Abstract
To elucidate the clinical importance of estrogen receptor (ER) β in breast cancer, 29 archival primary breast cancer specimens, six locally recurrent cancers, and five benign mammary tumors were examined histochemically for ERα, ERβ and the proliferation markers Ki67 and cyclin A. In benign tumors, most epithelial cells contained ERβ, but ERα was rare. In primary cancers, both ERα and ERβ occurred in epithelial cells, the presence of ERβ being associated with elevated expression of Ki67 and cyclin A, and ERα with decreased levels. Thus, the highest content of proliferation markers was seen in primary cancers that were ERα− ERβ+. Most Ki67-containing cells coexpressed ERβ, but few showed ERα. In locally recurring cancers, ERα, ERβ, and Ki67 were more highly expressed than in the corresponding primary tumors, and many cells containing ERβ, but few with ERα, expressed Ki67. Surprisingly, ERβ, but not ERα, was seen in the stromal cells of both primary and recurrent cancers. Because the response of breast cancers to tamoxifen therapy is correlated with the presence of ERα, cancer cells that lack ERα but contain ERβ and proliferation markers represent a novel population of apparently proliferating cells that probably are not targeted by the current antiestrogens. Thus, appropriate ERβ-specific ligands, perhaps in combination with tamoxifen, may be useful in improving the treatment of breast cancers.
Estrogen is a modulator of cellular growth and differentiation (1). Its major targets in females are the mammary gland and uterus, but in both males and females this hormone is essential for maintenance of bone, brain, the cardiovascular system, and the urogenital tract (2–4). Estrogen mediates most of its functions through two specific intracellular receptors, ERα and ERβ (5), which are hormone-dependent transcription regulators.
Estrogen also is associated with the induction and growth of breast cancer. Animal studies have shown that estrogen can induce and stimulate breast cancer, and ovariectomy or administration of antiestrogens opposes this action (6–9). Because ERα-containing epithelial cells in the normal breast do not express proliferation markers (10–12), the mechanisms through which estrogen induces epithelial growth are not clear. The prevailing concept is that it induces the secretion of growth factors from the stroma and that these agents stimulate epithelial cells to proliferate (13). This explanation leaves two questions: (i) why do ERα-containing normal cells not proliferate? and (ii) do ERα-containing cells in breast cancer epithelium proliferate in response to estrogen? There is no clear answer to the first question, but an untested possibility is that ERα-containing cells do receive proliferation signals and proliferate but that ERα must be restricted for progression through the cell cycle to occur. There is evidence that ERα is rapidly down-regulated in cell lines in response to estradiol treatment (14, 15).
In the rodent mammary gland, most of the cells that express the proliferating cell nuclear antigen contain neither ERα nor ERβ (16), which suggests that the presence of ERs in epithelial cells restricts their proliferation. Regulation of growth factor receptors in the breast supports this role for ER. In breast cancer cells in culture, loss of ERα is accompanied by an increase in growth factors and growth factor receptors (17–19), as well as higher levels of phosphotyrosine residues indicating increased tyrosine kinase activity (20).
Expression of ERβ mRNAs has been detected by reverse transcriptase–PCR in both normal and malignant human breast tissue (21), and full-length ERβ protein (ERβ1) has been identified in human breast tumors by Western blotting (22). Immunohistochemistry of breast cancer tissue shows that ERβ is often coexpressed with ERα and that the presence of this receptor is associated with negative axillary node status and low tumor grade (23). Although it has been suggested that ERβ contributes to the initiation and progression of carcinogenesis (24), is expressed in breast cancers of higher grades (21), and is a marker for estrogen responsiveness (25), its precise role in breast tissue remains to be defined.
Clinical studies show that beneficial response of breast cancer to tamoxifen (26), as well as to other endocrine therapies (27), is related to the presence of ERα. Furthermore, breast cancer prevention trials show that there is a reduced incidence of ERα-positive but not ERα-negative cancers in the tamoxifen-treated group (28). Because ERβ is present in breast tumors, it is pertinent to ask whether proliferation and/or survival of ERβ-expressing cells are affected by tamoxifen. To provide insight into this question, we have examined the cellular distribution of the two ERs in mammary neoplasms and investigated the relationship between cell proliferation markers and ER expression pattern.
Materials and Methods
Tissue Collection.
Paraffin-embedded breast tumor sections from 34 patients undergoing breast cancer surgery were provided by the Helsinki University Central Hospital and the Charing Cross Hospital, London. They were composed of 25 invasive ductal cancers, four lobular cancers, and five benign mammary tumors. Information was recorded concerning the patient's age and menopausal status, the pathological diagnosis and differentiation grade of the tumor, and ERα positivity as determined immunohistochemically. Of the 29 cancer patients, six developed local recurrence during or after adjuvant tamoxifen treatment for 1–5 years, and sections of the recurrent cancers were compared with those from the primary tumors.
Antibodies.
Monoclonal antibody to mouse ERα (6F11) was obtained from NovoCastra (Newcastle, U.K.); rabbit anti-human ERβ polyclonal (06–629) was from Upstate Biotechnology (Lake Placid, NY); Ki67 monoclonal (Mib-1, M7240) and Ki67 rabbit polyclonal (A0047) were from Dako, and cyclin A rabbit polyclonal (H432) was from Santa Cruz Biotechnology. Biotinylated secondary antibodies (goat anti-mouse IgG and goat anti-rabbit IgG) and avidin-biotin kits were obtained from Vector Laboratories. FITC-conjugated anti-rabbit and Cy3-conjugated anti-mouse antibodies were from Jackson ImmunoResearch.
Immunohistochemistry.
Paraffin sections (4 μm) were dewaxed in xylene and rehydrated through graduated ethanol to water. Endogenous peroxidase was blocked by incubation for 30 min with a solution of 1% hydrogen peroxide, and antigen retrieval was performed by microwaving sections in 0.01 M citrate buffer, pH 6.0, for 20 min at 800 W.
Single Antibody Immunostaining.
Tissue sections were incubated for 1 h at 4°C with normal goat serum diluted at 1/10 in PBS. Antibodies were diluted individually in PBS containing 3% BSA. Dilution was 1:100 for ERβ, Ki67, and cyclin A antibodies and 1:500 for ERα antibody. Sections were incubated with antibodies overnight at 4°C. For negative controls, the primary antibody was replaced with PBS alone or with primary antibody after absorption with the corresponding antigen. Before addition of secondary antibody, sections were rinsed in PBS containing 0.05% Tween 20. The ABC method was used to visualize the signal according to the manual provided by the manufacturer (Vector Laboratories). Sections were incubated in biotinylated goat anti-rabbit or goat anti-mouse Ig (1:200 dilution) for 2 h at room temperature, followed by washing with PBS and incubation in avidin-biotin-horseradish peroxidase for 1 h. After thorough washing in PBS, sections were developed with 3,3′-diaminobenzidine tetra-hydrochloride (Dako), slightly counterstained with Mayers hematoxylin, and dehydrated through an ethanol series, followed by exposure to xylene and mounting.
The percentage of positively stained cells is an average after counting the stained and the total number of cells from four high-magnification fields with the software IMAGE-PRO PLUS 4.1 (Media Cybernetics, Silver Spring, MD).
Double Antibody Immunostaining.
Tissue sections were incubated for 1 h at 4°C with normal donkey serum (Sigma) diluted 1:10 in antibody diluent. This was followed by an overnight incubation at 4°C with a mixture composed of antibodies to either ERα and ERβ, ERα and Ki67 (A0047), or ERβ and Ki67 (M7240). PBS alone was used in place of these mixtures in the negative controls. Before addition of secondary antibodies, sections were washed with PBS containing 0.05% Tween 20. Slides were incubated for 1 h with a mixture of FITC-conjugated donkey anti-rabbit (1:100) and Cy3-conjugated donkey anti-mouse (1:200) antibody. After washing with PBS for 30 min, the slides were incubated with 0.1 μg/ml of 4′,6-diamidino-2-phenylindole dihydrochloride in PBS for 30 s, washed three times in PBS, and mounted with Vectashield.
Statistical Analysis.
Statistical differences between groups were analyzed with Student's t test and ANOVA using SPSS (SPSS, Chicago). A value of P < 0.05 was considered significant.
Results
Single Antibody Staining.
The expression of ERα, ERβ, Ki67, and cyclin A was analyzed in paraffin sections of 29 invasive breast cancers and five benign tumors (Fig. 1). Specimens in which no positively stained cells were detected were taken as ERα− or ERβ−. In receptor-positive specimens, the abundance of cells containing ERα or ERβ varied from 33% to 86%. Ki67 and cyclin A were expressed in all samples, but the percentage of positive cells per section varied widely from 0.1% to 34%. Staining for ERα in this study was in agreement with ERα analyses done at the time of initial surgery.
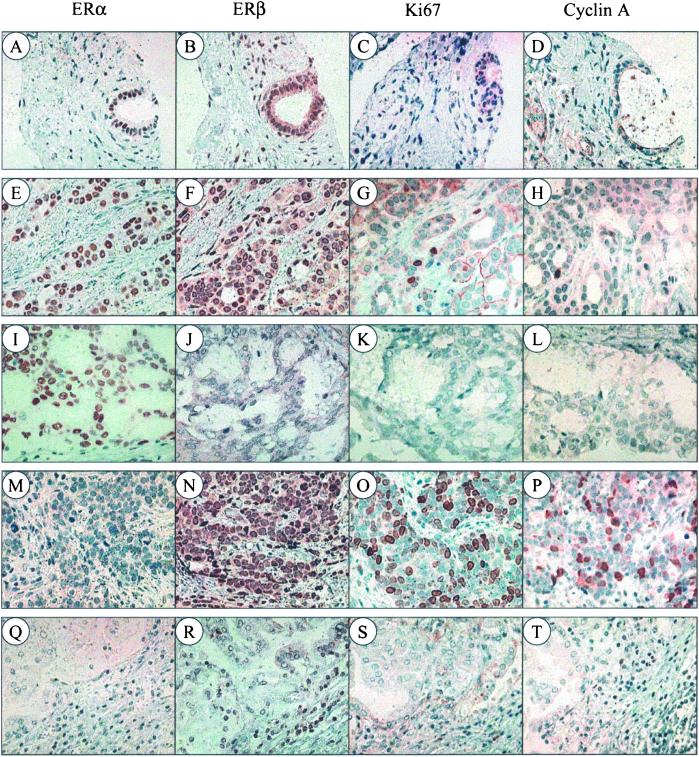
Figure 1.
Expression of ERα, ERβ, Ki67, and cyclin A in breast fibroadenoma and invasive ductal cancer. In fibroadenomas, ERα is seen only in epithelial cells (A), whereas ERβ is expressed in both epithelium and stroma (B). There was little Ki67 or cyclin A expression (C and D). In invasive ductal cancers, four patterns of ER expression can be identified: ERα+ ERβ+ (E and F), ERα+ ERβ− (I and J), ERα− ERβ+ (M and N), and ERα− ERβ− (Q and R). Some Ki67- and cyclin A-containing cells appear in ERα+ ERβ+ specimens (G and H), but ERα− ERβ+ cancers express much higher levels of these proliferation markers (O and P). In contrast, ERα+ ERβ− and ERα− ERβ− tumors contain little Ki67 or cyclin A (K, L, S, and T). (Magnification: ×200.)
Of the benign tumors, two fibroadenomas and one papilloma expressed both ERα and ERβ, with ERβ-positive cells being more abundant than ERα-positive. In one papilloma neither ERα nor ERβ was detectable. In fibroadenomas, there were few ERα-positive cells and these were exclusively epithelial (Fig. 1A), whereas ERβ-positive cells were abundant in both epithelium and stroma (Fig. 1B). Ki67 and cyclin A were present in less than 0.1% of the cells in all five benign tumors (Fig. 1 C and D).
In the 29 invasive cancers, 18 (62%) contained ERα and 19 (65%) ERβ (Table 1). In ERα+ cancers, Ki67 and cyclin A were expressed in 5% and 2% of the cells, respectively, whereas in ERα− cancers, the values were 17% and 12% (P < 0.05). In contrast to ERα, the expression of ERβ was associated with a higher content of Ki67 and cyclin A. The percentages of Ki67- and cyclin A-positive cells in ERβ+ cancers were 13% and 8%, respectively, significantly higher than the corresponding values of 2% and 1% seen in ERβ− cancers (P < 0.05).
Table 1.
Relation of Ki67 and cyclin A expression to ER pattern in breast cancer
| ER expression | Cases | Percentage of expression
|
|
|---|---|---|---|
| Ki67 | Cyclin A | ||
| ERα+ | 18 | 4.8 ± 1.6 | 1.9 ± 1.1 |
| ERα− | 11 | 17.2 ± 4.2* | 11.5 ± 3.8* |
| ERβ+ | 19 | 13.3 ± 2.8 | 8.0 ± 2.2 |
| ERβ− | 10 | 2.4 ± 0.8* | 0.9 ± 0.6* |
| ERα+ ERβ+ | 12 | 5.6 ± 1.4 | 2.3 ± 1.2 |
| ERα+ ERβ− | 6 | 3.5 ± 1.2 | 1.2 ± 0.4 |
| ERα− ERβ− | 4 | 0.8 ± 0.2 | 0.5 ± 0.1 |
| ERα− ERβ+ | 7 | 26.6 ± 2.8** | 17.8 ± 3.2** |
, P < 0.05.
, P < 0.01.
There were four patterns of ER expression in the epithelium of the 29 breast cancer samples, and these patterns occurred with different frequency. They were: ERα+ ERβ+ (12 = 41%), ERα+ ERβ− (6 = 21%), ERα− ERβ+ (7 = 24%), and ERα− ERβ− (4 = 14%). The combination of the presence of ERβ and the absence of ERα was associated with a substantially enhanced expression of Ki67 and cyclin A. In ERα− ERβ+ cancers, Ki67 and cyclin A contents were 27% and 18%, respectively. This was significantly higher than in the other three groups (P < 0.01). Ki67 and cyclin A were present in 6% and 2% of the cells in ERα+ ERβ+ cancers, but this difference was not significant when compared with ERα+ ERβ− or ERα− ERβ− cancers (P > 0.05). There was ERβ but no ERα staining in stromal cells, and little Ki67 or cyclin A was seen in the stroma (Fig. 1).
Although the expression of ERα and ERβ bore no relationship to differentiation grade (P > 0.05), Ki67 and cyclin A were more prevalent in cancers of higher grade. In cancers of grade 3, the average percentage of cells expressing Ki67 and cyclin A was 28% and 21%, respectively, as compared with 9% and 7% in grade 2, and 7% and 3% in grade 1 (P < 0.01). In cancers from the nine node-positive patients, 19% of the cells showed staining for Ki67 and 15% for cyclin A. This is a higher expression than that seen with the 14 node-negative patients, where the corresponding values were 8% and 5%, respectively (P < 0.05).
Coexpression of ERs with Ki67.
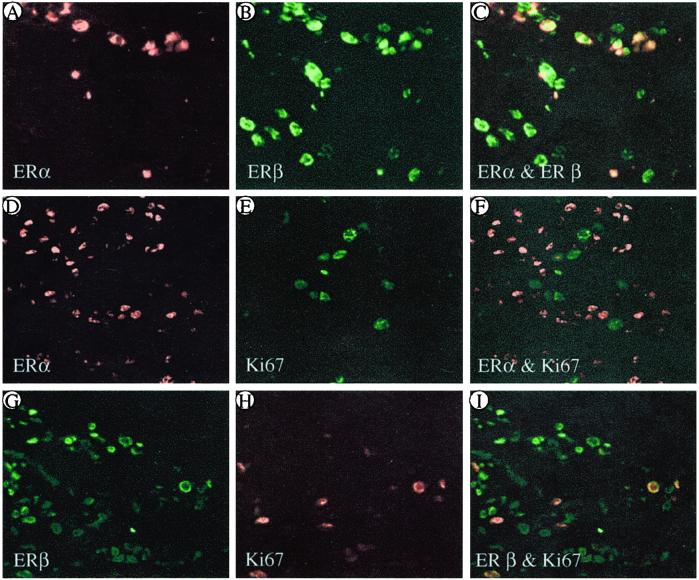
To determine whether ERs are expressed in proliferating cells, individual tumor sections were exposed to the following combinations of antibodies raised against the proteins indicated: ERα and ERβ; ERα and Ki67 (A0047); and ERβ and Ki67 (M7240). As shown in Fig. 2, in ERα+ ERβ+ cancers, all of the ERα-containing cells also expressed ERβ (Fig. 2 A–C). In ERα+ ERβ+ and ERα+ ERβ− cancers, some cells expressed Ki67, but in very few instances was this proliferation marker present in the same cells that contained ERα (Fig. 2 D–F). In specimens where ERβ was much more abundant than ERα, there were many Ki67-positive cells, 98% of which expressed ERβ (Fig. 2 G–I).
Figure 2.
Colocalization of ERα, ERβ, and Ki67 in invasive ductal breast cancer. Most of the ERα+ cells also contain ERβ (A–C). In ERα-dominant specimens, there are few cells containing Ki67, none of which coexpress ERα (D–F). In ERβ-dominant specimens, many cells express Ki67, and almost all of these coexpress ERβ (G–I). In C, F, and I, yellow indicates colocalization. (Magnification: ×200.)
ERs and Ki67 in Recurrent Cancers.
After initial breast cancer surgery, the patients received adjuvant tamoxifen therapy for 1–5 years. Six patients who had local recurrence were selected for study. In three of the patients, local breast cancer recurred during the tamoxifen treatment, whereas three other patients had recurrence 2, 8, and 9 years after cessation of tamoxifen therapy. ERα was expressed in all of the primary and recurrent tumors, whereas ERβ was present in four primary cancer specimens but in all six of the recurrent tumors. Thus the recurrent cancers were all ERα+ ERβ+ and showed an average percentage of Ki67-positive cells of 23% as compared with 7% in all of the primary malignancies and 5.6% in those of the ERα+ ERβ+ phenotype. This difference in proliferation index between ERα+ primary and recurrent cancers is significant (P < 0.01).
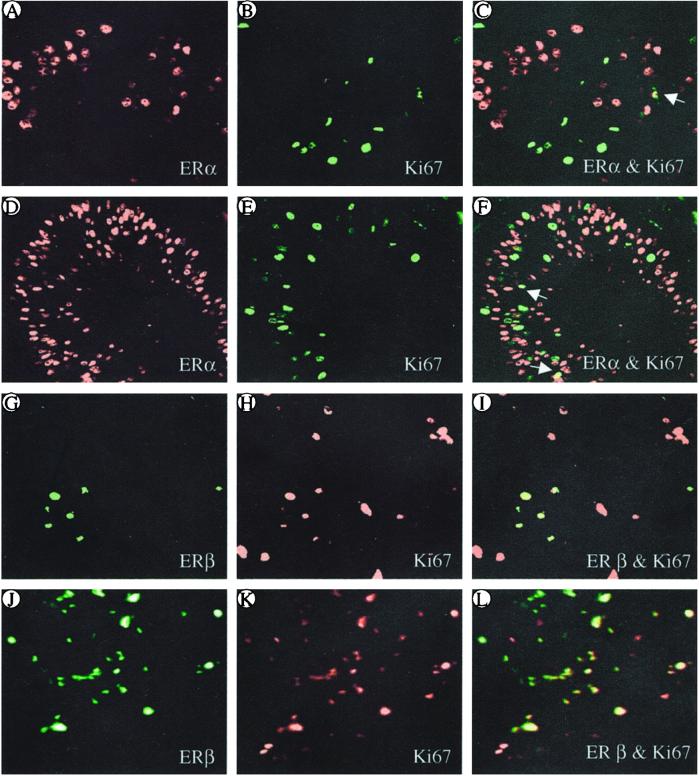
Primary and recurrent cancer specimens from individual patients were compared to determine whether there were changes in the populations of cells showing colocalization of ERα and ERβ, ERα and Ki67, and ERβ and Ki67. Although the contents of ERα and Ki67 were higher in recurrent (Fig. 3 D and E) than in primary cancers (Fig. 3 A and B), coexpression with ERα was seen in less than 5% of the Ki67+ cells (Fig. 3 C and F, arrow). However, in both primary (Fig. 3 G–I) and recurrent specimens (Fig. 3 J–L), 70–85% of the Ki67+ cells expressed ERβ whereas 15–29% did not contain either ER (Fig. 3 I and L, red).
Figure 3.
Comparison of primary and recurrent mammary cancer from the same patient in the expression and colocalization of Ki67 with either ERα (A–F) or ERβ (G–L). The levels of both ERα and Ki67 are higher in the recurrent cancer (D and E) than in the primary (A and B). Most of the ERα+ cells do not express Ki67, and only a few show coexpression (C and F, arrow). However, in both primary (G–I) and recurrent cancers (J–L), 71–85% of Ki67-positive cells also express ERβ (I and L, red). (Magnification: ×200.).
Discussion
The most striking findings of this study of ERα and ERβ in breast neoplasms are: (i) The presence of ERα in breast cancer epithelium is associated with a decreased expression of the proliferation markers, Ki67 and cyclin A, whereas ERβ expression is associated with elevated levels of these markers. The highest expression of either Ki67 or cyclin A is seen in cancers that are ERα− ERβ+. (ii) The only ER in the stroma of breast tumors is ERβ, whereas both ERα and ERβ are present in the epithelial cells. (iii) Both ERα and ERβ are expressed in recurrent breast cancers at higher levels than those in the corresponding primaries. (iv) In recurrent breast cancers, the presence of ERα is not associated with a lower levels of Ki67, as it is in primary cancers.
The presence of ERα in breast cancers is generally taken as an indication of hormone dependency (27), and, on this basis, treatment with antiestrogen (tamoxifen) is now the first-line therapy, both for metastatic disease and as adjuvant therapy after mastectomy (29, 30). Despite the initial benefits of tamoxifen, most patients eventually relapse with tumors that not only are tamoxifen-resistant but actually are stimulated by this agent (31).
In this study, we found that very few ERα-containing cells express proliferation markers and that cancers that are ERα− ERβ+ have higher percentage of cells expressing Ki67 and cyclin A, than do cancers that are ERα+ ERβ+. In ERβ− cancers, proliferation markers are the lowest regardless of whether or not they contain ERα. These results suggest that it might be ERβ, and not ERα, that is related to proliferation in breast cancer.
In the recurrent cancers, the expression of ERα, ERβ, and Ki67 was higher than that seen in the primary cancers from the same patients. All recurrence specimens were ERα+ ERβ+, with a level of Ki67 that was higher than that in ERα+ ERβ+ primary cancers. In both primary and recurrence specimens, less than 5% of the ERα+ cells expressed Ki67, but most of the Ki67-containing cells expressed ERβ.
It is known that only two-thirds of the patients with ERα-positive breast cancers respond to tamoxifen and that very few patients, classified as ERα-negative, benefit from tamoxifen therapy (29, 30). Clinically, ERα is measured either by estradiol binding, which detects both ERα and ERβ, and immunoassay, which detects only ERα. Because there is a clear relationship between response to tamoxifen and ERα content of the tumor, it may be that ERβ-positive, proliferating cells are insensitive to tamoxifen. Such cells may even depend on tamoxifen for growth, because it is known that ERβ in the presence of tamoxifen activates AP-1 response elements (32, 33). Other recent studies have suggested that ERβ is a prognostic marker in breast cancer, particularly for identifying tumors resistant to tamoxifen (23, 34, 35).
The beneficial effect of tamoxifen in breast cancer is probably caused by its effect on ERα-positive cells, very few of which are proliferating. Antagonism of ERα in these cells may result in reduction of the tumor burden and/or the removal of growth factors, which may be proliferative signals for ERβ-containing cells. Because the ERβ-containing, proliferating cells in the tumor probably are not targets of tamoxifen, addition of an ERβ antagonist to the therapeutic regimen may be useful in the clinical management of both primary and advanced breast cancers, particularly those that are stimulated by tamoxifen.
An unexpected finding in this study is that ERβ was the only ER detected in breast cancer stroma. The lack of ERα in human breast stroma has been observed previously (36). In rodents, ERα is present in the breast stroma and is responsible for secretion of growth factors (epithelial cellular mitogens) in response to estradiol. Tissue recombinants with stroma and epithelium from normal as compared to ERα knockout mice have demonstrated that ERα in the stroma is responsible for estrogen-induced epithelial growth in response to estradiol (37). Whether the distribution of ERs in different cellular compartments of breast tissue is different in rodents than in humans or whether there are variations between individual patients that have not been addressed in our specimens, are questions that need further examination.
From the foregoing data, it appears that to obtain a more complete picture of the action of antiestrogens in breast cancer therapy, both ERα and ERβ should be measured in the tumor. Although the number of patients is relatively small, from this limited study it is evident that the expression of ERβ is important in cell proliferation in breast cancers and, possibly, in their response to tamoxifen.
Acknowledgments
The skillful assistance of Christina Thulin-Andersson is gratefully acknowledged. Research was supported by grants from the Swedish Cancer Fund, Cancer Research Campaign, Deutsche Krebshilfe, and KaroBio AB, Sweden. S.S. has a fellowship from the Wenner-Gren Foundation and a research grant from the Scandinavia-Japan Sasakawa Foundation.
Abbreviation
- ER
estrogen receptor
References
- 1.Warner M, Nilsson S, Gustafsson J-Å. Curr Opin Obstet Gynecol. 1999;11:249–254. doi: 10.1097/00001703-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Simpson E R. Mol Cell Endocrinol. 1998;145:55–59. doi: 10.1016/s0303-7207(98)00169-5. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa S, Washburn T F, Taylor J, Lubahn D B, Korach K S, Pfaff D W. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 4.Wenger N K. Curr Opin Cardiol. 1999;14:292–297. doi: 10.1097/00001573-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao D Z, Pantazis C G, Hou X, Li S A. Carcinogenesis. 1998;19:2173–2180. doi: 10.1093/carcin/19.12.2173. [DOI] [PubMed] [Google Scholar]
- 7.Hilakivi-Clarke L, Clarke R, Lippman M. Nutrition. 1999;15:392–401. doi: 10.1016/s0899-9007(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 8.Hilakivi-Clarke L, Cho E, Onojafe I, Raygada M, Clarke R. Oncol Rep. 1999;6:1089–1095. doi: 10.3892/or.6.5.1089. [DOI] [PubMed] [Google Scholar]
- 9.Koibuchi Y, Sugamata N, Iino Y, Yokoe T, Andoh T, Maemura M, Takei H, Horiguchi J, Matsumoto H, Morishita Y. Int J Mol Med. 1999;4:145–148. doi: 10.3892/ijmm.4.2.145. [DOI] [PubMed] [Google Scholar]
- 10.Zeps N, Bentel J M, Papadimitriou J M, D'Antuono M F, Dawkins H J. Differentiation. 1998;62:221–226. doi: 10.1046/j.1432-0436.1998.6250221.x. [DOI] [PubMed] [Google Scholar]
- 11.Clarke R B, Howell A, Potten C S, Anderson E. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 12.Clarke R B, Howell A, Anderson E. Breast Cancer Res Treat. 1997;45:121–133. doi: 10.1023/a:1005805831460. [DOI] [PubMed] [Google Scholar]
- 13.Wiesen J F, Young P, Werb Z, Cunha G R. Development (Cambridge, UK) 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- 14.Lonard D M, Nawaz Z, Smith C L, O'Malley B W. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 15.Wijayaratne A L, McDonnell D P. J Biol Chem. 2001;276:35684–35692. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- 16.Saji S, Jensen E V, Nilsson S, Rylander T, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke R, Skaar T, Leonessa F, Branken H, James M, Brunner N, Lippman M E. Cancer Treat Res. 1996;87:263–283. doi: 10.1007/978-1-4613-1267-3_11. [DOI] [PubMed] [Google Scholar]
- 18.Wosikowski K, Schuurhuis D, Kops G J P L, Saceda M, Bates S E. Clin Cancer Res. 1997;3:2405–2414. [PubMed] [Google Scholar]
- 19.Nicholson R I, McClelland R A, Robertson J F R, Gee J M W. Endocrine-Related Cancer. 1999;6:373–387. doi: 10.1677/erc.0.0060373. [DOI] [PubMed] [Google Scholar]
- 20.El-Ashry D, Miller D L, Kharbanda S, Lippman M E, Kern F G. Oncogene. 1997;15:423–435. doi: 10.1038/sj.onc.1201198. [DOI] [PubMed] [Google Scholar]
- 21.Speirs V, Parkes A T, Kerin M J, Walton D S, Carleton P J, Fox J N, Atkin S L. Cancer Res. 1999;59:525–528. [PubMed] [Google Scholar]
- 22.Fuqua S A W, Schiff R, Parra I, Friedrichs W E, Su J L, McKee D D, Slentz-Kesler K, Moore L B, Wilson T M, Moore J T. Cancer Res. 1999;59:5425–5428. [PubMed] [Google Scholar]
- 23.Jarvein T A H, Pelti-Huikko M, Holli K, Isola J. Am J Pathol. 2000;156:29–35. doi: 10.1016/s0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y F, Lau K M, Ho S M, Russo J. Int J Oncol. 1998;12:1225–1228. doi: 10.3892/ijo.12.6.1225. [DOI] [PubMed] [Google Scholar]
- 25.Dotzlaw H, Leygue E, Watson P H, Murphy L C. Cancer Res. 1999;59:529–532. [PubMed] [Google Scholar]
- 26.Fisher B, Costanino J P, Wickerham D L, Redmond C K, Kavanah M, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, et al. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 27.DeSombre E R, Jensen E V. In: Cancer Medicine. 5th Ed. Holland J F, Frei E III, Bast R C Jr, Kufe D W, Pollock R E, Weichselbaum R R, editors. Hamilton, ON, Canada: Decker; 2000. pp. 706–714. [Google Scholar]
- 28.Leygue E, Dotzlaw H, Watson P H, Murphy L C. Cancer Res. 1999;59:1175–1179. [PubMed] [Google Scholar]
- 29.Furr B J A, Jordan V C. Pharmacol Ther. 1984;25:127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- 30.Early Breast Cancer Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 31.MacGregor J I, Jordan V C. Pharmocol Rev. 1998;50:151–196. [PubMed] [Google Scholar]
- 32.Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J-Å, Nilsson S. Mol Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 33.Paech K, Webb P, Kuiper G G, Nilsson S, Gustafsson J-Å, Kushner P J, Scanlan T S. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 34.Speirs V, Malone C, Walton D S, Kerin M J, Atkin S L. Cancer Res. 1999;59:5421–5424. [PubMed] [Google Scholar]
- 35.Speirs V, Kerin M J. Br J Surg. 2000;87:405–409. doi: 10.1046/j.1365-2168.2000.01402.x. [DOI] [PubMed] [Google Scholar]
- 36.McCarty K S, Jr, Szabo E, Flowers J L, Cox E B, Leight G S, Miller L, Konrath J, Soper J T, Budwit D A, Creasman W T, et al. Cancer Res Suppl. 1986;46:4244s–4248s. [PubMed] [Google Scholar]
- 37.Cunha G R, Young P, Hom Y K, Cooke P S, Taylor J A, Lubahn D B. J Mammary Gland Biol Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]