Abstract
Cemented fixation has been the gold standard in total knee arthroplasty (TKA). However, with younger and more active patients requiring TKA, cementless (pressfit) fixation has sparked renewed interest. Therefore, we investigated differences in (1) patient demographics, (2) inpatient costs, (3) short-term complications, and (4) discharge disposition between patients who underwent TKA with cemented and cementless fixation. The National Inpatient Sample database was queried for TKA patients with cement or cementless fixation between October 1 and December 31, 2015. Primary outcomes of interest included complications, length of stay (LOS), discharge disposition, and inpatient costs. Student’s t-test and chi-square analysis were used to assess continuous and categorical data, respectively. Multivariable analysis evaluated the effects of fixation type on the continuous and categorical dependent variables. Patients who received cementless fixation were more often younger (63.5 vs. 65.9 years), male (47.4 vs. 40.3%), Black (10.7 vs. 7.7%), from the Northeast census region (29.1 vs. 17.1%), and under private insurance (49.2 vs. 40.3%; p < 0.001 for all). Cementless fixation involved higher inpatient hospital costs (US$17,357 vs. US $16,888) and charges (US$67,366 vs. US$64,190; p < 0.001 for both), lower mean LOS (2.63 vs. 2.71 days; p < 0.001), and higher odds of being discharged to home (odds ratio = 1.99; p = 0.002). This study revisited the outcomes of TKA with cementless fixation and demonstrated higher inpatient charges and costs, shorter mean LOS, and higher odds of being discharged home. Future studies should arthroplasty investigate patient outcomes and complications past the inpatient period, evaluate long-term survivorship and failure rates, and implement a prospective study design.
Keywords: cementless fixation, cementless total knee arthroplasty, total knee arthroplasty, cost
Total knee arthroplasty (TKA) is a highly effective procedure for patients suffering from end-stage knee arthritis, with demand expected to grow nearly sevenfold by 2030.1 Despite its success, there have been increasing pressures to improve patient outcomes and modernize TKA procedures to reflect the newer demands of health care reform2–5 and accommodate a rapidly changing patient population. As such, several issues, including optimal surgical approach, type of prosthesis, efficacy of surgical assistive technologies, pain control, and infection prophylaxis, have surfaced as areas of contention.
Similarly, identifying the ideal fixation method has become a renewed topic of debate. While cemented fixation remains the gold standard due to its long reliable history, a growing demand for TKAs among younger and more active patients, in conjunction with growing concerns over cement-mediated osteolysis,6–8 has led to a revisiting of cementless fixation TKAs. The use of cementless prostheses was first implemented as a response to early total hip arthroplasty (THA) failures in young patients.9,10 While its success was demonstrated in THA recipients, early use of cementless TKA was less than promising due to reports of early tibial and patella component loosening.11,12 However, this failure has since been attributed to early design flaws, which were characterized by poor osteoconductive surfaces and inadequate fixation devices.13 Newer implants have incorporated effective solutions such as porous coatings, plasma spray, and rotating platforms that reduce stress conditions and micromotion at the bone-metal interface.
The reported benefits of cementless fixation are preservation of bone stock, decreased operating room time, ease of revision, and elimination of complications associated with cementing.13 However, anecdotal reports have hinted toward higher costs associated with the use of cementless prosthesis. Thus, with newer studies reporting excellent short- and midterm outcomes with cementless TKA,14–19 a comparison of costs and value outcomes between the two fixation methods is now warranted. This is increasingly more important as newer reimbursement models centered on cost reduction and quality improvement are being implemented in our nation’s health care system.2,3,20 Thus, the purpose of this study was to investigate differences in patient selection, costs, and quality outcomes between cemented and cementless TKA. More specifically, by using a large validated database, (1) patient demographics, (2) inpatient costs, (3) short-term complications, and (4) discharge disposition were assessed for patients who underwent a TKA procedure with either cemented or cementless TKA fixation.
Methods
Database and Patient Selection
The National Inpatient Sample (NIS) database was used to identify all patients who underwent a TKA procedure with either cemented (International Classification for Diseases, Tenth edition, procedure code [ICD-10 PCS]: 0SRD0J9 and 0SRC0J9) or cementless fixation (ICD-10 PCS: 0SRD0JA and 0SRC0JA). Exclusion criteria included patient age less than 18 years, patients who underwent TKA as a nonelective procedure, and patients without osteoarthritis as the primary diagnosis for TKA (►Fig. 1). This yielded a total of 167,930 cases (mean age = 65.83 years; women = 59.5%).
Fig. 1.
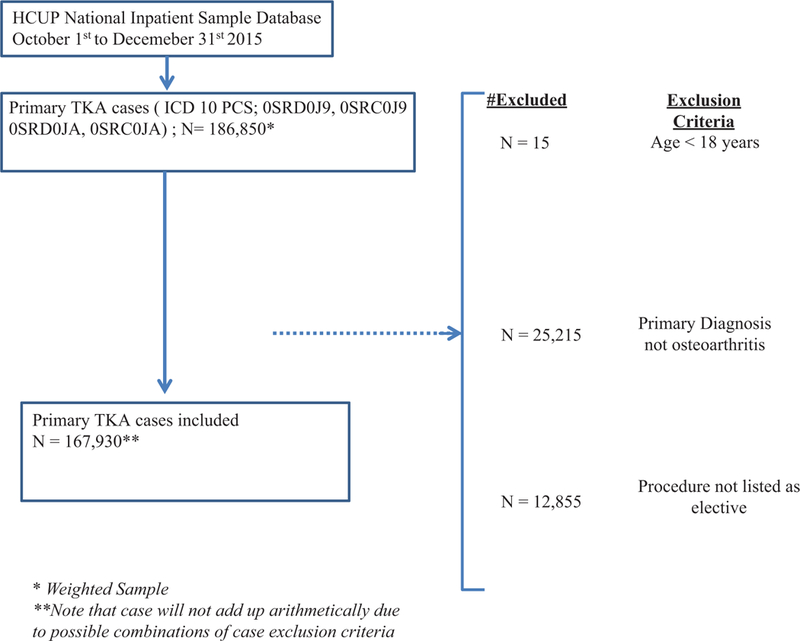
Exclusion criteria. TKA, total knee arthroplasty.
The NIS was created through a private and federal partnership between the Healthcare Cost and Utilization Project (HCUP) and the Agency for Healthcare Research and Quality. Currently, NIS is the largest all-payer inpatient care database in the United States, containing data on more than 7 million hospital stays annually.21 Weighted, it estimates more than 35 million hospitalizations annually22,23 and reliably reflects the U.S. demographics of all inpatient stays.24 Prior to 2015, patient episodes of care were identifiable using ICD-9-CM coding; however, as of October 1, 2015, the NIS was updated to include ICD-10 coding system.25
Independent Variables
Patient demographics (age, gender, race, and insurance status) and hospital characteristics (rural, urban nonteaching, urban teaching, small bed size vs. large bed size, medium bed size vs. large bed size) were used as variables in predictive models. Charlson Comorbidity Index (CCI),26–29 a validated predictor of inpatient mortality, hospital length of stay (LOS), 10-year mortality, and readmission, was used to assess patient preoperative health status.30–32
Risk Factors and Adverse Events
Complications were adopted from Martin et al,33,34 which was grouped as either major or minor. Major complications include evidence of deep infection (T84.50XA), sepsis (ICD-10: T81.12, T81.1), shock (ICD-10: T81.10), wound dehiscence (ICD-10: T81.3, T81.31), pulmonary embolism (ICD-10: I26.90, I26.92, I26.99, I26.01, I26.02, I26.09), postprocedural cardiac failure/ insufficiency (ICD-10: I97.121, I97.110), cerebrovascular accident (ICD-10: I97.8), and death. Minor complications included superficial surgical site infection (L03.119, T814), postoperative pneumonia (ICD-10: J95.1, J95.2, J95.3, J95.4),35 urinary tract infection (ICD-10: N99.89), peroneal nerve injury (S84.10XA), deep vein thrombosis (ICD-10: I82.401, I82.402 I82.403, I82.409), and acute renal failure (ICD-10: N17.0, N17.1, N17.2, N17.8, N17.9).36 Coding adopted from HCUP. net37 was used to distinguish between chronic and acute medical conditions. Discharge disposition was recoded as a binomial dependent variable, with “routine discharge” (i.e., transfer to home) coded as “1 “ and categories such as “transfer to short-term hospital” and “transfer other” (includes skilled nursing facility, intermediate care facility, or other type of facility, and home health care) coded as “0.”
Statistical Analysis
Continuous and categorical data were analyzed using Student’s t-test and chi-square analysis, respectively. All continuous data were assessed and corrected for violations to normality. Generalized mixed models were used to explore the effects of fixation methodology on continuous and categorical dependent variables. Firth’s log regression38 was used for categorical data for small sample events. A two-tailed p-value of 0.05 was set as the threshold for statistical significance. Statistical analysis was conducted using SPSS version 25 (IBM Corporation, Armonk, NY) and R version 3.4.1.
Results
Patient Demographics and Hospital Characteristics
Analysis of weighted data demonstrated a statistically significant difference in age (63.5 vs. 65.9 years; p < 0.001), gender (women = 52.6 vs. 59.7%; p < 0.001), race (African-American = 10.7 vs. 7.7%; p < 0.001), census region (Northeast = 29.1 vs. 17.1%; p < 0.001), and primary payer (private insurance= 49.2 vs. 40.3%; p < 0.001) distributions between episodes of cementless and cemented TKA (►Table 1). There was a significant difference in CCI scores between the groups (p < 0.001), in which a lower proportion of patients with a CCI of 2 or more underwent cementless TKA fixation when compared with cemented fixation (6.1 vs. 8.4%). There was no difference in hospital location/teaching status between the two groups (p = 0.103).
Table 1.
Demographic and hospital characteristic between cementless and cemented TKA cases
| Cementless (4,870) |
Cemented (163,060) |
p-Value | |
|---|---|---|---|
| Mean age in years (standard deviation) |
63.51 (9.45) | 65.9 (9.5) | <0.001 |
| Women | 52.6% | 59.7% | <0.001 |
| Morbid obesity (BMI over 40 kg/m2) |
9.8% | 9.4% | 0.378 |
| Charlson-Deyo comorbidity |
|||
| 0 | 73.8% | 71.4% | |
| 1 | 20.1% | 20.2% | <0.001 |
| 2 | 4.6% | 6.4% | |
| 3 | 1.5% | 2.0% | |
| Bed size | |||
| Small | 28.1% | 28.6% | |
| Medium | 37.3% | 29.0% | <0.001 |
| Large | 34.6% | 42.4% | |
| Location/teaching status of hospital |
|||
| Rural | 10.0% | 10.1% | |
| Urban nonteaching | 30.8% | 32.2% | 0.103 |
| Urban teaching | 59.2% | 57.7% | |
| Race | |||
| White | 83.8% | 83.2% | |
| Black | 10.7% | 7.7% | |
| Hispanic | 3.1% | 5.1% | <0.001 |
| Asian or Pacific Islander | 0.7% | 1.6% | |
| Native American | 0.0% | 0.4% | |
| Other | 1.8% | 2.0% | |
| Region | |||
| Northeast | 29.1% | 17.1% | |
| Midwest | 23.7% | 29.4% | |
| South | 32.2% | 34.1% | <0.001 |
| West | 14.9% | 19.5% | |
| Primary payer | |||
| Medicare | 41.2% | 53.0% | |
| Medicaid | 5.8% | 3.5% | |
| Private insurance | 49.2% | 40.3% | |
| Self-pay | 0.5% | 0.5% | <0.001 |
| No charge | 0.0% | 0.0% |
Abbreviations: BMI, body mass index; TKA, total knee arthroplasty. Note: Statistically significant values appear in bold.
Costs and Length of Stay
Analysis revealed higher total inpatient mean costs (US $16,010.43 vs. US$15,394.45; p = < 0.001) and charges (US$58,652.50 vs. US$57,383.35; p = 0.006) for episodes that involved cementless TKA fixation (►Table 2). Similarly, analysis demonstrated higher estimated mean costs (US $17,357.18 vs. US$16,887.92; p < 0.001) and charges (US $67,365.53 vs. US$64,190.12; p < 0.001) after adjusting for differences in patient demographics (age, gender, bed size, race, region, primary payer) (►Table 3).
Table 2.
Unadjusted comparisons for differences in costs, charges, and length of stay
| Cementless | Cemented | p-Value | |
|---|---|---|---|
| Mean charges (standard deviation) | US$58,652.50 (US$33,524.44) | US$57,383.35 (US$31,561.53) | 0.006 |
| Mean costs (standard deviation) | US$16,415.71 (US$6,557.79) | US$16,008.97 (US$6,790.50) | <0.001 |
| Mean length of stay (standard deviation) | 2.48 d (1.27 d) | 2.55 d (1.42 d) | <0.001 |
Table 3.
Comparison of costs, charges, and length of stay after adjusting for differences in patient and hospital characteristics
| Cementless | Cemented | p-Value | |
|---|---|---|---|
| Mean charges (standard error) | 67,365.53 (6,058.14) | 64,190.12 (6,041.335) | <0.001 |
| Mean costs (standard error) | 17,357.18 (1,208.66) | 16,887.92 (1,204.761) | <0.001 |
| Mean length of stay (standard error) | 2.63 (0.14) | 2.71 (0.14) | <0.001 |
There was a lower mean LOS for patients undergoing cementless TKAs (2.48 vs. 2.55 days; p < 0.001) (►Table 2). Even after adjusting for patient and regional level clustering, linear mixed effects modeling demonstrated a lower mean LOS for patients undergoing cementless TKAs (2.63 vs. 2.71 days; p < 0.001) (►Table 3).
Short-Term Complications and Discharge Disposition
General mixed models demonstrated no association between fixation methodology and the development of acute renal failure (adjusted odds ratio [OR]: 0.8344; p = 0.088), urinary tract infection (adjusted OR: 0.989; p = 0.898), or pulmonary embolism (adjusted OR: 1.247; p = 0.492) (►Table 4). Firth’s log regression revealed no association between fixation type and the development of surgical site infection (adjusted OR: 2.31; p = 0.99) postoperative pneumonia (adjusted OR: 2.26; p = 0.99), or deep vein thrombosis (adjusted OR: 1.19; p = 0.99). There were no ICD-10-identifiable cases of deep infection, sepsis, shock, wound dehiscence, postprocedural cardiac insufficiency, cerebrovascular accident, and peroneal nerve injury in the database during this time period, thereby preventing analyses of these endpoints. Mixed-effects log regression analysis revealed slightly higher odds of being discharged to home if having received cementless TKA (OR = 1.99; p = 0.002) after adjusting for patient- and hospital-specific variables.
Table 4.
Modela assessment for association between fixation type, adverse events, and discharge disposition
| Odds ratiob | p-Value | Confidence intervals | |
|---|---|---|---|
| Acute renal failure | 0.83 | 0.088 | 0.68 to 1.03 |
| Urinary tract infection | 0.99 | 0.898 | 0.83 to 1.17 |
| Pulmonary embolism | 1.25 | 0.492 | 0.66 to 2.34 |
| Superficial surgical site infection | 2.31 | 0.999 | −5.05 to 10.68 |
| Postoperative pneumonia | 2.27 | 0.999 | −5.01 to 10.31 |
| Discharge home | 1.99 | 0.002 | 1.29 to 3.08 |
There were no ICD-10 (International Classification for Diseases, Tenth edition) identifiable cases of deep infection, sepsis, shock, wound dehiscence, postprocedural cardiac insufficiency, cerebrovascular accident, and peroneal nerve injury in the database during this time period.
Reference = cemented total knee arthroplasty.
Discussion
Ideal fixation during TKA is a topic of contentious debate among arthroplasty surgeons. While cemented prostheses remain the current gold standard for fixation methodologies, evidence of an increasing demand among young and active patients, coupled with an increasing life expectancy, has spurred renewed interests in the use of press-fit options. This study used NIS data to explore patient demographics and regional fixation differences, inpatient costs differences, LOS, and complication fixation differences for TKA recipients during the final quarter of 2015.
The difference in patient demographics may indicate selective use of cementless fixation modalities by arthroplasty surgeons. This comes as no surprise as cementless fixation technology is predicated on good bone quality with high metabolic activity,39 which is negatively correlated with increasing patient age.40 Furthermore, a higher proportion of cementless TKA recipients among private insurance beneficiaries may be a function of private insurance policies that more readily permit the use of newer technologies.41 Still, it was interesting to note a higher proportion of African-Americans receiving cementless TKAs when compared with cemented TKA recipients (10.7 vs. 7.7%).
However, the increased acute care costs associated with cementless fixation (adjusted cost difference = +US$469.26) can spur some hesitation in its use. With increased government and private sector pressures to reduce costs, increased expenditure from cementless fixation may result unfavorably.2,3,42 As such, the use of cementless fixation modalities may warrant some bargaining between arthroplasty providers and implant companies to decrease implant costs. This was demonstrated by Elbuluk and Bosco43 in a continuous series involving 52 revision TKA episodes. The authors revealed an approximate US$7,000 and US$1,000 increase in acute care episode savings with “direct to hospital” and “fixed pricing” models, respectively, yielding a potential savings of US$8,000 per revision TKA based on implants alone.
Our mixed logit regression modeling revealed improved odds of discharge to home with cementless TKA when compared with cemented TKA (OR: 1.99). While this may demonstrate a small association between fixation choice and discharge status, it can translate to significant savings for high-volume arthroplasty institutions. This was illustrated by Zeng and Waldo in a retrospective review entailing 8,801 TKA surgeries.44 In their study, the authors reported significant increases in 90-day costs for inpatient rehabilitation (+US $22,921; p < 0.05), skilled nursing facility (+US$15,489; p < 0.05), and home health care (+US$6,620; p < 0.05). Similarly, in a retrospective review of 2,328 consecutive TKA recipients, Barad et al45 revealed that a decrease in mean LOS (2 days to 1.3 days) coupled with an increased discharge to home (9% to 53%) translated to a mean costs savings of US $3,245. Furthermore, the authors reported no change in readmission rates during the study duration (2009–2014).
Current studies have reported similar short- to long-term outcomes with cemented and cementless TKA. Prudhon and Verdier46 retrospectively compared 100 cemented TKA with 100 cementless TKA (mean follow-up = 11 years; range: 11–16 years). The authors reported no difference in survivorship between the two groups (90.2 vs. 95.4%; p = 0.32). Similarly, Miller et al15 compared midterm outcomes (mean follow- up = 5.3 years) in a case-control study of 400 primary TKAs (200 cemented TKAs vs. 200 cementless TKAs). The authors reported higher mean Knee Society functional scores (70 vs. 76; p = 0.016) and Knee Society knee scores (91.5 vs. 94.1; p = 0.007) at 2 years for the cementless TKA recipients. Interestingly, one study reports the superiority of using cementless fixation for obese patients. In a multicentered review of 298, of which 292 were morbidly obese (body mass index > 40 kg/m2), Bagsby et al47 reported a higher rate of revisions among cemented TKA recipients when compared with patients who received cementless TKA (13 vs. 0.7%; p < 0.05). Additionally, the authors revealed superior improvement in postoperative range of motion for the cementless group (+23.7° vs. +5.7°; p < 0.001) when compared with cemented TKA recipients at final follow-up.
This study is not without its limitations. Our study is retrospective by nature and thus limits the assertions of causality. As such, any differences in our results should be interpreted as an association and not causation. Also, the NIS database covers inpatient episodes, thus rendering us incapable of exploring differences in costs and outcomes that extend past the acute care period. This may have impacted our ability to detect adverse events in that a large portion of complications related to TKA procedure and costs often occur after the acute care period. However, such analysis is still warranted as reimbursement models such as the inpatient prospective payment system incorporate negative adjustments for adverse events and excessive costs that occur during inpatient care periods.48 Additionally, our analysis did not allow us to distinguish hybrid fixation options from fully cementless fixation. This limitation was due to lack of specificity in the ICD-10 coding to distinguish between the two modalities. Despite these limitations, the large representative sample size in our study allowed us to make well-powered comparisons and thus may be able to guide hospital administration policies and physician level of practice.
Conclusion
Although cemented fixation is presently the gold standard in TKA, a younger and more active patient population has reignited interest in the cementless technique. In this study, we demonstrate that TKA with cementless fixation is associated with higher inpatient charges and costs, shorter mean LOS, and higher odds of being discharged home relative to TKA with cemented fixation. Future areas of interest include a comparison of complications and patient outcomes occurring past hospital discharge, as well as long-term survivorship and failure rates of TKA with the present operative techniques and implant designs.
Footnotes
Conflict of Interest
None.
References
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89(04):780–785 [DOI] [PubMed] [Google Scholar]
- 2.Siddiqi A, White PB, Mistry JB, et al. Effect of bundled payments and health care reform as alternative payment models in total joint arthroplasty: a clinical review. J Arthroplasty 2017;32(08): 2590–2597 [DOI] [PubMed] [Google Scholar]
- 3.Chughtai M, Patel NK, Gwam CU, et al. Do Press Ganey Scores correlate with total knee arthroplasty-specific outcome questionnaires in postsurgical patients? J Arthroplasty 2017;32(9S):S109–S112 [DOI] [PubMed] [Google Scholar]
- 4.Delanois RE, Gwam CU, Mistry JB, et al. Does gender influence how patients rate their patient experience after total hip arthroplasty? Hip Int 2018;28(01):40–43 [DOI] [PubMed] [Google Scholar]
- 5.Gwam CU, Mistry JB, Mohamed N, et al. does age influence how patients rate their experience of care after total knee arthroplasty? J Knee Surg 2017;30(07):647–651 [DOI] [PubMed] [Google Scholar]
- 6.Kandahari AM, Yang X, Laroche KA, Dighe AS, Pan D, Cui Q. A review of UHMWPE wear-induced osteolysis: the role for early detection of the immune response. Bone Res 2016;4:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ML, Sharkey PF, Tuan RS. Particle bioreactivity and wear- mediated osteolysis. J Arthroplasty 2004;19(08):1028–1038 [DOI] [PubMed] [Google Scholar]
- 8.Gallo J, Goodman SB, Konttinen YT, Wimmer MA, Holinka M. Osteolysis around total knee arthroplasty: a review of pathogenetic mechanisms. Acta Biomater 2013;9(09):8046–8058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranawat CS, Atkinson RE, Salvati EA, Wilson PD Jr. Conventional total hip arthroplasty for degenerative joint disease in patients between the ages of forty and sixty years. J Bone Joint Surg Am 1984;66(05):745–752 [PubMed] [Google Scholar]
- 10.Springer BD, Connelly SE, Odum SM, et al. Cementless femoral components in young patients: review and meta-analysis of total hip arthroplasty and hip resurfacing. J Arthroplasty 2009;24(6, Suppl):2–8 [DOI] [PubMed] [Google Scholar]
- 11.Baech J, Kofoed H. Failure of metal-backed patellar arthroplasty. 47 AGC total knees followed for at least 1 year. Acta Orthop Scand 1991;62(02):166–168 [DOI] [PubMed] [Google Scholar]
- 12.Lombardi AVJr Engh GA, Volz RG Albrigo JL, Brainard BJ. Fracture/ dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am 1988;70 (05):675–679 [PubMed] [Google Scholar]
- 13.Aprato A, Risitano S, Sabatini L, Giachino M, Agati G, Massè A. Cementless total knee arthroplasty. Ann Transl Med 2016;4(07): 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, Henricson A, Nilsson KG. Cemented versus uncemented fixation of the femoral component of the NexGen CR total knee replacement in patients younger than 60 years: a prospective randomised controlled RSA study. Knee 2009;16(03): 200–206 [DOI] [PubMed] [Google Scholar]
- 15.Miller AJ,Stimac JD, Smith LS, Feher AW, Yakkanti MR, Malkani AL. Results of cemented vs cementless primary total knee arthroplasty using the same implant design. J Arthroplasty 2018;33 (04):1089–1093 [DOI] [PubMed] [Google Scholar]
- 16.Bercovy M, Beldame J, Lefebvre B, Duron A. A prospective clinical and radiological study comparing hydroxyapatite-coated with cemented tibial components in total knee replacement. J Bone Joint Surg Br 2012;94(04):497–503 [DOI] [PubMed] [Google Scholar]
- 17.Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg 2014;27(05):369–376 [DOI] [PubMed] [Google Scholar]
- 18.Harwin SF, Levin JM, Khlopas A, et al. Cementless posteriorly stabilized total knee arthroplasty: seven-year minimum follow- up report. J Arthroplasty 2018;33(05):1399–1403 [DOI] [PubMed] [Google Scholar]
- 19.Gerscovich D, Schwing C, Unger A. Long-term results of a porous tantalum monoblock tibia component: clinical and radiographic results at follow-up of 10 years. Arthroplast Today 2017;3(03): 192–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwam C, Mistry JB, Piuzzi N, et al. What influences how patients with depression rate hospital stay after total joint arthroplasty? Surg Technol Int 2017;30:373–378 [PubMed] [Google Scholar]
- 21.Database Documentation NIS. Available at: https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp.Accessed August 13, 2017
- 22.HCUP-US NIS Overview. Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp.Published 2015 Accessed August 13, 2017
- 23.Gwam CU, Mistry JB, Mohamed NS, et al. Current epidemiology of revision total hip arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J Arthroplasty 2017;32(07): 2088–2092 [DOI] [PubMed] [Google Scholar]
- 24.Steiner C, Elixhauser A, Schnaier J. The Healthcare Cost and Utilization Project: an overview. Eff Clin Pract 2002;5(03): 143–151 [PubMed] [Google Scholar]
- 25.Data Innovations - ICD-10-CM/PCS Resources. Available at: https://www.hcup-us.ahrq.gov/datainnovations/icd10_re-sources.jsp. Accessed December 11, 2017
- 26.Marchena-Gomez J, Acosta-Merida MA, Hemmersbach-Miller M, Conde-Martel A, Roque-Castellano C, Hernandez-Romero J. The age-adjusted Charlson Comorbidity Index as an outcome predictor of patients with acute mesenteric ischemia. Ann Vasc Surg 2009;23(04):458–464 [DOI] [PubMed] [Google Scholar]
- 27.Tian Y, Xu B, Yu G, Li Y, Liu H. Age-adjusted Charlson comorbidity index score as predictor of prolonged postoperative ileus in patients with colorectal cancer who underwent surgical resection. Oncotarget 2017;8(13):20794–20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 2008;112(11):2384–2392 [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–1139 [DOI] [PubMed] [Google Scholar]
- 30.Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res 2014;472(05):1638–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toson B, Harvey LA, Close JCT. The ICD-10 Charlson Comorbidity Index predicted mortality but not resource utilization following hip fracture. J Clin Epidemiol 2015;68(01):44–51 [DOI] [PubMed] [Google Scholar]
- 32.Rattanasompattikul M, Feroze U, Molnar MZ, et al. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol 2012;44(06):1813–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy 2016;32(07):1286–1292 [DOI] [PubMed] [Google Scholar]
- 34.Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am 2013;95(14):e98, 1–10 [DOI] [PubMed] [Google Scholar]
- 35.Ask ACDIS. Clarifying requirements for postoperative pneumonia and respiratory complications. Available at: http://www.hcpro.com/HOM-303637-5728/Ask-ACDIS-Clarifying-requirements-for-postoperative-pneumonia-and-respiratory-complications.html. Accessed December 18, 2017
- 36.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and ade-quacy,and measuring and reducing errors. Stat Med 1996;15(04): 361–387 [DOI] [PubMed] [Google Scholar]
- 37.HCUPnet. A tool for identifying, tracking, and analyzing national hospital statistics n.d. Available at: https://hcupnet.ahrq.gov/#setup. Accessed January 3, 2018
- 38.Firth D Bias reduction of maximum likelihood estimates. Biome- trika 1993;80:27 [Google Scholar]
- 39.Matassi F, Carulli C, Civinini R, Innocenti M. Cemented versus cementless fixation in total knee arthroplasty. Joints 2014;1(03): 121–125 [PMC free article] [PubMed] [Google Scholar]
- 40.Boskey AL, Coleman R. Aging and bone. J Dent Res 2010;89(12): 1333–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Why Innovation in Health Care Is So Hard. Available at: https://hbr.org/2006/05/why-innovation-in-health-care-is-so-hard Accessed January 15, 2018
- 42.Delanois RE, Gwam CU, Mistry JB, et al. Does gender influence how patients rate their patient experience after total hip arthroplasty? Hip Int 2018;28(01 ):40–43 [DOI] [PubMed] [Google Scholar]
- 43.Elbuluk A, Bosco JA. Private payer bundled payment arrangements. Semin Arthroplasty 2016;27:201–206 [Google Scholar]
- 44.Zeng F, Waldo D. Total knee arthroplasty post acute care costs by discharge status. Value Health 2016;19:A13–A14 [Google Scholar]
- 45.Barad SJ, Howell SM, Tom J. Is a shortened length of stay and increased rate of discharge to home associated with a low readmission rate and cost-effectiveness after primary total knee arthroplasty? Arthroplast Today 2015;4(01):107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prudhon J-L, Verdier R. Cemented or cementless total knee arthroplasty? - Comparative results of 200 cases at a minimum follow-up of 11 years. SICOT J 2017;3:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bagsby DT, Issa K, Smith LS, et al. Cemented vs cementless total knee arthroplasty in morbidly obese patients. J Arthroplasty 2016;31(08):1727–1731 [DOI] [PubMed] [Google Scholar]
- 48.The 2018 Inpatient Prospective Payment System final rule. The Bulletin. Available at: http://bulletin.facs.org/2017/12/the-2018-inpatient-prospective-payment-system-final-rule/#.Wly7fZM-eYU. Accessed January 15, 2018


