Abstract
Purpose
Leptin is a nutritional cytokine encoded by the obesity gene whose concentration in the tumor microenvironment is closely related to the occurrence and progression of cancer. However, previous evidence has suggested that there is no clear relationship between serum leptin concentrations and lung cancer progression. Cancer-associated fibroblasts (CAFs), the most abundant component of the tumor microenvironment in a variety of solid tumors, were recently reported to produce leptin. Therefore, it was inferred that leptin is most likely to affect non-small-cell lung cancer (NSCLC) through an autocrine and paracrine mechanism. In the current study, we investigated the paracrine effect and mechanism of leptin produced by CAFs on NSCLC by establishing a novel in vitro cell coculture system.
Methods
A noncontact coculture device was designed and made by 3D printing. CAFs and paired normal lung fibroblasts (NLFs) from 5 patients were successfully isolated and cocultured with two NSCLC cell lines in a coculture system. The background expression of leptin was detected by western blot. The in situ expression of leptin and its receptor (Ob-R) in NSCLC tissues and paired normal lung tissues was analyzed by immunohistochemistry. Furthermore, we downregulated the expression of leptin in CAFs and assessed changes in its promotion on NSCLC cells in the coculture system. Finally, changes in the phosphorylation of ERK1/2 and AKT were examined to investigate the molecular mechanisms responsible for the paracrine promotion of NSCLC cells by leptin.
Results
Leptin was overexpressed in nearly all five primary CAF lines compared with its expression in paired NLFs. IHC staining showed that the expression of leptin was high in NSCLC cells, slightly lower in CAF, and negative in normal lung tissue. Ob-R was strongly expressed in NSCLC cells. The ability of A549 and H1299 cells to proliferate and migrate was enhanced by high leptin levels in both the cocultured fibroblasts and the culture medium. Furthermore, western blot assays suggested that the MAPK/ERK1/2 and PI3K/AKT signaling pathways were activated by leptin produced by CAFs, which demonstrated that the functions of paracrine leptin in NSCLC are as those of the serum leptin to other cancers.
Conclusion
Leptin produced by CAF promotes proliferation and migration of NSCLC cells probably via PI3K/AKT and MAPK/ERK1/2 signaling pathways in a paracrine manner.
1. Introduction
Lung cancer is the most common malignancy worldwide [1]. Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers [2], and is usually diagnosed at an advanced stage, often with metastases [3]. Therefore, the elucidation of the mechanisms involved in occurrence and progress of NSCLC could lead to novel diagnostic and therapeutic approaches [4].
Obesity is considered to be a growing health problem worldwide [5]. Leptin, a 16 kDa peptide hormone encoded by LEP gene (an obesity gene) and a nutritional cytokine, is closely related to cancer [6]. The main function of leptin is to regulate energy metabolism and fat synthesis in the body, and it also amplifies inflammation and immunity signals [7]. Leptin exerts its effects through a specific transmembrane receptor on the surface of its target cells, the obesity receptor (Ob-R), which is assigned to the class I cytokine receptor family [8]. The binding of leptin to its receptor activates multiple downstream signaling pathways such as those involving JAK2-STAT3, mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase/ protein kinase B (PI3K/AKT) [9]. Therefore, leptin-mediated signaling pathways play an important role in cancer cell proliferation, invasion, and metastasis [10].
In recent years, crosstalk between the tumor microenvironment and cancer cells has received increasing attention [11]. The tumor microenvironment is the internal environment in which cancer cells intergrow and interact with the “nontumoral” components [12]. Cancer-associated fibroblasts (CAFs), the most abundant component of the tumor microenvironment of NSCLC, account for 70% of the total number of cells in solid tumors [13] and are most often identified by the expression of myofibroblastic biomarkers such as α-smooth muscle actin (α-SMA) [14]. Besides, fibroblast activating protein (FAP) and vimentin are also used as auxiliary markers to identify CAF [15]. CAFs can promote tumor progression through the secretion of various cytokines.
In addition to its synthesis by adipocytes, leptin can also be synthesized by epithelial cells or fibroblasts. Leptin is usually absent in nonneoplastic tissue; however, recent studies demonstrated that, leptin was also secreted by CAFs in breast cancer [16]. Thus, we inferred that CAF in NSCLC tissues can also produce leptin and affect the function of NSCLC in a paracrine manner. This hypothesis is supported by several recent pieces of evidence, as the increase of leptin concentration in the microenvironment was related to the occurrence and metastasis of several types of cancer [17–19]. On the other hand, a recent meta-analysis [20] suggested that there may be no clear relationship between the serum leptin concentration and lung cancer progression. This may be due to the special histological characteristics of alveoli and bronchi. Therefore, leptin is most likely to affect NSCLC through autocrine and paracrine effects. In the current study, we investigated the protumorigenic effect of leptin produced by CAFs on NSCLC by establishing a novel in vitro cell coculture system.
2. Methods
2.1. Cell Lines
Human embryonic lung fibroblasts (HLF) and the human NSCLC cell lines A549 and H1299 were obtained from the American type culture collection (ATCC), and all cell lines were cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and penicillin/streptomycin at 37°C in 5% CO2.
2.2. Culture of Primary Fibroblasts
Tissue samples from 5 patients who had a definitive pathological diagnosis of stage IIIA NSCLC were resected by thoracoscopic lobectomy in the 1st Hospital of Dalian Medical University in April 2018. The tissues were soaked in DMEM at 0°C and digested within 2 h of resection. Tumor tissues and their paired normal lung tissues (4~5 cm away from the incisal margin) were homogenized and digested for 2.5~4 h at 37°C in DMEM containing 0.1 mg/mL DNase I (Roche, Switzerland) and 1 mg/mL collagenase (Roche, Switzerland). The cells were filtered with a 75 μm filter and then resuspended and plated with DMEM containing 1% penicillin/streptomycin and 15% FBS. The cultures were maintained at 37°C in 5% CO2. After 5 passages, fibroblast purity was tested by RT-qPCR. Then, CAFs were immortalized with SV40-large T antigen (EX-SV40T-Lv105, GeneCopoeia, USA) following the recommended protocol.
2.3. Noncontact Coculture System
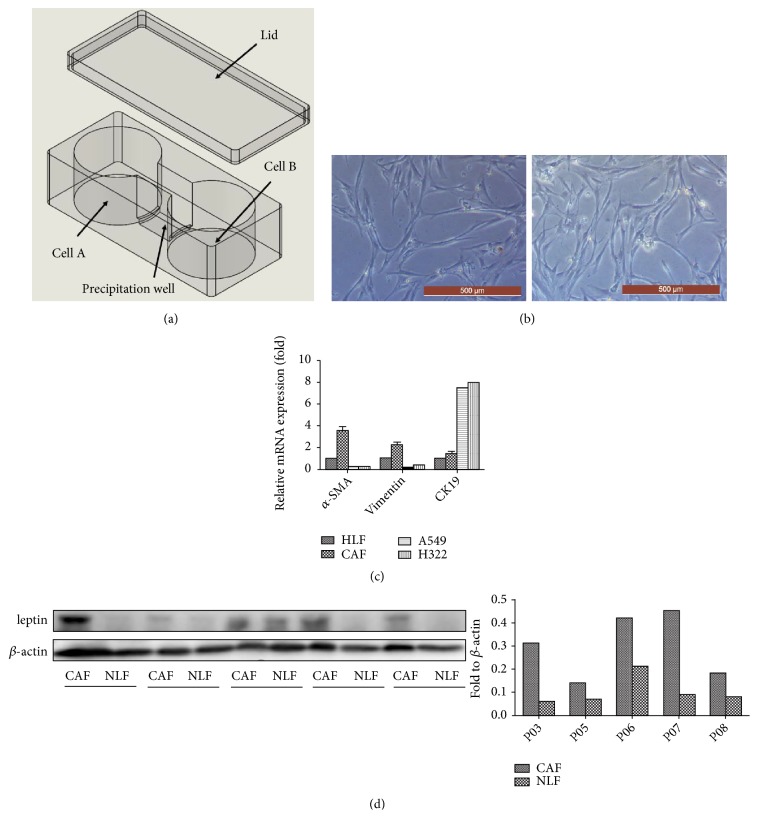
A noncontact coculture device was designed (already in the patent examination and approval process in China) and made by 3D printing (Wanwan 3D, Dongguan, China) with highly transparent nontoxic resin to simulate the internal environment. The device was a vessel consisting of two culture wells and a precipitation well (Figure 1(a)). The culture wells and the precipitation well were separated by two 2 mm-high partitions. During cell seeding, the level of the liquid in both culture wells should be kept below the height of the partition. Fibroblasts and NSCLC cells were seeded into the two culture wells, at a 2:1 ratio. After cell adherence, DMEM containing 10% FBS was added to the vessel until the level was above the partition. Based on the design of the coculture device, floating cells do not go directly into the contralateral culture well but are deposited into the precipitation well. Before anything was done to the cells, the medium in the precipitation well was drained in case any floating cells entered their contralateral culture well. Then, the two cell lines were isolated again, and 0.5% trypsin was used for separate passage/collection of the two cell lines.
Figure 1.
Isolation and characterization of CAFs and NLFs. (a) Three-dimensional model of the novel noncontact coculture device. (b) Morphology of primary fibroblasts under a microscope. Left: CAF, right: NLF. Scale bar = 500 μm. (c) The purity of the primary cells was verified by RT-qPCR to determine the levels of α-SMA, vimentin, and CK19. (d) Leptin expression in primary CAF and NLF cell lines was detected with western blot. The quantification of leptin is shown in the bar chart.
2.4. Conditioned Medium
Fibroblasts were cultured in DMEM with 10% FBS and routine antibiotics. Then, the medium was changed to FBS-free medium when the cells had grown to 80% confluence. After 48 h, the supernatant was extracted and centrifuged at 1600×g for 10 min. The conditioned medium (CM) was stored at –79°C and used for the colony formation assay.
2.5. Reagents and Antibodies
Recombinant human leptin was purchased from Pepro Tech (Rocky Hill, USA). A primary antibody against α-SMA was purchased from Abcam (Cambridge, UK). Primary antibodies against Ob-R were purchased from Wanleibio (Shenyang, China). A leptin antibody was obtained from Bioss (Beijing, China). Other primary antibodies against AKT, p-AKT, ERK, and p-ERK were obtained from Cell Signaling Technology (Massachusetts, USA). And other chemicals were purchased from Sigma (St. Louis, USA) and Vetec (St. Louis, USA).
2.6. Plasmids and Lentivirus
pLK0.1-PXR-shRNA targeting the LEP gene was acquired from the Institute of Cancer Stem Cell at Dalian Medical University and was packaged with the pMD2.G lentivirus packing system (GeneCopoeia, USA). Viral packaging was conducted based on the recommended protocols and previous articles.
2.7. RNA Extraction and Real-Time RT-PCR
Total RNA was extracted from CAFs and the NLFs using the TRIzol method according to previous articles [21]. TransScript One-step gDNA Removal and cDNA Synthesis Supermix (Transgene, Beijing, China) was used to synthesize first strand cDNA. SYBR Premix Ex Taq II (RR820A, Takara, Japan) was used for quantitative polymerase chain reaction according to the manufacturer's instructions. The primer sequences used were as follows: α-SMA:5'- ATTGCCGACCGAATGCAGA -3',5'- ATGGAGCCACCGATCCAGAC-3'; vimentin: 5'- TGCCGTTGAAGCTGCTAACTA -3', 5'- CCAGAGGGAGTGAATCCAGATTA -3'; CK-19: 5'- ACCAAGTTTGAGACGGAACAG -3', 5'- CCCTCAGCGTACTGATTTCCT -3'. The comparative Ct method was used to calculate relative changes in gene expression.
2.8. Immunohistochemistry Staining
IHC staining of leptin, Ob-R and α-SMA was performed according to the manufacturer's instructions. A streptavidin-peroxidase staining kit was purchased from ZSGB BIO (Beijing, China). The paraffin-embedded tissues were prepared by a pathology specialist, and dewaxed, and rehydrated in the lab. The experimental steps were carried out according to the instructions of the SP kit. The tissues were incubated with primary antibodies diluted to the recommended concentration overnight at 4°C with antibodies that was dilute to the recommended concentration. Then 3,3′-diaminobenzidine (DAB) staining was performed, and the results were observed under a microscope.
2.9. Western Blot Analysis
Total protein was collected according to the methods in our previous article [22]. The protein concentration of the cell lysis solution was assessed using a Thermo Fisher BCA kit. Then 30 μg of total protein was run on 10% SDS-PAGE and transferred to a PVDF membrane. Samples were incubated with primary antibodies diluted to the recommended concentration overnight at 4°C. The samples were incubated with HRP-conjugated secondary antibody at room temperature for 2 h and protein bands were detected with a chemiluminescence device.
2.10. Colony Formation Assay
Briefly, A549 cells or H1299 cells were seeded into six-well plates (2 × 103 per well) and incubated in complete DMEM for 24 h. Then, the medium was replaced by the abovementioned CM with 10% FBS, and the cells were cultured in a 37°C incubator with 5% CO2 for 2 weeks until they grew into macroscopic colonies. Finally, the medium was removed, and the cell colonies were stained with 0.1% crystal violet and counted.
2.11. Cell Migration Assay
Cell migration assays were performed in Transwell chambers with an 8.0μm pore size (Corning, US). The cells were starved in FBS-free DMEM overnight before they were collected and resuspended. A total of 1×105 A549 or H1299 cells were resuspended in 250 μL DMEM with 10% FBS and injected into the upper chamber. A total of 3×105 CAF cells were resuspended in 500 μL DMEM with 10% FBS and injected into the lower chamber. After 24 h, the upper chamber was washed with PBS, the nonpenetrated cells were removed with a cotton swab, and the Transwell chamber was dried at RT. The migrated cells at the back side of the Transwell membrane were stained with 0.1% crystal violet and then observed under a microscope.
3. Results
3.1. Isolation and Characterization of CAFs and NLFs
CAFs and paired normal lung fibroblasts (NLFs) from 5 patients were successfully established, their cell morphology was first observed under an inverted microscope (Figure 1(b)), and no significant difference was observed between the two cell lines. The purity of the primary cells was verified by biomarkers using RT-qPCR. α-SMA, vimentin, and FAP were more strongly expressed, while CK19 and E-cadherin were more weakly expressed in the 5 CAF lines compared with their expression in the respective paired normal fibroblasts (Figure 1(c)). Leptin was overexpressed in almost all five primary CAF lines compared to its expression in paired corresponding NLFs (Figure 1(d)).
3.2. A Noncontact Coculture Model Was Successfully Established
One of the five CAF cell lines (CAF03), whose leptin background expression level was the highest, was selected for further study. Since it was difficult to stably culture NLFs in vitro, HLF cells were used as an alternative to the NLFs in the coculture system. In the coculture device, CAFs and HLF were successfully cocultured with A549 and H1299 cells through 5 consecutive passages.
3.3. Leptin Produced by CAFs Promotes the Proliferation of NSCLC
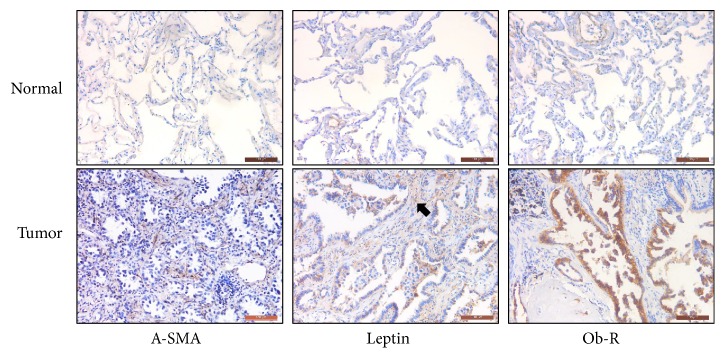
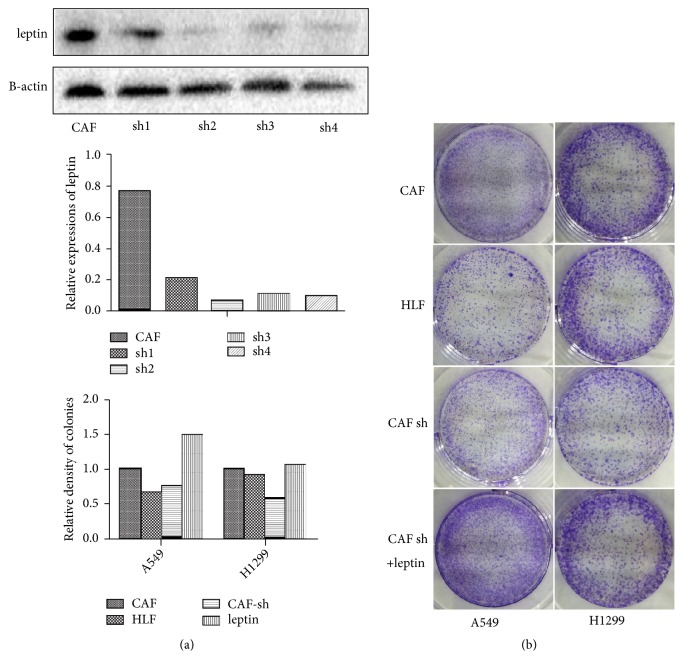
The original tumor tissue of the CAF03 cell line (from a 54-year-old female patient with stage IIIA acinar growth predominant lung adenocarcinoma) and its adjacent normal lung tissue were stained by immunohistochemistry, and α-SMA clearly marked the location of the stroma. IHC staining showed that leptin was highly expressed in epithelial cells and expressed at a slightly lower level in the cytoplasm of tumor stromal cells (mainly CAF). In contrast, both α-SMA and leptin were not expressed in normal lung tissues (Figure 2). Ob-R was strongly expressed in NSCLC cells and was expressed at low levels in both tumor stroma and normal tissues. Subsequently, the LEP gene in CAFs was stably downregulated by lentivirus transfection (sh4) with shRNA (Figure 3(a)). The ability of A549 and H1299 cells cultured with CAF-CM to form colonies was greater than that of cells cultured with HLF-CM. However, A549 and H1299 cells cultured with shLEP-CAF-CM showed inhibited proliferation, while this decrease in the ability to form colonies was reversed by the addition of 50 ng/ml recombinant leptin into the CM (Figure 3(b)).
Figure 2.
Immunohistochemical analyses of α-SMA, leptin and Ob-R in NSCLC. The expression of α-SMA, leptin, and Ob-R in the tumor and paired normal lung tissues of a 54-year-old male patient with acinar growth predominant lung adenocarcinoma was detected by IHC assay. The leptin overexpressed in the tumor stroma is marked with a black arrow in the corresponding position. Scale bar = 100 μm.
Figure 3.
The proliferation of NSCLC cells was inhibited by the downregulation of leptin in CAFs. (a) The efficiency of gene intervention by four different shRNA fragments was verified by western blot. (b) The proliferation of NSCLC cell lines treated with the CM of different fibroblasts was assessed with a colony formation assay. The quantification of cell colonies is shown in the bar chart.
3.4. Leptin Produced by CAFs Promotes Migration of NSCLC
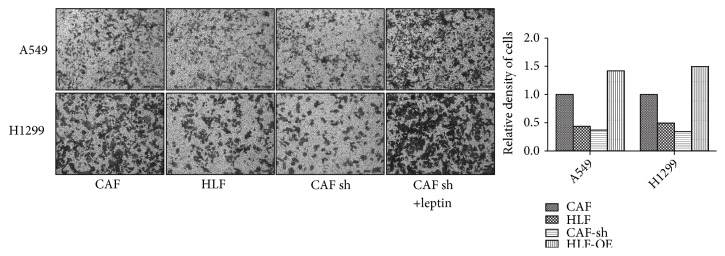
Similar to the results of the cloning assay, the migration of A549 and H1299 cells cocultured with CAFs was enhanced compared with that of cells cocultured with HLF cells. While the expression of leptin was downregulated by shLEP in CAFs, the cocultured A549 and H1299 cells showed inhibited migration. However, this decrease in migration was reversed by the addition of 50 ng/ml recombinant into the medium (Figure 4).
Figure 4.
The migration of NSCLC cells was inhibited by the downregulation of leptin in CAFs. The migration of NSCLC cell lines cocultured with different fibroblasts was assessed by Transwell migration assay. The quantification of the migrated cells is shown in the bar chart.
3.5. Leptin Produced by CAFs Mediates the Activation of Inflammatory Cytokine-Related Pathways in NSCLC
A549 cells grown with HLF, CAFs, CAF-shLEP, and CAF-shLEP+leptin were continuously cultured to four passages in the coculture system. Then the total proteins of the A549 cells were analyzed by western blot (Figure 5). The expression of Ob-Rb was no different in the four groups. Furthermore, changes in the phosphorylation of ERK1/2 and AKT were examined to investigate the molecular mechanisms responsible for the paracrine promotion of NSCLC cells by leptin. Similarly, leptin significantly increased the phosphorylation of ERK1/2 and AKT. This result suggested that these two pathways, which are closely related to the internal inflammatory malignant environment, were activated under conditions of high paracrine leptin secretion.
Figure 5.
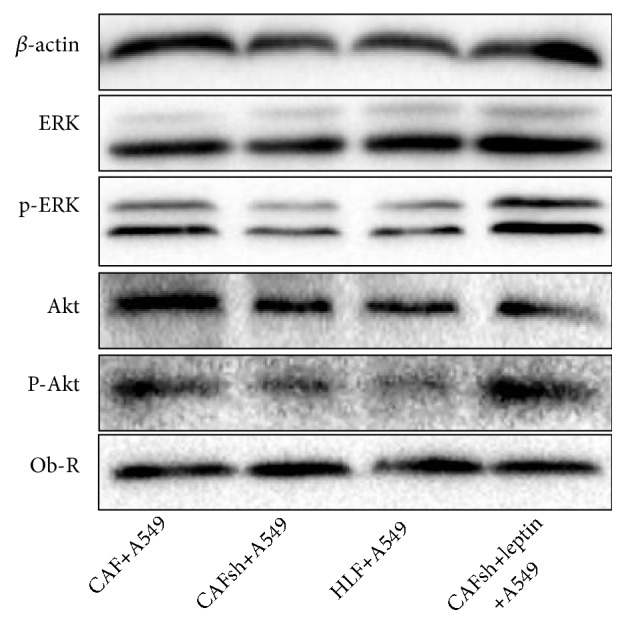
The phosphorylation of ERK1/2 and AKT depends on paracrine leptin. (a) Leptin significantly increased the phosphorylation of ERK1/2 and AKT. Three independent experiments were performed, and representative protein bands from one of the three tests are shown. Leptin treatment: 50 ng/ml, 1 hour.
4. Discussion
CAFs act on NSCLC cells by secreting a variety of cytokines, thereby affecting the proliferation, migration, invasion, and other biological functions of NSCLC cells. At the same time, cancer cells have positive feedback on the activation and maintenance of the cancer-associated properties of CAFs [23]. Researchers have tried many methods to simulate tumor microenvironment in both in vivo and in vitro experiments, especially to revive the interaction between CAFs and tumor cells. Classic models include conditioned medium, the Transwell system [24], the Boyden chamber system [25], and xenoanimal coinjection models [26]. In recent years, microfluidic chip technology [27] and the patient-derived xenograft mouse model [28] (PDX) have also become common research tools. In the current study, we introduced a novel noncontact coculture device, that solves the limitations of previous models in terms of cell quantity and passages, and its practicality was experimentally verified.
Leptin is a cytokine and hormone first shown to be secreted by adipose tissue [29]. Leptin is also produced and secreted by many nonadipose tissues, including gastric mucosal [30], breast [31], muscle [32], placenta [33], and lung tissues [34]. Studies have suggested that a leptin-enriched microenvironment promotes tumor cells [17–19, 35]. In the current study, we confirmed that leptin was overexpressed in NSCLC and was produced not only from epithelial cells, but also from CAFs. Furthermore, we found that the receptor of leptin, Ob-Rb, was only overexpressed in NSCLC cells, and was not expressed in CAFs. As previous studies have shown that serum leptin has no significant effect on lung cancer, we hypothesized that leptin most likely affects NSCLC through autocrine and paracrine mechanisms.
Furthermore, we observed the effect of paracrine leptin on NSCLC, and found that CAF-produced leptin reversibly promoted colony formation and migration of NSCLC cells. On this basis we found that the function of paracrine leptin was similar to that of the addition of recombinant leptin directly into the medium. Nevertheless, the downregulation of leptin produced by CAFs can offset this effect. This result confirmed that paracrine leptin promotes the proliferation and migration of NSCLC cells. However, given that NSCLC cells themselves also produce large amounts of leptin, whether autocrine leptin has an effect on NSCLC still needs to be explored in future studies.
The function of leptin depends on a specific receptor (leptin receptor, OB- Rb) on the surface of its target cells [36]. Ob-R is expressed at very low levels in epithelial cells from normal human mammary glands, but it is overexpressed in cancer cells [37]. A variety of inflammatory cytokines and inflammatory-mediating pathways are downstream of the leptin-OB-Rb signaling pathway, such as the PI3K/AKT, MAPK/ERK1/2, JAK2/STAT3, and insulin receptor substance (IRS) pathways [38]. In the current study, we investigated changes in key molecules from several signaling pathways in NSCLC cells cocultured with CAFs and found that the MAPK/ERK1/2 and PI3K/AKT signaling pathways were activated by leptin produced by CAFs, which demonstrated that the functions of paracrine leptin in NSCLC were similar to those in the same pattern with that of the serum leptin to in other cancers. Thus, it was concluded that leptin produced by CAFs promotes the proliferation and migration of NSCLC cells probably via the PI3K/AKT and MAPK/ERK1/2 signaling pathways in a paracrine manner.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81173453, 81774078, and 81803886) and Natural Science Foundation of Liaoning Province, China (201602227).
Contributor Information
Jinxiu Li, Email: lijinxiu@dmu.edu.cn.
Chundong Gu, Email: guchundong@dmu.edu.cn.
Data Availability
The experimental data used to support the findings of this study are included within the article. The 3D printing drawing data are available from the corresponding author upon request. Detailed data of the patients involved in the study are restricted by the Ethics Committee of the 1st Hospital of Dalian Medical University. Data are available from Dr. Gu (guchundong@dmu.edu.cn) for researchers who meet the criteria for access to confidential data.
Conflicts of Interest
The author reports no conflicts of interest in this work.
Authors' Contributions
All authors contributed to the design of the study and the preparation and critical revision of the manuscript and agreed to be accountable for all aspects of the study. The manuscript is approved by all authors for publication. Fengzhou Li and Shilei Zhao contributed equally to this study.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics. CA: A Cancer Journal for Clinicians. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Li F., Zhang S., Zhang Q., Li J., Zhao S., Gu C. CYP1B1 G199T polymorphism affects prognosis of NSCLC patients with the potential to be an indicator and target for precise drug intervention. BioMed Research International. 2017;2017 doi: 10.1155/2017/1529564.1529564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu T., Guo Z., Fan H., et al. Cancer-associated fibroblasts promote non-small cell lung cancer cell invasion by upregulation of glucose-regulated protein 78 (GRP78) expression in an integrated bionic microfluidic device. Oncotarget. 2016;7(18):25593–25603. doi: 10.18632/oncotarget.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shafiee M., Mohammadzadeh E., Shahidsales S., et al. Current status and perspectives regarding the therapeutic potential of targeting EGFR pathway by curcumin in lung cancer. Current Pharmaceutical Design. 2017;23(13):2002–2008. doi: 10.2174/1381612823666170123143648. [DOI] [PubMed] [Google Scholar]
- 5.Zhou X., He L., Zuo S., et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2018;1864(2):488–498. doi: 10.1016/j.bbadis.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Gui X., Chen H., Cai H., Sun L., Gu L. Leptin promotes pulmonary fibrosis development by inhibiting autophagy via PI3K/Akt/mTOR pathway. Biochemical and Biophysical Research Communications. 2018;498(3):660–666. doi: 10.1016/j.bbrc.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 8.Uddin S., Bu R., Ahmed M., et al. Leptin receptor expression and its association with PI3K/AKT signaling pathway in diffuse large B-cell lymphoma. Leukemia & Lymphoma. 2010;51(7):1305–1314. doi: 10.3109/10428191003802365. [DOI] [PubMed] [Google Scholar]
- 9.Giordano C., Chemi F., Panza S., et al. Leptin as a mediator of tumor-stromal interactions promotes breast cancer stem cell activity. Oncotarget . 2016;7(2):1262–1275. doi: 10.18632/oncotarget.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zabeau L., Lavens D., Peelman F., Eyckerman S., Vandekerckhove J., Tavernier J. The ins and outs of leptin receptor activation. FEBS Letters. 2003;546(1):45–50. doi: 10.1016/s0014-5793(03)00440-x. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y., Lin D., Taniguchi C. M. Hypoxia inducible factor (HIF) in the tumor microenvironment: friend or foe? Science China Life Sciences. 2017;60(10):1114–1124. doi: 10.1007/s11427-017-9178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berdiel-Acer M., Sanz-Pamplona R., Calon A., et al. Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information. Molecular Oncology. 2014;8(7):1290–1305. doi: 10.1016/j.molonc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vicent S., Sayles L. C., Vaka D., et al. Cross-species functional analysis of cancer-associated fibroblasts identifies a critical role for CLCF1 and IL-6 in non-small cell lung cancer in vivo. Cancer Research. 2012;72(22):5744–5756. doi: 10.1158/0008-5472.CAN-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goicoechea S. M., García-Mata R., Staub J., et al. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene. 2014;33(10):1265–1273. doi: 10.1038/onc.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao Z., Tan Z. W., Zhu P., Tan N. S. Cancer-associated fibroblasts in tumor microenvironment - Accomplices in tumor malignancy. Cellular Immunology. 2018 doi: 10.1016/j.cellimm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Barone I., Catalano S., Gelsomino L., et al. Leptin mediates tumor-stromal interactions that promote the invasive growth of breast cancer cells. Cancer Research. 2012;72(6):1416–1427. doi: 10.1158/0008-5472.CAN-11-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calgani A., Delle Monache S., Cesare P., Vicentini C., Bologna M., Angelucci A. Leptin contributes to long-term stabilization of HIF-1α in cancer cells subjected to oxygen limiting conditions. Cancer Letters. 2016;376(1):1–9. doi: 10.1016/j.canlet.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Li K., Wei L., Huang Y., et al. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. International Journal of Oncology. 2016;48(6):2479–2487. doi: 10.3892/ijo.2016.3483. [DOI] [PubMed] [Google Scholar]
- 19.Yousef A. I., El-Masry O. S., Yassin E. H. The anti-oncogenic influence of ellagic acid on colon cancer cells in leptin-enriched microenvironment. Tumor Biology. 2016;37(10):13345–13353. doi: 10.1007/s13277-016-5284-7. [DOI] [PubMed] [Google Scholar]
- 20.Tong X., Ma Y., Zhou Q., et al. Serum and tissue leptin in lung cancer: A meta-analysis. Oncotarget . 2017;8(12):19699–19711. doi: 10.18632/oncotarget.14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rio D. C., Ares M., Jr., Hannon G. J., Nilsen T. W. Purification of RNA using TRIzol (TRI Reagent) Cold Spring Harbor Protocols. 2010;2010(6) doi: 10.1101/pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S., Guo W., Li J., et al. High expression of Y-box-binding protein 1 correlates with poor prognosis and early recurrence in patients with small invasive lung adenocarcinoma. OncoTargets and Therapy. 2016;9:2683–2692. doi: 10.2147/OTT.S99939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W. J., Ho C. C., Chang Y. L. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nature Communications. 2014;5, article 3472 doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 24.Lv G., Tan Y., Lv H., et al. MXR7 facilitates liver cancer metastasis via epithelial-mesenchymal transition. Science China Life Sciences. 2017;60(11):1203–1213. doi: 10.1007/s11427-016-9042-y. [DOI] [PubMed] [Google Scholar]
- 25.Hebeiss I., Truckenmüller R., Giselbrecht S., Schepers U. Novel three-dimensional Boyden chamber system for studying transendothelial transport. Lab on a Chip . 2012;12(4):829–834. doi: 10.1039/c2lc20733h. [DOI] [PubMed] [Google Scholar]
- 26.Olumi A. F., Grossfeld G. D., Hayward S. W., Carroll P. R., Tlsty T. D., Cunha G. R. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Research. 1999;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q., Liu T., Qin J. A microfluidic-based device for study of transendothelial invasion of tumor aggregates in realtime. Lab on a Chip . 2012;12(16):2837–2842. doi: 10.1039/c2lc00030j. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi K., Murakami T., Kawaguchi K., et al. A patient-derived orthotopic xenograft (PDOX) mouse model of a cisplatinum-resistant osteosarcoma lung metastasis that was sensitive to temozolomide and trabectedin: Implications for precision oncology. Oncotarget . 2017;8(37):62111–62119. doi: 10.18632/oncotarget.19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Vincentis A., Pedone C., Vespasiani-Gentilucci U., et al. Effect of sibutramine on plasma C-reactive protein, leptin and adipon ectin concentrations: a systematic review and meta-analysis of randomized contr olled trials. Current Pharmaceutical Design. 2017;23(6):870–878. doi: 10.2174/1381612822666161006122934. [DOI] [PubMed] [Google Scholar]
- 30.González C. R., Caminos J. E., Gallego R., et al. Adiponectin receptor 2 is regulated by nutritional status, leptin and pregnancy in a tissue-specific manner. Physiology & Behavior. 2010;99(1):91–99. doi: 10.1016/j.physbeh.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 31.MacCiò A., Madeddu C., Gramignano G., et al. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: Preliminary results and therapeutic implications. Journal of Molecular Medicine. 2010;88(7):677–686. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Liu R., Hawkins M., Barzilial N., Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393(6686):684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 33.Basak S., Duttaroy A. K. Leptin induces tube formation in first-trimester extravillous trophoblast cells. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2012;164(1):24–29. doi: 10.1016/j.ejogrb.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 34.Zheng X.-J., Yang Z.-X., Dong Y.-J., et al. Downregulation of leptin inhibits growth and induces apoptosis of lung cancer cells via the Notch and JAK/STAT3 signaling pathways. Biology Open. 2016;5(6):794–800. doi: 10.1242/bio.017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullwinkle E. M., Parker M. D., Bonan N. F., Falkenberg L. G., Davison S. P., DeCicco-Skinner K. L. Adipocytes contribute to the growth and progression of multiple myeloma: Unraveling obesity related differences in adipocyte signaling. Cancer Letters. 2016;380(1):114–121. doi: 10.1016/j.canlet.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Lu W., Gao N., et al. Generation of obese rat model by transcription activator-like effector nucleases targeting the leptin receptor gene. Science China Life Sciences. 2017;60(2):152–157. doi: 10.1007/s11427-016-5049-y. [DOI] [PubMed] [Google Scholar]
- 37.Newman G., Gonzalez-Perez R. R. Leptin-cytokine crosstalk in breast cancer. Molecular and Cellular Endocrinology. 2014;382(1):570–582. doi: 10.1016/j.mce.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song W., Wang Z., Zhang X., Li Y. Ethanol extract from Ulva prolifera prevents high-fat diet-induced insulin resistance, oxidative stress, and inflammation response in mice. BioMed Research International. 2018;2018:9. doi: 10.1155/2018/1374565.1374565 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The experimental data used to support the findings of this study are included within the article. The 3D printing drawing data are available from the corresponding author upon request. Detailed data of the patients involved in the study are restricted by the Ethics Committee of the 1st Hospital of Dalian Medical University. Data are available from Dr. Gu (guchundong@dmu.edu.cn) for researchers who meet the criteria for access to confidential data.