Abstract
Background:
Approximately 50% of patients with chronic spontaneous urticaria (CSU) experience symptoms that are not fully controlled by antihistamines, indicating an unmet clinical need.
Objective:
To evaluate the effects of the selective CRTh2 antagonist AZD1981 on symptoms and targeted leukocytes in adults with persistent CSU despite treatment with H1- antihistamines.
Methods:
We performed a single center, randomized, placebo-controlled study involving adult CSU subjects with symptoms despite daily antihistamines. Subjects underwent a 2-week placebo run-in, 4 weeks of double-blinded therapy with either AZD1981 40 mg TID or placebo, followed by a 2-week placebo washout. The primary objective was the effect of AZD1981 on CSU signs and symptoms. Secondary objectives included the effects of AZD1981 on PGD2-induced eosinophil shape change, circulating leukocyte subsets, CRTh2 expression on blood leukocytes, and total blood leukocyte histamine content.
Results:
Twenty-eight subjects were randomized to AZD1981 or placebo, with 26 completing the study. Urticaria activity scores declined during the treatment phase for both groups, and were significantly reduced in the AZD1981 group at the end of washout. AZD1981 treatment increased circulating eosinophils and significantly impaired PGD2-mediated eosinophil shape change. CRTh2 surface expression rose significantly on blood basophils during active treatment. No serious adverse events were observed.
Conclusions:
This is the first study to examine the efficacy of a CRTh2 antagonist in antihistamine-refractory CSU. AZD1981 treatment was well-tolerated, effectively inhibited PGD2-mediated eosinophil shape change, shifted numbers of circulating eosinophils, and reduced weekly itch scores more than hives during treatment and into washout. Further studies are needed to determine whether inhibition of the PGD2/CRTh2 pathway will be an effective treatment for CSU.
Keywords: basophils, clinical trial, CRTh2, eosinophil, prostaglandin D2, urticaria
2. Introduction
Chronic spontaneous urticaria (CSU) is defined as pruritus and hives occurring daily or almost daily for at least 6 weeks without an underlying etiology [1]. Although second generation H1- antihistamines are first-line therapy for CSU, 30–50% of patients fail to achieve symptom control with antihistamines alone [2–4]. Cyclosporine, sulfasalazine, dapsone, plaquenil, and mycophenolate mofetil have been used in more severe cases of CSU, but each has the potential for significant toxicities that require frequent laboratory monitoring [4, 5]. Omalizumab is approved for antihistamine-refractory CSU by the U.S. Food and Drug Administration (FDA) at 150 mg or 300 mg every 4 weeks, and the European Medicines Agency (EMA) at 300 mg every 4 weeks. The optimal dose of omalizumab is 300 mg every 4 weeks [6, 7], yet 47–66% of patients treated fail to achieve complete symptom control (UAS7=0) at this dose[4, 6–8]. The disadvantages of omalizumab treatment include the high cost of a biologic agent, the requirement for subcutaneous injection in a physician’s office, and the need for an epinephrine auto-injector prescription due to the risk of drug-related anaphylaxis of up to 0.2% [9, 10]. Thus, there remains a need for safe, affordable, and convenient therapies for antihistamine-refractory CSU.
While the pathogenesis of CSU remains unknown, biopsies of CSU skin lesions reveal evidence of mast cell degranulation and infiltration by CRTh2-bearing leukocytes which include basophils, eosinophils, and Th2 lymphocytes [11]. The endogenous agonist for CRTh2 is prostaglandin D2 (PGD2) [12, 13], which is synthesized and released by activated mast cells [14–18]. We recently reported that CRTh2 levels were notably decreased on blood basophils and eosinophils from CSU subjects as compared to healthy controls [19]. This finding is consistent with ongoing in vivo receptor-ligand engagement, leading to CRTh2 receptor internalization [20, 21].
The cellular effects of CRTh2 activation include shape change, chemotaxis, upregulation of CD11b, Th2 cytokine production by Th2 cells and group 2 innate lymphoid cells (ILC2s), eosinophil cationic protein (ECP) release by eosinophils, and enhancement of allergen-induced basophil histamine release [21–28]. Animal studies have shown that PGD2 propagates eosinophil mobilization from bone marrow [29] and mediates eosinophil infiltration into the airway and skin [30–33]. PGD2 injection into healthy human skin has been shown to trigger wheal and flare responses [34, 35]. While the functional role of the CRTh2 activation in CSU is unknown, 2 single nucleotide polymorphisms in the CRTh2 promoter region are associated with antihistamineresistance in CSU [36].
AZD1981 is an oral, potent, selective, reversible antagonist of CRTh2 [37]. Preclinical studies have shown that AZD1981 blocks CRTh2-mediated shape change in human eosinophils and basophils in vitro, inhibits CRTh2-mediated chemotaxis of human Th2 cells and eosinophils, and blocks PGD2-mediated eosinophil release from bone marrow in guinea pigs [37]. In clinical trials, oral CRTh2 antagonists have reduced esophageal eosinophils in eosinophilic esophagitis [38] and provided an improvement in airway function and protection against allergen-induced symptoms in patients with allergic rhinoconjunctivitis and asthma [39–43]. Here we report the results of the first study to examine the safety and efficacy of the selective oral CRTh2 antagonist AZD1981 as add-on therapy to standard antihistamines in CSU.
3. Materials and Methods
3.1. Study Design and Participants
This is a phase II, single-center, randomized, double-blind, placebo-controlled, parallel group study which recruited patients with CSU, ages 18 to 65, who remained symptomatic despite treatment with standard doses of non-sedating Hrantihistamines (for details of inclusion and exclusion criteria, see the Online Supplemental Materials section). This study involved a 1- week screening period (days −7 to 0), a 2-week single-blind placebo run-in (days 1 to 14), a 4- week randomized double-blind treatment period (days 15 to 42), and a 2-week single-blind placebo washout (days 43 to 56) (Fig. 1).
Figure 1.
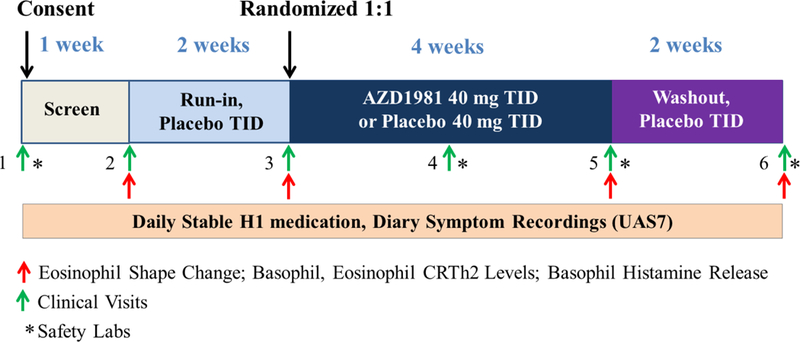
Study design.
During the screening and placebo run-in periods, subjects were required to have an in-clinic physician-assessed urticaria activity score (UAS) of 2 or greater (range 0–6) and demonstrate a UAS over 7 days (UAS7) of ≥ 8. The UAS is a validated measure of CSU disease activity which scores the intensity of pruritus (0–3) and number of hives (0–3) with a maximum value of 6 [44]. The UAS7 is the sum of the daily average UAS scores (average of a.m. and p.m.) for 7 days with a maximum value of 42 [45]. Participants were required to show compliance with twice-daily symptom recording in eDiaries throughout the study.
Subjects who met screening and run-in criteria for symptom activity were randomized on day 15 in a 1:1 ratio to receive either AZD1981 40 mg TID or matched placebo administered orally as 10 mg tablets. This dose was selected based on preclinical PK and PD studies comparing BID to TID dosing as well as safety considerations. Throughout the study, participants were required to maintain stable doses of their pre-randomization, Hrantihistamine treatment. Participants could take diphenhydramine 25 mg up to TID as a rescue medication. Subjects who required therapies other than diphenhydramine (e.g., prednisone) to treat disease symptoms were removed from the study.
All research team members were blinded to treatment assignments with the exception of the site pharmacists who dispensed the study drug, but did not interact with participants. The pharmacists randomized subjects in blocks of 4 to maintain a balanced randomization.
3.2. Study Endpoints
The primary efficacy endpoint was the change in diary-based clinical symptoms as measured by the UAS7 from baseline (placebo run-in, days 8 to 14) to the last week in the treatment period (days 36 to 42).
Secondary endpoints included the following: the safety of AZD1981; the pharmacodynamics (PD) of AZD1981 through an ex-vivo eosinophil shape change assay; the pharmacokinetics of AZD1981 through a serum bioassay; and quality of life benefit as measured by Dermatology Life Quality Index (DLQI) questionnaires. Additional efficacy endpoints included the change from baseline to the last week of treatment of the following: weekly itch severity scores (ISS), weekly score for number of hives, weekly score for the sleep interference, rescue medication use, and presence of angioedema. Safety analyses included adverse events (AEs), severe AEs (SAEs), laboratory abnormalities, and physical examination abnormalities.
Exploratory endpoints were as follows: the change in circulating leukocyte population numbers that are targeted by CRTh2 such as blood basophils, eosinophil and lymphocyte numbers by automated analysis; the change in basophil histamine release and whole blood histamine content; and pruritus-free and hive-free days during the treatment period. For details of laboratory experiments, please see the Methods section in this article’s Online Supplemental Materials.
3.3. Data Analysis
The analysis of the primary endpoint (change in UAS7 from baseline to week 4) was made using pairwise comparisons. The UAS7 obtained 1 week prior to randomization was used as the baseline score. The minimally important difference (MID) of the UAS7 for patients with CSU is estimated to be 9.5 to 10.5 [45]. The Wilcoxon matched-pairs signed-rank test (Prism 7.00) was used for intragroup comparisons between UAS7 values at baseline, the end of treatment, and the end of placebo washout. The secondary endpoints were analyzed using pairwise comparisons among the 2 study groups. Treatment comparisons of change from baseline to week 6 in the weekly ISS and number of hives score was made using the Wilcoxon signed-rank test. For missing patient-entered data (ISS and number of hives), the preceding recorded observation carried forward. Patient characteristics were analyzed using Chi-Square test and 2-sample t test for categorical and continuous variables, respectively. Any AEs or SAEs were tabulated by system organ class and preferred term, as well as by seriousness, severity, and treatment relatedness and reported by frequency. P values of ≤ .05 were considered significant.
4. Results
4.1. Subject Characteristics
A total of 38 subjects were consented and 28 subjects were randomized to treatment with AZD1981 or placebo. Following randomization, 1 subject withdrew due to CSU disease progression and 1 subject withdrew due to inability to comply with study visits, leaving 26 subjects (93%) who completed the study (See the Online Supplemental Materials, Fig. S1). Baseline characteristics were similar across treatment groups (Table 1). All CSU subjects were taking approved doses of H1-receptor antagonists. No subjects were taking leukotriene receptor antagonists (LTRAs), aspirin, or nonsteroidal anti-inflammatory drugs (NSAIDs) during the study.
TABLE 1.
Characteristics of subjects who completed the study
| Active (n = 14) | Placebo (n = 12) | P value | |
|---|---|---|---|
| Age (years) | 41.85 (23–65) | 45.17 (23–64) | .54 |
| Female (%) | 10 (71.4) | 10 (83.3) | .47 |
| Race/ Ethnicity (%) | |||
| Caucasian | 6 (42.9) | 4 (33.3) | .62 |
| African American | 4 (28.6) | 4 (33.3) | .79 |
| Asian | 3(21.4) | 2 (16.7) | .61 |
| Pacific Islander | 0 | 1 (8.3) | .31 |
| More than 1 Race | 1 (7.1) | 1 (8.3) | .91 |
| Hispanic | 0 | 0 | – |
| Baseline Symptom Scores (days 8–14) | |||
| UAS7 | 20.61 | 22.79 | .83 |
| Weekly Itch Scores | 9.71 | 10.17 | .81 |
| Weekly Hives Scores | 11.11 | 12.46 | .93 |
| Aspirin/NSAID Sensitivity † | |||
| Sensitive (%) | 6 (42.9) | 3 (25) | .34 |
| Tolerant (%) | 6 (42.9) | 9 (75) | .10 |
| Unknown (%) | 2 (14.3) | 0 (0) | .17 |
| Current Atopy | |||
| Allergic Rhinitis | 5 (35.7) | 2 (16.7) | .19 |
| Asthma | 3(21.4) | 0 | .06 |
| Atopic Dermatitis | 1 (7.1) | 2 (16.7) | .45 |
Values are expressed as % or median.
Aspirin/NSAID sensitivity was determined (by patient interview) if a subject reported increased symptoms within 24 hours of taking either an aspirin or NSAIDs, which led to avoidance.
P values are based on a X2 test for the categorical variables, and based on a 2-sample t test for the continuous variables.
4.2. Patient-reported Outcomes
During the course of the study, UAS7 scores decreased during the treatment phase for both active and placebo groups, but reached a significant reduction only in the AZD1981 group at the end of washout (Fig. 2). Active treatment had a greater effect on weekly itch severity scores (35.9% reduction) than on hives (20.5% reduction, not shown), but only itch was significantly reduced at the end of washout compared to baseline (P = .001 vs. P = .33).
Figure 2.
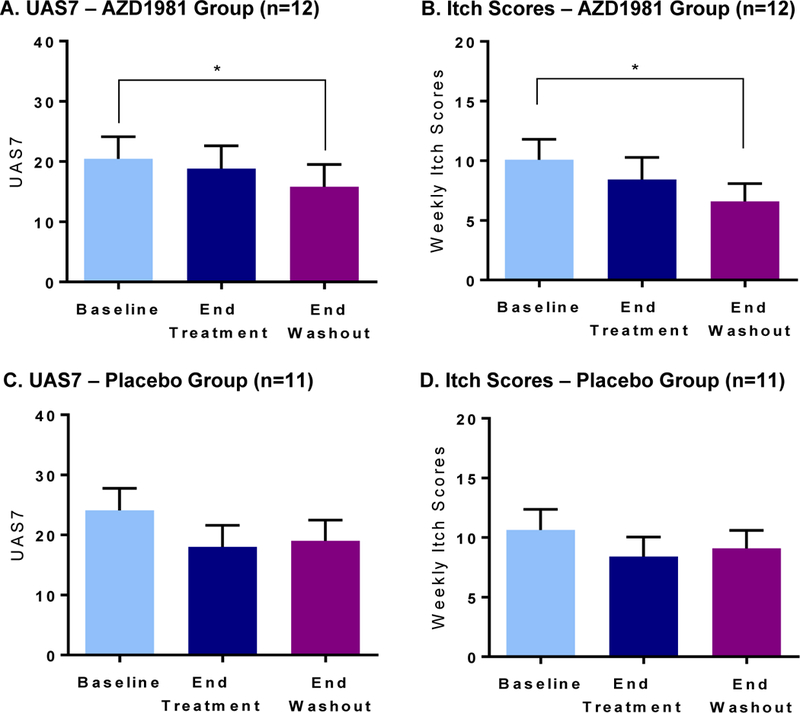
Effect of AZD1981 therapy and washout on UAS7 and weekly itch scores is shown in upper panels. Placebo is shown in lower panels. Values are shown as mean ± SEM. *P < .05.
This delayed effect raised the possibility of a carry-over effect as has been seen in prior studies with CRTh2 antagonists [46]. UAS7 scores also decreased in the placebo group from baseline to the end of treatment, but did not reach statistical significance. There was no difference in symptom response to AZD1981 based on aspirin/NSAID sensitivity or tolerance, baseline laboratory assessments (CRTh2 expression, blood eosinophil percent, eosinophil shape change response), baseline UAS scores, or medication compliance. There was no significant change in diphenhydramine use, DLQI, angioedema episodes, sleep disturbance, emergency room visits, or hive-free days with AZD1981 treatment relative to placebo.
Compliance with eDiary entry was high (>90%) throughout the study and was similar among groups. Of the 14 subjects who completed the study in the AZD1981 arm, 1 patient was excluded from the final data analysis due to a dispensing error which resulted in the patient receiving AZD1981 during the washout period. No other dispensing errors occurred. One active patient and placebo patient were excluded from symptom analysis due to malfunction or loss of eDiary device. Pill counts after each 2 week dispensation were performed by the research pharmacists and revealed an overall compliance level of 86.54% and 84.79% for active and placebo groups, respectively, during the 4 week randomized treatment period.
4.3. Effects on Eosinophil Shape Change
Eosinophil shape change after PGD2 stimulation is shown as mean increase in forward scatter above vehicle for subjects at randomization, the end of treatment, and the end of washout (Fig. 3). At baseline, the in vitro addition of AZD1981 significantly impaired PGD2-mediated eosinophil shape change at 0.1 μM dose (not shown) and 1 μM dose as calculated by AUC (Table S1). in vitro incubation with 1 μM AZD1981 significantly decreased the area under the curve (AUC) of PGD2-mediated eosinophil shape at baseline (prior to randomization) in both the active and placebo arms (P = .0005 and P = .002, respectively).
Figure 3.
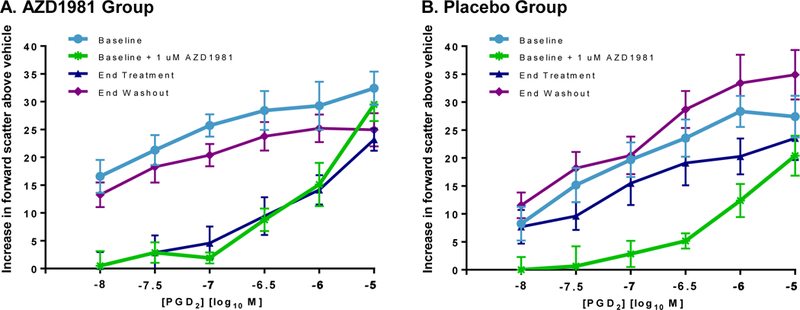
Effect of AZD1981 therapy on ex vivo eosinophil shape change is shown as net change (baseline value - no PGD2) for subjects at randomization, the end of treatment, and the end of washout. Values are shown as mean ± SEM. *P < .05.
At the end of the treatment period, ex vivo PGD2-induced eosinophil shape change was significantly inhibited in the AZD1981-treated group but not in the placebo group (Fig. 3). Treatment with oral AZD1981 showed similar impairment in PGD2-mediated eosinophil shape change to in vitro inhibition with 1 μM AZD1981 performed at baseline. Furthermore, peak serum drug levels achieved at end of AZD1981 therapy fell within or above this range (Table S2). This inhibitory effect of AZD1981 therapy trended toward baseline at the end of washout. In the placebo group, there were no significant changes observed at the end of treatment and washout relative to baseline (Table S1B).
4.5. Effects on CRTh2+ Leukocyte Percentages in Blood
The functional inhibition of eosinophil shape change observed at the end of AZD1981 treatment (Fig. 3A) occurred in parallel with a rise in circulating eosinophils at the end of treatment (Fig. 4A) that did not reach statistical significance (from 2.49 ± 0.37% to 3.5 ± 0.70%, P = .08). This rise in circulating eosinophils appeared to reverse at the end of washout (2.9 ± 0.46%, P = .40), supporting a transient impairment in eosinophil trafficking to the skin. Shifts in the percentage of blood basophils did not follow a similar pattern as eosinophils in the AZD1981 group, but assessments were limited by the presence of notable basopenia in subjects (Fig. 4B). No changes in these parameters were seen in the placebo group.
Figure 4.
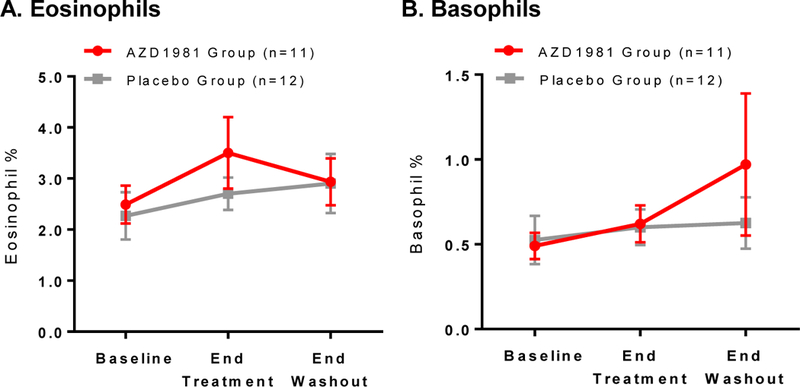
Circulating numbers of CRTh2+ leukocytes based on CBC. Shown is the % of peripheral blood (A) eosinophils and (B) basophils at baseline, end of treatment, and washout. Data is not shown for 3 active subjects (2 subjects lacked a CBC differential at the end of washout, and 1 subject was administered AZD1981 during the washout period). Values are displayed as mean ± SEM. *P < .05.
4.4. Effects on Basophil Surface Markers and Function
At the end of AZD1981 therapy, levels of CRTh2 surface expression rose significantly on blood basophils (P = .03) (Fig. 5A) and trended towards baseline at the end of washout (P = .13). Similar changes in basophil CRTh2 expression were not seen in the placebo group (Fig. 5B) or on blood eosinophils (Fig. 5C and 5D). We did not observe changes in the expression levels of other basophil chemoattractant surface receptors, including CCR1, CCR3, or CCR5 (not shown). There was also no significant change in CD11b expression on basophils or eosinophils, or CD203c expression on basophils, which are known to be enhanced after in vitro exposure to PGD2.[24] We did not observe significant changes in total blood leukocyte histamine content, which is a reflection of blood basophil presence, or changes in basophil histamine release (Fig. S2). Both of these measures for blood basophils were limited by the small numbers of subjects in this study.
Figure 5.
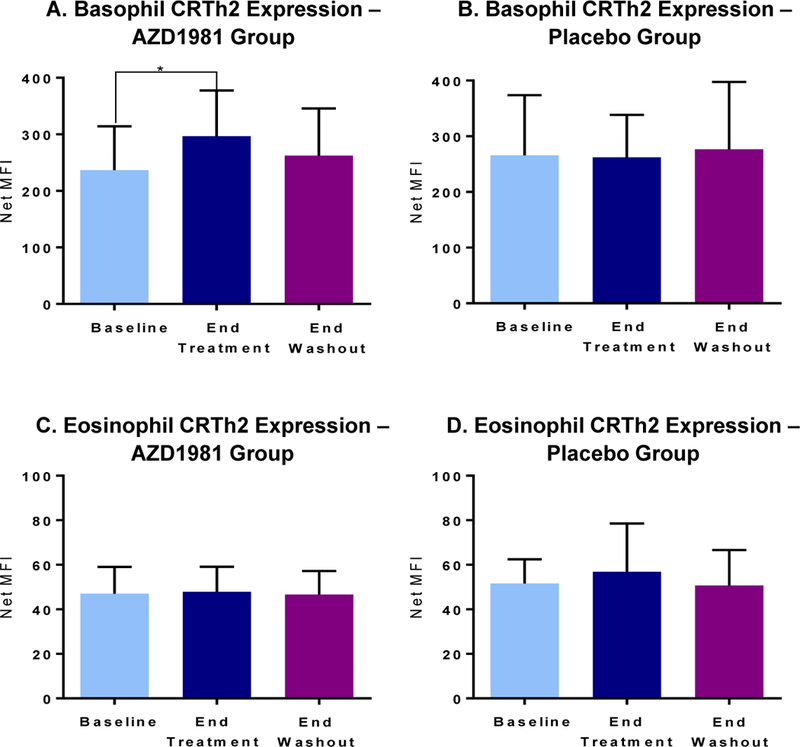
Expression of CRTh2 on blood basophils and eosinophils is shown at baseline, the end of treatment, and the end of washout. A,B. Upper panels: basophil CRTh2 levels in active (left) and placebo recipients. C,D. Lower panels: eosinophil CRTh2 levels in active (left) and placebo recipients. Values are shown as mean ± SEM. *P < .05.
4.6. Safety and Tolerability
During the treatment period and follow-up period, no significant safety AEs, CBC, or liver function test abnormalities occurred. A total of 9 patients (64.2 %) in the AZD1981 group experienced at least one AE compared with 8 patients (66.6 %) in the placebo group (Table 2). The majority of adverse events were mild and led to no patients withdrawing from the study. The most frequently reported adverse events among subjects were infection (5 active and 5 placebo).
TABLE 2.
Reported adverse events
| Adverse event, n (%) | Active (n = 14) | Placebo (n = 12) | P value |
|---|---|---|---|
| Headache | 0 | 2 (16.6) | .10 |
| Musculoskeletal | 3(21.4) | 1 (8.3) | .36 |
| Infection | 5 (35.7) | 5(41.7) | .76 |
| Sleep impairment | 1 (7.1) | 1 (8.3) | .91 |
| Respiratory disorders (non-infectious) | 0 | 0 | – |
| Gastrointestinal disorders | 5 (35.7) | 2 (16.6) | .34 |
| Blood disorders | 0 | 0 | – |
| Eye and vision disorders | 0 | 1 (8.3) | .27 |
| Cardiovascular disorders | 1 (7.1) | 1 (8.3) | .91 |
| Renal disorders | 0 | 0 | – |
| Reproductive disorders | 0 | 0 | – |
| Skin or hair disorders | 0 | 2 (16.6) | .10 |
| Immunologic disorders | 0 | 0 | – |
P values are based on Chi-Square test for the categorical variables
5. Discussion/Conclusion
This is the first study to examine the safety and efficacy of a CRTh2 antagonist in CSU. For this study, we allowed the use of antihistamines as a major goal of this our study was to evaluate the use of AZD1981 as an add-on therapy in antihistamine-refractory CSU patients. As such, we required all subjects to take a standard dose of H1 antihistamines throughout the study. Regardless, all patients continued to have moderate disease despite antihistamine use and we excluded subjects whose symptoms were controlled on antihistamines.
With AZD1981 treatment, UAS7 scores decreased during the treatment phase, but reached a significant decline from baseline at the end of drug washout. During this same time period, AZD1981-treated subjects experienced a 35.9% decline in itch component scores. This suggests the possibility of a carry-over effect as has been seen in other diseases targeted by a CRTh2 antagonist [46]. In the placebo group, decreases were observed in UAS7 and itch scores from baseline to the end of treatment which did not reach statistical significance. These findings were confirmed using one-way ANOVA, with significant differences (P<0.01) being seen only in the active group for UAS7 and itch. Active treatment had a greater effect on weekly itch severity scores than on hive scoring (Fig. 2). However, this study failed to meet the primary endpoint (UAS7 decrease ≥ 9.5). There was no significant change in DLQI, sleep interference, or in-clinic UAS scoring as a result of this brief 4-week treatment period. Rescue medications were rarely taken by our subjects and there was no significant impact of AZD1981 treatment on rescue medication use.
Consistent with the in vitro effects of AZD1981 on eosinophil migration, we found marked inhibition of ex vivo eosinophil PGD2-induced shape change in CSU subjects on active therapy (Fig. 3) along with a transient rise in circulating eosinophils (Fig. 4). Both changes trended towards baseline during drug washout suggesting a drug-induced effect on eosinophils. This suggests that PGD2 may be a central pathway for eosinophil migration to the skin. Surprisingly, these changes did not correspond with a significant change in CRTh2 surface expression on blood eosinophils. Although in vitro PGD2 binding to CRTh2 leads to receptor internalization [20, 21], our results suggest that the decrease in eosinophil CRTh2 observed in CSU subjects compared to healthy controls [19] may not solely reflect CRTh2 receptor activation by PGD2. For instance, in vitro IL-5 stimulation significantly reduces human eosinophil CRTh2 expression [20] and it was shown that IL-5 m-RNA and protein expression are increased in chronic urticaria lesions [11, 47].
Although eosinophils are a consistent infiltrating cell in urticarial skin biopsies [11, 48], their role in CSU disease is unknown. The changes seen in eosinophil shape change and circulating eosinophils with CRTh2 antagonism suggest a role for mast cell-derived PGD2 in the migration of eosinophils into skin lesions in CSU and contribution to itch. While eosinopenia is not a feature of CSU, there is data to suggest overall lower eosinophil counts in CSU as compared to healthy controls [49]. In contrast to this study, CRTh2 antagonism in subjects with persistent eosinophilic asthma led to reductions in sputum and tissue eosinophils and in asthma symptoms, but did not shift peripheral eosinophils counts [43]. Thus, the effects of PGD2 may be disease and tissue compartment specific.
In support of impairment of PGD2 actions on basophils, levels of CRTh2 surface expression rose significantly on blood basophils at the end of AZD1981 therapy (Fig. 5A). Unlike eosinophils, this finding in basophils supports the hypothesis that the reduced levels of basophil CRTh2 expression previously noted in active CSU subjects is due to ongoing PGD2-mediated receptor downregulation [19]. We were limited in the performance of additional functional assays of basophil CRTh2 inhibition such as basophil shape change due to the basopenia associated with CSU. However, the effects of AZD1981 on basophil receptor expression were specific to CRTh2, as we did not observe changes in the expression levels of other basophil chemoattractant surface receptors that may also have roles in basophil skin recruitment.
Interval improvement in clinical symptoms in CSU patients are reported to occur with rising blood basophil numbers [50]. After 4 weeks of AZD1981 treatment, we did not find significant changes in the percentage of basophils or a rise in total blood leukocyte histamine content. Given the wide variance of this latter measure in CSU disease, we were under-powered to detect basophil number increase based on past experience [51]. In addition, the lack of change in other basophil chemoattractant receptors may also explain the lack of change in measures of circulating basophils.
The reason for the decrease in itch from AZD1981 therapy is currently unknown. Intradermal PGD2 injections trigger wheal and flare responses but do not produce immediate itching [35]. We did not alter basophil histamine release mediated through the IgE receptor or spontaneous histamine release, consistent with past studies that showed that PGD2 enhances antigen mediated basophil histamine release but not that from polyclonal IgE receptor crosslinking [52]. Therefore, the improvements in CSU subjects’ itch in our AZD1981 trial may be through indirect actions on mast cells and/or basophils.
The late onset of itch reduction in AZD1981 recipients could be the result of reduction in eosinophil recruitment to the skin and/or eosinophil-derived granule proteins (EDGPs) that contribute to itch pathways. Previous studies have shown that PGD2 activation of eosinophils triggers ECP release [22]. Thus, it is possible that PGD2 may trigger the release of other EDGPs. Prior studies have revealed evidence of eosinophil activation CSU, specifically the presence of extracellular MBP and ECP in CSU skin lesions and elevated levels of ECP and major basic protein (MBP) in the serum of CSU patients despite normal peripheral eosinophil counts [53, 54]. MBP produces an immediate erythematous wheal when injected intradermally in humans and guinea pigs [55, 56]. Eosinophil MBP and eosinophil peroxidase (EPO) were recently shown to trigger histamine release from skin-derived mast cells via MrgX2 receptors which are increased in CSU skin mast cells compared to healthy controls [57]. Thus, there is a need for additional studies to fully understand the consequences of CRTh2 activation on eosinophil EDGP release.
Among the limitations of this study are the small number of subjects, short duration of therapy and washout periods, and the lack of skin tissue biopsies to correlate with peripheral blood leukocyte findings. In addition, the optimal dose of AZD1981 in CSU is unknown and we were limited to the use of a single dose (40 mg TID) in this study. AZD1981 was previously studied in allergic asthma at doses ranging from 10 mg BID to 400 mg BID, but failed to produce a clinically relevant improvement in lung function in patients with allergic asthma on ICS and LABA therapy [58]. This dose was selected based on preclinical PK and PD studies comparing BID to TID dosing and safety considerations. While it is possible that the use of a higher dose may have achieved the primary endpoint, a higher frequency of liver function abnormalities were previously noted in higher doses (400 mg BID) [58]. Despite this, we did observe a delayed yet modest reduction of disease activity particularly in itch, impairment in eosinophil shape change and potentially migration, and a rise in basophil CRTh2 expression, which would suggest that this pathway was inhibited by AZD1981.
In summary, this is the first study to examine the safety and efficacy of a CRTh2 antagonist in antihistamine-refractory CSU. Our findings of AZD1981 support the hypothesis that the CRTh2 pathway contributes to the formation of CSU skin lesions and symptoms, particularly for eosinophils. AZD1981 was well-tolerated with no significant safety issues. AZD1981 appeared to have a delayed positive effect on itch more so than wheals, although the study failed to meet the primary endpoint in UAS7 reduction at the dose studied. Given these findings, further studies are needed with longer treatment periods and higher dose ranges to determine whether inhibition of the PGD2/CRTh2 pathway will be an effective treatment for CSU.
Supplementary Material
8.1 Acknowledgement
We would like to thank our patients for their participation in this study, Tina Grace who managed our database, and Dr. Mark Liu who served as the medical monitor.
8.4. Funding Sources
This study was an investigator-sponsored study, with funding and clinical supplies from AstraZeneca. AstraZeneca had input into the dosing of study drug. AstraZeneca had no involvement in other aspects of study design, data collection, the analysis or interpretation of the data, the writing of the manuscript or the decision to submit to this journal. E.O. was supported in part by an institutional training grant funded by NHLBI (T32HL007534).
Abbreviations used:
- AE
Adverse event
- AUC
Area under the curve
- BID
Two times a day
- CBC
Complete blood count
- CRTh2
Chemoattractant receptor-homologous molecule expressed on Th2 cells
- CSU
Chronic spontaneous urticaria
- DLQI
Dermatology Life Quality Index
- ECP
Eosinophil cationic protein
- eDiary
Electronic diary
- EDGP
Eosinophil-derived granule proteins
- EMA
European Medicines Agency
- EPO
Eosinophil peroxidase
- FDA
United States Food and Drug Administration
- fMLP
N-Formyl-methionyl-leucyl-phenylalanine
- HR
Histamine release
- ILC2
Group 2 innate lymphoid cells
- ISS
Itch severity score
- LTRA
Leukotriene receptor antagonist
- MrgX2
Mas-related gene X2
- MBP
Major basic protein
- MID
Minimally important difference
- NSAID
Nonsteroidal anti-inflammatory drug
- PGD2
Prostaglandin D2
- SAE
Serious adverse event
- SD
Standard deviation
- SEM
Standard error of mean
- TID
Three times a day
- UAS
Urticaria activity score
- UAS7
Urticaria activity score over 7 days
6. Appendix
None
Footnotes
7. Supplementary Material
See Supplementary Materials File
8. Statements
8.2. Statement of Ethics
This study was conducted using the International Conference on Harmonization E6 Guidelines for Good Clinical Practice. All subjects gave their written informed consent. Before study initiation, the protocol and the written informed consent documents were approved by The Johns Hopkins Hospital Institutional Review Board (NA_00089252).
8.3. Disclosure Statement
Craig Wegner, PhD was in full-time employment with AstraZeneca during the design, initiation, and conduct of this study. He had no role in data collection or analysis. The remaining authors have no other conflicts of interest to declare.
8.6 References
- (1).Greaves M: Chronic urticaria. J.Allergy Clin.Immunol. 2000; 105: 664–672. [DOI] [PubMed] [Google Scholar]
- (2).Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, Church MK, Ensina LF, Gimenez-Arnau A, Godse K, Goncalo M, Grattan C, Hebert J, Hide M, Kaplan A, Kapp A, Abdul Latiff AH, Mathelier-Fusade P, Metz M, Nast A, Saini SS, Sanchez-Borges M, Schmid- Grendelmeier P, Simons FE, Staubach P, Sussman G, Toubi E, Vena GA, Wedi B, Zhu XJ and Maurer M: The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy 2014; 69: 868–887. [DOI] [PubMed] [Google Scholar]
- (3).Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, Sheikh J, Weldon D, Zuraw B, Bernstein DI, Blessing-Moore J, Cox L, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CR, Schuller DE, Spector SL, Tilles SA and Wallace D: The diagnosis and management of acute and chronic urticaria: 2014 update. J.Allergy Clin.Immunol. 2014; 133: 1270–1277. [DOI] [PubMed] [Google Scholar]
- (4).Kaplan AP and Popov TA: Biologic agents and the therapy of chronic spontaneous urticaria. Curr.Opin.Allergy Clin.Immunol. 2014; 14: 347–353. [DOI] [PubMed] [Google Scholar]
- (5).Morgan M and Khan DA: Therapeutic alternatives for chronic urticaria: an evidence-based review, Part 2. Ann.Allergy Asthma Immunol. 2008; 100: 517–26; quiz 526–8, 544. [DOI] [PubMed] [Google Scholar]
- (6).Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, Agarwal S, Doyle R, Canvin J, Kaplan A and Casale T: Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N.Engl.J.Med. 2013; 368: 924–935. [DOI] [PubMed] [Google Scholar]
- (7).Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bulbul Baskan E, Bradley MS, Canvin J, Rahmaoui A, Georgiou P, Alpan O, Spector S and Rosen K: Efficacy and Safety of Omalizumab in Patients with Chronic Idiopathic/Spontaneous Urticaria Who Remain Symptomatic on H Antihistamines: A Randomized, Placebo-Controlled Study. J.Invest.Dermatol. 2014; . [Google Scholar]
- (8).Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, Veith J, Kamath N, Staubach P, Jakob T, Stirling RG, Kuna P, Berger W, Maurer M and Rosen K: Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J.Allergy Clin.Immunol. 2013; 132: 101–109. [DOI] [PubMed] [Google Scholar]
- (9).Cox L, Platts-Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV, American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma and Immunology: American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J.Allergy Clin.Immunol. 2007; 120: 1373–1377. [DOI] [PubMed] [Google Scholar]
- (10).Cox L, Lieberman P, Wallace D, Simons FE, Finegold I, Platts-Mills T and Schwartz L: American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma & Immunology Omalizumab-Associated Anaphylaxis Joint Task Force follow-up report. J.Allergy Clin.Immunol. 2011; 128: 210–212. [DOI] [PubMed] [Google Scholar]
- (11).Ying S, Kikuchi Y, Meng Q, Kay AB and Kaplan AP: TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J.Allergy Clin.Immunol. 2002; 109: 694–700. [DOI] [PubMed] [Google Scholar]
- (12).Sedej M, Schroder R, Bell K, Platzer W, Vukoja A, Kostenis E, Heinemann A and Waldhoer M: D-type prostanoid receptor enhances the signaling of chemoattractant receptor-homologous molecule expressed on T(H)2 cells. J.Allergy Clin.Immunol. 2012; 129: 492–500, 500.e1–9. [DOI] [PubMed] [Google Scholar]
- (13).Arima M and Fukuda T: Prostaglandin D(2) and T(H)2 inflammation in the pathogenesis of bronchial asthma. Korean J.Intern.Med. 2011; 26: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Benyon RC, Robinson C and Church MK: Differential release of histamine and eicosanoids from human skin mast cells activated by IgE-dependent and non-immunological stimuli. BrJ.Pharmacol. 1989; 97: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dahlen SE and Kumlin M: Monitoring mast cell activation by prostaglandin D2 in vivo. Thorax 2004; 59: 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Nantel F, Fong C, Lamontagne S, Wright DH, Giaid A, Desrosiers M, Metters KM, O’Neill GP and Gervais FG: Expression of prostaglandin D synthase and the prostaglandin D2 receptors DP and CRTH2 in human nasal mucosa. Prostaglandins Other Lipid Mediat. 2004; 73: 87–101. [DOI] [PubMed] [Google Scholar]
- (17).Urade Y, Eguchi N, Aritake K and Hayaishi O: Functional analyses of lipocalin-type and hematopoietic prostaglandin D synthases. Nihon Yakurigaku Zasshi. 2004; 123: 5–13. [DOI] [PubMed] [Google Scholar]
- (18).Urade Y and Eguchi N: Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 2002; 68–69: 375–382. [DOI] [PubMed] [Google Scholar]
- (19).Oliver ET, Sterba PM, Devine K, Vonakis BM and Saini SS: Altered expression of chemoattractant receptor-homologous molecule expressed on T(H)2 cells on blood basophils and eosinophils in patients with chronic spontaneous urticaria. J.Allergy Clin.Immunol. 2016; 137: 304–306. [DOI] [PubMed] [Google Scholar]
- (20).Hamada K, Yamada Y, Kamada Y, Ueki S, Yamaguchi K, Oyamada H, Fujita M, Usami A, Chiba T, Kanda A, Kayaba H and Chihara J: Prostaglandin D2 and interleukin-5 reduce CRTH2 surface expression on human eosinophils. Allergology international : official journal of the Japanese Society of Allergology 2004; 53: 179–184. [Google Scholar]
- (21).Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, Klenerman P and Ogg G: Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor- homologous molecule expressed on TH2 cells. J.Allergy Clin.Immunol. 2014; 133: 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, Metters KM and O’neill GP: Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J.Allergy Clin.Immunol. 2001; 108: 982–988. [DOI] [PubMed] [Google Scholar]
- (23).Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, Sugamura K, Nakamura M, Takano S and Nagata K: Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J.Exp.Med. 2001; 193: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Monneret G, Boumiza R, Gravel S, Cossette C, Bienvenu J, Rokach J and Powell WS: Effects of prostaglandin D(2) and 5-lipoxygenase products on the expression of CD203c and CD11b by basophils. J.Pharmacol.Exp.Ther. 2005; 312: 627–634. [DOI] [PubMed] [Google Scholar]
- (25).Kostenis E and Ulven T: Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol.Med. 2006; 12: 148–158. [DOI] [PubMed] [Google Scholar]
- (26).Townley RG and Agrawal S: CRTH2 antagonists in the treatment of allergic responses involving TH2 cells, basophils, and eosinophils. Ann.Allergy Asthma Immunol. 2012; 109: 365–374. [DOI] [PubMed] [Google Scholar]
- (27).Kupczyk M and Kuna P : Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives. Drugs 2017; 77: 1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Charlesworth EN, Hood AF, Soter NA, Kagey-Sobotka A, Norman PS and Lichtenstein LM: Cutaneous late-phase response to allergen. Mediator release and inflammatory cell infiltration. J.Clin.Invest. 1989; 83: 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Heinemann A, Schuligoi R, Sabroe I, Hartnell A and Peskar BA: Delta 12-prostaglandin J2, a plasma metabolite of prostaglandin D2, causes eosinophil mobilization from the bone marrow and primes eosinophils for chemotaxis. J.Immunol. 2003; 170: 4752–4758. [DOI] [PubMed] [Google Scholar]
- (30).Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, Yokozeki H, Hirai H, Nagata K, Hara T, Utsuyama M, Hirokawa K, Sugamura K, Nishioka K and Nakamura M: Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J.Immunol. 2006; 177: 2621–2629. [DOI] [PubMed] [Google Scholar]
- (31).Spik I, Brenuchon C, Angeli V, Staumont D, Fleury S, Capron M, Trottein F and Dombrowicz D: Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J.Immunol. 2005; 174: 3703–3708. [DOI] [PubMed] [Google Scholar]
- (32).Shiraishi Y, Asano K, Nakajima T, Oguma T, Suzuki Y, Shiomi T, Sayama K, Niimi K, Wakaki M, Kagyo J, Ikeda E, Hirai H, Yamaguchi K and Ishizaka A: Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J.Pharmacol.Exp.Ther. 2005; 312: 954–960. [DOI] [PubMed] [Google Scholar]
- (33).Almishri W, Cossette C, Rokach J, Martin JG, Hamid Q and Powell WS: Effects of prostaglandin D2, 15-deoxy-Delta12,14-prostaglandin J2, and selective DP1 and DP2 receptor agonists on pulmonary infiltration of eosinophils in Brown Norway rats. J.Pharmacol.Exp.Ther. 2005; 313: 64–69. [DOI] [PubMed] [Google Scholar]
- (34).Soter NA, Lewis RA, Corey EJ and Austen KF: Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J.Invest.Dermatol. 1983; 80: 115–119. [DOI] [PubMed] [Google Scholar]
- (35).Beasley R, Hovel C, Mani R, Robinson C, Varley J and Holgate ST: Comparative vascular effects of histamine, prostaglandin (PG) D2 and its metabolite 9 alpha,11 beta-PGF2 in human skin. Clin.Allergy 1988; 18: 619–627. [DOI] [PubMed] [Google Scholar]
- (36).Palikhe NS, Kim SH, Ye YM, Hur GY, Cho BY and Park HS: Association of CRTH2 gene polymorphisms with the required dose of antihistamines in patients with chronic urticaria. Pharmacogenomics 2009; 10: 375–383. [DOI] [PubMed] [Google Scholar]
- (37).Schmidt JA, Bell FM, Akam E, Marshall C, Dainty IA, Heinemann A, Dougall IG, Bonnert RV and Sargent CA: Biochemical and pharmacological characterization of AZD1981, an orally available selective DP2 antagonist in clinical development for asthma. Br.J.Pharmacol. 2013; 168: 1626–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, Payton M, Pettipher R, Hunter M, Steiner J and Simon HU: Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy 2013; 68: 375–385. [DOI] [PubMed] [Google Scholar]
- (39).Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Collins LP, Hunter MG, Steiner J, Lewis T, Payton MA, Perkins CM and Pettipher R: The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy 2012; 67: 1572–1579. [DOI] [PubMed] [Google Scholar]
- (40).Barnes N, Pavord I, Chuchalin A, Bell J, Hunter M, Lewis T, Parker D, Payton M, Collins LP, Pettipher R, Steiner J and Perkins CM: A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin.Exp.Allergy 2012; 42: 38–48. [DOI] [PubMed] [Google Scholar]
- (41).Diamant Z, Sidharta PN, Singh D, O’Connor BJ, Zuiker R, Leaker BR, Silkey M and Dingemanse J: Setipiprant, a selective CRTH2 antagonist, reduces allergen-induced airway responses in allergic asthmatics. Clin.Exp.Allergy 2014; 44: 1044–1052. [DOI] [PubMed] [Google Scholar]
- (42).Busse WW, Wenzel SE, Meltzer EO, Kerwin EM, Liu MC, Zhang N, Chon Y, Budelsky AL, Lin J and Lin SL: Safety and efficacy of the prostaglandin D2 receptor antagonist AMG 853 in asthmatic patients. J.Allergy Clin.Immunol. 2013; 131: 339–345. [DOI] [PubMed] [Google Scholar]
- (43).Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MF, Bacher G, Holzhauer B, Bourne M, Mistry V, Pavord ID, Mansur AH, Wardlaw AJ, Siddiqui SH, Kay RA and Brightling CE: Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir.Med. 2016; 4: 699–707. [DOI] [PubMed] [Google Scholar]
- (44).Mlynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T and Maurer M: How to assess disease activity in patients with chronic urticaria? Allergy 2008; 63: 777–780. [DOI] [PubMed] [Google Scholar]
- (45).Mathias SD, Crosby RD, Zazzali JL, Maurer M and Saini SS: Evaluating the minimally important difference of the urticaria activity score and other measures of disease activity in patients with chronic idiopathic urticaria. Ann.Allergy Asthma Immunol. 2012; 108: 20–24. [DOI] [PubMed] [Google Scholar]
- (46).Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Collins LP, Hunter MG, Steiner J, Lewis T, Payton MA, Perkins CM and Pettipher R: The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy 2012; 67: 1572–1579. [DOI] [PubMed] [Google Scholar]
- (47).Kay AB, Clark P, Maurer M and Ying S: Elevations in Th2-initiating cytokines (IL-33, IL-25, TSLP) in lesional skin from Chronic Spontaneous (“Idiopathic”) Urticaria. Br.J.Dermatol. 2014; . [DOI] [PubMed] [Google Scholar]
- (48).Kay AB, Ying S, Ardelean E, Mlynek A, Kita H, Clark P and Maurer M: Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. Br.J.Dermatol. 2014; 171: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Grattan CE, Dawn G, Gibbs S and Francis DM: Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin.Exp.Allergy 2003; 33: 337–341. [DOI] [PubMed] [Google Scholar]
- (50).Oliver ET, Sterba PM and Saini SS: Interval shifts in basophil measures correlate with disease activity in chronic spontaneous urticaria. Allergy 2015; 70: 601–603. [DOI] [PubMed] [Google Scholar]
- (51).Saini SS, Omachi TA, Trzaskoma B, Hulter HN, Rosen K, Sterba PM, Courneya JP, Lackey A and Chen H: Effect of Omalizumab on Blood Basophil Counts in Patients with Chronic Idiopathic/Spontaneous Urticaria. J.Invest.Dermatol. 2017; 137: 958–961. [DOI] [PubMed] [Google Scholar]
- (52).Atkins PC, Schwartz LB, Adkinson NF, von Allmen C, Valenzano M and Zweiman B: In vivo antigen-induced cutaneous mediator release: simultaneous comparisons of histamine, tryptase, and prostaglandin D2 release and the effect of oral corticosteroid administration. J.Allergy Clin.Immunol. 1990; 86: 360–370. [DOI] [PubMed] [Google Scholar]
- (53).Haas N, Motel K and Czarnetzki BM: Comparative immunoreactivity of the eosinophil constituents MBP and ECP in different types of urticaria. Arch.Dermatol.Res. 1995; 287: 180–185. [DOI] [PubMed] [Google Scholar]
- (54).Peters MS, Schroeter AL, Kephart GM and Gleich GJ: Localization of eosinophil granule major basic protein in chronic urticaria. J.Invest.Dermatol. 1983; 81: 39–43. [DOI] [PubMed] [Google Scholar]
- (55).Gleich GJ, Schroeter AL, Marcoux JP, Sachs MI, O’Connell EJ and Kohler PF: Episodic angioedema associated with eosinophilia. N.Engl.J.Med. 1984; 310: 1621–1626. [DOI] [PubMed] [Google Scholar]
- (56).Plager DA, Davis MD, Andrews AG, Coenen MJ, George TJ, Gleich GJ and Leiferman KM: Eosinophil ribonucleases and their cutaneous lesion-forming activity. J.Immunol. 2009; 183: 4013–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, Kuroda K, Nunomura S, Hayama K, Terui T, Ra C and Okayama Y: Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J.Allergy Clin.Immunol. 2014; 134: 622–633.e9. [DOI] [PubMed] [Google Scholar]
- (58).Bateman ED, O’Brien C, Rugman P, Luke S, Ivanov S and Uddin M: Efficacy and safety of the CRTh2 antagonist AZD1981 as add-on therapy to inhaled corticosteroids and long-acting beta2-agonists in patients with atopic asthma. Drug Des.Devel.Ther. 2018; 12: 1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


