Abstract
Deficits in cognition and motivation are common and debilitating aspects of psychiatric disorders, yet still go largely untreated. The neuropeptide oxytocin (OT) is a potential novel therapeutic for deficits in social cognition and motivation in psychiatric patients. However, the effects of OT on clinically relevant domains of non-social cognition and motivation remain under studied. The present study investigated the effects of acute and chronic (21-day) administration of subcutaneous OT (0.04, 0.2, and 1 mg/kg) in cross-species translatable operant paradigms of reward learning and effortful motivation in male and female Brown Norway (BN) rats (n=8-10/group). Reward learning was assessed using the probabilistic reversal learning task (PRLT) and effortful motivation was measured using the progressive ratio breakpoint task (PRBT). As predicted, BN rats exhibited baseline deficits in the detection of reversals of reward contingency in the PRLT relative to Long Evans (LE) rats. The two strains performed equally in the PRBT. Thirty minutes after a single OT injection (1 mg/kg), measures of both initial probabilistic learning (trials to first criterion) and subsequent reversal learning (contingency switches) were significantly improved to levels comparable with LE rats. The OT effect on switches persisted in male, but not female, BN rats 30 minutes, 24 hours, and 6 days after long-term OT administration, suggesting the induction of neuroplastic changes. OT did not affect effortful motivation at any time-point. The beneficial effects of OT on reward learning in the absence of increased effortful motivation support the development of OT as a novel therapeutic to improve cognitive functioning.
Keywords: Oxytocin, Reward Learning, Motivation, Probabilistic Learning, Progressive Ratio
Graphical Abstract
1. Introduction
Cognitive deficits in psychiatric conditions are associated with poor functional outcome, defined as a patient’s ability to reintegrate into society (Duarte et al., 2016; Green, 2006; Green et al., 2000; McIntyre et al., 2013; Sole et al., 2017; Woo et al., 2016). Unfortunately, there remains a conspicuous absence of reliable treatments for such symptoms (Sole et al., 2017; Young et al., 2012; Young and Geyer, 2015). Currently approved drugs to treat psychiatric conditions – e.g., antipsychotics (Shilling and Feifel, 2016; Young and Geyer, 2015) and antidepressants (Bortolato et al., 2014; McIntyre et al., 2013) – are mostly, if not entirely, ineffective in treating cognitive deficits. Novel pharmacological interventions are therefore needed to specifically address cognitive dysfunction.
Oxytocin (OT), a nine-amino acid neuropeptide, is a potential novel therapeutic for psychiatric conditions. To date, OT has reduced positive and negative symptoms in patients with schizophrenia when used as an add-on therapeutic to antipsychotics in some clinical trials (Feifel et al., 2010; Modabbernia et al., 2013; Pedersen et al., 2011) (but see (Cacciotti-Saija et al., 2015; Dagani et al., 2016; Horta de Macedo et al., 2014), consistent with its potential described in preclinical rodent and human studies (Caldwell et al., 2009; Feifel and Reza, 1999; Feifel et al., 2015; Rubin et al., 2011). OT has also been shown to remediate some cognitive deficits of patients with schizophrenia (Davis et al., 2013; Gibson et al., 2014; Goldman et al., 2011; Woolley et al., 2014), though these investigations have largely focused on improving social cognition. Studies of OT in the context of non-social cognition are few, though positive effects of OT on such domains as verbal memory (Feifel et al., 2012a), working memory (Michalopoulou et al., 2015), and olfactory identification (Lee et al., 2013) have been observed; such findings suggest that the neuropeptide may in fact play a larger role in general cognition than previously assumed. It is therefore necessary to address the sizeable knowledge gap in the non-social behavioral effects of OT. The clinically relevant and as-yet unexplored domain of non-social reward-related learning is a prime target for such investigation.
Deficits in reward-associated learning have been observed in major depression, bipolar disorder, and schizophrenia (Whitton et al., 2015). This behavior is important in part because cognitive therapies primarily use positive reinforcement to facilitate learning in patients and encourage treatment engagement (Acheson et al., 2013). One method used to quantify reward-learning is the probabilistic reversal learning task (PRLT), in which subjects must choose between two stimuli with different reward schedules and integrate response feedback into subsequent decision-making. Subjects’ learning is gauged by accuracy across trials, while other in-task measures provide insight into motoric impulsivity (premature responses)(Cope et al., 2016) and reward- and punishment-sensitivity (Young and Markou, 2015).
Deficits in motivation are another common feature across psychiatric disorders. Patients with schizophrenia (Bismark et al., 2017; Strauss et al., 2016) and patients with unipolar and bipolar depression (Hershenberg et al., 2016) demonstrate significantly lower motivation than healthy controls, as measured by the progressive ratio breakpoint task (PRBT). In the PRBT, subjects must expend progressively more effort across successive testing trials in order to earn the same reward. The testing session progresses until the subject’s “breakpoint” is reached – i.e. until the subject decides that the reward is no longer worth the effort and stops responding. In a recent study, low breakpoints (i.e. poor effortful motivation) accounted for approximately 25% of variance in global cognitive test scores in patients with schizophrenia (Bismark et al., 2017). Hence, deficient motivation may also impact cognition in tasks like the PRLT. OT plays a role in regulating dopamine function in the mesolimbic system, a pathway strongly involved in reward and motivation (Love, 2014); however, its impact on non-social reward and motivation have not been well studied (although it may reduce responding for sucrose reward in rats (Zhou et al., 2015)).
The PRLT and PRBT can be readily administered to both humans (Bismark et al., 2017; Wolf et al., 2014) and animals (Milienne-Petiot et al., 2017; Young and Markou, 2015), and thereby provide the means by which to study the effects of OT on reward learning and motivation using rodents. Another key advantage of these tasks is their relative simplicity; both are easy to administer, which enables the repeated testing of animals at different time-points. Studies that only investigate acute drug effects (as do a large percentage of extant OT studies) have limited predictive validity in that they do not simulate treatment course – valid prediction of clinical outcomes requires chronic assessment (Feifel et al., 2007). This may be particularly true for OT, as some recent animal studies have found the initial therapeutic-like effects of acute OT on social behavior to be absent – and in some cases reversed - following chronic daily administration (Bales et al., 2013; Huang et al., 2014). Other studies, meanwhile, have found acute effects of OT that persisted, and even grew stronger, with chronic administration (Janezic et al., 2016). This possibility of differential effects of acute vs. chronic OT necessitates assessment of the peptide at both time-points. Post-treatment time-points also require consideration, however. Chronic administration can often extend the effects of a drug beyond its primary pharmacological action via the induction of neoplastic processes; such neoplastic changes can continue to affect the system several days after discontinuation of treatment, and may account for certain long-term effects of OT (Janezic et al., 2016). Therefore, testing the effects of chronic OT after its cessation is also necessary in behavioral studies.
In addition to an apparent dependence upon treatment time-point, the central and peripheral effects of endogenous OT are highly sex-specific, with many of the effects of exogenously administered OT exhibiting sexual dimorphism (Bredewold and Veenema, 2018). Rat and mouse studies have also revealed appreciable sex differences in OT and OT receptor activity in key brain areas implicated in learning and decision making (Dumais et al., 2013). It is therefore important to test any putative cognitive effects of OT on both males and females.
The cognitive and motivational effects of acute and chronic administration of various doses of OT on male and female Brown Norway (BN) rats were assessed using the PRLT and PRBT. BN rats were selected for these studies because, relative to other rat strains (e.g., Long Evans), they perform poorly on several cross-species behavioral/cognitive tasks relevant to psychiatric disorders (Conti et al., 2001; Didriksen and Christensen, 1993; Feifel et al., 2012b; Feifel et al., 2015; Keiser et al., 2014). The strain has also been proposed as a model for schizophrenia-like deficits in prepulse inhibition (Feifel et al., 2011). We therefore hypothesized that BN rats would display similarly poor performance in the PRLT compared to other strains. The biological basis for BN rats’ cognitive performance decrement relative to other strains is currently unknown, so they were therefore not intended to model psychiatric pathology; however, their baseline cognitive inferiority to other strains suggests that they have “room for improvement” in certain domains that may be acted upon by OT.
We hypothesized that OT would improve reward learning in BN rats, as measured by the PRLT. To determine whether BN rats do indeed perform poorly in the PRLT relative to other strains, and whether any dosage of OT would be sufficient to mitigate said deficit, Long Evans (LE) rats were administered vehicle and tested alongside the BN cohorts at all time-points.
2. Materials and Methods
2.1. Animals
Male and female Brown Norway (BN) and Long Evans (LE) rats were used in this study (BN: 16 per group; males: 200-275 g at training initiation; females: 130-170 g at training initiation; Harlan Laboratories, San Diego) (LE: 8 per group; males: 300-325 grams at training initiation; females: 210-225 grams at training initiation; Harlan Laboratories, San Diego). Rats were housed in dyads in clear plastic chambers in a climate-controlled room on a 12h light/dark schedule (lights on at 7:00 AM). Training began at approximately 10 weeks of age. Rats were food restricted to 85% of their free feeding weight, with feed calculated to maintain normal growth. Water was provided ad libitum throughout training and testing phases, except when in the testing chambers. All training and testing was conducted during the light period of the rats’ light/dark schedule. All rats were maintained in an animal facility meeting all federal and state requirements for animal care and approved by the American Association for Accreditation of Laboratory Animal Care. All procedures were approved by the University of California San Diego (UCSD) Animal Care and Use Committee.
2.2. Apparatus
All training and testing was conducted in 9-choice operant testing chambers housed in ventilated, sound-attenuating cabinets (Med Associates Inc., St. Albans, VT and Lafayette Instrument Company, Lafayette, IN). Nine apertures were located laterally along the rear wall of each testing chamber. Six of these apertures were covered by metal inserts, leaving three evenly-spaced open apertures. Stimuli were delivered by LED lights recessed in each aperture. Nosepoke responses were detected by infrared beams located at the entrance of each aperture. Liquid reinforcement (strawberry Nesquik® plus non-fat milk, 40 μl) was delivered via peristaltic pump into a magazine located on the opposite wall of the chamber in conjunction with magazine illumination. Reward collection was monitored by an infrared beam located at the opening of the magazine. Each testing chamber contained a house light, centered on the ceiling. Stimulus outputs and response inputs were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc., St. Albans, VT) using custom programming.
2.3. Training
Rats were first trained in MAG1, designed to build association between the magazine light and reward delivery. MAG1 operated on a fixed interval schedule in which 40 μL of strawberry Nesquik® was delivered every 15 seconds into the magazine in conjunction with magazine illumination. Reward collection was recorded for each 15 s bin. Once all animals were performing reliably (3 days), rats were trained in FR1 – a basic training program in which rewards were delivered immediately after a nosepoke to any one of three illuminated stimulus apertures on an FR1 reward schedule. Responses were recorded and training continued until >70 correct responses were recorded for 2 consecutive days for each rat, after which stability of responding was confirmed over 4 days.
2.4. Chronic Treatment and Testing Schedule
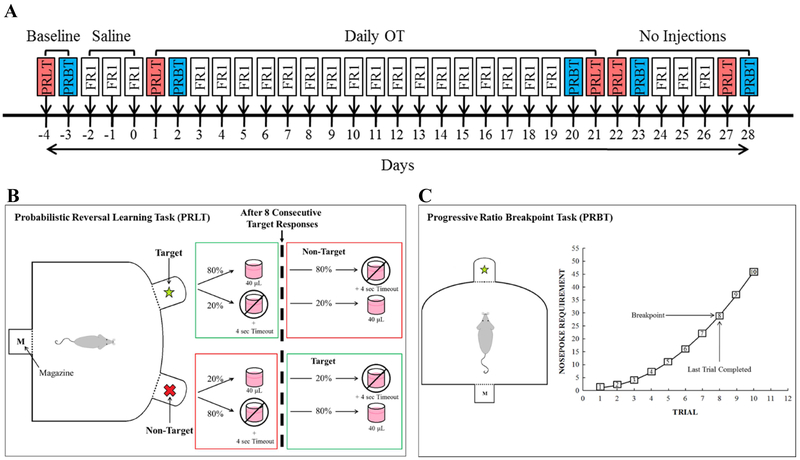
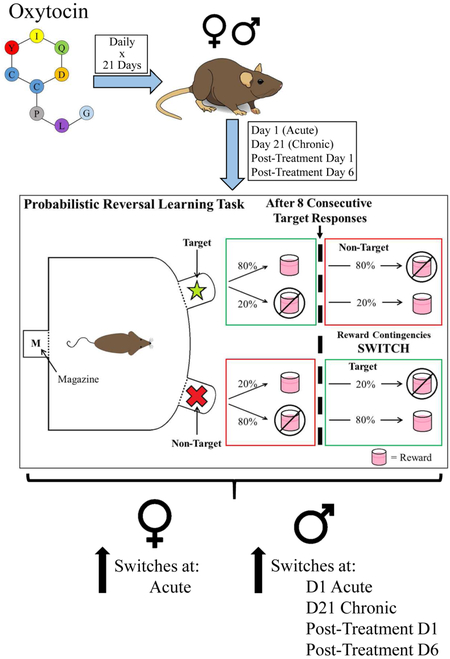
The testing and treatment regimen is outlined in Figure 1A. Baseline performance on the Probabilistic Reversal Learning Task (PRLT) and Progressive Ratio Breakpoint Task (PRBT), respectively, was determined over two days following initial FR1 training completion. The BN rats were then counter-balanced into 4 cohorts based on primary outcome variables from the two tasks (see below). The rats then underwent a three-day saline acclimation period in which they received saline injections 30 minutes prior to FR1 training. Effects of acute OT treatment on reward learning were assessed by testing rats in the PRLT 30 min after administration of their first subcutaneous (s.c.) injection of either saline or OT (0.04, 0.2, or 1 mg/kg, according to assigned drug cohort). All LE rats were allocated to a single cohort and were administered saline 30 min prior to the PRLT. The following day, rats were administered the same dose of OT (or saline) as before and tested 30 minutes later in the PRBT. Every day for the next 17 days, rats were injected with s.c. OT (or saline). On each day, rats were trained in FR1 to maintain consistency in training and testing. In order to thoroughly characterize the effects of three weeks of daily OT administration on reversal learning, PRLT performance was assessed at three clinically relevant time-points after the final (twenty-first) daily injection of drug: 30 minutes (D21), 24 hours (D22), and 6 days (D27). These time-points represent, respectively: 1) the point by which exogenously administered OT is present in the blood and CNS at pharmacological levels, 2) the maximum time between OT administrations in a once-daily regimen, and 3) a post-treatment time-point at which any observed effects would represent treatment-induced neuroplasticity. PRBT was tested at similar time-points, but starting after the 20th (penultimate) drug injection.
Figure 1. Experiment design schematics.
Experiment schedule (A). Male and female Brown Norway (BN) Rats’ PRLT & PRBT performance was first assessed at baseline with no injections. Cohorts were counter-balanced into 4 groups to receive vehicle or OT (0.04, 0.2, or 1 mg/kg). Rats were then tested following acute and chronic (21 day) administration of OT. No injections were administered during the acute and chronic withdrawal phases of the study (D22-D28). Injections were administered 30 minutes prior to testing on each day of the study. On non-PRLT/PRBT days, rats were tested in the FR1 training module. Performance was compared with that of male and female Long Evans rats, which received vehicle injections throughout. Schematic of PRLT (B). At the beginning of each trial, rats nose poked in one of two lit apertures and were either rewarded with strawberry milkshake (~40 μL) delivered into the magazine or punished with a 4 second timeout and house light illumination. Rates of reward/punishment differed between the two stimuli, whereby target stimulus responses resulted in an 80% chance of reward and 20% chance of punishment, while the inverse was true for responses to the non-target stimulus. Following 8 consecutive target responses, the reward ratios of the two stimuli switched. Schematic of PRBT (C). Rats were rewarded after making a number of responses to a single stimulus. The number of responses required for a reward increased as a function of trial number. PRLT: Probabilistic Reversal Learning Task; PRBT: Progressive Ratio Breakpoint Task; FR1: FR1 training module; OT: Oxytocin
Throughout testing, a cohort of male and female LE rats was trained and tested concurrently with the above BN rats, but only received saline. This allowed for the assessment of any relative baseline abnormalities in BN rats.
2.5. Probabilistic Reversal Learning Task (PRLT)
The PRLT (Fig. 1B) utilized two recessed apertures at the rear of the operant chamber. Trials were initiated by the illumination and subsequent extinction of the magazine light via nosepoke. Following a 2 s inter-trial interval (ITI), the two apertures were illuminated, and rats were given 10 s to make a selection via nosepoke. One of the two stimuli was designated as the target stimulus, to which nosepokes were rewarded 80% of the time and punished 20% of the time. The other stimulus was designated as the non-target stimulus, to which responses were rewarded 20% of the time and punished 80% of the time. Rewards consisted of 40 μL milkshake delivered into the magazine, and were immediately preceded by aperture light extinction and magazine illumination. Punishments consisted of a 4 s timeout period following aperture light extinction; timeout periods were accompanied by illumination of the house light, which, as per the previously documented effects of illumination on open-field behavior (Walsh and Cummins, 1976), was included as an aversive stimulus. Initial target and non-target locations alternated among the eight testing chambers such that the first target of the session was the left aperture in four of the eight chambers, and the right in the others. Criterion for demonstrating acquisition was designated as 8 consecutive responses to the target stimulus (Fig. 1B). After first criterion acquisition, the target and non-target designations and reward schedules were reversed (Fig. 1B). The subject then had to recognize that this change, or "switch," in apertures’ reward schedules had occurred, and adjust its responses accordingly. After another 8 consecutive correct responses, the reward ratios were switched again. This pattern was repeated for the remainder of the 1-hour session. See Table 1 for outcome descriptions. The number of switches the subject was able to complete within the testing period was the primary outcome variable of reversal learning in the PRLT. An additional primary outcome measure, trials required to attain the first criterion, provided a measure of initial reinforcement learning without the confound of reversal learning.
Table 1.
Description of primary and secondary outcome variables of PRLT.
| Outcome Variables | Description |
|---|---|
| Switches | Total number of times within the 1-hour testing session that the reward contingencies of the two stimuli reversed |
| Trials to First Criterion | Number of trials completed by the subject before attaining the first criterion (8 consecutive target responses) |
| Trials for Reversal 1 | Trials taken by the subject to make 8 consecutive correct responses following the first reversal of reward contingency (i.e. number of trials completed by the subject within the second block of testing) |
| Premature Responses | Total number of responses made before presentation of stimuli across all trials |
| Omissions | Total number of trials in which no response was made |
| Mean Target Latency | Mean latency to respond to the target stimulus |
| Mean Non-Target Latency | Mean latency to respond to the non-target stimulus |
| Mean Reward Latency | Mean latency to collect rewards |
| Target Win-Stay Ratio | Metric of subjects’ tendency to choose the target (more frequently rewarded) stimulus again after choosing, and being rewarded by, that stimulus in the preceding trial |
| Target Lose-Shift Ratio | Metric of subjects’ tendency to choose the non-target (less frequently rewarded) stimulus after being punished by the target stimulus in the preceding trial |
| Non-Target Win-Stay Ratio | Metric of subjects’ tendency to choose the non-target stimulus again after being rewarded by that stimulus in the preceding trial. |
| Non-Target Lose-Shift Ratio | Metric of subjects’ tendency to choose the target stimulus after being punished by the non-target stimulus in the preceding trial |
| Total Trials | Total number of trials completed within entire testing session |
Note: Bold = primary outcome variable
Secondary outcome variables included omissions (no response to stimuli within 10 s of presentation) and premature responses (nosepokes in stimulus apertures during the ITI), both of which resulted in a 4 s punitive time out and house light illumination. Several latency measures included mean target latency (ms taken to respond to the target stimulus), mean non-target latency (ms taken to respond to the non-target stimulus), and mean reward latency (ms taken to collect a reward). Learning via rewards or punitive time outs was quantified using standard win-stay/lose-shift metrics for the target and non-target locations throughout the session; elevations in either could indicate a change in sensitivity to reward and/or punishment (i.e. reward- vs. punishment-sensitivity). Finally, the trials taken to complete the first reversal (trials for reversal 1) were also quantified.
2.6. Progressive Ratio Breakpoint Task (PRBT)
The PRBT utilized only the central stimulus aperture, with the other apertures in the chamber remaining unlit. The subjects responded to this stimulus via nosepoke, with the requisite number of responses to earn a reward increasing as a function of trial number – i.e. only 1 response was required for Trial 1, 2 were required for Trial 2, 4 for Trial 3, then 7, 11, 16, 22, 29, 37, 46, etc. (Figure 1C). The primary outcome variable was the ‘breakpoint’ – the total number of trials which a subject completed for a reward within 60 min (or before 5 min of inactivity). This measure provided a metric of effortful motivation (i.e. how hard subjects were willing to work for a fixed reward, and at what point they decided that the reward was no longer worth the effort).
2.7. Drug Treatment
After baseline matching into testing cohorts, Brown Norway (BN) rats received s.c. injections of either vehicle or OT (0.04, 0.2, or 1 mg/kg) every day for 21 consecutive days, as described above. The solutions were prepared every 3-4 days by dissolving OT (VWR International, Bachem Americas, Inc.) in saline to a concentration of 1 mg/mL, which was then diluted to 0.2 and 0.04 mg/mL via serial dilution. Long Evans (LE) rats received only vehicle injections. All injections were administered at 1 ml/kg. OT doses were based on those previously reported to increase prepulse inhibition in BN rats (Feifel and Reza, 1999; Feifel et al., 2012b).
2.8. Statistical Analyses
After confirming homogeneity of variance, primary outcome variables were analyzed using a one- or two-way analysis of variance (ANOVA). For baseline assessment, data from BN rats were compared to LE rats using a two-factor ANOVA, with sex and strain as between-subjects factors. Following this analysis, rats were assigned to cohorts that dictated OT dosage for the remainder of the study; all LE rats were grouped into a single cohort, and received vehicle injections only. Task performance data following drug administrations were analyzed across the entire session of treatment using a three-factor ANOVA with sex and cohort as between-subjects factors and test day as a within-subjects factor. Each individual test session was analyzed using a 2-way ANOVA with sex and cohort as between-subjects factors. Any significant main or interactive effects were subjected to further analyses using Tukey post hoc comparisons. As this study was designed to explore differences in acute vs. chronic OT administration in BN rats and potential differential OT effects across sex, planned separate analyses were performed for each test day on males and females. For all time-points, the inclusion criterion for primary outcome variable analysis was completion of the first block of the PRLT (i.e. attainment of the first criterion for reversal of reward contingency (Table 1)); primary outcome data (both PRLT and PRBT) from rats that did not complete the first block of PRLT testing on a given day were excluded from analysis of that time-point. Inactivity, as determined by total trials completed within a testing session, was used as an exclusion criterion for secondary outcome variable analysis. Similarly, inactivity during one or more PRLT sessions necessitated the exclusion of certain subjects from time-course analysis of switches and breakpoint data. All data were analyzed using SPSS 24.0 (Chicago, IL) and were represented by mean and standard error of the mean.
3. Results
3.1. Baseline (Days −4 and −3)
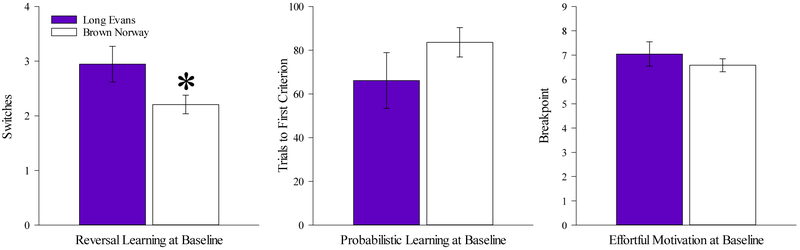
10 rats failed to meet the inclusion criterion for primary outcome variable analysis (see Section 2.8) and were excluded. A two-factor ANOVA (sex X strain) applied to the switches data revealed a significant effect of strain [F(1,65=4.05, p<0.05], but no significant effect of sex or sex X strain interaction. BN rats completed significantly fewer switches than LE rats (Fig. 2A). There were no significant main or interactive effects revealed for trials to first criterion (Fig. 2B), or for the PRBT (Fig. 2C) data. After counter-balancing, there were no significant effects of drug cohort or drug X sex interactions on any measure (Supplemental Fig 1).
Figure 2. Baseline performance on reward learning and effortful motivation.
BN rats completed fewer switches than LE rats within the session (A), but there was no significant difference between the strains in the number of trials to first criterion (B) in the PRLT. Similarly, no effect of strain or group was observed on breakpoint (C) in the PRBT. Data presented as mean ±S.E.M. * = p <0.05 relative to LE.
3 rats were excluded from analysis of secondary outcome variables on grounds of inactivity. There were no significant effects of strain or sex on any secondary measure [F(1,69)<2.9, ns]. No interaction between strain and sex was observed for any secondary measure (F<1.4, ns), including trials for reversal 1 (i.e. number of trials completed within the first testing block following the first reversal of reward contingency) (Table 2).
Table 2.
Selected PRLT secondary outcome data by time-point and OT dose.
| Time Period |
Outcome Measure |
BN Rats Mean (±SEM) | LE rats VEH | Sex | Cohort | Interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| VEH | 0.04 mg/kg | 0.2 mg/kg | 1 mg/kg | Mean(±SEM) | F(1,58), p | F(4,58), p | F(4,58), p | ||
|
Baseline No Injections |
TargWS | 0.457 (0.046) | 0.479 (0.043) | 0.539 (0.042) | 0.488 (0.042) | 0.489 (0.037) | <1, ns | <1, ns | <1.2, ns |
| TargLS | 0.445 (0.053) | 0.436 (0.050) | 0.380 (0.049) | 0.512 (0.049) | 0.431 (0.043) | <1, ns | <1, ns | <1, ns | |
| NonTWS | 0.493 (0.070) | 0.513 (0.066) | 0.549 (0.065) | 0.539 (0.065) | 0.597 (0.056) | <1.3, ns | <1, ns | <1, ns | |
| NonTLS | 0.458 (0.032) | 0.481 (0.030) | 0.444 (0.030) | 0.399 (0.030) | 0.393 (0.026) | <1.9, ns | <1.7, ns | <1, ns | |
| TrialsforRev1 | 58.2 (18.0) | 76.1 (16.5) | 100.6 (14.9) | 83.3 (15.9) | 91.4 (14.4) | <1.4, ns | <1, ns | <1.5, ns | |
|
Aoute OT (D1) |
TargWS | 0.499 (0.033) | 0.448 (0.032) | 0.436 (0.031) | 0.582 (0.030) | 0.556 (0.032) | 2.4, p=0.127 | 4.1, p<0.01 | <1.9, ns |
| TargLS | 0.491 (0.046) | 0.468 (0.044) | 0.481 (0.043) | 0.491 (0.043) | 0.380 (0.037) | <1, ns | 1.4, ns | <1, ns | |
| NonTWS | 0.544 (0.073) | 0.586 (0.069) | 0.605 (0.067) | 0.644 (0.067) | 0.543 (0.058) | <1, ns | <1, ns | <1, ns | |
| NonTLS | 0.385 (0.031) | 0.446 (0.030) | 0.400 (0.029) | 0.481 (0.029) | 0.418 (0.025) | <1, ns | 1.7, ns | <1, ns | |
| TrialsforRev1 | 94.8 (25.4) | 58.3 (28.4) | 99.1 (15.4) | 63.0 (14.5) | 103.0 (14.1) | <1, ns | <1.4, ns | <1, ns | |
|
Chronic OT (D21) |
TargWS | 0.482 (0.034) | 0.493 (0.032) | 0.504 (0.031) | 0.622 (0.031) | 0.559 (0.027) | <1, ns | <1.8, ns | <1.6, ns |
| TargLS | 0.373 (0.037) | 0.353 (0.035) | 0.374 (0.034) | 0.434 (0.034) | 0.465 (0.030) | <1, ns | 2.5, p<0.1 | <1.5, ns | |
| NonTWS | 0.548 (0.056) | 0.525 (0.053) | 0.604 (0.052) | 0.562 (0.052) | 0.533 (0.045) | <1, ns | F<1, ns | <1, ns | |
| NonTLS | 0.396 (0.028) | 0.483 (0.028) | 0.400 (0.027) | 0.496 (0.026) | 0.421 (0.024) | <1, ns | 3.1, p1<0.05 | <1.1, ns | |
| TrialsforRev1 | 98.7 (16.9) | 81.4 (16.0) | 81.3 (14.0) | 63.5 (15.5) | 93.0 (13.5) | <1, ns | <1, ns | <1.5, ns | |
|
Aoute Withdrawal OT(D22) |
TargWS | 0.475 (0.032) | 0.557 (0.031) | 0.546 (0.031) | 0.583 (0.030) | 0.593 (0.028) | 2.2, p=0.140 | 2.3, p<0.1 | <1.6, ns |
| TargLS | 0.440 (0.050) | 0.416 (0.049) | 0.460 (0.048) | 0.448 (0.046) | 0.414 (0.043) | <1, ns | <1, ns | <1.7, ns | |
| NonTWS | 0.542 (0.060) | 0.511 (0.059) | 0.533 (0.057) | 0.538 (0.056) | 0.523 (0.052) | <1, ns | <1, ns | <1, ns | |
| NonTLS | 0.389 (0.030) | 0.474 (0.029) | 0.348 (0.028) | 0.400 (0.028) | 0.461 (0.026) | <1, ns | 3.5, p<0.05 | <1, ns | |
| TrialsforRev1 | 113.2 (21.4) | 78.8 (19.4) | 111.9 (17.0) | 85.7 (16.4) | 92.1 (14.5) | <1, ns | <1, ns | <1.1, ns | |
|
Chronic Withdrawal OT (D27) |
TargWS | 0.485 (0.032) | 0.527 (0.031) | 0.552 (0.030) | 0.543 (0.029) | 0.594 (0.027) | <1, ns | <1.8, ns | <1.1, ns |
| TargLS | 0.429 (0.038) | 0.485 (0.037) | 0.467 (0.036) | 0.406 (0.035) | 0.411 (0.033) | <1, ns | <1.5, ns | <1, ns | |
| NonTWS | 0.601 (0.047) | 0.523 (0.046) | 0.550 (0.045) | 0.615 (0.043) | 0.517 (0.040) | <1.1, ns | <1.2, ns | <1.7, ns | |
| NonTLS | 0.394 (0.029) | 0.493 (0.029) | 0.322 (0.028) | 0.413 (0.027) | 0.445 (0.025) | 2.3, p<0.130 | 5.1, p<0.01 | <1, ns | |
| TrialsforRev1 | 85.2 (17.3) | 98.7 (15.1) | 82.3 (14.7) | 92.0 (15.1) | 99.3 (13.3) | <1, ns | <1, ns | <1, ns | |
Bolded text and box denotes p<0.05 compared with BN vehicle-treated rats. Bolded box indicates main effect. OT=Oxytocin, BN=Brown Norway rats, LE=Long Evans rats, Targ=Target, NonT=Non-Target, WS=Win-Stay, LS=Lose-Shift.
3.2. Overall Time-Course Analysis of Oxytocin Treatment Effects on BN rats
3.2.1. D(−4)-D27 for PRLT
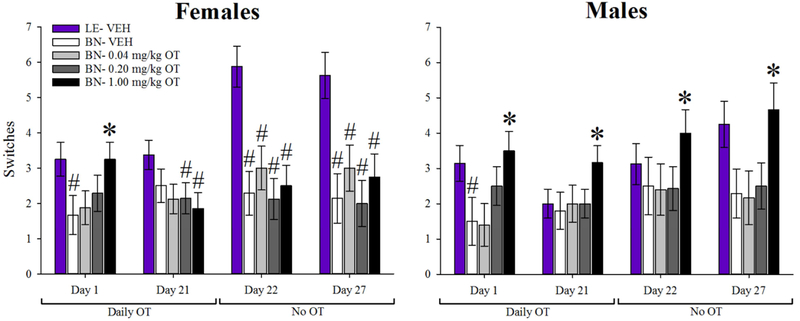
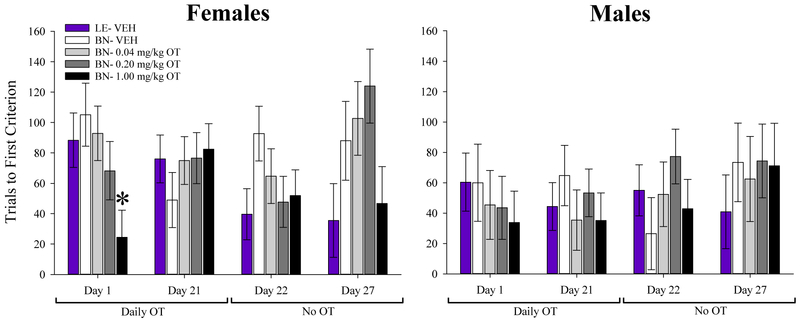
Inactivity during one or more PRLT sessions necessitated the exclusion of 4 subjects from time-course analysis of switches data. Failure to complete the first block of testing necessitated the exclusion of 24 subjects from time-course analysis of trials to first criterion data. When examined across days for the primary outcome variables, main effects of test day [F(4,280)=9.5, p<0.001] and cohort [F(4,70)=6.8, p<0.001] were observed for switches, as was a test day X cohort interaction [F(16,280)=2.46, p<0.005]. No main effect of sex or interaction with test day or cohort was observed. Post hoc analyses revealed that vehicle-treated BN rats completed fewer switches than both BN rats treated with 1 mg/kg OT (p<0.01) and vehicle-treated LE rats (p<0.001; Fig. 3). A main effect of test day was also observed on trials to first criterion [F(4,180)=3.6, p<0.01], with no interaction with cohort (F<1.6, ns). Overall, vehicle-treated BN rats required more trials to attain first criterion than BN rats treated with OT at 1 mg/kg and vehicle-treated LE rats (p<0.05), although the main effect of cohort failed to reach statistical significance. [F(4,70)=2.2, p=0.08; Fig. 4] Specific within-test day analyses are provided below. Correlational analysis of vehicle-treated BN and LE rats between each time-point based on switches enabled the assessment of test-retest reliability of this primary outcome variable. This analysis revealed significant consistency of performance in both strains (Supplemental Fig. 2).
Figure 3. Effects of oxytocin (OT) on probabilistic reversal learning.
Acute OT (1 mg/kg) increased the total number of switches completed by male and female BN rats vs. vehicle in the PRLT. Vehicle-treated BN rats exhibited fewer switches than vehicle-treated LE rats within both sexes at this time-point. Chronic OT at 1 mg/kg increased the number of switches completed by male, but not female, BN rats relative to saline and lower OT-dosed BN rats 30 minutes after OT administration. This effect of chronic 1 mg/kg OT on male BN rats persisted after cessation of daily OT administration, with improved performance observed at both the one- and six-day post treatment time-points. Male BN rats showed no statistical difference from LE rats in terms of switches at any time-point. BN females that had received 0.2 and 1 mg/kg OT chronically completed fewer switches than LE females at the day 21 time-point. All female BN cohorts completed fewer switches than the female LE cohort at the day 22 and 27 time-points. Data presented as mean ± S.E.M. * = p <0.05 relative to BN- vehicle group; # = p <0.05 relative to LE- vehicle group.
Figure 4. Effects of oxytocin (OT) on probabilistic learning.
Acute OT (1 mg/kg) decreased the number of trials required by female BN rats to reach the first criterion relative to vehicle-treated BN females. No effect of OT was seen on this measure in male rats at this time-point although there was a dose-related trend in the same direction observed in females. OT had no effect on trials to first criterion at any other time-point in either sex. Data presented as mean ± S.E.M. * = p<0.05 vs BN VEH,
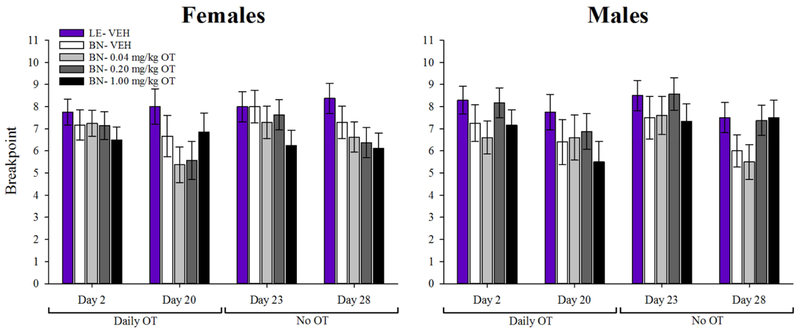
3.2.2. D(−3)-D28 for PRBT
3 subjects had to be excluded from time-course analysis of PRBT data (Section 2.8). When examined across days, a main effect of test day was observed for breakpoint [F(4,284)=3.8, p<0.005], but there was no interaction with cohort (F<1, ns). A main effect of cohort on breakpoint was observed however, with vehicle-treated LE rats exhibiting higher breakpoints than BN rats at every dose [F(4,71)=2.8, p<0.05].
3.3. The Effects of Acute and Chronic Administration of Oxytocin on the PRLT
3.3.1. Effects of acute OT treatment on the PRLT (D1):
14 subjects were excluded from D1 primary outcome analysis due to failure to complete the first block of testing. On day 1 (D1) of drug treatment, a main effect of cohort on switches was observed [F(4,55)=4.8, p<0.01], with post hoc analyses revealing that OT-treated BN rats (1 mg/kg) completed more switches than vehicle-treated BN rats (p<0.005). Vehicle-treated LE rats completed significantly more switches than BN rats treated with vehicle or the lowest dose of OT (p<0.001). There was no significant effect of sex, nor was there a sex X cohort interaction. When males and females were analyzed separately, the OT effect on switches failed to reach statistical significance in both sexes’ data [Females: F(4,32)=2.3, p=0.076; Males: F(4,32)=2.5, p=0.073]. Pairwise comparisons revealed that in both sexes, the 1 mg/kg OT groups and Long Evans groups completed more switches than the respective vehicle groups (p<0.05; Fig. 3). No difference was observed between 1 mg/kg OT and Long Evans groups within either sex.
Although a main cohort effect on trials to first criterion did not reach statistical significance [F(4,55)=2.6, p=0.086; Fig.4], overall, OT-treated BN rats (1 mg/kg) required fewer trials to achieve first criterion than vehicle-treated LE and BN rats (p<0.05). Despite a main effect of sex (F(1,55)=4.5, p<0.05), there was no significant sex X cohort interaction. When males and females were analyzed separately, no significant effect of cohort was observed for either sex (Fs<2.1, p’s>0.102), although females treated with 1 mg/kg OT still required fewer trials for the first criterion than vehicle-treated BN rats (p<0.05).
4 subjects were excluded from analysis of secondary variables on grounds of inactivity. No significant main or interactive effects were observed on latency measures such as target, non-target, or reward collection latency (F<1, ns). A main effect of cohort was observed on premature responses [F(4,65)=3.5, p<0.05], with vehicle-treated LE rats making more premature responses (36.563 ±6.227; Mean ± S.E.M.) than all BN cohorts (VEH: 15.598 ±6.227; 0.04 mg/kg OT: 19.438 ±6.498; 1 mg/kg OT: 8.917 ±6.498; p<0.05), except those treated with 0.2 mg/kg OT (32.188 ±6.016; p>0.1). A main effect of cohort on target win-stay ratio [F(4,65), p<0.05] was observed, with 1 mg/kg OT increasing this ratio in BN rats relative to vehicle. No effects of cohort (F<1, ns), sex (F<1.4, ns), or sex X cohort interaction (F<1.5, ns) were observed on trials for reversal 1.
3.3.2. Effects of chronic OT treatment on the PRLT 30 minutes after final injection (D21)
Rats were treated with the same doses of OT daily for 21 days. On D21, rats were tested in the PRLT 30 min after injection. 11 rats failed to complete the first block of testing, and were excluded from primary analysis (Supplementary Table 1). No significant main effects of cohort or sex were observed for switches, though there was a near-significant sex X cohort interaction [F(4,58)=2.5, p=0.054; Fig. 3]. Planned post-hoc analyses revealed that male BN rats treated with 1 mg/kg OT completed more switches than the vehicle group (p<0.05). In contrast, female rats did not exhibit an OT-induced increase in switches at any dose. 1 mg/kg and 0.2 mg/kg OT-treated female BN rats completed significantly fewer switches than the vehicle-treated LE rats (p<0.05 and p<0.01, respectively). There was a main effect of sex on trials to first criterion, with females requiring more trials to complete the first testing block than males [F(1,58)=5.3, p<0.05; Fig. 4]. There was no effect of cohort on this measure, nor was there a sex X cohort interaction.
4 subjects were excluded from analysis of secondary variables on grounds of inactivity. A main effect of sex was observed on total trials completed within a testing session [F(1,65)=6.143, p<0.001], with females (218.225 ±13.880; Mean ± S.E.M.) completing more trials than males (168.350 ±14.570). Females (26.164 ±3.859) completed more premature responses than males (18.917 ±4.051), though the main effect of sex on this behavior did not reach statistical significance [F(1,65)=2.931, p=0.092]. Although a main effect of cohort did not reach statistical significance [F(1,65)=2.185, p=0.080], overall, the vehicle-treated LE cohort and all OT-treated BN cohorts exhibited faster target latencies (LE: 272.616 ±358.342 ms; BN-0.04 mg/kg OT: 385.808 ±387.053 ms; BN-0.2 mg/kg OT: 294.010 ±358.342 ms; BN-1 mg/kg OT: 347.129 ±387.053 ms) than vehicle-treated BN rats (BN-VEH: 1544.341 ±370.919 ms). A main effect of cohort was observed on non-target lose-shift ratio [F(4,65)=3.1, p<0.05], with 1 mg/kg OT-treated BN rats having a higher ratio than vehicle-treated BN rats (Table 2). No effects of sex (F<1, ns), cohort (F<1.4, ns), or sex X cohort interaction (F<1, ns) were observed on trials for reversal 1 (Table 2).
3.3.3. Effects of chronic OT treatment on the PRLT 24.5 hours after final injection (D22)
After 21 days of consecutive OT treatment, rats were tested in the PRLT 24.5 hours after their final injection (D22). 11 rats failed to complete the first block of testing, and were excluded from primary analysis (Supplementary Table 1). A significant main effect of cohort [F(4,58)=4.5, p<0.005] was observed on switches, as was a significant sex × cohort interaction [F(4,58)=3.5, p<0.05]. There was no significant main effect of sex. Post-hoc analysis indicated that in BN males, 1 mg/kg OT significantly increased the number of switches completed compared to vehicle-treated BN rats (p<0.05); however, in females, no OT dose significantly altered switches, and all OT groups completed fewer switches than the LE group (p’s<0.05). There were no main effects of cohort or sex on trials to first criterion (F<1, ns), nor any interaction between the two factors (F<1, ns).
6 rats were excluded from analysis of secondary outcome variables on grounds of inactivity. A main effect of cohort was observed on mean non-target latency [F(4,63)=2.613, p<0.05], with vehicle-treated BN rats (868.306 ±176.897 ms; Mean ±S.E.M.) taking considerably longer to respond than vehicle-treated LE rats (164.829 ±165.472 ms) and all OT-treated BN groups (0.04 mg/kg OT: 314.913 ±188.668 ms; 0.2 mg/kg OT: 246.266 ±165.472 ms; 1 mg/kg OT: 265.773 ±178.731 ms). A main effect of cohort was also detected on total trials completed within the testing session [F(4,63)=2.7, p<0.05], with LE rats completing more trials (289.312 ±23.154) than vehicle-treated BN rats (187.214 ±24.752). A main effect of sex was observed on this measure as well [F(1,63)=5.4, p<0.05], with females completing more trials (251.346 ±14.851) than males (200.516±16.143). A main effect of cohort on target win-stay ratio did not reach statistical significance [F(4,63)=2.3, p<0.1]. No effects of sex (F<1, ns), cohort (F<1, ns), or sex X cohort interaction (F<1.5, ns) were observed on trials for reversal 1 (Table 2).
3.3.4. Effects of chronic OT treatment on the PRLT 6 days after final injection (D27)
6 days after their final injections, rats were tested in the PRLT (D27). 5 rats were excluded from primary analysis due to failure to complete the first block of testing (Supplementary Table 1). A main effect of cohort was observed on switches [F(4,64)=6.2, p<0.001; Fig. 3], with no effects of sex or sex X cohort interaction (F<1.7, ns). Planned post hoc analyses revealed that vehicle-treated LE rats completed significantly more switches than BN rats treated with vehicle, 0.04, and 0.2 mg/kg OT (p’s<0.05); LE rats also completed more switches than those BN rats treated with 1 mg/kg OT, though the results of this comparison did not reach statistical significance (p=0.076). Additionally, BN rats treated with 1 mg/kg OT completed more switches than those treated with vehicle (p<0.05). No main or interactive effects were observed on trials to first criterion (Fig. 4). When data from the two sexes were analyzed separately, male rats treated with 1 mg/kg OT completed more switches than the vehicle group (p<0.05). OT failed to affect female performance, however, and all BN groups completed significantly fewer switches than the LE group.
4 rats were excluded from analysis of secondary outcome variables on grounds of inactivity. A main effect of cohort was observed on total trials completed [F(4,65)=3.2, p<0.05], with LE rats completing more trials (304.375 ±20.280; Mean ±S.E.M.) than BN rats treated with vehicle (210.330 ±20.992), 0.04 mg/kg OT (218.521 ±21.905), and 1 mg/kg OT (242.167 ±21.905). There was also a main effect of sex on total trials [F(1,65)=6.0, p<0.05], with females (269.207 ±13.008) completing more trials than males (222.825 ±13.655). A main effect of sex was observed on mean reward latency [F(4,65)=6.7, p<0.05], with females taking less time (124.274 ±2.840 ms) than males (134.956 ±2.981 ms) to collect rewards. There were no main effects on any other secondary outcome variable (F<1.7, ns), nor were there any sex X cohort interactions (F<1.5, ns). No effects of sex (F<1, ns), cohort (F<1, ns), or sex X cohort interaction (F<1, ns) were observed on trials for reversal 1 (Table 2).
3.3.5. The Effects of Acute and Chronic Administration of Oxytocin on the PRBT
The effects of OT on PRBT performance at each time-point are illustrated by Figure 5. On D2, no main effects of cohort or sex, nor interactions between the two were observed [F’s<1.6, ns]. Similarly, no main effects of sex, cohort, or sex X cohort interaction were observed on D20 (F’s<1, ns), D23 (F’s<1.7), or D28 (F’s<1.8).
Figure 5. Effects of oxytocin (OT) on effortful motivation.
No significant differences in breakpoint were observed between cohorts or sexes at any time-point. Data presented as mean ±S.E.M.
4. Discussion
As predicted, vehicle-treated Brown Norway (BN) rats exhibited impaired reward learning, but not effortful motivation, relative to vehicle-treated Long Evan (LE) rats, as measured by total switches in the probabilistic reversal learning task (PRLT; Fig. 2). Acute OT (D1; 1.0 mg/kg) improved both initial probabilistic learning (decreased the number of trials required to reach the first criterion, primarily in females), as well as subsequent reversal learning (increased the number of switches completed) in BN rats relative to vehicle (D1; Figs. 3,4). This latter improvement was sufficiently large such that this group was no longer distinguishable from vehicle-treated LE rats, which exhibited better reversal learning than BN rats at baseline. Interestingly, chronic OT treatment continued to improve male PRLT performance (D21), though its effects in females disappeared. OT-induced improvement in males persisted even after daily treatment was discontinued (D22 and D27), an effect not observed in female BN rats. None of these effects were not driven by an overall increase in motivation, as revealed by a lack of effect in the progressive ratio breakpoint task at all time-points (PRBT; Fig. 5). Given that psychiatric patients exhibit impaired PRLT performance (Dickstein et al., 2010; Dombrovski et al., 2010; Reddy et al., 2016; Waltz and Gold, 2007; Young and Geyer, 2015; Young and Markou, 2015), the data presented here suggest that incorporation of OT into existing treatment plans may improve deficient reward-learning. The continued improvement with chronic treatment observed in males indicates that OT could be a viable, albeit sex-specific, long-term therapeutic.
The OT-induced improvement in PRLT performance on D1 was likely driven by improved reward association, given that 1 mg/kg OT increased target win-stay ratio (a measure of reward-sensitivity) relative to vehicle (Table 2). Interestingly, with chronic treatment this group’s behavior shifted toward increased punishment-sensitivity, as demonstrated by an elevation in non-target lose-shift ratio on D21 (Table 2). This shift may relate as to why enduring effects were observed only in male rats (e.g., shifting them to be punish-sensitive), but since no main effects of sex or sex X cohort interaction were observed in these secondary measures the link is not clear. Elevation of reward-sensitivity (increased target win-stay) reemerged on D22 (Table 2), but without concomitant task improvement in female rats. The apparent sex dependence of this effect may indicate an OT-induced change in receptor expression/activity that is specific to sex, in addition to presence of OT (see below). Although these possibilities require further examination, the primary driver of OT-induced improvement of PRLT performance appears to be improved reward-related learning. These findings may not be ubiquitous across all forms of reinforcement learning, however. When integrated with social stimuli and speculation of future rewards, OT may in fact impair feedback-based learning in human males (Ide et al., 2018). Confirmation of the effects of OT in human PRLT performance remains a necessity.
Importantly, in the present study, OT exerted no significant effect on effortful motivation as assessed by the PRBT. This result suggests that the OT-induced improvement in reward learning is domain-specific, and not driven by increased motivational/reward-seeking levels. This finding also supports the separation of reward learning and effortful motivation in rats as dissociable aspects of reward processing, consistent with a recent clinical study reporting no correlation between PRBT and PRLT performance in schizophrenia patients (Bismark et al., 2017). Hence, OT specifically improves reward-associated learning in rats, without affecting motivation.
Since acute OT improved both initial (trials to first criterion) and reversal learning (switches), it is difficult to determine whether OT affected both domains directly, or whether the increased number of switches was simply a consequence of improved initial learning. It is possible that the improvement in switches observed in 1 mg/kg OT-treated BN rats was driven by their having progressed through initial trials more quickly, and by consequentially having had more time left in the hour-long session to complete extra reversals. Given that total trials completed within the entire testing session did not differ between OT-treated groups however, improvements were unlikely a result of completing more trials. The conclusion that OT indirectly increased switch completion as a result of improving initial learning is supported by observations that 1 mg/kg OT-treated BN rats did not differ from any other cohort on trials to criterion within the second block (i.e. following the first reversal). Conceivably, had OT directly affected the domain of reversal learning, this cohort would have required fewer trials to progress from the second to third testing block, as they would have detected the reversal of reward contingency earlier than the vehicle BN group. The effect profile of acute OT (non-significantly faster initial learning irrespective of sex) may have been transient however, given that on days 21, 22, and 27, significant improvement in males was specific to switches. Thus, the chronic and persistent effects of OT on switches in male rats occurred in the absence of enhanced initial learning.
The most parsimonious explanation for the observed effects would be that the improvement in probabilistic learning observed on D1 was simply an initial effect of OT. Improvement in reversal learning after discontinuation of the daily OT regimen, meanwhile, may have been a result of neuroplastic changes in the brain caused by chronic OT administration. Such a relationship between neuroplasticity and experimental time point has been observed previously in an in vitro study of the effects of OT in the infralimbic medial prefrontal cortex (IL-mPFC)(Ninan, 2011). OT initially mediated suppression of glutamatergic transmission in pyramidal neurons after 25 minutes of perfusion, but then enhanced NMDA receptor-dependent synaptic potentiation in IL-mPFC slices after 60 minutes, likely as a consequence of time-dependent OT receptor-induced intracellular changes. While in vitro perfusion of brain slices is clearly not an ideal model of chronic drug/body interaction in vivo, it does illustrate that OT can have variable and long-lasting effects at the neuronal level depending on duration of administration. Considered alongside a growing number of rodent studies implicating OT as a mediator of cognitively relevant neuroplastic processes in such areas as the hippocampus (Lee et al., 2015; Park et al., 2017), olfactory bulb (Fang et al., 2008), and auditory cortex (Marlin et al., 2015), it remains plausible that OT produced its effects on reversal learning through such a mechanism. Delineating these mechanisms, and characterizing any sexual dimorphism, is a vital step in understanding the behavioral effects of OT. However, as the current study design included no assay of neuroplastic activity, this task can only be formally addressed by future research.
While the site of action of OT was not assessed here, the orbitofrontal cortex (OFC) and nucleus accumbens (NAc) are promising areas for examination. The importance of these two regions in reversal learning is well established – OFC disruption impairs initial probabilistic learning and subsequent reversal learning in rodents (Dalton et al., 2016) and humans (Tsuchida et al., 2010), and similar deficits have been observed in rats after NAc shell inactivation (Dalton et al., 2014). These brain regions are particularly relevant to the present study because of their relationship with OT – the NAc shell (but not the core) is densely populated by OT binding sites in the rat (Kremarik et al., 1993; Veinante and Freund-Mercier, 1997), and intranasal OT influences OFC activity in fMRI studies of healthy human subjects (Ide et al., 2018; Preckel et al., 2015; Scheele et al., 2014). Intranasally and peripherally administered OT can, through some as-yet unknown mechanism of transport, cross the blood-brain-barrier in rodents and macaques (Lee et al., 2018; Neumann et al., 2013), so it is therefore possible that exogenous OT can directly act on these systems. Though these relationships of course in no way affirm that OT-augmented activity in one or both of these structures is responsible for the observed improvement in reward learning, they do offer justification for more targeted studies in the future.
Tolerance to the pro-learning effects of OT was observed following 21 days of daily administration in female rats only. Tolerance in response to repeated administration is consistent with the OT literature. Like most G protein-coupled receptors, the OT receptor (OTR) undergoes desensitization and internalization following stimulation, with evidence of endocytosis in vitro as early as five minutes after agonist application (Conti et al., 2009). However, chronic OT administration alters receptor activity beyond this classic agonist-receptor dynamic. The OTR exhibits significantly reduced binding activity following 15 days of daily intracranial OT in mice (Peters et al., 2014). Significant reductions in binding site number have also been observed following 7 days of twice-daily nasal OT in mice (Huang et al., 2014), and after 21 days of daily intracranial OT in rats (Freeman et al., 2018). While such phenomena may account for loss of an initial OT effect following 21 days of chronic administration, these findings do not explain why the present study observed tolerance in female rats but not males. Expression of the OTR varies significantly between sexes in both mice and rats. According to a recent meta-analysis of murine brain areas, OTR expression and binding density is higher in males (though OT expression is generally higher in females) (Dumais and Veenema, 2016). Specific areas include the NAc and the amygdala, both of which are implicated in learning and decision-making (Dumais et al., 2013). Unfortunately, the current paucity of chronic OT studies makes it difficult to predict the cognitive and behavioral consequences of such sexual dimorphism across a long-term treatment course. Also complicating matters, most behavioral studies that look at OTR activity together with sex predominantly do so in a strictly social context. It is therefore difficult to extrapolate established correlations between OTR activity and animal behavior to the present findings on non-social probabilistic learning. It is also important to note that effects of sex were not observed in certain studies of chronic OT on positive, negative, and cognitive symptoms of schizophrenia in humans (Feifel et al., 2012a; Feifel et al., 2010); these negative findings suggest that OT and sex may interact differently across species and across cognitive/neuropsychiatric domains. Indeed, the relationship between OTR binding, density, and sex found in rodents by Dumais and Veenema does not appear to extend to humans (Dumais and Veenema, 2016), so while this general interaction may inform results of rat and mouse studies, it remains to be seen if the question of sexually dimorphic OTR dynamics would be relevant to human reward learning. With this in mind, the positive effects observed by the present study, as well as the apparent absence of deleterious side effects or withdrawal symptoms in either sex (e.g., reduction in effortful motivation or elevation in motoric impulsivity), recommend further study of OT as a viable therapeutic.
OT-induced improvement of probabilistic reversal learning would be beneficial in the treatment of many disorders involving impaired reward learning, but may be of particular importance to schizophrenia. Impaired probabilistic learning is predictive of poor performance in subsequent reversal learning in patients with schizophrenia, while a schizophrenia diagnosis in the absence of initial probabilistic learning deficits does not seem to correlate with deficient reversal learning (Reddy et al., 2016). This apparent reliance of reversal learning upon initial probabilistic task performance in the schizophrenia disease state could potentially be addressed by acute OT treatment. The present findings support a large body of existing literature reporting OT as a potential adjunct treatment for the cognitive symptoms of schizophrenia (Feifel et al., 2010; Modabbernia et al., 2013; Pedersen et al., 2011), adding reward learning to the list of possible applications.
It remains unclear from these data whether OT would be beneficial for the negative symptoms (e.g., amotivation) of schizophrenia. Patients with schizophrenia exhibit significantly lower breakpoint than healthy comparison subjects (Bismark et al., 2017; Wolf et al., 2014). Such amotivation may negatively impact cognitive outcomes (Markou et al., 2013), as seen where breakpoints predicted 24% of the variance of global cognitive scores in patients (Bismark et al., 2017). The lack of effect of OT on BN rats could however, be a result of ceiling effects given that BN rats exhibited comparable breakpoints with LE rats throughout testing. It would behoove future studies to test the potential benefits of OT on motivation in animals with reduced breakpoint vs. control subjects, e.g., Sp4 hypomorphic mice (Young et al., 2015). As a positive however, these data support the premise that OT is unlikely to exacerbate pre-existing substance abuse disorders in clinical populations. The high comorbidity rate between schizophrenia and substance disorders (47% as of 1990 (Regier et al., 1990)) makes abuse potential a concern when assessing novel therapeutics for this patient population. As stated above, OT did not increase breakpoint in the PRBT, in contrast with drugs of abuse such as cocaine (Sizemore et al., 2003), or phencyclidine (Amitai et al., 2017). Furthermore, OT actually attenuated drug seeking behavior in rodents for methamphetamine (Carson et al., 2010), cocaine (Kohtz et al., 2018), and alcohol (King et al., 2017); hence it is under separate investigation as a possible treatment for drug addiction (McGregor and Bowen, 2012). Although peripherally and centrally administered OT produced conditioned place preference in rats (Liberzon et al., 1997) and mice (Kent et al., 2013), this effect is not consistent across studies (Kosaki and Watanabe, 2016; Qi et al., 2009; Ramos et al., 2015). Since the rewarding effects of OT administration cannot be reliably demonstrated in rodents, any rewarding effects of OT in a clinical context would likely be minimal. Taken together, while OT may not remediate amotivation seen in schizophrenia, the present findings predict an overall low abuse potential for prescription OT.
The categorization of OT as a potential treatment for psychiatric conditions draws attention to additional limitations of this study. In addition to its inability to definitively identify specific mechanisms and areas of action of OT effects, this study is limited in that it did not incorporate any manipulations relevant to disease etiology (hence limited construct validity). Although the incomplete knowledge of the etiology of psychiatric disorders may support the use of strain comparisons to model cognitive deficits, no specific assumptions of diseased circuitry can be made. While the present study hypothesized, and later demonstrated, that BN rats are naturally inferior to LE rats on the PRLT, such cognitive limitation is a characteristic of the strain itself, and not the result of pathology. Future studies demonstrating OT-induced improvement in disease models would prove beneficial to the development of OT as a targeted therapeutic. It is also of critical importance that, regardless of the results of such studies, it is highly unlikely that OT would be able to completely replace established medication for psychiatric disorders – in the “best case scenario,” OT would be an adjunct therapeutic only. OT effects on reward learning must therefore be assessed when coadministered with primary treatment medications (antidepressants, antipsychotics, etc.). OT has previously conferred benefits to medicated schizophrenia patients in some clinical studies (summarized by (Shilling and Feifel, 2016)), so such an investigation may be regarded optimistically.
5. Conclusion
The results described herein provide evidence for OT as a safe and effective therapeutic to improve reward learning in patient populations. Specifically, OT improved probabilistic reversal learning in healthy BN rats without affecting effortful motivation/pleasure-seeking behavior. Our findings demonstrate both tolerance (females) and persistence (males) of the acute effects of OT with chronic administration. Effects persisted beyond the direct pharmacological influence of OT, consistent with previous studies (Bales et al., 2013; Conti et al., 2009; Huang et al., 2014; Janezic et al., 2016). Importantly, acute improvements were observed irrespective of sex, while long-term improvements were specific to males. Acute treatment is therefore likely to be beneficial across genders, though long-term efficacy may be restricted to males. No deleterious side effects of OT were observed on either males or females at any point during the present study. While the design of the study did not include any means of determining exact mechanism of action of OT, the cognitive enhancement induced by OT and the apparent safety of the drug strongly recommend further effort towards its development as a novel therapeutic.
Supplementary Material
Highlights.
Rats received daily OT for 21 days and tested in a reward learning task (PRLT).
Rats also assessed in a effortful motivation task (PRBT).
OT improved PRLT performance in males throughout testing
Only acute OT improved PRLT in females.
Neither acute nor chronic OT had any significant effect on PRBT.
Acknowledgments
We thank Ms. Gilia Melendez and Mahalah Buell as well as Mr. Richard Sharp for their support. This research was supported by NIMH grant R01MH103421 (DF), and R01DA044909 (JY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson DT, Twamley EW, Young JW, 2013. Reward learning as a potential target for pharmacological augmentation of cognitive remediation for schizophrenia: a roadmap for preclinical development. Front Neurosci 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Powell SB, Young JW, 2017. Phencyclidine increased while isolation rearing did not affect progressive ratio responding in rats: Investigating potential models of amotivation in schizophrenia. Behav Brain Res. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP, 2013. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry 74, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismark AW, Thomas ML, Tarasenko M, Shiluk AL, Rackelmann SY, Young JW, Light GA, 2017. Relationship between effortful motivation and neurocognition in schizophrenia. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato B, Carvalho AF, McIntyre RS, 2014. Cognitive dysfunction in major depressive disorder: a state-of-the-art clinical review. CNS Neurol Disord Drug Targets 13, 1804–1818. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Veenema AH, 2018. Sex differences in the regulation of social and anxiety-related behaviors: insights from vasopressin and oxytocin brain systems. Curr Opin Neurobiol 49, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, Moore L, Alvares GA, Redoblado Hodge MA, Guastella AJ, 2015. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophr Bull 41, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Stephens SL, Young WS 3rd, 2009. Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry 14, 190–196. [DOI] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS, 2010. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology 58, 38–43. [DOI] [PubMed] [Google Scholar]
- Conti F, Sertic S, Reversi A, Chini B, 2009. Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a Rab4/Rab5 "short cycle". Am J Physiol Endocrinol Metab 296, E532–542. [DOI] [PubMed] [Google Scholar]
- Conti LH, Palmer AA, Vanella JJ, Printz MP, 2001. Latent inhibition and conditioning in rat strains which show differential prepulse inhibition. Behav Genet 31, 325–333. [DOI] [PubMed] [Google Scholar]
- Cope ZA, Powell SB, Young JW, 2016. Modeling neurodevelopmental cognitive deficits in tasks with cross-species translational validity. Genes Brain Behav 15, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagani J, Sisti D, Abelli M, Di Paolo L, Pini S, Raimondi S, Rocchi MB, Saviotti FM, Scocco P, Totaro S, Balestrieri M, de Girolamo G, 2016. Do we need oxytocin to treat schizophrenia? A randomized clinical trial. Schizophr Res 172, 158–164. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Phillips AG, Floresco SB, 2014. Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. J Neurosci 34, 4618–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Wang NY, Phillips AG, Floresco SB, 2016. Multifaceted Contributions by Different Regions of the Orbitofrontal and Medial Prefrontal Cortex to Probabilistic Reversal Learning. J Neurosci 36, 1996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, Marder SR, 2013. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res 147, 393–397. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E, 2010. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med 40, 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didriksen M, Christensen AV, 1993. Differences in performance in three strains of rats in a 5-choice serial reaction time task. Pharmacol Toxicol 72, 66–68. [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Clark L, Siegle GJ, Butters MA, Ichikawa N, Sahakian BJ, Szanto K, 2010. Reward/Punishment reversal learning in older suicide attempters. Am J Psychiatry 167, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte W, Becerra R, Cruise K, 2016. The Relationship Between Neurocognitive Functioning and Occupational Functioning in Bipolar Disorder: A Literature Review. Eur J Psychol 12, 659–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH, 2013. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex-specific ways. Horm Behav 64, 693–701. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH, 2016. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol 40, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang LY, Quan RD, Kaba H, 2008. Oxytocin facilitates the induction of long-term potentiation in the accessory olfactory bulb. Neurosci Lett 438, 133–137. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Cobb P, Minassian A, 2012a. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res 139, 207–210. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A, 2010. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry 68, 678–680. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Priebe K, Shilling PD, 2007. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res 181, 278–286. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza T, 1999. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology (Berl) 141, 93–98. [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Belcher AM, 2012b. The effects of oxytocin and its analog, carbetocin, on genetic deficits in sensorimotor gating. Eur Neuropsychopharmacol 22, 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Hillman J, Maisel M, Winfield J, Melendez G, 2015. Peripherally administered oxytocin modulates latent inhibition in a manner consistent with antipsychotic drugs. Behav Brain Res 278, 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Melendez G, 2011. Clozapine and PD149163 elevate prepulse inhibition in Brown Norway rats. Behav Neurosci 125, 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Ngo J, Singh B, Masnaghetti M, Bales KL, Blevins JE, 2018. Effects of Chronic Oxytocin Administration and Diet Composition on Oxytocin and Vasopressin 1a Receptor Binding in the Rat Brain. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA, 2014. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr Res 156, 261–265. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R, 2011. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl) 216, 101–110. [DOI] [PubMed] [Google Scholar]
- Green MF, 2006. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 67, e12. [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J, 2000. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull 26, 119–136. [DOI] [PubMed] [Google Scholar]
- Hershenberg R, Satterthwaite TD, Daldal A, Katchmar N, Moore TM, Kable JW, Wolf DH, 2016. Diminished effort on a progressive ratio task in both unipolar and bipolar depression. J Affect Disord 196, 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta de Macedo LR, Zuardi AW, Machado-de-Sousa JP, Chagas MH, Hallak JE, 2014. Oxytocin does not improve performance of patients with schizophrenia and healthy volunteers in a facial emotion matching task. Psychiatry Res 220, 125–128. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni ML, Papaleo F, 2014. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 39, 1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Nedic S, Wong KF, Strey SL, Lawson EA, Dickerson BC, Wald LL, La Camera G, Mujica-Parodi LR, 2018. Oxytocin attenuates trust as a subset of more general reinforcement learning, with altered reward circuit functional connectivity in males. Neuroimage 174, 35–43. [DOI] [PubMed] [Google Scholar]
- Janezic EM, Uppalapati S, Nagl S, Contreras M, French ED, Fellous JM, 2016. Beneficial effects of chronic oxytocin administration and social co-housing in a rodent model of post-traumatic stress disorder. Behav Pharmacol 27, 704–717. [DOI] [PubMed] [Google Scholar]
- Keiser AA, Matazel KS, Esser MK, Feifel D, Prus AJ, 2014. Systemic administration of the neurotensin NTS(1)-receptor agonist PD149163 improves performance on a memory task in naturally deficient male brown Norway rats. Exp Clin Psychopharmacol 22, 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K, Arientyl V, Khachatryan MM, Wood RI, 2013. Oxytocin induces a conditioned social preference in female mice. J Neuroendocrinol 25, 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC, 2017. Oxytocin Reduces Ethanol Self-Administration in Mice. Alcohol Clin Exp Res 41, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz AS, Lin B, Smith ME, Aston-Jones G, 2018. Attenuated cocaine-seeking after oxytocin administration in male and female rats. Psychopharmacology (Berl) 235, 2051–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaki Y, Watanabe S, 2016. Conditioned social preference, but not place preference, produced by intranasal oxytocin in female mice. Behav Neurosci 130, 182–195. [DOI] [PubMed] [Google Scholar]
- Kremarik P, Freund-Mercier MJ, Stoeckel ME, 1993. Histoautoradiographic detection of oxytocin- and vasopressin-binding sites in the telencephalon of the rat. J Comp Neurol 333, 343–359. [DOI] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, Leggio L, Averbeck BB, 2018. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry 23, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Wehring HJ, McMahon RP, Linthicum J, Cascella N, Liu F, Bellack A, Buchanan RW, Strauss GP, Contoreggi C, Kelly DL, 2013. Effects of adjunctive intranasal oxytocin on olfactory identification and clinical symptoms in schizophrenia: results from a randomized double blind placebo controlled pilot study. Schizophr Res 145, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Park SH, Chung C, Kim JJ, Choi SY, Han JS, 2015. Oxytocin Protects Hippocampal Memory and Plasticity from Uncontrollable Stress. Sci Rep 5, 18540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Trujillo KA, Akil H, Young EA, 1997. Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology 17, 353–359. [DOI] [PubMed] [Google Scholar]
- Love TM, 2014. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav 119, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Salamone JD, Bussey TJ, Mar AC, Brunner D, Gilmour G, Balsam P, 2013. Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. Neurosci Biobehav Rev 37, 2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D'Amour J A, Chao MV, Froemke RC, 2015. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT, 2012. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav 61, 331–339. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, Alsuwaidan M, Baskaran A, 2013. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety 30, 515–527. [DOI] [PubMed] [Google Scholar]
- Michalopoulou PG, Averbeck BB, Kalpakidou AK, Evans S, Bobin T, Kapur S, Shergill SS, 2015. The effects of a single dose of oxytocin on working memory in schizophrenia. Schizophr Res 162, 62–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milienne-Petiot M, Kesby JP, Graves M, van Enkhuizen J, Semenova S, Minassian A, Markou A, Geyer MA, Young JW, 2017. The effects of reduced dopamine transporter function and chronic lithium on motivation, probabilistic learning, and neurochemistry in mice: Modeling bipolar mania. Neuropharmacology 113, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SM, Tajdini M, Ghaleiha A, Akhondzadeh S, 2013. Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia : an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs 27, 57–65. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R, 2013. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38, 1985–1993. [DOI] [PubMed] [Google Scholar]
- Ninan I, 2011. Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J Neurochem 119, 324–331. [DOI] [PubMed] [Google Scholar]
- Park SH, Kim YJ, Park JC, Han JS, Choi SY, 2017. Intranasal Oxytocin following Uncontrollable Stress Blocks Impairments in Hippocampal Plasticity and Recognition Memory in Stressed Rats. Int J Neuropsychopharmacol 20, 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL, 2011. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res 132, 50–53. [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID, 2014. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 42, 225–236. [DOI] [PubMed] [Google Scholar]
- Preckel K, Scheele D, Eckstein M, Maier W, Hurlemann R, 2015. The influence of oxytocin on volitional and emotional ambivalence. Soc Cogn Affect Neurosci 10, 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF, 2009. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology 56, 856–865. [DOI] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Caminer A, Goodwin J, McGregor IS, 2015. Oxytocin and MDMA ('Ecstasy') enhance social reward in rats. Psychopharmacology (Berl) 232, 2631–2641. [DOI] [PubMed] [Google Scholar]
- Reddy LF, Waltz JA, Green MF, Wynn JK, Horan WP, 2016. Probabilistic Reversal Learning in Schizophrenia: Stability of Deficits and Potential Causal Mechanisms. Schizophr Bull 42, 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK, 1990. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 264, 2511–2518. [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM, 2011. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res 130, 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Kendrick KM, Khouri C, Kretzer E, Schlapfer TE, Stoffel-Wagner B, Gunturkun O, Maier W, Hurlemann R, 2014. An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology 39, 2078–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling PD, Feifel D, 2016. Potential of Oxytocin in the Treatment of Schizophrenia. CNS Drugs 30, 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore GM, Cannon DG, Smith JE, Dworkin SI, 2003. The effects of acutely administered cocaine on responding maintained by a progressive-ratio schedule of food presentation. Behav Pharmacol 14, 33–40. [DOI] [PubMed] [Google Scholar]
- Sole B, Jimenez E, Torrent C, Reinares M, Del Mar Bonnin C, Torres I, Varo C, Grande I, Valls E, Salagre E, Sanchez-Moreno J, Martinez-Aran A, Carvalho AF, Vieta E, 2017. Cognitive Impairment in Bipolar Disorder: Treatment and Prevention Strategies. Int J Neuropsychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Whearty KM, Morra LF, Sullivan SK, Ossenfort KL, Frost KH, 2016. Avolition in schizophrenia is associated with reduced willingness to expend effort for reward on a Progressive Ratio task. Schizophr Res 170, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK, 2010. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci 30, 16868–16875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ, 1997. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol 383, 305–325. [PubMed] [Google Scholar]
- Walsh RN, Cummins RA, 1976. The Open-Field Test: a critical review. Psychol Bull 83, 482–504. [PubMed] [Google Scholar]
- Waltz JA, Gold JM, 2007. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res 93, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA, 2015. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry 28, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA, Ruparel K, 2014. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull 40, 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YS, Rosenblat JD, Kakar R, Bahk WM, McIntyre RS, 2016. Cognitive Deficits as a Mediator of Poor Occupational Function in Remitted Major Depressive Disorder Patients. Clin Psychopharmacol Neurosci 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Lam O, Lai W, O'Donovan A, Rankin KP, Mathalon DH, Vinogradov S, 2014. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology 47, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Amitai N, Geyer MA, 2012. Behavioral animal models to assess pro-cognitive treatments for schizophrenia. Handb Exp Pharmacol, 39–79. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA, 2015. Developing treatments for cognitive deficits in schizophrenia: the challenge of translation. J Psychopharmacol 29, 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kamenski ME, Higa KK, Light GA, Geyer MA, Zhou X, 2015. GlyT-1 Inhibition Attenuates Attentional But Not Learning or Motivational Deficits of the Sp4 Hypomorphic Mouse Model Relevant to Psychiatric Disorders. Neuropsychopharmacology 40, 2715–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Markou A, 2015. Translational Rodent Paradigms to Investigate Neuromechanisms Underlying Behaviors Relevant to Amotivation and Altered Reward Processing in Schizophrenia. Schizophr Bull 41, 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM, 2015. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behav Brain Res 283, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.