Abstract
Objectives:
HPV-positive oropharyngeal cancer (OPC) patients have been observed to be younger than patients with HPV-negative OPC at diagnosis. We evaluated recent trends in age at OPC diagnosis, and whether older age attenuates the survival benefit of HPV-positive tumor status.
Materials and methods:
Patients diagnosed with OPC from 2004 to 2014 represented in the National Cancer Database were included. HPV tumor status was available after 2010. Trends in age by calendar year were compared using linear regression. Overall survival was compared using Cox Proportional Hazards models.
Results:
The mean age of OPC patients (N=119,611) increased significantly from 2004 to 2014 (ß=0.21 years of age per calendar year, 95% confidence interval [CI]=0.19–0.23). The increase in age from 2010 to 2014 was similar for HPV-positive (N=21,880; ß=0.63, 95%CI=0.53–0.72) and HPV-negative (N=11,504; ß=0.59, 95%CI=0.45–0.74) patients. Between 2010 and 2014, the proportion of OPCs that were HPV-positive increased significantly for all age groups, including for patients ≥70 years old (from 45% to 60%, ptrend < 0.001). Although patients ≥70 years with HPV-OPC had improved survival compared to those with HPV-negative OPC (adjusted hazard ratio [aHR]=0.65, 95%CI=0.55–0.76), the survival benefit of HPV-positive tumor status was significantly attenuated compared to younger HPV-OPC patients (50–59 years: aHR=0.45, 95%CI=0.39–0.51; pinteraction < 0.001).
Conclusion:
The age at OPC diagnosis is increasing for both HPV-positive and HPV-negative patients, and a rising proportion of older patients have HPV-positive tumors. These findings dispel the notion that HPV-positive OPC is a disease of younger patients, identify a growing elderly population of HPV-positive OPC patients with reduced survival, and have implications for evolving treatment paradigms.
Keywords: Oropharynx cancer, Head and neck cancer, Human papillomavirus, Elderly, Aging, Survival
Introduction
Human papillomavirus (HPV) is responsible for a unique and growing subset of oropharyngeal cancers (OPCs) in the United States (U.S.) [1]. These HPV-positive OPCs (HPV-OPCs) arise primarily from the immunologically specialized reticulated stratified squamous lymphoepithelium investing the lymphoid tissues of the palatine and lingual tonsils, rather than the continuous stratified squamous epithelium of the soft palate and posterior pharyngeal walls that more often harbors HPV-negative OPCs [2,3]. Many earlier studies have observed that patients with HPV-OPCs have a distinct epidemiology when compared to patients with HPV-unrelated OPCs: in addition to being statistically younger, they are more likely to be male, have fewer comorbidities, and report less tobacco exposure but higher numbers of sexual partners [1,4,5]. Population-based research has demonstrated dramatic increases in the incidence of OPC in the U.S and abroad among younger age cohorts and males that are attributable to HPV [1]. However, a recent analysis of the U.S. Surveillance, Epidemiology and End Results (SEER) data shows analogous increases in incidence of OPSCC among individuals older than 65 years [6]. It remains unknown whether HPV is also responsible for this change.
In addition to conferring a unique clinical-demographic profile upon patients, HPV-OPCs have improved overall and progression-free survival as compared with HPV-unrelated OPCs [7]. Presently, clinical trials for OPC are focused on patients with HPV-related disease, with a goal of maintaining the excellent prognosis while decreasing treatment-related toxicities [8,9]. Such so-called deintensification trials are motivated in part by the younger age of HPV-OPC patients and the many years that most are expected to survive after treatment. However, should the population of elderly OPC patients continue to expand, deintensification clinical trial design must take into account whether HPV continues to confer the same prognostic advantage among older individuals that is observed for their younger counterparts who historically comprised the vast majority of HPV-OPC patients.
This study was designed to evaluate age-related changes in OPC over time, to determine whether the prevalence of HPV-related tumors is increasing among older OPC patients in analogous fashion as with younger patients, and to determine whether HPV continues to be associated with improved overall survival among older individuals.
Methods
Data source and patient population
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. It captures newly diagnosed cancers from over 1500 hospitals, encompassing approximately 70% of incident U.S. cancer cases [10]. The data used in this study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. This study was deemed exempt from review by the Johns Hopkins Medicine Institutional Review Board.
Primary tumor sites were classified by International Classification of Disease for Oncology, 3rd edition (ICD-O-3) site codes as follows: OPC, comprised of ‘lymphoid OPC’, including the palatine tonsils (C090-091, C098-099) and lingual tonsils/tongue base (C019, C024), and ‘other OPC’, including all oropharynx tumors not specifically designated as tonsil or tongue base/lingual tonsil (C051-052, C100-104, C108-109, C142); and oral cavity, C000-006, C008-009, C020-0234, C030-031, C039-041, C048-050, C060-062, C068-069. ICD-O-3 histology codes were limited to squamous cell carcinomas (8052, 8070-8076, 8078, 8083).
OPCs that tested positive for low-risk HPV types only were considered HPV-negative, and tumors without HPV testing reported were considered HPV-unknown. OPCs were considered HPV-positive if they were positive for any high-risk HPV type, or were reported as “HPV-positive, not otherwise specified, risk and type not stated”. A sensitivity analysis was performed that considered “HPV-positive, not otherwise specified, risk and type not stated” tumors as HPV-unknown, and the conclusions of the analysis were unchanged (data not shown). Therefore, these tumors were retained in the HPV-positive group.
Trends in age at diagnosis over time from 2004 to 2014 were explored and compared by tumor site (OPC and oral cavity). Comparison of trends in age at diagnosis over time by HPV tumor status was limited to OPC cases from 2010 to 2014, as there were very few tumors (N=112) tested for HPV prior to 2010. Survival analysis by HPV tumor status was further limited to OPC cases from 2010 to 2013, as vital status was not available for 2014 diagnoses. Staging for tumors with known HPV status was adjusted to be consistent with the American Joint Committee on Cancer (AJCC) Eighth Edition clinical staging system [11,12]. HPV-positive tumors without nodal laterality information available were assigned an ‘unknown’ Eight Edition N stage. AJCC Seventh Edition T0, Tx, and Tis tumors were assigned an ‘unknown’ Eighth Edition T stage. HPV-negative tumors without clinical extranodal extension information available were assigned their original AJCC Seventh Edition N stage. Patients with missing AJCC Eighth Edition stage information and those who were treated with palliative intent were excluded from survival analysis.
Statistical analysis
Descriptive statistics were reported as N (%) and mean (standard deviation [SD]). Characteristics were compared by HPV status among OPCs, and by age group for HPV-OPCs, using chi-squared tests for categorical variables and t-tests for continuous variables. Trends in mean age over calendar year were analyzed using linear regression, reporting ß and 95% confidence intervals (CI) and p-values for interaction terms. Trends in prevalence of HPV-positive OPCs were analyzed using nonparametric tests of trend across ordered groups [13]. Overall survival (OS) was calculated using the Kaplan-Meier method, and unadjusted and adjusted hazard ratios (HR and aHR) with 95% CIs generated using Cox Proportional Hazards models. Interaction terms were tested and p-values reported. The proportional hazards assumptions were confirmed using log-log plots. Two-sided p < 0.05 was considered significant. Statistical analysis was performed using Stata 14 (College Station, TX).
Results
Trends in age at diagnosis of oropharyngeal cancer
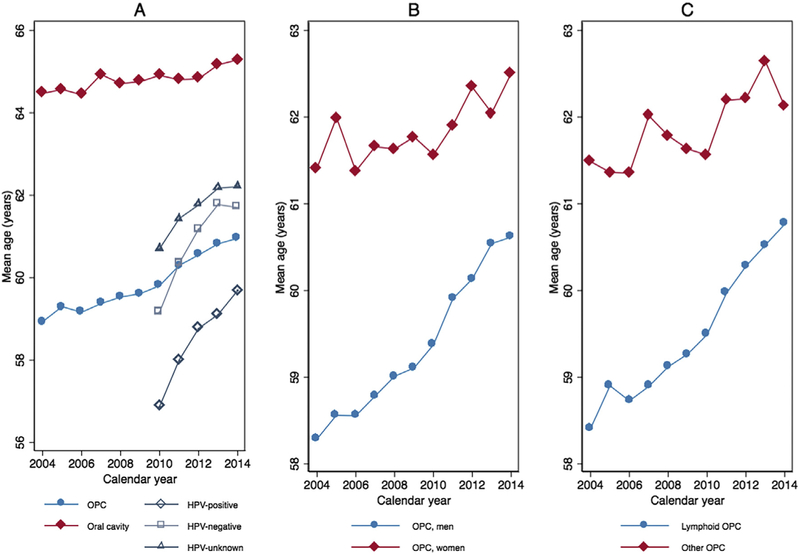
To evaluate trends in mean age at diagnosis, all patients with a diagnosis of OPC between 2004 and 2014 (N=119,611) were considered. The average age of diagnosis with OPC increased steadily from 58.9 (SD=11.1) in 2004 to 61.0 (SD=10.0) in 2014 (ß 0.21, 95%CI=0.19–0.23; ß represents years of age per calendar year; Fig. 1A and Supplemental Table 1). In comparison, the mean age of oral cavity cancer diagnosis, representative of HPV-negative head and neck cancers, only increased from 64.5 (SD=13.7) in 2004 to 65.3 (SD=13.1) in 2014 (ß=0.07, 95%CI=0.04–0.10). The magnitude of increase in mean age per calendar year was approximately three-fold higher for OPC relative to oral cavity cancer (pinteraction < 0.001 for tumor site and calendar year).
Fig. 1.
Trends in mean age at diagnosis by calendar year stratified by tumor site and, among OPCs, by HPV status (Panel A), a sex (Panel B) and lymphoid vs. other OPC subsite (Panel C). aHPV tumor status was only included for OPCs 2010–2014 due to limited availability prior to 2010. Abbreviations: HPV, human papillomavirus; OPC, oropharyngeal cancer.
Given established sex-related differences in the epidemiology of OPC, trends in age of diagnosis over time were examined by sex (Fig. 1B). As expected, most OPC patients were men (N=96,205, 80%). Men were ~2.4 years younger than women (59.5 versus 61.9 years old, p < 0.001). While age increased for both sexes the rate of increase among men (ß 0.25, 95%CI=0.23–0.27) was significantly faster than that among women (ß 0.09, 95%CI=0.04–0.13, pinteraction < 0.001 for sex and calendar year).
Finally, considering that the lymphoid oropharyngeal subsites of the palatine and lingual tonsils are uniquely susceptible to HPV-OPC, trends in age were examined for lymphoid (N=101,696, 85%) and other OPC (N=17,915, 15%) tumors (Fig. 1C). Patients with lymphoid OPCs were 2.3 years younger than those with other OPCs (59.6 versus 61.9 years, p < 0.001), and exhibited a significantly more rapid increase in age over time compared with other OPCs (lymphoid versus other OPC: ß 0.24, 95%CI=0.22–0.26 versus ß 0.10, 95%CI=0.05–0.15; pinteraction < 0.001 for OPC subsite and calendar year).
With further stratification by sex and subsite, the most dramatic age increase was among men with lymphoid OPCs (N=82,899; ß 0.27, 95%CI=0.24–0.29). Moderate increases were observed for men with other OPCs (N=13,307; ß 0.17, 95%CI=0.11–0.23) and women with lymphoid OPCs (N=18,797; ß 0.13, 95%CI=0.08–0.19). A nonsignificant decrease in age over time was seen for women with other OPCs (N=4608; ß −0.08, 95%CI=−0.19–0.02).
Age increase amongst HPV-positive, HPV-negative and HPV-unknown OPC patients
To determine whether the increase in age among OPC patients was attributable to HPV, further analyses were limited to 2010–2014, during which time HPV tumor status was available. Characteristics of the OPC patients stratified by HPV tumor status are described in Table 1. Among 63,187 OPCs, 33,384 (53%) were tested for HPV. Of these, the majority was HPV-positive (N=21,880, 66%). Patients with HPV testing were on average 2.0 years younger than those without testing (59.6 versus 61.6, p < 0.001), and HPV-positive patients were on average 2.3 years younger than HPV-negative patients (58.8 versus 61.1, p < 0.001).
Table 1.
Characteristics of OPC patients with HPV-positive, HPV-negative and HPV-unknown tumors from 2010 to 2014.
| OPCs included in analysis of HPV tumor status 2010–2014 | |||||
|---|---|---|---|---|---|
| Characteristic | Total No. (%)a | HPV-positive No. (%)a | HPV-negative No. (%)a | HPV-unknown No. (%)a | p-valueb |
| Total | 63,187 | 21,880 (35) | 11,504 (18) | 29,803 (47) | |
| Age in years, mean (SD) | 60.5 (10.2) | 58.8 (9.5) | 61.1 (10.5) | 61.6 (10.5) | <0.001 |
| Sex | <0.001 | ||||
| Male | 51,248 (81) | 13,729 (86) | 8752 (76) | 23,767 (80) | |
| Female | 11,939 (19) | 3151 (14) | 2752 (24) | 6036 (20) | |
| Race | <0.001 | ||||
| White | 55,922 (89) | 20,316 (93) | 9760 (85) | 25,846 (87) | |
| Black | 5498 (9) | 1030 (5) | 1380 (12) | 3088 (10) | |
| Other/unknown | 1767 (3) | 534 (2) | 364 (3) | 869 (3) | |
| Residence | <0.001 | ||||
| Metro | 51,144 (81) | 18,032 (82) | 9561 (83) | 23,551 (79) | |
| Urban | 9,357 (15) | 2975 (14) | 1514 (13) | 4868 (16) | |
| Rural | 1075 (2) | 347 (2) | 140 (1) | 588 (2) | |
| Unknown | 1611 (3) | 526 (2) | 289 (3) | 796 (3) | |
| Facility type | <0.001 | ||||
| Community cancer program | 5671 (9) | 1529 (7) | 968 (9) | 3174 (11) | |
| Comprehensive community cancer program | 22,858 (37) | 6934 (32) | 3948 (35) | 11,976 (41) | |
| Academic /research | 27,335 (44) | 10,598 (49) | 5179 (46) | 11,558 (39) | |
| Integrated network | 6529 (10) | 2473 (11) | 1243 (11) | 2813 (10) | |
| Insurance | <0.001 | ||||
| Not insured | 3424 (5) | 839 (4) | 683 (6) | 1902 (6) | |
| Private | 29,579 (47) | 13,125 (60) | 4877 (42) | 11,577 (39) | |
| Medicare, Medicaid, other government | 28,235 (45) | 7645 (35) | 5744 (50) | 14,846 (50) | |
| Unknown | 1953 (3) | 271 (1) | 201 (2) | 1481 (5) | |
| Charleson-Deyo Score | <0.001 | ||||
| 0 | 51,359 (81) | 18,324 (84) | 9097 (79) | 23,938 (80) | |
| 1 | 9144 (14) | 2834 (13) | 1838 (16) | 4472 (15) | |
| 2+ | 2684 (4) | 722 (3) | 569 (5) | 1393 (5) | |
| Year of diagnosis | <0.001 | ||||
| 2010 | 11,261 (18) | 2111 (10) | 1326 (12) | 7824 (26) | |
| 2011 | 12,154 (19) | 3340 (15) | 2154 (19) | 6660 (22) | |
| 2012 | 12,637 (20) | 4622 (21) | 2518 (22) | 5497 (18) | |
| 2013 | 13,356 (21) | 5524 (25) | 2710 (24) | 5122 (17) | |
| 2014 | 13,779 (22) | 6283 (29) | 2796 (24) | 4700 (16) | |
| Primary subsite | <0.001 | ||||
| Tonsil | 28,866 (46) | 11,902 (54) | 4839 (42) | 12,125 (41) | |
| Tongue base | 25,249 (40) | 8435 (39) | 4470 (39) | 12,344 (41) | |
| Other oropharynx | 9072 (14) | 1543 (7) | 2195 (19) | 5334 (18) | |
| Clinical T stagec | <0.001 | ||||
| T1 | 13,642 (22) | 5558 (25) | 2320 (20) | 5764 (19) | |
| T2 | 21,479 (34) | 8190 (37) | 3734 (32) | 9555 (32) | |
| T3 | 10,374 (16) | 3131 (14) | 2154 (19) | 4089 (17) | |
| T4 | 9111 (14) | 2192 (10) | 2000 (17) | 4919 (17) | |
| Unknown | 8585 (14) | 2809 (13) | 1297 (11) | 4479 (15) | |
| Clinical N stagec | <0.001 | ||||
| N0 | 13,034 (21) | 2979 (14) | 3069 (27) | 6986 (23) | |
| N1 | 19,392 (31) | 12,728 (58) | 1872 (16) | 4792 (16) | |
| N2 | 22,567 (36) | 2892 (13) | 5187 (45) | 14,488 (49) | |
| N3 | 3145 (5) | 802 (4) | 823 (7) | 1520 (5) | |
| Unknown | 5050 (8) | 2479 (11) | 554 (5) | 2017 (7) | |
| Clinical M stagec | <0.001 | ||||
| M0 | 56,266 (89) | 20,196 (92) | 10,139 (88) | 25,931 (87) | |
| M1 | 2226 (4) | 435 (2) | 436 (4) | 1355 (5) | |
| Unknown | 4695 (7) | 1249 (6) | 929 (8) | 2517 (8) | |
| Treatment | <0.001 | ||||
| Surgery | 6910 (11) | 1861 (9) | 1539 (13) | 3510 (12) | |
| Surgery + radiation | 4955 (8) | 2327 (11) | 844 (7) | 1784 (6) | |
| Surgery + chemoradiation | 10,739 (17) | 5134 (23) | 1802 (16) | 3803 (13) | |
| Radiation | 4743 (8) | 1315 (6) | 904 (8) | 2524 (8) | |
| Chemoradiation | 28,594 (45) | 10,006 (46) | 5024 (44) | 13,564 (46) | |
| Palliative only | 1266 (2) | 253 (1) | 282 (2) | 731 (2) | |
| Other/unknown | 5984 (9) | 984 (5) | 1110 (10) | 3890 (13) | |
Abbreviations: HPV, human papillomavirus; OPC, oropharyngeal cancer; SD, standard deviation; No., number.
Percentages may not sum to 100% because of rounding.
p-values were calculated using t-test for age, and chi2 tests for other variables.
American Joint Committee on Cancer Eighth Edition clinical staging was used for HPV-positive and HPV-negative tumors, but Seventh Edition staging was used for HPV-unknown tumors because Eighth Edition requires known HPV tumor status.
OPC patients exhibited significant increases in mean age over time from 2010 to 2014, whether HPV-positive or HPV-negative (Fig. 1A and Supplemental Table 1). Both experienced dramatic and remarkably similar rates of age increase from 2010 to 2014 (HPV-positive K 0.63, 95%CI=0.53–0.72; HPV-negative ß 0.59, 95%CI=0.45–0.74; Fig. 1A). HPV-unknown patients sustained a significantly slower, but still notable, increase of ß 0.39, (95%CI=0.31–0.48; pinteraction < 0.001 for HPV status [known versus unknown] and calendar year). Importantly, the age difference between patients with known compared with unknown HPV tumor status decreased significantly from 2.9 years in 2010 to 1.9 years in 2014 (p=0.004).
Increasing proportion of older individuals among HPV-positive and HPV-negative OPCs
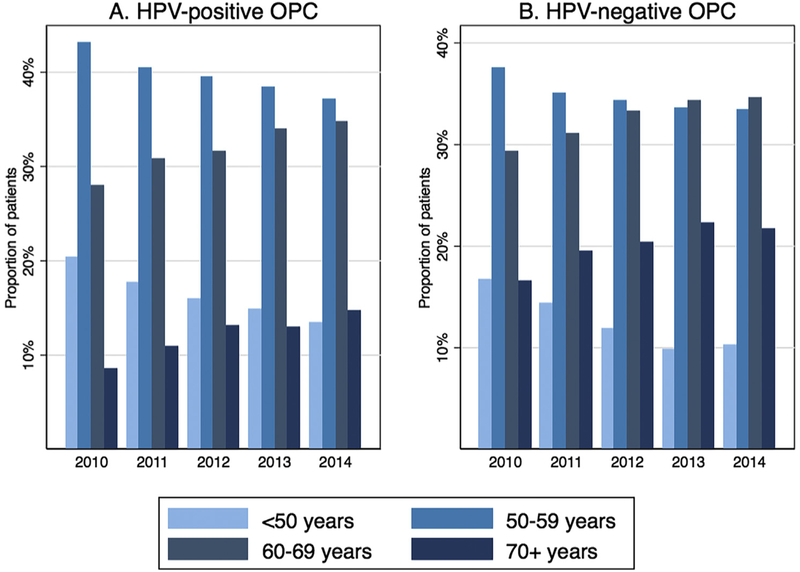
To understand how the observed increases in mean age impacted the demographic profile of OPC patients at diagnosis, the distribution of age categories was considered in consecutive calendar periods. The proportion of HPV-OPC patients ≥70 years old increased steadily from just 9% (181 of 2111) in 2010 to 15% (925 of 6283) in 2014, while the proportion of patients <50 years old decreased from 20% (429 of 2111) in 2010 to 13% (840 of 6283) in 2014 (Fig. 2A). Similarly, the proportion of HPV-negative patients ≥70 years old increased from 17% (219 of 1326) in 2010 to 22% (607 of 2,796) in 2014, and the proportion of <50 year olds decreased from 17% (221 of 1326) in 2010 to 10% (287 of 2796) in 2014 (Fig. 2B).
Fig. 2.
Proportion of each age group for HPV-positive OPCs (Panel A) and HPV-negative OPCs (Panel B) by calendar year. Abbreviations: HPV, human papillomavirus; OPC, oropharyngeal cancer.
Increasing proportion of HPV-positive OPCs in older age groups
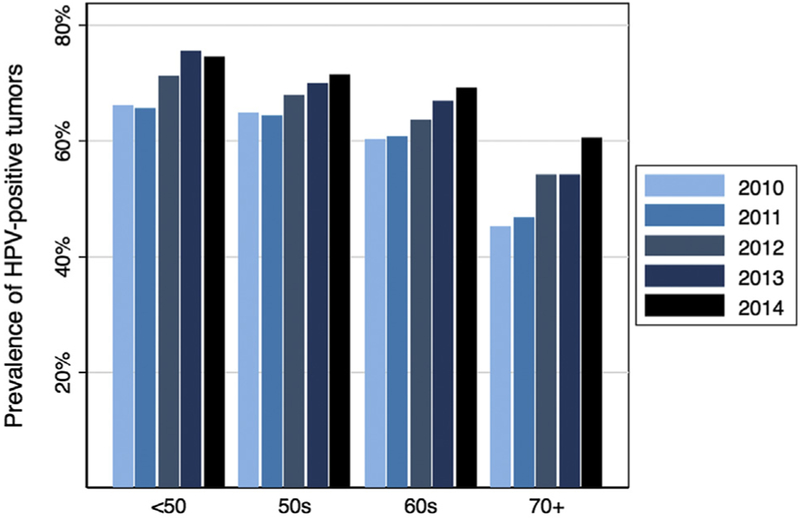
Given the increasing mean age of both HPV-positive and HPV-negative OPC patients, prevalence trends of HPV-positive tumor status were examined across age groups among patients with known HPV tumor status. The proportion of HPV-positive tumors was observed to increase significantly from 2010 to 2014 in all age groups, including those 60–69 and ≥70 years of age (ptrend < 0.001 for all; Fig. 3, Supplemental Table 2). Indeed, the increase in HPV prevalence among patients ≥70 years old was more dramatic than that observed for younger age groups (from 45% in 2010 to 60% in 2014 for ≥70 year olds, compared with 60–69% for 60–69 year olds and 65–71% for 50–59 year olds).
Fig. 3.
Proportion of HPV-positive oropharynx carcinomas by age group and calendar year among OPCs with known HPV status. Abbreviations: HPV, human papillomavirus; OPC, oropharyngeal cancer.
Characteristics of older HPV-OPC patients
In order to better understand the implications of an aging OPC population with an increasing predominance of HPV-related disease, characteristics of HPV-OPC patients were compared across age groups (Supplemental Table 3). Several clinically relevant patterns were observed. Older patients had higher Charleson-Deyo Comorbidity scores (6% versus 2% of ≥70 versus <50 year-olds with scores of 2+, p < 0.001). They also tended to present with higher T-stages (29% versus 20% of ≥70 versus <50 year olds with T3-T4 disease, p < 0.001) and lower N-stages (20% versus 11% of ≥70 versus <50 year-olds with N0 disease, p < 0.001). Older patients were significantly less likely to undergo primary surgical treatment (35% versus 52% of ≥70 versus <50 year-olds), a trend that held true across all tumor stages (data not shown). Finally, although just 1.2% of patients overall were treated with palliative intent, the proportion of patients receiving palliative treatment was higher among older age groups (1.8% of ≥70 versus 0.9% of <50 years olds, ptrend < 0.001; Supplemental Table 3).
Survival advantage associated with HPV-positive tumor status is attenuated by older age
With the increasing age of OPC diagnosis, we investigated whether the survival advantage of HPV-positive tumor status was attenuated among older OPC patients, as they are at increased risk for competing causes of mortality. 18,713 patients with known tumor HPV status and median follow-up time of 27.6 months (IQR=17.5–40.1 months) were included in this survival analysis.
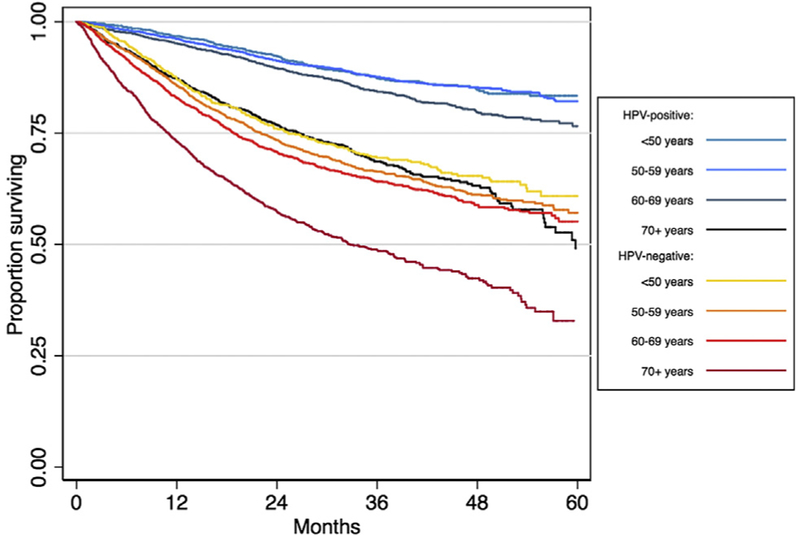
Overall, HPV-positive tumor status was associated with a 65% reduced risk of death (HR 0.35, 95%CI=0.33–0.38) for patients diagnosed from 2010 to 2013. Among patients 70 years and older, HPV-positive tumor status was still associated with improved survival (HR 0.51, 95%CI=0.45–0.57; Table 2 and Fig. 4), however this was significantly attenuated when compared to the HPV-related survival differences observed amongst younger age groups. Hazard ratios for younger patients ranged from 0.32 to 0.37, and there was a significant interaction of age group with HPV tumor status in predicting survival (pinteraction < 0.001; Table 2). This pattern persisted after adjustment for other prognostic factors (Supplemental Table 4), resulting in an aHR of 0.65 (95%CI=0.55–0.76) for patients 70 years and older, compared with aHRs of 0.45–0.55 for younger age groups (pinteraction < 0.001; Table 2).
Table 2.
Overall survival for HPV-positive compared with HPV-negative OPC patients, stratified by age group.
| Risk of death for patients with HPV-positive compared with HPV-negative OPC |
|||||
|---|---|---|---|---|---|
| No. | Unadjusted HR (95%CI) | p-value | Adjusted HR (95%CI)a | p-value | |
| Overall | 18,713 | 0.35 (0.33–0.38) | <0.001 | 0.54 (0.49–0.58) | <0.001 |
| Age group: | |||||
| <50 years | 2701 | 0.35 (0.29–0.42) | <0.001 | 0.55 (0.43–0.72) | <0.001 |
| 50–59 years | 7049 | 0.32 (0.28–0.35) | <0.001 | 0.45 (0.39–0.51) | <0.001 |
| 60–69 years | 6074 | 0.37 (0.33–0.41) | <0.001 | 0.53 (0.46–0.61) | <0.001 |
| 70+ years | 2889 | 0.51 (0.45–0.57) | <0.001 | 0.65 (0.55–0.76) | <0.001 |
| Pinteraction age group with HPV status | <0.001 | <0.001 | |||
Abbreviations: HPV, human papillomavirus; OPC, oropharyngeal cancer; HR, hazard ratio; No., number.
Adjusted for: age (continuous), sex, race, income, education, Charleson comorbidity index, facility type, insurance status, year of diagnosis, primary tumor subsite, treatment, and American Joint Committee on Cancer Eighth Edition clinical T stage, clinical N stage, and M stage. See Supplemental Table 4 for covariate HR and aHR estimates.
Fig. 4.
Overall survival for OPC patients stratified by HPV tumor status and age group. Abbreviations: HPV, human papillomavirus; OPC, oropharyngeal cancer.
Discussion
The appreciation that HPV is responsible for a distinct subset of OPC patients with unique characteristics, including younger age and better survival, has afforded the possibility of therapeutic de-intensification, currently under investigation in multiple clinical trials. However, this analysis highlights that the epidemiologic characteristics of OPC patients, including the unique phenotype of HPV-OPC relative to HPV-negative OPC, may have evolved. The age of diagnosis for HPV-positive OPC has increased rapidly and is accompanied by a contemporaneous notable rise in the proportion of OPCs caused by HPV among all age groups – especially elderly adults. Of particular clinical importance is the attenuated survival advantage afforded by HPV-positive tumor status among the growing population of older individuals with OPC, which should be accounted for in future clinical trial design and evolving treatment paradigms.
The increase in age at diagnosis of OPC is consistent with a recent analysis of nationally representative SEER data that showed significant increases in the age-adjusted incidence of OPC among older (65 years and older) individuals from 2000 to 2012, contrasted with a concomitant decrease in incidence for other head and neck tumors [6]. In our study, the age at diagnosis of OPC increased dramatically from 2004 to 2014 at a significantly greater rate than oral cavity cancers, further supporting a distinct epidemiology for OPC compared with other head and neck cancers.
Although the age increase was not surprising given the previously reported SEER data, the fact that it was not modified by HPV tumor status was unexpected. We had hypothesized that the aging of OPC patients at diagnosis would largely be driven by HPV-positive OPCs, as the cohort of individuals at highest risk for OPC – men with a history of multiple oral sexual partners [5,16,17], e.g., those who became adults during and after the sexual revolution of the 1960s – aged into later adulthood. Indeed, individuals older than 70 years of age sustained a dramatic increase in the proportion of OPCs caused by HPV. Surprisingly, while the age increase was clearly driven by men with tumors of the lymphoid tissues that harbor HPV-OPC, the mean age of patients diagnosed with HPV-positive and HPV-negative OPCs increased at essentially the same rate. Although there was clear evidence of an early bias towards referring younger patients for HPV testing before it became standard of care that almost certainly exaggerated these findings, there was also a dramatic, albeit slower, age increase among HPV-unknown patients.
Reasons for the age increase for both HPV-negative and HPV-positive OPCs are unclear. In addition to a likely age cohort effect for HPV-positive OPCs related to generational norms in sexual behaviors, other factors that may modify the age-related epidemiology of OPC include tobacco smoking prevalence trends and the aging of the U.S. population in general. However, these two latter forces would also be expected to strongly influence the epidemiology of oral cavity cancers, which demonstrated markedly slower age increases than OPC.
The palatine and lingual tonsils which comprise the lymphoid OPCs are clearly driving the observed aging trends, as opposed to their mucosal counterparts of other OPC sites. Indeed, the rate of change for these non-lymphoid OPCs was nearly identical to that of oral cavity cancers. In the context of rising age for both HPV-negative as well as HPV-positive OPCs, this raises the question of whether other unrecognized lymphoid tissue-specific influences are contributing to the trends that we describe. One such possible factor is Epstein-Barr Virus (EBV), which also infects these tissues [18,19] and has been detected in many tonsil and tongue base OPCs, frequently as a coinfection with HPV [20]. The role for EBV in both HPV-positive and HPV-negative OPC is not well understood but warrants further investigation.
HPV-OPC has been considered a disease of younger individuals, based on many earlier reports [5,21]. However, data from this and several other recent studies [6,14,15] portend that the population of elderly patients with HPV-OPC is expanding and will likely continue to do so, signaling a shift in the paradigm of a ‘typical’ HPV-OPC patient. There are several important clinical implications of such a shift. First, keeping in mind that older head and neck cancer patients have historically been underrepresented in clinical trials [22], every effort should be made to recruit older OPC patients into the trials that will shape future treatment algorithms for HPV-OPC patients. This is of particular importance when considering that in this study the survival benefit derived from HPV-positive tumor status was significantly attenuated for patients over 70 years of age when compared with their younger counterparts, and was in fact essentially similar to survival for the young HPV-negative patients (Fig. 3). Second, older head and neck cancer patients in general have unique characteristics that differentiate them from younger patients. Older patients in our study exhibited higher comorbidity scores and distinct disease characteristics, were less likely to undergo surgery and more likely to undergo palliative treatment; others have shown that older patients have higher rates of treatment-related toxicities [23–25], decreased survival benefit from various proposed treatment intensification strategies [22,26,27], and increased risk of non-cancer deaths [28]. While there is a focus upon therapeutic deintensification for younger HPV-OPC patients with the goal to reduce long-term potential toxicities in anticipation of long-term survival, taken together these data suggest that similar considerations should be applied to older HPV-OPC patients, but with careful regard for their distinct clinical characteristics and prognosis. Although long-term survivorship is less likely, optimizing quality of life and reducing the short-term toxicities could be warranted in this patient population.
This study drew from a robust national database with a large patient cohort, allowing for a detailed analysis of aging trends. However, the NCDB is not population-based and therefore is not necessarily generalizable to the U.S. population. This study was also limited by a lack of cause-of-death data, precluding a thorough disease-specific survival analysis. It should also be highlighted that older individuals are expected to have worse overall survival due to competing causes of mortality. Additionally, tobacco use is an established risk factor for overall survival but is not included in this dataset, so that it could not be accounted for it in our survival models.
Conclusions
HPV-positive OPC has been considered a disease of younger individuals, however this analysis shows that the average age of HPV-positive (and negative) patients at diagnosis is increasing. The proportion of OPCs caused by HPV is expanding in all age groups, however the survival advantage of HPV-positivity is attenuated in older age groups. These trends may herald a growing population of elderly HPV-OPC patients with unique needs and treatment-related considerations.
Supplementary Material
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- [1].Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26(4):612–9. [DOI] [PubMed] [Google Scholar]
- [2].Fossum CC, Chintakuntlawar AV, Price DL, Garcia JJ. Characterization of the oropharynx: anatomy, histology, immunology, squamous cell carcinoma and surgical resection. Histopathology 2017;70(7):1021–9. [DOI] [PubMed] [Google Scholar]
- [3].Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Seminars Oncol 2004;31(6):744–54. [DOI] [PubMed] [Google Scholar]
- [4].Rischin D, Young RJ, Fisher R, Fox SR, Le QT, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 2010;28(27):4142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008;100(6):407–20. [DOI] [PubMed] [Google Scholar]
- [6].Zumsteg ZS, Cook-Wiens G, Yoshida E, Shiao SL, Lee NY, Mita A, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol 2016;2(12):1617–23. [DOI] [PubMed] [Google Scholar]
- [7].Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100(4):261–9. [DOI] [PubMed] [Google Scholar]
- [8].Holsinger FC, Ferris RL. Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: robotics, lasers, and clinical trials. J Clin Oncol 2015;33(29):3285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bhatia A, Burtness B. Human Papillomavirus-associated oropharyngeal cancer: defining risk groups and clinical trials. J Clin Oncol 2015;33(29):3243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15(3):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA: A Cancer J Clin 2017;67(2):122–37. [DOI] [PubMed] [Google Scholar]
- [12].Horne ZD, Glaser SM, Vargo JA, Ferris RL, Balasubramani GK, Clump DA, et al. Confirmation of proposed human papillomavirus risk-adapted staging according to AJCC/UICC TNM criteria for positive oropharyngeal carcinomas. Cancer 2016;122(13):2021–30. [DOI] [PubMed] [Google Scholar]
- [13].Cuzick J A Wilcoxon-type test for trend. Statistics Med 1985;4(1):87–90. [DOI] [PubMed] [Google Scholar]
- [14].Rettig EM, Fakhry C, Khararjian A, Westra WH. Age profile of patients with oropharyngeal squamous cell carcinoma. JAMA Otolaryngol– Head Neck Surgery 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Windon MJ, D'Souza G, Rettig EM, Westra WH, van Zante A, Wang SJ, et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].D'Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PloS One 2014;9(1):e86023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007;356(19):1944–56. [DOI] [PubMed] [Google Scholar]
- [18].Seishima N, Kondo S, Wakisaka N, Kobayashi E, Imoto T, Moriyama-Kita M, et al. EBV infection is prevalent in the adenoid and palatine tonsils in adults. J Med Virol 2017;89(6):1088–95. [DOI] [PubMed] [Google Scholar]
- [19].Assadian F, Sandstrom K, Bondeson K, Laurell G, Lidian A, Svensson C, et al. distribution and molecular characterization of human adenovirus and Epstein-Barr virus infections in tonsillar lymphocytes isolated from patients diagnosed with tonsillar diseases. PloS One 2016;11(5):e0154814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiang R, Ekshyyan O, Moore-Medlin T, Rong X, Nathan S, Gu X, et al. Association between human papilloma virus/Epstein-Barr virus coinfection and oral carcinogenesis. J Oral Pathol Med: Official Public Int Assoc Oral Pathol Am Acad Oral Pathol 2015;44(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pignon JP, le Maitre A, Maillard E, Bourhis J. Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92(1):4–14. [DOI] [PubMed] [Google Scholar]
- [23].O'Neill CR, Baxi SS, Atoria CL, O'Neill JP, Henman MC, Sherman EJ, et al. Treatment-related toxicities in older adults with head and neck cancer: a population-based analysis. Cancer 2015;121(12):2083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Michal SA, Adelstein DJ, Rybicki LA, Rodriguez CP, Saxton JP, Wood RG, et al. Multi-agent concurrent chemoradiotherapy for locally advanced head and neck squamous cell cancer in the elderly. Head Neck 2012;34(8):1147–52. [DOI] [PubMed] [Google Scholar]
- [25].Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008;26(21):3582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a metaanalysis. Lancet 2006;368(9538):843–54. [DOI] [PubMed] [Google Scholar]
- [27].Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11(1):21–8. [DOI] [PubMed] [Google Scholar]
- [28].van Monsjou HS, Schaapveld M, Hamming-Vrieze O, de Boer JP, van den Brekel MW, Balm AJ. Cause-specific excess mortality in patients treated for cancer of the oral cavity and oropharynx: a population-based study. Oral Oncol 2016;52:37–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.