Abstract
Beta-glucan (β-glucan) is a macromolecule structure where glucose unit has bonded through β-glycosidic bond at 1 and 3 positions. It is well known as a natural immunomodulator without exhibiting any side effects via enhancing immunity. Mushroom contains a large amount of β-glucan and it has anticancerous and antioxidant efficacy. Structure and physical properties of β-glucan are highly influenced by the types of mushroom. In particular, Grifola frondosa has β-1, 3 and β-1, 6 bonds in their structure. It has been noted that β-glucan content also depends upon the size of mushroom particles. The exact content of β-glucan and their immunological activity by a particle size of G. frondosa have yet to be fully elucidated. Herein, β-glucan contents were analyzed according to the particle size of leaf mushroom followed by cell activation and immunoactivity analysis. The highest β-glucan content was observed at a particle size of 20-30 μm (27.65 ± 0.30 w/w). All samples showed ~ 103% cell activation compared to the control and greater cell activity was observed at higher concentration. The significant increase in cytokines secretion was observed in the presence of 20-30 μm particle size of G. frondosa compared to the control. This study suggested that 20-30 μm size is the suitable size of G. frondosa that can be used as a health supplement and food additive to act as an immune booster, hypotensive agent, and hypoglycemic agent.
1. Introduction
Much research on immune enhancement from natural products has been carried out. Notably, there is a growing interest in the efficacy of specific physiologically active substances from natural products, and many studies on natural substances with anticancer, antioxidant, and immunological activity effects have been reported [1]. Mushrooms are one of the most frequently used natural products for immunological activity [2]. Many polysaccharides extracted from mushrooms are effective as anticancer drugs and for immunity control [3]. A lot of edible mushrooms have become attractive as “functional foods” and as source materials for immunomodulators, antitumor agents, antibiotics, and antihypertensive drugs [4]. G. frondosa, one of the outstanding medicinal mushrooms with reported anticancer effects, contains various free amino acids and vitamins B1, B2, C, and D. G. frondosa is widely used in Japan, China, and Korea as a traditional food additive due to its texture, delicious taste, and excellent aroma [5]. Unlike other mushrooms, β-1, 3 bonds are added to β-1, 6 bonds in G. frondosa [6]. Furthermore, β-glucan extracted from G. frondosa has been reported to enhance antitumor activity and bone marrow toxicity by strengthening the immune system [7]. The key ingredient in improving the immune system by affecting cellular immune recovery is known as β-glucan in edible mushrooms [8]. It is a valuable dietary fibre and is a polysaccharide of D‐glucose monomers linked by β-glycosidic bonds [9]. The capacity of β-glucan to activate immune effector response varies with its structure and particle size. It has also been reported that the size of β-glucan particles controls the production of specific cytokines [10]. As interest in β-glucan increased, various studies into solvent extraction [11], pH condition [12], and air classification [13] were conducted to improve the content of β-glucan. In particular, a mushroom extract prepared from superfine particles has been reported to be useful for immunity enhancement because the activity of lymphocytes was effectively activated in nonclinical experiments [14]. G. frondosa induces enhanced production of cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor α [15]. Besides, it has been reported that D-Fraction extracted from G. frondosa activates interleukin-12 production and also for the T helper-1 cytokine interferon-γ and the T helper-2 cytokines interleukin-4 and interleukin-10, thereby eliciting antitumor activity [16]. Studies on the effect of various particle sizes of G. frondosa on immunity have continued, but few have investigated the evaporation effect of β-glucan in microparticulated G. frondosa. In this study, the content of β-glucan was analyzed according to the particle size of G. frondosa (10-20, 20-30, and 30-40 μm, respectively). The optimized microsize should be examined and compared to the control sample. Pulverizing G. frondosa using an air classifying mill could be an effective way to concentrate β-glucan content.
2. Materials and Methods
2.1. Preparation of Materials
The mushrooms (G. frondosa, Republic of Korea) were treated by an air classifying mill (ACM-AL, Daega, Republic of Korea) that had a pulverizer and a separator. Mushroom samples were pulverized after passing an impeller rotating at high speed in the air classifying milling machine, and a classifier analysis software (Mastersizer S v3.10, Malvern, UK) was used to classify particle size by centrifugal force and drag force. Three fractions labeled as G1, G2, and G3 (10-20, 20-30, and 30-40 μm, respectively) were obtained through air classification. In the process, the feed mass was kept constant at 13 kg, and the rotating speed was set at 3,600 g.
2.2. β-Glucan Analysis
The quantitative analysis of β-glucan was performed using a β-glucan kit (Megazyme Inc., Chicago) [17]. The content of β-glucan was calculated from the difference between total glucan (β-glucan and α-glucan) and α-glucan. To measure total glucan, 2.0 mL of 12 M sulfuric acid was added to each of the three samples and left for 2 h. Over this period, 10 mL of water was added to each sample and boiled in a water bath at 100°C for 5 min. The samples were then cooled at room temperature. Each sample was subsequently transferred to a 100 mL volumetric flask and 6 mL of 10M potassium hydroxide (KOH) was added. The samples adjust the pH 5 using 200 mM sodium acetate buffer. The samples were centrifuged at 1,500 g for 10 min. The obtained supernatants were transferred into a test tube, and 0.1 mL mixture solution (exo-1, 3-β-glucanase/β-glucosidase in 200 mM sodium acetate buffer) was added. The samples were incubated at 40°C for 60 min and 3.0 mL of GOPOD Reagent was added. Absorbance was measured at 510 nm. To measure α-glucan, 100 mg of each sample was added to a tube with 2 mL of 2 M KOH and stirred for 20 min in an ice bath. Then, 8 mL of 1.2 M sodium acetate buffer (pH 3.8) and 0.2 mL of amyloglucosidase/invertase were added. The samples were incubated at 40°C for 30 min. Next, 0.1 mL of the sodium acetate buffer and 3.0 mL of GOPOD reagent were added before incubation at 40°C for 20 min. Absorbance was measured at 510 nm.
2.3. Isolation and Structural Characterization of β-Glucan
Isolation of β-glucan from G. frondosa was done as described by Chakraborty et al. [18]. In brief, washing of G. frondosa particles (20-30 μm) was done with water followed by the ethanol and dried it in an air oven at 45°C for 48 h. After this, extraction of β-glucan was performed by boiling the dried particles in 4% NaOH solution for 30 min and kept it overnight at 4°C followed by centrifugation at 4000 rpm at 8°C for 1 h. The supernatant was separated and precipitated with 1:10 (v/v) ethanol and kept it for 48 h at 4°C. The precipitate was collected by centrifugation and repeated wash with ethanol and acetone. The obtained material was extensively dialyzed with cellulose bag to remove the small molecules as well as alkali. The precipitate was collected by centrifugation and freeze-dried. The structural properties of the extracted material were characterized by 1H-NMR measurement (400 MHz, JNM-ECZ400S/L1) in Me2SO-D2O (6:1) and matrix-assisted laser deposition/ionization (time-of-flight), MALDI-TOF mass spectrometry (Bruker Autoflex speed TOF/TOF) as described earlier [19].
2.4. Cytotoxicity Test
For the study of cytotoxicity, cell viability experiment was performed with human mesenchymal stem cells (hMSCs). The hMSCs isolated from bone (Korean Cell Line Bank, Republic of Korea) were cultured with proliferative medium (90% DMEM, 10% FBS, and 1% antibiotics) in a culture plate. Cell viability was analyzed using WST-1 assay (EZ-Cytox Cell Viability Assay Kit, Daeillab Service Co., Ltd., Republic of Korea). Before adding the samples (G1, G2, and G3), the hMSCs were cultured in 96-well plates for 24 h. The samples were added to each well at different concentrations (10, 50, and 100 μg/ml). The viability of cultured hMSCs at 24 h after sample adding was assayed using EZ-Cytox according to the described procedure. The cell medium with the untreated sample was set as a control. The absorbance was measured at 450 nm (OD450) using the spectrophotometer.
2.5. Antibody-Based Cytokine Array System
Human Cytokine Array C kit was purchased from Ray Biotech (AAH-CYT-1-2, Norcross, GA). The experiment was performed according to the manufacturer's instructions. The array membranes could detect 23 different growth factors/cytokines at a time. To investigate the immune effects of G1, G2, and G3 on cells, 2.2 × 106 cells/well were seeded into 100 mm culture plate. Cell medium without samples served as the control. Cells were grown with samples for 14 days. The cells were then starved to exclude the influence of serum cells for 24 h. Cell-free supernatants were obtained and analyzed by the human cytokine arrays. The membranes were treated with a blocking buffer and then treated with a sample at 4°C overnight. The membranes were then washed with wash buffer, and biotinylated antibody cocktail was added. The membranes were incubated at 4°C overnight and then washed, followed by incubation with the HRP-Streptavidin at 4°C overnight. Finally, the membranes were rewashed and developed by incubation with detection buffer for 5 min. Chemiluminescence of the membranes was measured using the Chemidoc XRS system (BR170-8265, Bio-Rad, USA). Relative protein expression was obtained by comparing the signal intensities.
2.6. Statistical Analysis
Data are presented as mean ± standard deviation. All statistical analyses were carried out using SPSS Statistics (IBM SPSS Statistics 23, IBM Inc., USA). Statistical significance between control and treatment groups was compared with a one-way analysis of variance (ANOVA). Statistical significance was considered ∗p < 0.05.
3. Results and Discussion
3.1. Analysis of β-Glucan Content in Different Particle Sizes
The three different sizes of G. frondosa studied in this work were obtained by air classifying mill. Figure 1 shows the granulometric distribution of the three different particle sizes by Malvern software. The accompanying 10, 50, and 90% values from the cumulative undersize curves are presented in Table 1. The d50 of samples (G1, G2, and G3) was 18.3, 22.8, and 32.7 μm, respectively. Also, high yields were maintained at 97% in the pulverization.
Figure 1.
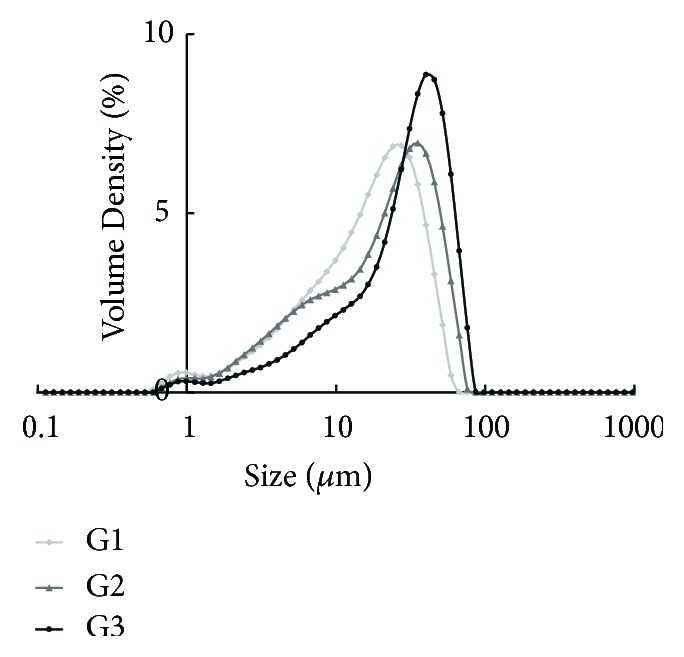
Particle size analysis of the G. frondosa powder by air classifying mill.
Table 1.
Granulometric distribution of G. frondosa samples.
| Sample | Parameters on Particle Size | Yield (%) | ||
|---|---|---|---|---|
| <d10 (μm) | <d50 (μm) | <d90 (μm) | ||
| G1 | 3.94 | 18.3 | 66.8 | 97 |
| G2 | 4.09 | 22.8 | 85.8 | 97 |
| G3 | 6.67 | 32.7 | 90.2 | 97 |
β-glucan contents of three different particle sizes in microparticulated G. frondosa are shown in Table 2. The experiment was repeated three times for each sample. The β-glucan contents of G1 and G3 were 18.80 ± 0.78% and 17.67 ± 0.33%, respectively. The highest average β-glucan content was found in a particle size of 20-30 μm (G2, 27.65 ± 0.30%). The β-glucan content of G. frondosa was generally reported to be 6.52% [20]. Furthermore, the β-glucan contents of G. frondosa were measured as 25.991% in a recent study [21]. These results indicated that the microsized mushroom could concentrate the β-glucan contents. Besides, it was shown that the β-glucan content in a particle size of 20-30 μm was more significant than the β-glucan content of various mushrooms pulverized to a capacity of 100 μm or less [22].
Table 2.
β-glucan contents via particle size of G. frondosa.
| Sample | β-glucan (%, w/w)(1) | RSD (%)(2) |
|---|---|---|
| G1 | 18.80 ± 0.78 | 4.16 |
| G2 | 27.65 ± 0.30 | 1.09 |
| G3 | 17.67 ± 0.33 | 1.90 |
(1)All values are expressed as mean ± SD in triplicate.
(2)Relative standard deviation.
3.2. Structural Analysis of β-Glucan
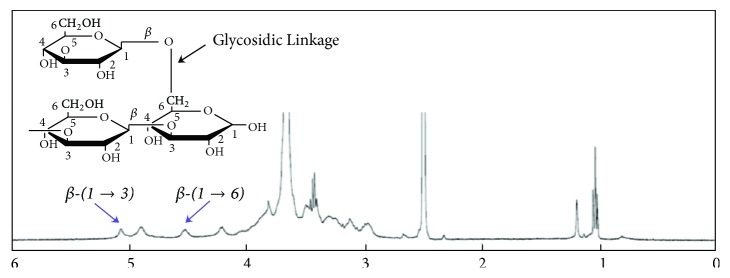
A 1H-NMR spectrum of β-glucan extracted from G. frondosa has been presented in Figure 2. It is well known that anomeric proton signal of the β-glucan has occurred in the range of 4-6 ppm depending upon the chemical moieties present in their structure [23]. The appearance of the peaks at 5 and 4.5 ppm in the 1H-NMR spectrum confirms the presence of the β-(1→3) and β-(1→6)-D-glucan linkage in the extracted material, respectively.
Figure 2.
A 1H-NMR spectrum of β-glucan extracted from G. frondosa.
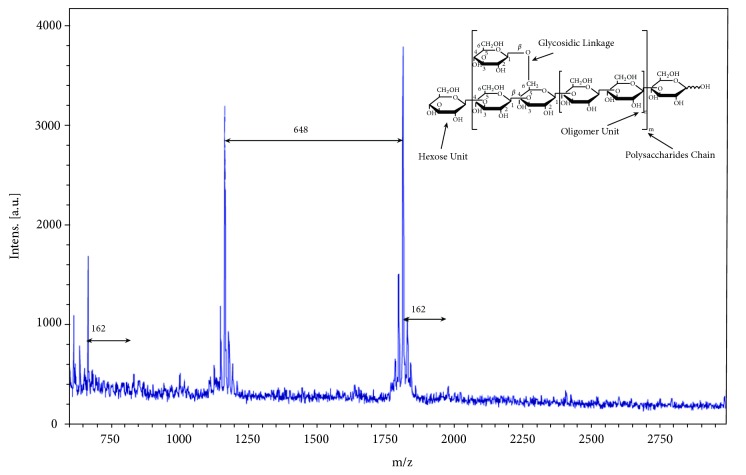
MALDI-TOF mass spectrum of β-glucan extracted from G. frondosa has been presented in Figure 3. MALDI-TOF mass analysis confirmed the presence of the β-(1→3) glucan structure in the extract with the molecular mass gap of 162 Da (one hexose unit) as observations have been reported earlier [24, 25]. 2,5-Dihydroxybenzoic acid (2,5-DHB) matrix improved the polysaccharide signals intensity in the MALDI-TOF mass spectrometry. However, it was interesting to note that in-between some peaks molecular gap was 648 Da. This was 4-fold higher than one hexose unit suggesting the presence of oligomer structures in the polymer chain. Mass spectrum of β-glucan extracted from the G. frondosa indicates that glucans are composed of a mixture of glucose units with molecular weights around 667.8 ~ 2406.8 Da with the degree of polymerization (DP) = 4 ~ 15 which is similar to the earlier reported values of glucan extracted from mushrooms [26]. On the basis of these experiments (NMR and Mass data), we concluded that extracted material has composed of hexose units with β-(1→3) and β-(1→6)-D-glucan linkage and presumable structure of glucans is given inside the mass spectrum.
Figure 3.
MALDI-TOF mass spectrum of G. frondosa glucans using 2, 5-DHB as a matrix. The gap with 162 Da between peaks represents a hexose unit in the extract. Fragmented ions appeared at DP = 4-15 with 667.8, 830.0, 1166.0, 1814.5, 1976.6, and 2406.0 Da, respectively.
3.3. Cytotoxicity Test
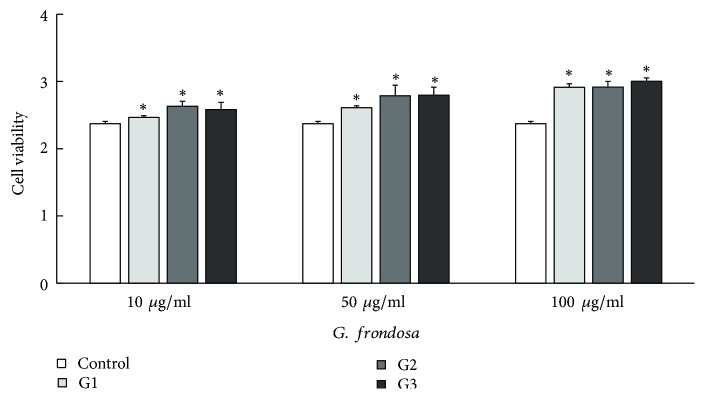
The in vitro cell culture experiments were performed with hMSCs (bone cell) to investigate the effect of sample concentration and the three different particle sizes on cell proliferation responses. The concentration of samples was set at 10, 50, and 100 μg/ml. The WST-1 assaying of hMSCs cells on an individual has been carried out after incubating bone cells for 2 days (24 h after addition) in a proliferative medium at 37°C. The results of the WST-1 cytotoxicity test for different particle sizes are shown in (Figure 4). From this figure, each of the microparticulated mushroom samples induced a concentration-dependent increase in cell proliferation. G. frondosa samples caused a significant increase (∗p < 0.05) in cell viability at 50 and 100 μg/ml. The cell viability at 10 μg/ml of G1 was almost the same (p = 0.611) but was higher at the other conditions (50 and 100 μg/ml). In this regard, the proliferation of cells was most accelerated at 100 μg/ml of G3 (increase 126% compared with the control group). These results have confirmed noncytotoxicity for the three different concentrations of G1, G2, and G3.
Figure 4.
WST-1 assay of hMSCs (bone cell) cultured for different particle sizes of G. frondosa. Values are means ± SD for three concentrations per sample. ∗p < 0.05 compared with the control group.
3.4. Protein Expression of Antibody-Based Cytokine Array
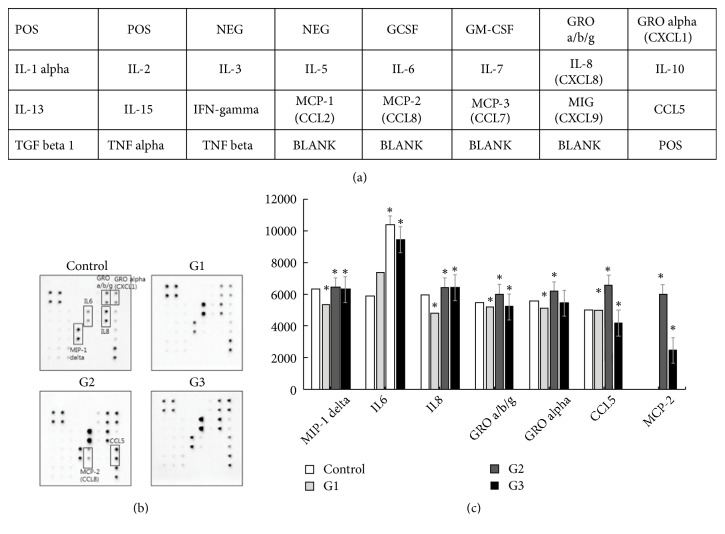
The cytokine expression profile using a human cytokine protein membrane array is shown in Figure 5. The membrane was exposed only to the culture supernatant of hMSCs. By using the antibody array (Figure 5(a)), the expression of 23 different antibodies in cell-free supernatants with or without G. frondosa treatment. The quantitation of cytokine was determined by calculating the intensity of the spot. Seven cytokines were detected as spots on the membranes (IL-6, IL-8, GRO a/b/g, GRO alpha, MCP-2, CCL5, and MIP-1) (Figure 5(b)). The mean for the signal densities for each of the membranes was calculated (Figure 5(c)). Interleukin 6 (IL-6) was upregulated about 126-177% in hMSCs with G. frondosa treatment. Although interleukin-8 (IL-8) was decreased by the addition of G1, it was increased by about 108% by addition of G2 and G3. Furthermore, the addition of G2 was induced that the three cytokines (chemokine ligand 1 (CXCL1), CC-chemokine ligand 5 (CCL5), and GRO a/b/g) were increased compared to control. When G2 and G3 were added to the cells, they induced monocyte chemotactic protein-2 (MCP-2) production. These results showed the immunomodulation effect on various cytokine levels expressed in the hMSCs with G. frondosa treatment. The characteristic features of the cytokines are their functional pleiotropy and redundancy which include interferons, interleukins, stimulating factors, and growth factors, and their regulating activities such as proliferation and differentiation are highly depended upon the nature of the cells involved [27]. These cytokines may exhibit the proinflammatory or anti-inflammatory activities or both, depending upon the target microenvironments [28]. IL-1β, TNFα, IL-6, IL-15, IL-17, and IL-18 are the most crucial group of cytokines that exhibit inflammatory activities, whereas IL-4, IL-10, and IL-13 are known as anti-inflammatory cytokines. It was noted that the role of inflammatory and anti-inflammatory cytokines in various inflammation concerning their signaling pathways is still ambiguous [29–31].
Figure 5.
Representative profile of the release of cytokines from hMSCs activated by microparticulated G. frondosa. (a) Template showing the location of antibodies for protein spotted onto the human cytokine antibody array kit. (b) Representative expression of various antibodies in the cell-free supernatants with or without G. frondosa treated. (c) Relative protein levels detected with the cytokine array. Values are means ± SD for three concentrations per sample. ∗p < 0.05 compared with the control group.
4. Conclusions
G. frondosa has various physiological activities. β-glucan, an essential component of G. frondosa, affects immune activity. β-glucan content in G. frondosa depends on particle size, raw materials, and extraction conditions. In this study, the particle size of G. frondosa was microsized to concentrate on β-glucan content (10-20, 20-30, and 30-40 μm). Furthermore, the immunostimulatory activity of G. frondosa was verified through cell activation ability and cytokine expression. β-glucan contents of the microsized G. frondosa were higher than generally known β-glucan contents for other particle sizes. It was confirmed that the microsized G. frondosa effectively concentrated the contents of β-glucan. There was also a significant difference in cell proliferation in the treated cells compared to the control. Higher cytokine production was observed in the treated cells than in control. In particular, the addition of G2 tended to increase all seven cytokines (IL-6, IL-8, GRO a/b/g, GRO alpha, MCP-2, CCL5, and MIP-1) expressed. The addition of G. frondosa of particle sizes at 20-30, 30-40 μm induced MCP-2 production, but the addition of particle sizes of 10-20 μm did not lead to expression. The expression of cytokines showed substantially higher cytokine production in the treated cells than in control. As a result, the microsized G. frondosa promoted cytokine production and is considered to be an excellent medicinal mushroom in terms of enhancing immunity. It is suggested that the increased expression of various cytokines could be useful in the control of immunity through anticancer and antitumor effects.
Acknowledgments
This research was supported by Cooperative Research Program for Fostering Local Specialized Industries (no. R0006141), Ministry of Trade, Industry and Energy, and Cooperative Research Program for Agriculture Science and Technology Development (no. PJ012854012 017), Rural Development Administration. The authors also acknowledge the Ministry of Education (no. 2018R1A6A1A03025582), Republic of Korea, for providing the financial support in the Basic Science Research Program through the National Research Foundation of Korea (NRF). This research was also supported by the BK 21 Plus through the National Research Foundation of korea (KRF) funded by the Ministry of Education (no. C1010546-010-07), Republic of Korea.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Yu-Ri Seo and Dinesh K. Patel are equivalent authors.
Conflicts of Interest
The authors state no conflicts of interest.
References
- 1.Wagner H. Search for plant derived natural products with immunostimulatory activity (recent advances) Pure and Applied Chemistry. 1990;62(7):1217–1222. doi: 10.1351/pac199062071217. [DOI] [Google Scholar]
- 2.Patel S., Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2012;2(1):1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reis F. S., Martins A., Vasconcelos M. H., et al. Functional foods based on extracts or compounds derived from mushrooms. Trends in Food Science and Technology. 2017;66:48–62. doi: 10.1016/j.tifs.2017.05.010. [DOI] [Google Scholar]
- 4.Minato K.-I., Ohara A., Mizuno M. A proinflammatory effect of the β-Glucan from Pleurotuscornucopiae mushroom on macrophage action. Mediators of Inflammation. 2017;2017:9. doi: 10.1155/2017/8402405.8402405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klaus A., Kozarski M., Vunduk J., et al. Biological potential of extracts of the wild edible basidiomycete mushroom grifola frondosa. Food Research International. 2015;67:272–283. doi: 10.1016/j.foodres.2014.11.035. [DOI] [Google Scholar]
- 6.Ohno N., Sato K., Adachi Y., Suzuk I., Yadoma T., Oikawa S. Characterization of the antitumor glucan obtained from liquid-cultured grifola frondosa. Chemical & Pharmaceutical Bulletin. 1986;34(4):1709–1715. doi: 10.1248/cpb.34.1709. [DOI] [PubMed] [Google Scholar]
- 7.Masuda Y., Inoue M., Miyata A., Mizuno S., Nanba H. Maitake β-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. International Immunopharmacology. 2009;9(5):620–626. doi: 10.1016/j.intimp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Ayeka P. A. Potential of mushroom compounds as immunomodulators in cancer immunotherapy: a review. Evidence-Based Complementary and Alternative Medicine. 2018;2018:9. doi: 10.1155/2018/7271509.7271509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du B., Bian Z., Xu B. Skin health promotion effects of natural Beta-Glucan derived from cereals and microorganisms: a review. Phytotherapy Research. 2014;28(2):159–166. doi: 10.1002/ptr.4963. [DOI] [PubMed] [Google Scholar]
- 10.Elder M. J., Webster S. J., Chee R., Williams D. L., Hill Gaston J. S., Goodall J. C. β-glucan size controls dectin-1-mediated immune responses in human dendritic cells by regulating IL-1β production. Frontiers in Immunology. 2017;8:p. 791. doi: 10.3389/fimmu.2017.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saulnier L., Gévaudan S., Thibault J.-F. Extraction and partial characterisation of β-glucan from the endosperms of two barley cultivars. Journal of Cereal Science. 1994;19(2):171–178. doi: 10.1006/jcrs.1994.1023. [DOI] [Google Scholar]
- 12.Temelli F. Extraction and functional properties of barley β-glucan as affected by temperature and pH. Journal of Food Science. 1997;62(6):1194–1201. doi: 10.1111/j.1365-2621.1997.tb12242.x. [DOI] [Google Scholar]
- 13.Wu Y., Stringfellow A., Inglett G. Protein and β-glucan enriched fractions from high-protein, high β-glucan barleys by sieving and air classification. Cereal Chemistry. 1994;71(3):220–223. [Google Scholar]
- 14.Shen J., Ren H., Tomiyama-Miyaji C., et al. Potentiation of intestinal immunity by micellary mushroom extracts. Journal of Biomedical Research. 2007;28(2):71–77. doi: 10.2220/biomedres.28.71. [DOI] [PubMed] [Google Scholar]
- 15.Adachi Y., Okazaki M., Ohno N., Yadomae T. Enhancement of cytokine production by macrophages stimulated with (l→3)-β-D-glucan, grifolan (GRN), isolated from grifola frondosa. Biological and Pharmaceutical Bulletin. 1994;17(12):1554–1560. doi: 10.1248/bpb.17.1554. [DOI] [PubMed] [Google Scholar]
- 16.Kodama N., Murata Y., Nanba H. Administration of a polysaccharide from Grifola frondosa stimulates immune function of normal mice. Journal of Medicinal Food. 2004;7(2):141–145. doi: 10.1089/1096620041224012. [DOI] [PubMed] [Google Scholar]
- 17.Mcclear B. V., Glennie‐Holmes M. Enzymic quantification OF (1→3) (1→4)‐β‐D‐glucan in barley and malt. Journal of the Institute of Brewing. 1985;91(5):285–295. doi: 10.1002/j.2050-0416.1985.tb04345.x. [DOI] [Google Scholar]
- 18.Chakraborty I., Mondal S., Rout D., Islam S. S. A water-insoluble (1→3)-β-d-glucan from the alkaline extract of an edible mushroom Termitomyces eurhizus. Carbohydrate Research. 2006;341(18):2990–2993. doi: 10.1016/j.carres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Tada R., Harada T., Nagi-Miura N., et al. NMR characterization of the structure of a β-(1→3)-d-glucan isolate from cultured fruit bodies of Sparassis crispa. Carbohydrate Research. 2007;342(17):2611–2618. doi: 10.1016/j.carres.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Seoane P., González-Muñoz M. J., Falqué E., Domínguez H. Pressurized hot water extraction of β-glucans from Cantharellus tubaeformis. Electrophoresis. 2018 doi: 10.1002/elps.201700399. [DOI] [PubMed] [Google Scholar]
- 21.Sari M., Prange A., Lelley J. I., Hambitzer R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chemistry. 2017;216:45–51. doi: 10.1016/j.foodchem.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Özcan Ö., Ertan F. Beta-glucan content, antioxidant and antimicrobial activities of some edible mushroom species. Food Science and Technology. 2018;6(2):47–55. doi: 10.13189/fst.2018.060201. [DOI] [Google Scholar]
- 23.Park H.-G., Shim Y. Y., Choi S.-O., Park W.-M. New method development for nanoparticle extraction of water-soluble β-(1→3)-D-glucan from edible mushrooms, sparassis crispa and Phellinus linteus. Journal of Agricultural and Food Chemistry. 2009;57(6):2147–2154. doi: 10.1021/jf802940x. [DOI] [PubMed] [Google Scholar]
- 24.Chan T.-W. D., Tang K. Y. Analysis of a bioactive β-(1 → 3) polysaccharide (Curdlan) using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2003;17(9):887–896. doi: 10.1002/rcm.991. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Jiang G., Vasanthan T., Sporns P. MALDI-MS characterization of maltooligo/polysaccharides from debranched starch amylopectin of corn and barley. Starch - Stärke. 1999;51(7):243–248. doi: 10.1002/(SICI)1521-379X(199907)51:7<243::AID-STAR243>3.0.CO;2-B. [DOI] [Google Scholar]
- 26.Hung W.-T., Wang S.-H., Chen C.-H., Yang W.-B. Structure determination of β-glucans from Ganoderma lucidum with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Molecules. 2008;13(8):1538–1550. doi: 10.3390/molecules13081538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Su D., Lu Z., Shen M., Li X., Sun L. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. Journal of Biomedicine and Biotechnology. 2012;2012:15. doi: 10.1155/2012/347141.347141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojdasiewicz P., Poniatowski Ł. A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of Inflammation. 2014;2014:19. doi: 10.1155/2014/561459.561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D. G., Seo S. W., Cho B. K., et al. Review of current approaches for implementing metabolic reconstruction. Journal of Biosystems Engineering. 2018;43:45–58. [Google Scholar]
- 31.Seo Y. R., Kim J. W., Seonwoo H., et al. Cellulose-based nanocrystals: sources and applications via agricultural byproducts. Journal of Biosystems Engineering. 2018;43:59–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.