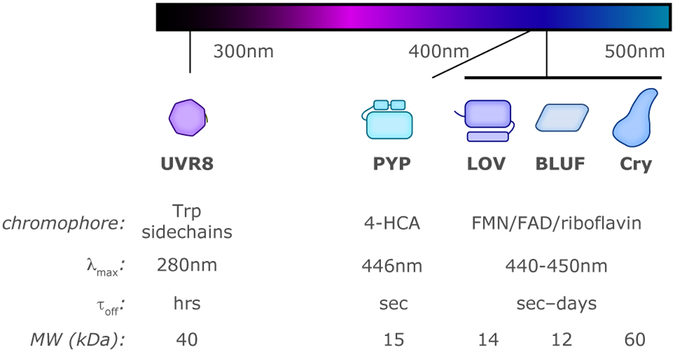
Our focus here is on photoreceptors which sense light in the UV and blue regions of the electromagnetic spectrum, roughly spanning 250-500 nm. While such proteins have very diverse origins – including disparate host organisms, kinds of biology they control, and methods used to originally identify them – they share several common themes:
modularity: these photoreceptors are relatively small proteins or protein domains, often under 20 kDa in size (all except the cryptochromes and UVR8 in this chapter). These can be found in a wide variety of settings with other enzymatic and non-enzymatic effectors, either in cis in the same polypeptide or in trans with other components. While the in cis combinations are obviously easier to identify by sequence analyses, a substantial number of standalone “short” proteins which contain only photosensor domains suggest that many in trans sensor/effector pairs remain to be identified.
chromophores: fundamental to photoreception are chromophores which absorb electromagnetic radiation in the appropriate section of the spectrum, using photochemical reactions of different kinds to initiate signaling processes. The bulk of the systems described in this chapter are blue-light-sensitive through the binding of flavin chromophores – flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD) and riboflavin (Fig. 1) – to take advantage of their maximal absorption near 440-450 nm (and substantial absorbance across a broader range, ca. 390-490 nm) in the oxidized state. UVR8, which absorbs much shorter wavelength (UV-B, 280-315 nm) utilizes tryptophan sidechains instead of small ligands.
allosteric signal transmission: the photochemical initiation of the photosensory process leads to a variety of changes in protein/chromophore interactions, sometimes including substantial configurational changes to the chromophore itself (e.g., the formation of novel covalent protein/chromophore adducts or double bond Z/E isomerization). These conformational transitions in the protein structure immediately surrounding the chromophores are subsequently amplified by allosteric networks within the photoreceptors. The resulting changes in protein dynamics and structure – which can be as dramatic as light-driven protein (un)folding events, protein/peptide binding interactions, and changes in quaternary structure – provide the molecular foundation of signaling in biological and engineered systems.
thermal reversion: post-excitation, the light-adapted conformations of all systems detailed here will spontaneously revert back to the initial resting state upon the cessation of illumination. The relaxation times for these processes vary widely among the different photoreceptor systems, and within specific proteins among the different families. These structure/function variations have been fruitful at both revealing insights into the mechanisms of the reversion process and enabling the rational tuning of the kinetics of such processes. Notably, the light-adapted state of certain photoreceptors can be catalyzed to revert to the dark-adapted state by illumination at wavelengths absorbed by the light-adapted state chromophores, making them photochromic switches.
Figure 1.
Overview of the five types of soluble blue-/UV-light-sensitive photosensory proteins and protein domains utilized in optogenetic applications. Typical characteristics for members of each family are listed, including chromophores, wavelength of maximum sensitivity (γmax), typical time constants for thermal reversion of photoactivated state (τoff) and molecular weight (MW) of minimal sensory fragment minus effectors.
2.1.1. Light-Oxygen-Voltage (LOV) Proteins
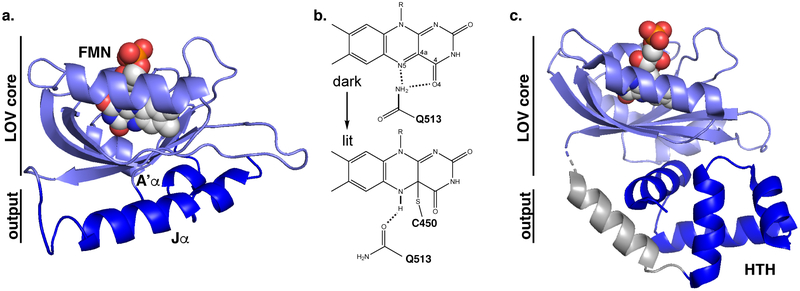
LOV domains were initially identified in the phototropins,9,10 a group of plant and algal serine/threonine kinases activated by blue light, giving them the primary sensory role in the process of phototropism (Fig. 2). Soon afterwards, these LOV domains were discovered in fungal and bacterial systems, including transcription factors, histidine kinases and standalone “short” LOV proteins containing only the photosensor domain itself. Continuing large-scale sequencing efforts of genomic and metagenomics samples have led to over 7,000 LOV domains being identified to date.16 Most of these proteins contain effectors C-terminally attached of the LOV sensor itself.
Figure 2.
Fundamental aspects of photoactivation of LOV domains. (A) Structure of AsLOV2,19,20 showing the location of the LOV α/β “core” domain surrounding the FMN chromophore and effector A’α and Jα helices on adjacent faces of the β-sheet. (B) Simplified LOV photocycle, demonstrating the effect of blue light to interconvert between noncovalent protein complex with oxidized flavin and covalently-attached, reduced flavin. Residue numberings apply to AsLOV2. (C) Example of a more complex LOV-effector arrangement within EL222,21 a LOV-helix-turn-helix (HTH) protein, where an effector helix (more distantly located within the primary structure) from within the HTH domain structurally and functionally replaces the Jα helix of AsLOV2.
At a molecular level, LOV domains are a subset of the broader family of PAS (Period-ARNT-Singleminded) domains of environmental sensory domains.17,18 All of these domains are approximately 110 amino acid residues long in their minimal forms and adopt a mixed α/β protein fold, with several α-helices located on one face of an antiparallel β-sheet. Many PAS domains are involved in protein/protein interactions, often regulating the strength of these interactions via the presence of small, internally-bound cofactors or ligands which have environmentally-sensitive concentrations or configurations. Different subsets of PAS domains are capable of preferentially interacting with different ligands, thus giving rise to collections of sensors specific for diverse stimuli.18
Within this broader context, LOV domains achieve their photosensory functionality via their specific binding to flavin chromophores. While FMN most commonly serves in this role, FAD and riboflavin-bound forms have also been reported in the literature.22 The oxidized quinone forms of these cofactors non-covalently bind within LOV domains in the dark, nestled within the helices mentioned above. Photochemically-triggered radical chemistry23 leads to the specific formation of a novel covalent adduct between the γ sulfur atom of a conserved cysteine residue (Cys 450 in the widely-studied Avena sativa phototropin 1 LOV2 domain, often referred to as “AsLOV2”) and the C4a position of the flavin isoalloxazine ring. This modification can be trivially followed by visible absorption spectroscopy, as it leads to the elimination of substantial absorption in the blue around 450 nm with a simultaneous increase in near-UV absorption around 390 nm. Coupled with the concomitant protonation of the adjacent N5 position, this change effectively serves as the photochemical trigger for a range of subsequent structural transitions. Most importantly, this includes a reversal of hydrogen bonding activity of a conserved Gln residue (Gln 513 in AsLOV2) in the LOV β sheet, switching it from donating an H-bond to a deprotonated N5 to accepting an H-bond from the protonated N5. In combination with other structural modifications, a wider range of larger allosteric changes are triggered. In the arguably best-known case of AsLOV2, these changes culminate in the reversible unfolding of a C-terminal α helix, termed Jα.19 Subsequent work also implicated an N-terminal helix, A’α, in the light-induced signal transduction process.20,24 Notwithstanding the widespread use of AsLOV2 in photoreceptor engineering (detailed in section 4.), signaling in the parental plant phototropin receptor remains incompletely understood, as different experimental approaches suggest different reliance on Jα unfolding for activation of the intact receptor.25,26 The photoadduct spontaneously (i.e. thermally) decays to regenerate the dark-adapted, non-covalently-bound state over a timescale of seconds to hours, depending on protein sequence and structure surrounding the chromophore.
Notably, the ample mechanistic information available for LOV domains has led to the collection of a wide range of useful point mutations that work across many comparable systems. These include residue exchanges that lock the photoreceptor in its dark-adapted state. In the corresponding “constitutively dark” variants the critical cysteine is replaced by an alanine or serine residue that is incapable of progressing through adduct formation. Of note, recent reports have shown that reduced flavins can bind into some cysteine-free LOV domains, activating these systems via a redox process rather than via light-induced formation of the covalent thioether bond.27 Complementing such variants are “constitutively lit” variants that are locked in their light-adapted state, many of which replace the Gln 513 residue with an asparagine or directly perturb LOV-effector interactions. Finally, a suite of mutations is available for controlling the kinetics of the spontaneous dark-state reversion process by up to three orders of magnitude, many of which were initially inspired by variations in such rates evident in natural LOV domains.28 We underscore that such mutations provide a starting point for regulating the signaling properties of any new LOV receptor, but their efficacy in doing so must be checked in each setting.
2.1.2. Sensors of Blue Light Using Flavin Adenine Dinucleotide (BLUF)
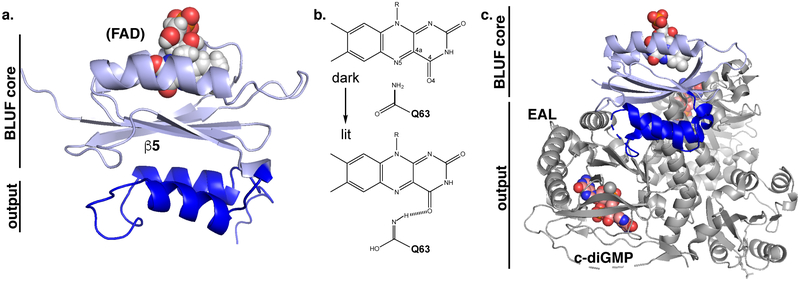
Similar to the LOV receptors, BLUF domains sense blue light through flavin chromophores non-covalently bound within a mixed α/β fold, but with substantial differences in origin and mechanism worth noting (Fig. 3). The vast majority of the presently-known ca. 900 BLUF domains are from proteobacteria, with a few notable exceptions from eukaryotic fungi and flagellates.29 Most BLUF-containing proteins are “short” BLUF-only photoreceptors, although a number contain covalently attached effectors that are either enzymatic (typically involved in cyclic-nucleotide biosynthesis or degradation) or non-enzymatic (including DNA-binding).
Figure 3.
Fundamental aspects of photoactivation of BLUF domains. (A) Structure of the BLUF domain of Klebsiella pneumoniae BlrP1,30,31 exemplifying location of the BLUF α/β “core” domain surrounding the FAD chromophore and effector C-terminal helices. (B) Simplified BLUF photocycle, showing how photochemically-driven effects including altered hydrogen-bonding of a nearby glutamine lead to altered protein/FAD interactions. (C) Example of a more complex BLUF-effector arrangement within full-length BlrP1,31 a BLUF-EAL enzyme involved in c-di-GMP breakdown.
Structurally, BLUF domains adopt a ferredoxin-type fold of about 100 amino acids, placing two α- helices on one face of an antiparallel β-sheet.30–33 Within the gap between these two helices, the isoalloxazine ring of an oxidized FAD chromophore is non-covalently bound. In contrast to the photochemistry of either the LOV domains or cryptochromes, blue light illumination near the absorption maximum at 450 nm does not elicit a change in oxidation state of the flavin. Instead, a relatively subtle electronic change is triggered, accompanied by a 10-nm red shift seen by visible absorption spectroscopy upon the dark to lit state conversion.34–36 The precise nature of the BLUF signaling state is still under debate. In one model, a conserved glutamine residue is thought to undergo tautomerization of its amide side chain, thereby triggering subsequent allosteric transitions that culminate in changes across the β-sheet (including a conserved Trp residue which interconverts between inward- and outward-pointing states).37–39 In a competing view, the same glutamine residue is proposed to undergo a 180° flip of its sidechain and to thus elicit the described allosteric changes.35,40 Given the limited light-induced structural changes that either model proposes, the relatively long persistence of the signaling state (between seconds and minutes depending upon BLUF protein) is puzzling. Regardless of the precise mechanism, the light-induced changes appear to alter flavin/protein hydrogen-bonding patterns and trigger conformational shifts across the central β-sheet that propagate to moderately conserved α-helices on the far side and onwards to effector modules.
2.1.3. Cryptochromes (CRYs)
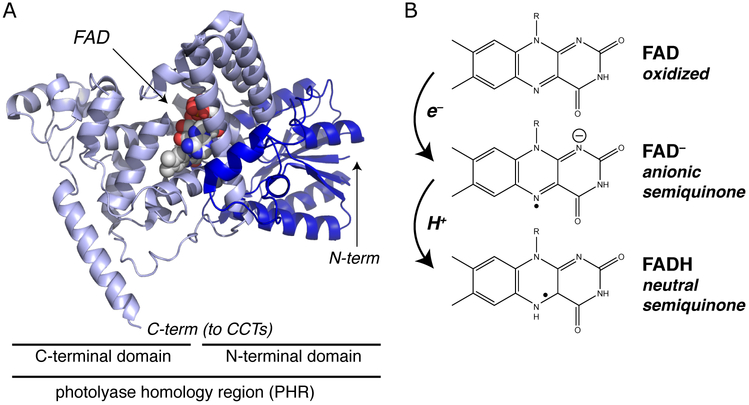
The third and final class of flavin-containing photoreceptors we cover here are the cryptochromes (Fig. 4). First postulated as a class of blue-light sensors controlling plant growth, they were subsequently found as regulators of circadian processes in mammals and insects.41 While this broad group of proteins has evolved into several different phylogenic families, all maintain a homology to the photolyase class of DNA-repair enzymes. Whereas most cryptochromes are incapable of catalyzing DNA repair, at least some representatives retain this ability.42 The cryptochrome/photolyase homology displays a similar two-domain structural organization, including N-terminal α/β and C-terminal all-helical domains, together constituting a “photolyase homology region” (PHR).43 Fundamental to blue light sensing is a FAD chromophore bound within the C-terminal domain of the PHR.
Figure 4.
Fundamental aspects of photoactivation of cryptochromes. (A) Structure of the photolyase homology region (PHR) of A. thaliana CRY1,44 showing the location of the bound FAD chromophore within the highly-helical C-terminal domain. The locations of the N- and C-termini are also indicated, as these have been implicated in CIB1 binding and homooligomerization45 in the homologous A. thaliana CRY2 (AtCRY2) protein widely used for optogenetic applications. CCT = CRY C-Terminal region. (B) Simplified cryptochrome photocycle, showing the oxidized FAD chromophore present in the inactive dark-adapted state and photochemically-generated anionic and neutral semiquinone states.
Cryptochrome photochemistry is an area of active research, and some debate, at the time of this review. This contrasts with the photolyases, where three photochemical excitation mechanisms are well understood: a photophysical energy transfer from an antenna pigment to FADH−, electron transfer from the flavin to the DNA damage, and photochemical activation involving three conserved Trp residues (“Trp triad”). The applicability of either route to cryptochromes remain somewhat in question, given the apparent utilization of a flavin semiquinone in Cry signaling along with the differential effects of Trp triad mutations in signaling.46–49
Regardless of the precise activation mechanism, converting these photochemical changes to altered protein/protein interactions requires the involvement of C-terminal extensions (CRY C-termini, or “CCTs”) which vary among the cryptochromes. For the optogenetic uses detailed below, these lead to light-controlled heterotypic interactions of the cryptochromes – usually constructs of A. thaliana CRY2 (“AtCRY2”) encoding the PHR alone or with short CCTs – with the cryptochrome-interacting basic-helix-loop-helix proteins (“CIBs”)50 involving Cry N-terminal regions or homotypic CRY:CRY interactions via CCTs.45,51–53 In both cases, the dark-adapted state does not participate in these interactions while the light-adapted state does.
2.1.4. Non-Flavin Alternatives: Xanthopsins and UVR8
Two additional classes of soluble biological photosensors detect electromagnetic radiation in the blue and UV regions of the spectrum without using flavin chromophores: the xanthopsins, including their best-known member photoactive yellow protein (PYP),14 and the plant photoreceptor UVR8.15 Like the LOV domains, xanthopsins are members of the PAS domain family of environmental sensors. While LOV and xanthopsin receptors also share the sensing of blue-light stimuli, they do so with substantially different chromophores and mechanisms. PYP and the other xanthopsins rely on 4-hydroxycinnamic acid (4-HCA, also termed p-coumaric acid) chromophores, attached to the photoreceptor through a thioester linkage to a conserved cysteine. Notably, this chromophore is not routinely available in most heterologous systems, necessitating either the expression of biosynthetic enzymes or feeding of precursor compounds to enable the use of PYP-based optogenetic tools in living cells. In the dark-adapted state, this covalently-tethered chromophore exhibits a trans configuration of the C7-C8 double bond and establishes a series of hydrogen-bond interactions to stabilize the phenolate state which absorbs in the blue (ca. 430-460 nm). Illumination with BL leads to protonation of 4-HCA and isomerization about the double bond, producing a cis configuration and substantial change in the structure and dynamics of the protein.54–58 These principles have been best examined in PYP, a standalone photoreceptor approximately 17 kDa in size, originally isolated from halophilic bacteria and believed to be involved in a negative phototactic response to blue light.14,59 As with most of the other photoreceptor types here, other xanthopsin domains have been found in a handful more complex proteins with different sensory and effector domains.
The last component we review here is UVR8, part of the UV-B (280-320 nm) response pathway in Arabidopsis thaliana and other plants. Initial biochemical identification of a light-dependent interaction of UVR8 with the COP1 protein in the same signaling pathway led to further biophysical characterization.15,60 These studies revealed that UVR8 interconverts between a dark-adapted homodimeric state and a light-adapted monomeric state which interacts with COP1 – all strikingly without the use of any small-molecule chromophores like the flavins or hydroxycinnamic acid described above. The mechanistic basis of this phenomenon was revealed by a crystal structure of a UVR8 dark-state dimer, showing a collection of aromatic sidechains arranged in close proximity to each other at the protein/protein interface and facilitating an excitonic coupling excitation mechanism.61–63 In addition, the rest of the interface involves a network of salt bridges which laid the foundation for point mutations which can be used to generate constitutively monomerized (= “constitutively lit state”) UVR8.
We note that numerous members of the rhodopsin photoreceptor family also serve as blue-light-sensitive receptors. However, as the focus of the present treatise is on the soluble classes of UV-B/BL-sensitive photoreceptors, we refer to the accompanying reviews on rhodopsin photoreceptors by Bamberg, Engelhard, and Kandori.
2.2. Allostery and Signal Transduction by Blue-Light-Responsive Photoreceptors
To exert control over biological function, the photochemical changes initiated at chromophores and immediately-surrounding protein residues must be relayed via allosteric pathways to affect protein conformation more globally. While a comprehensive discussion of these processes is outside the scope of this review, we can broadly categorize them into four groups:
Intramolecular effector release: Light induces the release of an intramolecular interaction between the photosensory and effector domains, often converting an autoinhibited dark state into an activated lit state. Examples include the AsLOV2 domain mentioned above, where N- and C-terminal helices (A’α and Jα) are freed from interactions with the LOV core domain upon illumination,19 thus allowing them to freely interact with other partners.
Rearrangement of preformed dimer/oligomer: Commonly seen among LOV and BLUF domains, these proteins undergo light-triggered conformational changes in a dimer which exists regardless of illumination state. In these cases, rotations and/or translations between the subunits are utilized to move effectors between different functional states, as exhibited by the engineered YF1 LOV-histidine kinase system.64
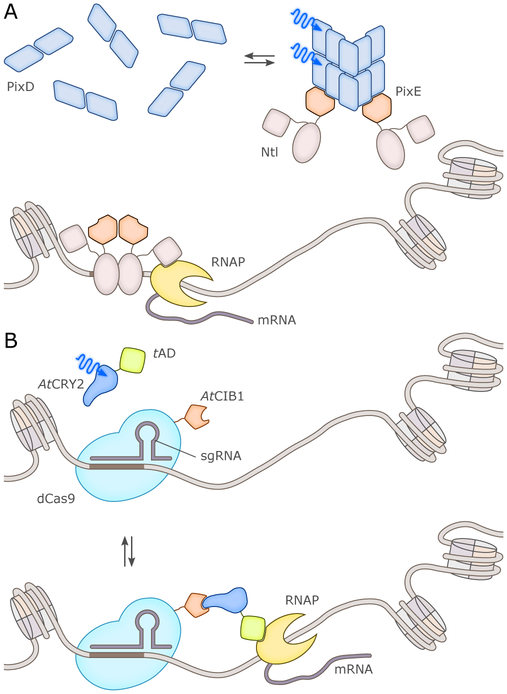
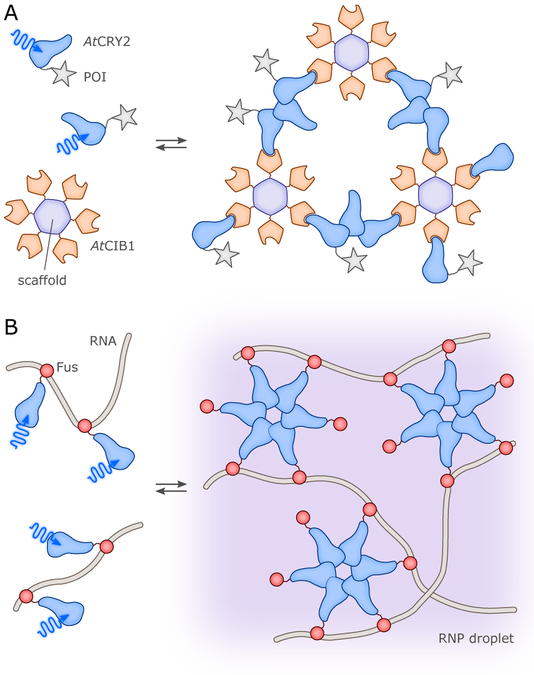
Change in oligomerization state: Most commonly, this involves a light-dependent change from monomer to dimer, or dimer to monomer, as observed in UVR8.60,61 However, higher-order changes have also been observed in natural systems (e.g., in BLUF PixD,33 and in cryptochrome photobody formation50,65), to the point that light-dependent phase separation can occur.66
Recruitment of heterologous partner: A number of blue-light receptors bind to other proteins selectively in either dark- or light-adapted states. The blue-light-activated interaction observed between plant cryptochromes and the CIB1 interacting partner has been most actively used for optogenetic applications,51 but other examples exist as well. A similar concept is realized in the red-light-sensitive plant phytochromes (reviewed in this issue by Gärtner) with the light-dependent recruitment of phytochrome-interacting factors (PIFs).
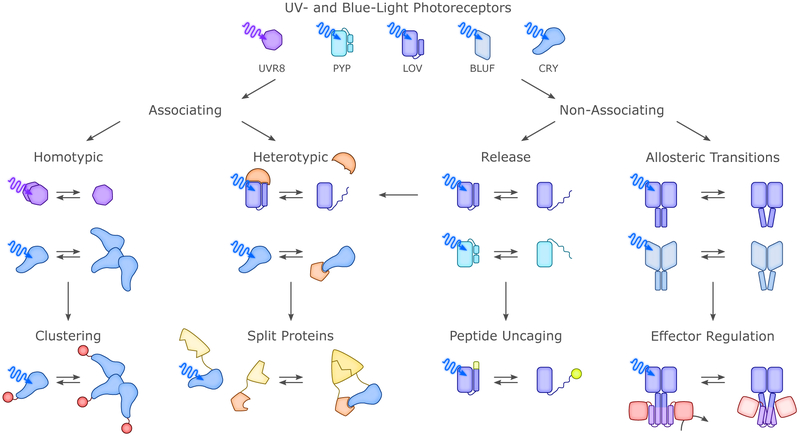
Notably, photoreceptors using the first two mechanisms maintain their oligomeric states upon illumination, while those using the latter two undergo substantial changes. Hence, photoreceptors can alternatively be grouped into “non-associating” and “associating” types (Fig. 5).67 We underscore that several of these elementary mechanisms are often combined with each other, rather than acting individually. The UVR8 UV-B plant photoreceptor mentioned above provides an excellent example of this principle, with illumination triggering the dissociation of a dark-adapted dimer into light-adapted monomers capable of recruiting the heterotypic partner COP1;15,60,61 analogously, the BLUF PixD system interacts in similarly-controlled manner with PixE.33,68 Likewise, BL-induced rearrangements in the LOV proteins Vivid and EL222 lead to dimerization via the unmasking of protein segments (an N-terminal cap segment for Vivid,22,69 a C-terminal helix-turn-helix DNA-binding effector for EL22221,70) from the surface of the photosensory LOV domain.
Figure 5.
Allostery and engineering of UV-light- and BL-sensitive photoreceptors. Despite the rich diversity of these photoreceptors, their signal transduction mechanisms largely fall into but a few classes. In case of the associating photoreceptors, the transition between dark-adapted and light-adapted states entails a change in oligomeric state, either in homotypic or heterotypic manner. Light-modulated oligomerization has proven a particularly versatile approach for engineering novel optogenetic actuators as detailed in section 4. Among the non-associating photoreceptors, we identify light-modulated order-disorder transitions, as exemplified by the Jα helix unfolding in AsLOV2,19 and changes in tertiary and quaternary structure as prevalent mechanisms. Both types of mechanisms have lent themselves to the engineering of novel photoreceptors (cf. sec. 4.).
More broadly, it is important to appreciate that different members of the same family of photosensory domain can use different mechanisms from among these four groups. Similarly, different variants of the same system – such as truncations – can exhibit differences as well.25,71 These idiosyncrasies stem in large part from the relatively small changes in protein structure needed to switch structural and functional states, where “off” and “on” states exhibit slightly different sets of non-covalent interactions within or between proteins. As reflected by the moderate 10- to 100-fold switches in function seen in many blue-light photoreceptor systems,67 these differences translate into small energetic differences on the order of 5-10 kJ mol−1 which can be easily modulated by differences in domain context, sequence or point mutations. While this feature opens up opportunities for rational structure-based tuning of important functional parameters like background activation and dynamic range,72 it also underscores the importance of validating signaling mechanisms within full-length native proteins and engineered optogenetic systems.
3. Photoreceptor Engineering
At a phenomenological level, biological processes responsive to light have long been known, e.g., flowering onset and tropic growth in plants, as well as diurnal rhythmicity and vision in diverse organisms. Although these and related light-dependent responses in nature already display the pertinent traits we now cherish in optogenetics, that is, genetic encoding, precision in time and space, non-invasiveness, and often reversibility (cf. sec. 1.), an analysis of the underlying light-sensitive cellular circuits, let alone their rational construction and practical application, had long been precluded. This situation changed dramatically with the molecular identification of the sensory photoreceptors underpinning many of these responses (cf. sec. 2.), which enabled more detailed study and eventual application. In a parallel key development, researchers pinpointed light as the ideal perturbatory stimulus for the acute and precise control and monitoring of living systems,73 thus laying the conceptual groundwork for the later implementation of optogenetics.74 Certain sensory photoreceptors, exemplified by the channelrhodopsins75,76 and photoactivated adenylate cyclases11, proved of immediate optogenetic utility upon expression in heterologous cells and organisms.5–8 Not only did these naturally-occurring photoreceptors facilitate the interrogation of biological systems in unprecedented ways, but they also validated the principal concept and feasibility of optogenetics. At the same time, the molecular description of the structure of the archetypical phototropin LOV photosensor module77,78 and the light-dependent allosteric transition it undergoes19 constituted key events towards the engineering of novel UV-light- and BL-sensitive photoreceptors. Aside from earlier work on PYP,56 these studies provided the first atomic view of how light signals are detected by a soluble, autonomously assembling photoreceptor and translated into protein structural transitions, here the reversible unfolding of the ancillary Jα helix of the LOV photosensor. In combination with the ongoing revolutionary success of optogenetics in the neurosciences,79,80 these findings provided the impetus for researchers to explore how other protein activities might be subjected to light control in genetically encodable fashion.67,81–89 As we illustrate in this and the ensuing section 4., the engineering of light-regulated protein actuators that serve as tools in optogenetics has been nothing but amazingly successful. Section 3.1. considers the principal and most successful design strategies which have spawned the plethora of optogenetic actuators now available for controlling cellular metabolism and parameters (cf. sec. 4.). In the subsequent section 3.2., we discuss attributes of light-regulated actuators that are relevant for optogenetic application and that are hence often optimized during the engineering of novel photoreceptors.
3.1. Optogenetic Application of Photoreceptors
3.1.1. Applications of Natural Photoreceptors
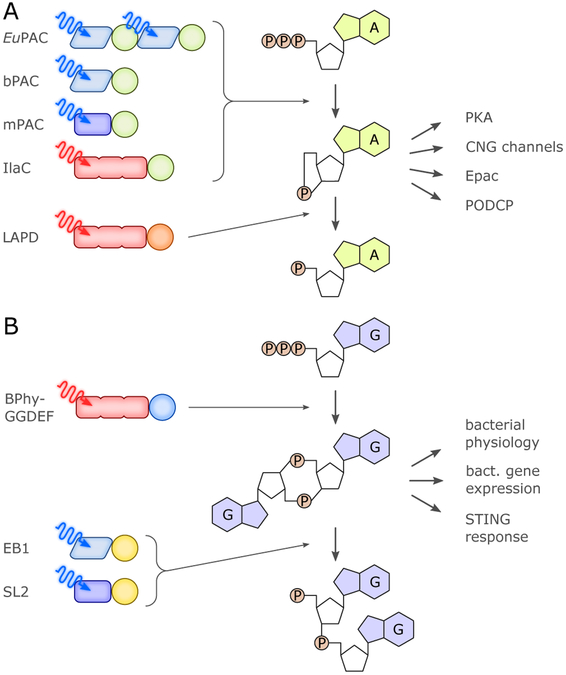
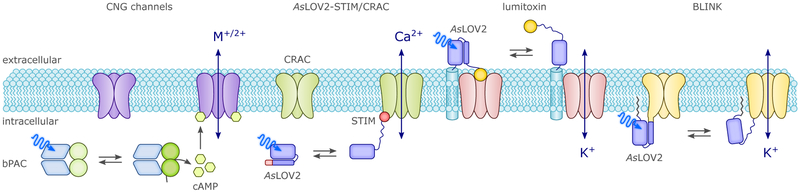
As a manifestation of their intrinsic modularity,16,90 the photoreceptors of the BL-sensitive classes BLUF and LOV occur in conjunction with a diverse set of effector modules, certain of which can be exploited as optogenetic actuators essentially in their naturally occurring forms. Prominent examples of this approach are LOV- and BLUF-based nucleotide cyclases (cf. sec. 4.6.1.),91 and the transcriptional regulator EL222 (cf. sec. 4.1.1.).92 These and a limited number of related receptors can often be optogenetically deployed in heterologous hosts with minimal prior engineering or modification. For example, the bacterial light-activated adenylate cyclase bPAC is readily expressed in animal host cells where it regulates by light cAMP-dependent processes such as the opening of cyclic-nucleotide-gated ion channels in neurons.93,94 Certain properties of naturally occurring photoreceptors, e.g., photocycle dynamics or substrate specificity,94 can be modified as dictated by application via the introduction of appropriate residue modifications. The considerable potential of natural BL-sensing systems has only been tapped to limited extent, often on account of practical issues such as low dynamic range (e.g., LOV-EAL and BLUF-EAL enzymes that regulate the turnover of the second messenger c-di-GMP, cf. sec. 4.6.1.), large size and unwieldy architecture (e.g., the fungal WC-1:WC-2 complex, cf. sec. 4.1.2.), or complications of using the effector output in a heterologous setting. As the number of known BL receptors continues to steadily increase, additional protein architectures and functions are likely to emerge and find application in optogenetics.16,95
3.1.2. Engineering Novel Photoreceptors
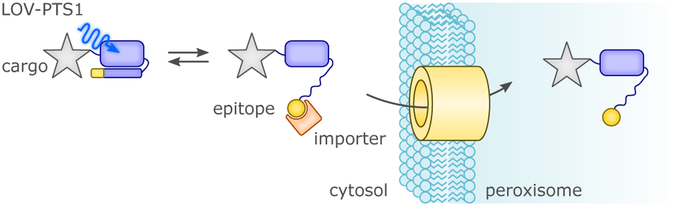
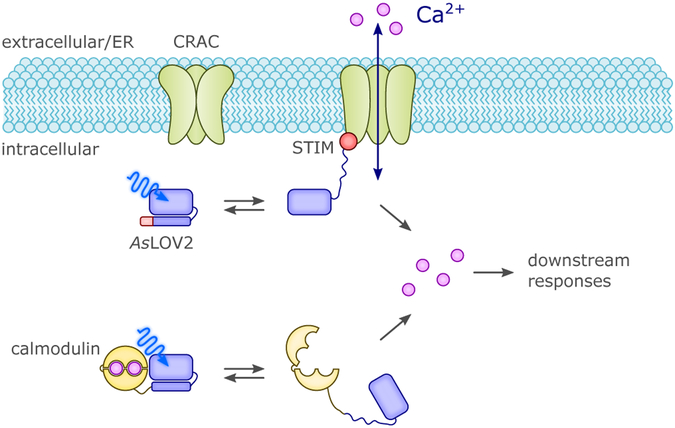
As diverse and ingenious as the design approaches are that underlie novel light-regulated protein actuators (detailed in section 4.), they share a common foundation in the mechanistic knowledge on naturally occurring photoreceptors. Perplexingly, this knowledge is often incomplete, a prime example being plant phototropins where the light-dependent signal transduction mechanism still awaits full elucidation.96 That notwithstanding, the allosteric principles realized in natural receptors and laid out above (cf. sec. 2.), have been employed in numerous creative ways for the construction of novel photoreceptors. Despite the rich versatility of engineered optogenetic actuators now available, we identify in the following recurring themes which span natural and artificial systems (Fig. 5). Surveying these systems, we see the two broad categories of non-associating and associating forms proposed by Ziegler & Möglich67 which can be further branched into detailed molecular mechanisms (Fig. 5). All of these mechanisms rely on light-triggered conformational changes that affect the activity of tethered effector domains, binding to other macromolecules, hetero- and homo-oligomerization, or compartmentalization within cells. This principle is general for the UV-light- and BL-responsive optogenetic tools treated here, with the exceptions described in section 5., e.g., derivative LOV photosensors for use as fluorophores or for production of reactive oxygen species (ROS). To enable the light-dependent control of cellular activity in scenarios where no suitable naturally-evolved photoreceptor already exists, a cohort of artificial photoreceptors has been engineered as section 4. discusses in depth. Specifically, the intrinsic modularity and mechanistic versatility of BL-sensitive photoreceptors, together with the ubiquitous availability of flavins in vivo, make these photoreceptors invaluable tools for a growing number of applications, including light-controlled gene expression, gene modification, protein activity and localization, and regulation of signaling networks.67,81–89 Compared to the membrane-embedded rhodopsins, soluble photoreceptors necessitate different engineering strategies for optogenetics (Fig. 5),67,81,85 on account of differences in their modular organization16 and allosteric signaling mechanisms.97 As mentioned in section 2.2., BL-regulated actuators can be broadly classed into associating and non-associating forms. Associating photoreceptors such as AtCRY2:AtCIB151 usually offer predictable and successful engineering strategies when target proteins and processes are regulated via oligomerization (i.e. light-dependent recruitment and colocalization). This concept can be extended to the reconstitution of split proteins or functions relying on a two-hybrid strategy by linking one polypeptide to a photosensing module and the second one to its interacting partner.67,86,87 As illustrated in section 2., physiological modes of action for associating photoreceptors are often well understood and at hand. Generally, there are minimal requirements for the linker between photosensing and effector domain(s), in that protein-protein interactions themselves drive the process. As a potential disadvantage, such association/dissociation equilibria strongly depend on several factors that may require optimization, including local concentration, self-association and limited dynamic range of the off-kinetics. By contrast, nonassociating photoreceptors keep their oligomeric state upon light activation, generally monomeric (e.g., AsLOV2)20 or dimeric (e.g., Bacillus subtilis YtvA [BsYtvA]).98,99 Notably, the oligomeric state that a photosensor assumes may depend on protein fragment size; for example, a construct of A. thaliana phototropin 1 LOV2 that included a more extended A’α helix than previously used in AsLOV219,20 crystallized as a homodimer.100 Non-associating photoreceptors form the basis of chimeric proteins where a molecular or cellular function is put under light control by fusing light-sensing modules to various effectors. The design is in many cases inspired by the natural and variegate architecture of BL receptors, with particular emphasis on LOV proteins.16 In this category, many applications explore and exploit the order-disorder transitions induced by BL-triggered detachment and unfolding of the Jα-linker in AsLOV2.101
Despite reasonably mature engineering strategies and many case studies to draw upon, the construction of novel light-gated actuators for optogenetic application remains challenging. To help surmount such challenges, efficient experimental and computational protocols have been devised. Whereas an exhaustive survey is beyond the current scope, we present several vignettes. At the experimental level, eventual success of photoreceptor engineering often depends on being able to create and then screen sizeable collections (or, libraries) of candidate construct variants. Techniques for generating libraries of desired size and diversity are well established in the protein-design field.102 A classic approach that pertains to both associating and non-associating photoreceptors is provided by random mutagenesis, followed by efficient functional in vitro and in vivo screening, cf. below.67 In particular for non-associating photoreceptors, the linker segment connecting photosensor and effector modules can severely impact on photoreceptor activity and degree of regulation by light.103–106 To aid evaluation of the best length and sequence for such linkers, a strategy was designed for the construction of hybrid-gene libraries with defined linker distributions.106,107 Regardless of the strategy by which candidate photoreceptor libraries are obtained, these libraries must be efficiently screened to identify the (few) variants displaying the desired property of robustly light-regulated function. Again, the protein design field has developed efficient approaches for this purpose.102,108 The best-suited strategy differs on a case-by-case basis, but generally speaking, screening is most efficient if light-dependent photoreceptor activity can be tied to cell survival, to a colorimetric or fluorogenic output, or to binding of a substrate molecule (e.g., another protein, a small molecule, or nucleic acids).
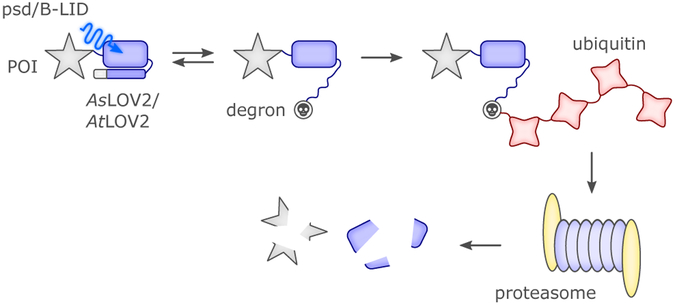
In addition to experimental protocols, new computational methods for the rational design of photoactivatable proteins can provide a solid base for the construction of optimized BL-sensitive actuators.72,86 A prominent example of computationally aided design has been recently provided by Dagliyan et al.109 Informed by molecular dynamics simulations, the AsLOV2 photosensor was inserted into non-conserved surface loops of target proteins that are allosterically coupled to the active site of these proteins (e.g., kinases, phosphatases, guanine exchange factors). The idea was prompted by the fact that the N- and C-terminal parts of AsLOV2 are close in space19,20 and therefore suited for insertion into surface loops: light-triggered undocking of Jα hence imposes a larger flexibility in the spacing between the AsLOV2 N- and C-termini, thus disordering portions of host proteins and inducing functional inhibition. With this powerful and generalizable approach, diverse photo-inhibited (PI) proteins were designed, as covered in more detail in sections 4.4. and 4.9.109 We note that a related approach was proposed based on a circular permutant of PYP, obtained by linking its N- and C-termini via a short peptide. Given that this BL sensor partially unfolds at its N terminus when forming the signaling state,110 this “circularization” system was proposed as a general approach to control conformation and activity of host proteins, albeit somewhat limited by the fact that PYP has a chromophore foreign to most organisms.
Other computational methods used to date include large-scale molecular dynamics simulations aimed at improving the dynamic range of BL-gated actuators by identifying key residues,111 and differential network analysis.16 This latter approach correctly identified several residues within the LOV core which affect a large number of distant nodes (single amino acids) and edges (connections). Importantly, this network-like behavior continues during signal transmission, in which linker regions flanking the LOV core (and particularly, connecting it to effectors) play a pivotal role. Moreover, the detailed bioinformatics analysis of more than 6,700 proteins exhibited clusters of conserved linker lengths, to some extent related to a common ancestry and to the type of effector.112 The important parameter required for maintaining intact signaling is the preservation of heptad repeats in helical linkers, rendering the distribution of linker lengths in nature highly discretized, at least for some effectors.16,17,105,113
3.1.3. Pioneering Examples of Engineered Photoreceptors
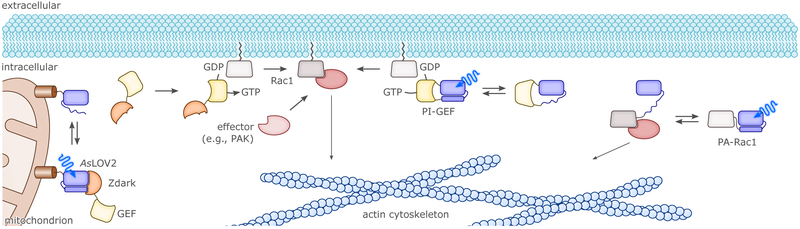
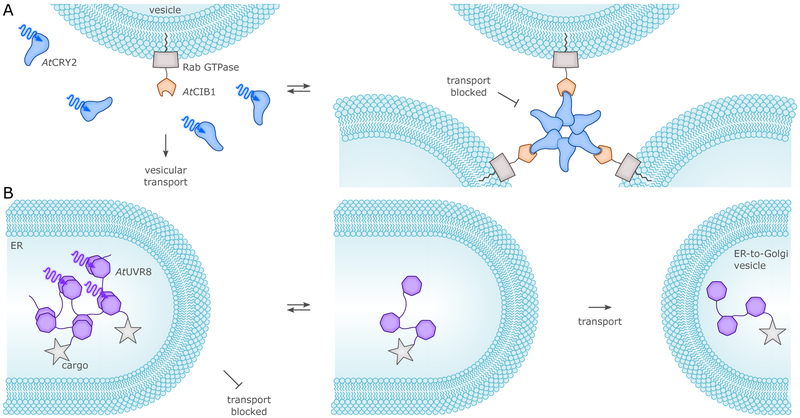
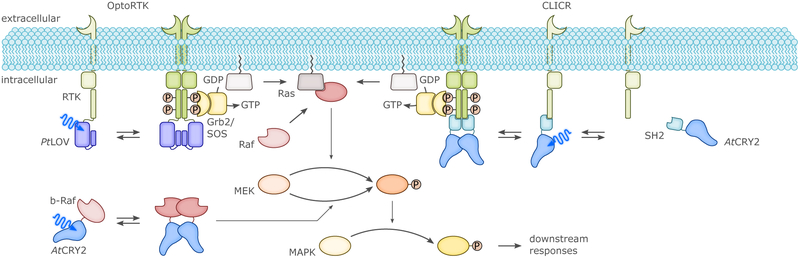
Prior to comprehensively surveying in section 4. the UV-light- and BL-sensitive optogenetic actuators available for manipulation of cellular physiology, we start by highlighting pioneering examples of photoreceptor engineering and the general principles they exhibit. By exploiting the intrinsic modularity of LOV receptors and their light-controlled changes in protein structure or oligomerization, several useful types of chimeric proteins were devised. The first example linked the AsLOV2 domain to the E. coli Trp repressor protein (TrpR) to build a light-regulated DNA-binding protein (cf. sec. 4.1.1.), thereby making use of the light-induced undocking of the Jα helix as an allosteric photoswitch.104 Initially, the degree of light activation in the AsLOV2-TrpR hybrid protein was modest due to the docked-undocked equilibrium of Jα being shifted towards the undocked state, thus rendering the protein mostly functionally active even in the dark. Subsequent introduction of residue exchanges which increased LOV2–Jα affinity in the dark led to a considerable improvement in dynamic range of light regulation, thereby paving the general way to enhanced LOV-based actuators.72 The light-induced unfolding of the AsLOV2 Jα helix also provided the foundation for a photo-activatable form of the small GTPase Rac1 that served to control cytoskeletal dynamics by BL (cf. sec. 4.4.1.).103 To subject Rac1 activity to BL control, the GTPase was linked to AsLOV2 such that steric occlusion resulted in the dark but could be relieved upon illumination. Another LOV protein, FKF1 from A. thaliana, was used to develop the light-activated-dimerization (LAD) technology which capitalizes on the light-activated binding of AtFKF1 to its interacting protein GIGANTEA (AtGI).114 Using LAD, Rac1 could be recruited to the cell membrane via a membrane-anchored GI, thus eliciting cytoskeletal rearrangements upon BL (cf. sec. 4.4.1.). The LAD system was also adapted to generate a light-activated transcription factor (cf. sec. 4.1.2.).114 A major drawback of the AtFKF1:AtGI dimerizing system was the slow kinetics of association (tens of minutes) and, especially, of dissociation (tens of hours). This latter aspect rendered the interaction effectively irreversible on most physiologically relevant timescales, underscoring the importance of off-kinetics.28 In another early application, the LOV domain of BsYtvA replaced the O2-sensing PAS-B domain of the histidine kinase FixL from Bradyrhizobium japonicum, thus generating the hybrid YF1 protein (cf. sec. 4.1.1.).105 BL regulation of histidine-kinase activity in the constitutively dimeric YF1115 apparently relies on left-handed supercoiling of a coiled-coil linker between photosensor and effector, which in turn induces internal repositioning within the effector unit.64
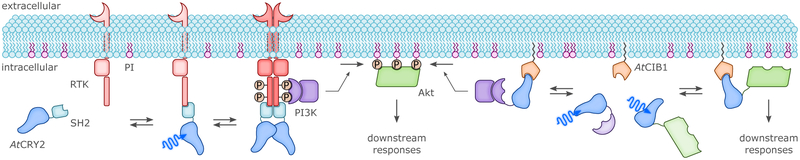
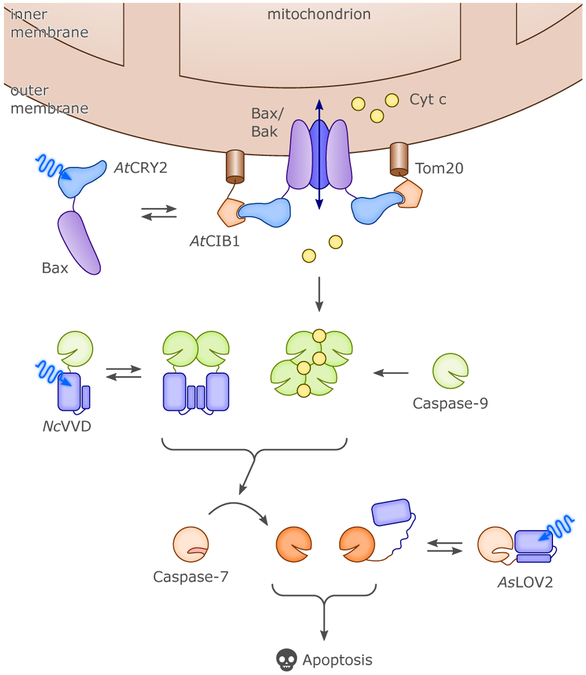
CRYs came into optogenetics as an alternative option for BL-induced dimerization (cf. sec. 4., esp. sec. 4.1.2.). By fusing proteins of interest (or, parts of split proteins) to either AtCRY2 or its partner AtCIB1, it was possible to control gene expression and subcellular protein localization.51 Heterodimerization of AtCRY2:AtCIB1 occurred significantly faster (in seconds) than with AtFKF1:AtGI, whereas dissociation was in the minutes range. Importantly it was demonstrated that the PHR domain of AtCRY2 suffices for forming heterodimers with full-length CIB1 or a truncated version lacking the basic helix-loop-helix DNA-binding domain, and that the system could also be triggered with two-photon excitation at 860 nm.51 Beyond heterodimerization with AtCIB1, the AtCRY2-PHR domain can also independently homooligomerize to give large clusters, within a few seconds after illumination.65 PYP was first fused to the basic-zipper protein GCN4, in an attempt to put under light control the binding of GCN4-PYP to DNA (cf. sec. 4.1.2.).116 The weak, two-fold increase in DNA affinity induced by BL was later improved to some extent by mutations.117 In a further application the N- and C-terminal ends of PYP were linked by means of a short peptide, introducing into BL-regulated actuators the concept of caging by circularization as a strategy for light-dependent control (cf. sec. 3.1.2.).110
3.2. Traits in Photoreceptor Engineering
The performance and eventual success of UV-light-/BL-sensitive actuators within a specific application setting depend on several aspects including genetic encoding, spatial and temporal resolution, light sensitivity and magnitude of light-induced effect,67,81 as summarized below. The photoreceptor engineering (cf. sec. 3.1.) and implementation stages commonly strive to optimize performance regarding these parameters. Because these considerations generally apply to optogenetics, they have been recently discussed in detail.67,81 In the present article, we mainly focus on sensory photoreceptors themselves and their engineering, and hence only touch upon these practical aspects.
3.2.1. Genetic Encoding and Spatial Resolution
Genetic encodability is usually a given for the three flavin-based photoreceptor classes (LOV, BLUF, CRY) because upon expression and folding in situ they autonomously incorporate their flavin chromophores which universally occur as essential metabolic cofactors. A quantitative analysis revealed riboflavin, FMN and FAD to be present in mammalian cell lines in attomole quantities per cell.118 The ready tissue availability of flavin chromophores contrasts with the situation for several other photoreceptor families, specifically the xanthopsin and many bilin-based photoreceptors, which require chromophores that are specific to certain organisms and that hence need be added exogenously to assemble the functional holo receptor in a heterologous cell context.
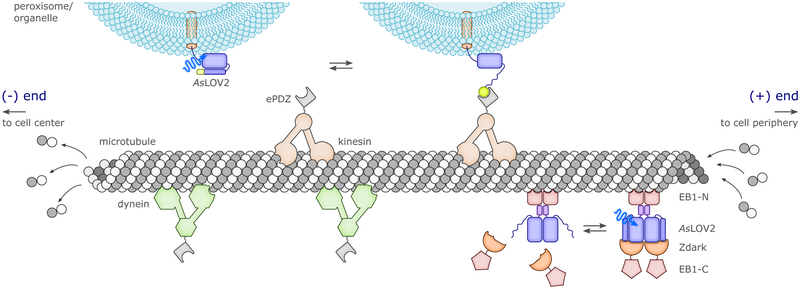
Spatial control in optogenetics is commonly exerted at the levels of gene expression and illumination protocols. For the former, tailored gene-delivery methods and specific promoters can target the expression of photoreceptors (and hence, light sensitivity) to specific cells, cell types, tissues or organs. Moreover, flavin-based photoreceptors have been successfully directed to different cellular compartments119 and organelles to achieve subcellular spatial resolution. An additional layer of spatial control can be achieved by using spatially confined light (as opposed to wide-field illumination) to specifically actuate photoreceptors within a given region of interest. Depending upon photoreceptor, the targeted cellular process and the timeframe of the experiment, diffusive events post illumination can degrade the attainable spatial resolution. In contrast to chemical means of controlling cellular metabolism, optogenetics at least offers the benefit of using a trigger, i.e. light, which is not diffusive itself, although light scattering may limit the achievable spatial resolution.
3.2.2. Light Sensitivity
Compared to other photoreceptor classes, UV-light/BL receptors are generally sensitive to relatively short wavelengths and feature low absorption cross sections, e.g., ε450 = 12,500 M−1 cm−1 for FMN in water120, or ε280 ≈ 5,500 M−1 cm−1 for the tryptophan indole group. A potential impediment to optogenetic application stems from light of short wavelengths not penetrating living tissue as deeply as red/near-infrared light does.67,121,122 Moreover, UV and blue light are potentially phototoxic because of absorption by endogenous photosensitizers for reactive oxygen species, such as flavins themselves (cf. sec. 5.) and iron-free porphyrins.123 In some studies, the shallow tissue penetration and phototoxicity of BL was bypassed by two-photon excitation or by using upconverting nanoparticles to convert near-infrared light into visible light, but the latter approach suffers from the need of delivering the particles to target sites.87,124,125 On the other hand, BL-responsive optogenetic circuits are largely insensitive to wavelengths larger than 500 nm and can hence be readily combined with fluorescent reporters with more red-shifted absorption spectra. This offers a particular advantage if an experiment requires just a short perturbation via BL excitation, while long-term effects can be probed with a red-absorbing reporter.
Short of introducing chemically-modified chromophores,126 the tuning of the spectral sensitivity of flavin-binding photoreceptors has proven to be a very difficult task.123 As a case in point, in the BsYtvA LOV receptor, the absorption maxima of the dark-adapted state ranged between 445 and 448 nm across a wide range of protein variants bearing different residue exchanges near the chromophore.127 Owing to the rigid scaffold of the flavin isoalloxazine ring, the tuning of absorption of flavin chromophores in their oxidized quinone state to substantially longer wavelengths, let alone to the attractive near-infrared ‘transparent’ window (650–900 nm) where light readily penetrates mammalian tissue,121,122 is likely impossible.123,128,129 By contrast, the partially reduced semiquinone radical states of flavin chromophores are known to absorb at longer wavelengths.130 Recently, an animal-type cryptochrome from Chlamydomonas reinhardtii has extended the spectral range of BL receptors to yellow and red light, given that its dark-adapted state contains the neutral semiquinone radical form of FAD.131–133 It is currently unclear if this is a rare exception and whether flavin-binding photoreceptors can be deliberately modified to assume a partially reduced flavin in their dark-adapted states, cf. sec. 5.
3.2.3. In Situ Activity and Dynamic Range
The in situ activity of an optogenetic actuator and accordingly the response of the system under study will depend on the applied light dose, as well as on the expression levels, the spatiotemporal distribution and the specific activity of the underlying photoreceptor.67 As cellular circuits often display threshold and amplification effects, the system response to optogenetic perturbation may be highly nonlinear and hence its quantitative prediction challenging. This particularly applies to actuators embedded in signaling cascades that amplify the response, e.g., for enzymes engaged in second-messenger signaling, cf. sec. 4.6. Likewise, this is true for associating photoreceptors, e.g., in case of BL-induced clustering of AtCRY2, the response of which is expected to display a strong dependence on the spatiotemporal concentration of activated receptor.67
Beyond the overall activity, the difference in activity between the dark-adapted and light-adapted states of photoreceptors and derived optogenetic circuits is of prime interest. Commonly, the ratio of activities in these two states is referred to as the dynamic range, and photoreceptor engineering often attempts to maximize this quantity. The maximally achievable dynamic range is strongly governed by how well activity can be suspended in the low-activity state of a receptor, i.e. in darkness for light-activated actuators, or under light for light-repressed receptors.67 Whereas the membrane-integral rhodopsin photoreceptors often feature exquisitely low dark-state activities and accordingly high dynamic ranges, e.g., references75,134,135, the soluble BL-sensitive photoreceptors frequently display substantial residual activity in their low-activity state and correspondingly smaller dynamic ranges. As previously discussed in a thermodynamic framework,67 these soluble photoreceptors fundamentally rely on equilibria between low-activity and high-activity conformations136 which are modulated by illumination.101 Put another way, BL receptors usually do not behave as digital on/off switches but as analog switches. Non-binary switching of optogenetic circuits may incur high dark-state (background) activity and limited extent of activation by illumination. Potentially, the energy content in visible light, e.g., ca. 250-300 kJ mol−1 for BL in the range of 480 to 400 nm, suffices to substantially shift the equilibrium between low- and high-activity states and to thereby achieve much larger dynamic range. However, to the extent it is known, only a fraction of the photon energy is converted into useable free energy changes (ΔG). For example, the unfolding of the Jα helix in the widely-used AsLOV2 photosensor is associated with a ΔG of only around 16 kJ mol−1, and accordingly the maximally achievable dynamic range is inherently limited.67,101 Notably, judiciously-chosen residue exchanges within the photosensor can shift the equilibrium between low- and high-activity states and hence the attainable dynamic range.67,72,101 In addition, dynamic range may be enhanced by embedding photoreceptors into signaling cascades or by exploiting cooperativity effects in oligomeric receptors.105,137
3.2.4. Temporal Resolution
Depending on the timescale of biological processes one desires to interrogate, the kinetics of activation and deactivation of optogenetic circuits are relevant. Generally, photoreceptor activation occurs well under a second, making it fast compared to many cellular events (excepting the millisecond and faster timescale processes common in the neurosciences). For example, the detachment and unfolding of Jα in AsLOV2 is complete within 0.3-1.0 ms.138–141 Comparable structural perturbations in associating photoreceptor systems are equally fast, occurring on the sub-millisecond timescale in plant cryptochromes.142–144 Similarly, the photodissociation of multimeric BLUF proteins takes place within 4 to 45 ms,145–147 and the light-induced dimerization of Neurospora crassa Vivid (NcVivid) is complete within 20 ms, compatible with a diffusion-limited process under the conditions tested.148 As such, aspects other than the inherent photochemical mechanisms typically limit the on-kinetics with which an optogenetic response can be triggered. For example, light is strongly absorbed and scattered by tissue, cf. sec. 3.2.2., and hence in some scenarios only a relatively low dose may effectively be delivered to the target site. As a corollary, the accumulation of sufficient amounts of activated photoreceptor molecules in time and space to trigger the desired physiological response, cf. threshold effects mentioned above, can become time-limiting. Furthermore, the triggered cellular function may be inherently slow, e.g., gene expression, thus limiting response dynamics.
In addition to the activation kinetics, the off-kinetics with which an optogenetic circuit deactivates once illumination ceases greatly bear on optogenetic application. As detailed in section 2., photoexcitation of BL-sensitive and UV-sensitive photoreceptors leads to population of a metastable signaling state that thermally (i.e. passively) decays back to the dark-adapted state with kinetics that are governed by receptor identity, solvent accessibility and environment of the chromophore, oxygen concentrations and temperature.28,97,123,149–153 A range of residue exchanges modulating these dark-recovery kinetics have been identified, especially for LOV proteins.28 To a considerable extent, such exchanges are transferable between related photoreceptors and thus provide a ready means of adjusting recovery kinetics for a given optogenetic application. As a word of caution, we note that such exchanges can potentially impair proper signal transduction within the photoreceptor,154 as is the case for a conserved glutamine in LOV domains152,155 or for hydrogen-bond forming histidines in cryptochromes.156 Faster off-kinetics and resultant enhancement of the temporal resolution can be effected by photochromic photoreceptors that are toggled back and forth between two photochemical and activity states by light of different colors. Photochromicity is a general feature of bilin-based photoreceptors (reviewed by Gärtner) and frequently occurs among rhodopsins. By contrast, BL-sensitive photoreceptors are usually not photochromic. However, we note that the covalent thioether bond formed in LOV receptors upon BL absorption (cf. sec. 2.) can be photolyzed by UV-A/violet radiation.157–159 Due to the low quantum yield for this process and the requirement for potentially phototoxic UV-A/violet illumination, this effect has to date not been taken up in optogenetic applications. In cryptochrome photoreceptors, BL absorption leads to population of the partially reduced semiquinone radical state of the flavin chromophore (cf. sec. 2.1.3.). At least in certain cases,131,160 secondary absorption of photons between 450 and 600 nm promotes complete reduction to the hydroquinone state and thereby toggles the effector output of the photoreceptor.
Optogenetic experiments often resort to prolonged illumination, such that photoreceptors undergo repeated cycles of photoactivation to their light-adapted states and thermal recovery to their dark-adapted states. As a consequence, a photostationary state is assumed in which on average a constant fraction of the photoreceptor ensemble resides in its dark-adapted state and the remainder in the signaling state. While the absolute light sensitivity of a photoreceptor (cf. sec. 3.2.2.) cannot be modified much, the effective light sensitivity at photostationary state can be conveniently modified by altering recovery kinetics via the above strategies.67 Knowledge of the recovery kinetics of a given optogenetic circuit can be exploited for the optimization of illumination protocols and for the parallel deployment of actuators that respond to the same light color but differ in their sensitivity.161
4. Photoreceptors as Actuators in Optogenetics
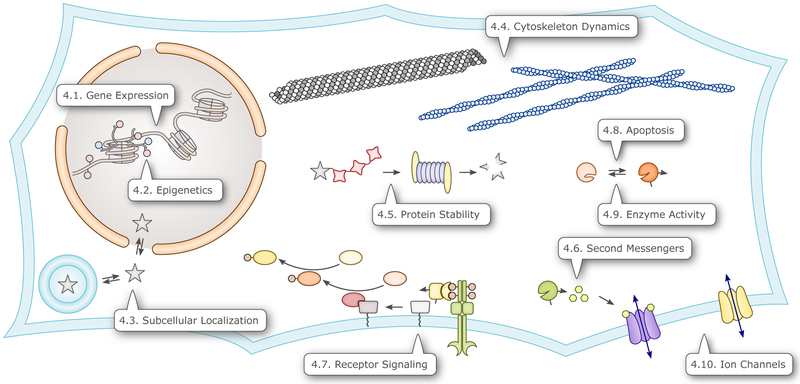
Galvanized by the ready and far-reaching impact of the initial optogenetic applications in the neurosciences5–8 that employed rhodopsin photoreceptors to act on membrane potential,75,76 researchers also explored the suitability of other photoreceptor classes for optogenetics. To this end, a small set of naturally-occurring photoreceptors with immediate optogenetic applicability have been complemented by a much larger suite of engineered photoreceptors devised by the strategies covered in section 3.67,81–89 To date, these engineering efforts have been most successful with blue-light-sensitive photoreceptors, particularly in the cryptochrome and LOV classes. As discussed in this section, natural and engineered UV- and BL-sensitive photoreceptors together have now unlocked numerous cellular parameters and processes for optogenetic intervention, including protein-protein interactions, transcription (sec. 4.1.), recombination and epigenetic modification (sec. 4.2.), subcellular localization (sec. 4.3.), cytoskeleton dynamics (sec. 4.4.), protein stability (sec. 4.5.), signaling by second messengers (sec. 4.6.), receptor signaling (sec. 4.7.), apoptosis (sec. 4.8.), enzyme activity (sec. 4.9.), and membrane potential (sec. 4.10.) (Fig. 6).
Figure 6.
Overview of cellular processes that have been optogenetically controlled via photoreceptors sensitive to UV and blue light. The callouts direct to the sections that discuss the corresponding applications.
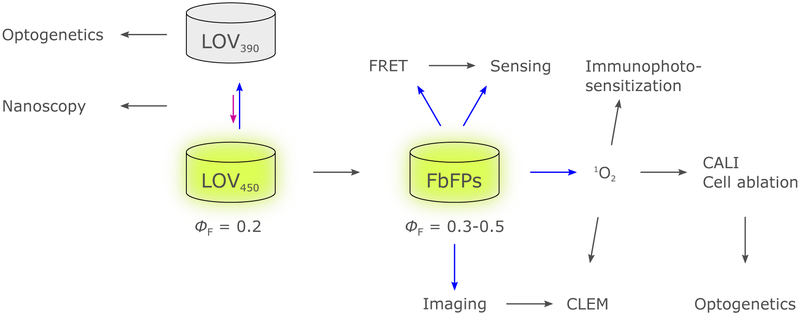
Moreover, as we illustrate in section 5., photoreceptor proteins, once suitably conditioned, are not only restricted to their conventional role of regulating effector output in response to light, but can also serve other purposes. Pertinent applications generally exploit the genetic encodability of photoreceptor proteins and their ability to autonomously and specifically bind their respective chromophores, even within living cells. In this manner, several flavin-based photoreceptor variants have been developed that function as fluorescent proteins or as light-driven generators of reactive oxygen species. Among the UV-B/BL-sensitive receptors, these ‘off-label’ applications have to date been realized for LOV receptors, but conceptually they should extend to at least the other flavin-based photoreceptors, too. Moreover, there is mounting evidence that flavin-based photoreceptors can double as sensors of intracellular oxygen and redox potential under physiological conditions, and we discuss both the intended and the unintended implications of these properties.
In the following, we survey cellular processes and parameters which have been controlled by light via optogenetic actuators based on the UV-/blue-light-sensitive photoreceptors introduced in section 2. (Fig. 6). We loosely group these applications and photoreceptors based on the cellular process targeted. In doing so, we focus on the original development and initial optogenetic application(s) of a given photoreceptor, as a comprehensive treatise of each subsequent application of each tool is beyond the scope of the current review. For an up-to-date overview, we refer to a web resource that records available optogenetic actuators in incremental manner.162 On the whole, the optogenetic deployment of blue-light-sensitive photoreceptors displays impressive versatility and ingenuity of the approaches chosen. As perhaps best exemplified by the recurring use of the AsLOV2 domain, even a single photosensor unit can be configured such that it regulates by light the activity of a broad palette of highly disparate effectors. Evidently, the underlying allosteric principles of light-dependent signal transduction, treated in sections 2. and 3., far transcend sensor-effector combinations realized in nature and can be extended to even completely unrelated effector moieties.
4.1. Transcription
Going by the sheer number of different examples, the regulation of gene expression by light represents one of the most successful optogenetic application areas afforded by BL-sensitive photoreceptors. Most often, control over gene expression is exerted at the level of transcription initiation, but select photoreceptors intervene in later stages as well. There are at least three principal reasons for the relative popularity of light-regulated transcription: first, gene expression is of profound biological significance and lends itself as a highly versatile leverage point for optogenetic intervention; second, the biological process of transcription is well understood and many transcription factors (TF) are inherently modular which benefits photoreceptor engineering (cf. sec. 3.); third, expression of (fluorescent) reporter genes provides a ready means for engineering and optimizing novel photoreceptors, cf. sec. 3.1.2. Light-gated actuators have been constructed for the regulation of transcription initiation in both prokaryotes and eukaryotes, and we will cover them in turn. Certain representatives straddle this divide in that they are of optogenetic utility in both domains of life.
4.1.1. Prokaryotic Transcription
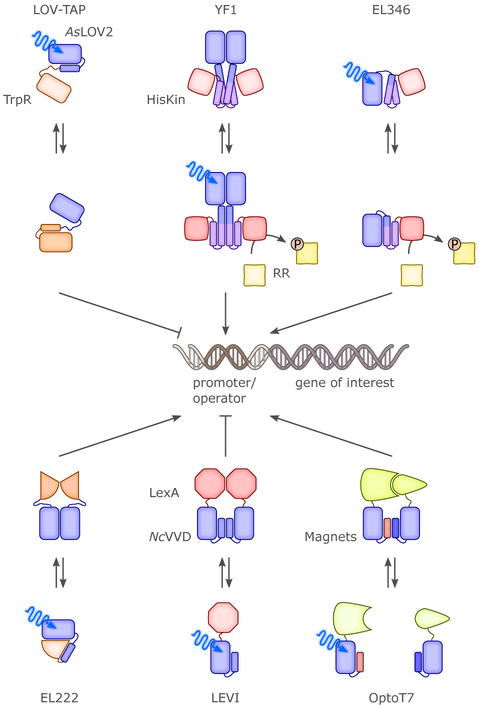
In one of the earliest examples of photoreceptor engineering, the activity of the E. coli Trp repressor (TrpR) was put under light control by fusing its N-terminal helix with the C-terminal Jα helix of the AsLOV2 module104 such that steric overlap would result between the two entities (Fig. 7), cf. sec. 3.1.3. Within such fusions, the TrpR and AsLOV2 domains thus engage in a tug-of-war for the intervening Jα linker helix. As the Jα conformation and its affinity for the AsLOV2 core are modulated by light, the correct folding and function of TrpR is thus regulated. Fusion constructs between TrpR and AsLOV2 were prepared according to this rationale and tested for light-regulated binding to the TrpR DNA operator sequence in nuclease-protection assays. In one variant, denoted LOVTAP, DNA affinity was enhanced by ~6-fold by BL. While subsequent stabilization of the AsLOV2:Jα interface by site-directed mutagenesis72 improved the dark/light difference in DNA affinity to around 65-fold, LOVTAP has not been widely deployed, arguably because its DNA affinity is much weaker than that of wild-type (wt) TrpR.
Figure 7.
Several optogenetic systems achieved BL control over transcription in prokaryotes. In LOV-TAP104, the E. coll Trp repressor and the AsLOV2 were fused such that mutually exclusive folding of a shared α helix resulted; BL exposure allowed the repressor to correctly fold and bind to DNA. The homodimeric YF1105 and the monomeric EL346163 are LOV histidine kinases that phosphorylate cognate response regulators in BL-dependent manner; when phosphorylated, the response regulators bind DNA and activate transcription. In EL222,21 BL absorption by a LOV photosensor prompts dimerization and DNA binding of an associated helix-turn-helix effector, leading to transcriptional activation. The LEVI approach164 is based on the NcVivid LOV sensor; BL-induced homodimerization rescues the repressional activity of the truncated LexA repressor. Based on the Magnets system for BL-induced heterodimerization165, BL-activated split variants of the phage T7 polymerase were engineered.166,167
Complementing this fusion approach, domain-exchange strategies into existing transcriptional control systems have also been successful. In the most highly used application of this approach, the YF1 light-regulated sensor histidine kinase (SHK) was generated105 by replacing the oxygen-sensitive PAS-B sensor domain of the B. japonicum FixL SHK by the structurally homologous LOV photosensor of B. subtilis YtvA (Fig. 7).168 Notably, SHKs form part of two-component systems (TCS)169 that mediate transcriptional responses to cognate stimuli in bacteria and in certain plants and fungi, and the architecture of YF1 closely corresponds to that of naturally occurring LOV-SHKs.16,170–172 Net phosphorylation of the cognate response regulator BjFixJ by YF1 was repressed by more than 1000-fold in blue light compared to in darkness. Two portable plasmids, denoted pDusk and pDawn, assembled on the basis of YF1 and BjFixJ, afford BL-activated and BL-repressed gene expression, respectively, and have been widely used.107,173–176 A derivative version of YF1, that combined the original PAS-B domain of BjFixL with BsYtvA-LOV rather than replacing it, integrated the signals blue light and molecular oxygen in positive cooperative manner.137 Catalytic activity and response to light of YF1 variants crucially depended on the length of the linker that connects sensor and effector moieties and that adopts parallel α-helical coiled-coil conformation in the dimeric receptor.105,106 This dependence on linker length hinted at the structural mechanism for signal transduction in YF1 which was recently borne out in biophysical measurements.64,177,178 BL absorption evidently promotes left-handed supercoiling of the coiled-coil linker, thereby triggering reconfiguration of the effector module. Insertion of single residues in said linker sufficed for inversion of the response to light,105,106 as did certain residue exchanges within the LOV sensor.115,179 Whereas canonical SHKs, such as YF1, adopt homodimeric structure, a bona fide monomeric LOV-SHK, denoted EL346, was discovered in the marine bacterium Erythrobacter litoralis (Fig. 7).163 In EL346, a LOV photosensor forms an intramolecular complex with the effector moiety; upon light absorption, this complex dissociates, the effector is liberated and its activity increased for both autophosphorylation and phosphotransfer to cognate response regulators.163,170,180,181 EL346 represents an important paradigm for SHKs and LOV receptors alike and could be used as a light-gated actuator in optogenetics, but to date it has not been deployed in this manner.
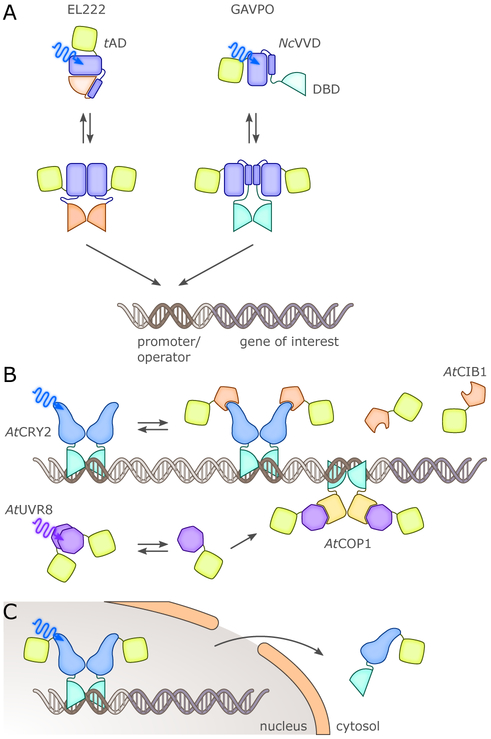
Two other BL-sensitive photoreceptor systems afford a simpler architecture than the above TCSs in that they are realized as single protein entities. First, in the EL222 receptor, also from E. litoralis, a LOV photosensor associates intramolecularly with a helix-turn-helix effector via a helical connector (Fig. 7).21 Light absorption promotes dissociation of the effector from the LOV sensor and allows receptor dimerization. In its dimeric state, EL222 binds to a cognate operator sequence to activate transcription from the corresponding genetic loci.182 As discussed below, EL222 underpins an efficient system for light-activated gene expression in eukaryotes,92 but more recently it was also deployed in E. coli.183 By placing the cognate operator sequence at different positions relative to the −35 and −10 regions of bacterial promoters, EL222 either served as a light-activated transcriptional activator or repressor. This approach recently provided the basis for a cell-free optogenetic expression system.184 Second, a light-regulated transcriptional repressor, termed LEVI, was generated through fusion of the E. coli LexA repressor with the NcVivid LOV sensor,164 conceptually similar to the LightON system (cf. below) which affords light-activated gene expression in eukaryotes (Fig. 7).185 In LEVI, the LexA effector was truncated such that it lost its ability to dimerize and to bind to DNA; light-promoted association of NcVivid rescued dimerization, DNA binding and transcriptional repression. The LEVI system exceled in its compact architecture and highly stringent response to blue light.
Recently, bacterial expression was also optogenetically regulated at the level of the RNA polymerase itself. In two closely similar approaches,166,167 the phage T7 polymerase was split into two fragments which could be reconstituted in BL-activated manner by linking the split parts to the LOV-based Magnets photoreceptors for heterodimerization (Fig. 7).165 By varying the split site within the T7 polymerase and the (relative) abundancies of the resultant fragments, expression of target genes could be induced by BL by up to several hundredfold. As the wild-type T7 polymerase can be functionally expressed in mammalian cells,186 the split, BL-regulated variants may also unlock optogenetic control of transcription in eukaryotic cells.
4.1.2. Eukaryotic Transcription
Natural BL-regulated transcription factors have been identified in several eukaryotic organisms, most prominently the fungal white-collar (WC) proteins, e.g., from N. crassa,187 and the aureochromes, first identified in stramenopile algae188 but later also in diatoms. The fungal WC system is involved in regulating circadian rhythm in response to BL and consists of several components. One protein, WC-1, comprises a LOV sensor and a zinc finger DNA-binding domain (DBD). Upon light absorption, WC-1 forms a heterodimeric complex with WC-2 which also contains a zinc finger but lacks a LOV photosensor. The WC-1:WC-2 complex can then bind to cognate operator sequences and activate transcription from associated promoters.187 Activation of the WC complex drives the expression of several genes, including one encoding another LOV receptor (NcVivid) that also contributes to light adaptation. Despite its relatively early discovery and functional annotation, the WC system has not been widely deployed in optogenetics, presumably because of the heterodimeric nature of the system and the considerable size of its constitutive components. By contrast, aureochromes188 feature a more compact architecture with a basic-zipper DNA-binding module succeeded by a LOV photosensor domain. In the alga Vaucheria frigida, two aureochrome receptors regulate development and morphogenesis in response to BL. Although no endogenous operators/promoters have been reported, an artificially-selected DNA consensus sequence was identified from a random pool of DNA fragments that the aureochromes bind to. Sequence homology searches also identified aureochrome receptors in diatoms, e.g., in Phaeodactylum tricornutum and Thalassiosira pseudonana.188 The isolated LOV photosensors of several aureochromes have been shown to undergo light-regulated homodimerization,189 prompting their subsequent use as building blocks in photoreceptor engineering.190 By contrast, intact aureochromes themselves have not yet played a significant role in optogenetic applications despite their small size (perhaps due to the limited degree of light-dependent switching of DNA binding affinity191,192).
To address the need for efficient light-regulated gene expression in eukaryotes, a cohort of photoreceptor systems, many of which respond to blue light, have been engineered. Following its original characterization21 and identification of its DNA target sequences,182 the prokaryotic LOV receptor EL222 has been converted into an eukaryotic transcription factor via C-terminal appendage of a viral trans-activating domain (tAD) (Fig. 8A).92 The expression of transgenes from promoters that contained several repeats of the EL222 target operator sequence could be upregulated by more than 100-fold by BL illumination. For applications in zebrafish, an optimized version of the system with lower cytotoxicity was developed by exchanging the tAD for another.197 Recently, light-regulated gene expression via EL222-tAD in yeast was deployed in single cells453 or to optogenetically control metabolic flux in bulk culture.198 Application of BL induced yeast cells to switch from growth to production phase at desired time points and thus enabled the overall increase of biosynthesis yields by several folds. While EL222 has essentially been used as an intact protein as provided by nature, other systems for light-regulated gene expression generally recombine photosensor, tAD and DNA-binding modules. As a case in point, in the LightON approach a truncated Gal4 DBD, the NcVivid LOV photosensor and a tAD were fused to yield a monomeric chimera, denoted GAVPO, that in darkness had low affinity for the Gal operator sequence (Fig. 8A). BL absorption by the NcVivid photosensor domain triggered dimerization of the chimeric receptor and thereby restored DNA affinity. Using GAVPO, transgenes could be expressed in strongly BL-regulated manner from promoters that contained several copies of the Gal operator sequence. In mammalian cell culture, upregulation of luminescent reporters by several hundredfold was achieved, and the LightON system also showed good performance in a mouse model. Introduction of a mutation in the NcVivid LOV domain that increases dimerization propensity yielded a variant of GAVPO that supported higher absolute transgene expression levels, albeit at the cost of a reduced dynamic range by increased dark-state binding.199 Whereas EL222 and GAVPO are single polypeptide chain designs, several other systems for light-regulated gene expression rely on a two-hybrid strategy, employing split transcription factors that are composed of two separate polypeptide components. Although details differ, these systems generally utilize photoreceptor pairs that undergo light-driven association/dissociation reactions involving separate DNA-binding and transcriptional-activation components (Fig. 8B). Light prompts association of the two components and thereby recruits the tAD to the DNA site specified by the DBD, and transcription is initiated. An early implementation, denoted light-activated dimerization (LAD), of this concept was achieved on the basis of the LOV receptor FKF1 from Arabidopsis thaliana that associates with its partner protein GIGANTEA (AtGI), or N-terminal fragments thereof, under blue light,114 cf. sec. 3.1.3. When fused to the Gal4 DBD and a viral tAD, respectively, the AtGI:AtFKF1 pair enabled expression of transgenes in mammalian cells that could be enhanced by BL by up to around 5-fold. Of note, this BL-induced activation of expression was essentially irreversible on physiologically relevant time scales due to the exceedingly slow dark-recovery reaction of the AtFKF1 LOV receptor.200 Recently, the performance of this system for light-regulated gene expression was significantly enhanced by random mutagenesis of AtFKF1 and construct optimization. The improved setup enabled transgene expression in cell culture and in mice that could be upregulated under BL by around two orders of magnitude.201 In a different LOV-based strategy, Lungu et al.202 interwove short peptide epitopes in the Jα helix of AsLOV2 such that upon light-induced unfolding of Jα, they become more accessible and able to specifically bind to partner proteins. The modified AsLOV2 sensor and the partner protein were connected to the DBD and tAD of Gal4, respectively, to furnish a system that achieved around 10-fold upregulation of a reporter gene in yeast under BL. Although not implemented yet, the performance of the gene-expression system could conceivably be improved by resorting to enhanced versions of the AsLOV2-based, photo-associating protein pair that were developed in a later study.203
Figure 8.
BL-dependent of control of transgene transcription in eukaryotes was realized with single-chain constructs (panels A and C) and with split transcription factors (TF) (panel B). (A) The bacterial LOV receptor EL222 was linked to a eukaryotic trans-activating domain (tAD) to achieve light-dependent control of transgenes in eukaryotic cells.92 The GAVPO approach makes use of the homodimerization reaction NcVivid undergoes upon BL exposure.185 By linking a DNA-binding domain (DBD) and a tAD to NcVivid, a chimeric TF was obtained that in darkness is monomeric and unable to bind to the DNA operator sequence. BL induced dimerization, DNA binding and transcriptional activation. (B) In several studies, split-TF systems were generated, as exemplarily shown for two specific scenarios. (top) Several approaches relied on linking AtCRY2 to a DBD such that upon BL application a tAD, linked to AtCIB1, could be recruited to induce gene expression.51,193 (bottom) Conceptually similar approaches were realized for AtUVR8 which forms a homodimer in the dark but dissociates upon UV-light exposure.194,195 In its monomerized form, AtUVR8 can then bind to AtCOP1. By linking the two proteins to a DBD and tAD, respectively, UV-light-dependent control of transcription was achieved. (C) AtCRY2 was fused with both a DBD and a tAD to yield a single-chain TF.196 BL induced nuclear clearing of this TF, accompanied by downregulation of transcription.
Other systems for light-regulated gene expression have been based on cryptochromes, most notably cryptochrome 2 from Arabidopsis thaliana (AtCRY2). As discussed in section 3., AtCRY2 undergoes light-dependent association with the full-length AtCIB1 protein or N-terminal parts of it.50 (Unless explicitly stated otherwise, in the following the abbreviation AtCIB1 refers to the N-terminal fragment of the protein. Likewise, the abbreviation AtCRY2 denotes the N-terminal PHR portion rather than the entire protein.) Early on,51 the AtCRY2:AtCIB1 pair was linked with the DBD and tAD of Gal4, respectively, to drive gene expression of transgenes in yeast that could be strongly upregulated by blue light (Fig. 8B). Unexpectedly, the same system failed to achieve meaningful degrees of light-regulated gene expression when applied in mammalian cells.196 A careful investigation revealed that blue light promotes clearing from the nucleus of the AtCRY2-DBD that could eventually be pinpointed to the presence of a dimerization motif within the DBD. Removal of this dimerization motif abolished BL-induced nuclear export of AtCRY2-DBD and, in combination with AtCIB1-tAD, enabled robust BL-activated gene expression; variation of the tAD further enhanced the system up to more than 100-fold induction by BL. In addition, Tucker and colleagues realized that the phenomenon of BL-induced nuclear clearing can be capitalized on and devised a single-chain transcription factor that comprised DBD, AtCRY2 and tAD modules (Fig. 8C). In the dark, this TF predominantly resided in the nucleus and drove expression of transgenes, but upon illumination with blue light, it translocated to the cytosol, and expression could hence be repressed by up to 50-fold. Similar to the original approach by Kennedy et al.,51 a system for light-regulated gene expression in zebrafish was established by connecting AtCRY2 and AtCIB1 to the DBD and tAD of Gal4, respectively.193 Interestingly and in line with the above work, the performance of the system in zebrafish lagged behind that in yeast. In a related setup,204 AtCIB1 and AtCRY2 were fused to the LexA-DBD and a tAD, respectively, to allow BL-induced expression of transgenes in Drosophila. In a similar vein, AtCIB1 was combined with the widely used TetR-DBD to enable BL-induced recruitment of AtCRY2 connected to a strong tAD that enabled transcriptional activation of target transgenes.205 Because the TetR-DBD has been widely employed in cell biology, this implementation of the AtCIB1:AtCRY2 system unlocks scores of additional systems for optogenetic intervention.
As these examples compellingly illustrate, the performance and function of light-regulated gene expression systems may drastically vary between hosts and contexts, often for (initially) poorly understood reasons. As it is challenging to systematically compare the various systems, let alone in a number of heterologous host systems, only few efforts have been undertaken to this end.206,207 Against this backdrop, we regard it an advantage that several systems are now in place from which can be selected the best suited for a given application.
Photoreceptors other than LOV and cryptochromes have also provided building blocks for light-regulated gene expression. In two related studies,194,195 the UV-light induced dissociation of the AtUVR8 homodimer into monomers and their subsequent association with the AtCOP1 protein was harnessed (Fig. 8B). In one report,194 AtCOP1 was fused with the Gal4-DBD, and AtUVR8 with a tAD to drive expression of transgenes in mammalian cells in strongly UV-B-dependent manner. In the other study,195 AtUVR8 was covalently linked to the DBD of the macrolide-responsive repressor E, and the WD40 domain of AtCOP1 was linked with a tAD to achieve expression of transgenes in mammalian cells that could be up-regulated by up to several-hundredfold by UV-B light. Notably, the combination of the UV-sensitive AtUVR8:AtCOP1 systems with a BL-sensitive and a red/far-red-light-sensitive system enabled the sequential light-triggered expression of three separate transgenes.195 In a different approach,116,117 PYP was employed to control the GCN4 TF. The DNA affinity could be modestly upregulated by BL via linkage of the C terminus of GCN4 to an N-terminally truncated variant of PYP. A biophysical characterization indicated that in the dark the GCN4 moiety folds back onto PYP, and a monomeric protein results. Light-induced refolding of the PYP N terminus liberates GCN4 and thus promotes dimerization and DNA binding. Arguably, due to the limited enhancement of DNA affinity by light and due to the requirement for the specific chromophore p-coumaric acid, the system has to date not been applied in optogenetics.
The combination of split transcription factors with photoassociating photoreceptors that underpins many of the above strategies is not limited to regulating transcriptional initiation alone, but instead extends to other processes. As demonstrated by Cao et al.,208 AtCIB1 can be linked to the λN RNA-binding domain that binds to a specific motif embedded in the 5’-untranslated region of a target mRNA; blue light allowed recruitment of a fusion protein between AtCRY2 and the eukaryotic translation initiation factor eIF4E. In turn, other components of the translational machinery could be assembled, and expression of a transgene was upregulated by BL by up to around threefold. An alternative means of regulating gene expression is provided by BL-controlled nuclear import and export, discussed in detail in section 4.3.1. Briefly, in pertinent setups,209–212 the transcriptional activity of target TFs is regulated by sequestering them in the cytosol in BL-dependent manner.
4.1.3. Eukaryotic Transcription from Endogenous Promoters
The above approaches have in common that they permit light-regulated expression of transgenes from synthetic promoters. As versatile and powerful these approaches are, they suffer from the requirement of delivering to host cells a suitable promoter-transgene cassette in addition to the photoreceptor setup per se. As such, the copy number and expression strength of the transgene may substantially differ from the corresponding endogenous genes. Moreover, depending upon the research question pursued, the host system may need to be configured beforehand, e.g., by attenuating or suspending expression of certain endogenous genes. These potential problems may be circumvented by a set of optogenetic actuators that operate on the cellular expression machinery in a dominant way, thus obviating delivery of transgene cassettes or prior modification of the host cell.
In the PICCORO approach,213 a dominant-negative version of the zebrafish transcriptional repressor Ntl was constructed and linked to the N-terminal portion of the SsPixE protein from Synecchocystis sp. PCC6803 (Fig. 9A). The chimeric Ntl-SsPixE protein was expressed in zebrafish alongside the BLUF photoreceptor SsPixD. Notably, in the dark, SsPixD formed a homodecamer capable of strongly interacting with SsPixE, but BL promoted dissociation of SsPixD into homodimers and concomitant dissociation from SsPixE. Complex formation between SsPixD and Ntl-SsPixE in the dark lowered the DNA affinity of Ntl and relieved transcriptional repression of endogenous genes involved in tail development of zebrafish. The authors suggested that PICCORO may be a widely applicable strategy to regulate expression from endogenous promoters.216 A recent study217 achieved light-dependent regulation of the so-called ‘repressor element 1-silencing transcription factor’ (REST) and downstream genes. REST naturally acts in concert with co-repressory factors, among them mSin3a, to repress expression of target genes. A two-pronged strategy was chosen to interfere with function of endogenous REST in light-dependent manner. First, a REST epitope that mediates interaction with mSin3a was fused to the C terminus of AsLOV2 such that its affinity to mSin3a was subject to BL. Following illumination, the resultant AsLOV2-PAH1 construct competed with REST for mSin3a binding and thereby relieved transcriptional repression. Second, in the construct AsLOV2-RILP, AsLOV2 was C-terminally fused with the interaction domain of a REST inhibitor such that BL absorption allowed inhibition of DNA binding by REST and relief of transcriptional repression. Both approaches succeeded in upregulating REST-target genes in response to BL in both neuronal cell culture and primary neurons. In a recent approach,218 the Drosophila morphogen Bicoid that acts as a transcription factor and key regulator of development was fused with AtCRY2 such that BL attenuated Bicoid activity, presumably due to AtCRY2-mediated protein clustering. Interestingly, the Bicoid-AtCRY2 chimera acted in dominant-negative manner, and BL also suspended the transcriptional activity of endogenous Bicoid. Fly development could hence be precisely studied in time and space. Another group of optogenetic actuators combine photoassociating photoreceptors with DNA-binding proteins that can be programmed to specifically bind (almost) arbitrary unique target sequences within eukaryotic genomes. Polstein and Gersbach219 introduced the LITEZ system by connecting a zinc-finger DNA-binding protein to AtGI, and a tAD to the AtFKF1-LOV module.114 BL stimulated recruitment of the tAD to the DBD and allowed expression of a reporter gene in mammalian cells to be strongly up-regulated under blue light. Although the proof-of-concept was achieved for a transgene, zinc fingers220 can be reprogrammed to target diverse, defined DNA sequences, and therefore light-regulated expression of endogenous genes appears feasible with the LITEZ system as well. In the conceptually similar LITE approach,221 the ‘transcription activator like effectors’ (TALE)220 served as a programmable DNA-binding platform to which AtCRY2 was covalently linked. Light-triggered association with AtCIB1 allowed the recruitment of a palette of effectors to the target DNA site specified by the TALE. By using transcriptional activators and repressors as effectors, the expression of endogenous genes could be up- or downregulated in response to blue light.
Figure 9.
BL-dependent expression from endogenous eukaryotic promoters. (A) In the PICCORO approach,213 an endogenous transcription factor, e.g., Ntl, was fused to the PixE protein which in the dark associates with the homodecameric BLUF photoreceptor PixD. Upon BL absorption, PixD disassembled into homodimers and dissociated from the PixE-Ntl fusion protein, thus allowing Ntl to bind its endogenous operator site and activate transcription. (B) Programmable DNA-binding proteins, e.g., the TALEs or the cleavage-deficient dCas9, allow to specifically designate endogenous promoters. Transcriptional activation of these promoters was achieved by BL-dependent recruitment of a trans-activating domain (tAD), for example via the AtCRY2:AtCIB1 pair.214,215
The DNA sequence specificity of both zinc fingers and TALEs is rooted in modular protein domains, and reprogramming to different DNA targets therefore entails laborious production of new protein variants. By contrast, the DNA endonuclease Cas9 from Streptococcus pyogenes encodes its DNA specificity in one of two bound RNA molecules, which can easily be adapted to new targets. For practical applications, the two RNAs are routinely combined into a so-called single guide RNA or sgRNA (Fig. 9B)222. SpCas9 belongs to the CRISPR-Cas (clustered regularly interspaced short palindromic repeats-CRISPR associated) system that mediates adaptive immunity.223,224 A cleavage-deficient variant, denoted dCas9, that harbors two mutations serves as an inert, programmable DNA-binding platform that has been exploited for the construction of systems for BL-regulated expression of endogenous genes.225 In the LACE setup,214 dCas9 was linked to two copies of the N-terminal part of AtCIB1, whereas AtCRY2 was fused with a strong tAD. Directed to the promoter regions of different endogenous genes via suitable sgRNAs, the LACE system enabled upregulation by BL of expression by up to several hundredfold in mammalian cells. In a closely similar approach, Sato and coworkers fused dCas9 with one copy of AtCIB1 to which an AtCRY2-tAD fusion protein could be recruited under blue light.215 As in the LACE system, strong upregulation of endogenous genes by BL was achieved in mammalian cells. Compared side-by-side, the degree of light regulation appears slightly higher in LACE which could be due to the fusion of two AtCIB1 copies to dCas9.214 In a related recent approach,226 unmodified dCas9 was combined with an extended sgRNA that harbored MS2 aptamers at its 3’ end. AtCIB1 was fused with the MS2 phage coat protein which strongly binds to these aptamers; AtCRY2-linked effectors could hence be recruited upon BL exposure, and transcription could be strongly activated. Sato also pursued an orthogonal strategy by which dCas9 activity could be subjected to light control.227 To this end, dCas9 was split into two fragments which were connected to derivatives of NcVivid, denoted Magnets, that afford BL-stimulated heterodimerization.165 The split halves of dCas9, denoted pa-dCas9, could thus be reassembled in light-dependent manner and DNA-binding be restored. When one dCas9 fragment was linked with a tAD, strong upregulation of endogenous genes was achieved under BL. In a strategy inspired by the CRISPRi concept for transcriptional regulation in bacteria,228 pa-dCas9 could also be directed to the coding region of target genes such that the expression was attenuated as a function of BL. As in the approach by Konermann et al.,221 the degree of light-induced downregulation was relatively modest, much lower than that achievable with CRISPRi in bacteria.228 As is also evident in the LACE method,214 the degree of regulating gene expression by dCas9-dependent approaches can be significantly improved by recruiting to the same site and by thereby multiplexing several DNA effectors.229 The underlying rationale principally applies to optogenetic applications as well and should help to further improve their efficiency.
4.2. Epigenetics & Recombination
Given the modularity of the above dCas9-based recruitment strategies, these approaches are also suited for facilitating BL-regulated addition or removal of epigenetic markers (Fig. 10). Employing this rationale, different effector functionalities could be recruited to specific genomic sites to induce, for example, histone (de)acetylation, histone and DNA (de)methylation, or chromatin remodeling.221 This strategy was recently implemented by using as locus-specific DNA-binding proteins TALEs to which AtCIB1 was fused.230 Via covalent linkage to AtCRY2, either a CpG DNA methylase or a methylation-editing enzyme could be recruited to the specified DNA target site in BL-activated manner. Resultant changes in DNA methylation were confined to the immediate genomic vicinity of the TALE binding site and elicited changes in the expression of a nearby gene. Regulation of epigenetic modifications was also achieved with a BL-induced nuclear export system,212 described in section 4.3.1., that enabled optogenetic control of the subcellular localization of histone-modifying enzymes.
Figure 10.
Optogenetic control of epigenetics. TALE proteins and cleavage-deficient dCas9 serve as inert DNA-binding modules that can be programmed to specifically locate to unique target sites in eukaryotic genomes.230 Linkage of dCas9 with AtCIB1 allows BL-dependent recruitment of DNA effector enzymes that are covalently coupled to AtCRY2. Suitable effectors include DNA and histone methylases, histone (de)acetylases as well as chromatin remodeling enzymes. For example, acetylation and methylation (indicated by ‘A’ and ‘M’, respectively) serve as epigenetic marks and modulate transcriptional activity. For clarity, histone N-terminal tails are only drawn for one nucleosome unit.
In addition to the cleavage-deficient dCas9 variant (cf. above), the original cleavage-competent Cas9 form has also been the subject of photoreceptor engineering (Fig. 11A). Current approaches towards regulating Cas9 cleavage activity by external trigger signals are generally realized at the levels of DNA and sgRNA binding, rather than at the level of DNA cleavage per se.225 As a corollary, strategies that put Cas9 activity under control of an external trigger should directly apply to dCas9-based applications as well but the opposite is not necessarily true. As a case in point, the above split-Cas9 approach by Sato227 not only enabled light-regulated gene expression but also supported BL-activated generation of double-strand breaks (DSBs) at target DNA sites. In turn, DSBs trigger cellular repair mechanisms,231 principally non-homologous end joining and homology-directed repair, to promote deletions/insertions and recombination, respectively, at specified genomic loci. In another study,232 (d)Cas9 was put under light control by inserting in its sequence at varying positions the homodimeric LOV sensor from Rhodobacter sphaeroides (RsLOV) that undergoes light-induced subunit dissociation.233 In one of the resultant chimeric proteins, denoted paRC9, in the dark the (d)Cas9 enzyme was presumably sequestered in a homodimeric complex such that binding to the DNA target sequence was sterically hindered.232 Light absorption enhanced DNA affinity of paRC9 and increased site-specific cleavage activity, albeit to relatively modest extent. Unexpectedly, insertion of the RsLOV sensor conferred a pronounced temperature sensitivity to (d)Cas9; whereas robust activity was observed at 29°C, almost none was detected at 37°C.
Figure 11.
Optogenetic control of DNA recombination. (A) The programmable DNA endonuclease Cas9 was split into two parts which could be reassembled in BL-dependent manner via the Magnets LOV receptors.227 The reconstituted Cas9 enzyme mediated double-strand breaks at defined genomic sites, thus triggering non-homologous end joining and homology-directed repair. (B) Light-regulated recombination was also achieved by splitting the Cre recombinase into two fragments which were linked with AtCRY2 and AtCIB1, respectively.51,53,234 BL induced fragment assembly and restoration of activity, thereby enabling recombination at loxP sites.
Light-regulated DNA recombination has also been accomplished via photosensitive variants of the Cre recombinase (Fig. 11B).51 Earlier work had shown that Cre can be split in two fragments that have little mutual affinity and thus very low catalytic activity.235 By linking the two fragments to the FKBP protein and the FRB domain, respectively, they could be reconstituted upon rapamycin addition, and recombinase activity was restored. To render activity of the split Cre BL-sensitive, the FKBP:FRB pair was replaced by the photoassociating AtCRY2:AtCIB1 pair51 and a photoactivatable Cre (PA-Cre) was obtained. In mammalian cells, DNA recombination by PA-Cre could be activated by BL by more than hundredfold with low dark activity. PA-Cre and derived variants were also deployed in vivo, e.g., to achieve BL-induced recombination for the regulation of angiogenesis in mice.236 Another study employed PA-Cre for BL-activated recombination and concomitant activation of a reporter gene in mice brain.237 To achieve high frequencies of recombination events, BL had to be applied for extended periods of time, up to several hours. Tucker and colleagues53 reasoned that a PA-Cre variant with a longer-lived signaling state of the AtCRY2 photoreceptor would be more light-sensitive at photostationary state and might hence support efficient recombination at lower light doses. Using a screening assay based on light-regulated gene expression in yeast, a longer-lived variant of AtCRY2 was identified and implemented in the second generation of PA-Cre. Consistent with the design rationale, PA-Cre 2.0 indeed required lower BL doses for activation; unexpectedly, PA-Cre 2.0 also displayed lower recombination activity in the dark than observed for the original PA-Cre, and thus PA-Cre 2.0 had a higher dynamic range than its predecessor. A split Cre was also established using the Magnets LOV receptors (cf. above) that heterodimerize upon BL exposure.234 The resultant light-regulated Cre recombinase, confusingly also denoted PA-Cre, supported DNA recombination in mammalian cells that could be enhanced by BL by up to several hundredfold. Notably, the Magnet-based split Cre showed enhanced light sensitivity and accompanying efficient activation by comparatively low BL doses to the point that Cre recombination could be activated within the internal organs of mice.
4.3. SUBCELLULAR LOCALIZATION
Numerous biological processes, e.g., transport into or out of organelles, cell polarization and motility, require the precisely orchestrated localization of cellular constituents to specific compartments or sites within cells. Optogenetics is well suited to perturb and study such processes because many photoreceptors, especially BL-sensitive ones, grant ready control over the subcellular localization of near-arbitrary target proteins. The most common, and arguably most easily implementable approach, relies on photoreceptors that undergo light-dependent protein-protein interactions (PPI) with partner proteins, cf. sections 2 and 3. To this end, one component of the interacting photoreceptor:partner pair is constitutively directed to the cellular location of interest, often via immobilization, e.g., by a membrane anchor. Passive diffusion or active transport mechanisms bring into spatial vicinity the other component and allow it to be retained, provided the photoreceptor is in its binding-component state (depending on photoreceptor, either in the presence or absence of light). For example, this design principle of light-modulated PPIs underpins many optogenetic approaches for controlling cytoskeleton dynamics, cf. section 4.4. To a first approximation, every photoreceptor that undergoes light-dependent PPIs lends itself to control subcellular localization. An alternative strategy capitalizes on trafficking signal epitopes that the cell uses to control localization, e.g., nuclear import and export signals. By modulating as a function of light the exposure of these epitopes, optogenetic control over subcellular localization was achieved as well.
4.3.1. Nuclear Import & Export
The control by blue light of the exposure and hence the activity of trafficking epitopes underpins BL-regulated systems for nuclear import and export (Fig. 12).209–212 In the LINuS system,209 a nuclear localization signal (NLS) peptide was embedded in or appended to the Jα helix of the AsLOV2 photosensor to regulate by BL epitope accessibility and cellular activity. In darkness, the NLS was predominantly masked and the target protein of interest (POI) preferentially resided in the cytosol but upon BL application, Jα unfolded, the NLS was exposed and the POI distributed to the nucleus. In this manner, the nuclear localization of fluorescent reporters could be controlled by BL in mammalian cells. A suite of LINuS variants offered different degrees of BL regulation and NLS versions of varying strengths which benefits the coupling of LINuS to arbitrary POIs with different intrinsic propensities of residing in the nucleus or the cytosol. The nucleocytoplasmic distribution can further be modulated by including constitutive NLS and nuclear export signals (NES). Using LINuS, mitotic entry and gene expression in mammalian cells was controlled by BL. The LANS approach211 is related to LINuS in that it is also based on interleaving the AsLOV2 Jα helix with NLS peptides. BL-induced nuclear translocation by LANS was demonstrated for mammalian cells, yeast and in Caenorhabditis elegans embryos. Based on LANS, a light-inducible transcription factor was devised and expressed in transgenic C. elegans to control by BL development. Recently, the dynamic range of the LANS system was improved by combining it with the LOVTRAP238 strategy (cf. sec. 4.4.1.1.).239 Moreover, employing the same general strategy underpinning LINuS and LANS, two systems were engineered that mediate BL-induced nuclear export.210,212 In LEXY,210 an NES peptide was embedded in the AsLOV2 Jα helix to allow BL-activated depletion of target POIs from the nucleus of mammalian cells. The technique was used to regulate by BL gene expression and activity of the tumor suppressor protein p53. LINX212 realized a similar setup to achieve BL-induced nuclear export in mammalian cells, yeast and C. elegans. Using LINX, gene expression and epigenetic modifications in yeast were optogenetically controlled. As an alternative discussed in section 4.1.2., the versatile AtCRY2 photoreceptor can also respond to BL exposure by nuclear clearing.196 Hence, AtCRY2 may provide another means for light-induced nuclear export of POIs.
Figure 12.
BL control of nuclear import and export processes was achieved in the LINuS209/LANS211 and LINX212/LEXY210 approaches by embedding corresponding trafficking signal peptides in the Jα helix of the AsLOV2 photosensor. BL-induced unfolding prompted exposure of the signal peptides and caused nuclear import and export, respectively, of cargo proteins.
4.3.2. Peroxisomal Import
A strategy similar to that for the BL-induced nuclear import and export209–212 gave rise to the LOV-PTS1 system for optogenetic control of peroxisomal import (Fig. 13).240 A pertinent trafficking signal peptide was appended to Jα of AsLOV2 and thereby caged in BL-dependent fashion. In mammalian cells, LOV-PTS1 mediated the peroxisomal import of fluorescent reporter proteins upon BL exposure.
Figure 13.
The LOV-PTS1 strategy240 is based on the AsLOV2 photosensor to which a peroxisomal trafficking epitope was appended. BL-induced Jα unfolding relieved caging of the epitope and promoted peroxisomal import of cargo proteins.
4.3.3. Optically Induced Compartments
Several optogenetic actuators exploit the propensity of AtCRY2 to associate with AtCIB151 or to form higher-order oligomers under BL (Fig. 14).65,241 Via conjugation to AtCRY2, target effector proteins of interest can hence be assembled into clusters upon BL, and protein-based microcompartments can be formed inside the cell. For improved efficiency, often the E490G variant of AtCRY2, denoted Cry2olig,52 is employed as it shows enhanced clustering propensity. The protein-based compartments were used to analyze PPIs between proteins of interest inside of cells,52 and to modulate by BL the activity of target effectors.52,242 In the LINC setup,52 the interaction between two fluorescently labeled proteins of interest was assessed by connecting one of them to AtCRY2. BL induced AtCRY2-mediated cluster formation of this protein, and a possible interaction with a second POI could be detected by coclustering of the two interacting proteins. The LARIAT method242 pursued a slightly different strategy in that protein clusters were formed via the BL-induced heterodimerization of AtCRY2:AtCIB1 instead of the homooligomerization of AtCRY2 (Fig. 14A). In this approach, AtCIB1 was fused with a multimeric scaffold protein that assumes a homododecamer. BL induced the association of AtCRY2 and the multimeric AtCIB1 conjugate such that clusters were formed. While little BL-induced clustering was observed with AtCRY2 alone, it is conceivable that robust clusters would be obtained if using Cry2olig. By conjugating AtCRY2 with a GFP-specific nanobody, GFP-tagged target proteins were sequestered into the BL-induced clusters formed by AtCRY2 and the multimeric AtCIB1. In principle, the LARIAT technique could be adapted to target for BL-induced sequestration and concomitant attenuation other endogenous proteins via substitution of the GFP-specific nanobody for another one. Similar to the applications of LARIAT, LINC52 was used to disrupt via BL-induced AtCRY2 clustering endocytosis, cf. section 4.4.3.
Figure 14.
Optically induced compartments. (A) In the LARIAT method,242 AtCIB1 is conjugated to a multimeric scaffold protein such that upon BL-induced association with AtCRY2 clusters formed. Proteins of interest (POI) can be sequestered into said clusters either via direct coupling to AtCRY2 or via adapter proteins. For clarity, not all AtCRY2 molecules are shown with attached POI. The related LINC approach52 does away with AtCIB1 and instead exploits the ability of AtCRY2 to form homooligomers upon BL absorption. (B) The BL-induced clustering of AtCRY2 also underpins a strategy for optogenetically controlling ribonucleoprotein (RNP) droplets.66 To this end, AtCRY2 was fused to the unstructured RNA-binding protein FUS to allow light-induced liquid-liquid phase transition and formation of RNP droplets.
In recent years, it has become increasingly evident that membrane-less organelles formed by assemblies of ribonucleoproteins (RNP) are engaged in important biological processes,243 prominent examples being the Cajal bodies and nucleoli. At sufficiently high local concentration, the RNP complexes display liquid-liquid phase separation to form distinct RNP droplets that are dynamic and in constant flux with the surroundings. The formation and size of RNP droplets inside mammalian cells was optogenetically controlled by connecting to AtCRY2 the intrinsically disordered protein FUS known to bind RNA and capable of forming droplets (Fig. 14B).66 Upon BL exposure, AtCRY2 assembled into clusters, leading to an increased local concentration of the disordered protein with bound RNA and to the appearance of RNP droplets as evidenced by speckle formation. By varying expression levels and applied BL dose, the number and average size of the droplets could be varied. Cry2olig greatly enhanced clustering and droplet formation. An alternative to Cry2olig might be provided by the recent observation244 that either ligation of AtCRY2 to oligomeric fluorescent proteins or C-terminal appendage of a short peptide to AtCRY2 enhanced BL-induced clustering propensity. Arguably, these later strategies may be combined with the E490G mutation that gave rise to Cry2olig.
4.3.4. Light-induced Interactions Among Organelles and Cells
Optogenetic approaches based on BL receptors have also enabled the control of interactions among organelles and entire cells. In one study,245 the iLID system203 for BL-activated heterodimerization was deployed to trigger the formation of contact sites between the endoplasmic reticulum and mitochondria. To this end, the two components of iLID were directed to the ER membrane and the mitochondrial outer membrane, respectively. The tethering sites that were formed between ER and mitochondrion upon BL exposure may resemble the naturally occurring contact structures that are implicated in cellular signaling and apoptosis, among other processes.246 The strategy employed in this work readily extends to other organelles and may be used to induce spatial contacts among them. Recently, the surface attachment, cell-cell contacts and biofilm formation of E. coli bacteria were regulated in BL-dependent manner by LOV-mediated expression of a specific membrane protein.247 A different system relies on LOV receptors for BL-induced heterodimerization to also control by light bacterial adhesion to a substrate.248 In this approach, the Magnet photoreceptors were deposited on a solid support and expressed on the surface of E. coli cells, respectively. BL promoted interaction of the Magnet components and resulted in bacterial attachment. As demonstrated in this study, LOV BL receptors can apparently retain flavin chromophore binding and light sensitivity upon cell-surface expression. Prospectively, this approach may be adapted to eukaryotic cells to control interactions among them and their spatial arrangement on substrates, with potential applications in tissue engineering.
4.4. Cytoskeleton Dynamics
The optogenetic toolkit for manipulating cytoskeleton structure and dynamics by BL is similarly rich as that for regulating gene expression, cf. sec. 4.1. The large number of pertinent light-regulated actuators equally reflects the biological significance of the cytoskeleton and the relative ease with which it can be optogenetically controlled. Inside the cell, the structure and dynamics of the cytoskeleton are governed by an intricate network of factors that mutually interact in spatially and temporally defined manner. Approaches for regulating by light PPIs and subcellular localization are hence particularly applicable for optogenetic control of the cytoskeleton and associated processes. Indeed, optogenetics provides an unprecedented means of precisely interrogating individual nodes and connectivity of the signaling networks underlying cytoskeleton dynamics.
4.4.1. Actin & Myosin
4.4.1.1. Cytoskeleton Remodeling
Actin (or, intermediate) filaments impart mechanical strength to eukaryotic cells and facilitate their motility.249 The flexible actin filaments are formed by polymerization of globular monomeric actin and are organized in bundles, fibers and mesh-like networks. These higher-order assemblies and the constituent actin filaments are highly dynamic entities, constantly undergoing assembly and disassembly. These reactions are orchestrated in time and space by complex signaling networks which feature small GTPases of the Rho family as key nodes.250,251 Specifically, the Rho GTPases Rac1, RhoA and Cdc42 are anchored to the plasma membrane and integrate inputs from upstream factors. These GTPases display low(er) activity in their GDP-bound forms, but when binding GTP, they activate a set of downstream effectors that modulate actin filament structure and dynamics. To optogenetically control the activity of Rac1, its membrane anchor was removed and it was coupled to AtFKF1.114 BL exposure prompted association of AtFKF1 with membrane-anchored AtGI and resulted in the recruitment of Rac1 to the plasma membrane which sufficed for triggering actin polymerization. Spatially confined BL illumination led to the formation of lamellipodia in mammalian cells. In a similar vein, Rac1 activity was controlled via the LARIAT method242 by sequestration of the GTPase into AtCRY2:AtCIB1-based clusters and concomitant attenuation upon BL exposure. Vice versa, in a different study65 AtCRY2-mediated, BL-induced clustering of Rac1 resulted in its translocation to the plasma membrane and concomitant activation. A different strategy towards controlling Rac1 activity by BL was employed by Hahn and colleagues (Fig. 15).103 In the engineered PA-Rac1 photoreceptor (cf. sec. 3.1.3.), Rac1 was linked to the C-terminal Jα helix of AsLOV2 and its interaction with downstream effectors, e.g., PAK1, was thus sterically impeded. BL absorption triggered Jα unfolding, prompted dissociation of AsLOV2 from Rac1 and thereby restored activity. Local illumination of mammalian cells elicited spatially defined actin remodeling, membrane ruffling and formation of lamellipodia. Fibroblasts could thus be induced to migrate in the direction of a focused BL laser spot. Introduction of a dominant-negative mutation into Rac1 sufficed to prompt fibroblasts to migrate away from a BL spot, instead of towards it. As two of many applications of PA-Rac1, migrating neutrophil cells within developing zebrafish embryos could be steered by BL,252 and dendritic spines could be both selectively labeled and shrunk in mice.253 Using the design strategy underpinning PA-Rac1, a photoactivatable variant of Cdc42, denoted PA-Cdc42, was obtained that mediated membrane ruffling and formation of filopodia in mammalian cells under BL.103
Figure 15.
Optogenetic control of cytoskeleton dynamics. The activity of a soluble form of Rac1 was put under BL control via linkage to the AsLOV2 photosensor such that steric occlusion of the active site resulted.103 In the resultant PA-Rac1, BL-induced Jα unwinding triggered dissociation of the AsLOV2 and Rac1 moieties, thus restoring access to the active site and eliciting downstream effects on the cytoskeleton. Other approaches targeted the guanine nucleotide exchange factors (GEF) that act on Rac1, drive the exchange of bound GDP for GTP and thereby activate Rac1. Several strategies, including the BL-dependent interaction between AsLOV2 and Zdark,238 were harnessed to regulate access to the plasma membrane and exchange activity of the GEFs. In the PI-GEF strategy,109 several GEFs were also subjected to BL control via insertion of AsLOV2 in surface-exposed protein loops. Note that the examples shown here are paradigmatic for numerous related approaches by which the activity of the Rho-family GTPases Rac1, Cdc42 and RhoA was put under BL control, cf. sec. 4.4. for details.
Rather than by optogenetically targeting Rac1 directly, actin cytoskeleton dynamics have also been controlled by regulating by BL factors upstream of Rac1. As for other small GTPases, the activity of Rho GTPases is regulated by guanine nucleotide exchange factors (GEF) that promote the exchange of bound GDP for GTP, and by GTPase-activating proteins (GAP) that stimulate GTP hydrolysis to GDP.250 Accordingly, optogenetic control over cytoskeleton dynamics could be exerted by controlling the subcellular localization and activity of GEFs as a function of BL. The LARIAT approach for optogenetic trapping242 was used to sequester the Rac1 GEFs Tiam1 and Vav2 upon BL illumination, resulting in a decrease of GTPase activity and membrane retraction in mammalian cells. In the CAD method,254 based on the Magnets LOV photoreceptors for BL-induced heterodimerization,165 Tiam1 was recruited to the plasma membrane upon BL exposure and elicited actin reorganization, membrane ruffling and formation of lamellipodia. CAD improved the efficiency of these BL-induced responses by conjugating Tiam1 with several copies of the Magnet LOV receptors. A versatile sequestration-based technique for optogenetically controlling the cytoskeleton was realized in the LOVTRAP method (Fig. 15).238 Via phage display, affibodies,255 named Zdark, were developed that strongly bind the dark-adapted state of the AsLOV2 photosensor with its Jα helix folded and docked onto the core domain. BL exposure prompted Jα undocking and resulted in an about 150-fold decrease in the affinity of the best Zdark affibody variant for AsLOV2. By anchoring AsLOV2 to the outer mitochondrial membrane, target proteins conjugated to Zdark were sequestered away from the plasma membrane in the absence of light. BL exposure triggered dissociation of the AsLOV2:Zdark complex and enabled the target proteins to reach the plasma membrane. In this way, the intracellular localization and activity of the GTPases Rac1 and RhoA, and of the GEF Vav2 could be controlled by BL with downstream effects similar to those described for the above optogenetic actuators. In a landmark approach,109 several players engaged in regulating cytoskeleton dynamics were allosterically regulated by BL (Fig. 15). Informed by molecular dynamics simulations, the AsLOV2 photosensor was inserted into target proteins at surface loops that are mechanically connected to their active sites (cf. sec. 3.1.2.). BL-induced unfolding of the AsLOV2 Jα helix was thus coupled to a decrease in activity of the target protein. Using this generalizable strategy, photoinhibited (PI) variants of the soluble tyrosine kinase Src, of the GTPases Rac1, RhoA and Cdc42, and of the GEFs Vav2, GEF-H1 and Intersectin were generated. These and related optogenetic tools in hand, the complex processes determining actin cytoskeleton structure and reorganization can now be deciphered in ever more precise and detailed manner.
The GTPase RhoA was also subjected to BL control by fusing it with AtCRY2 such that upon BL exposure clusters formed that translocated to the plasma membrane of mammalian cells.65 RhoA then induced actin rearrangements that resulted in membrane spreading. BL-triggered inhibition of the related GTPases RhoG and Cdc42 was achieved with the LARIAT method via sequestration into AtCRY2:AtCIB1-based clusters.242 In two related studies,256,257 Cdc42 was optogenetically regulated by controlling via BL application the subcellular localization of the Cdc42-targeting GEF Intersectin. Using the iLID system for BL-activated heterodimerization,203 Intersectin was recruited to the plasma membrane under BL and promoted local Cdc42 activation. In budding yeast, Cdc42 is involved in governing cell polarization and division by budding. Based on the TULIP setup for light-induced heterodimerization,258 either the Cdc42-specific GEF Cdc24 or the scaffold protein Bem1 that mediates the interaction between Cdc24 and Cdc42 were recruited to the plasma membrane upon BL exposure.259 In either manner, cell polarity and the situation of the budding site could be optogenetically controlled via BL and mechanistically studied.
Optogenetic control over cytoskeleton reorganization was also exerted at levels other than the Rho GTPases and their GEFs. On the one hand, several transmembrane signal receptors impinge upon the actin cytoskeleton. BL-regulated versions of several such receptors, discussed in section 4.7., can hence be used to regulate actin dynamics. On the other hand, actin polymerization was directly triggered via AtCRY2-mediated BL-induced clustering of the SH3 domains of the Nck protein.52 Local BL stimulation and resultant actin reorganization prompted the retraction of membrane protrusions in mammalian cells.
4.4.1.2. Actin/Myosin-Based Transport
Myosin motor proteins move along actin filaments and thereby mediate diverse motility processes, including muscle contraction and intracellular transport.249 In the LOVDab design,260 a short peptide derived from Dab2, a cargo protein for myosin VI-mediated transport, was appended to the Jα helix of AsLOV2 such that its exposure was governed by BL. Light-induced Jα unfolding allowed the Dab peptide epitope to bind to myosin VI which translocates to the minus end of actin filaments. By anchoring LOVDab to the membrane of peroxisomes, the intracellular transport of these organelles could be stalled in BL-activated manner. In a related strategy,261 the TULIP system258 was harnessed to control by BL the interaction between a peroxisome-located protein and myosin Vb, also resulting in interference with intracellular organelle transport.
4.4.2. Microtubules
Microtubules (MT) are composed of α- and β-tubulin that polymerize to form hollow cylinders.249 As is the case for actin filaments (cf. sec. 4.4.1.), MTs are highly dynamic and constantly undergo assembly and disassembly reactions. At the so-called minus end, disassembly outweighs assembly, and a net shrinkage of the MT results; vice versa, at the plus end, assembly dominates, and the MTs display net growth. MT dynamics are subject to the regulation by various factors which offer toeholds for optogenetics. As a case in point, end-binding proteins (EB) mediate the interaction between the plus end of MTs and a diverse set of tip-interacting proteins (TIP). To control these interactions by BL,262 the N-terminal half of a split EB1 that binds to the MT plus end was fused with the AsLOV2 photosensor and the C-terminal half of EB1 that mediates interactions with TIPs was fused to the interacting affibody Zdark (Fig. 16).238 In darkness, AsLOV2 and Zdark associated and an active EB1 thus resulted; BL exposure triggered dissociation and rendered EB1 unable to recruit TIPs. BL illumination hence resulted in local attenuation of MT growth at their plus ends which could culminate in MT depolymerization.
Figure 16.
Optogenetic control of microtubule stability and transport. Using the TULIP system for BL-induced heterodimerization,258 kinesin motors could be recruited to desired organelles, e.g., peroxisomes, which were then transported to the (+) end of microtubules.261 The principal concept extends to dyneins which move to the (−) end and to myosins which move along actin filaments (not shown). The polymerization dynamics of microtubules was modulated by using a split version of the end-binding protein EB1.262 In darkness, the two halves of split-EB1 were held together via the AsLOV2:Zdark interaction but BL prompted AsLOV2 Jα unwinding and dissociation of the EB1 fragments.
Transport along MTs is mediated by kinesin and dynein motor proteins which (mostly) walk towards the plus and minus ends, respectively.249 Employing the TULIP system,258 the interaction between a peroxisome-anchored protein and a kinesin motor could be turned on by BL, resulting in translocation of the organelles towards the MT plus ends and accumulation in the periphery of mammalian cells (Fig. 16).261 Owing to its modularity, the method could be extended to myosin (cf. sec. 4.4.1.2.) and dynein motor proteins. When dynein was thus recruited in BL-activated manner, the peroxisomes instead translocated to the minus ends of MTs, i.e. to the cell center. Likewise, the intracellular localization of mitochondria was regulated by BL using this technique. A conceptually similar strategy263 employed the photoassociating AtCRY2:AtCIB1 pair rather than the TULIP system. Via BL-induced recruitment of kinesin and dynein motors to peroxisomes, lysosomes and mitochondria, these organelles could be moved to the cell center or periphery, respectively.
4.4.3. Endocytosis & Exocytosis
Vesicular transport in mammalian cells was also targeted by optogenetics. The dominant endocytic pathway in eukaryotes is mediated by clathrin which polymerizes at the plasma membrane as a cage-like structure and thereby allows membrane vesicles to pinch off.249 To optogenetically control endocytosis, the light chain of clathrin was connected to AtCRY2 such that under BL clusters formed.52 Said clusters impaired clathrin assembly and slowed down endocytosis. By contrast, in a recent study,264 clathrin-mediated endocytosis could be stimulated by BL. To this end, the TULIP system258 for BL-activated heterodimerization was employed to recruit a clathrin-binding protein to the plasma membrane which in turn triggered endocytosis. AtCRY2 also underpins the IM-LARIAT strategy for controlling by BL vesicular transport (Fig. 17).265 Small GTPases of the Rab family are integral to coordinating vesicular trafficking between membrane-surrounded organelles and the cell membrane. Individual Rab proteins were fused with AtCIB1 and coexpressed in mammalian cells with AtCRY2.265 BL induced AtCRY2 clustering and binding of the Rab-AtCIB1 conjugates; vesicles were thus gummed up and transport impaired. By targeting different Rab GTPases, various branches of the trafficking pathway could be subjected to BL control, including different stages of endocytosis (early and late endosomes) and exocytosis (vesicles trafficking between endoplasmic reticulum and Golgi apparatus, or between Golgi and plasma membrane, as well as secretory vesicles). The trafficking from the endoplasmic reticulum to the Golgi could also be optogenetically regulated by linking target cargo proteins to one or several copies of AtUVR8266 that in darkness, forms a homodimer but that dissociates following UV-light exposure. Conjugation of target cargo to AtUVR8 promoted formation of aggregates in the endoplasmic reticulum and effectively suspended vesicular trafficking of the cargo to the Golgi. Under UV light, AtUVR8 dissociated, the aggregates dissolved and vesicular transport of the cargo protein to the Golgi and onward proceeded. Lastly, the secretion of vesicles in pancreatic cells could be optogenetically perturbed via BL control of phosphatidylinositol signaling, cf. sec. 4.7.2.267
Figure 17.
Optogenetic control of vesicular transport. (A) AtCIB1 was linked to different Rab GTPases that orchestrate vesicular transport.265 BL induced AtCRY2 to form clusters and to bind AtCIB1, thereby gumming up the vesicular transport machinery. (B) The secretory export of cargo proteins could be modulated in UV-light-dependent manner by linking them to one or several copies of the homodimeric AtUVR8.266 Formation of higher-order assemblies resulted in retention in the endoplasmic reticulum. UV light prompted AtUVR8 dissociation and resolution of these assemblies, and transport of the cargo ensued.
4.5. Intracellular Protein Half-Life & Proteolytic Cleavage
In many optogenetic applications, the target effector output and downstream cellular responses are up-regulated by light absorption. Provided a given application scenario only requires slow time resolution, light-regulated expression provides an easily implementable and highly versatile means of optogenetic activation of desired cellular responses. At the same time, there are use cases where shutting-off or down-regulation of cellular activities in response to light stimuli are demanded. The LARIAT system,242 described in section 4.3.3., offers a general path towards BL-induced reversible down-regulation of cellular activities via sequestration of the proteins into microcompartments. Alternatively, the cellular activity levels of a target effector may be irreversibly reduced by prompting its active degradation via the proteasome system.268 Inside eukaryotic cells, proteins can be tagged for destruction by ubiquitination, followed by proteolytic cleavage at the proteasome. Proteins to be destroyed are recognized by the cellular ubiquitination machinery via specific degradation signals, denoted degrons, that often amount to short peptide epitopes. Two systems269,270 for triggering intracellular protein degradation via the ubiquitin:proteasome machinery were implemented based on the widely used AsLOV2 photosensor or its homolog AtLOV2 from Arabidopsis thaliana phototropin 1 (Fig. 18). To this end, degron sequences were interleaved with or appended to the AsLOV2/AtLOV2 Jα helix such that they were largely sequestered in the darkness when Jα is mostly folded. BL-promoted Jα unfolding exposed the degron epitopes, thereby prompting the proteasomal degradation of AsLOV2/AtLOV2 and covalently linked target proteins. These co-called photosensitive degrons (psd) allowed the intracellular half-life in yeast of suitably tagged effector proteins to be decreased by BL by around sixfold.271 Notably, assuming single-exponential kinetics for the degradation process, a sixfold difference in half-life can translate into much higher differences in actual target protein concentrations between dark and BL conditions. Using the psd strategy, the steady-state levels of metabolic enzymes and progression through the yeast cell cycle were controlled by BL. Subsequently,271 a suite of psd systems were generated that included variants with up to tenfold decrease of protein half-life by BL. A related approach, called B-LID,270 was implemented in mammalian cells where the steady-state levels of a fluorescent reporter protein could be lowered following BL exposure by around five- to tenfold. Similarly, B-LID was applied in zebrafish to induce by BL the degradation of a reporter protein. In a later study,272 B-LID was covalently linked to a protein fragment, denoted Med25VBD, of the eukaryotic mediator complex to bestow light sensitivity on the widely used Tet-ON/Tet-OFF gene-regulatory systems. In these systems, the Tet repressor is linked to a trans-activating domain that recruits components of the eukaryotic transcriptional machinery and thereby induces gene expression. As Med25VBD competes for binding to the tAD, it effectively represses expression from the Tet-ON promoter. When linked to B-LID, Med25VBD can be degraded in BL-stimulated manner, resulting in relief of transcriptional repression. Notably, the Med25VBD-B-LID complex does not perturb DNA binding of the Tet repressor nor its regulation by tetracycline analogs, and it can therefore be employed as an optogenetic upgrade to existing Tet-ON systems. Recently, the LovD approach for BL-induced protein degradation closely recapitulated the psd setup273 except for employing the AsLOV2 rather than the AtLOV2 photosensor. Using LovD, the abundance and intracellular half-life of reporter proteins in mammalian cells could be controlled by BL.
Figure 18.
The intracellular half life of POIs was optogenetically regulated with the psd271 and B-LID270 strategies. BL stimulated unfolding of the Jα helix of AtLOV2 or AsLOV2, thereby increasing the exposure of an embedded degron epitope. The cellular ubiquitin/proteasome machinery then degraded the POI and the attached LOV2 module.
Whereas in the psd, B-LID and LovD systems the AsLOV2/AtLOV2 module is used to regulate by BL the accessibility of degron epitopes interwoven with the Jα helix, in two similar approaches, denoted Cal-Light274 and FLARE,275 the cleavage sequence of the TEV protease was embedded in this helix. BL prompted exposure of this sequence and allowed cleavage by the TEV protease to occur. To enhance regulatory efficiency, the Cal-Light method was further combined with a split-TEV protease activated by BL.276 To this end, TEV protease fragments were linked with AtCRY2 and AtCIB1, respectively; BL hence induced association of the split parts and increase in protease activity. As another class of proteases, caspases that mediate the programmed cell death have been put under BL control, see section 4.8.199,277
4.6. Second Messengers
Widely distributed in nature as components of signal transduction cascades, second messengers serve to amplify and relay signals inside cells.278 Upon perception of a suitable stimulus, second messengers are released from storage compartments or produced enzymatically; vice versa, signaling is eventually suspended by sequestration or enzymatic degradation of the second messengers. As the production/release and degradation/removal processes are often regulated in spatiotemporally precise manner, intracellular microdomains of elevated second messenger concentration result in time and space. Given inherent amplification, spatiotemporal dynamics and a wide range of physiological responses regulated, second-messenger signaling has been a prime subject for optogenetic intervention.
4.6.1. Cyclic Nucleotides
4.6.1.1. Cyclic Mononucleotides
3’,5’-cyclic nucleotide monophosphates (cNMPs) are versatile second messengers engaged in the regulation of multiple physiological responses in both prokaryotes and eukaryotes.278 Nucleotide cyclases catalyze the formation of cNMPs from the corresponding nucleotide triphosphates, and phosphodiesterases catalyze the hydrolytic breakdown to the (non-cyclic) nucleotide monophosphates (Fig. 19). In eukaryotic cells, the two most widespread cNMPs, 3’,5’-cyclic adenosine monophosphate (cAMP) and 3’,5’-cyclic guanosine monophosphate (cGMP), bind to and thereby regulate the activity of cyclic-nucleotide-gated (CNG) ion channels, protein kinases A or G (PKA or PKG), Epac (exchange protein directly activated by cAMP) and popeye-domain-containing proteins (PODCP). Inside eukaryotic cells, the activity of adenylate cyclases that produce cAMP is primarily controlled by intracellular calcium concentrations and by G-protein coupled receptors (GPCRs).
Figure 19.
Optogenetic actuators for controlling cyclic-nucleotide second messengers. (A) A palette of photoactivated adenylate cyclases (PACs) responsive to BL93,94,279,280 or red light catalyze the formation of cAMP or cGMP. In eukaryotic cells, cAMP binds to and thus activates CNG channels, PKA, Epac and popeye-domain-containing proteins (PODCP).278 The red-light-activated PDE LAPD mediates the hydrolytic breakdown of cAMP and cGMP.281 (B) C-di-GMP is a versatile second messenger involved in numerous physiological adaptations of bacteria. Red-light-activated GGDEF enzymes produce c-di-GMP and achieve optogenetic control over physiology and gene expression in bacteria.282,283 In eukaryotes, c-di-GMP triggers the STING response as part of the vertebrate innate immune system. BL-activated EAL enzymes31,284,285 catalyze the hydrolysis of c-di-GMP. For clarity, all photoreceptors in panels (A) and (B) are drawn as monomers although they are active as homodimers. BLUF, LOV and bacteriophytochrome photosensors are denoted as parallelograms, rectangles and tripartite shapes, respectively; colored circles denote cyclases and phosphodiesterases specific for cyclic nucleotides.
Sensory photoreceptors acting at the molecular level as photoactivated nucleotide cyclases have been identified in several organisms (Fig. 19A). Chronologically first, a BLUF photoreceptor, denoted as a photoactivated adenylate cyclase (PAC), was discovered in Euglena gracilis where it mediates a photophobic reaction in response to strong BL exposure.11 Enzymatic analysis of the purified E. gracilis PAC (EuPAC) revealed its adenylate cyclase activity to be upregulated by around 80-fold under BL compared to darkness. EuPAC assumes heterotetrameric state with two copies each of the chains EuPACα and EuPACβ. Each α and β chain comprises two BLUF photosensor and two class III adenylate cyclase effector modules. Remarkably, the initial discovery of EuPAC occurred around the same time as that of the channelrhodopsins (ChR),75,76 thus predating the advent of optogenetics.4 Notwithstanding its early discovery, EuPAC was not immediately deployed in optogenetics, arguably owing to its considerable molecular size and heterotetrameric architecture. The path towards optogenetic application was paved when it was realized that both EuPACα and EuPACβ mediate BL-stimulated adenylate cyclase activity on their own in the absence of the respective other PAC chain.279 Notably, EuPACα was around hundredfold more active than EuPACβ but also showed higher basal activity in the dark. Heterologous expression of either EuPACα or EuPACβ enabled BL-stimulated cAMP production as demonstrated for frog oocytes, mammalian cells and Drosophila melanogaster flies.279 Transgenic flies expressing EuPACα in their brains showed BL-dependent behavioral responses, e.g., hyperactivity or freezing. Despite later efforts at optimization, the application of EuPAC has remained limited, presumably due to the significant dark activity. A related bacterial PAC (denoted bPAC93 or BlaC94), discovered in the bacterium Beggiatoa sp. by sequence homology, has largely superseded EuPAC. Compared to EuPAC, bPAC is smaller in size, consisting of single BLUF and type-III cyclase domains only, features a longer lifetime of the signaling state and displays a higher degree of regulation by BL (up to 300-fold).93 Consequently, bPAC proved more efficient at activating CNG ion channels in frog oocytes than EuPAC.93 Whereas bPAC/BlaC is specific for the BL-induced formation of cAMP, site-directed mutagenesis yielded the variant BlaG that produced cGMP around five times more efficiently than cAMP.94 bPAC and BlaG have been used in a number of optogenetics studies, e.g., to control by BL flagellar beating of murine sperm.91 In another example,286 BlaG was derivatized by mutagenesis to alter the basal levels and BL-induced increases of cAMP/cGMP production in mammalian cells. An optimized variant, denoted EROS, was transfected into male rats where it supported BL-induced cGMP production, ensued by smooth muscle relaxation and penile erection. The recently elucidated three-dimensional structures of bPAC287 and of a related PAC from the cyanobacterium Oscillatoria acuminata (oPAC)288 revealed a homodimeric arrangement with the BLUF photosensor and the cyclase domains forming two dimers that are connected by a two-helix bundle. Diffraction data on BL-exposed PAC crystals hinted at the structural mechanism underpinning regulation of cyclase activity and stand to inform the engineering of improved bPAC/oPAC variants.287,289 Another PAC, termed mPAC, was discovered in the cyanobacterium Microcoleus chthonoplastes PCC 7420 and uses a LOV rather than a BLUF photosensor.280 Compared to bPAC, mPAC is somewhat larger in size and has a similar lifetime of the signaling state but a less pronounced BL-induced enhancement of catalytic activity. mPAC was deployed in a cyclase-deficient Dictyostelium discoideum strain where it partially restored fruiting-body formation that could be enhanced by BL.290
Distinct from the BL-regulated PACs, the photoreceptor BeGC1 from the fungus Blastocladiella emersonii employs a rhodopsin photosensor and produces cGMP in response to green light.291 As dark activity is exceedingly low and specificity for cGMP over cAMP is high, BeGC1 has already found optogenetic application.134,135 Furthermore, based on bacterial phytochromes, red-light-activated PACs292,293 and a red-light-activated cAMP/cGMP-specific PDE, denoted LAPD,281 were engineered. In a similar vein, a recent study reported a naturally occurring, light-regulated adenylate cyclase that is based on a CBCR photosensor unit.294 Lastly, a recently reported photoreceptor from Salpingoeca rosetta comprises rhodopsin and PDE modules but showed only minute light-induced enhancement of catalytic activity.295 In combination with genetically encoded sensors for the intracellular detection of cNMP levels, e.g., reference,296 PACs and light-regulated PDEs enable the precise and online optogenetic control of these second messengers.
Optogenetic control of cNMP-dependent cellular responses was also accomplished via a BL-regulated version of PKA. An impaired kinase variant with attenuated catalytic activity was tethered to AtCRY2 and could be recruited via BL to the AtCIB1 protein immobilized at the outer mitochondrial membrane.297 The resultant increase in local kinase concentration promoted the phosphorylation of target proteins associated with this organelle.
4.6.1.2. Cyclic Dinucleotides
Prokaryotes use as second messengers not only cAMP and (to much lesser extent) cGMP but also the cyclic dinucleotides c-di-GMP (cyclic diguanylate) and c-di-AMP (cyclic diadenylate).298 In particular, c-di-GMP is engaged in the regulation of numerous processes in bacteria, including biofilm formation, motility and virulence.299 Cyclic diguanlyate is produced from two molecules of GTP by GGDEF diguanylate cyclases and is hydrolyzed to 5′-phosphoguanylyl-(3′-5′)-guanosine by EAL phosphodiesterases. Given the wide range of processes regulated by c-di-GMP, it comes as no surprise that light-regulated variants of GGDEF and EAL enzymes exist in nature (Fig. 19B). Whereas GGDEF effectors are frequently regulated by bacteriophytochromes, EAL effectors are often found in conjunction with BLUF photosensors. As a case in point, the BlrP1 receptor from Klebsiella pneumoniae consists of a BLUF photosensor connected to an EAL effector.31 Hydrolysis of c-di-GMP catalyzed by BlrP1 was modestly upregulated by BL but more strongly by pH changes. In a recent study, a fragment, termed EB1, of the Magnetococcus marinus BldP protein comprising BLUF and EAL domains was generated.285 C-di-GMP hydrolysis activity of EB1 was upregulated by more than 30-fold under BL. Certain EAL effectors are connected to LOV rather than BLUF photosensors, e.g., in a photoreceptor from Synechococcus elongatus denoted SL2.284 However, BL exposure only triggered a modest increase in EAL activity of SL2. Rather than by subjecting EAL activity under direct BL control, optogenetic perturbation of c-di-GMP-mediated processes, e.g., biofilm formation, was recently achieved by expression of a constitutively active EAL protein in light-dependent manner.300 In combination with red-light-regulated GGDEF enzymes, BlrP1, EB1, SL2 and related BL-regulated EAL enzymes unlock optogenetic perturbation of diverse physiological processes in bacteria that are mediated by c-di-GMP.299 Beyond targeting these processes, c-di-GMP-dependent genetic circuits were built that allow regulation of gene expression as a function of red light.282,283 Moreover, c-di-GMP and c-di-AMP trigger the STING response which forms part of the vertebrate innate immune system.301 Briefly, the presence of double-stranded DNA in the cytosol indicates the presence of a pathogen and leads to activation of the eukaryotic cGAMP synthase which produces the mixed cyclic dinucleotide cyclic GMP-AMP (cGAMP).302 cGAMP, c-di-GMP and c-di-AMP bind to STING and thereby activate a number of downstream immune responses including induction of interferon β. Light-regulated enzymes of prokaryotic provenance that make or break cyclic dinucleotides thus hold immediate optogenetic potential for interrogating the vertebrate innate immune system.
4.6.2. Calcium Ions
Calcium is one of the most widely used second messengers that impacts on multiple physiological processes, among them gene expression, allosteric regulation of enzyme activity, nerve excitability, muscle contraction and apotosis.303 Although calcium is present in the extracellular space in millimolar concentrations, in the cytosol of eukaryotic cells the concentration is kept at sub-micromolar levels via the action of Ca2+-ATPases and Ca2+ antiporters that actively transport calcium ions to the outside of the cell or into intracellular storage compartments, in particular the endoplasmic (or, sarcoplasmic) reticulum (ER). During signal transduction, Ca2+-specific ion channels in the plasma or ER membrane are opened to allow passive influx of calcium ions along the electrochemical gradient. Calcium-dependent signaling generally involves spatial and temporal microdomains of elevated Ca2+ concentration.303 Given the preeminent role of Ca2+ as a second messenger, several optogenetic approaches have been implemented to manipulate by BL its intracellular concentration (Fig. 20). Despite differences in strategy, all approaches have in common that Ca2+ ions are released from extracellular or intracellular reservoirs. In three studies,124,304,305 the AsLOV2 photosensor was used to regulate by BL the activity of a peptide epitope derived from the STIM protein which serves as an ER-situated Ca2+ sensor. The STIM peptide can bind to CRAC (Ca2+-release-activated Ca2+) channels, specifically to the Orai1 pore-forming subunit, to induce their opening and allow calcium influx into the cytosol. In the original implementation,304 the LOVS1K photoreceptor was constructed by appending the STIM peptide to the Jα helix of AsLOV2. BL hence allowed to control exposure of the STIM epitope and interaction with Orai1. When co-transfected with Orai1 into mammalian cells, LOVS1K mediated BL-induced release of Ca2+ from the ER. The same underlying concept was further developed by generating a suite of AsLOV2-STIM fusions,305 certain of which displayed lower dark activity and more pronounced BL-induced effect than LOVS1K. Specifically, tandem duplication of AsLOV2-STIM and replacement of the STIM epitope and the Orai1 protein for their Drosophila homologs resulted in faster and stronger Ca2+ increases upon BL exposure. The improved AsLOV2-STIM receptors were used to drive gene expression in BL-dependent manner in mammalian cell culture and to evoke electrophysiological responses in the murine olfactory nervous system. In a third application of this concept,124 AsLOV2-STIM variants were generated and used to control by BL a slew of physiological responses including gene expression in mammalian cells and in mice, and immunomodulatory responses. Recently, these AsLOV2-STIM variants were further improved and combined with a calcium-responsive dCas9 construct to achieve light-regulated expression of endogenous genes with high dynamic range.306 Kyung et al.307 also exploited the activation of CRAC channels by STIM but chose a different route towards subjecting this interaction to light control. In the OptoSTIM chimeric receptor, a soluble fragment of STIM was fused with AtCRY2 such that oligomeric clusters were formed upon BL exposure. These clusters translocated to the plasma membrane where they induced opening of endogenous CRAC channels. Of particular advantage, use of OptoSTIM obviated the need for co-expressing Orai1. OptoSTIM was used in zebrafish and embryonic stem cells to transiently increase by BL intracellular calcium concentrations, and in the mouse hippocampus to modulate memory formation. In the recent optoRGK strategy,308 ion flux through voltage-gated calcium channels was controlled by BL-induced recruitment of an RGK GTPase to the plasma membrane via the iLID system for light-activated heterodimerization.203 Upon BL exposure, the GTPase translocated to the membrane and thereby inhibited ion flux through the calcium channels.
Figure 20.
Intracellular calcium concentrations could be perturbed with BL-sensitive photoreceptors. On the one hand, the opening of Ca2+-specific CRAC channels in the plasma membrane or the endoplasmic/sarcoplasmic reticulum was gated via interactions with the STIM peptide.124,304,305 When interleaved with Jα of AsLOV2, the exposure of STIM could be controlled by BL exposure. Alternatively, the STIM epitope was fused to AtCRY2 such that BL-induced clustering resulted in translocation to the membrane and CRAC gating (not shown307). A different strategy was pursued in the construction of a fusion protein between the AsLOV2 photosensor and the calcium-binding calmodulin.309 BL prompted Jα unfolding, destabilization of the calmodulin module and release of bound Ca2+ ions.
In contrast to the previous approaches, Nagai and colleagues constructed a soluble Ca2+-binding protein that sequesters calcium ions in the darkness but releases them under BL.309 In the PACR construct, the AsLOV2 photosensor was inserted into calmodulin (CaM) which was fused C-terminally with a peptide, termed M13, that enhances the calcium affinity of CaM. BL induced Jα unfolding and decreased the Ca2+ affinity of PACR by around 200-fold. When expressed in mammalian cells, PACR elicited local calcium increases upon BL illumination. In transgenic C. elegans stably expressing PACR, a BL-dependent behavorial response could be evoked. Notably, PACR releases Ca2+ in stoichiometric amounts whereas in the above STIM:Orai1-based approaches larger BL-induced increases in intracellular calcium levels can be effected. Conceptually similar to PACR, a photoreceptor was engineered that specifically releases Zn2+ ions under BL.310 A chimeric protein was constructed in which two copies of the NcVivid LOV photosensor bracket a tandem fusion of the Atox1 and WD4 proteins which bind at their interface a Zn2+ ion with picomolar affinity. BL induced homodimerization of NcVivid that in turn resulted in splaying apart of the Atox1:WD4 interface and concomitant release of the bound zinc ion. A palette of protein variants offered different zinc dissociation constants and BL-induced decreases of ion affinity by up to around 50-fold.
4.7. Receptor Signaling Cascades
In mammalian cells, an interconnected network of receptor signaling pathways couples extracellular stimuli to intracellular responses.278 Ligand binding to the extracellular portion of a transmembrane receptor alters the activity of its intracellular portion and leads to the triggering of signal cascades. Several of the major signaling pathways in mammalian cells have now been unlocked for optogenetics. While GPCRs were rendered light-responsive mostly by employing rhodopsin photoreceptors (reviewed in this issue by Bamberg), other receptors and pathways have been put under BL control by using LOV and cryptochrome photoreceptors.
4.7.1. Mitogen-Activated Protein Kinase Pathways
4.7.1.1. MAPK/ERK Pathway
The mitogen-activated protein kinase (MAPK) pathway is usually triggered by binding of extracellular ligands, e.g., epidermal growth factor (EGF), to a cognate transmembrane receptor tyrosine kinase (RTK).278 In response, the RTK autophosphorylates and thus turns on adapter proteins that act as a nucleotide exchange factor for the Ras GTPase. GTP-bound Ras then activates a MAP3K (MAPK kinase kinase), e.g., c-Raf, which in turn phosphorylates and thereby activates a MAP2K (MAPK kinase), e.g., MEK. Phosphorylated MAP2K acts as a kinase on the MAPK, e.g., ERK, which then activates by phosphorylation downstream effectors that usually exert gene-regulatory function, e.g., the transcription factor Fos.
Several research groups realized that ligand binding to RTKs often entails receptor dimerization as part of the activation mechanism which provides a leverage point for optogenetics (Fig. 21).190,311,312 Grusch et al.190 substituted the extracellular ligand-binding domain of the fibroblast growth factor (FGF) receptor tyrosine kinase for an intracellularly placed photoassociating LOV photosensor from the V. frigida or P. tricornutum aureochromes. BL could trigger dimerization of the modified RTK, termed opto-mFGFR1, and activation of the downstream MAPK/ERK pathway in mammalian cells, leading for example to gene-regulatory and cell-morphological responses. In at least one cell type, the PI3K/Akt pathway, cf. section 4.7.2., was activated in addition. Interestingly, no light regulation of RTK activity was obtained when employing photoassociating LOV domains other than the ones from the aureochromes. This finding could reflect that in the natural aureochrome receptors the LOV photosensor is situated C-terminally of the effector, thus resembling the arrangement in opto-mFGFR1, whereas the other LOV photosensors are invariably N-terminally situated in their parental receptors. The underlying modular design strategy giving rise to opto-mFGFR1 proved portable and could endow the EGF and hRET RTKs with BL sensitivity, too. BL-regulated RTKs may empower drug development as they enable all-optical screening of candidate compounds affecting receptor signaling pathways, as recently demonstrated.313,314 Following a rationale similar to that of Grusch et al., a BL-controlled variant of the FGF receptor, denoted optoFGFR1, was obtained by fusing the intracellular C terminus of the FGF RTK to AtCRY2.312 BL promoted AtCRY2 association and activation of the downstream MAPK/ERK, PI3K/Akt and phospholipase Cγ pathways. Using optoFGFR1, cytoskeleton dynamics, polarity and formation of lamellipodia in mammalian cells were controlled by BL, cf. section 4.4. Repetitive localized illumination induced cells to undergo phototaxis. A third approach311 also subjected RTK signaling to BL control via intracellular fusion with AtCRY2. Corresponding light-regulated variants of the tropomyosin-related kinases (Trk) A, B and C, denoted optoTrkA-C, triggered the MAPK/ERK and PI3K/Akt pathways in BL-activated manner. OptoTrkB allowed to control by BL the morphology of neuronal cells. Because in the optoFGFR1312 and optoTrk311 approaches the extracellular portions of the RTKs were left intact, the resultant optogenetic actuators retained sensitivity to the original extracellular ligands. All three approaches190,311,312 have in common that a suitably modified BL-sensitive RTK needs to be transfected into target cells, potentially leading to non-physiological expression levels and background stemming from the endogenous RTK repertoire. To overcome this deficiency, the CLICR approach allows the optogenetic control of endogenous receptors and RTKs (Fig. 21).315 In this strategy, AtCRY2 was fused to a SH2 domain that specifically binds to the intracellular part of several RTKs. BL-induced AtCRY2 clustering thus promoted association of RTKs, autophosphorylation and activation of the MAPK/ERK and PI3K/Akt pathways. Similar to the above studies, CLICR mediated BL-promoted formation of lamellipodia and phototaxis of mammalian cells. By exchanging the SH2 domain for other interacting domains, different subsets of RTKs and other receptors were targeted.
Figure 21.
Receptor tyrosine kinase (RTK) signaling was subjected to BL-dependent optogenetic control as exemplarily illustrated for the MAPK/ERK pathway. In several approaches,190,311,312 OptoRTKs were constructed by appending an associating photoreceptor, e.g., the LOV domain of P. tricornutum aureochrome, to the intracellular C terminus of an RTK. BL then induced homodimerization of the chimeric receptor and activation of the downstream signaling cascade. In the CLICR strategy,315 endogenous RTKs could be activated upon BL exposure via an adapter protein consisting of AtCRY2 and an SH2 domain that specifically binds to the C termini of RTKs. The MAPK/ERK pathway was also targeted at lower tiers.316,317 On the one hand, the Raf kinase can be activated by recruiting it in BL-dependent manner to the plasma membrane (not shown). On the other hand, the B-Raf isoform can be activated away from the membrane in the cytosol by homodimerization or association with the isoform c-Raf. To optogenetically control these processes, the BL-dependent oligomerization of AtCRY2 or its interaction with AtCIB1 was harnessed.
Optogenetic control of MAPK pathways was also realized at downstream nodes, thus effectively bypassing the RTKs and facilitating dissection and more precise interrogation of signaling cascades. In two similar approaches,316,317 AtCIB1 was directed to the plasma membrane via a lipid anchor, and c-Raf was fused with AtCRY2. BL hence promoted translocation of c-Raf to the membrane which triggered its activation and that of the downstream MAPK/ERK pathway. Similar responses were elicited by BL as for the above light-regulated RTKs, including cell differentiation and proliferation. In a later report,318 a similar system was applied in Xenopus embryos where it allowed to control by BL the MAPK/ERK pathway and developmental processes. The activation of c-Raf can also be accomplished remote from the plasma membrane via homodimerization or heterodimerization with the isoform B-Raf (Fig. 21).319 To this end, AtCRY2 was fused with c-Raf to allow BL-driven clustering and kinase activation. As an alternative to the homooligomerization of AtCRY2, its BL-induced interaction with AtCIB1 was exploited to assemble c-Raf:c-Raf homodimers or c-Raf:B-Raf heterodimers. By all approaches, BL activation of the MAPK/ERK pathway was achieved, thus for example providing a platform for the characterization of inhibitors of different Raf isoforms.320
4.7.1.2. Other MAPK Pathways
Two other mammalian RTK/MAPK pathways lead to the activation of the MAPKs JNK and p38 which are involved in cell differentiation, stress adaptation and apoptosis.278 As JNK and p38 are regulated by several joint MAP3K and MAP2K enzymes, independent activation and detailed study of the interlocked pathways is challenging. As a possible remedy, light-activated specific inhibitors of JNK and p38 were established as optogenetic actuators.321 By appending different peptide epitopes to the Jα helix of the AsLOV2 photosensor, their solvent exposure and inhibitory effect on JNK and p38, respectively, could be regulated by BL.
To control by BL the MAPK mating pathway in yeast, the TULIP system for light-induced PPIs was engineered based on AsLOV2.258 Using TULIP, the scaffolding protein Ste5 that spatially arranges the individual pathway components was recruited to the plasma membrane in BL-activated manner and MAPK signaling thus turned on. Alternatively, activation of the MAPK mating pathway was accomplished by recruiting the MAP3K of this pathway, Ste11, to the membrane upon BL stimulation.
4.7.2. PI3K/Akt Pathway
As another signaling cascade that is activated by RTKs and GPCRs, the PI3K/Akt pathway mediates cell proliferation, survival and migration, among other responses.278 Upon activation by membrane receptors, PI3K (phosphatidylinositol 3-kinase) catalyzes the phosphorylation of the phospholipid phosphatidylinositol (PI) at several positions to yield various phosphoinositides. Diverse physiological and metabolic processes,322 e.g., endocytosis, exocytosis, cell motility, cell adhesion and regulation of ion channels, are regulated by phosphoinositides, often in concert with other signaling pathways. Successive phosphorylation of PI by PI3K generates PI(3,4,5)P3 which binds to Akt (also known as protein kinase B), thus allowing its membrane association and activation through phosphorlyation. In turn, activated Akt phosphorylates and thereby regulates a series of downstream effectors. PI3K is counteracted by phosphatases that remove phosphoryl groups from PI(3,4,5)P3 and other phosphoinositides. Moreover, the hydrolysis of PI(4,5)P2 by phospholipase C yields the second messengers IP3 (inositol-1,4,5-trisphosphate) and DAG (diacyl glycerol).278
Optogenetic control over the PI3K/Akt cascade has been established at several levels (Fig. 22). Owing to the interconnectedness of signaling networks, several of the above approaches for regulation by BL of MAPK pathways could also elicit activation of the PI3K/Akt pathway, e.g., the CLICR method.315 Alternatively, optogenetic intervention in PI signaling was achieved by regulating the activity of PIPx phosphatases and kinases in BL-dependent manner.323,324 By tethering AtCIB1 to the plasma membrane, fusions between AtCRY2 and desired effector enzymes acting on PIPx could be recruited to the plasma membrane and hence activated.323 Light-induced activation of a PIPx phosphatase locally depleted PIP3 and PIP2 with effects on clathrin-mediated endocytosis, cytoskeleton dynamics and ion-channel activity in mammalian cells. Vice versa, recruitment of PI3K locally increased PIP3 and PIP2 amounts and impacted on cytoskeleton dynamics (cf. sec. 4.4.). In a closely similar approach,267 the iLID system served to recruit upon BL exposure a PIPx phosphatase to the plasma membrane of pancreatic cells. The resultant local depletion of PIPx caused membrane-docked secretory vesicles to detach, thereby interfering with insulin secretion from these vesciles. Employing a shared principal design strategy, three groups325–327 targeted Akt, downstream of PI3K. AtCIB1 was tethered to the plasma membrane, and an Akt-AtCRY2 chimera could be recruited under BL. Membrane localization of Akt prompted its phosphorylation, resulting in activation of the PI3K/Akt pathway,326 e.g., to elicit vesicle transport in adipocytes.325 Notably, one of the downstream targets of Akt is Bad that is engaged in eliciting apoptosis; optogenetic control over the PI3K/Akt pathway may hence provide the means of controlling cell survival, cf. sec. 4.8.
Figure 22.
Optogenetic control of phosphatidylinositol signaling. In the CLICR strategy,315 endogenous receptor tyrosine kinases (RTKs) were put under BL control via AtCRY2-mediated clustering, and the PI3K/Akt signal pathway could be optogenetically manipulated. Once activated by the RTK, the PI3K kinase phosphorylates phosphatidylinositol (PI) to produce the phosphoinositides PIP2 and PIP3. In turn, the Akt kinase binds to PIP3, is thereby activated and elicits downstream responses. Optogenetic intervention in the pathway was also accomplished at the level of PI3K via AtCRY2:AtCIB1-mediated membrane recruitment and concomitant activation.323,324 Likewise, the Akt kinase could be directly controlled by translocating it to the membrane upon BL exposure, again using the AtCRY2:AtCIB1 system.325–327
4.7.3. Other Receptor Signaling Pathways
The Wnt signaling pathway is triggered by binding of a Wnt-family glycoprotein to a complex formed by a GPCR of the Frizzled family and coreceptors, e.g., LRP6.278 Activation of the so-called canonical Wnt/β-catenin branch of the pathway depends on signal-induced clustering of the Frizzled:LRP6 complex which promotes intracellular stabilization of β-catenin. In turn, β-catenin accumulates, translates to the nucleus, forms higher-order oligomers and activates gene expression. By linking AtCRY2 to a C-terminal fragment of LRP6, clusters of this fragment could be formed under BL and downstream β-catenin-mediated responses be triggered in mammalian cells.65 In another study, the activity of β-catenin was optogenetically controlled via fusion to AtCRY2328 to achieve BL-induced protein clustering. Conceptually similar to the LARIAT strategy,242 sequestration of β-catenin into the photodynamically-formed clusters reduced its activity and allowed modulation of Drosophila development.
As transmembrane receptors, integrins are engaged in the bidirectional signaling between the cell exterior, i.e. cell-matrix and cell-cell interactions, and the cell interior.278 Upon activation, integrins interact with intracellular kindlin and talin adapter proteins to trigger a series of downstream responses including activation of the focal adhesion kinase (FAK). To control these signaling processes by light, the interaction between integrin and kindlin was first disrupted via C-terminal truncation of the integrin.329 The integrin:kindlin interaction and downstream signaling could then be rescued in light-activated manner by employing the TULIP system for heterodimerization.258 Using this strategy, cell adhesion and migration of endothelial cells could be promoted and controlled by BL, cf. sec. 4.4. A related study optogenetically targeted FAK downstream of the integrin receptor.330 FAK was C-terminally connected to AtCRY2 to allow for BL-induced clustering, resultant autophosphorylation and concomitant activation of this kinase. The BL-activated FAK variant is suited to study the lower branches of the signaling network without upstream input by the integrin receptors. Similar to integrins, ephrin RTKs are also involved in bidirectional signaling between adjacent cells. Employing the same rationale as for the OptoTrks,311 light sensitivity was bestowed on the ephrin receptor via C-terminal fusion with the Cry2olig variant of AtCRY2.331 BL accordingly prompted RTK clustering, autophosphorylation and activation. Using the light-regulated ephrin RTK variant, actin cytoskeleton rearrangements and formation of filopodia could be triggered, cf. sec. 4.4. A related rationale also underpins the engineering of a BL-regulated variant of the transforming-growth-factor (TGF) β receptor.332 The heterooligomeric TGF receptors are naturally activated upon ligand-induced association and resultant phosphorylation.278 To control heteroligomer formation and downstream pathway activation by BL, the transmembrane and intracellular segments of one TGF receptor subunit was fused with AtCIB1, and the cytosolic portion of the other subunit was connected to AtCRY2. BL illumination triggered subunit association and activation of the TGF β pathway.
Lastly, pattern recognition receptors (PRR) are parts of the innate immune system and mediate the detection of pathogen-associated molecular patterns. As one PRR, DAI resides in the cytosol and homodimerizes upon binding double-stranded DNA (of pathogenic origin). Dimerization of DAI prompts expression of target genes and downstream immune responses, in particular programmed necrosis of the infected cell.333 To subject DAI activity to BL control, two copies of DAI were fused with AtCRY2 and AtCIB1, respectively.334 In mammalian cells, BL triggered DAI dimerization and thereby induced expression of target genes. Owing to homooligomerization of AtCRY2, a BL effect could also be elicited with DAI-AtCRY2 in the absence of DAI-AtCIB1.
4.8. Apoptosis
Apoptosis denotes the programmed cell death in multicellular eukaryotic organisms335 and involves the successive activation of caspase cysteine proteases, mostly via proteolytic cleavage of pro-caspase precursors. Several intrinsic and extrinsic pathways lead to activation of initiator caspases, in particular caspase-9, and thereby initiate apoptosis. Once turned on, initiator caspases activate downstream executioner caspases, e.g., caspase-3 and caspase-7, by proteolytic cleavage. In turn, the executioner caspases operate on a number of targets, eventually culminating in the controlled destruction of the entire cell. Optogenetic control over apoptosis has been implemented at several stages (Fig. 23). A possible avenue towards controlling apoptosis by BL is provided by the PI3K/Akt pathway, cf. sec. 4.7.2., because one of the targets of this pathway is the proapoptotic protein Bad which upon phosphorylation is inhibited in mediating apoptosis. Another strategy directly targeted the initiation of apoptosis by the proapoptotic protein Bax.336 Once activated by upstream events, Bax translocates to the outer mitochondrial membrane where it oligomerizes and contributes to perforating the mitochondrion.335 Resultant outflow of cytochrome c from the mitochondrial intermembrane compartment causes activation of the cytosolic initiator caspase-9. By fusing AtCIB1 to a protein within the outer mitochondrial membrane, AtCRY2-linked Bax could be recruited there in BL-dependent fashion and downstream apoptotic events be initiated. Using this approach, a 3.5-fold increase in the concentrations of activated executioner caspase-3 could be elicited in mammalian cells by BL. Optogenetic control was also exerted at the level of the initiator and executioner caspases. By connecting the catalytic domain of caspase-9 to the NcVivid photosensor, this caspase could be dimerized and its activity increased under BL,215 even in the absence of cytosolic cytochrome c. In a viability assay, apoptosis was observed for few cells in darkness but for around half of the cells under BL. Furthermore, the catalytic activity of the executioner caspase-3 was directly regulated via insertion of the AsLOV2 domain into a linker connecting two caspase subunits.337 In the resultant enzyme, BL promoted a 2- to 3-fold enhancement of caspase activity, and studies in Drosophila demonstrated that the caspase-3 variant elicited apoptosis in BL-stimulated manner. In another approach, executioner caspase activity was regulated in BL-dependent manner by fusing the AsLOV2 photosensor domain to the catalytic domain of caspase-7.277 In darkness, steric occlusion between the two domains led to impairment of enzymatic activity; light-induced Jα unfolding presumably promoted domain dissociation and increase of caspase activity. Although the engineered photoreceptor induced apoptosis even in darkness, the efficiency of doing so could be upregulated by BL.
Figure 23.
Apoptosis, the programmed cell death, was optogenetically controlled at several tiers with BL-responsive photoreceptors. Covalent fusion of Bax with AtCRY2 allowed its BL-regulated recruitment to AtCIB1 which was connected to the Tom20 protein residing in the outer membrane of the mitochondrion.336 Oligomerization and assembly with Bak contributed to pore formation and outflow of cytochrome c from the mitochrondrial intermembrane compartment; in the cytosol, cytochrome c promoted oligomerization and activation of the initiator caspase-9. In an alternative approach, the activity of caspase-9 was directly controlled via coupling to the NcVivid photosensor which undergoes BL-induced homodimerization.215 Activated caspase-9 proteolytically activated downstream executioner caspases, e.g., caspase-7. The latter could be subjected to direct BL control by linkage to the AsLOV2 photosensor such that steric hindrance of the active site resulted.277 BL promoted AsLOV2 Jα unfolding and restored catalytic activity of caspase-7. Executioner caspases then acted on numerous downstream targets to elicit apoptosis.
As a possible alternative to the above methods for BL-induced apoptosis, one may resort to optogenetic actuators for cell ablation that are based on incapacitated LOV sensors that produce ROS upon BL illumination (cf. sec. 5.). However, cell ablation may lead to uncontrolled cell death (necrosis) as opposed to apoptosis.
4.9. Enzyme Activity
The previous sections have encountered a palette of BL-regulated enzymes in the context of epigenetic chromatin modifications (sec. 4.2.), cyclic nucleotides (section 4.6.1.), signaling cascades (section 4.7.) and apoptosis (section 4.8.). This section now explores how BL can be harnessed to modulate the activity of other enzymes. Specifically, metabolic pathways could thus be regulated by BL, conceivably leading to innovative biotechnological applications. Depending on the spatial and temporal resolution a given application demands, several general albeit mostly slower-acting strategies may apply. Short of directly regulating by BL enzymatic activity, one could instead regulate the expression of the enzyme of interest as a function of BL by resorting to well-established and versatile optogenetic actuators (cf. sec. 4.1.).198 Vice versa, BL-promoted protein degradation (cf. sec. 4.5.) provides an avenue for downregulating enzymatic activity as has been demonstrated for a biosynthetic pathway in yeast.271 Rather than degrading target enzymes, their activity levels may also be reduced reversibly by BL-induced sequestration into organelles and protein-based compartments (cf. sec. 4.3.3.). For example, the LARIAT approach242 is suited for sequestering proteins of interest into clusters which may well entail attenuation of enzymatic activity. Beyond these general strategies for optogenetic actuation, several directly BL-regulated enzymes have been engineered.
In one of the early examples of photoreceptor engineering,338 the catalytic activity of the E. coli dihydrofolate reductase enzyme that regenerates the important metabolic cofactor tetrahydrofolate was modestly regulated by BL. To achieve light sensitivity, the AsLOV2 photosensor was inserted via its N-terminal A’α and its C-terminal Jα helices into a surface loop of dihydrofolate reductase known to be sensitive to modifications. One variant showed up to 2-fold BL-induced enhancement of enzymatic turnover albeit at the cost of a 103-fold decrease in overall catalytic activity and a strong reduction of substrate affinity. Also via insertion of AsLOV2 into a surface loop, the catalytic activity of a mammalian pyruvate kinase that catalyzes the final step in glycolysis was subjected to BL control.339 In the resultant chimeric enzyme, BL exposure elicited a 40% enhancement of substrate affinity as measured by steady-state kinetics. When expressed in mammalian cells, a modest BL-induced increase of catalytic activity could be detected. Although to date the performance of BL-regulated metabolic enzymes evidently leaves wanting, recent progress in photoreceptor engineering, especially reference109, could greatly benefit future efforts in this area.
Although beyond the scope of the present review, we note that, rather than being regulated by BL, a small set of enzymes directly harvest the energy contained in BL to drive demanding chemical conversions. Put another way, photons are to be considered a substrate in these enzymatic reactions. The best-known representatives are the DNA photolyases which are homologous to cryptochromes (cf. sec. 2.1.3.), absorb BL via FAD cofactors and revert certain types of UV-induced damage, e.g., thymine dimers.340 Excitingly, a ground-breaking report recently identified a metabolic enzyme from Chlorella variabilis that bears a FAD chromophore and harnesses BL energy to catalyze the decarboxylation of fatty acids to long-chain alkanes and alkenes.3 Even earlier, an artificial system was constructed in which the FAD cofactor of a monooxygenase enzyme could be regenerated in light-driven manner in the presence of sacrificial electron donors.341,342
4.10. Ion Channels & Synaptic Communication
A vast body of optogenetic experiments in the neurosciences rely on channelrhodopsins that serve as light-gated channels for cations75,76 and, more recently, for anions.343–345 Despite the immense utility of ChRs, there is scope for diversifying and improving light-gated ion channels, in particular with regards to their ion selectivity and conductivity. First, ChRs show little ion specificity, with the conventional ChRs75,76 largely indiscriminately conducting several different mono- and divalent cations and protons. Second, the unitary conductance of a single ChR channel is low compared to other ion channels, particularly those engaged in the nervous system. Against this backdrop, several efforts have been undertaken to bestow light sensitivity on ion channels that possess high conductance and ion selectivity but that are normally light-inert (Fig. 24). Because certain ion channels are gated by second messengers, optogenetic actuators that modify the intracellular concentration of these second messengers can be harnessed to control by light ion-channel activity. For this application, PACs93,94,279,280 are combined with CNG channels to trigger by BL channel opening, as illustrated above (cf. sec. 4.6.1., Fig. 24). Similarly, certain ion channels that are modulated in their activity by phosphoinositides322 hold potential for optogenetic perturbation.
Figure 24.
Optogenetic regulation of membrane potential and ion flux. BL-sensitive photoreceptors mediate optogenetic perturbation of membrane potential and thus supplement the light-gated channelrhodopsins. Several PACs catalyze the formation of cAMP and cGMP upon BL exposure,93,94,279,280 cf. Fig. 19, and can be combined with CNG channels to optogenetically control ion flux across the plasma membrane. Depending upon CNG channel, different mono- and divalent cations (M+/2+) are specifically conducted. The gating of CRAC channels was optogenetically controlled by embedding a stimulatory peptide derived from the STIM protein into the Jα helix of AsLOV2, cf. Fig. 20.124,304,305 In the lumitoxin method,346 the AsLOV2 photosensor was anchored to the outer leaflet of the plasma membrane and connected to a peptide toxin that blocked potassium channels. Light-induced Jα unfolding granted enhanced diffusional space to the toxin, resulting in its dissociation from the channel and relieve of inhibition. In the BLINK receptor,347 the AsLOV2 photosensor was fused via its C-terminal Jα helix to the N terminus of a minimal potassium channel. BL exposure resulted in an increased potassium conductance of BLINK.
As already discussed in section 4.6.2., BL photoreceptors have been used to control gating of the Ca2+-specific CRAC channels and of voltage-gated calcium channels (Figs. 20 and 24).124,304,305,307,308 An optogenetic approach348 was also used to modulate by BL the Ca2+ conduction through the voltage-dependent CaV1.2 channel which is expressed in smooth muscle. Cluster formation of CaV1.2 channels allows mutual interactions that modulate the gating dynamics and ion conductivity. The linkage of two copies of CaV1.2 to either AtFKF1 or AtGI allowed the average CaV1.2 cluster size to be increased via the BL-induced formation of the AtFKF1:AtGI heterodimer. In ventricular myocytes, the voltage dependence of channel gating was thus altered by BL, the coupling between adjacent channels strengthened and thereby the overall Ca2+ currents increased. In addition to Ca2+-specific channels, light-gated variants of ion channels selective for K+ are of key interest because they could facilitate optogenetic silencing of excitable cells (Fig. 24). To this end, the so-called lumitoxins were constructed which connect a peptide toxin to a membrane-anchored AsLOV2 photosensor.346 In darkness, the toxin can bind to voltage-dependent potassium channels (KV) and thereby block ion conductance. BL-promoted Jα unfolding increased the average distance between the toxin and the membrane anchor such that the toxin could dissociate from the KV channel and thereby relieve channel blocking. In mammalian cells, the lumitoxins mediated the BL-dependent unblocking of specific KV channels. By exchanging the toxin domain for other variants, different subsets of KV channels could be selectively targeted. A different strategy was pursued in the engineering of the BL-gated K+ channel BLINK (Fig. 24).347 The AsLOV2 photosensor was linked via its C-terminal Jα helix to the N terminus of a minimal K+ channel of viral origin. Different linker and mutant variants of the chimeric protein were selected in yeast for light-regulated channel activity. The best performing construct, denoted BLINK, showed around 3-fold BL-induced increases of K+ conductivity in both frog oocytes and mammalian cell culture. Transient expression of BLINK in zebrafish embryos allowed modulation of their touch-induced escape response by BL. Although both the lumitoxins and BLINK offer room for improvement, their successful construction clearly demonstrates that light-induced channel gating is not restricted to rhodopsin photoreceptors nor to unspecific ion channels. Improved versions stand to become important optogenetic tools for the neurosciences.
Finally, BL-responsive photoreceptors were also employed to perturb and investigate neurotransmission through chemical synapses.349 To this end, AtCRY2 was directed to the postsynaptic density (PSD) via fusion to PSD scaffold proteins. BL was then applied to induce recruitment of AtCIB1-linked AMPA-type glutamate receptors to the PSD. Resultant BL-induced elevated concentrations of AMPA receptors at the PSD were found to enhance excitatory neurotransmission. In addition, previous work52 had shown that AtCRY2 directed to the PSD can induce protein clustering at this site which could also provide an avenue towards modulating synaptic transmission.
5. Off-Label Use of Photoreceptors
Optogenetics in general and its numerous specific manifestations covered in section 4. capitalize on the ability of sensory photoreceptors to autonomously bind and functionally reconstitute their chromophores in situ. Flavin compounds, utilized by the BL-sensitive photoreceptors discussed here, ubiquitously recur as essential metabolic cofactors across many cell types and organisms, therefore allowing the versatile deployment of genetically-encoded optogenetic tools without the exogenous addition of non-native chromophores. These favorable attributes also enable modified versions of the BL-sensitive photoreceptors to serve in ‘off-label’ applications as fluorescent proteins (cf. sec. 5.1.) and generators of reactive oxygen species (cf. sec. 5.2.) rather than as signal receptors (Fig. 25). In sec. 5.3., we discuss mounting evidence that flavin-based receptors can be sensitive to oxygen and redox potential under physiological conditions, with consequences both intended, e.g., when deliberately using them as sensors for these parameters, and unintended, e.g., when these parameters influence the outcomes of optogenetics experiments.
Figure 25.
Overview of the properties and biophysical applications of flavin-binding fluorescent proteins (FbFPs). Constitutively fluorescent FbFPs are engineered from wild-type LOV domains, by substituting the active-site cysteine to abrogate canonical LOV photochemistry, and by introducing other mutations to increase the fluorescence quantum yield ΦF. They can be used for imaging in fluorescence microscopy, as donors in FRET and as fluorescence-based sensors.350–353 The photochromicity of cysteine-retaining LOV domains can be exploited in cellular super-resolution microscopy (nanoscopy),157–159 while formation of the thioadduct in LOV domains underlies conventional optogenetic applications. In addition, FbFPs can function as genetically-encoded photosensitizers for 1O2, with a range of further applications.354,355 Blue and purple arrows indicate excitation with blue or violet light, respectively.
5.1. Photoreceptors as Fluorophores
The relatively high fluorescence quantum yield of LOV domains (ΦF ca. 0.1-0.5 in the dark-adapted state for wt proteins) allows cellular applications based on fluorescence.350,352,353,356–358 Collectively called flavin-mononucleotide-binding Fluorescent Proteins (FbFPs), LOV domains have been first introduced as fluorescent reporters of choice for anaerobic or microaerobic environments.359 The ability to functionally incorporate their flavin chromophores in the absence of oxygen and their smaller size represent substantial advantages over GFP (Green Fluorescent Protein) and structurally related FPs, which are significantly larger and require O2-dependent chromophore maturation.351 FbFPs also form the basis of the genetically-encoded photosensitizers for the generation of singlet oxygen, the development and optogenetic applications of which are discussed in section 5.2. The fluorescence in LOV domains is lost upon formation of the thioadduct-containing signaling state LOV390, and if this last process is prevented by removal of the reactive cysteine, a permanently fluorescent molecule is yielded with ΦF between 0.13 and 0.51, and a molar brightness between 1,850 and 6,380 M−1 cm−1.360,361 The main photophysical parameters of FbFPs are summarized in Table 1.
Table 1.
Photophysical parameters of FbFPs
| Proteina | ΦF | Brightness/ M−1 cm−1 | Absmax/nm | Fluomax/nm |
|---|---|---|---|---|
| BsFbFP357,359 | 0.39 | 5,420 | 449 | 495 |
| EcFbFP357,359,366 | 0.39; 0.44; 0.34 | 6,380; 4,250 | 448 | 496 |
| Pp2FbFP357,359,366 | 0.17; 0.22 | 2,125; 3,120 | 449 | 495 |
| Pp2FbFP-F37Sb,451 | 0.30 | 4,260c | 450 | 497 |
| Pp2FbFP-F37T451 | 0.24 | 3,400c | 450 | 498 |
| Pp2FbFP-Q116V357 | 0.26 | 3,930 | 439 | 485 |
| Pp2FbFP-Y112L357 | 0.30 | 4,200 | 449 | 496 |
| Pp2FbFP-L30M | 0.25403 | 3,550c | 448 | 494 |
| Pp1FbFP357 | 0.27 | 3,750 | 450 | 496 |
| DsFbFP357 | 0.35 | 5,000 | 449 | 498 |
| iLOV128,362,366 | 0.32; 0.33; 0.34 | 4,880; 4,250 | 447 | 493 |
| iLOV-Q489K128 | 0.35 | 5,630 | 440 | 489 |
| miniSOG357,391 | 0.41; 0.37 | 5,820 | 447 | 497 |
| phiLOV2.1365 | 0.20 | 2,500 | 450 | 496 |
| MrFbFP360 | 0.22 | 3,340 | 448 | 498 |
| TeFbFP360 | 0.13 | 1,850 | 445 | 494 |
| YNP1FbFP360 | 0.31 | 4,120 | 446 | 496 |
| YNP2FbFP360 | 0.33 | 4,690 | 449 | 497 |
| YNP3FbFP360 | 0.20 | 2,840 | 449 | 498 |
| YNP3FbFP-Y116F360 | 0.26 | 3,590 | 449 | 498 |
| YNP4FbFP360 | 0.33 | 4,720 | 446 | 496 |
| VfLOV361 | 0.23 | 2,875 | 450 | 498 |
| CrLOV361 | 0.51 | 6,375 | 450 | 498 |
| rsLOV1379 | 0.17 | 1,850 | 450 | 498 |
| rsLOV2379 | 0.31 | 3,530 | 450 | 498 |
| phiSOG408 | 0.36 | 4800d | 449 | 498 |
| phiSOG-Q103V408 | 0.35 | 4670d | 444 | 497 |
Organism labels: Bs = Bacillus subtilis; Ec = Escherichia coli; Pp = Pseudomonas putida; Ds = Dinoreoseobacter shibae; Mr = Meiothermus ruber; Te = Thermosynechococcus elongatus; YNP = metagenomic sequences; Vf = Vaucheria frigida; Cr = Chlamydomonas reinhardtii
phenylalanine in this position is conserved in the LOV series.90 This residue, localized on helix Cα, is in hydrophobic contact with the chromophore.77,452
calculated using the absorption coefficient of Pp2FbFP, ε450 = 14,200 M−1 cm−1, reference357
In the seminal work of Drepper et al.,359 two bacterial proteins – YtvA from B. subtilis (Uniprot code O34627) and SB2 from Pseudomonas putida (Q88JB0) – served as starting points to engineer BsFbFP (261 aa) and PpFbFP (149 aa), respectively. In both cases, the reactive cysteine was changed into alanine; in addition, BsFbFP was truncated to solely encompass its LOV domain (aa 1-137) and codon-optimized for E. coli expression to yield the well-known EcFbFP. These novel FP were tested in the facultative aerobe Rhodobacter capsulatus and in mammalian cells, and were found to be fluorescent under both standard and O2-depleted conditions. Soon afterwards, iLOV was engineered based on the LOV2 domain from A. thaliana phototropin 2 and was used for studying the dynamics of viral infections in plants and animal cells.362,363 iLOV bears six mutations (R386F, S394T, S409G, C426A, I452T, F470L) which cumulatively enhanced fluorescence to ΦF = 0.32 and minimized irreversible photobleaching. Notably, tagging viruses with GFP-derivatives often resulted in decreased infectivity and loss of FP through recombination events because of the limited size of viral genomes and high recombination rate. The smaller size of iLOV (ca. 11 kDa, ca. 55% the size of GFP) apparently overcame this problem and allowed optimal packing within the viral genome.362 Furthermore, distinct from GFP, the lack of an obligate post-translational maturation step permitted visualization of the infection dynamics on the minutes timescale. Generally, the small size of FbFPs permits applications where steric constraints might impair protein translocation, for example allowing them to be applied to studies of E. coli infections.364 In other words, this first wave of FbFPs demonstrated that LOV domains can be engineered into oxygen-independent, small and minimally perturbative fluorescent reporters.121 Several variants of iLOV were later developed that bear additional mutations, with the aim of increasing photostability. Among these, phiLOV2.1 revealed that the N390S and N401Y changes are crucial for attaining this goal, likely by indirectly anchoring and rigidifying the FMN chromophore.365 Strictly related to iLOV are miniSOG and derived genetically-encoded photosensitizers for reactive oxygen discussed in section 5.2. Extensive characterization of iLOV, Pp2FbFP and EcFbFP demonstrated further useful qualities of LOV-based FPs: a broad functional pH range with fluorescence largely retained between pH 4 and 11; high thermal stability of up to 60°C for iLOV; persistence of fluorescence under strongly reducing conditions up to a reduction potential of −660 mV; retention of oligomeric state with iLOV being a monomer and the other two proteins stable dimers; reliable detection of protein expression kinetics thanks to fast and complete maturation, even in bioprocesses that have semi-aerobic or anaerobic stages.366
Since, researchers have tried to further improve FbFPs by addressing one of several shortcomings. The relatively low molar brightness, generally one order of magnitude below GFP-related FPs, has been slightly improved by engineering novel proteins from the algae C. reinhardtii (CrLOV) and V. frigida (VfLOV). CrLOV has a large ΦF = 0.51 and the largest relative brightness (6,375 M−1 cm−1) so far reported for any FbFP, but the relatively low absorption of flavins intrinsically limits this parameter.361 Thermal and photostability were further improved with novel FbFPs from thermostable bacteria, among which the most promising specimens were derived from Meiothermus ruber (MrFbFP) and a metagenomic sequence from Yellowstone National Park (“Chocolate Pots”, YNP3FbFP). The group of newly characterized FbFPs from thermostable organisms showed an array of different fluorescence lifetimes, from 1.5 to 4.6 ns, thus making them promising candidates for multitarget labeling in a fluorescence lifetime imaging (FLIM) approach.361
The strongest limitations of FbFP are represented by their relatively low brightness, as mentioned above, and by the difficulties in tuning their absorption and fluorescence maxima towards the red flank of the visible spectrum, that is considered the most useful for animal applications.121 Despite many attempts by mutagenesis approaches, the natural chromophores of LOV domains have not been successfully red-shifted, cf. section 3.2.2. Rational optimization of spectral properties is complicated by the difficulty of predicting fluorescence excitation and emission spectra. As a case in point, the Q489K mutation in iLOV was calculated to have 52 and 97 nm red-shifts in the fluorescence excitation and emission spectra, respectively,367 but experimental characterization of iLOV-Q489K showed instead an 8 nm blue-shift.128 Recently, it was proposed that PFbFBs could be spectrally tuned and enhanced in fluorescence by means of structurally-modified chromophores, such as lumichrome and 7-methyl-8-chloro-riboflavin.126 The apoprotein W619_1-LOV from Pseudomonas putida (strain W619) bound to lumichrome increased its ΦF to 0.4 and was ca. 30 nm blue-shifted relative to the riboflavin-bound form. This approach is extremely interesting for elucidating the structural and local chemical factors that affect the photophysical parameters of FbFPs. By contrast, optogenetic use appears limited given the requirement for non-natural chromophores.
Fluorescence applications based on FbFPs have become numerous, mostly related to anaerobic and micro-aerobic environments, metabolic stages and hypoxic niches.350–353 An extensive survey of these applications goes beyond the scope of this review, but it is worth underscoring the utility of FbFPs as real-time reporters for cell processes and host-microbe interactions in anaerobic, facultative aerobic and microaerobic microorganisms of great medical or technological importance, e.g., Listeria,368 Porphyromonas gingivalis,369 Saccharomyces cerevisiae and Candida albicans,370 Synechocystis sp.,371 Trichomonas vaginalis,372 Clostridium difficile,373 and Campylobacter jejuni.374 Another FbFP-based application is the in-cell sensing of heavy metals ions, such as mercury375 and arsenic.376 Exploiting their intrinsic photochromicity (cf. section 3.2.2.),157,377 wt LOV domains with intact cysteine residues can also function as LOV-based FPs. The photochromicity of BsYtvA has been employed for localization-based super-resolution microscopy, where blue light completely switched off fluorescence and violet light recovered fluorescence at the single-molecule level, thus achieving ca. 35 nm resolution.378 More recently, rsLOV1 and rsLOV2 have been developed from BsYtvA-LOV for RESOLFT (reversible saturable/switchable optical linear fluorescence transitions) and STED (stimulated emission depletion) nanoscopy respectively: the new variants, encompassing aa 1-137, bear several mutations; rsLOV1 can be photoswitched with ten-fold better efficiency as wt BsYtvA, while rsLOV2 is more brightly fluorescent (Table 1) and has a high photostability.379 Illumination with UV/violet light drove the LOV domains into a photoequilibrium by exciting both the adduct-containing LOV390 and the adduct-free LOV447 states, whereas BL fully converted LOV447 into LOV390.159 The in vivo relevance of such UV/violet-driven photoconversion is unknown for LOV proteins,380 but this property could be useful for visualizing LOV proteins within their natural host without labeling. Doing so would combine the power of super-resolution fluorescence microscopy with optogenetics, taking advantage of the ability of LOV domains to photoactivate different biological functions (cf. sec. 4.). FbFPs can function as donors in Förster resonance energy transfer (FRET) pairs, an approach that has been used for visualizing intracellular changes in oxygen levels381 and pH.382 In the former case, a tandem construct, denoted FluBO, was built with EcFpFB as the donor and enhanced yellow fluorescent protein (EYFP) as the acceptor. Notably, EYFP only forms the chromophore and hence becomes fluorescent when O2 is present. FluBO was calibrated in E.coli cells and changed its fluorescence properties depending on the oxygen concentration at the time that the fluorophore matures.381 In a similar approach, EcFbFP was fused to a palette of EYFPs having different pKa values. Given that EcFbFP fluorescence is tolerant towards acidic conditions, it was fused as a donor domain to EYFPs with pKa values of 5.7, 6.1 and 7.5. This FRET toolbox, called FluBpH, was characterized and calibrated both in solution and in vivo.382 Finally, a fused protein comprising the LOV domain of BsYtvA-C62S and a bilin-binding, photochromic (red/green absorbing form) CBCR GAF domain was recently characterized and found to constitute a good and minimal FRET pair, with three-color fluorescence.383 Perspectively, these FRET pairs could take advantage of photoswitchable LOV domains retaining their native photoreactive cysteine, and could thus be used in super-resolution microscopy, cf. above.
As a whole, LOV-based FbFPs offer several advantages over GFP-related proteins owing to their smaller size, pH and thermal tolerance, utility under anaerobic conditions and ability to generate reactive oxygen species, detailed below. Nevertheless, to date FbFPs have intrinsic limitations especially in terms of relatively low molar brightness and limited spectral tunability.
5.2. Photoreceptors as Generators of Reactive Oxygen Species
For organisms that use flavins for photoreception, an aerobic environment represents a potential risk, because flavins are efficient photosensitizers (PS) towards molecular oxygen, leading to the formation of reactive oxygen species.384 To effectively prevent harmful ROS generation inside of the cell, prokaryotes and eukaryotes accordingly implement homeostatic and protective mechanisms for preserving the integrity of the flavin cellular pool.385 For example, in archaea and bacteria dodecins sequester free flavins that are liable to photo-induced degradation and ROS production; once the flavins are bound to dodecins, the excitation energy of absorbed light quanta can be rapidly dissipated via ultrafast proton and electron transfer mechanisms.386 Similar mechanisms might be operating in BL photoreceptors as well. Essentially, the generation of reactive oxygen species by photosensitizers proceeds via one of two general mechanisms: in type I, a PS donates an electron to O2 thereby forming the superoxide anion radical (O2−.); in type II photosensitization, the triplet state of a PS performs energy transfer (ET) to O2 via the Dexter mechanism, leading to transition from the triplet ground state O2 to the strong oxidant, excited singlet state 1O2 (short 1OΔ).387 Both mechanisms are diffusion-limited and require bimolecular collision between a PS excited state and oxygen; therefore, long-lived excited states of a chromophore are particularly relevant. The triplet states of both free and LOV-bound FMN (3FMN) have an energy level of ca. 200 kJ mol−1,168,388 a perfect situation to perform efficient ET to oxygen and generate 1OΔ, that lies 94 kJ mol−1 above the ground state O2 triplet.389 ET between triplet states is allowed, and for free FMN in solution it results in quite a high quantum yield for singlet-oxygen formation ΦΔ = 0.51 - 0.65.384,390 In wt LOV domains, formation of the thioadduct with the substrate cysteine is relatively fast (2-4 μs) and 1OΔ formation is negligible. However, recently researchers became interested in deliberately modifying LOV domains such that the triplet lifetime is extended, competitive triplet quenching reactions are minimized and the yield for 1OΔ formation is enhanced. In this manner, LOV domains became genetically encoded photosensitizers for a variety of applications, with the added values of fluorescence and of small size. The first implementation arrived with the so-called miniSOG (mini Singlet Oxygen Generator)391 and derived/related proteins. This seminal work also demonstrated that a simple substitution of the reactive cysteine (e.g., with serine or alanine) is not sufficient to generate 1OΔ, even if the triplet lifetime considerably increases and becomes oxygen dependent,392 meaning that FbFPs are poor 1OΔ sensitizers if they are not further engineered (cf. below). The topic of LOV-based photosensitizers has been excellently reviewed recently,354,355 and we will hence summarize applications more closely related to optogenetics as well as most recent updates.
MiniSOG was first designed based on the LOV2 domain from A. thaliana phototropin 2 (Atphot2- LOV2, UniProt P93025, residues 387492)391 to be employed for CLEM (Correlative Light Electron Microscopy) (cf. sec. 5.1.).393,394 In this application, miniSOG sensitized sufficient 1OΔ to locally precipitate diaminobenzidine and to allow staining with osmium contrast agents, thus combining fluorescence imaging with the high resolution of electron microscopy (EM). Six mutations were introduced into Atphot2-LOV2 (numbering refers to the 106 aa sequence of miniSOG; to recover original numbering in Atphot2 one must add 386): C40G, that abolishes light-induced thioether formation, to provide constitutive fluorescence and to give more space for O2 diffusion to the FMN cavity; I1M, N4S, S8T, S23G and F84L to increase brightness. By using miniSOG, Shu et al. succeeded in discriminating the localization of two closely situated synaptic cell-adhesion molecules in cultured neurons and in intact mouse brain, thus overcoming problems arising with conventional antibody staining. CLEM applications of miniSOG have become countless and excel in terms of target discrimination and spatial resolution compared to immunolabeling and chemical staining.354,395–399 In particular, miniSOG is substantially smaller than GFP-based fluorescent proteins, has low toxicity, and produces strong EM contrast.394 A key question is the value of ΦΔ for miniSOG, initially reported as 0.47391 and later corrected to 0.03 as directly detected by 1OΔ phosphorescence.400 The original overestimation of ΦΔ was probably due the use of anthracene-9,10-dipropionic acid as the 1Oδ sensor, a molecule that can be also oxidized by other ROS; furthermore, prolonged illumination of miniSOG likely resulted in degradation of FMN and/or protein, with a further apparent increase in Φδ.401,402 A second LOV-based photosensitizer with ΦΔ = 0.09 was later designed from Pp2FbFP by introducing the L30M mutation.403
Notwithstanding the relatively low value for ΦΔ, miniSOG served as a novel generator for intracellular 1OΔ in E. coli, thus confirming the in vivo efficiency of this photosensitizing, LOV-derived protein.404 A substantial improvement of ΦΔ from 0.03 to 0.25 was achieved with the Q103L mutation, a substitution that removes hydrogen bonds between residue 103 and position C=O(4) of FMN.402 The new derivative was called SOPP (singlet oxygen protein photosensitizer), and proved more efficient than miniSOG for both in vitro and in vivo studies.399,405 The Q103V variant of SOPP had an even larger ΦΔ = 0.39.399 Partial disruption of the hydrogen-bond network around FMN in SOPP resulted in a protein matrix that facilitates O2 diffusion, in a less efficient 3FMN quenching by electron transfer from the protein (due to larger electron density on the chromophore) and in a reduced rate of 3FMN deactivation by electronic-to-vibrational energy transfer.390,402 Nevertheless, even if ΦΔ for SOPP increased with temperature between 10 and 43°C (up to 0.27), it did not reach the value of ca. 0.6 for free FMN: encapsulation of the protein within a protein matrix obviously hinders bimolecular collision with O2.390 Among the latest developed is miniSOG2, involving seven novel mutations: G22S, G40P, Q44R, R57H, L84F, H85R, M89I, some of which affect residues in close proximity to the chromophore (residues 40, 44, 57, 84) and are likely responsible for the 20 nm blue-shift in absorption and fluorescence spectra.406 The value of ΦΔ for miniSOG2 remains to be determined. Very recently, Westberg and collaborators managed to rationally engineer very efficient variants of SOPP, named SOPP2 (W81L/L103V) and SOPP3 (W81L/H85N/M89I/Y98A/L103V).407 Notably, residue W81 was identified as the most efficient electron donor for quenching of 3FMN in SOPP. The new variants have ΦΔ = 0.57 and 0.6 respectively, reaching the value for free FMN in air (21 % O2). Most importantly, at 5 % O2, that is closer to cellular conditions, they keep high ΦΔ values of 0.27 (SOPP2) and 0.50 (SOPP3). SOPP3 is presently the best photosensitizing protein at hand, and also brightly fluorescent with ΦF = 0.41.407 A very recent development combines miniSOG or its Q103V variant, with phiLOV2.1 to produce phiSOG heterodimers that combine efficient DAB photo-oxidation (miniSOG-Q103V) and photostability (phiLOV2.1) (see Table 1).408
Beyond CLEM, optogenetic applications of LOV-derived PS for ROS production include chromophore-assisted light inactivation (CALI) of biological macromolecules, photo-induced cell ablation and immunophotosensitization.354 In CALI, a biological macromolecule of interest is tagged with a PS and illuminated by light.409 Genetically encoded PS are advantageous over free chromophores (e.g., malachite green or fluorescein) because they do not suffer from background labeling and do not require exogenous chemicals. Nevertheless, applications are still limited, mainly due to low Φδ, such as in the GFP-derived Killer Red protein,410 and secondary photodamage. LOV-derived PS are promising tools for CALI, but require blue light that penetrates poorly in tissues and has potential harmful effects on cells and their components, possibly rendering the interpretation of experimental results challenging. MiniSOG-based CALI was first employed in C. elegans in an approach called InSynC.411 By fusing miniSOG to SNARE proteins in cultured neurons, hippocampal slices and entire organisms, it was possible to inhibit synaptic release and influence C. elegans movement with light. Further work with the same nematode and using heterologous SNARE proteins fused to miniSOG confirmed that the technique works, with the limitation that both fused and nontarget synaptic proteins were damaged by ROS, causing complex and multifaceted phenotypes.412 Nevertheless, the InSynC technology is uniquely able to efficiently inhibit a specific axonal projection, as demonstrated by fusing miniSOG to presynaptic active zone proteins of the UNC-13 family.413 To target DNA, miniSOG was fused to a histone where it could stimulate mutagenesis induced by ROS after blue-light illumination.414 Another application of CALI is light-mediated inactivation of the mitochondrial electron transport chain of C. elegans by fusing miniSOG to a subunit of complex II.415 The obtained phenotypes demonstrated crucial features of complex II and its selective importance for different cell types.
Cell ablation is a powerful tool in the study of eukaryotic developmental biology and in selectively killing cells for therapeutic purposes. It can be achieved by several methods,416–418 recently also taking advantage of optogenetic approaches with KillerRed and LOV-derived proteins.419–422 In such approaches, miniSOG can be fused to different cell compartments, e.g., mitochondria, cell membranes, but in each case cell death is induced by light-generated ROS that trigger apoptosis, necrosis and phagocytic pathways.354 Phototoxic effects can be modulated with light intensity and exposure times. CALI worked with a few minutes of BL irradiation in the 0.5-3 W cm−2 range,411,413 whereas cell ablation required light intensities of 50 mW cm−2 and a prolonged time of irradiation (above 30 min), or, even more efficiently, pulsed light.419,421 MiniSOG was successfully employed for cell ablation in C. elegans,405,419–421 where a distinct trait of this approach emerged. The phototoxic effects depended on intracellular targeting, with low level of toxicity in the cytoplasm, but high photodamage when miniSOG is targeted to mitochondria, resulting in complete destruction of the cells. Most importantly, photodamage was not induced in neighboring cells, thus making miniSOG-based cell ablation a promising tool. Owing to its precision, efficiency and selectivity, the above-mentioned miniSOG2 recently allowed to inactivate single neurons in larvae of D. melanogaster.406 Very recently, photoablation of selected neurons in C. elegans with miniSOG showed that excitatory class A motor neurons have intrinsic and oscillatory activity.423
A major interest of cell ablation is the precise killing of tumor cells, a process that works very well with miniSOG in cultured cells, but much less so in vivo,424 arguably due to poor transparency of skin to blue light and low oxygen concentration in the analyzed tumors.354 Another factor could be related to the photosensitizing activity of miniSOG that, as discussed above, can also act via a type I mechanism, generating ROS that are deactivated by enzymes such as superoxide dismutase.425,426 The use of LOV-derived proteins for killing tumor cells is still in its infancy, but advantages of these genetically encoded PS over protein-free chromophores employed in conventional photodynamic therapy, such as porphyrins, are emerging: higher solubility in non-membrane compartments ensures low toxicity in the long term, while precise targeting by fusion to selected proteins and compartments should improve efficiency. This latter aspect is also related to the development of fully genetically encoded immunophotosensitizers, where a targeting antibody is fused to a protein PS.354 To produce so-called phototoxins, miniSOG was fused to antibodies and DARPins (designed ankyrin repeat proteins), specifically directed against HER2 tumor cell lines and were shown to have efficient phototoxicity, which was further enhanced by coupling the treatment with antimitotic drugs.427,428 Intriguingly, recent reports demonstrated the BL-induced production of ROS by A. thaliana CRY2 under physiological conditions.429–431 The resultant accumulation of ROS and hydrogen peroxide in the nucleus triggered the transcription of genes engaged in plant responses to abiotic and biotic stresses. Taken together, these observations imply that even unmodified photoreceptors (here, cryptochromes) can produce significant quantities of ROS at physiological conditions. Apparently, nature has harnessed this process as a parallel mechanism for transducing light signals.
5.3. Photoreceptors as Sensors of Oxygen and Redox Potential
The redox properties of flavins in solutions are well known.432 The quinone form is fully oxidized (ox), one-electron reduction leads to the semiquinone form (sq), while the doubly reduced form is called hydroquinone (hq). The reduction of ox to hq follows the “ece” sequence: electrochemical step (electron transfer, eT), chemical step (H+ transfer), eT step to give hq as the only observed final product.433 Redox titration of free FMN revealed the overlapping of the two eT steps, giving an overall value for the midpoint potential reported as Eox/hq = −205 mV434, −207 mV,433 −2 1 9 mV435 or −224 mV.436 Dissecting the single redox steps with different methodologies yielded more contrasting values of Eox/sq = −238 mV,437 −246/−314/−313 mV,433 and Esq/hq = −172 mV,437 −166/−124/−101 mV.433 Protonation equilibria of the sq state, further complicate the scenario.433
Upon photoexcitation of flavins, Eox/hq strongly changes: given the singlet and triplet excited states energy level of 2.5 and 2.1 eV, respectively,168 we can estimate 1Eox/hq = +2.3 V and 3Eox/hq = +1.9 V.168 This dramatic shift in redox potentials promotes all flavin-based photochemical reactions described in section 2., which are initiated from the ox state in LOV, BLUF438 and most CRY proteins.132,439,440 This suggests that flavin-based photoreceptors might become light-insensitive at cellular redox potentials close to their own value of Eox/hq. The intracellular redox potential of gram-negative bacteria was estimated as −270 mV, but may become more negative under oxygen-depletion conditions,441 and similar values were reported for eukaryotic cells.442 The relevant question for optogenetics is whether LOV, BLUF and CRY proteins are robust against reducing conditions and keep their photochemically competent state against intracellular redox variations. The other biologically relevant aspect is the possibility that photoreceptors can also function as redox sensors.
As mentioned in section 5.1., in vitro FbFPs partially retain their fluorescence even under strongly reducing conditions, up to a redox potential of ca. −660 mV.366 This reflects the quite negative values of Eox/hq measured for LOV proteins at the ground state of −290 mV for CrLOV-wt and −280 mV for CrLOV-C57S;436 −303 mV for BsYtvA; −308 mV for Asphot1-LOV2;443 and −258 mV for the LOV histidine kinase protein of Caulobacter crescentus11 In agreement with observations on FbFPs,366 only 20% of CrLOV-C57S is reduced at a redox potential as low as −428mV.436 Chemical reduction of LOV proteins resulted in production of the hq form only (i.e. a two-electron reduction), while photochemical reduction of CrLOV-C57S (where the FMN-Cys adduct cannot be formed) using EDTA as sacrificial electron donor, produced a neutral sq stable under deoxygenated conditions, that slowly recovered to ox after O2 admission.436 BLUF proteins showed a value of Eox/hq intermediate between free chromophores and LOV domains, localized at −260 mV for R. sphaeroides AppA, i.e. 50 mV more negative than free FAD in solution.443 The midpoint potential can be increased by the Q63H mutation and, more dramatically, by the double exchange Y21F/W104F, but surprisingly not by Y21F alone. As for LOV proteins, the sq was not detected for BLUF proteins during chemical reduction.443 For cryptochromes the scenario is more complicated: for A. thaliana CRY1 a value of Eox/hq = −161 mV was reported, but for this protein also the sq species was detected with similar Esq/hq = −153 mV,444 quite close to a previously determined value Esq/hq = −181 mV and Eox/sq = −143 mV.445 In the photolyases (PLs), a class of photoenzymes that are closely related to cryptochromes (cf. sec. 2.1.3.), the midpoint potential is less negative with Esq/hq = −39/−48 mV444 or even +16 mV446 for the enzyme alone (no detection of the ox state during oxidative titration of the PL active form), and increasing to +28444/+81446 mV when the enzyme is bound to damaged DNA. This ensures that bound PL remains in its competent form for DNA photorepair, while the more delicate redox equilibria of cryptochromes account for their quite complex photochemical properties,132,440 and possibly renders these photoreceptors more susceptible to intracellular redox conditions.
Following the consideration of midpoint potentials of flavin-based photoreceptors, we turn to their possible, flavin-centered, double role as light and redox sensors. This was discussed, yet not demonstrated for CrLovK71 but some intriguing findings emerged during the last years. For example, the Trichoderma reesei TrENV1 protein integrated both light and oxidative stress sensing by the LOV domain. Functional dimerization of TrENV1 required both BL and oxidative conditions, where redox sensing relied on an additional cysteine residue that enabled cross-linking within the dimer.447 This cysteine is localized in a hinge region between the N-terminal cap and the first β strand of the LOV core, a region that undergoes light-driven conformational changes.
In LOV receptors, photoadduct formation is the key event initiating signaling, but is the FMN-Cys covalent bond necessary to trigger signal propagation, or is protonation of N5 (promoting flipping of a conserved glutamine) possibly sufficient? Yee and co-authors elegantly demonstrated that even in the absence of the reactive cysteine light- and chemically-driven conformational changes of LOV proteins do indeed occur, via the formation of a flavin sq.27 Experimentally, this was readily demonstrated by removing the conserved functional cysteine in well-characterized LOV proteins. However, a LOV-like domain that does not possess the substrate cysteine, BAT-LOV from the archaeon Halorubrum hochstenium (HhBAT-LOV), could not be photo-reduced without replacing several aromatic amino acids close to the chromophore, because the excited state of HhBAT-LOV was efficiently quenched by these amino acids. An attempt to convert HhBAT-LOV to a canonical LOV domain by introduction of an active-site cysteine residue failed to produce any photoadduct, although the photo-reduction rate was increased. The authors speculated that canonical, adduct-forming LOV domains arose from ancestral redox-active flavoproteins via the introduction of a cysteine residue that rendered these proteins less susceptible to changes in cellular redox potential, less prone to photodamage, but more effective in photosensing.27 Recently, Magerl et al. succeeded in blocking the canonical photocycle of a cysteinebearing LOV domain, by introducing a tyrosine in the vicinity of the FMN, thereby inducing proton-coupled electron transfer towards the flavin chromophore with no formation of the adduct.448 Transient absorption spectroscopy indicated that a radical FMN.−:Tyr.+ pair was formed which decayed on the microsecond time-scale, with concomitant protonation of N5. MD simulations also implied that this tyrosine is the likely proton donor for this reaction.
As a whole, the data available for LOV and BLUF proteins currently do not support a physiologically relevant role as dual light and redox sensors centered solely on the flavin chromophore. However, the redox properties of flavins can be modulated to elucidate some critical aspects of the photocycle, and to understand evolution and natural abundance of these photoreceptors. When light and redox or O2 sensing are found in the same proteins, additional elements are present, as mentioned above. In the more nuanced scenario of cryptochromes, the redox state of the cell could have a role in their functionality and alternative photo-induced pathways of activation. The interplay of BL and O2 is one of the most intriguing issue in the field of flavin-based photoreceptors.123
6. Conclusions
As the preceding sections conclusively illustrate, within a strikingly short time span UV-light-/BL-sensitive receptors have unlocked numerous cellular activities and parameters for reversible, non-invasive and spatiotemporally precise optogenetic intervention. UV-light- and BL-sensitive photoreceptors have thus greatly contributed to decisively expanding the application scope of optogenetics beyond the light-triggered perturbation of membrane potential and the neurosciences. In particular, the engineering of novel photoreceptors has been nothing but amazingly successful, and the set of available optogenetic actuators has greatly grown, with new additions standing to arrive in the near future. Notably, photoreceptor engineering is rooted in a detailed although often incomplete molecular characterization of the underlying natural photoreceptors. The modular architecture of many natural photoreceptors, prominently evidenced in the LOV, BLUF and phytochrome classes, already hints at inherent versatility of the underlying mechanisms of light-dependent allostery. This versatility has indeed been borne out and duly exploited in photoreceptor engineering, strikingly so for the near-ubiquitous AsLOV2 photosensor; empowered by human ingenuity and creativity, even this single building block has sufficed for regulating by light numerous effectors and cellular pathways. Light-regulated order-disorder transitions as embodied by the C-terminal Jα helix of AsLOV2 represent one of the two most successful engineering concepts, with the other being light-dependent association/dissociation reactions. By resorting to these versatile (and, other) photoreceptor engineering strategies, additional effector activities will be subjected to light control in due course. In fact, photoreceptor engineering has become so successful that comprehensive optogenetic application often lags behind the actual design of a given light-regulated actuator. By fully capitalizing on the already existing and additionally upcoming photoreceptors, the inner workings of cellular systems can be interrogated and hopefully disentangled in unprecedented and ever more exact and efficient manner. While the current treatise has been deliberately restricted in scope to soluble UV-light- and BL-sensitive photoreceptors, we note in closing that many of the concepts and considerations also apply to other classes of soluble photoreceptors. In addition to phytochromes treated in this issue by Gärtner, the more recently described vitamin-B12-based photoreceptors449 and the orange-carotenoid protein450 also appear as attractive building blocks for photoreceptor engineering.
7. Acknowledgements
We thank our coworkers and colleagues for collaboration and many inspiring interactions. Funding by the FIL 2016 program of the University of Parma (A.L.), National Institutes of Health (R01 GM106239 to K.H.G.), the Alexander-von-Humboldt Foundation (A.M.) and the Deutsche Forschungsgemeinschaft (A.M.) is gratefully acknowledged.
ABBREVIATIONS
- 4-HCA
4-hydroxycinnamic acid
- Φ
quantum yield
- aa
amino acids
- BL
blue light
- BLUF
sensors of blue light using flavin adenine dinucleotide
- CALI
chromophore-assisted light inactivation
- cAMP
3’,5’-cyclic adenosine monophosphate
- Cas
CRISPR associated
- CBCR
cyanobacteriochrome
- CCT
cryptochrome C terminus
- c-di-AMP
cyclic diadenylate
- c-di-GMP
cyclic diguanylate
- cGMP
3’,5’-cyclic guanosine monophosphate
- ChR
channelrhodopsin
- CIB
cryptochrome-interacting basic-helix-loop-helix protein
- CLEM
correlative light electron microscopy
- CNG
cyclic-nucleotide-gated
- cNMP
3’,5’-cyclic nucleotide monophosphate
- CRAC
Ca2+-release-activated Ca2+ channels
- CRISPR
clustered regularly interspaced short palindromic repeats
- Cry
cryptochrome
- DARPin
designed ankyrin repeat protein
- DBD
DNA-binding domain
- dCas
cleavage-deficient variant of Cas
- DSB
double-strand break
- EB
end-binding (protein)
- EGF
epidermal growth factor
- EM
electron microscopy
- Epac
exchange protein directly activated by cAMP
- ER
endoplasmic reticulum
- eT
electron transfer
- ET
energy transfer
- EYFP
enhanced yellow fluorescent protein
- FAD
flavin adenine dinucleotide
- FAK
focal adhesion kinase
- FbFP
flavin-mononucleotide-binding fluorescent protein
- FLIM
fluorescence lifetime imaging
- FMN
flavin mononucleotide
- FP
fluorescent protein
- FRET
Förster resonance energy transfer
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- GPCR
G-protein coupled receptor
- hq
fully reduced hydroquinone state of flavin
- HTH
helix-turn-helix
- LAD
light-activated dimerization
- LOV
light-oxygen-voltage
- MAP2K
mitogen-activated protein kinase kinase
- MAP3K
mitogen-activated protein kinase kinase kinase
- MAPK
mitogen-activated protein kinase
- miniSOG
mini Singlet Oxygen Generator
- MT
microtubules
- MW
molecular weight
- NES
nuclear export signal
- NLS
nuclear localization signal
- ox
oxidized quinone state of flavin
- PA
photoactivated
- PAC
photoactivated adenylate cyclase
- PDE
phosphodiesterase
- PHR
photolyase homology region
- PI
photoinhibited
- PI
phosphatidylinositol
- PIF
phytochrome-interacting factor
- PKA
protein kinase A
- PKG
protein kinase G
- PODCP
popeye-domain-containing protein
- POI
protein of interest
- PPI
protein-protein interaction
- PRR
pattern recognition receptor
- PS
photosensitizer
- PYP
photoactive yellow protein
- REST
repressor element 1-silencing transcription factor
- RNP
ribonucleoprotein
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- sec.
section
- sgRNA
single guide RNA
- SHK
sensor histidine kinase
- SOPP
singlet oxygen protein photosensitizer
- sq
partially reduced semiquinone radical state of flavin
- tAD
trans-activation domain
- TALE
transcription factor like effector
- TCS
two-component system
- TF
transcription factor
- TGF
transforming growth factor
- TIP
tip-interacting protein
- TrpR
tryptophan repressor
- WC
white collar
- wt
wild type
Biography
Aba Losi
Aba Losi received her Ph.D. in biophysics in 1997 at the University of Parma (Italy). During her post-doctoral training with Silvia Braslavsky (Max Planck Institute for Radiation Chemistry, Germany), she explored the energy landscape of photoreceptors by means of pulsed photoacoustics. She is presently enrolled as associate professor at the University of Parma (Italy), lecturing physics, photobiophysics and photobiology. Her research is focused on functional aspects of blue-light photoreceptors in bacteria and their applications in biophysics, as well as their evolution and physiological role.
Kevin Gardner
Kevin Gardner received his training in biochemistry and biophysics with undergraduate work at UC Davis (B.S., Biochemistry, 1989), graduate work at Yale (Ph.D., Molecular Biophysics & Biochemistry, 1995) and postdoctoral research at the University of Toronto. After establishing his independent research group at UT Southwestern Medical Center, he moved his lab in 2014 to the CUNY Advanced Science Research Center to establish and direct the new Structural Biology Initiative there. In parallel, he also started as the Einstein Professor of Chemistry & Biochemistry at the City College of New York. Using a combination of structural biology, biochemistry and cell-biology approaches, his research examines the atomic-level signaling mechanisms of proteins used by cells to sense and respond to the environment around them, with the goal of understanding the natural regulation of these systems and artificially controlling them.
Andreas Möglich
Andreas Möglich studied biochemistry at the University of Regensburg (Germany) and obtained his diploma degree in 2001. Following his graduation in biophysics at the Biozentrum of the University of Basel (Switzerland) in 2005, he moved to the University of Chicago for postdoctoral studies under Dr. Keith Moffat’s guidance. On the back of a Sofja-Kovalevskaya Award by the Alexander-von-Humboldt Foundation, he returned to Germany in 2010 to assume a professorship in Biophysical Chemistry at the Humboldt University Berlin. In spring 2015, he became a full professor for Biochemistry at the University of Bayreuth (Germany). His research group focuses on the structure, mechanism, function, engineering and optogenetic application of sensory photoreceptors, in particular of light-oxygen-voltage and phytochrome photoreceptors.
8. References
- (1).Möglich A; Yang X; Ayers RA; Moffat K Structure and Function of Plant Photoreceptors. Annu. Rev. Plant Biol 2010, 61, 21–47. [DOI] [PubMed] [Google Scholar]
- (2).Hegemann P Algal Sensory Photoreceptors. Annu. Rev. Plant Biol. 2008, 59, 167–189. [DOI] [PubMed] [Google Scholar]
- (3).Sorigué D; Légeret B; Cuiné S; Blangy S; Moulin S; Billon E; Richaud P; Brugière S; Couté Y; Nurizzo D; et al. An Algal Photoenzyme Converts Fatty Acids to Hydrocarbons. Science 2017, 357, 903–907. [DOI] [PubMed] [Google Scholar]
- (4).Deisseroth K; Feng G; Majewska AK; Miesenböck G; Ting A; Schnitzer MJ Next-Generation Optical Technologies for Illuminating Genetically Targeted Brain Circuits. J. Neurosci. 2006, 26, 10380–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Li X; Gutierrez DV; Hanson MG; Han J; Mark MD; Chiel H; Hegemann P; Landmesser LT; Herlitze S Fast Noninvasive Activation and Inhibition of Neural and Network Activity by Vertebrate Rhodopsin and Green Algae Channelrhodopsin. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 17816–17821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bi A; Cui J; Ma Y-P; Olshevskaya E; Pu M; Dizhoor AM; Pan Z-H Ectopic Expression of a Microbial-Type Rhodopsin Restores Visual Responses in Mice with Photoreceptor Degeneration. Neuron 2006, 50, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Nagel G; Brauner M; Liewald JF; Adeishvili N; Bamberg E; Gottschalk A Light Activation of Channelrhodopsin-2 in Excitable Cells of Caenorhabditis Elegans Triggers Rapid Behavioral Responses. Curr. Biol. 2005, 15, 2279–2284. [DOI] [PubMed] [Google Scholar]
- (8).Boyden ES; Zhang F; Bamberg E; Nagel G; Deisseroth K Millisecond-Timescale, Genetically Targeted Optical Control of Neural Activity. Nat. Neurosci. 2005, 8, 1263–1268. [DOI] [PubMed] [Google Scholar]
- (9).Huala E; Oeller PW; Liscum E; Han IS; Larsen E; Briggs WR Arabidopsis NPH1: A Protein Kinase with a Putative Redox-Sensing Domain. Science 1997, 278, 2120–2123. [DOI] [PubMed] [Google Scholar]
- (10).Christie JM; Reymond P; Powell GK; Bernasconi P; Raibekas AA; Liscum E; Briggs WR Arabidopsis NPH1: A Flavoprotein with the Properties of a Photoreceptor for Phototropism. Science 1998, 282, 1698–1701. [DOI] [PubMed] [Google Scholar]
- (11).Iseki M; Matsunaga S; Murakami A; Ohno K; Shiga K; Yoshida K; Sugai M; Takahashi T; Hori T; Watanabe M A Blue-Light-Activated Adenylyl Cyclase Mediates Photoavoidance in Euglena Gracilis. Nature 2002, 415, 1047–1051. [DOI] [PubMed] [Google Scholar]
- (12).Gomelsky M; Klug G BLUF: A Novel FAD-Binding Domain Involved in Sensory Transduction in Microorganisms. Trends Biochem Sci 2002, 27, 497–500. [DOI] [PubMed] [Google Scholar]
- (13).Ahmad M; Lin C; Cashmore AR Mutations throughout an Arabidopsis Blue-Light Photoreceptor Impair Blue-Light-Responsive Anthocyanin Accumulation and Inhibition of Hypocotyl Elongation. Plant J 1995, 8, 653–658. [DOI] [PubMed] [Google Scholar]
- (14).Meyer TE Isolation and Characterization of Soluble Cytochromes, Ferredoxins and Other Chromophoric Proteins from the Halophilic Phototrophic Bacterium Ectothiorhodospira Halophila. Biochim. Biophys. Acta 1985, 806, 175–183. [DOI] [PubMed] [Google Scholar]
- (15).Brown BA; Cloix C; Jiang GH; Kaiserli E; Herzyk P; Kliebenstein DJ; Jenkins GI A UV-B-Specific Signaling Component Orchestrates Plant UV Protection. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 18225–18230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Glantz ST; Carpenter EJ; Melkonian M; Gardner KH; Boyden ES; Wong GK-S; Chow BY Functional and Topological Diversity of LOV Domain Photoreceptors. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Möglich A; Ayers RA; Moffat K Structure and Signaling Mechanism of Per-ARNT-Sim Domains. Structure 2009, 17, 1282–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Henry JT; Crosson S Ligand-Binding PAS Domains in a Genomic, Cellular, and Structural Context. Annu. Rev. Microbiol. 2011, 65, 261–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Harper SM; Neil LC; Gardner KH Structural Basis of a Phototropin Light Switch. Science 2003, 301, 1541–1544. [DOI] [PubMed] [Google Scholar]
- (20).Halavaty AS; Moffat K N- and C-Terminal Flanking Regions Modulate Light-Induced Signal Transduction in the LOV2 Domain of the Blue Light Sensor Phototropin 1 from Avena Sativa. Biochemistry 2007, 46, 14001–14009. [DOI] [PubMed] [Google Scholar]
- (21).Nash AI; McNulty R; Shillito ME; Swartz TE; Bogomolni RA; Luecke H; Gardner KH Structural Basis of Photosensitivity in a Bacterial Light-Oxygen-Voltage/helix-Turn-Helix (LOV-HTH) DNA-Binding Protein. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 9449–9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zoltowski BD; Schwerdtfeger C; Widom J; Loros JJ; Bilwes AM; Dunlap JC; Crane BR Conformational Switching in the Fungal Light Sensor Vivid. Science 2007, 316, 1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Alexandre MT; Domratcheva T; Bonetti C; van Wilderen LJ; van Grondelle R; Groot ML; Hellingwerf KJ; Kennis JT Primary Reactions of the LOV2 Domain of Phototropin Studied with Ultrafast Mid-Infrared Spectroscopy and Quantum Chemistry. Biophys J 2009, 97, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zayner JP; Antoniou C; Sosnick TR The Amino-Terminal Helix Modulates Light-Activated Conformational Changes in AsLOV2. J. Mol. Biol. 2012, 419, 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Pfeifer A; Mathes T; Lu Y; Hegemann P; Kottke T Blue Light Induces Global and Localized Conformational Changes in the Kinase Domain of Full-Length Phototropin. Biochemistry 2010, 49, 1024–1032. [DOI] [PubMed] [Google Scholar]
- (26).Harper SM; Christie JM; Gardner KH Disruption of the LOV-Jα Helix Interaction Activates Phototropin Kinase Activity. Biochemistry 2004, 43, 16184–16192. [DOI] [PubMed] [Google Scholar]
- (27).Yee EF; Diensthuber RP; Vaidya AT; Borbat PP; Engelhard C; Freed JH; Bittl R; Möglich A; Crane BR Signal Transduction in Light–oxygen–voltage Receptors Lacking the Adduct-Forming Cysteine Residue. Nat. Commun. 2015, 6, 10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Pudasaini A; El-Arab KK; Zoltowski BD LOV-Based Optogenetic Devices: Light-Driven Modules to Impart Photoregulated Control of Cellular Signaling. Front. Mol. Biosci. 2015, 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Finn RD; Mistry J; Schuster-Böckler B; Griffiths-Jones S; Hollich V; Lassmann T; Moxon S; Marshall M; Khanna A; Durbin R; et al. Pfam: Clans, Web Tools and Services. Nucleic Acids Res 2006, 34 (Database issue), D247–D251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wu Q; Gardner KH Structure and Insight into Blue Light-Induced Changes in the BlrP1 BLUF Domain. Biochemistry 2009, 48, 2620–2629. [DOI] [PubMed] [Google Scholar]
- (31).Barends TR; Hartmann E; Griese JJ; Beitlich T; Kirienko NV; Ryjenkov DA; Reinstein J; Shoeman RL; Gomelsky M; Schlichting I Structure and Mechanism of a Bacterial Light-Regulated Cyclic Nucleotide Phosphodiesterase. Nature 2009, 459, 1015–1018. [DOI] [PubMed] [Google Scholar]
- (32).Jung A; Domratcheva T; Tarutina M; Wu Q; Ko W; Shoeman RL; Gomelsky M; Gardner KH; Schlichting I Structure of a Bacterial BLUF Photoreceptor: Insights into Blue Light-Mediated Signal Transduction. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 12350–12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Yuan H; Bauer CE PixE Promotes Dark Oligomerization of the BLUF Photoreceptor PixD. Proc. Natl. Acad. Sci. 2008, 105, 11715–11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kraft BJ; Masuda S; Kikuchi J; Dragnea V; Tollin G; Zaleski JM; Bauer CE Spectroscopic and Mutational Analysis of the Blue-Light Photoreceptor AppA: A Novel Photocycle Involving Flavin Stacking with an Aromatic Amino Acid. Biochemistry 2003, 42, 6726–6734. [DOI] [PubMed] [Google Scholar]
- (35).Gauden M; Yeremenko S; Laan W; van Stokkum IHM; Ihalainen JA; van Grondelle R; Hellingwerf KJ; Kennis JTM Photocycle of the Flavin-Binding Photoreceptor AppA, a Bacterial Transcriptional Antirepressor of Photosynthesis Genes. Biochemistry 2005, 44, 3653–3662. [DOI] [PubMed] [Google Scholar]
- (36).Laan W; van der Horst MA; van Stokkum I; Hellingwerf KJ Initial Characterization of the Primary Photochemistry of AppA, Ablue-Light-Using Flavin Adenine Dinucleotide-Domain Containing Transcriptionalantirepressor Protein from Rhodobacter Sphaeroides: A Key Role for Reversibleintramolecular Proton Transfer from. Photochem Photobiol 2003, 78, 290–297. [DOI] [PubMed] [Google Scholar]
- (37).Stelling AL; Ronayne KL; Nappa J; Tonge PJ; Meech SR Ultrafast Structural Dynamics in BLUF Domains: Transient Infrared Spectroscopy of AppA and Its Mutants. J. Am. Chem. Soc. 2007, 129, 15556–15564. [DOI] [PubMed] [Google Scholar]
- (38).Domratcheva T; Grigorenko BL; Schlichting I; Nemukhin AV Molecular Models Predict Light-Induced Glutamine Tautomerization in BLUF Photoreceptors. Biophys. J. 2008, 94, 3872–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Domratcheva T; Hartmann E; Schlichting I; Kottke T Evidence for Tautomerisation of Glutamine in BLUF Blue Light Receptors by Vibrational Spectroscopy and Computational Chemistry. Sci. Rep. 2016, 6, 22669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Gauden M; Grinstead JS; Laan W; Van Stokkum IHM; Avila-Perez M; Toh KC; Boelens R; Kaptein R; Van Grondelle R; Hellingwerf KJ; et al. On the Role of Aromatic Side Chains in the Photoactivation of BLUF Domains. Biochemistry 2007, 46, 7405–7415. [DOI] [PubMed] [Google Scholar]
- (41).Yu X; Liu H; Klejnot J; Lin C The Cryptochrome Blue Light Receptors. Arab. B. 2010, 8, e0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Coesel S; Mangogna M; Ishikawa T; Heijde M; Rogato A; Finazzi G; Todo T; Bowler C; Falciatore A Diatom PtCPF1 Is a New Cryptochrome/photolyase Family Member with DNA Repair and Transcription Regulation Activity. EMBO Rep. 2009, 10, 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Zoltowski BD; Gardner KH Tripping the Light Fantastic: Blue-Light Photoreceptors as Examples of Environmentally Modulated Protein–Protein Interactions. Biochemistry 2011, 50, 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Brautigam CA; Smith BS; Ma Z; Palnitkar M; Tomchick DR; Machius M; Deisenhofer J Structure of the Photolyase-like Domain of Cryptochrome 1 from Arabidopsis Thaliana. Proc. Natl. Acad. Sci. 2004, 101, 12142–12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Duan L; Hope J; Ong Q; Lou HY; Kim N; McCarthy C; Acero V; Lin MZ; Cui B Understanding CRY2 Interactions for Optical Control of Intracellular Signaling. Nat. Commun. 2017, 8, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Zeugner A; Byrdin M; Bouly JP; Bakrim N; Giovani B; Brettel H; Ahmad M Light-Induced Electron Transfer in Arabidopsis Cryptochrome-1 Correlates with in Vivo Function. J. Biol. Chem. 2005, 280, 19437–19440. [DOI] [PubMed] [Google Scholar]
- (47).Ahmad M Action Spectrum for Cryptochrome-Dependent Hypocotyl Growth Inhibition in Arabidopsis. PLANT Physiol. 2002, 129, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Banerjee R; Schleicher E; Meier S; Viana RM; Pokorny R; Ahmad M; Bittl R; Batschauer A The Signaling State of Arabidopsis Cryptochrome 2 Contains Flavin Semiquinone. J. Biol. Chem. 2007, 282, 14916–14922. [DOI] [PubMed] [Google Scholar]
- (49).VanVickle-Chavez SJ; Van Gelder RN Action Spectrum of Drosophila Cryptochrome. J. Biol. Chem. 2007, 282, 10561–10566. [DOI] [PubMed] [Google Scholar]
- (50).Liu H; Yu X; Li K; Klejnot J; Yang H; Lisiero D; Lin C Photoexcited CRY2 Interacts with CIB1 to Regulate Transcription and Floral Initiation in Arabidopsis. Science 2008, 322, 1535–1539. [DOI] [PubMed] [Google Scholar]
- (51).Kennedy MJ; Hughes RM; Peteya LA; Schwartz JW; Ehlers MD; Tucker CL Rapid Blue-Light-Mediated Induction of Protein Interactions in Living Cells. Nat. Methods 2010, 7, 973–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Taslimi A; Vrana JD; Chen D; Borinskaya S; Mayer BJ; Kennedy MJ; Tucker CL An Optimized Optogenetic Clustering Tool for Probing Protein Interaction and Function. Nat. Commun. 2014, 5, 4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Taslimi A; Zoltowski B; Miranda JG; Pathak GP; Hughes RM; Tucker CL Optimized Second-Generation CRY2-CIB Dimerizers and Photoactivatable Cre Recombinase. Nat. Chem. Biol. 2016, 12, 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Ramachandran PL; Lovett JE; Carl PJ; Cammarata M; Lee JH; Jung YO; Ihee H; Timmel CR; van Thor JJ The Short-Lived Signaling State of the Photoactive Yellow Protein Photoreceptor Revealed by Combined Structural Probes. J. Am. Chem. Soc. 2011, 133, 9395–9404. [DOI] [PubMed] [Google Scholar]
- (55).Yang C; Kim SO; Kim Y; Yun SR; Choi J; Ihee H Photocycle of Photoactive Yellow Protein in Cell-Mimetic Environments: Molecular Volume Changes and Kinetics. J. Phys. Chem. B 2017, 121, 769–779. [DOI] [PubMed] [Google Scholar]
- (56).Genick UK; Borgstahl GE; Ng K; Ren Z; Pradervand C; Burke PM; Srajer V; Teng TY; Schildkamp W; McRee DE; et al. Structure of a Protein Photocycle Intermediate by Millisecond Time-Resolved Crystallography. Science 1997, 275, 1471–1475. [DOI] [PubMed] [Google Scholar]
- (57).Ihee H; Rajagopal S; Srajer V; Pahl R; Anderson S; Schmidt M; Schotte F; Anfinrud PA; Wulff M; Moffat K From The Cover: Visualizing Reaction Pathways in Photoactive Yellow Protein from Nanoseconds to Seconds. Proc. Natl. Acad. Sci. 2005, 102, 7145–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Imamoto Y; Kataoka M Structure and Photoreaction of Photoactive Yellow Protein, a Structural Prototype of the PAS Domain Superfamily. Photochem. Photobiol. 2007, 83, 40–49. [DOI] [PubMed] [Google Scholar]
- (59).Sprenger WW; Hoff WD; Armitage JP; Hellingwerf KJ The Eubacterium Ectothiorhodospira Halophila Is Negatively Phototactic, with a Wavelength Dependence That Fits the Absorption Spectrum of the Photoactive Yellow Protein. J. Bacteriol. 1993, 175, 3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Favory JJ; Stec A; Gruber H; Rizzini L; Oravecz A; Funk M; Albert A; Cloix C; Jenkins GI; Oakeley EJ; et al. Interaction of COP1 and UVR8 Regulates UV-B-Induced Photomorphogenesis and Stress Acclimation in Arabidopsis. EMBO J. 2009, 28, 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Rizzini L; Favory JJ; Cloix C; Faggionato D; O’Hara A; Kaiserli E; Baumeister R; Schafer E; Nagy F; Jenkins GI; et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 2011, 332, 103–106. [DOI] [PubMed] [Google Scholar]
- (62).Di Wu; Hu Q; Yan Z; Chen W; Yan C; Huang X; Zhang J; Yang P; Deng H; Wang J; et al. Structural Basis of Ultraviolet-B Perception by UVR8. Nature 2012, 484, 214–219. [DOI] [PubMed] [Google Scholar]
- (63).Christie JM; Arvai AS; Baxter KJ; Heilmann M; Pratt AJ; O’Hara A; Kelly SM; Hothorn M; Smith BO; Hitomi K; et al. Plant UVR8 Photoreceptor Senses UV-B by Tryptophan-Mediated Disruption of Cross-Dimer Salt Bridges. Science 2012, 335, 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Berntsson O; Diensthuber RP; Panman MR; Björling A; Gustavsson E; Hoernke M; Hughes AJ; Henry L; Niebling S; Takala H; et al. Sequential Conformational Transitions and α-Helical Supercoiling Regulate a Sensor Histidine Kinase. Nat. Commun 2017, 8, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Bugaj LJ; Choksi AT; Mesuda CK; Kane RS; Schaffer DV Optogenetic Protein Clustering and Signaling Activation in Mammalian Cells. Nat. Methods 2013, 10, 249–252. [DOI] [PubMed] [Google Scholar]
- (66).Shin Y; Berry J; Pannucci N; Haataja MP; Toettcher JE; Brangwynne CP Spatiotemporal Control of Intracellular Phase Transitions Using Light-Activated optoDroplets. Cell 2017, 168, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Ziegler T; Möglich A Photoreceptor Engineering. Front. Mol. Biosci. 2015, 2, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Ren S; Sato R; Hasegawa K; Ohta H; Masuda S A Predicted Structure for the PixD-PixE Complex Determined by Homology Modeling, Docking Simulations, and a Mutagenesis Study. Biochemistry 2013, 52, 1272–1279. [DOI] [PubMed] [Google Scholar]
- (69).Vaidya AT; Chen C-H; Dunlap JC; Loros JJ; Crane BR Structure of a Light-Activated LOV Protein Dimer That Regulates Transcription. Sci. Signal. 2011, 4, ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Takakado A; Nakasone Y; Terazima M Photoinduced Dimerization of a Photosensory DNA-Binding Protein EL222 and Its LOV Domain. Phys. Chem. Chem. Phys. 2017, 19, 24855–24865. [DOI] [PubMed] [Google Scholar]
- (71).Purcell EB; McDonald CA; Palfey BA; Crosson S An Analysis of the Solution Structure and Signaling Mechanism of LovK, a Sensor Histidine Kinase Integrating Light and Redox Signals. Biochemistry 2010, 49, 6761–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Strickland D; Yao X; Gawlak G; Rosen MK; Gardner KH; Sosnick TR Rationally Improving LOV Domain-Based Photoswitches. Nat. Methods 2010, 7, 623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Crick F The Impact of Molecular Biology on Neuroscience. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 2021–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Zemelman BV; Lee GA; Ng M; Miesenböck G Selective Photostimulation of Genetically ChARGed Neurons. Neuron 2002, 33, 15–22. [DOI] [PubMed] [Google Scholar]
- (75).Nagel G; Ollig D; Fuhrmann M; Kateriya S; Musti AM; Bamberg E; Hegemann P Channelrhodopsin-1: A Light-Gated Proton Channel in Green Algae. Science 2002, 296, 2395–2398. [DOI] [PubMed] [Google Scholar]
- (76).Nagel G; Szellas T; Huhn W; Kateriya S; Adeishvili N; Berthold P; Ollig D; Hegemann P; Bamberg E Channelrhodopsin-2, a Directly Light-Gated Cation-Selective Membrane Channel. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Crosson S; Moffat K Structure of a Flavin-Binding Plant Photoreceptor Domain: Insights into Light-Mediated Signal Transduction. Proc Natl Acad Sci U S A 2001, 98, 2995–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Crosson S; Moffat K Photoexcited Structure of a Plant Photoreceptor Domain Reveals a Light-Driven Molecular Switch. Plant Cell 2002, 14, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Deisseroth K Optogenetics. Nat Methods 2011, 8, 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Hegemann P; Möglich A Channelrhodopsin Engineering and Exploration of New Optogenetic Tools. Nat. Methods 2011, 8, 39–42. [DOI] [PubMed] [Google Scholar]
- (81).Repina NA; Rosenbloom A; Mukherjee A; Schaffer DV; Kane RS At Light Speed: Advances in Optogenetic Systems for Regulating Cell Signaling and Behavior. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 13–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Eleftheriou C; Cesca F; Maragliano L; Benfenati F; Maya-Vetencourt JF Optogenetic Modulation of Intracellular Signalling and Transcription: Focus on Neuronal Plasticity. J. Exp. Neurosci 2017, 11, 1179069517703354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Fan LZ; Lin MZ Optical Control of Biological Processes by Light-Switchable Proteins. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Kolar K; Weber W Synthetic Biological Approaches to Optogenetically Control Cell Signaling. Curr. Opin. Biotechnol. 2017, 47, 112–119. [DOI] [PubMed] [Google Scholar]
- (85).Endo M; Ozawa T Strategies for Development of Optogenetic Systems and Their Applications. J. Photochem. Photobiol. C Photochem. Rev 2017, 30, 10–23. [Google Scholar]
- (86).Liu Q; Tucker CL Engineering Genetically-Encoded Tools for Optogenetic Control of Protein Activity. Curr. Opin. Chem. Biol. 2017, 40, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Khamo JS; Krishnamurthy VV; Sharum SR; Mondal P; Zhang K Applications of Optobiology in Intact Cells and Multicellular Organisms. J. Mol. Biol. 2017, 429, 2999–3017. [DOI] [PubMed] [Google Scholar]
- (88).Tischer D; Weiner OD Illuminating Cell Signalling with Optogenetic Tools. Nat. Rev. Mol. Cell Biol. 2014, 15, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Ueda Y; Sato M Induction of Signal Transduction Using Non-Channelrhodopsin-Type Optogenetic Tools. Chembiochem A Eur. J. Chem. Biol 2018, in press, 10.1002/cbic.201700635. [DOI] [PubMed] [Google Scholar]
- (90).Losi A; Mandalari C; Gärtner W From Plant Infectivity to Growth Patterns: The Role of Blue-Light Sensing in the Prokaryotic World. Plants 2014, 3, 70–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Jansen V; Jikeli JF; Wachten D How to Control Cyclic Nucleotide Signaling by Light. Curr. Opin. Biotechnol. 2017, 48, 15–20. [DOI] [PubMed] [Google Scholar]
- (92).Motta-Mena LB; Reade A; Mallory MJ; Glantz S; Weiner OD; Lynch KW; Gardner KH An Optogenetic Gene Expression System with Rapid Activation and Deactivation Kinetics. Nat. Chem. Biol. 2014, 10, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Stierl M; Stumpf P; Udwari D; Gueta R; Hagedorn R; Losi A; Gärtner W; Petereit L; Efetova M; Schwarzel M; et al. Light-Modulation of Cellular cAMP by a Small Bacterial Photoactivated Adenylyl Cyclase, bPAC, of the Soil Bacterium Beggiatoa. J. Biol. Chem. 2011, 286, 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Ryu M-H; Moskvin OV; Siltberg-Liberles J; Gomelsky M Natural and Engineered Photoactivated Nucleotidyl Cyclases for Optogenetic Applications. J. Biol. Chem. 2010, 285, 41501–41508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Park S-Y; Tame JRH Seeing the Light with BLUF Proteins. Biophys. Rev. 2017, 9, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Fankhauser C; Christie JM Plant Phototropic Growth. Curr. Biol. 2015, 25, R384–389. [DOI] [PubMed] [Google Scholar]
- (97).Pennacchietti F; Abbruzzetti S; Losi A; Mandalari C; Bedotti R; Viappiani C; Zanacchi FC; Diaspro A; Gärtner W The Dark Recovery Rate in the Photocycle of the Bacterial Photoreceptor YtvA Is Affected by the Cellular Environment and by Hydration. PLoS One 2014, 9, e107489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Jurk M; Dorn M; Kikhney A; Svergun D; Gärtner W; Schmieder P The Switch That Does Not Flip: The Blue-Light Receptor YtvA from Bacillus Subtilis Adopts an Elongated Dimer Conformation Independent of the Activation State as Revealed by a Combined AUC and SAXS Study. J. Mol. Biol. 2010, 403, 78–87. [DOI] [PubMed] [Google Scholar]
- (99).Engelhard C; Raffelberg S; Tang Y; Diensthuber RP; Möglich A; Losi A; Gärtner W; Bittl R A Structural Model for the Full-Length Blue Light-Sensing Protein YtvA from Bacillus Subtilis, Based on EPR Spectroscopy. Photochem. Photobiol. Sci. 2013, 12, 1855–1863. [DOI] [PubMed] [Google Scholar]
- (100).Halavaty AS; Moffat K Coiled-Coil Dimerization of the LOV2 Domain of the Blue-Light Photoreceptor Phototropin 1 from Arabidopsis Thaliana. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Yao X; Rosen MK; Gardner KH Estimation of the Available Free Energy in a LOV2-Jα Photoswitch. Nat. Chem. Biol. 2008, 4, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Jäckel C; Kast P; Hilvert D Protein Design by Directed Evolution. Annu. Rev. Biophys. 2008, 37, 153–173. [DOI] [PubMed] [Google Scholar]
- (103).Wu YI; Frey D; Lungu OI; Jaehrig A; Schlichting I; Kuhlman B; Hahn KM A Genetically Encoded Photoactivatable Rac Controls the Motility of Living Cells. Nature 2009, 461, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Strickland D; Moffat K; Sosnick TR Light-Activated DNA Binding in a Designed Allosteric Protein. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 10709–10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Möglich A; Ayers RA; Moffat K Design and Signaling Mechanism of Light-Regulated Histidine Kinases. J. Mol. Biol. 2009, 385, 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Ohlendorf R; Schumacher CH; Richter F; Möglich A Library-Aided Probing of Linker Determinants in Hybrid Photoreceptors. ACS Synth. Biol. 2016, 5, 1117–1126. [DOI] [PubMed] [Google Scholar]
- (107).Ohlendorf R; Vidavski RR; Eldar A; Möffat K; Möglich A From Dusk till Dawn: One-Plasmid Systems for Light-Regulated Gene Expression. J. Mol. Biol. 2012, 416, 534–542. [DOI] [PubMed] [Google Scholar]
- (108).Longwell CK; Labanieh L; Cochran JR High-Throughput Screening Technologies for Enzyme Engineering. Curr. Opin. Biotechnol. 2017, 48, 196–202. [DOI] [PubMed] [Google Scholar]
- (109).Dagliyan O; Tarnawski M; Chu P-H; Shirvanyants D; Schlichting I; Dokholyan NV; Hahn KM Engineering Extrinsic Disorder to Control Protein Activity in Living Cells. Science 2016, 354, 1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Kumar A; Burns DC; Al-Abdul-Wahid MS; Woolley GA A Circularly Permuted Photoactive Yellow Protein as a Scaffold for Photoswitch Design. Biochemistry 2013, 52, 3320–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Zhou H; Zoltowski BD; Tao P Revealing Hidden Conformational Space of LOV Protein VIVID Through Rigid Residue Scan Simulations. Sci. Rep. 2017, 7, 46626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Glantz ST; Carpenter EJ; Melkonian M; Gardner KH; Boyden ES; Wong GK-S; Chow BY Functional and Topological Diversity of LOV Domain Photoreceptors. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Rockwell NC; Ohlendorf R; Möglich A Cyanobacteriochromes in Full Color and Three Dimensions. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 806–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Yazawa M; Sadaghiani AM; Hsueh B; Dolmetsch RE Induction of Protein-Protein Interactions in Live Cells Using Light. Nat. Biotechnol. 2009, 27, 941–945. [DOI] [PubMed] [Google Scholar]
- (115).Diensthuber RP; Bommer M; Gleichmann T; Möglich A Full-Length Structure of a Sensor Histidine Kinase Pinpoints Coaxial Coiled Coils as Signal Transducers and Modulators. Structure 2013, 21, 1127–1136. [DOI] [PubMed] [Google Scholar]
- (116).Morgan S-A; Al-Abdul-Wahid S; Woolley GA Structure-Based Design of a Photocontrolled DNA Binding Protein. J. Mol. Biol. 2010, 399, 94–112. [DOI] [PubMed] [Google Scholar]
- (117).Fan HY; Morgan S-A; Brechun KE; Chen Y-Y; Jaikaran ASI; Woolley GA Improving a Designed Photocontrolled DNA-Binding Protein. Biochemistry 2011, 50, 1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Hühner J; Ingles-Prieto Á; Neusüß C; Lämmerhofer M; Janovjak H Quantification of Riboflavin, Flavin Mononucleotide, and Flavin Adenine Dinucleotide in Mammalian Model Cells by CE with LED-Induced Fluorescence Detection. Electrophoresis 2015, 36, 518–525. [DOI] [PubMed] [Google Scholar]
- (119).Mühlhäuser WWD; Fischer A; Weber W; Radziwill G Optogenetics - Bringing Light into the Darkness of Mammalian Signal Transduction. Biochim. Biophys. Acta - Mol. Cell Res. 2017, 1864, 280–292. [DOI] [PubMed] [Google Scholar]
- (120).van den Berg PA; Widengren J; Hink MA; Rigler R; Visser AJ Fluorescence Correlation Spectroscopy of Flavins and Flavoenzymes: Photochemical and Photophysical Aspects. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2001, 57, 2135–2144. [DOI] [PubMed] [Google Scholar]
- (121).Shcherbakova DM; Shemetov AA; Kaberniuk AA; Verkhusha VV Natural Photoreceptors as a Source of Fluorescent Proteins, Biosensors, and Optogenetic Tools. Annu. Rev. Biochem 2015, 84, 519–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Kobayashi H; Ogawa M; Alford R; Choyke PL; Urano Y New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Losi A; Gärtner W Solving Blue Light Riddles: New Lessons from Flavin-Binding LOV Photoreceptors. Photochem. Photobiol. 2017, 93, 141–158. [DOI] [PubMed] [Google Scholar]
- (124).He L; Zhang Y; Ma G; Tan P; Li Z; Zang S; Wu X; Jing J; Fang S; Zhou L; et al. Near-Infrared Photoactivatable Control of Ca(2+) Signaling and Optogenetic Immunomodulation. Elife 2015, 4, e10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Di Ventura B; Kuhlman B Go in! Go out! Inducible Control of Nuclear Localization. Curr. Opin. Chem. Biol. 2016, 34, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Arinkin V; Granzin J; Röllen K; Krauss U; Jaeger K Structure of a LOV Protein in Apo-State and Implications for Construction of LOV-Based Optical Tools. Sci. Rep. 2017, 7, 42971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Raffelberg S; Gutt A; Gärtner W; Mandalari C; Abbruzzetti S; Viappiani C; Losi A The Amino Acids Surrounding the Flavin 7a-Methyl Group Determine the UVA Spectral Features of a LOV Protein. Biol. Chem. 2013, 394, 1517–1528. [DOI] [PubMed] [Google Scholar]
- (128).Davari MD; Kopka B; Wingen M; Bocola M; Drepper T; Jaeger K-E; Schwaneberg U; Krauss U Photophysics of the LOV-Based Fluorescent Protein Variant iLOV-Q489K Determined by Simulation and Experiment. J. Phys. Chem. B 2016, 120, 3344–3352. [DOI] [PubMed] [Google Scholar]
- (129).Khrenova MG; Meteleshko YI; Nemukhin AV Mutants of the Flavoprotein iLOV as Prospective Red-Shifted Fluorescent Markers. J. Phys. Chem. B 2017, 121, 10018–10025. [DOI] [PubMed] [Google Scholar]
- (130).Bury A; Hellingwerf KJ On the in Vivo Redox State of Flavin-Containing Photosensory Receptor Proteins. Methods Mol. Biol. 2014, 1146, 177–190. [DOI] [PubMed] [Google Scholar]
- (131).Beel B; Prager K; Spexard M; Sasso S; Weiss D; Muller N; Heinnickel M; Dewez D; Ikoma D; Grossman AR; et al. A Flavin Binding Cryptochrome Photoreceptor Responds to Both Blue and Red Light in Chlamydomonas Reinhardtii. Plant Cell 2012, 24, 2992–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Kottke T; Oldemeyer S; Wenzel S; Zou Y; Mittag M Cryptochrome Photoreceptors in Green Algae: Unexpected Versatility of Mechanisms and Functions. J. Plant Physiol. 2017, 217, 4–14. [DOI] [PubMed] [Google Scholar]
- (133).Spexard M; Thöing C; Beel B; Mittag M; Kottke T Response of the Sensory Animal-like Cryptochrome aCRY to Blue and Red Light As Revealed by Infrared Difference Spectroscopy. Biochemistry 2014, 53, 1041–1050. [DOI] [PubMed] [Google Scholar]
- (134).Scheib U; Stehfest K; Gee CE; Körschen HG; Fudim R; Oertner TG; Hegemann P The Rhodopsin–guanylyl Cyclase of the Aquatic Fungus Blastocladiella Emersonii Enables Fast Optical Control of cGMP Signaling. Sci. Signal. 2015, 8, rs8. [DOI] [PubMed] [Google Scholar]
- (135).Gao S; Nagpal J; Schneider MW; Kozjak-Pavlovic V; Nagel G; Gottschalk A Optogenetic Manipulation of cGMP in Cells and Animals by the Tightly Light-Regulated Guanylyl-Cyclase Opsin CyclOp. Nat. Commun. 2015, 6, 8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Monod J; Wyman J; Changeux JP On the Nature of Allosteric Transitions: A Plausible Model. J Mol Biol 1965, 12, 88–118. [DOI] [PubMed] [Google Scholar]
- (137).Möglich A; Ayers RA; Moffat K Addition at the Molecular Level: Signal Integration in Designed Per-ARNT-Sim Receptor Proteins. J. Mol. Biol. 2010, 400, 477–486. [DOI] [PubMed] [Google Scholar]
- (138).Konold PE; Mathes T; Weiβenborn J; Groot ML; Hegemann P; Kennis JTM Unfolding of the C-Terminal Jα Helix in the LOV2 Photoreceptor Domain Observed by Time-Resolved Vibrational Spectroscopy. J. Phys. Chem. Lett 2016, 7, 3472–3476. [DOI] [PubMed] [Google Scholar]
- (139).Freddolino PL; Gardner KH; Schulten K Signaling Mechanisms of LOV Domains: New Insights from Molecular Dynamics Studies. Photochem. Photobiol. Sci. 2013, 12, 1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Takeda K; Nakasone Y; Zikihara K; Tokutomi S; Terazima M Dynamics of the Amino-Terminal and Carboxyl-Terminal Helices of Arabidopsis Phototropin 1 LOV2 Studied by the Transient Grating. J. Phys. Chem. B 2013, 117, 15606–15613. [DOI] [PubMed] [Google Scholar]
- (141).Choi S; Nakasone Y; Hellingwerf KJ; Terazima M Photochemical Reactions of the LOV and LOV-Linker Domains of the Blue Light Sensor Protein YtvA. Biochemistry 2016, 55, 3107–3115. [DOI] [PubMed] [Google Scholar]
- (142).Thöing C; Oldemeyer S; Kottke T Microsecond Deprotonation of Aspartic Acid and Response of the A/β Subdomain Precede C-Terminal Signaling in the Blue Light Sensor Plant Cryptochrome. J. Am. Chem. Soc. 2015, 137, 5990–5999. [DOI] [PubMed] [Google Scholar]
- (143).Sommer C; Dietz MS; Patschkowski T; Mathes T; Kottke T Light-Induced Conformational Changes in the Plant Cryptochrome Photolyase Homology Region Resolved by Selective Isotope Labeling and Infrared Spectroscopy. Photochem. Photobiol. 2017, 93, 881–887. [DOI] [PubMed] [Google Scholar]
- (144).Kondoh M; Shiraishi C; Müller P; Ahmad M; Hitomi K; Getzoff ED; Terazima M Light-Induced Conformational Changes in Full-Length Arabidopsis Thaliana Cryptochrome. J. Mol. Biol. 2011, 413, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Tanaka K; Nakasone Y; Okajima K; Ikeuchi M; Tokutomi S; Terazima M Oligomeric-State-Dependent Conformational Change of the BLUF Protein TePixD (Tll0078). J. Mol. Biol. 2009, 386, 1290–1300. [DOI] [PubMed] [Google Scholar]
- (146).Kuroi K; Tanaka K; Okajima K; Ikeuchi M; Tokutomi S; Terazima M Anomalous Diffusion of TePixD and Identification of the Photoreaction Product. Photochem. Photobiol. Sci. 2013, 12, 1180–1186. [DOI] [PubMed] [Google Scholar]
- (147).Tanaka K; Nakasone Y; Okajima K; Ikeuchi M; Tokutomi S; Terazima M Light-Induced Conformational Change and Transient Dissociation Reaction of the BLUF Photoreceptor Synechocystis PixD (Slr1694). J. Mol. Biol. 2011, 409, 773–785. [DOI] [PubMed] [Google Scholar]
- (148).Lamb JS; Zoltowski BD; Pabit SA; Crane BR; Pollack L Time-Resolved Dimerization of a PAS-LOV Protein Measured with Photocoupled Small Angle X-Ray Scattering. J. Am. Chem. Soc. 2008, 130, 12226–12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Zoltowski BD; Vaccaro B; Crane BR Mechanism-Based Tuning of a LOV Domain Photoreceptor. Nat. Chem. Biol. 2009, 5, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).El-Arab KK; Pudasaini A; Zoltowski BD Short LOV Proteins in Methylocystis Reveal Insight into LOV Domain Photocycle Mechanisms. PLoS One 2015, 10, e0124874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Lokhandwala J; Silverman Y de la Vega RI; Hopkins HC; Britton CW; Rodriguez-Iglesias A; Bogomolni R; Schmoll M; Zoltowski BD A Native Threonine Coordinates Ordered Water to Tune Light-Oxygen-Voltage (LOV) Domain Photocycle Kinetics and Osmotic Stress Signaling in Trichoderma Reesei ENVOY. J. Biol. Chem. 2016, 291, 14839–14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Raffelberg S; Mansurova M; Gärtner W; Losi A Modulation of the Photocycle of a LOV Domain Photoreceptor by the Hydrogen-Bonding Network. J. Am. Chem. Soc. 2011, 133, 5346–5356. [DOI] [PubMed] [Google Scholar]
- (153).Zayner JP; Sosnick TR Factors That Control the Chemistry of the LOV Domain Photocycle. PLoS One 2014, 9, e87074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Diensthuber RP; Engelhard C; Lemke N; Gleichmann T; Ohlendorf R; Bittl R; Möglich A Biophysical, Mutational, and Functional Investigation of the Chromophore-Binding Pocket of Light-Oxygen-Voltage Photoreceptors. ACS Synth. Biol. 2014, 3, 811–819. [DOI] [PubMed] [Google Scholar]
- (155).Ganguly A; Thiel W; Crane BR Glutamine Amide Flip Elicits Long Distance Allosteric Responses in the LOV Protein Vivid. J. Am. Chem. Soc. 2017, 139, 2972–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Ganguly A; Manahan CC; Top D; Yee EF; Lin C; Young MW; Thiel W; Crane BR Changes in Active Site Histidine Hydrogen Bonding Trigger Cryptochrome Activation. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 10073–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Kennis JTM; van Stokkum IHM; Crosson S; Gauden M; Moffat K; van Grondelle R The LOV2 Domain of Phototropin: A Reversible Photochromic Switch. J. Am. Chem. Soc. 2004, 126, 4512–4513. [DOI] [PubMed] [Google Scholar]
- (158).Losi A; Gärtner W; Raffelberg S; Cella Zanacchi F; Bianchini P; Diaspro A; Mandalari C; Abbruzzetti S; Viappiani C A Photochromic Bacterial Photoreceptor with Potential for Super-Resolution Microscopy. Photochem. Photobiol. Sci. 2013, 12, 231–235. [DOI] [PubMed] [Google Scholar]
- (159).Song S-H; Madsen D; van der Steen JB; Pullman R; Freer LH; Hellingwerf KJ; Larsen DS Primary Photochemistry of the Dark- and Light-Adapted States of the YtvA Protein from Bacillus Subtilis. Biochemistry 2013, 52, 7951–7963. [DOI] [PubMed] [Google Scholar]
- (160).Bouly J-P; Schleicher E; Dionisio-Sese M; Vandenbussche F; Van Der Straeten D; Bakrim N; Meier S; Batschauer A; Galland P; Bittl R; et al. Cryptochrome Blue Light Photoreceptors Are Activated through Interconversion of Flavin Redox States. J. Biol. Chem. 2007, 282, 9383–9391. [DOI] [PubMed] [Google Scholar]
- (161).Hennemann J; Iwasaki RI; Grund TN; Diensthuber RP; Richter F; Möglich A Optogenetic Control by Pulsed Illumination. ChemBioChem 2018, 10.1002/cbic.201800030. [DOI] [PubMed] [Google Scholar]
- (162).Kolar K; Stork H; Knobloch C; Žnidarič M; Weber W OptoBase. www.optobase.org. [DOI] [PubMed] [Google Scholar]
- (163).Rivera-Cancel G; Ko W; Tomchick DR; Correa F; Gardner KH Full-Length Structure of a Monomeric Histidine Kinase Reveals Basis for Sensory Regulation. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 17839–17844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Chen X; Liu R; Ma Z; Xu X; Zhang H; Xu J; Ouyang Q; Yang Y An Extraordinary Stringent and Sensitive Light-Switchable Gene Expression System for Bacterial Cells. Cell Res. 2016, 26, 854–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Kawano F; Suzuki H; Furuya A; Sato M Engineered Pairs of Distinct Photoswitches for Optogenetic Control of Cellular Proteins. Nat. Commun. 2015, 6, 6256. [DOI] [PubMed] [Google Scholar]
- (166).Han T; Chen Q; Liu H Engineered Photoactivatable Genetic Switches Based on the Bacterium Phage T7 RNA Polymerase. ACS Synth. Biol. 2017, 6, 357–366. [DOI] [PubMed] [Google Scholar]
- (167).Baumschlager A; Aoki SK; Khammash M Dynamic Blue Light-Inducible T7 RNA Polymerases (Opto-T7RNAPs) for Precise Spatiotemporal Gene Expression Control. ACS Synth. Biol. 2017, 6, 2157–2167. [DOI] [PubMed] [Google Scholar]
- (168).Losi A; Polverini E; Quest B; Gärtner W First Evidence for Phototropin-Related Blue-Light Receptors in Prokaryotes. Biophys J 2002, 82, 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Capra EJ; Laub MT Evolution of Two-Component Signal Transduction Systems. Annu. Rev. Microbiol 2012, 66, 325–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Swartz TE; Tseng TS; Frederickson MA; Paris G; Comerci DJ; Rajashekara G; Kim JG; Mudgett MB; Splitter GA; Ugalde RA; et al. Blue-Light-Activated Histidine Kinases: Two-Component Sensors in Bacteria. Science 2007, 317, 1090–1093. [DOI] [PubMed] [Google Scholar]
- (171).Purcell EB; Siegal-Gaskins D; Rawling DC; Fiebig A; Crosson S A Photosensory Two-Component System Regulates Bacterial Cell Attachment. Proc Natl Acad Sci U S A 2007, 104, 18241–18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Herrou J; Crosson S Function, Structure and Mechanism of Bacterial Photosensory LOV Proteins. Nat. Rev. Microbiol. 2011, 9, 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Fernandez-Rodriguez J; Moser F; Song M; Voigt CA Engineering RGB Color Vision into Escherichia Coli. Nat. Chem. Biol. 2017, 13, 706–708. [DOI] [PubMed] [Google Scholar]
- (174).Magaraci MS; Veerakumar A; Qiao P; Amurthur A; Lee JY; Miller JS; Goulian M; Sarkar CA Engineering Escherichia Coli for Light-Activated Cytolysis of Mammalian Cells. ACS Synth. Biol. 2014, 3, 944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Farzadfard F; Lu TK Genomically Encoded Analog Memory with Precise in Vivo DNA Writing in Living Cell Populations. Science 2014, 346, 1256272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Tang W; Liu DR Rewritable Multi-Event Analog Recording in Bacterial and Mammalian Cells. Science 2018, 360, eaap8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Engelhard C; Diensthuber RP; Möglich A; Bittl R Blue-Light Reception through Quaternary Transitions. Sci. Rep 2017, 7, 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Berntsson O; Diensthuber RP; Panman MR; Björling A; Hughes AJ; Henry L; Niebling S; Newby G; Liebi M; Menzel A; et al. Time-Resolved X-Ray Solution Scattering Reveals the Structural Photoactivation of a Light-Oxygen-Voltage Photoreceptor. Structure 2017, 25, 933–938. [DOI] [PubMed] [Google Scholar]
- (179).Gleichmann T; Diensthuber RP; Möglich A Charting the Signal Trajectory in a Light-Oxygen-Voltage Photoreceptor by Random Mutagenesis and Covariance Analysis. J. Biol. Chem. 2013, 288, 29345–29355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Correa F; Ko WH; Ocasio V; Bogomolni RA; Gardner KH Blue Light Regulated Two-Component Systems: Enzymatic and Functional Analyses of Light-Oxygen-Voltage (LOV)-Histidine Kinases and Downstream Response Regulators. Biochemistry 2013, 52, 4656–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Corrêa F; Gardner KH Basis of Mutual Domain Inhibition in a Bacterial Response Regulator. Cell Chem. Biol. 2016, 23, 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Rivera-Cancel G; Motta-Mena LB; Gardner KH Identification of Natural and Artificial DNA Substrates for Light-Activated LOV–HTH Transcription Factor EL222. Biochemistry 2012, 51, 10024–10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Jayaraman P; Devarajan K; Chua TK; Zhang H; Gunawan E; Poh CL Blue Light-Mediated Transcriptional Activation and Repression of Gene Expression in Bacteria. Nucleic Acids Res. 2016, 44, 6994–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Jayaraman P; Yeoh JW; Jayaraman S; Teh AY; Zhang J; Poh CL Cell-Free Optogenetic Gene Expression System. ACS Synth. Biol. 2018, 7, 986–994. [DOI] [PubMed] [Google Scholar]
- (185).Wang X; Chen X; Yang Y Spatiotemporal Control of Gene Expression by a Light-Switchable Transgene System. Nat. Methods 2012, 9, 266–269. [DOI] [PubMed] [Google Scholar]
- (186).Fuerst TR; Niles EG; Studier FW; Moss B Eukaryotic Transient-Expression System Based on Recombinant Vaccinia Virus That Synthesizes Bacteriophage T7 RNA Polymerase. Proc. Natl. Acad. Sci. U. S. A. 1986, 83, 8122–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Froehlich AC; Liu Y; Loros JJ; Dunlap JC White Collar-1, a Circadian Blue Light Photoreceptor, Binding to the Frequency Promoter. Science 2002, 297, 815–819. [DOI] [PubMed] [Google Scholar]
- (188).Takahashi F; Yamagata D; Ishikawa M; Fukamatsu Y; Ogura Y; Kasahara M; Kiyosue T; Kikuyama M; Wada M; Kataoka H AUREOCHROME, a Photoreceptor Required for Photomorphogenesis in Stramenopiles. Proc Natl Acad Sci U S A 2007, 104, 19625–19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Herman E; Sachse M; Kroth PG; Kottke T Blue-Light-Induced Unfolding of the Jα Helix Allows for the Dimerization of Aureochrome-LOV from the Diatom Phaeodactylum Tricornutum. Biochemistry 2013, 52, 3094–3101. [DOI] [PubMed] [Google Scholar]
- (190).Grusch M; Schelch K; Riedler R; Reichhart E; Differ C; Berger W; Inglés-Prieto Á; Janovjak H Spatio-Temporally Precise Activation of Engineered Receptor Tyrosine Kinases by Light. EMBO J. 2014, 33, 1713–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Heintz U; Schlichting I Blue Light-Induced LOV Domain Dimerization Enhances the Affinity of Aureochrome 1a for Its Target DNA Sequence. Elife 2016, 5, e11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Banerjee A; Herman E; Kottke T; Essen L-O Structure of a Native-like Aureochrome 1a LOV Domain Dimer from Phaeodactylum Tricornutum. Structure 2016, 24, 171–178. [DOI] [PubMed] [Google Scholar]
- (193).Liu H; Gomez G; Lin S; Lin S; Lin C Optogenetic Control of Transcription in Zebrafish. PLoS One 2012, 7, e50738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Crefcoeur RP; Yin R; Ulm R; Halazonetis TD Ultraviolet-B-Mediated Induction of Protein-Protein Interactions in Mammalian Cells. Nat. Commun. 2013, 4, 1779. [DOI] [PubMed] [Google Scholar]
- (195).Müller K; Engesser R; Schulz S; Steinberg T; Tomakidi P; Weber CC; Ulm R; Timmer J; Zurbriggen MD; Weber W Multi-Chromatic Control of Mammalian Gene Expression and Signaling. Nucleic Acids Res. 2013, 41, e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Pathak GP; Spiltoir JI; Höglund C; Polstein LR; Heine-Koskinen S; Gersbach CA; Rossi J; Tucker CL Bidirectional Approaches for Optogenetic Regulation of Gene Expression in Mammalian Cells Using Arabidopsis Cryptochrome 2. Nucleic Acids Res. 2017, 45, e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Reade A; Motta-Mena LB; Gardner KH; Stainier DY; Weiner OD; Woo S TAEL: A Zebrafish-Optimized Optogenetic Gene Expression System with Fine Spatial and Temporal Control. Development 2017, 144, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Zhao EM; Zhang Y; Mehl J; Park H; Lalwani MA; Toettcher JE; Avalos JL Optogenetic Regulation of Engineered Cellular Metabolism for Microbial Chemical Production. Nature 2018, 555, 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Nihongaki Y; Suzuki H; Kawano F; Sato M Genetically Engineered Photoinducible Homodimerization System with Improved Dimer-Forming Efficiency. ACS Chem. Biol. 2014, 9, 617–621. [DOI] [PubMed] [Google Scholar]
- (200).Zikihara K; Iwata T; Matsuoka D; Kandori H; Todo T; Tokutomi S Photoreaction Cycle of the Light, Oxygen, and Voltage Domain in FKF1 Determined by Low-Temperature Absorption Spectroscopy. Biochemistry 2006, 45, 10828–10837. [DOI] [PubMed] [Google Scholar]
- (201).Quejada JR; Park S-HE; Awari DW; Shi F; Yamamoto HE; Kawano F; Jung JC; Yazawa M Optimized Light-Inducible Transcription in Mammalian Cells Using Flavin Kelch-Repeat F-box1/GIGANTEA and CRY2/CIB1. Nucleic Acids Res. 2017, 45, e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Lungu OI; Hallett RA; Choi EJ; Aiken MJ; Hahn KM; Kuhlman B Designing Photoswitchable Peptides Using the AsLOV2 Domain. Chem. Biol. 2012, 19, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Guntas G; Hallett RA; Zimmerman SP; Williams T; Yumerefendi H; Bear JE; Kuhlman B Engineering an Improved Light-Induced Dimer (iLID) for Controlling the Localization and Activity of Signaling Proteins. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Chan Y-B; Alekseyenko OV; Kravitz EA Optogenetic Control of Gene Expression in Drosophila. PLoS One 2015, 10, e0138181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Rademacher A; Erdel F; Trojanowski J; Schumacher S; Rippe K Real-Time Observation of Light-Controlled Transcription in Living Cells. J Cell Sci 2017, 130, 4213–4224. [DOI] [PubMed] [Google Scholar]
- (206).Pathak GP; Strickland D; Vrana JD; Tucker CL Benchmarking of Optical Dimerizer Systems. ACS Synth. Biol. 2014, 3, 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Benedetti L; Barentine AES; Messa M; Wheeler H; Bewersdorf J; De Camilli P Light-Activated Protein Interaction with High Spatial Subcellular Confinement. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E2238–E2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Cao J; Arha M; Sudrik C; Bugaj LJ; Schaffer DV; Kane RS Light-Inducible Activation of Target mRNA Translation in Mammalian Cells. Chem. Commun. 2013, 49, 8338–8340. [DOI] [PubMed] [Google Scholar]
- (209).Niopek D; Benzinger D; Roensch J; Draebing T; Wehler P; Eils R; Di Ventura B Engineering Light-Inducible Nuclear Localization Signals for Precise Spatiotemporal Control of Protein Dynamics in Living Cells. Nat. Commun. 2014, 5, 4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Niopek D; Wehler P; Roensch J; Eils R; Di Ventura B Optogenetic Control of Nuclear Protein Export. Nat. Commun. 2016, 7, 10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Yumerefendi H; Dickinson DJ; Wang H; Zimmerman SP; Bear JE; Goldstein B; Hahn K; Kuhlman B Control of Protein Activity and Cell Fate Specification via Light-Mediated Nuclear Translocation. PLoS One 2015, 10, e0128443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Yumerefendi H; Lerner AM; Zimmerman SP; Hahn K; Bear JE; Strahl BD; Kuhlman B Light-Induced Nuclear Export Reveals Rapid Dynamics of Epigenetic Modifications. Nat. Chem. Biol. 2016, 12, 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Masuda S; Nakatani Y; Ren S; Tanaka M Blue Light-Mediated Manipulation of Transcription Factor Activity In Vivo. ACS Chem. Biol. 2013, 8, 2649–2653. [DOI] [PubMed] [Google Scholar]
- (214).Polstein LR; Gersbach CA A Light-Inducible CRISPR-Cas9 System for Control of Endogenous Gene Activation. Nat. Chem. Biol. 2015, 11, 198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Nihongaki Y; Yamamoto S; Kawano F; Suzuki H; Sato M CRISPR-Cas9-Based Photoactivatable Transcription System. Chem. Biol. 2015, 22, 169–174. [DOI] [PubMed] [Google Scholar]
- (216).Masuda S; Tanaka M PICCORO. Methods Cell Biol. 2016, 135, 289–295. [DOI] [PubMed] [Google Scholar]
- (217).Paonessa F; Criscuolo S; Sacchetti S; Amoroso D; Scarongella H; Pecoraro Bisogni F; Carminati E; Pruzzo G; Maragliano L; Cesca F; et al. Regulation of Neural Gene Transcription by Optogenetic Inhibition of the RE1-Silencing Transcription Factor. Proc. Natl. Acad. Sci. 2016, 113, E91–E100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Huang A; Amourda C; Zhang S; Tolwinski NS; Saunders TE Decoding Temporal Interpretation of the Morphogen Bicoid in the Early Drosophila Embryo. Elife 2017, 6, e26258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Polstein LR; Gersbach CA Light-Inducible Spatiotemporal Control of Gene Activation by Customizable Zinc Finger Transcription Factors. J. Am. Chem. Soc. 2012, 134, 16480–16483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Gaj T; Gersbach CA; Barbas CF ZFN, TALEN and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Konermann S; Brigham MD; Trevino AE; Hsu PD; Heidenreich M; Cong L; Platt RJ; Scott DA; Church GM; Zhang F Optical Control of Mammalian Endogenous Transcription and Epigenetic States. Nature 2013, 500, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Jinek M; Chylinski K; Fonfara I; Hauer M; Doudna JA; Charpentier E A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (223).Makarova KS; Wolf YI; Alkhnbashi OS; Costa F; Shah SA; Saunders SJ; Barrangou R; Brouns SJJ; Charpentier E; Haft DH; et al. An Updated Evolutionary Classification of CRISPR-Cas Systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Shmakov S; Smargon A; Scott D; Cox D; Pyzocha N; Yan W; Abudayyeh OO; Gootenberg JS; Makarova KS; Wolf YI; et al. Diversity and Evolution of Class 2 CRISPR-Cas Systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Richter F; Fonfara I; Gelfert R; Nack J; Charpentier E; Möglich A Switchable Cas9. Curr. Opin. Biotechnol. 2017, 48, 119–126. [DOI] [PubMed] [Google Scholar]
- (226).Nihongaki Y; Furuhata Y; Otabe T; Hasegawa S; Yoshimoto K; Sato M CRISPR-Cas9-Based Photoactivatable Transcription Systems to Induce Neuronal Differentiation. Nat. Methods 2017, 10, 963–966. [DOI] [PubMed] [Google Scholar]
- (227).Nihongaki Y; Kawano F; Nakajima T; Sato M Photoactivatable CRISPR-Cas9 for Optogenetic Genome Editing. Nat. Biotechnol. 2015, 33, 755–760. [DOI] [PubMed] [Google Scholar]
- (228).Qi LS; Larson MH; Gilbert LA; Doudna JA; Weissman JS; Arkin AP; Lim WA Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (229).Konermann S; Brigham MD; Trevino AE; Joung J; Abudayyeh OO; Barcena C; Hsu PD; Habib N; Gootenberg JS; Nishimasu H; et al. Genome-Scale Transcriptional Activation by an Engineered CRISPR-Cas9 Complex. Nature 2015, 517, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Lo C-L; Choudhury SR; Irudayaraj J; Zhou FC Epigenetic Editing of Ascl1 Gene in Neural Stem Cells by Optogenetics. Sci. Rep. 2017, 7, 42047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Doudna JA; Charpentier E The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [DOI] [PubMed] [Google Scholar]
- (232).Richter F; Fonfara I; Bouazza B; Schumacher CH; Bratovič M; Charpentier E; Möglich A Engineering of Temperature- and Light-Switchable Cas9 Variants. Nucleic Acids Res. 2016, 44, 10003–10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Conrad KS; Bilwes AM; Crane BR Light-Induced Subunit Dissociation by a Light-Oxygen-Voltage Domain Photoreceptor from Rhodobacter Sphaeroides. Biochemistry 2013, 52, 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Kawano F; Okazaki R; Yazawa M; Sato M A Photoactivatable Cre-loxP Recombination System for Optogenetic Genome Engineering. Nat. Chem. Biol. 2016, 12, 1059–1064. [DOI] [PubMed] [Google Scholar]
- (235).Jullien N; Sampieri F; Enjalbert A; Herman J Regulation of Cre Recombinase by Ligand-Induced Complementation of Inactive Fragments. Nucleic Acids Res. 2003, 31, e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (236).Polstein L; Juhas M; Hanna G; Bursac N; Gersbach CA An Engineered Optogenetic Switch for Spatiotemporal Control of Gene Expression, Cell Differentiation, and Tissue Morphogenesis. ACS Synth. Biol. 2017, 6, 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Schindler SE; McCall JG; Yan P; Hyrc KL; Li M; Tucker CL; Lee J-M; Bruchas MR; Diamond MI Photo-Activatable Cre Recombinase Regulates Gene Expression in Vivo. Sci. Rep. 2015, 5, 13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Wang H; Vilela M; Winkler A; Tarnawski M; Schlichting I; Yumerefendi H; Kuhlman B; Liu R; Danuser G; Hahn KM LOVTRAP: An Optogenetic System for Photoinduced Protein Dissociation. Nat. Methods 2016, 13, 755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Yumerefendi H; Wang H; Dickinson DJ; Lerner AM; Malkus P; Goldstein B; Hahn K; Kuhlman B Light-Dependent Cytoplasmic Recruitment Enhances the Dynamic Range of a Nuclear Import Photoswitch. Chembiochem A Eur. J. Chem. Biol. 2018, 10.1002/cbic.201700681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (240).Spiltoir JI; Strickland D; Glotzer M; Tucker CL Optical Control of Peroxisomal Trafficking. ACS Synth. Biol. 2016, 5, 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (241).Yu X; Sayegh R; Maymon M; Warpeha K; Klejnot J; Yang H; Huang J; Lee J; Kaufman L; Lin C Formation of Nuclear Bodies of Arabidopsis CRY2 in Response to Blue Light Is Associated with Its Blue Light-Dependent Degradation. Plant Cell 2009, 21, 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Lee S; Park H; Kyung T; Kim NY; Kim S; Kim J; Heo W.Do. Reversible Protein Inactivation by Optogenetic Trapping in Cells. Nat. Methods 2014, 11, 633–636. [DOI] [PubMed] [Google Scholar]
- (243).Brangwynne CP Phase Transitions and Size Scaling of Membrane-Less Organelles. J. Cell Biol. 2013, 203, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Park H; Kim NYN; Lee S; Kim NYN; Kim J; Heo W. Do. Optogenetic Protein Clustering through Fluorescent Protein Tagging and Extension of CRY2. Nat. Commun 2017, 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (245).Shi F; Kawano F; Park SE; Komazaki S; Hirabayashi Y; Polleux F; Yazawa M Optogenetic Control of Endoplasmic Reticulum–Mitochondria Tethering. ACS Synth. Biol. 2018, 7, 2–9. [DOI] [PubMed] [Google Scholar]
- (246).Kornmann B; Currie E; Collins SR; Schuldiner M; Nunnari J; Weissman JS; Walter P An ER-Mitochondria Tethering Complex Revealed by a Synthetic Biology Screen. Science 2009, 325 (5939), 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (247).Jin X; Riedel-Kruse IH Biofilm Lithography Enables High-Resolution Cell Patterning via Optogenetic Adhesin Expression. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (248).Chen F; Wegner SV Blue Light Switchable Bacterial Adhesion as a Key Step toward the Design of Biofilms. ACS Synth. Biol 2017, 6, 2170–2174. [DOI] [PubMed] [Google Scholar]
- (249).Alberts B; Johnson A; Lewis J; Morgan D; Raff M; Roberts K; Walter P Molecular Biology of the Cell 6th Ed.; Garland Science, 2014. [Google Scholar]
- (250).Hodge RG; Ridley AJ Regulating Rho GTPases and Their Regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [DOI] [PubMed] [Google Scholar]
- (251).Hanna S; El-Sibai M Signaling Networks of Rho GTPases in Cell Motility. Cell. Signal. 2013, 25, 1955–1961. [DOI] [PubMed] [Google Scholar]
- (252).Yoo SK; Deng Q; Cavnar PJ; Wu YI; Hahn KM; Huttenlocher A; Takuwa Y; Sugimoto N; Mitchison T; Bourne HR; et al. Differential Regulation of Protrusion and Polarity by PI3K during Neutrophil Motility in Live Zebrafish. Dev. Cell 2010, 18, 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Hayashi-Takagi A; Yagishita S; Nakamura M; Shirai F; Wu YI; Loshbaugh AL; Kuhlman B; Hahn KM; Kasai H Labelling and Optical Erasure of Synaptic Memory Traces in the Motor Cortex. Nature 2015, 525, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Furuya A; Kawano F; Nakajima T; Ueda Y; Sato M Assembly Domain-Based Optogenetic System for the Efficient Control of Cellular Signaling. ACS Synth. Biol. 2017, 6, 1086–1095. [DOI] [PubMed] [Google Scholar]
- (255).Binz HK; Amstutz P; Pluckthun A Engineering Novel Binding Proteins from Nonimmunoglobulin Domains. Nat. Biotechnol. 2005, 23, 1257–1268. [DOI] [PubMed] [Google Scholar]
- (256).ONeill PR; Kalyanaraman V; Gautam N Subcellular Optogenetic Activation of Cdc42 Controls Local and Distal Signaling to Drive Immune Cell Migration. Mol. Biol. Cell 2016, 27, 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (257).Zimmerman SP; Asokan SB; Kuhlman B; Bear JE Cells Lay Their Own Tracks – Optogenetic Cdc42 Activation Stimulates Fibronectin Deposition Supporting Directed Migration. J Cell Sci 2017, 130, 2971–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Strickland D; Lin Y; Wagner E; Hope CM; Zayner J; Antoniou C; Sosnick TR; Weiss EL; Glotzer M TULIPs: Tunable, Light-Controlled Interacting Protein Tags for Cell Biology. Nat. Methods 2012, 9, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (259).Witte K; Strickland D; Glotzer M Cell Cycle Entry Triggers a Switch between Two Modes of Cdc42 Activation during Yeast Polarization. Elife 2017, 6, e26722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (260).French AR; Sosnick TR; Rock RS Investigations of Human Myosin VI Targeting Using Optogenetically Controlled Cargo Loading. Proc. Natl. Acad. Sci. 2017, 114, E1607–E1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).van Bergeijk P; Adrian M; Hoogenraad CC; Kapitein LC Optogenetic Control of Organelle Transport and Positioning. Nature 2015, 518, 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).van Haren J; Ettinger A; Wang H; Hahn K; Wittmann T Local Control of Intracellular Microtubule Dynamics by End Binding Protein 1 (EB1) Photo-Dissociation. Nat. Cell Biol. 2018, 20, 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (263).Duan L; Che D; Zhang K; Ong Q; Guo S; Cui B Optogenetic Control of Molecular Motors and Organelle Distributions in Cells. Chem. Biol. 2015, 22, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (264).Wood LA; Larocque G; Clarke NI; Sarkar S; Royle SJ New Tools for “hot-Wiring” Clathrin-Mediated Endocytosis with Temporal and Spatial Precision. J Cell Biol 2017, 216, 4351–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (265).Nguyen MK; Kim CY; Kim JM; Park BO; Lee S; Park H; Heo W. Do. Optogenetic Oligomerization of Rab GTPases Regulates Intracellular Membrane Trafficking. Nat. Chem. Biol. 2016, 12, 431–436. [DOI] [PubMed] [Google Scholar]
- (266).Chen D; Gibson ES; Kennedy MJ A Light-Triggered Protein Secretion System. J. Cell Biol. 2013, 201, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Ji C; Fan F; Lou X Vesicle Docking Is a Key Target of Local PI(4,5)P2 Metabolism in the Secretory Pathway of INS-1 Cells. Cell Rep. 2017, 20, 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (268).Komander D; Rape M The Ubiquitin Code. Annu. Rev. Biochem. 2012, 81, 203–229. [DOI] [PubMed] [Google Scholar]
- (269).Renicke C; Schuster D; Usherenko S; Essen L-O; Taxis C A LOV2 Domain-Based Optogenetic Tool to Control Protein Degradation and Cellular Function. Chem. Biol. 2013, 20, 619–626. [DOI] [PubMed] [Google Scholar]
- (270).Bonger KM; Rakhit R; Payumo AY; Chen JK; Wandless TJ General Method for Regulating Protein Stability with Light. ACS Chem. Biol. 2014, 9, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (271).Usherenko S; Stibbe H; Muscó M; Essen L-O; Kostina EA; Taxis C Photo-Sensitive Degron Variants for Tuning Protein Stability by Light. BMC Syst. Biol. 2014, 8, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Müller K; Zurbriggen MD; Weber W An Optogenetic Upgrade for the Tet-OFF System. Biotechnol. Bioeng. 2015, 112, 1483–1487. [DOI] [PubMed] [Google Scholar]
- (273).Sun W; Zhang W; Zhang C; Mao M; Zhao Y; Chen X; Yang Y Light-Induced Protein Degradation in Human-Derived Cells. Biochem. Biophys. Res. Commun. 2017, 487, 241–246. [DOI] [PubMed] [Google Scholar]
- (274).Lee D; Hyun JH; Jung K; Hannan P; Kwon H-B A Calcium- and Light-Gated Switch to Induce Gene Expression in Activated Neurons. Nat. Biotechnol. 2017, 35, 858–863. [DOI] [PubMed] [Google Scholar]
- (275).Wang W; Wildes CP; Pattarabanjird T; Sanchez MI; Glober GF; Matthews GA; Tye KM; Ting AY A Light- and Calcium-Gated Transcription Factor for Imaging and Manipulating Activated Neurons. Nat. Biotechnol. 2017, 35, 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Lee D; Creed M; Jung K; Stefanelli T; Wendler DJ; Oh WC; Mignocchi NL; Lüscher C; Kwon H-B Temporally Precise Labeling and Control of Neuromodulatory Circuits in the Mammalian Brain. Nat. Methods 2017, 14, 495–503. [DOI] [PubMed] [Google Scholar]
- (277).Mills E; Chen X; Pham E; Wong S; Truong K Engineering a Photoactivated Caspase-7 for Rapid Induction of Apoptosis. ACS Synth. Biol. 2012, 1, 75–82. [DOI] [PubMed] [Google Scholar]
- (278).Krauss G Biochemistry of Signal Transduction and Regulation.; Wiley-VCH, 2014. [Google Scholar]
- (279).Schröder-Lang S; Schwärzel M; Seifert R; Strünker T; Kateriya S; Looser J; Watanabe M; Kaupp UB; Hegemann P; Nagel G Fast Manipulation of Cellular cAMP Level by Light in Vivo. Nat. Methods 2007, 4, 39–42. [DOI] [PubMed] [Google Scholar]
- (280).Raffelberg S; Wang L; Gao S; Losi A; Gärtner W; Nagel G A LOV-Domain-Mediated Blue-Light-Activated Adenylate (Adenylyl) Cyclase from the Cyanobacterium Microcoleus Chthonoplastes PCC 7420. Biochem. J 2013, 455, 359–365. [DOI] [PubMed] [Google Scholar]
- (281).Gasser C; Taiber S; Yeh C-M; Wittig CH; Hegemann P; Ryu S; Wunder F; Möglich A Engineering of a Red-Light-Activated Human cAMP/cGMP-Specific Phosphodiesterase. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 8803–8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Ryu M-H; Gomelsky M Near-Infrared Light Responsive Synthetic c-di-GMP Module for Optogenetic Applications. ACS Synth. Biol 2014, 3, 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Folcher M; Oesterle S; Zwicky K; Thekkottil T; Heymoz J; Hohmann M; Christen M; Daoud El-Baba M; Buchmann P; Fussenegger M Mind-Controlled Transgene Expression by a Wireless-Powered Optogenetic Designer Cell Implant. Nat. Commun. 2014, 5, 5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Cao Z; Livoti E; Losi A; Gärtner W A Blue Light-Inducible Phosphodiesterase Activity in the Cyanobacterium Synechococcus Elongatus. Photochem. Photobiol. 2010, 86, 606–611. [DOI] [PubMed] [Google Scholar]
- (285).Ryu M-H; Fomicheva A; Moskvin OV; Gomelsky M Optogenetic Module for Dichromatic Control of c-di-GMP Signaling. J. Bacteriol. 2017, 199, e00014–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (286).Kim T; Folcher M; Baba MD-E; Fussenegger M A Synthetic Erectile Optogenetic Stimulator Enabling Blue-Light-Inducible Penile Erection. Angew. Chemie Int. Ed. 2015, 54, 5933–5938. [DOI] [PubMed] [Google Scholar]
- (287).Lindner R; Hartmann E; Tarnawski M; Winkler A; Frey D; Reinstein J; Meinhart A; Schlichting I Photoactivation Mechanism of a Bacterial Light-Regulated Adenylyl Cyclase. J. Mol. Biol. 2017, 429, 1336–1351. [DOI] [PubMed] [Google Scholar]
- (288).Ohki M; Sugiyama K; Kawai F; Tanaka H; Nihei Y; Unzai S; Takebe M; Matsunaga S; Adachi S; Shibayama N; et al. Structural Insight into Photoactivation of an Adenylate Cyclase from a Photosynthetic Cyanobacterium. Proc. Natl. Acad. Sci. 2016, 113, 6659–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Ohki M; Sato-Tomita A; Matsunaga S; Iseki M; Tame JRH; Shibayama N; Park S-Y Molecular Mechanism of Photoactivation of a Light-Regulated Adenylate Cyclase. Proc. Natl. Acad. Sci. 2017, 114, 8562–8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (290).Chen Z; Raffelberg S; Losi A; Schaap P; Gärtner W A Cyanobacterial Light Activated Adenylyl Cyclase Partially Restores Development of a Dictyostelium Discoideum, Adenylyl Cyclase a Null Mutant. J. Biotechnol. 2014, 191, 246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (291).Avelar GM; Schumacher RI; Zaini PA; Leonard G; Richards TA; Gomes SL A Rhodopsin-Guanylyl Cyclase Gene Fusion Functions in Visual Perception in a Fungus. Curr. Biol. 2014, 24, 1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Ryu M-H; Kang I-H; Nelson MD; Jensen TM; Lyuksyutova AI; Siltberg-Liberles J; Raizen DM; Gomelsky M Engineering Adenylate Cyclases Regulated by near-Infrared Window Light. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 10167–10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Etzl S; Lindner R; Nelson MD; Winkler A Structure-Guided Design and Functional Characterization of an Artificial Red Light-Regulated Guanylate/adenylate Cyclase for Optogenetic Applications. J. Biol. Chem. 2018, 293, 9078–9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (294).Blain-Hartung M; Rockwell NC; Moreno MV; Martin SS; Gan F; Bryant DA; Lagarias JC Cyanobacteriochrome-Based Photoswitchable Adenylyl Cyclases (cPACs) for Broad Spectrum Light Regulation of cAMP Levels in Cells. J. Biol. Chem. 2018, 293, 8473–8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (295).Yoshida K; Tsunoda SP; Brown LS; Kandori H A Unique Choanoflagellate Enzyme Rhodopsin Exhibits Light-Dependent Cyclic Nucleotide Phosphodiesterase Activity. J. Biol. Chem. 2017, 292, 7531–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (296).Mukherjee S; Jansen V; Jikeli JF; Hamzeh H; Alvarez L; Dombrowski M; Balbach M; Strünker T; Seifert R; Kaupp UB; et al. A Novel Biosensor to Study cAMP Dynamics in Cilia and Flagella. Elife 2016, 5, e14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (297).O’Banion CP; Priestman MA; Hughes RM; Herring LE; Capuzzi SJ; Lawrence DS Design and Profiling of a Subcellular Targeted Optogenetic cAMP-Dependent Protein Kinase. Cell Chem. Biol. 2018, 25, 100–109.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (298).Gomelsky M cAMP, c-di-GMP, c-di-AMP and Now cGMP: Bacteria Use Them All! Mol. Microbiol. 2011, 79, 562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (299).Jenal U; Reinders A; Lori C Cyclic di-GMP: Second Messenger Extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [DOI] [PubMed] [Google Scholar]
- (300).Pu L; Yang S; Xia A; Jin F Optogenetics Manipulation Enables Prevention of Biofilm Formation of Engineered Pseudomonas Aeruginosa on Surfaces. ACS Synth. Biol. 2018, 7, 200–208. [DOI] [PubMed] [Google Scholar]
- (301).Burdette DL; Vance RE STING and the Innate Immune Response to Nucleic Acids in the Cytosol. Nat. Immunol. 2012, 14, 19–26. [DOI] [PubMed] [Google Scholar]
- (302).Sun L; Wu J; Du F; Chen X; Chen ZJ Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).Clapham DE; Yeromin AV; Zhang XH; Yu Y; Safrina O; Penna A; Roos J; Stauderman KA; Cahalan MD; Takemori H; et al. Calcium Signaling. Cell 2007, 131, 1047–1058.18083096 [Google Scholar]
- (304).Pham E; Mills E; Truong K A Synthetic Photoactivated Protein to Generate Local or Global Ca(2+) Signals. Chem. Biol. 2011, 18, 880–890. [DOI] [PubMed] [Google Scholar]
- (305).Ishii T; Sato K; Kakumoto T; Miura S; Touhara K; Takeuchi S; Nakata T Light Generation of Intracellular Ca(2+) Signals by a Genetically Encoded Protein BACCS. Nat. Commun. 2015, 6, 8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (306).Nguyen NT; He L; Martinez-Moczygemba M; Huang Y; Zhou Y Rewiring Calcium Signaling for Precise Transcriptional Reprogramming. ACS Synth. Biol. 2018, 7, 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (307).Kyung T; Lee S; Kim JE; Cho T; Park H; Jeong Y-M; Kim D; Shin A; Kim S; Baek J; et al. Optogenetic Control of Endogenous Ca(2+) Channels in Vivo. Nat. Biotechnol. 2015, 33, 1092–1096. [DOI] [PubMed] [Google Scholar]
- (308).Ma G; Liu J; Ke Y; Liu X; Li M; Wang F; Han G; Huang Y; Wang Y; Zhou Y Optogenetic Control of Voltage-Gated Calcium Channels. Angew. Chem. Int. Ed. Engl. 2018, 57, 7019–7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (309).Fukuda N; Matsuda T; Nagai T Optical Control of the Ca2+ Concentration in a Live Specimen with a Genetically Encoded Ca2+ -Releasing Molecular Tool. ACS Chem. Biol. 2014, 9, 1197–1203. [DOI] [PubMed] [Google Scholar]
- (310).Aper SJA; Merkx M Rewiring Multidomain Protein Switches: Transforming a Fluorescent Zn2+ Sensor into a Light-Responsive Zn2+ Binding Protein. ACS Synth. Biol 2016, 5, 698–709. [DOI] [PubMed] [Google Scholar]
- (311).Chang K-Y; Woo D; Jung H; Lee S; Kim S; Won J; Kyung T; Park H; Kim N; Yang HW; et al. Light-Inducible Receptor Tyrosine Kinases That Regulate Neurotrophin Signalling. Nat. Commun. 2014, 5, 4057. [DOI] [PubMed] [Google Scholar]
- (312).Kim N; Kim JM; Lee M; Kim CY; Chang K-Y; Heo W.Do. Spatiotemporal Control of Fibroblast Growth Factor Receptor Signals by Blue Light. Chem. Biol. 2014, 21, 903–912. [DOI] [PubMed] [Google Scholar]
- (313).Inglés-Prieto Á; Reichhart E; Muellner MK; Nowak M; Nijman SMB; Grusch M; Janovjak H Light-Assisted Small-Molecule Screening against Protein Kinases. Nat. Chem. Biol. 2015, 11, 952–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (314).Agus V; Janovjak H Optogenetic Methods in Drug Screening: Technologies and Applications. Curr. Opin. Biotechnol. 2017, 48, 8–14. [DOI] [PubMed] [Google Scholar]
- (315).Bugaj LJ; Spelke DP; Mesuda CK; Varedi M; Kane RS; Schaffer DV Regulation of Endogenous Transmembrane Receptors through Optogenetic Cry2 Clustering. Nat. Commun. 2015, 6, 6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (316).Aoki K; Kumagai Y; Sakurai A; Komatsu N; Fujita Y; Shionyu C; Matsuda M Stochastic ERK Activation Induced by Noise and Cell-to-Cell Propagation Regulates Cell Density-Dependent Proliferation. Mol. Cell 2013, 52, 529–540. [DOI] [PubMed] [Google Scholar]
- (317).Zhang K; Duan L; Ong Q; Lin Z; Varman PM; Sung K; Cui B Light-Mediated Kinetic Control Reveals the Temporal Effect of the Raf/MEK/ERK Pathway in PC12 Cell Neurite Outgrowth. PLoS One 2014, 9, e92917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Krishnamurthy VV; Khamo JS; Mei W; Turgeon AJ; Ashraf HM; Mondal P; Patel DB; Risner N; Cho EE; Yang J; et al. Reversible Optogenetic Control of Kinase Activity during Differentiation and Embryonic Development. Development 2016, 143, 4085–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (319).Wend S; Wagner HJ; Müller K; Zurbriggen MD; Weber W; Radziwill G Optogenetic Control of Protein Kinase Activity in Mammalian Cells. ACS Synth. Biol. 2014, 3, 280–285. [DOI] [PubMed] [Google Scholar]
- (320).Chatelle CV; Hövermann D; Müller A; Wagner HJ; Weber W; Radziwill G Optogenetically Controlled RAF to Characterize BRAF and CRAF Protein Kinase Inhibitors. Sci. Rep. 2016, 6, 23713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (321).Melero-Fernandez de Mera RM; Li L-L; Popinigis A; Cisek K; Tuittila M; Yadav L; Serva A; Courtney MJ A Simple Optogenetic MAPK Inhibitor Design Reveals Resonance between Transcription-Regulating Circuitry and Temporally-Encoded Inputs. Nat. Commun. 2017, 8, 15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (322).Di Paolo G; De Camilli P Phosphoinositides in Cell Regulation and Membrane Dynamics. Nature 2006, 443, 651–657. [DOI] [PubMed] [Google Scholar]
- (323).Idevall-Hagren O; Dickson EJ; Hille B; Toomre DK; De Camilli P Optogenetic Control of Phosphoinositide Metabolism. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, E2316–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Kakumoto T; Nakata T Optogenetic Control of PIP3: PIP3 Is Sufficient to Induce the Actin-Based Active Part of Growth Cones and Is Regulated via Endocytosis. PLoS One 2013, 8, e70861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Xu Y; Nan D; Fan J; Bogan JS; Toomre D Optogenetic Activation Reveals Distinct Roles of PIP3 and Akt in Adipocyte Insulin Action. J. Cell Sci. 2016, 129, 2085–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (326).Katsura Y; Kubota H; Kunida K; Kanno A; Kuroda S; Ozawa T An Optogenetic System for Interrogating the Temporal Dynamics of Akt. Sci. Rep. 2015, 5, 14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Mühlhäuser WWD; Hörner M; Weber W; Radziwill G Light-Regulated Protein Kinases Based on the CRY2-CIB1 System. In Methods in molecular biology (Clifton, N.J.); 2017; Vol. 1596, pp 257–270. [DOI] [PubMed] [Google Scholar]
- (328).Kaur P; Saunders TE; Tolwinski NS Coupling Optogenetics and Light-Sheet Microscopy, a Method to Study Wnt Signaling during Embryogenesis. Sci. Rep 2017, 7, 16636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (329).Liao Z; Kasirer-Friede A; Shattil SJ Optogenetic Interrogation of Integrin αVβ3 Function in Endothelial Cells. J Cell Sci. 2017, 130, 3532–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Hörner M; Chatelle C; Mühlhäuser WWD; Stocker DR; Coats M; Weber W; Radziwill G Optogenetic Control of Focal Adhesion Kinase Signaling. Cell. Signal. 2018, 42, 176–183. [DOI] [PubMed] [Google Scholar]
- (331).Locke C; Machida K; Wu Y; Yu J Optogenetic Activation of EphB2 Receptor in Dendrites Induced Actin Polymerization by Activating Arg Kinase. Biol. Open 2017, 6, 1820–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (332).Li Y; Lee M; Kim N; Wu G; Deng D; Kim JM; Liu X; Heo W. Do.; Zi Z Spatiotemporal Control of TGF-β Signaling with Light. ACS Synth. Biol. 2018, 7, 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (333).Broz P; Monack DM Newly Described Pattern Recognition Receptors Team up against Intracellular Pathogens. Nat. Rev. Immunol. 2013, 13, 551–565. [DOI] [PubMed] [Google Scholar]
- (334).Moser BA; Esser-Kahn AP A Photoactivatable Innate Immune Receptor for Optogenetic Inflammation. ACS Chem. Biol. 2017, 12, 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Taylor RC; Cullen SP; Martin SJ Apoptosis: Controlled Demolition at the Cellular Level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [DOI] [PubMed] [Google Scholar]
- (336).Hughes RM; Freeman DJ; Lamb KN; Pollet RM; Smith WJ; Lawrence DS Optogenetic Apoptosis: Light-Triggered Cell Death. Angew. Chemie Int. Ed. 2015, 54, 12064–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (337).Smart AD; Pache RA; Thomsen ND; Kortemme T; Davis GW; Wells JA Engineering a Light-Activated Caspase-3 for Precise Ablation of Neurons in Vivo. Proc. Natl. Acad. Sci. 2017, 114, E8174–E8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (338).Lee J; Natarajan M; Nashine VC; Socolich M; Vo T; Russ WP; Benkovic SJ; Ranganathan R Surface Sites for Engineering Allosteric Control in Proteins. Science 2008, 322, 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (339).Gehrig S; Macpherson JA; Driscoll PC; Symon A; Martin SR; MacRae JI; Kleinjung J; Fraternali F; Anastasiou D An Engineered Photoswitchable Mammalian Pyruvate Kinase. FEBS J. 2017, 284, 2955–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Sancar A Mechanisms of DNA Repair by Photolyase and Excision Nuclease (Nobel Lecture). Angew. Chemie Int. Ed 2016, 55, 8502–8527. [DOI] [PubMed] [Google Scholar]
- (341).Hollmann F; Taglieber A; Schulz F; Reetz MT A Light-Driven Stereoselective Biocatalytic Oxidation. Angew. Chemie Int. Ed. 2007, 46, 2903–2906. [DOI] [PubMed] [Google Scholar]
- (342).Taglieber A; Schulz F; Hollmann F; Rusek M; Reetz MT Light-Driven Biocatalytic Oxidation and Reduction Reactions: Scope and Limitations. Chembiochem A Eur. J. Chem. Biol. 2008, 9, 565–572. [DOI] [PubMed] [Google Scholar]
- (343).Wietek J; Wiegert JS; Adeishvili N; Schneider F; Watanabe H; Tsunoda SP; Vogt A; Elstner M; Oertner TG; Hegemann P Conversion of Channelrhodopsin into a Light-Gated Chloride Channel. Science 2014, 344, 409–412. [DOI] [PubMed] [Google Scholar]
- (344).Berndt A; Lee SY; Ramakrishnan C; Deisseroth K Structure-Guided Transformation of Channelrhodopsin into a Light-Activated Chloride Channel. Science 2014, 344, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (345).Govorunova EG; Sineshchekov OA; Janz R; Liu X; Spudich JL Natural Light-Gated Anion Channels: A Family of Microbial Rhodopsins for Advanced Optogenetics. Science 2015, 349, 647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (346).Schmidt D; Tillberg PW; Chen F; Boyden ES A Fully Genetically Encoded Protein Architecture for Optical Control of Peptide Ligand Concentration. Nat. Commun. 2014, 5, 3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (347).Cosentino C; Alberio L; Gazzarrini S; Aquila M; Romano E; Cermenati S; Zuccolini P; Petersen J; Beltrame M; Van Etten JL; et al. Optogenetics. Engineering of a Light-Gated Potassium Channel. Science 2015, 348, 707–710. [DOI] [PubMed] [Google Scholar]
- (348).Dixon RE; Yuan C; Cheng EP; Navedo MF; Santana LF Ca2+ Signaling Amplification by Oligomerization of L-Type Cav1.2 Channels. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 1749–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Sinnen BL; Bowen AB; Forte JS; Hiester BG; Crosby KC; Gibson ES; Dell’Acqua ML; Kennedy MJ Optogenetic Control of Synaptic Composition and Function. Neuron 2017, 93, 646–660.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (350).Sanford L; Palmer A Recent Advances in Development of Genetically Encoded Fluorescent Sensors. Methods Enzym. 2017, 589, 1–49. [DOI] [PubMed] [Google Scholar]
- (351).Thorn K Genetically Encoded Fluorescent Tags. Mol. Biol. Cell 2017, 28, 848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (352).Buckley AM; Petersen J; Roe AJ; Douce GR; Christie JM LOV-Based Reporters for Fluorescence Imaging. Curr. Opin. Chem. Biol. 2015, 27, 39–45. [DOI] [PubMed] [Google Scholar]
- (353).Rodriguez EA; Campbell RE; Lin JY; Lin MZ; Miyawaki A; Palmer AE; Shu X; Zhang J; Tsien RY The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins. Trends Biochem. Sci. 2017, 42, 111–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (354).Souslova EA; Mironova KE; Deyev SM Applications of Genetically Encoded Photosensitizer miniSOG: From Correlative Light Electron Microscopy to Immunophotosensitizing. J. Biophotonics 2017, 10, 338–352. [DOI] [PubMed] [Google Scholar]
- (355).Jiang HN; Li Y; Cui ZJ Photodynamic Physiology-Photonanomanipulations in Cellular Physiology with Protein Photosensitizers. Front. Physiol. 2017, 8, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (356).Drepper T; Gensch T; Pohl M; T. W. Gadella J; Coppey-Moisan M; Bertrand E; Biskup C; Michiels J; Vanderleyden J; Heberle J; et al. Advanced in Vivo Applications of Blue Light Photoreceptors as Alternative Fluorescent Proteins. Photochem. Photobiol. Sci. 2013, 12, 1125–1134. [DOI] [PubMed] [Google Scholar]
- (357).Wingen M; Potzkei J; Endres S; Casini G; Rupprecht C; Fahlke C; Krauss U; Jaeger K-E; Drepper T; Gensch T; et al. The Photophysics of LOV-Based Fluorescent Proteins – New Tools for Cell Biology. Photochem. Photobiol. Sci. 2014, 13, 875–883. [DOI] [PubMed] [Google Scholar]
- (358).Mukherjee A; Schroeder CM Flavin-Based Fluorescent Proteins: Emerging Paradigms in Biological Imaging. Curr. Opin. Biotechnol. 2015, 31, 16–23. [DOI] [PubMed] [Google Scholar]
- (359).Drepper T; Eggert T; Circolone F; Heck A; Krauß U; Guterl J-K; Wendorff M; Losi A; Gärtner W; Jaeger K-E Reporter Proteins for in Vivo Fluorescence without Oxygen. Nat. Biotechnol. 2007, 25, 443–445. [DOI] [PubMed] [Google Scholar]
- (360).Wingen M; Jaeger K-E; Gensch T; Drepper T Novel Thermostable Flavin-Binding Fluorescent Proteins from Thermophilic Organisms. Photochem. Photobiol. 2017, 93, 849–856. [DOI] [PubMed] [Google Scholar]
- (361).Mukherjee A; Weyant KB; Agrawal U; Walker J; Cann IKO; Schroeder CM Engineering and Characterization of New LOV-Based Fluorescent Proteins from Chlamydomonas Reinhardtii and Vaucheria Frigida. ACS Synth. Biol. 2015, 4, 371–377. [DOI] [PubMed] [Google Scholar]
- (362).Chapman S; Faulkner C; Kaiserli E; Garcia-Mata C; Savenkov EI; Roberts AG; Oparka KJ; Christie JM The Photoreversible Fluorescent Protein iLOV Outperforms GFP as a Reporter of Plant Virus Infection. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 20038–20043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (363).Seago J; Juleff N; Moffat K; Berryman S; Christie JM; Charleston B; Jackson T An Infectious Recombinant Foot-and-Mouth Disease Virus Expressing a Fluorescent Marker Protein. J. Gen. Virol. 2013, 94 (Pt 7), 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Christie JM; Gawthorne J; Young G; Fraser NJ; Roe AJ LOV to BLUF: Flavoprotein Contributions to the Optogenetic Toolkit. Mol. Plant 2012, 5, 533–544. [DOI] [PubMed] [Google Scholar]
- (365).Christie JM; Hitomi K; Arvai AS; Hartfield KA; Mettlen M; Pratt AJ; Tainer JA; Getzoff ED Structural Tuning of the Fluorescent Protein iLOV for Improved Photostability. J. Biol. Chem. 2012, 287, 22295–22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (366).Mukherjee A; Walker J; Weyant KB; Schroeder CM; Stahl U Characterization of Flavin-Based Fluorescent Proteins: An Emerging Class of Fluorescent Reporters. PLoS One 2013, 8, e64753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (367).Khrenova MG; Nemukhin AV; Domratcheva T Theoretical Characterization of the Flavin-Based Fluorescent Protein iLOV and Its Q489K Mutant. J. Phys. Chem. B 2015, 119, 5176–5183. [DOI] [PubMed] [Google Scholar]
- (368).Landete JM; Peirotén Á; Medina M; Arques JL Short Communication: Labeling Listeria with Anaerobic Fluorescent Protein for Food Safety Studies. J. Dairy Sci. 2017, 100, 113–117. [DOI] [PubMed] [Google Scholar]
- (369).Choi CH; DeGuzman JV; Lamont RJ; Yilmaz Ö; Berche P Genetic Transformation of an Obligate Anaerobe, P. Gingivalis for FMN-Green Fluorescent Protein Expression in Studying Host-Microbe Interaction. PLoS One 2011, 6, e18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (370).Eichhof I; Ernst JF Oxygen-Independent FbFP: Fluorescent Sentinel and Oxygen Sensor Component in Saccharomyces Cerevisiae and Candida Albicans. Fungal Genet. Biol. 2016, 92, 14–25. [DOI] [PubMed] [Google Scholar]
- (371).Immethun CM; DeLorenzo DM; Focht CM; Gupta D; Johnson CB; Moon TS Physical, Chemical, and Metabolic State Sensors Expand the Synthetic Biology Toolbox for Synechocystis Sp. PCC 6803. Biotechnol. Bioeng. 2017, 114, 1561–1569. [DOI] [PubMed] [Google Scholar]
- (372).Wang SE; Brooks AES; Cann B; Simoes-Barbosa A The Fluorescent Protein iLOV Outperforms eGFP as a Reporter Gene in the Microaerophilic Protozoan Trichomonas Vaginalis. Mol. Biochem. Parasitol. 2017, 216, 1–4. [DOI] [PubMed] [Google Scholar]
- (373).Buckley AM; Jukes C; Candlish D; Irvine JJ; Spencer J; Fagan RP; Roe AJ; Christie JM; Fairweather NF; Douce GR Lighting Up Clostridium Difficile: Reporting Gene Expression Using Fluorescent Lov Domains. Sci. Rep. 2016, 6, 23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (374).Elgamoudi BA; Ketley JM Lighting up My Life: A LOV-Based Fluorescent Reporter for Campylobacter Jejuni. Res. Microbiol. 2018, 169, 108–114. [DOI] [PubMed] [Google Scholar]
- (375).Ravikumar Y; Nadarajan SP; Lee C-S; Jung S; Bae D-H; Yun H FMN-Based Fluorescent Proteins as Heavy Metal Sensors Against Mercury Ions. J. Microbiol. Biotechnol. 2016, 26, 530–539. [DOI] [PubMed] [Google Scholar]
- (376).Ravikumar Y; Nadarajan SP; Lee C; Yun H Engineering an FMN-Based iLOV Protein for the Detection of Arsenic Ions. Anal. Biochem. 2017, 525, 38–43. [DOI] [PubMed] [Google Scholar]
- (377).Chang X-P; Gao Y-J; Fang W-H; Cui G; Thiel W Quantum Mechanics/Molecular Mechanics Study on the Photoreactions of Dark- and Light-Adapted States of a Blue-Light YtvA LOV Photoreceptor. Angew. Chemie Int. Ed 2017, 56, 9341–9345. [DOI] [PubMed] [Google Scholar]
- (378).Losi A; Gärtner W; Raffelberg S; Cella Zanacchi F; Bianchini P; Diaspro A; Mandalari C; Abbruzzetti S; Viappiani C; Schwarzel M; et al. A Photochromic Bacterial Photoreceptor with Potential for Super-Resolution Microscopy. Photochem. Photobiol. Sci. 2013, 12, 231–235. [DOI] [PubMed] [Google Scholar]
- (379).Gregor C; Sidenstein SC; Andresen M; Sahl SJ; Danzl JG; Hell SW Novel Reversibly Switchable Fluorescent Proteins for RESOLFT and STED Nanoscopy Engineered from the Bacterial Photoreceptor YtvA. Sci. Rep. 2018, 8, 2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (380).van der Steen JB; Hellingwerf KJ Activation of the General Stress Response of Bacillus Subtilis by Visible Light. Photochem. Photobiol. 2015, 91, 1032–1045. [DOI] [PubMed] [Google Scholar]
- (381).Potzkei J; Kunze M; Drepper T; Gensch T; Jaeger K-E; Buechs J Real-Time Determination of Intracellular Oxygen in Bacteria Using a Genetically Encoded FRET-Based Biosensor. BMC Biol. 2012, 10, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).Rupprecht C; Wingen M; Potzkei J; Gensch T; Jaeger K-E; Drepper T A Novel FbFP-Based Biosensor Toolbox for Sensitive in Vivo Determination of Intracellular pH. J. Biotechnol. 2017, 258, 25–35. [DOI] [PubMed] [Google Scholar]
- (383).Simon J; Losi A; Zhao K-H; Gärtner W FRET in a Synthetic Flavin- and Bilin-Binding Protein. Photochem. Photobiol. 2017, 93, 1057–1062. [DOI] [PubMed] [Google Scholar]
- (384).Baier J; Maisch T; Maier M; Engel E; Landthaler M; Bäumler W Singlet Oxygen Generation by UVA Light Exposure of Endogenous Photosensitizers. Biophys. J. 2006, 91, 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (385).Bourdeaux F; Hammer CA; Vogt S; Schweighöfer F; Nöll G; Wachtveitl J; Grininger M Flavin Storage and Sequestration by Mycobacterium Tuberculosis Dodecin. ACS Infect. Dis. 2018, in press, 10.1021/acsinfecdis.7b00237. [DOI] [PubMed] [Google Scholar]
- (386).Scheurer M; Brisker-Klaiman D; Dreuw A Molecular Mechanism of Flavin Photoprotection by Archaeal Dodecin: Photoinduced Electron Transfer and Mg2+ -Promoted Proton Transfer. J. Phys. Chem. B 2017, 121, 10457–10466. [DOI] [PubMed] [Google Scholar]
- (387).Baptista MS; Cadet J; Di Mascio P; Ghogare AA; Greer A; Hamblin MR; Lorente C; Nunez SC; Ribeiro MS; Thomas AH; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (388).Brunk GR; Martin KA; Nishimura AM A Study of Solvent Effects on the Phosphorescence Properties of Flavins. Biophys. J. 1976, 16, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (389).Bregnhøj M; Blázquez-Castro A; Westberg M; Breitenbach T; Ogilby PR Direct 765 nm Optical Excitation of Molecular Oxygen in Solution and in Single Mammalian Cells. J. Phys. Chem. B 2015, 119, 5422–5429. [DOI] [PubMed] [Google Scholar]
- (390).Westberg M; Bregnhøj M; Etzerodt M; Ogilby PR Temperature Sensitive Singlet Oxygen Photosensitization by LOV-Derived Fluorescent Flavoproteins. J. Phys. Chem. B 2017, 121, 2561–2574. [DOI] [PubMed] [Google Scholar]
- (391).Shu X; Lev-Ram V; Deerinck TJ; Qi Y; Ramko EB; Davidson MW; Jin Y; Ellisman MH; Tsien RY A Genetically Encoded Tag for Correlated Light and Electron Microscopy of Intact Cells, Tissues, and Organisms. PLoS Biol. 2011, 9, e1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (392).Kottke T; Heberle J; Hehn D; Dick B; Hegemann P Phot-LOV1: Photocycle of a Blue-Light Receptor Domain from the Green Alga Chlamydomonas Reinhardtii. Biophys. J. 2003, 84, 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (393).Caplan J; Niethammer M; Taylor RM; Czymmek KJ The Power of Correlative Microscopy: Multi-Modal, Multi-Scale, Multi-Dimensional. Curr. Opin. Struct. Biol. 2011, 21, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Hauser M; Wojcik M; Kim D; Mahmoudi M; Li W; Xu K Correlative Super-Resolution Microscopy: New Dimensions and New Opportunities. Chem. Rev. 2017, 117, 7428–7456. [DOI] [PubMed] [Google Scholar]
- (395).Hirabayashi Y; Tapia JC; Polleux F Correlated Light-Serial Scanning Electron Microscopy (CoLSSEM) for Ultrastructural Visualization of Single Neurons in Vivo. bioRxiv 2017, 10.1101/148585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (396).Lee D; Huang T-H; De La Cruz A; Callejas A; Lois C Methods to Investigate the Structure and Connectivity of the Nervous System. Fly (Austin). 2017, 11, 224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Follain G; Mercier L; Osmani N; Harlepp S; Goetz JG Seeing Is Believing – Multi-Scale Spatio-Temporal Imaging towards in Vivo Cell Biology. J. Cell Sci. 2017, 130, 23–38. [DOI] [PubMed] [Google Scholar]
- (398).Ellisman MH; Deerinck TJ; Shu X; Sosinsky GE Picking Faces out of a Crowd: Genetic Labels for Identification of Proteins in Correlated Light and Electron Microscopy Imaging. Methods Cell Biol. 2012, 111, 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (399).Rodríguez-Pulido A; Cortajarena AL; Torra J; Ruiz-González R; Nonell S; Flors C Assessing the Potential of Photosensitizing Flavoproteins as Tags for Correlative Microscopy. Chem. Commun. 2016, 52, 8405–8408. [DOI] [PubMed] [Google Scholar]
- (400).Ruiz-González R; Cortajarena AL; Mejias SH; Agut M; Nonell S; Flors C Singlet Oxygen Generation by the Genetically Encoded Tag miniSOG. J. Am. Chem. Soc. 2013, 135, 9564–9567. [DOI] [PubMed] [Google Scholar]
- (401).Pimenta FM; Jensen RL; Breitenbach T; Etzerodt M; Ogilby PR Oxygen-Dependent Photochemistry and Photophysics of “MiniSOG,” a Protein-Encased Flavin. Photochem. Photobiol. 2013, 89, 1116–1126. [DOI] [PubMed] [Google Scholar]
- (402).Westberg M; Holmegaard L; Pimenta FM; Etzerodt M; Ogilby PR Rational Design of an Efficient, Genetically Encodable, Protein-Encased Singlet Oxygen Photosensitizer. J. Am. Chem. Soc. 2015, 137, 1632–1642. [DOI] [PubMed] [Google Scholar]
- (403).Torra J; Burgos-Caminal A; Endres S; Wingen M; Drepper T; Gensch T; Ruiz-González R; Nonell S; Drepper T; Gensch T; et al. Singlet Oxygen Photosensitisation by the Fluorescent Protein Pp2FbFP L30M, a Novel Derivative of Pseudomonas Putida Flavin-Binding Pp2FbFP. Photochem. Photobiol. Sci. 2015, 14, 280–287. [DOI] [PubMed] [Google Scholar]
- (404).Ruiz-González R; Bresolí-Obach R; Gulías Ò; Agut M; Savoie H; Boyle RW; Nonell S; Giuntini F NanoSOSG: A Nanostructured Fluorescent Probe for the Detection of Intracellular Singlet Oxygen. Angew. Chemie Int. Ed 2017, 56, 2885–2888. [DOI] [PubMed] [Google Scholar]
- (405).Xu S; Chisholm AD Highly Efficient Optogenetic Cell Ablation in C. Elegans Using Membrane-Targeted miniSOG. Sci. Rep. 2016, 6, 21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (406).Makhijani K; To T-L; Ruiz-González R; Lafaye C; Royant A; Shu X Precision Optogenetic Tool for Selective Single- and Multiple-Cell Ablation in a Live Animal Model System. Cell Chem. Biol. 2017, 24, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (407).Westberg M; Bregnhøj M; Etzerodt M; Ogilby PR No Photon Wasted: An Efficient and Selective Singlet Oxygen Photosensitizing Protein. J. Phys. Chem. B 2017, 121, 9366–9371. [DOI] [PubMed] [Google Scholar]
- (408).Rodríguez-Pulido A; Torra J; Mejías SH; Cortajarena AL; Ruiz-González R; Nonell S; Flors C Fluorescent Flavoprotein Heterodimers: Combining Photostability with Singlet Oxygen Generation. ChemPhotoChem 2018, in press, 10.1002/cptc.201800002. [DOI] [Google Scholar]
- (409).Serebrovskaya EO; Lukyanov KA Chromophore-Assisted Light Inactivation: A Powerful Tool to Study Protein Functions. In Singlet Oxygen: Applications in Biosciences and Nanosciences , Volume 2; 2016; pp 185–203. [Google Scholar]
- (410).Sano Y; Watanabe W; Matsunaga S Chromophore-Assisted Laser Inactivation – towards a Spatiotemporal–functional Analysis of Proteins, and the Ablation of Chromatin, Organelle and Cell Function. J. Cell Sci. 2014, 127, 1621–1629. [DOI] [PubMed] [Google Scholar]
- (411).Lin JY; Sann SB; Zhou K; Nabavi S; Proulx CD; Malinow R; Jin Y; Tsien RY Optogenetic Inhibition of Synaptic Release with Chromophore-Assisted Light Inactivation (CALI). Neuron 2013, 79, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (412).Hermann A; Liewald JF; Gottschalk A A Photosensitive Degron Enables Acute Light-Induced Protein Degradation in the Nervous System. Current Biology. 2015, pp R749–R750. [DOI] [PubMed] [Google Scholar]
- (413).Zhou K; Stawicki T; Goncharov A; Jin Y Position of UNC-13 in the Active Zone Regulates Synaptic Vesicle Release Probability and Release Kinetics. Elife 2013, 2, e01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (414).Noma K; Jin Y Optogenetic Mutagenesis in Caenorhabditis Elegans. Nat. Commun. 2015, 6, 8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (415).Wojtovich A; Wei A; Sherman T; Foster T Chromophore-Assisted Light Inactivation of Mitochondrial Electron Transport Chain Complex II in Caenorhabditis Elegans. Sci. Rep. 2016, 6, 29695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (416).Sweeney ST; Hidalgo A; de Belle JS; Keshishian H Hydroxyurea Ablation of Mushroom Bodies in Drosophila. Cold Spring Harb. Protoc. 2012, 2012, 231–234. [DOI] [PubMed] [Google Scholar]
- (417).Sweeney ST; Hidalgo A; de Belle JS; Keshishian H Genetic Systems for Functional Cell Ablation in Drosophila. Cold Spring Harb. Protoc. 2012, 2012, 950–956. [DOI] [PubMed] [Google Scholar]
- (418).Sweeney ST; Hidalgo A; de Belle JS; Keshishian H Embryonic Cell Ablation in Drosophila Using Lasers. Cold Spring Harb. Protoc. 2012, 2012, 691–693. [DOI] [PubMed] [Google Scholar]
- (419).Leinwand SG; Chalasani SH Neuropeptide Signaling Remodels Chemosensory Circuit Composition in Caenorhabditis Elegans. Nat. Neurosci. 2013, 16, 1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (420).Fry AL; Laboy JT; Norman KR VAV-1 Acts in a Single Interneuron to Inhibit Motor Circuit Activity in Caenorhabditis Elegans. Nat. Commun. 2014, 5, 5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (421).Qi YB; Garren EJ; Shu X; Tsien RY; Jin Y Photo-Inducible Cell Ablation in Caenorhabditis Elegans Using the Genetically Encoded Singlet Oxygen Generating Protein miniSOG. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 7499–7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (422).Williams DC; El Bejjani R; Ramirez PM; Coakley S; Kim SA; Lee H; Wen Q; Samuel A; Lu H; Hilliard MA; et al. Rapid and Permanent Neuronal Inactivation In Vivo via Subcellular Generation of Reactive Oxygen with the Use of KillerRed. Cell Rep. 2013, 5, 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Gao S; Guan SA; Fouad AD; Meng J; Huang Y-C; Li Y; Alcaire S; Hung W; Kawano T; Lu Y; et al. Excitatory Motor Neurons Are Local Central Pattern Generators in an Anatomically Compressed Motor Circuit for Reverse Locomotion. eLife 2018, 7, e29915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (424).Ryumina AP; Serebrovskaya EO; Shirmanova MV; Snopova LB; Kuznetsova MM; Turchin IV; Ignatova NI; Klementieva NV; Fradkov AF; Shakhov BE; et al. Flavoprotein miniSOG as a Genetically Encoded Photosensitizer for Cancer Cells. Biochim. Biophys. Acta - Gen. Subj 2013, 1830, 5059–5067. [DOI] [PubMed] [Google Scholar]
- (425).Barnett M; Baran T; Foster T; Wojtovich A The Genetically-Encoded Photosensitizer Mini Singlet Oxygen Generator Produces Superoxide. Free Radic. Biol. Med. 2017, 112, 61–62. [Google Scholar]
- (426).Barnett ME; Baran TM; Foster TH; Wojtovich AP Quantification of Light-Induced miniSOG Superoxide Production Using the Selective Marker, 2-Hydroxyethidium. Free Radic. Biol. Med. 2018, 116, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (427).Mironova KE; Proshkina GM; Ryabova AV; Stremovskiy OA; Lukyanov SA; Petrov RV; Deyev SM Genetically Encoded Immunophotosensitizer 4D5scFv-miniSOG Is a Highly Selective Agent for Targeted Photokilling of Tumor Cells in Vitro. Theranostics 2013, 3, 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (428).Proshkina GM; Shilova ON; Ryabova AV; Stremovskiy OA; Deyev SM A New Anticancer Toxin Based on HER2/neu-Specific DARPin and Photoactive Flavoprotein miniSOG. Biochimie 2015, 118, 116–122. [DOI] [PubMed] [Google Scholar]
- (429).Consentino L; Lambert S; Martino C; Jourdan N; Bouchet P-E; Witczak J; Castello P; El-Esawi M; Corbineau F; d’Harlingue A; et al. Blue-light Dependent Reactive Oxygen Species Formation by Arabidopsis Cryptochrome May Define a Novel Evolutionarily Conserved Signaling Mechanism. New Phytol. 2015, 206, 1450–1462. [DOI] [PubMed] [Google Scholar]
- (430).Jourdan N; Martino CF; El-Esawi M; Witczak J; Bouchet P-E; D’Harlingue A; Ahmad M Blue-Light Dependent ROS Formation by Arabidopsis Cryptochrome-2 May Contribute toward Its Signaling Role. Plant Signal. Behav 2015, 10, e1042647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (431).El-Esawi M; Arthaut L-D; Jourdan N; D’Harlingue A; Link J; Martino CF; Ahmad M Blue-Light Induced Biosynthesis of ROS Contributes to the Signaling Mechanism of Arabidopsis Cryptochrome. Sci. Rep. 2017, 7, 13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (432).Massey V The Chemical and Biological Versatility of Riboflavin. Biochem. Soc. Trans. 2000, 28, 283–296. [PubMed] [Google Scholar]
- (433).Mayhew SG The Effects of pH and Semiquinone Formation on the Oxidation-Reduction Potentials of Flavin Mononucleotide. A Reappraisal. Eur. J. Biochem. 1999, 265, 698–702. [DOI] [PubMed] [Google Scholar]
- (434).Hemmerich P; Veeger C; Wood HCS Progress in the Chemistry and Molecular Biology of Flavins and Flavocoenzymes. Angew. Chemie Int. Ed. English 1965, 4, 671–687. [DOI] [PubMed] [Google Scholar]
- (435).Wilson GS Determination of Oxidation-Reduction Potentials. 1978, 54, 396–410. [DOI] [PubMed] [Google Scholar]
- (436).Nöll G; Hauska G; Hegemann P; Lanzl K; Nöll T; von Sanden-Flohe M; Dick B Redox Properties of LOV Domains: Chemical versus Photochemical Reduction, and Influence on the Photocycle. ChemBioChem 2007, 8, 2256–2264. [DOI] [PubMed] [Google Scholar]
- (437).Draper RD; Ingraham LL A Potentiometric Study of the Flavin Semiquinone Equilibrium. Arch. Biochem. Biophys. 1968, 125, 802–808. [DOI] [PubMed] [Google Scholar]
- (438).Losi A Flavin-Based Blue-Light Photosensors: A Photobiophysics Update. Photochem. Photobiol. 2007, 83, 1283–1300. [DOI] [PubMed] [Google Scholar]
- (439).Chaves I; Pokorny R; Byrdin M; Hoang N; Ritz T; Brettel K; Essen L-O; van der Horst GTJ; Batschauer A; Ahmad M The Cryptochromes: Blue Light Photoreceptors in Plants and Animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [DOI] [PubMed] [Google Scholar]
- (440).Ahmad M Photocycle and Signaling Mechanisms of Plant Cryptochromes. Curr. Opin. Plant Biol. 2016, 33, 108–115. [DOI] [PubMed] [Google Scholar]
- (441).Hwang C; Sinskey AJ; Lodish HF Oxidized Redox State of Glutathione in the Endoplasmic Reticulum. Science 1992, 257, 1496–1502. [DOI] [PubMed] [Google Scholar]
- (442).López-Mirabal HR; Winther JR Redox Characteristics of the Eukaryotic Cytosol. Biochim. Biophys. Acta - Mol. Cell Res 2008, 1783, 629–640. [DOI] [PubMed] [Google Scholar]
- (443).Arents JC; Perez MA; Hendriks J; Hellingwerf KJ On the Midpoint Potential of the FAD Chromophore in a BLUF-Domain Containing Photoreceptor Protein. FEBS Lett. 2011, 585, 167–172. [DOI] [PubMed] [Google Scholar]
- (444).Balland V; Byrdin M; Eker APM; Ahmad M; Brettel K What Makes the Difference between a Cryptochrome and DNA Photolyase? A Spectroelectrochemical Comparison of the Flavin Redox Transitions. J. Am. Chem. Soc. 2009, 131, 426–427. [DOI] [PubMed] [Google Scholar]
- (445).Lin C; Robertson DE; Ahmad M; Raibekas AA; Jorns MS; Dutton PL; Cashmore AR Association of Flavin Adenine Dinucleotide with the Arabidopsis Blue Light Receptor CRY1. Science 1995, 269, 968–970. [DOI] [PubMed] [Google Scholar]
- (446).Gindt YM; Schelvis JPM; Thoren KL; Huang TH Substrate Binding Modulates the Reduction Potential of DNA Photolyase. J. Am. Chem. Soc. 2005, 127, 10472–10473. [DOI] [PubMed] [Google Scholar]
- (447).Lokhandwala J; Hopkins HC; Rodriguez-Iglesias A; Dattenböck C; Schmoll M; Zoltowski BD Structural Biochemistry of a Fungal LOV Domain Photoreceptor Reveals an Evolutionarily Conserved Pathway Integrating Light and Oxidative Stress. Structure 2015, 23, 116–125. [DOI] [PubMed] [Google Scholar]
- (448).Magerl K; Stambolic I; Dick B; Balland V; Getzoff ED; Ritz T; Brettel K; Nonell S; Tonge PJ; Meech SR; et al. Switching from Adduct Formation to Electron Transfer in a Light–oxygen–voltage Domain Containing the Reactive Cysteine. Phys. Chem. Chem. Phys. 2017, 19, 10808–10819. [DOI] [PubMed] [Google Scholar]
- (449).Ortiz-Guerrero JM; Polanco MC; Murillo FJ; Padmanabhan S; Elías-Arnanz M Light-Dependent Gene Regulation by a Coenzyme B12-Based Photoreceptor. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 7565–7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (450).Wilson A; Punginelli C; Gall A; Bonetti C; Alexandre M; Routaboul JM; Kerfeld CA; van Grondelle R; Robert B; Kennis JT; et al. A Photoactive Carotenoid Protein Acting as Light Intensity Sensor. Proc Natl Acad Sci U S A 2008, 105, 12075–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (451).Mukherjee A; Weyant KB; Walker J; Schroeder CM Directed Evolution of Bright Mutants of an Oxygen-Independent Flavin-Binding Fluorescent Protein from Pseudomonas Putida. J. Biol. Eng. 2012, 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (452).Möglich A; Moffat K Structural Basis for Light-Dependent Signaling in the Dimeric LOV Domain of the Photosensor YtvA. J. Mol. Biol. 2007, 373, 112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (453).Rullan M; Benzinger D; Schmidt GW; Milias-Argeitis A; Khammash M An optogenetic platform for real-time, single-cell interrogation of stochastic transcriptional regulation. Mol. Cell 2018, 70, 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]