Peripartum cardiomyopathy (PPCM) is a rare form of potentially fatal heart disease that develops toward the end of pregnancy or during early post-partum phase. It is marked by significant left ventricular (LV) systolic dysfunction and limited data indicate that up to 38% of peripartum fatalities are attributable to sudden cardiac death, suggesting a high burden of arrhythmia1. In addition, endothelial dysfunction has been found to be of prognostic value in predicting adverse outcomes even in the absence of coronary artery disease2. Previous research has suggested that vascular dysfunction triggered by late-gestational hormones plays a key role in the pathogenesis of PPCM3. More recently, it has been suggested that PCCM may share genetic traits with dilated cardiomyopathy (DCM)4. However, the exact mechanism remains unknown due to the lack of adequate experimental models and available patient tissues.
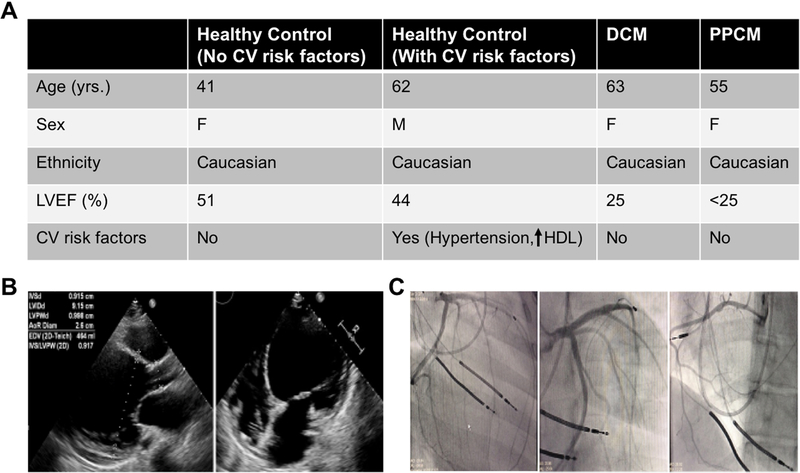
Here we describe a case of PPCM with progressive systolic heart failure that eventually required a heart transplant. The patient, a 55 year-old female, was diagnosed with PPCM at age 29 when significant ventricular ectopic beats were observed at the time of emergent caesarean section. She was subsequently managed with optimal medical and device therapies until she became refractory to available maximal therapies, ultimately requiring an orthotopic heart transplant. Review of the chart showed no apparent epigenetic insults till the time of her transplant to negatively influence her coronary vascular function and she was free of conventional cardiovascular risk factors including dyslipidemia, obesity, diabetes, hypertension, and substance abuse (Figure 1A). Despite no pre-existing heart condition, her echocardiogram showed marked LV dilatation with severely reduced LV ejection fraction (<20%) (Figure 1B). Importantly, her angiogram revealed coronaries without significant obstructive disease (Figure 1C).
Figure 1. Clinical features of patients.
(A) Table showing medical history of healthy controls (with or without CV risk factors), DCM control, and PPCM patients. (B) Echocardiogram of PPCM patient showing stably reduced LV systolic function without any significant changes. (C) Angiogram of PPCM patient showing coronaries without any significant obstruction.
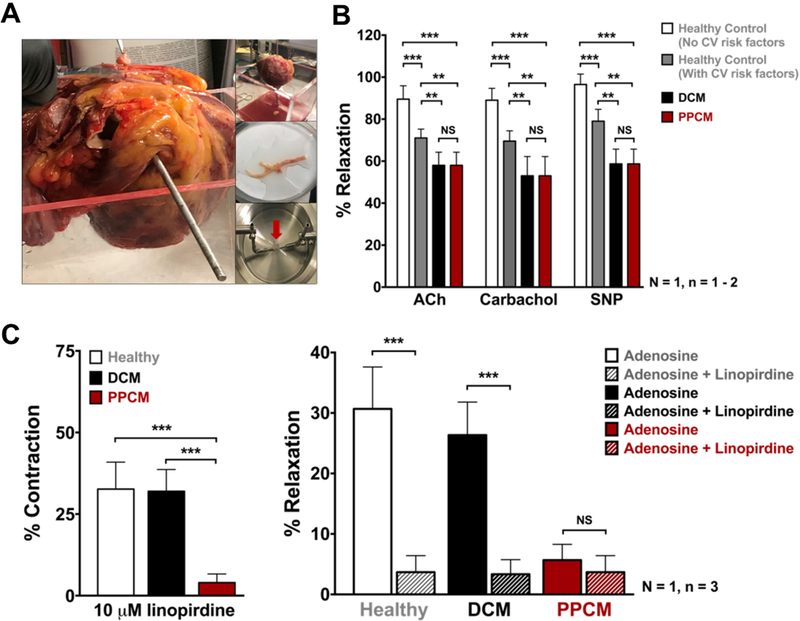
Knowing that systemic angiogenic imbalance could lead to PPCM3, we hypothesized that PPCM patients may suffer from coronary vascular dysfunction. Using isometric tension recordings of vessel reactivity, we investigated the coronary vasculature ex vivo utilizing explanted hearts from the PPCM patient (Figure 2A), an age/gender-matched DCM patient, and a gender-matched healthy donor patient. Of note, the healthy donor died of a non-cardiac cause (massive hemorrhagic stroke) and had no pre-existing conventional cardiac risk factors. Furthermore, gross examination of the donor heart revealed no palpable calcification to suggest any significant coronary artery disease. The DCM control heart was used, as PPCM is often considered a sub-type of DCM. Wire-myograph measurements found impaired endothelial cell function and nitric oxide production in PPCM and DCM as compared to the healthy control (Figure 2B).
Figure 2. Vascular reactivity in PPCM heart.
(A) Explanted PPCM heart, dissected coronary arteries and wire-myograph. (B) Endothelium function in PPCM heart. Isometric tension recordings of relaxation to Acetylcholine (10μM), Carbachol (10μM), and NO donor SNP (3μM) upon pre-constriction with U46619. (C) Tension recordings of PPCM LAD segments showing less contraction at basal tone upon application of 10μM linopirdine when compared to DCM and healthy control (left panel); impaired adenosine response in PPCM or in presence of 10μM linopirdine (right panel). Statistical analyses were done on three segments (n=3) from one coronary vessel per patient (N=1) using two-way ANOVA. *p< 0.5, **p< 0.05, ***p< 0.01.
Next, we investigated the vascular smooth muscle cell compartment, which is known to play a critical role in regulating vascular tone5. Our initial screen for the possible involvement of ion channels found that only voltage-gated K+ (Kv7) channels, which are activated by depolarization and key for maintaining the coronary circulation5 were impaired in the diseased PPCM coronary arteries. Pre-constricted PPCM left anterior descending arteries (LADs) showed significantly reduced relaxation compared to controls in response to retigabine, a KV7.2–7.5 channel activator, with this relaxation being completely abrogated in presence of linopirdine, a specific Kv7 inhibitor (data not shown). Also, responses to linopirdine failed to elicit vasoconstriction in PPCM LADs at basal tone as compared to both healthy control and DCM LADs (Figure 2C, left panel). This clearly suggests that KV7 activity is impaired in coronary vasculature of the PPCM patient. Furthermore, we ascertained the role of KV7 channels in response to adenosine, an intrinsic vasodilator. In healthy LADs, adenosine induced ~30% dilation, but this effect was absent in PPCM coronaries incubated with linopirdine (Figure 2C, right panel).
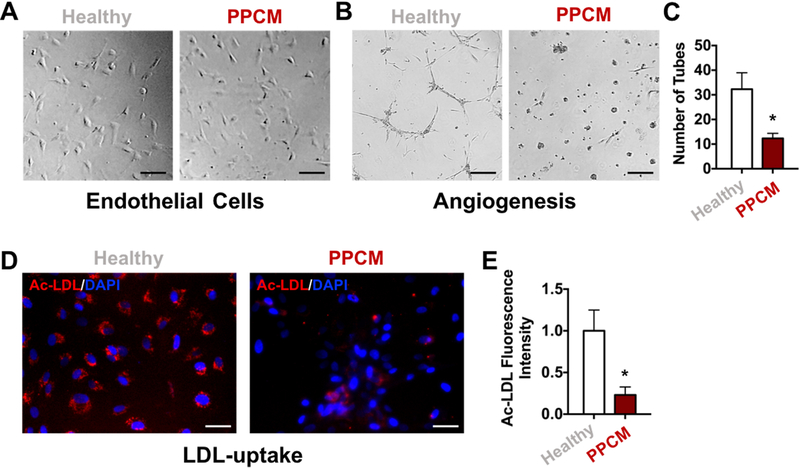
Since our wire-myograph measurements showed impairment of endothelial function in PPCM hearts, we next investigated the molecular characteristics of the endothelium in the PPCM patient to gain more insights into the vascular dysfunction. For this, we isolated primary ECs from the control and PPCM heart’s coronary vasculature (Figure 3A), and then subjected them to functional assays to assess their endothelial phenotype. Specifically, we tested the inherent ability of ECs to form three-dimensional vascular networks or uptake acetylated low-density lipoprotein (Ac-LDL)6. Consistent with our wire myography data, primary ECs isolated from the PPCM patient’s LADs showed a decreased capacity to form networks of tubular structures when placed on matrigel compared to primary ECs isolated from healthy controls (Figure 3B-C). Similarly, the PPCM patient’s ECs incorporated significantly less Ac-LDL when compared to control patient (Figure 3D-E). Taken together, these results suggest that ECs isolated from the PPCM patient show abnormal EC function, further strengthening our observation of marked vascular dysfunction in this PPCM patient.
Figure 3. Functional characteristics of primary endothelial cells from PPCM heart.
(A) Representative images of primary endothelial cells isolated from healthy and PPCM hearts. (B) Representative images of capillary-like networks formed by primary ECs showing impaired tube formation by PPCM ECs compared to healthy controls. (C) Bar graph showing quantification of the number of tubes formed by the primary ECs. (D) Representative fluorescent images of Ac-LDL uptake by primary ECs showing reduced capacity to incorporate Ac-LDL by PPCM ECs compared to healthy controls. (E) Bar graph showing quantification of Ac-LDL fluorescence intensity in primary ECs. All data represented as mean ± SEM, n=3, *p<0.05. Statistical analyses were done using standard Student t test and Mann-Whitney nonparametric test.
In summary, our report shows that the PPCM patient exhibits marked coronary vascular dysfunction using direct reactivity assays on blood vessels of explanted human hearts. We observed endothelial dysfunction with a clear impairment in nitric oxide responses in PPCM and ECs isolated from these PPCM coronaries showed functional impairment. Moreover, PPCM coronaries exhibited an apparent lack of adenosine-mediated vasorelaxation, related to impaired KV7 channel activity. While previous animal studies have suggested vascular dysfunction related to PPCM3, 7, this is the first human study to show a direct-link between PPCM and impaired coronary vascular function. Whether this observed dysfunction is a bystander effect of PPCM or a significant contributor to the underlying pathology remains unknown. Pre-eclampsia is considered to be a risk factor for PPCM8 and is often associated with vascular dysfunction, however only a small percentage of women with pre-eclampsia eventually develop PPCM, suggesting other possible mechanisms. Indeed, our patient did not have pre-eclampsia prior to developing PPCM and moreover did not show any signs of conventional cardiovascular risk factors. We speculate that the observed coronary artery dysfunction results in myocardial under-perfusion and compromised reactive hyperemia, especially during the peripartum phase of marked hormonal and metabolic changes, possibly leading to ischemic insult to myocardium and subsequent myocardial dysfunction. This might explain in part why early treatment with bromocriptine, a dopamine agonist known to vasodilate, resulted in improved outcome of PPCM9.
Even though our study brings forth a novel understanding of PPCM that is scientifically and clinically important, there are some limitations that needs to be acknowledged. Our report is representative of one patient that was diagnosed based on the timing of LV dysfunction related to pregnancy and in the absence of other competing factors. Moreover, as PPCM can occur months before or after pregnancy, not all PPCM patients present with similar phenotypes, making it difficult to compare results from different studies10. Further studies are warranted to elucidate the role of coronary vascular dysfunction in the pathogenesis of PPCM.
ACKNOWLEDGEMENTS
The authors gratefully thank all the patients for donating their hearts for research. We also thank Dr. Joseph C. Wu for his guidance and mentorship on this project. We acknowledge Drs. Y. Joseph Woo, Yasuhiro Shudo, and Jack Boyd who performed the orthotopic heart transplantations.
FUNDING SOURCES
This publication was supported in part by research grants from the National Institutes of Health (NIH) K01HL135455 and Stanford Translational Research and Applied Medicine (TRAM) pilot grant to Dr. Sayed, NIH F32 HL134221 to Dr. Rhee, and Carlsberg Foundation CF16-0345 to Dr. Khanamiri.
Footnotes
DISCLOSURE STATEMENT
The authors have nothing to disclose
REFERENCES
- 1.Arany Z and Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397–1409. [DOI] [PubMed] [Google Scholar]
- 2.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R and Carli MFD. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, Monte Fd, Hilfiker-Kleiner D, Karumanchi SA and Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, Tsai EJ, Hilfiker-Kleiner D, Kamiya CA, Mazzarotto F, Cook SA, Halder I, Prasad SK, Pisarcik J, Hanley-Yanez K, Alharethi R, Damp J, Hsich E, Elkayam U, Sheppard R, Kealey A, Alexis J, Ramani G, Safirstein J, Boehmer J, Pauly DF, Wittstein IS, Thohan V, Zucker MJ, Liu P, Gorcsan J, McNamara DM, Seidman CE, Seidman JG and Arany Z. Shared genetic predisposition in peripartum and dilated cardiomyopathies. New England Journal of Medicine. 2016;374:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, Greenwood IA and Olesen S-P. Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension. 2013;62:1090–1097. [DOI] [PubMed] [Google Scholar]
- 6.Sayed N, Wong WT, Ospino F, Meng S, Lee J, Jha A, Dexheimer P, Aronow BJ and Cooke JP. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation. 2015;131:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricke-Hoch M, Bultmann I, Stapel B, Condorelli G, Rinas U, Sliwa K, Scherr M and Hilfiker-Kleiner D. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovascular Research. 2014;101:587–596. [DOI] [PubMed] [Google Scholar]
- 8.Sliwa K, Fett J and Elkayam U. Peripartum cardiomyopathy. The Lancet. 2006;368:687–693. [DOI] [PubMed] [Google Scholar]
- 9.Hilfiker-Kleiner D, Haghikia A, Berliner D, Vogel-Claussen J, Schwab J, Franke A, Schwarzkopf M, Ehlermann P, Pfister R, Michels G, Westenfeld R, Stangl V, Kindermann I, Kühl U, Angermann CE, Schlitt A, Fischer D, Podewski E, Böhm M, Sliwa K and Bauersachs J. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. European Heart Journal. 2017;38:2671–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A and Shotan A. Pregnancy-associated cardiomyopathy. Circulation. 2005;111:2050–2055. [DOI] [PubMed] [Google Scholar]