Abstract
Background:
Schizophrenia is associated with progressive white matter changes, but it is unclear if antipsychotic medications contribute to these. Our objective was to characterize effects of short-term treatment with risperidone on white matter diffusion indices.
Methods:
We recruited 42 schizophrenia patients (30 never-treated, 12 currently untreated) and 42 matched healthy controls in this prospective case-control neuroimaging study. Patients received a six week trial of risperidone. Using diffusion tensor imaging, we assessed microstructural (fractional anisotropy [FA], mean diffusivity [MD], and radial diffusivity [RD]) and macrostructural (radial fiber trophy) white matter integrity deficits in unmedicated patients compared to controls and change in white matter integrity in patients before and after antipsychotic treatment (mean risperidone dose at endpoint was 3.73+/−1.72mg).
Results:
At baseline, FA was decreased in the left medial temporal white matter (cluster extent: 123 voxels, peak: x= −51; y= −44; z= −7; α< .05) and MD was increased in the fusiform/ lingual gyrus white matter extending to the hippocampal part of the cingulum (cluster extent 185 voxels, peak: x= −27; y= −49; z= 2; α< .04) in patients compared to controls. RD and macrostructure were not abnormal. None of the diffusion indices showed a significant change after six weeks of treatment, both with voxel-wise and whole brain white matter analyses.
Conclusions:
We demonstrate microstructural white matter integrity abnormalities in the absence of macrostructural impairment in unmedicated patients with, primarily early stage, schizophrenia. In our data, we found no significant white matter changes after short-term treatment with risperidone.
Keywords: diffusion tensor imaging, fractional anisotropy, mean diffusivity, radial diffusivity, treatment response, longitudinal
INTRODUCTION
Schizophrenia is a complex neuropsychiatric syndrome associated with subtle changes in brain structure that progress over the course of the illness (1, 2). While causes underlying this phenomenon remain poorly understood, a number of mechanisms including accelerated aging (1), glutamate related excitotoxicity (3, 4), and a variety of environmental influences are being contemplated (5). Antipsychotic medication exposure is probably the most widely debated putative environmental factor (6, 7). However, only a limited number of studies have directly investigated antipsychotic medication effects on gray matter volume, and even fewer have examined effects on white matter integrity, perhaps because imaging sequences optimized to examine white matter have only more recently become widely available.
With diffusion tensor imaging (DTI) scalar diffusion indices can be derived to describe white matter microstructural properties, and to characterize pathological processes in the microstructural composition white matter (8, 9). Fractional anisotropy (FA) measures the degree of orientational coherence of water diffusion within a voxel and is a non-specific biomarker of microstructural architecture and neuropathology, whereas mean diffusivity (MD) measures the extent to which water is able to diffuse into any direction within the voxel and is typically elevated in inflammatory states and with edema (10), whereas cell proliferation and neoplasia can decrease MD (8). Radial diffusivity (RD) quantifies diffusion perpendicular to the principal diffusion direction (i.e. the axon) and is thought to be increased in dysmyelination and demyelination (11). However, because changes in fiber diameter and tract spacing may also affect these measures, it is important to interpret microstructural alterations in the context of macrostructure (i.e. radial fiber trophy, a surrogate marker of white matter atrophy/ hypertrophy) (12).
While there is a general theme of decreased FA in schizophrenia (13, 14) and evidence of increased MD and RD (15, 16), findings in the literature are variable. Similarly, prospective studies investigating antipsychotic effects on white matter integrity have been inconsistent. Szeszko and colleagues report FA reduction and increased RD following twelve weeks of treatment with risperidone or aripiprazole in minimally treated first episode psychosis patients (17). Another group reported increased FA, decreased MD and RD, but unchanged white matter volume after twelve weeks of first or second generation antipsychotic treatment in first episode psychosis patients that were medicated at the time of enrollment (18). Wang and colleagues reported a significant decrease in absolute FA of white matter around the anterior cingulate gyrus and corona radiata in drug-naïve first episode patients compared to healthy controls after six weeks of treatment with various clinician selected antipsychotic medications (19). Similarly, Meng and colleagues found a widespread decrease in FA after six weeks of treatment with different second generation antipsychotic medications, in this study the medication dose expressed in chlorpromazine equivalents was correlated the extent of FA reduction during the treatment trial (20). Yet others found no medication related changes in FA or MD after eight weeks of treatment with different second generation antipsychotics (21), and in FA after various timeframes of treatment with a number of medications (22). The within-subject design in longitudinal studies controls for a number of subject level confounding factors, but it is possible that discrepancies in findings may be secondary to differential effects of a variety of antipsychotic drugs and to differences in data analysis and quality control techniques mitigating DTI measurement error.
The goal of this study was to characterize the effects of short term treatment with a single antipsychotic medication on white matter diffusion indices. We scanned a group of patients with schizophrenia and schizoaffective disorder (SZ) before and after six weeks of treatment with risperidone and scanned a group of matched healthy controls (HC) twice, approximately six weeks apart. We examined if (1) FA would be decreased, but MD and RD would be increased in never-treated and currently unmedicated SZ compared to HC, (2) if macrostructural white matter integrity would differ between HC and never-treated and currently unmedicated SZ, and (3) if six weeks of treatment with risperidone would affect micro- and macrostrcutural white matter integrity. In addition, we tested the hypothesis that greater duration of untreated psychosis (DUP) would be associated with poorer microstructural white matter integrity.
METHODS AND MATERIALS
Participants and study design
SZ were recruited from outpatient clinics, inpatient units, and the emergency room at the University of Alabama at Birmingham (UAB). HC matched on age, sex, and parental occupation were recruited by advertisements. Written informed consent was obtained prior to enrollment in this UAB Institutional Review Board approved study.
Diagnoses were established by review of medical records, the Diagnostic Interview for Genetic Studies (DIGS), and consensus of two board certified psychiatrists (ACL and NVK). The Brief Psychiatric Rating Scale (BPRS)(23) and Repeatable Battery for The Assessment of Neuropsychological Status (RBANS)(24) were used to assess symptom severity and cognition, respectively. Participants were excluded if they had major neurological or medical conditions, history of head trauma with loss of consciousness, substance use disorders (excluding nicotine) within six months of imaging, were prescribed medications known to affect brain function, were pregnant or breastfeeding, or had MRI contraindications. Controls with a personal or family history in a first-degree relative of an Axis I disorder were excluded.
We enrolled 42 SZ who were medication naïve or off antipsychotic medications for at least two weeks (determined by self report; patients were not taken off medications to meet this criterion) in a six-week trial of oral risperidone using a flexible dosing regimen. We chose this medication because it is commonly prescribed, now available as generic medication and thus one of the more affordable second generation antipsychotic medications in the US, and is considered first line treatment in schizophrenia, specifically first episode patients (25). Scans were obtained prior to treatment and after six weeks of treatment. Nine SZ dropped out before study completion. Medication was managed by ACL and NVK. Risperidone was started at 1–3 milligrams and titrated in 1–2 milligram increments; dosing was based on therapeutic and side effects. Use of concomitant medications was permitted as clinically indicated. Fourteen patients were prescribed benztropine, two trazodone, two fluoxetine, one sertraline, one desvenlafaxine, one mirtazapine, one amitriptyline, and one clonazepam. Compliance was monitored with pill counts at each visit. We also enrolled 42 HC, 38 of those were scanned twice, on average 51.5 +/− 19.8 days apart.
Data from 5 HC (one time point), and 14 SZ baseline as well as 5 SZ endpoint scans were included in a prior publication (16). No longitudinal DTI data has been included in prior publications.
Data acquisition
Imaging was performed on a 3 T head-only MRI scanner (Siemens Magnetom Allegra, Erlangen, Germany) using a circularly polarized transmit/receive head coil. A T1 weighted scan was acquired for anatomical reference (MPRAGE, TR/TE/TI= 2300/3.93/1100ms, flip angle 12°, phase encoding direction A>P, field of view= 256× 256mm, 1mm isotropic voxel size). Two diffusion-weighted runs were acquired, each non-collinearly distributed along 30 directions [b= 1000 s/mm2, TR/TE=9200/96ms, field of view=246× 246mm, matrix= 112× 112, 60 slices, interleaved acquisition, 2.2mm isotropic voxel size]. Both runs were concatenated to increase the signal-to-noise ratio (for three SZ datasets only one run was acquired). Five images with no diffusion gradients (b0; b= 0 s/mm2) were also acquired.
Data processing
In preparation for DTI registration in TORTOISE (version 2.5.2)(26), the T1 weighted images were skull stripped and processed through FATCAT in AFNI (27, 28) to create a volume with an approximate T2-weighted contrast. This “imitation T2” image was spatially aligned with the DTI images and used solely for providing an anatomical reference with requisite contrast similar to the DTI b0 volume (29).
After visual inspection of raw images, we calculated bulk motion indices. The root-mean-square (RMS) of the 6 motion parameters (translations and rotations) was calculated both for absolute (RMSabs; average of absolute displacement of each image and the first b0 image) and relative (RMSrel; average of absolute displacement between adjacent images, which reduces the likelihood of a few large movements biasing the outcome of motion estimates (30)) movement(31). Datasets with RMSabs of greater than half the voxel edge length (1.1mm) or RMSrel of >0.05mm per run were excluded from further analyses. One SZ baseline and one SZ week six datasets were rejected based on these criteria.
Artifacts due to between-volume bulk body motion, eddy currents, and EPI susceptibility-induced geometric distortions were removed using DIFF_PREP with a single interpolation step (32). For each dataset, the first b0 image was selected as the reference for registration; subsequent b0 images were affine registered to the initial b0 image. Prior to registration, diffusion weighted and structural images were upsampled at a factor two using a bicubic algorithm and smoothed with a Perona-Malik anisotropic edge favoring gradient based filter (33) to compute the transformations from moving to fixed images. After computation of transformations, original images were used to create the registered images. Bspline correction was done using the imitation T2 image. Diffusion images were resampled to 1.5mm isotropic voxels. Gradient tables were rotated along with motion correction (34, 35). Tensors were computed with DIFF_CALC using a linear fitting algorithm. We computed maps for FA, MD, and RD. Resulting maps were visually inspected for anomalies in scalar diffusion parameters. After quality control, a total of ten datasets were excluded from further analysis (one HC and three SZ baseline as well as two HC and four SZ week 6 scans); in addition five datasets were processed without imitation T2 images.
To spatially normalize diffusion images to the Illinois Institute of Technology atlas (IIT2) space (36), we implemented an optimized non-linear image registration procedure using a modified version of 3dQwarp in AFNI (37). The warping optimization implements an iterative refinement, where an input image is repeatedly processed through an optimizer in smaller and smaller patches, incorporating convergence criteria at each level to better resolve artifacts, with a final patch size of 5× 5× 5 millimeters. Resulting deformation maps were then used together with diffusion images to calculate radial fiber trophy, a surrogate marker of macrostructural white matter change (12). Briefly, stretch of the deformation field in the plane perpendicular to the predominant direction of diffusion is computed using slight modifications of traditional tensor based morphometry, a method for identifying macroscopic differences in brain structure based on the Jacobian determinant of the deformation matrix (38, 39). Before statistical analyses, a 5mm median filter was applied with 3dLocalstat to improve the signal to noise ratio while maintaining edges (40).
Statistical analyses
HC were considered negative controls, facilitating descriptive characterization of abnormalities in SZ and assessing temporal stability of diffusion indices. To examine whole brain voxel-wise group differences in scalar diffusion indices and atrophy measures within a IIT2 based white matter mask we used AFNI’s 3dttest++; a priori defined co-variates included age and gender (15, 41). Because baseline head motion differed between groups (RMSabs baseline: HC 0.56+/− 0.10mm; SZ 0.62+/− 0.14mm; t= −2.18; p= 0.03; RMSabs week 6: HC 0.56+/− 0.10mm; SZ 0.62+/− 0.16mm; t= −1.83; p= .09; RMSrel baseline: HC 0.14+/− 0.002mm; SZ 0.17+/− 0.005mm; t= −3.24; p= .002; RMSrel week 6: HC 0.15+/− 0.03mm; SZ 0.16+/− 0.05mm; t= −1.33; p= .19) we included RMSrel as additional covariate in analyses. To examine change over time in voxelwise analyses, we used AFNI’s 3dttest++ to compare diffusion indices at baseline and after six weeks in each group separately. Clustsim, an algorithm that uses randomization/permutation simulation to produce 10,000 iterations of noise only generated t-tests and determine global cluster-level threshold values, was implemented to control for the false positive rate (voxelwise threshold Bonferroni corrected p= .0008 [accounting for 12 comparisons at puncorrected= .01]; cluster threshold α= 0.05) (42). As a post hoc analysis, we extracted whole brain white matter scalar diffusion indices for each participant and compared baseline group differences and changes over time in each group using linear models, with age, gender and RMSrel included as covariates. We also examined the relationship between DUP (in patients who were antipsychotic medication-naïve) and voxelwise white matter integrity in the subgroup of medication-naïve patients with 3dttest++ including the log transformed DUP as regressor. In an exploratory fashion we also examined partial correlations between clinical measures in SZ and whole brain white matter scalar diffusion indices as well as diffusion indices in areas of abnormal white matter integrity, controlling for age, gender and RMSrel.
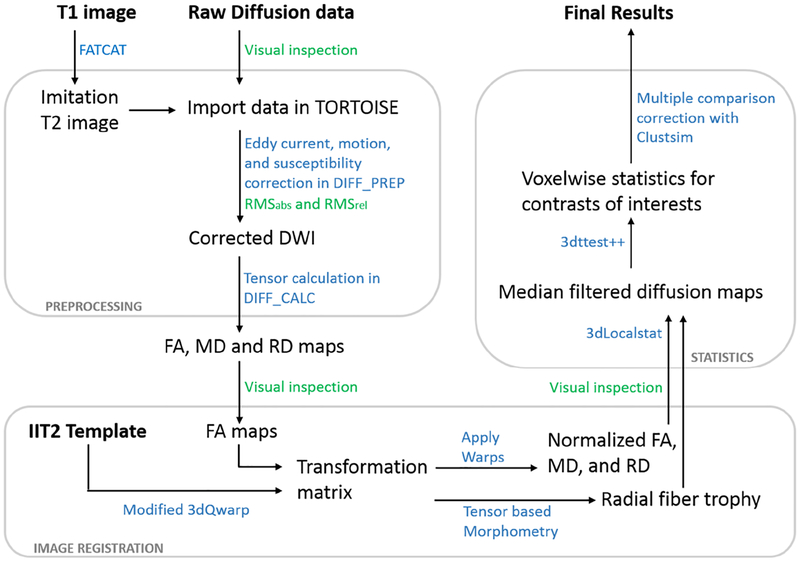
A schematic of our data analysis pipeline can be found in Figure 1.
Figure 1:
Schematic of the diffusion data processing pipeline. Major steps in preprocessing, image registration and statistical analyses are depicted here. The blue color signifies software programs used/ data processing steps applied, the green color signifies quality control steps. Abbreviations: DWI, diffusion weighted image; RMSabs and RMSrel, absolute and relative head motion; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity
Final analyses included good quality datasets from 41 HC and 38 SZ at baseline, and 36 HC and 28 SZ at week 6. A total of 36 HC and 27 SZ had good quality datasets for both baseline and week 6 scans.
RESULTS
HC and SZ did not differ in gender, age, or parental socioeconomic status (Table 1). BPRS scores significantly decreased after six weeks of treatment.
Table 1:
Demographics and clinical measuresa
| SZ (n=42) | HC (n=42) | t/X2/F | p value | |
|---|---|---|---|---|
| Gender (% male) | 61.9 | 61.9 | 0.00 | 1.0 |
| Age | 26.62 (9.00) | 27.88 (9.43) | 0.63 | .53 |
| Parental Occupationb | 7.16 (6.13) | 5.45 (4.18) | 16.28 | .30 |
| Smoking (packs per day) | 0.38 (0.53) | 0.23 (0.43) | −1.47 | .15 |
| Diagnosis | ||||
| Schizophrenia | 34 | |||
| Schizoaffective Disorder | 5 | |||
| Brief Psychotic Disorder | 3 | |||
| Illness Duration (years; mean, SD, median, range)c | 15.00 (8.67; 18.00; 1–25) | |||
| Prior antipsychotic treatment | ||||
| Antipsychotic naive (yes/ no) | 30/ 12 | |||
| Antipsychotic free interval (months; mean, SD, median, range)c | 17.33; (36.91; 3.25; 0.5–120) | |||
| Risperidone dose at week 6 (in mg; mean, SD, median, range) | 3.73 (1.72; 4.0; 1–8) | |||
| BPRSd | ||||
| Total | ||||
| Baseline | 50.26 (9.30) | |||
| Week 6e | 32.36 (9.60)g | |||
| Positive | ||||
| Baseline | 10.93 (3.46) | |||
| Week 6e | 5.24 (2.55)h | |||
| Negative | ||||
| Baseline | 7.19 (3.36) | |||
| Week 6e | 5.73 (2.41)i | |||
| RBANSf | ||||
| Total index | 71.82 (14.37) | 89.67 (12.73) | 5.89 | < .01 |
| Immediate memory | 77.42 (16.35) | 97.07 (15.33) | 5.55 | < .01 |
| Visuospatial | 73.24 (16.52) | 82.48 (15.41) | 2.59 | .01 |
| Language | 85.50 (12.82) | 96.64 (14.04) | 3.76 | < .01 |
| Attention span | 80.39 (19.67) | 94.07 (18.45) | 3.21 | < .01 |
| Delayed memory | 71.79 (20.57) | 91.38 (10.13) | 5.32 | < .01 |
SZ, schizophrenia; HC, healthy control
Mean (SD) unless indicated otherwise
Ranks determined from Diagnostic Interview for Genetic Studies (1 – 18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status
includes only patients who are not antipsychotic naïve (n=12), illness duration since first diagnosis
Brief Psychiatric Rating Scale (1 – 7 scale); positive (conceptual disorganization, hallucinatory behavior, and unusual thought content); negative (emotional withdrawal, motor retardation, and blunted affect)
n= 33
Repeatable Battery for the Assessment of Neuropsychological Status
t= 11.19; p< .01
t= 9.45; p< .01
t= 2.98; p< .01
White matter integrity in medication-naïve and unmedicated SZ compared to HC
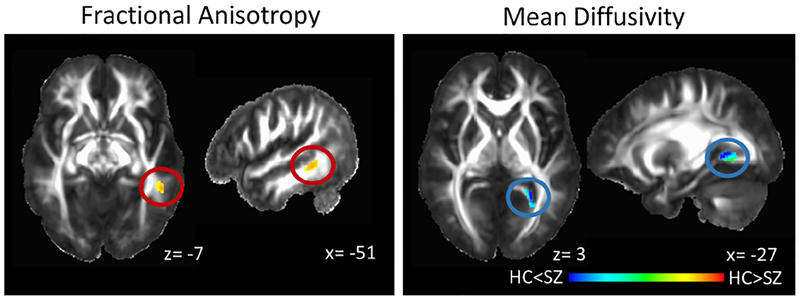
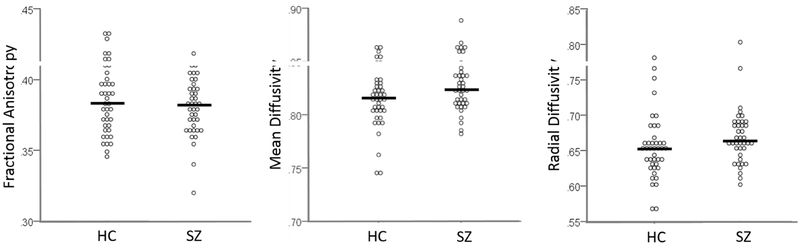
At baseline, FA was reduced in the left medial temporal white matter and MD was increased in the left fusiform/ lingual gyrus white matter extending to the hippocampal part of the cingulum in SZ compared to HC (Figure 2). RD and radial fiber trophy did not differ between groups, both when the entire SZ group was considered and when only antipsychotic medicationnaïve subjects were considered. Post hoc analysis did not show group differences in whole brain white matter scalar diffusion indices (Figure 3). We found no associations between DUP and FA, MD, or RD.
Figure 2:
White matter microstructural integrity abnormalities in never-treated and currently unmedicated patients with schizophrenia (SZ) compared to healthy controls (HC). Fractional anisotropy is decreased in the left medial temporal white matter (cluster extent: 123 voxels, peak: x= −51; y= −44; z= −7; α< .05) and mean diffusivity is increased in the fusiform/ lingual gyrus white matter extending to the hippocampal part of the cingulum (cluster extent 185 voxels, peak: x= −27; y= −49; z= 2; α< .04) in SZ compared to HC. Clusters are projected on the IIT2 white matter atlas template. Numbers adjacent to slices indicate x, y, and z coordinates in Talairach convention. Left= Right. Color bar indicates z scores.
Figure 3:
White matter scalar diffusion indices in healthy controls (HC; n= 41) and never treated and currently unmedicated patients with schizophrenia (SZ; n=38). We observed no significant group differences in fractional anisotropy, mean diffusivity, or radial diffusivity. Dots represent individual measurements, and the horizontal lines represent the group mean.
In a secondary analysis, we repeated the same computations but included only patients who were antipsychotic naïve and their matched controls. Findings in this subgroup were largely consistent with those in the larger group, where FA was is decreased in the left medial temporal white matter (cluster extent: 80 voxels, peak: x= −51; y= −36; z= −14; α< .10) and MD was increased in the fusiform/ lingual gyrus white matter extending to the hippocampal part of the cingulum (cluster extent 199 voxels, peak: x= −28; y= −49; z= 2; α< .04) in antipsychotic-naïve SZ compared to HC. No group differences were seen in RD.
Changes in white matter integrity after six weeks
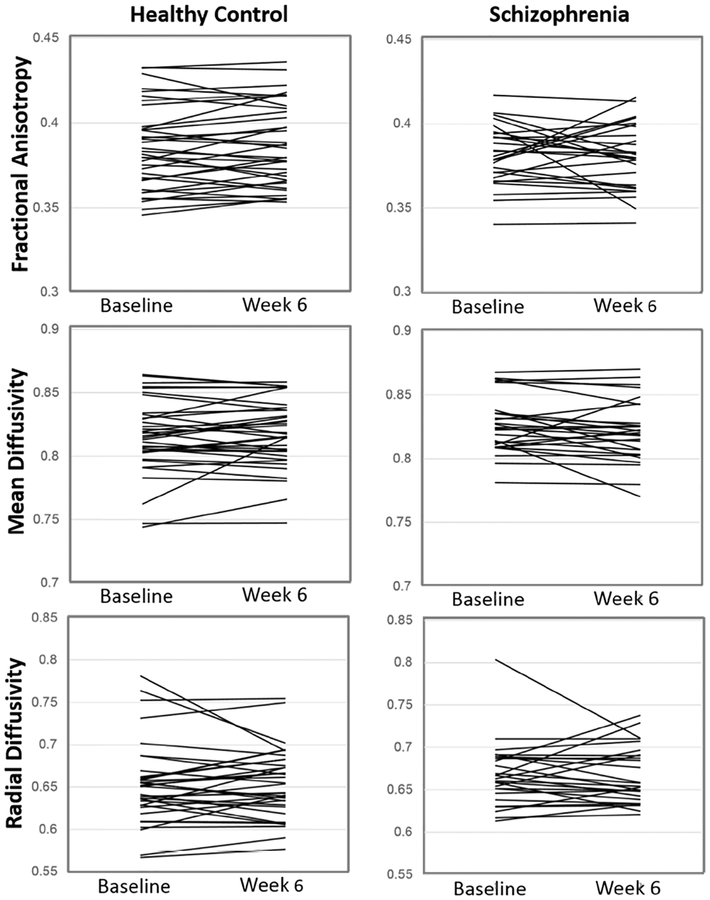
In HC, none of the white matter measures changed over time. We also observed no changes in micro- or macrostructural white matter integrity after six weeks of treatment in SZ. This was true even when lowering the statistical threshold to puncorrected< .001. Post hoc analyses did not show significant change in whole brain white matter scalar diffusion indices after six weeks in either group (Table 2, Figure 4). When we examined change in diffusion indices over time in each group separately with paired-sample t-tests, we again did not find significant changes over time in HC (FA: t= −1.00; p= .32 MD: t= −0.96; p= .34 ; RD: t= − 0.01; p= .99) and after six weeks of treatment in SZ (FA: t= 0.21; p= .76; MD: 1.45; p= .16; RD: t= 0.14; p= .89).
Table 2:
Whole brain white matter microstructural integrity measurementsa
| SZ (n= 39) | HC (n= 41) | F | p value | |
|---|---|---|---|---|
| Baselineb | ||||
| FA | 0.38 ± 0.02 | 0.39 ± 0.02 | 0.23 | 0.63 |
| MD | 0.83 ± 0.02 | 0.82 ± 0.03 | 1.72 | 0.19 |
| RD | 0.67 ± 0.04 | 0.65 ± 0.05 | 0.99 | 0.32 |
| Change from Baseline to Week 6c | ||||
| FA | 0.001 ± 0.018 | −0.002 ± 0.010 | 0.32 | 0.57 |
| MD | 0.004 ± 0.015 | −0.002 ± 0.013 | 2.99 | 0.09 |
| RD | 0.001 ± 0.030 | −0.000 ± 0.027 | 0.03 | 0.86 |
SZ, schizophrenia; HC, healthy control; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity.
Mean (SD)
All linear models controlled for age, gender, and relative movement of the participant at baseline.
All linear models controlled for age, gender, and relative movement of the participant at both baseline and Week 6.
Figure 4:
Individual changes in whole brain white matter scalar diffusion indices in healthy controls (left column; n= 36) after six weeks and patients with schizophrenia (right column, n= 27) after six weeks of treatment with risperidone. We observed no significant changes in fractional anisotropy, mean diffusivity, or radial diffusivity.
Secondary analyses in the antipsychotic naïve sample again were consistent with those in the larger group, showing no significant change in voxelwise FA, MD and RD after six weeks of treatment with risperidone. This again was true even when lowering the statistical threshold to puncorrected< .001.
Relationship between white matter integrity and clinical variables
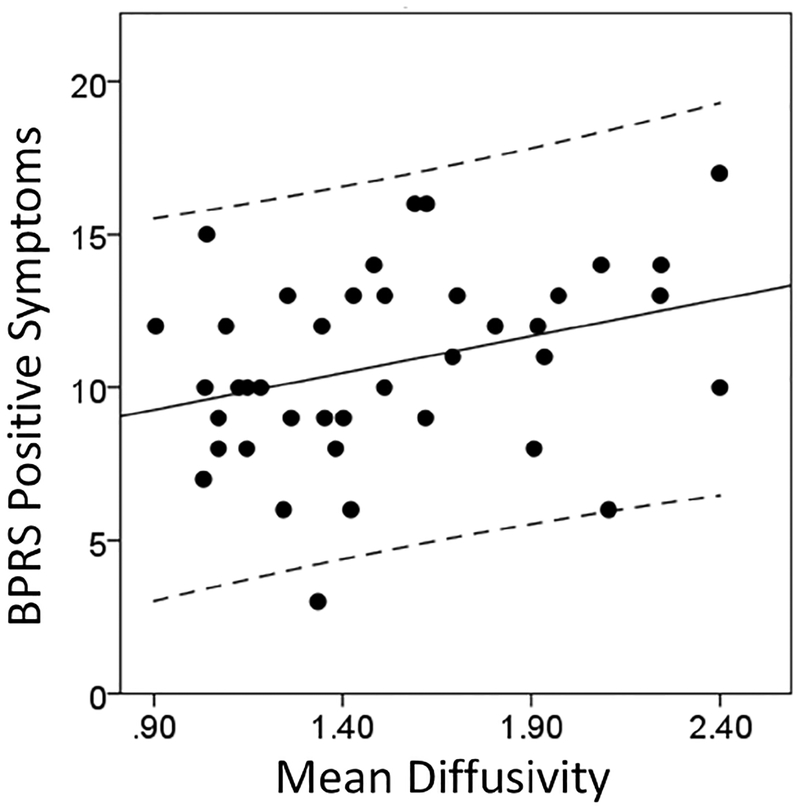
Exploratory analyses revealed a modest positive correlation between MD in left fusiform/ lingual gyrus white matter and BPRS positive symptoms in SZ at baseline (r= 0.34; p= 0.04 when age and RMSrel were included as covariates; r= 0.36; p= 0.07 when age, RMSrel and gender were included as covariates; Figure 5), but no relationship between whole brain scalar diffusion indices at baseline and clinical symptom severity. Risperidone dose was not associated with baseline diffusion indices or change in diffusion indices (FA, MD, RD). We also did not find correlations between whole brain diffusion indices (baseline and week 6) and RBANS total scores in either HC or SZ.
Figure 5:
Relationship between Mean Diffusivity in the left fusiform/ lingual gyrus white matter extending to the hippocampal part of the cingulum bundle (cluster extent: 185 voxels, Montreal Neurological Institute (MNI) peak coordinates: x= −27; y= −49; z= 2) in never-treated and currently unmedicated patients with schizophrenia and Brief Psychiatric Rating Scale (BPRS) positive symptom severity.
DISCUSSION
The purpose of this longitudinal, prospective study was to examine whole brain micro-and macrostructural white matter integrity in schizophrenia and to study effects of short-term antipsychotic treatment on these measures. We report focal decreased FA and increased MD in never-treated and currently unmedicated SZ; baseline MD abnormalities showed a modest association with positive symptom severity. No macrostructural white matter or RD alterations were observed at baseline; the latter was inconsistent with our a priori hypotheses. In addition, we did not observe a relationship between DUP and white matter microstructure in medicationnaïve patients. While six weeks of treatment with risperidone did improve psychosis severity, we did not observe significant changes associated with short-term risperidone treatment in any of the white matter indices.
Our report of decreased FA localized in the left medial temporal white matter in a group of mostly early stage schizophrenia patients replicates findings of a recent activation likelihood estimation meta-analysis showing that abnormalities in the left temporal and the right frontal lobe in first episode psychosis patients (43), and is in agreement with a recent cross-sectional study suggesting that whole-brain white matter integrity deficits are only found later in the illness (1). MD elevations in the fusiform/ lingual gyrus white matter extending to the hippocampal part of the cingulum suggest a possible inflammatory process in this area. Consistent with this, a small Positron Emission Tomography study reported evidence of hippocampal neuro-inflammation accompanied by a non-significant increase of tracer binding potential in whole-brain white matter in patients recovering from a psychotic episode (44). Furthermore, post-mortem studies in white matter revealed increased density (45) and activation (46) of microglia, the resident innate immune cells of the brain.
We did discern a modest positive relationship between positive symptom severity and MD abnormalities in the lingual/ fusiform gyrus white matter extending to the hippocampal part of the cingulum bundle), but no such association was seen with FA deficits. Similarly, Filippi et al. report an (albeit negative) relationship between MD, but not FA, and positive symptom severity in antipsychotic naïve patients with schizophrenia (47). The lack of spatial overlap between decreased FA and increased MD and the dissimilar relationships between these indices and symptom severity in our study points towards a multifactorial contribution to white matter pathology in schizophrenia. Based on findings from a twin study (48) and a study in schizophrenia patients and first degree relatives, Clark et al. argue that reduced FA may be related to genetic vulnerability for schizophrenia, while elevated MD may more directly be related to the disease processes. Pasternak and colleagues propose neuro-inflammation and axonal degeneration as two likely mechanisms of white matter alterations, with inflammation rather than degeneration as the predominant pathology at illness onset (49). This is further supported by a recent study showing widespread extracellular free water elevations, suggestive of neuro-inflammation, but only limited FA abnormalities in minimally treated first episode patients (50).
We did not detect changes in white matter macrostructure associated with six weeks of risperidone treatment which is consistent with the only other diffusion imaging study that has examined macrostructural integrity in this context (18). Using T1 weighted imaging, Emsley et al. report no white matter volume changes after one year of treatment in a small group of antipsychotic naïve patients (51), but others suggest that white matter volume trajectories in chronic patients differ based on the treatment received (52). Interestingly, higher antipsychotic doses were associated with white matter loss and lower doses were associated with volume increase, even after accounting for illness severity (53). In a rare study of chronically ill, never-medicated patients, Xiao and colleagues found that these patients showed an accelerated and clinically relevant age-related reduction of FA in the genu of the corpus callosum compared to antipsychotic treated patients, suggesting a potential beneficial medication effect on white matter integrity (54).
Notably, we also observed no significant changes in microstructural diffusion indices after six weeks of treatment with risperidone at the voxel level and averaged across the whole brain. However, as seen in figure 3, results were variable, especially in FA. Assuming a 4% FA change (the average decrease in FA reported by Szeszko et al. (17)) to be meaningful, a subset of patients showed either an increase (four patients) or decrease (five patients) in FA after six weeks of treatment. These results could help reconcile discrepancies in findings of prior studies investigating short term antipsychotic medication effects on white matter microstructural integrity, by demonstrating heterogeneity in changes with measurements on the individual subject level. However, a definitive attribution of this heterogeneity in changes in diffusion indices to differential, possibly even dose related, medication effects rather than to divergence in disease progression between patients is impossible in human studies. Here, we did not observe any clinical correlates of change in diffusion images. It will be important for future large-scale studies to investigate the clinical correlates of this heterogeneity at the level of brain structural change in response to antipsychotic exposure in an effort to better inform our pathophysiological understanding of the illness. Another question that remains unanswered is if different antipsychotic medications affect white matter structure differentially. A recent exploratory study in a small sample of patients directly compared effects of risperidone and aripiprazole on gray matter and white matter volumes as well as neurometabolite levels and found a non-significant increase in prefrontal white matter volume in those treated with aripiprazole but not risperidone, suggesting that these drugs may have differential effects (55). However, these results need to be considered preliminary at this time, and larger scale studies making head to head comparisons of medication effects not only on macrostructural but also microstructural white matter integrity are direly needed in the field. Here, clozapine could be an especially interesting compound to compare to other drugs, as showed differential effects on FA compared to other typical antipsychotics and typical antipsychotics (56) and a small study reported a widespread increase in FA in patients that corresponded to improvement in semantic fluency after a twelve week trial of clozapine (57).
Our findings have to be seen in the context of a number of strengths and limitations. To minimize variance in data, we enrolled currently unmedicated SZ, many of them without any prior antipsychotic exposure, carefully matched groups on key demographic characteristics, and used a single antipsychotic medication in this prospective longitudinal study. We took a number of steps to assure data quality, chose a non-linear image registration algorithm, and carefully controlled for false positive rates with state of the art cluster thresholding methods (42). Ideally, we would have obtained a T2 weighted scan to aid co-registration, but were not able to do so due to time constraints. To assess for possible effects of time on diffusion indices, we included HC that were scanned twice six weeks apart. It was not possible to follow medication-free or placebo treated SZ longitudinally, as it is not ethically permissible to withhold known effective treatments from patients. Additionally, we did not have information available on prior nonresponse to risperidone in the 12 patients who had prior antipsychotic exposure, precluding us from assessing how medication history affected findings.
In summary, we demonstrate microstructural white matter integrity abnormalities in the absence of macrostructural impairment in never treated and currently unmedicated patients with schizophrenia. Our data also empirically support a multifactorial model contributing to white matter pathology in this phenotypically heterogeneous syndrome, but the underlying mechanisms remain elusive. Importantly, we found no significant white matter changes after short-term treatment with risperidone in our data.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Mental Health (R01MH081014 and R01MH102951, ACL; K23MH106683, NVK). We would like to thank UAB IT Research Computing for providing the HPC resources (compute, storage and networking) for this project. Cheaha is supported in part by the National Science Foundation under Grant No. OAC-1541310, the University of Alabama at Birmingham, and the Alabama Innovation Fund.
We would like to thank the UAB neuroimaging community for their support, especially their flexibility in making same day scanning for this protocol possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
ACL has received an investigator initiated grant from Janssen Pharmaceuticals. All other authors reported no biomedical financial interests or potential conflicts of interest.
Trial registration: ClinicalTrials.gov: NCT02034253
REFERENCES
- 1.Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, et al. (2017): Accelerated Gray and White Matter Deterioration With Age in Schizophrenia. Am J Psychiatry. 174:286–295. [DOI] [PubMed] [Google Scholar]
- 2.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, et al. (2015): Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraguljac NV, White DM, Reid MA, Lahti AC (2013): Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plitman E, Patel R, Chung JK, Pipitone J, Chavez S, Reyes-Madrigal F, et al. (2016): Glutamatergic Metabolites, Volume and Cortical Thickness in Antipsychotic-Naive Patients with First-Episode Psychosis: Implications for Excitotoxicity. Neuropsychopharmacology. 41:2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeRosse P, Ikuta T, Peters BD, Karlsgodt KH, Szeszko PR, Malhotra AK (2014): Adding insult to injury: childhood and adolescent risk factors for psychosis predict lower fractional anisotropy in the superior longitudinal fasciculus in healthy adults. Psychiatry Res. 224:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roiz-Santianez R, Suarez-Pinilla P, Crespo-Facorro B (2015): Brain Structural Effects of Antipsychotic Treatment in Schizophrenia: A Systematic Review. Curr Neuropharmacol. 13:422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navari S, Dazzan P (2009): Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 39:1763–1777. [DOI] [PubMed] [Google Scholar]
- 8.Alexander AL, Lee JE, Lazar M, Field AS (2007): Diffusion tensor imaging of the brain. Neurotherapeutics. 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 15:435–455. [DOI] [PubMed] [Google Scholar]
- 10.Assaf Y, Pasternak O (2008): Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 34:51–61. [DOI] [PubMed] [Google Scholar]
- 11.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 17:1429–1436. [DOI] [PubMed] [Google Scholar]
- 12.Marstrander JR, Anthony T, Powel VL, Brook RG, Horton MD, Skidmore FM (2017): Radial fiber atrophy: a new metric for tensor-based morphometry Society for Desing and Process Science 2017. Birminhgam, AL, pp in press. [Google Scholar]
- 13.Karlsgodt KH (2016): Diffusion Imaging of White Matter In Schizophrenia: Progress and Future Directions. Biol Psychiatry Cogn Neurosci Neuroimaging. 1:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. (2017): Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwehm A, Robinson DG, Gallego JA, Karlsgodt KH, Ikuta T, Peters BD, et al. (2016): Age and Sex Effects on White Matter Tracts in Psychosis from Adolescence through Middle Adulthood. Neuropsychopharmacology. 41:2473–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid MA, White DM, Kraguljac NV, Lahti AC (2016): A combined diffusion tensor imaging and magnetic resonance spectroscopy study of patients with schizophrenia. Schizophr Res. 170:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szeszko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, et al. (2014): White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology. 39:1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis Marques T, Taylor H, Chaddock C, Dell’acqua F, Handley R, Reinders AA, et al. (2014): White matter integrity as a predictor of response to treatment in first episode psychosis. Brain. 137:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Cheung C, Deng W, Li M, Huang C, Ma X, et al. (2013): White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychol Med. 43:2301–2309. [DOI] [PubMed] [Google Scholar]
- 20.Meng L, Li K, Li W, Xiao Y, Lui S, Sweeney JA, et al. (2018): Widespread white-matter microstructure integrity reduction in first-episode schizophrenia patients after acute antipsychotic treatment. Schizophr Res. [DOI] [PubMed] [Google Scholar]
- 21.Zeng B, Ardekani BA, Tang Y, Zhang T, Zhao S, Cui H, et al. (2016): Abnormal white matter microstructure in drug-naive first episode schizophrenia patients before and after eight weeks of antipsychotic treatment. Schizophr Res. 172:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Serpa MH, Doshi J, Erus G, Chaim-Avancini TM, Cavallet M, van de Bilt MT, et al. (2017): State-dependent microstructural white matter changes in drug-naive patients with first-episode psychosis. Psychol Med.1–15. [DOI] [PubMed] [Google Scholar]
- 23.Woerner MG, Mannuzza S, Kane JM (1988): Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull. 24:112–117. [PubMed] [Google Scholar]
- 24.Randolph C, Tierney MC, Mohr E, Chase TN (1998): The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 20:310–319. [DOI] [PubMed] [Google Scholar]
- 25.Robinson DG, Schooler NR, John M, Correll CU, Marcy P, Addington J, et al. (2015): Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 172:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierpaoli C, Walker L, Irfanoglu MO, Barnett A, Basser P, Chang L-C, et al. (2010): TORTOISE: An integrated software package for processing of diffusion MRI data ISMRM 18th Annual Meeting. Stockholm, Sweden, pp p.1597. [Google Scholar]
- 27.Taylor PA, Saad ZS (2013): FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain connectivity. 3:523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 29:162–173. [DOI] [PubMed] [Google Scholar]
- 29.Taylor PA, Alhamud A, van der Kouwe A, Saleh MG, Laughton B, Meintjes E (2016): Assessing the performance of different DTI motion correction strategies in the presence of EPI distortion correction. Human brain mapping. 37:4405–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling J, Merideth F, Caprihan A, Pena A, Teshiba T, Mayer AR (2012): Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Human brain mapping. 33:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theys C, Wouters J, Ghesquiere P (2014): Diffusion tensor imaging and resting-state functional MRI-scanning in 5- and 6-year-old children: training protocol and motion assessment. PloS one. 9:e94019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C (2004): Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magnetic resonance in medicine. 51:103–114. [DOI] [PubMed] [Google Scholar]
- 33.Perona P, Malik J (1990): Scale-space and edge detection using anisotropic diffusion IEEE transactions on pattern analysis and machine intelligence. 12:629–639. [Google Scholar]
- 34.Leemans A, Jones DK (2009): The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic resonance in medicine. 61:1336–1349. [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Chang L-C, Walker L, Lemaitre H, Barnett AS, Marenco S, et al. (2008): Comparison of EPI distortion correction methods in diffusion tensor MRI using a novel framework. MICCAI, pp 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Peng H, Dawe RJ, Arfanakis K (2011): Enhanced ICBM diffusion tensor template of the human brain. NeuroImage. 54:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin J, Liu Y, Crosby LD, Anthony T, Burdyshaw C, Brook RG, et al. (2016): Optimization of nonlinear image registration in AFNI XSEDE. Miami, Florida: ACM. [Google Scholar]
- 38.Ashburner J, Good C, Friston KJ (2000): Tensor Based Morphometry. NeuroImage Clinical. 11:805–821. [DOI] [PubMed] [Google Scholar]
- 39.Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, et al. (2008): Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. NeuroImage. 43:458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R (2002): Processing and visualization for diffusion tensor MRI. Medical image analysis. 6:93–108. [DOI] [PubMed] [Google Scholar]
- 41.Kochunov P, Ganjgahi H, Winkler A, Kelly S, Shukla DK, Du X, et al. (2016): Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp. 37:4673–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017): fMRI clustering and false-positive rates. Proceedings of the National Academy of Sciences of the United States of America. 114:E3370–E3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA, et al. (2013): White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 45:100–106. [DOI] [PubMed] [Google Scholar]
- 44.Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC (2009): Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 50:1801–1807. [DOI] [PubMed] [Google Scholar]
- 45.Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. (2013): Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 18:206–214. [DOI] [PubMed] [Google Scholar]
- 46.Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD (2011): Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treatment. 2011:325789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filippi M, Canu E, Gasparotti R, Agosta F, Valsecchi P, Lodoli G, et al. (2014): Patterns of brain structural changes in first-contact, antipsychotic drug-naive patients with schizophrenia. AJNR Am J Neuroradiol. 35:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark KA, Nuechterlein KH, Asarnow RF, Hamilton LS, Phillips OR, Hageman NS, et al. (2011): Mean diffusivity and fractional anisotropy as indicators of disease and genetic liability to schizophrenia. J Psychiatr Res. 45:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, et al. (2012): Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 32:17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyall AE, Pasternak O, Robinson DG, Newell D, Trampush JW, Gallego JA, et al. (2017): Greater extracellular free-water in first-episode psychosis predicts better neurocognitive functioning. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emsley R, Asmal L, du Plessis S, Chiliza B, Phahladira L, Kilian S (2017): Brain volume changes over the first year of treatment in schizophrenia: relationships to antipsychotic treatment. Psychol Med. 47:2187–2196. [DOI] [PubMed] [Google Scholar]
- 52.Bartzokis G, Lu PH, Nuechterlein KH, Gitlin M, Doi C, Edwards N, et al. (2007): Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophr Res. 93:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011): Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao Y, Sun H, Shi S, Jiang D, Tao B, Zhao Y, et al. (2018): White Matter Abnormalities in Never-Treated Patients With Long-Term Schizophrenia. Am J Psychiatry.appiajp201817121402. [DOI] [PubMed] [Google Scholar]
- 55.Liemburg EJ, Sibeijn-Kuiper A, Knegtering H, Aleman A (2018): The effect of aripiprazole versus risperidone on prefrontal brain metabolite levels and brain volume in psychotic disorders: an exploratory study Neuropsychiatry (London). 8:176–185. [Google Scholar]
- 56.Leroux E, Vandevelde A, Trehout M, Dollfus S (2018): Abnormalities of fronto-subcortical pathways in schizophrenia and the differential impacts of antipsychotic treatment: a DTI-based tractography study. Psychiatry Res Neuroimaging. 280:22–29. [DOI] [PubMed] [Google Scholar]
- 57.Ozcelik-Eroglu E, Ertugrul A, Oguz KK, Has AC, Karahan S, Yazici MK (2014): Effect of clozapine on white matter integrity in patients with schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 223:226–235. [DOI] [PubMed] [Google Scholar]