Abstract
Although prolonged hypercortisolism is associated with increased mortality and substantial morbidity, the clinical signs and symptoms are wide ranging and often nonspecific, contributing to challenges in diagnosis, as well as treatment delays. Greater awareness is needed among clinicians to help identify which patients should undergo biochemical screening for excess cortisol. Several biochemical tests are available, each with important caveats that should be considered in the context of the individual patient. Cortisol secretion varies widely, further complicating the biochemical diagnosis of hypercortisolism, which relies on the use of definitive cutoff values. Patients with hypercortisolism resulting from adrenal adenomas, including those discovered incidentally, often do not present with overt Cushingoid features (plethora, striae, muscle weakness, moon facies, etc.). However, the consequences of prolonged exposure to even slight elevations in cortisol levels are profound, including increased risk of diabetes, hypertension, fractures, cardiovascular events, and mortality. Because most cases of hypercortisolism resulting from an adrenal adenoma can be managed, it is imperative to identify patients at risk and initiate testing early for the best outcomes. The aim of this report is to increase awareness of the indications for screening for hypercortisolism and to review the biochemical screening tests and diagnosis for hypercortisolism associated with adrenal adenomas.
Keywords: hypercortisolism, Cushing syndrome, adrenal adenoma, cushingoid, cortisol
Adrenal adenomas are common causes of autonomous cortisol secretion, and patients often present without Cushingoid features (e.g., bruising, proximal muscle weakness, purple striae, etc.). Even without manifestation of overt clinical phenotypes, the consequences of hypercortisolism can be profound. Prolonged exposure to slight elevation in cortisol levels is associated with increased risk of diabetes, hypertension, fragility fractures, cardiovascular events, and mortality [1–5]. Because progression to overt Cushing syndrome is relatively rare [6, 7], these patients are often described separately as having “subclinical Cushing syndrome.” However, this term is inadequate because it does not accurately define or convey the significances of the disease [6] or the importance of identifying at-risk patients. Instead, we have chosen to use the more general term, “hypercortisolism,” as we recognize that the clinical spectrum of Cushing syndrome is broad and that the diagnosis of hypercortisolism is challenging regardless of the classification.
The prevalence of hypercortisolism is estimated to be 5% to 30% in patients with adrenal masses, serendipitously found by imaging for unrelated diseases (adrenal “incidentalomas”), such as kidney stones or abdominal or back pain [6]. Adrenal incidentalomas are thought to be present in 4% to 7% of adults, and therefore, the prevalence of hypercortisolism in adults may be between 0.2% and 2.0% [6]. However, patients with incidentally discovered adrenal adenomas frequently do not receive biochemical workups to detect hypercortisolism, so this incidence rate may be underreported. Thus, this review will focus on hypercortisolism caused by adrenal adenomas and the screening and diagnosis of this patient population. Imaging recommendations to differentiate adrenal adenoma from carcinoma or to support a biochemical diagnosis and direct treatment will not be included.
1. Indications for Screening
The signs and symptoms of hypercortisolism are nonspecific and are shared with many conditions that affect the general population. These include hypertension, glucose intolerance/type 2 diabetes mellitus (T2DM), obesity, dyslipidemia, and osteoporosis [8–10] (Table 1). The determination of whether these comorbidities are related to cortisol excess in a given patient is not clinically straightforward. Additionally, because of the perceived rarity of Cushing syndrome, clinical suspicion of hypercortisolism as the primary cause of disease often is not considered in the differential diagnosis. The Endocrine Society guidelines recommend screening for hypercortisolism in patients who present with the following: adrenal incidentaloma compatible with adenoma, conditions inconsistent with the patient’s age (e.g., diabetes, hypertension, or osteoporosis), or multiple and progressive clinical features (e.g., weight gain, diabetes, depression, acne, facial fullness, etc.) [11].
Table 1.
Signs and Symptoms of Hypercortisolism: Clinical Features [8–10]
| General | Neuropsychiatric |
| Obesity | Emotional lability |
| Hypertension | Euphoria |
| Skin | Depression |
| Hirsutism | Psychosis |
| Plethora | Gonadal dysfunction |
| Striae | Menstrual disorders |
| Acne | Impotence/decreased libido |
| Bruising | Metabolic |
| Musculoskeletal | Glucose intolerance |
| Low BMD for age and sex | Diabetes |
| Weakness | Hyperlipidemia |
| Backache | Polyuria |
| Fragility fracture | Kidney stones |
Abbreviation: BMD, bone mineral density.
A. Adrenal Adenomas Incidentally Discovered
Various radiology and clinical guidelines have suggested routine clinical and hormonal workup for incidentally discovered adrenal adenomas [12]. However, adherence to such guidelines is inadequate, with only 30% of patients with adrenal adenomas referred for hormonal workup [13]. Although most adrenal incidentalomas are benign, clinical and hormonal evaluations are essential to assessment of the functionality and clinical impact of the adenomas. Clinicians should review radiology reports for mentions of adrenal adenomas and if found, should conduct a thorough clinical and biochemical workup as outlined below.
B. High Clinical Suspicion
B-1. Unusual features for the patient’s age
A patient younger than 50 years old with poorly controlled or resistant diabetes or hypertension should prompt further evaluation for hypercortisolism [11, 14]. A young patient with unexplained osteoporosis or a history of nontraumatic or fragility fractures should also be evaluated, as cortisol plays a major role in the bone and calcium homeostasis [14, 15].
B-2. Multiple progressive features
Because hypercortisolism can result in the entire clinical spectrum of metabolic syndrome, it is easy to overlook the contribution of cortisol and to view the progression of signs and symptoms as the natural progression of metabolic syndrome. Hypercortisolism should be suspected if patients have multiple uncontrolled conditions, such as “resistant” diabetes, hypertension, osteoporosis, or depression, regardless of age [15–17].
Chronic hypercortisolism, even in the so-called “subclinical” stage, can have pleiotropic effects on major peripheral tissues responsible for glucose homeostasis [18]. Consideration for patients to undergo secondary biochemical workups for hypercortisolism in the setting of resistant diabetes should be undertaken when the need for antiglycemic therapies and/or insulin therapy is significantly high (e.g., ≥2 units/kg/day) or incongruent with the expected degree of insulin resistance or obesity that a patient has. Whereas we do not advocate routine screening of all patients with T2DM for hypercortisolism, meta-analyses and multiple single-center studies have consistently demonstrated the prevalence of hypercortisolism to be ∼2% to ∼9% in these patients in the absence of any discriminatory “classic” clinical features (easy bruising, facial plethora, proximal muscle weakness, and/or striae) [19–21]. Whereas these percentages seem small, they translate to an estimate between 575,000 and >2.5 million people with T2DM in the United States possibly with secondary diabetes as a result of underlying hypercortisolism.
The prevalence of hypercortisolism in the general hypertensive patient population has been reported as ∼1% [22], although a higher rate (8%) has been reported in patients with resistant hypertension (i.e., uncontrolled with three antihypertensives, including a diuretic) [23]. In patients with resistant hypertension, workup for primary sources should include assessment of cortisol.
The prevalence of hypercortisolism in patients with osteoporosis varies from 0.6% to 4.8% [24–27], and a prevalence rate up to 10.8% has been reported in patients with low bone mineral density (BMD) and fragility fractures [27]. Clinicians should consider screening for hypercortisolism in patients with low BMD for their age or weight. Screening may also be considered in patients with greater than expected declines in BMD or with BMD that is resistant to treatment [27].
2. Biochemical Evaluation
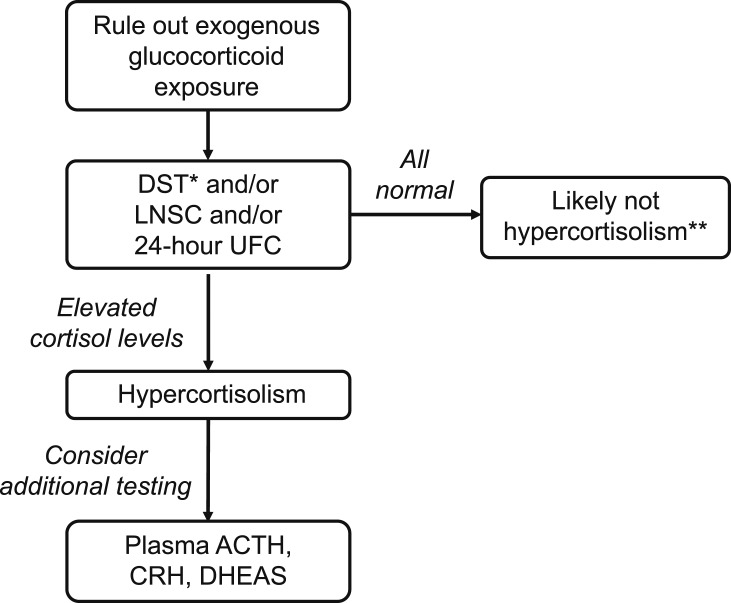
Patients with newly discovered adrenal adenomas and/or signs and symptoms leading to a suspicion of hypercortisolism should undergo biochemical screening [11]. Ideally, diagnostic assays should be used in combination with a degree of clinical suspicion for hypercortisolism to reduce the likelihood of false-positive results. A flowchart may be used as a practical approach (Fig. 1).
Figure 1.
Biochemical evaluation of suspected adrenal hypercortisolism. Assessment of hypercortisolism, based on biochemical assays, should include a degree of clinical suspicion to reduce the likelihood of false-positive results. *DST, dexamethasone suppression test; to be used as a unique, first-line screening test in the absence of overt signs and/or symptoms of hypercortisolism. **Repeat every 6 months if clinically suggestive of hypercortisolism. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; DHEAS, dehydroepiandrosterone sulfate; LNSC, late-night salivary cortisol; UFC, urinary-free cortisol.
The first step in the evaluation of patients for hypercortisolism is to exclude any exogenous causes of cortisol excess, such as corticosteroid medications. Other medications may include glucocorticoid-containing skin creams (including bleaching agents), some herbal medications, joint/nerve injections, and medroxyprogesterone acetate [11]. Next, it is important to select and perform the appropriate screening test to optimize diagnostic sensitivity and specificity without “over testing” the patient. After ruling out exogenous causes, the dexamethasone suppression test (DST), late-night salivary cortisol (LNSC), and 24-hour urinary-free cortisol (UFC) are used to diagnose hypercortisolism. They all rely on the pathophysiology of the hypothalamic-pituitary-adrenal axis; they also all possess limitations related to their sensitivity, specificity, and/or convenience [11] (Table 2). In addition, cortisol secretion fluctuates, and thus, assessments are subject to variability over time [6]. Because no single test is perfect, the performance of more than one test, either sequentially or simultaneously, is a common practice. In any case, in patients without the classical signs and symptoms of overt cortisol excess and in the absence of possible interference caused by other medications, it is the authors’ opinion that the DST test should be preferred for diagnosis of the presence of hypercortisolism [14].
Table 2.
Comparison of Different Testing Platforms
| Test | Basis of Test | Methodology | Convenience | Sensitivity and Specificity (%)[11] | Cautions |
|---|---|---|---|---|---|
| LNSC | Measures the disruption in the normal circadian rhythm seen with hypercortisolism | Salivary sample is collected between 2300 h and 2400 h; test should be repeated. | ✓✓✓✓ | Sensitivity: 92-100 Specificity: 93-100 | •Not taking the sample late at night significantly affects results. |
| •Cortisol levels can be abnormally high in those with altered sleep patterns (e.g., shift workers) and in those who smoke. | |||||
| •Lower sensitivity for less severe hypercortisolism | |||||
| 24-h UFC | Measures UFC over the course of a day | All urine is collected over a 24-h period, discarding the first void. | ✓ | Sensitivity: 80-98 Specificity: 45-98 | •Lower specificity and sensitivity compared with other methods for less severe hypercortisolism |
| •High fluid intake, contamination, incomplete urine collection, certain drugs, and decreased glomerular filtration rate (<60 mL/min) may affect accuracy. | |||||
| ODST (using 1.8 µg/dL cutoff) | Measures the amount of suppression by dexamethasone of ACTH and thereby, cortisol secretion; suppression is not observed in those with hypercortisolism | Oral dexamethasone (1 mg) is administered between 2300 h and 2400 h, and serum cortisol is collected between 0800 h and 0900 h the next morning. | ✓✓✓ | Sensitivity: 85-90 Specificity: 95-99 | •The fixed doses used in the tests do not take into consideration differences in dexamethasone absorption, volume of distribution, and metabolism, all of which are influenced by gastrointestinal function, body weight/composition, and hepatic and renal function. |
| •Falsely assumes all corticotroph adenomas have decreased sensitivity to dexamethasone, all ectopic ACTH-secreting tumors are insensitive to dexamethasone, and cortisol-secreting adenomas cannot be inhibited by dexamethasone | |||||
| LDDST | Measures the amount of suppression by dexamethasone of ACTH, and thereby, cortisol secretion; suppression is not observed in those with hypercortisolism | Oral dexamethasone (0.5 mg) is administered beginning at 0900 h on day 1 with repeated administration at 6-h intervals for 48 h (1500, 2100, 0300 h); serum cortisol is collected 6 h after the last dose (0900 h). | ✓✓ | Sensitivity: 91-98 | •The fixed doses used in the tests do not take into consideration differences in dexamethasone absorption, volume of distribution, and metabolism, all of which are influenced by gastrointestinal function, body weight/composition, and hepatic and renal function. |
| Specificity: 70-95 | •Falsely assumes all corticotroph adenomas have decreased sensitivity to dexamethasone, all ectopic ACTH-secreting tumors are insensitive to dexamethasone, and cortisol-secreting adenomas cannot be inhibited by dexamethasone |
Abbreviations: ✓, indicates lowest convenience; ✓✓✓✓, indicate highest convenience; LDDST, low-dose DST; ODST, overnight DST.
A. DST
The recommended screening test for evaluation of biochemical hypercortisolism in most patients is the 1-mg overnight DST (ODST) with a diagnostic cutoff of 1.8 μg/dL (50 nM) or greater. Whereas some clinicians have cited a historic cutoff of ≤5 μg/dL (138 nM) as “normal,” the use of this historic cutoff misclassified up to 15% of patients with false-negative results [6, 28]. The current cutoff of 1.8 μg/dL (50 nM) enhances test sensitivity to >95% and test specificity of 80% [14, 29]. Noting the challenges in the establishment of a single diagnostic cutoff, some guidelines have also recommended interpretation of the 1-mg ODST result as a continuous rather than strictly categorical variable [8].
With ODST, 1 mg oral dexamethasone is taken before bedtime (∼2300 hours). Serum cortisol levels are measured the next morning (between 0800 and 0900 hours) [11]. Advantages of this modality include drug availability, ease of testing, and patient tolerance.
Alternatively, particularly in patients with T2DM and/or obesity or in patients with equivocal results, a 2-day, low-dose DST (LDDST) can be used where 0.5 mg dexamethasone is taken every 6 hours (starting from ∼0900 hours) for 2 days, followed by cortisol measurement, 6 hours after the last dose was given. This test is cumbersome and more difficult to perform. In these patients, a prolonged dexamethasone administration may be needed to inhibit corticotropin-releasing hormone (CRH) release. A concordant result of ≥1.8 μg/dL (50 nM) is confirmation of hypercortisolism [14]. An LDDST should be validated with a serum dexamethasone level of >220 ng/dL (5.6 nM) to avoid the possibility of a false-positive result [30].
These tests have high diagnostic specificity when serum cortisol levels are >1.8 µg/dL (50 nM). It is important to keep in mind that these fixed dexamethasone doses do not take into consideration differences in dexamethasone absorption, volume of distribution, and metabolism, all of which are influenced by gastrointestinal function, body weight, and hepatic and renal function [9]. Therefore, we recommend evaluation of serum dexamethasone levels with all DSTs (or reflexively obtaining a dexamethasone level in patients with an initial positive DST).
B. LNSC
This test assesses the circadian rhythm of the hypothalamic-pituitary-adrenal axis [9]. Cortisol levels are expected to peak upon awakening in the morning (0700 to 0900 hours) and taper off during the day and are at their lowest during the late night (2300 to 0100 hours) [31, 32]. Hence, it was hypothesized that taking a late-night cortisol reading would help to identify those with abnormal elevated levels when cortisol should be at its lowest. For patients with overt cortisol excess, the LNSC has a high sensitivity and specificity [11] (see Table 2). However, the use of the LNSC may not be as accurate as previously thought in patients with de novo and recurrent/persistent Cushing disease, as shown by the wide fluctuations in sequentially measured LNSC in a recent prospective analysis [33]. Unfortunately, in patients with suspected hypercortisolism associated with adrenal incidentalomas, the LNSC test does not add much information because of its lack of sensitivity (22.7%) [34]. A recent study by Ceccato et al. [35], showed that daily cortisol exposure, evaluated using area under the curve from multiple saliva collections, was increased in the morning in patients with adrenal adenomas and a serum cortisol >1.8 mg/dL (50 nM) after DST, leading to reduced corticotropin levels. However, cortisol rhythm was preserved in these patients, offering further support that LNSC is not a useful screening test for less severe hypercortisolism [35]. However, it could be used as a confirmative test if the first results are abnormal [34, 36, 37].
The best validated assays used in the United States are an ELISA and an assay performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) [11]. For either of these assay techniques, late-night (2300 to 2400 hours) values above 145 ng/dL (4 nM) suggest hypercortisolism, although individual diagnostic thresholds may apply. Patients are advised to collect saliva samples on two separate evenings. A saliva sample is easy to obtain in the privacy of the patient’s home, substantially reducing ambient stress levels. Furthermore, the sample can be stored in the refrigerator for several days and mailed to the laboratory at room temperature [11, 38, 39].
Nonetheless, adherence to the timing of sample collection has been shown to be an issue [40]. Not following the collection protocol to take the sample late at night significantly affects results. Another confounder is that normal circadian rhythms are disrupted in persons with altered sleep patterns, such as shift workers, which causes abnormally elevated levels [41]. In these cases, samples should be taken at a time that correlates with the middle of their sleep cycle [11]. Additionally smokers have been shown to have higher LNSC levels than nonsmokers, and stress immediately before collection can cause cortisol levels to spike [11, 42–45] (Table 3).
Table 3.
| LNSC | UFC | DST | |
|---|---|---|---|
| Drugs/conditions that give false positives | |||
| Exercise/stress | X | X | X |
| Smoking | X | ||
| Disrupted sleep (e.g., shift work) | X | ||
| Topical hydrocortisone (contamination of sample) | X | ||
| Proteinuria | X | ||
| Polyuria | X | ||
| Anticonvulsants | X | X | |
| Fenofibrate | X | ||
| Synthetic glucocorticoids | X | ||
| Pregnancy | X | X | X |
| Alcoholism | X | X | X |
| Depression | X | X | X |
| Estrogen therapy | X | ||
| Drugs that induce CYP3A4 | X | ||
| Drugs that inhibit 11β-hydroxysteroid dehydrogenase type 2 (e.g., licorice, carbenoxolone) | X | X | |
| Drugs/conditions that give false negatives | |||
| Renal disease | X | X | |
| Urinary tract infection | X | ||
| Liver failure | X | ||
| Drugs that inhibit CYP3A4 | X |
Abbreviation: CYP3A4, cytochrome P450 3A4.
C. 24-Hour UFC
The 24-hour UFC test measures cortisol levels in urine over the course of a day. Normal, 24-hour UFC is a common laboratory finding in patients with less severe hypercortisolism, because the dysregulated and increased production of cortisol does not typically exceed the plasma-binding capacity of free cortisol, limiting the appearance of cortisol in the urine [46]. As a result of the lower specificity and sensitivity compared with other methods, especially for less severe disease, the use of this test is secondary to other testing assays [9]. Patients are instructed to discard the first void of the day and then collect all urine over the course of 24 hours, with the final collection being the first void of the next day. This aspect of the test can be inconvenient for those who cannot stay home for an entire day. The sample should be stored in the refrigerator until it can be brought to the clinic, which should occur within a few days. Samples are measured for cortisol by either an antibody-based immunoassay or LC-MS/MS, with thresholds for diagnosis dependent on the specific test used [11]. LC-MS/MS provides the most accurate assessment, but its use in routine practice is limited. Given that UFC testing only measures free cortisol, it is not affected by conditions or medications (i.e., oral estrogen) that increase bound cortisol levels found in serum [11]. However, high fluid intake, contamination, incomplete urine collection, certain drugs, and decreased glomerular filtration rate (<60 mL/min) may affect the accuracy of this test [9, 11, 47, 48]. Diagnostic accuracy can be improved by the concurrent measurement of urinary creatinine, with low levels indicating incomplete collection [48]. Urine collection should be repeated when creatinine levels are <1.5 g/day (<13.3 mmol/day) in men and <1 g/day (8.8 mmol/day) in women [49].
D. Plasma ACTH
ACTH is often used to differentiate ACTH-dependent (Cushing disease or ectopic ACTH) from ACTH-independent (adrenal) hypercortisolism. In ACTH-independent hypercortisolism, the negative-feedback suppression of pituitary corticotrophs by cortisol leads to reduced plasma ACTH levels.
In patients with plasma ACTH levels between 5 pg/mL (1.1 pM) and 20 pg/mL (4.4 pM), a CRH stimulation test may be recommended [9]. The premise is that normal corticotrophs are suppressed as a result of mild cortisol excess, and therefore, levels of ACTH will not elevate after provocative stimulation. In this setting, an ACTH rise above 30 pg/mL (6.6 pM) is diagnostic for an ACTH-dependent form of hypercortisolism. This test is particularly useful in patients with bilateral adrenal hyperplasia, which may also be sustained by an increased ACTH secretion (generally by a pituitary tumor). In these cases, the CRH test may be useful for evidencing the tendency of the adrenal masses toward an autonomous cortisol secretion, therefore helping to determine the treatment of choice. However, it must be noted that the CRH test is an expensive procedure, and therefore, it is not widely performed, especially in nonacademic centers. The need for a CRH test should always be ascertained by physicians with expertise in diagnosing Cushing syndrome.
E. DHEAS
Dehydroepiandrosterone sulfate (DHEAS) is an androgen precursor that is regulated, in part, by ACTH [50, 51]. Low-serum DHEAS levels have been shown to be a marker of excess cortisol in patients with adrenal adenomas (ACTH-independent hypercortisolism) [50, 52]. In a retrospective analysis of patients with adrenal adenomas conducted by Yener et al. [50], low DHEAS [<40 µg/dL (1.1 µM)] was found to be significantly predictive of hypercortisolism. In a more recent retrospective analysis of patients with adrenal adenomas, age-adjusted DHEAS assessment demonstrated comparable sensitivity (100%) and greater specificity (91.9%) than the 1-mg DST (>99% and 88.6%, respectively) for the diagnosis of hypercortisolism [52]. Although not recommended as an initial diagnostic test, DHEAS may be considered as a supportive assessment [11]. Considerations for this test include its long half-life and that it is not subject to circadian variations [51]. DHEAS levels also decrease with age.
3. Considerations in Making a Diagnosis
As the signs and symptoms of hypercortisolism are indistinguishable from a variety of other conditions, a differential diagnosis is often only realized after other etiologies have been exhausted. In fact, a definitive diagnosis in patients with either adrenal or pituitary etiologies can be up to be 2 years from onset of symptoms [53–55]. On average, patients wait ∼1 year from symptom onset before they seek medical advice [53]. Diagnosis is complicated by the selection of symptoms that patients report. Patients tend to report only those symptoms related to the specialty of the practitioner and use nonspecific layperson’s terms (e.g., “trouble with joints”) [53]. In addition, although 83% of patients initially go to their family physician to address their symptoms, almost 70% of patients ultimately are diagnosed by an endocrinologist, and substantial differences are observed between the number of consulted physicians in rural and urban places of living, suggesting differences in familiarity with hypercortisolism among specialties [53]. Another study found that, among physicians attending endocrinology meetings, the probability of diagnosing hypercortisolism was based on number of years in practice [56].
Whereas the most recent clinical guidelines regarding diagnosis of hypercortisolism in patients with adrenal incidentalomas, from 2016, suggest that patients undergo screening with the ODST [8], a recent study of 57 centers from 26 European countries found that testing protocols varied considerably across practices [57]. In addition to test-platform availability, clinical experience was found to contribute to the choice of diagnostic test. It was also found that testing protocols changed over time. In fact, some authors have suggested that multiple tests may be the best approach to diagnose hypercortisolism accurately [58].
4. Conclusion
General screening of the population at large for hypercortisolism is not recommended. However, for those with signs or symptoms indicative of hypercortisolism, appropriate testing is critical, as quality of life is significantly affected in those with the condition, and early initiation of treatment has been shown to relieve the burden of disease [59, 60]. Hypercortisolism represents a spectrum of cortisol levels and associated morbidity that challenges clinicians in making a diagnosis. Signs and symptoms are vague and nonspecific and do not always correlate with biochemical results. Nevertheless, suspicion should be raised when patients present with nondescript symptoms that resist conventional therapy.
A variety of biochemical tests are available to assist with making a diagnosis; however, they each have limitations, and the results can sometimes be equivocal. Given the detrimental effects associated with prolonged hypercortisolism, clinicians must consider interpretation of biochemical tests in the context of the patient’s medical history and clinical presentation. As most cases can be managed or even cured [61], it is imperative that clinicians learn to identify patients at risk and initiate testing as early as possible to afford the best outcomes.
Acknowledgments
The authors thank Meredith Rogers, The Lockwood Group (Stamford, CT); Sarah Mizne, MedVal Scientific Information Services, LLC (Princeton, NJ); and Mark R. Vogel and Dat Nguyen, Corcept Therapeutics (Menlo Park, CA), for providing editorial support.
Financial Support: Editorial and manuscript preparation support was funded by Corcept Therapeutics (Menlo Park, CA) in accordance with Good Publication Practice (or GPP3) guidelines (www.ismpp.org/gpp3).
Disclosure Summary: The published viewpoints are those of the individual authors and do not represent the official stance or statements of the respective academic and/or governmental agencies with which the authors are affiliated. I.C. is a consultant with Italfarmaco and speaker for Sandoz. A.O.M. is a consultant with Amgen and Regeneron and a speaker for AbbVie, Corcept, and MannKind. H.Y. is a consultant and speaker with Corcept. A.R.-R. has nothing to disclose.
Glossary
Abbreviations:
- ACTH
adrenocorticotropic hormone
- BMD
bone mineral density
- CRH
corticotropin-releasing hormone
- DHEAS
dehydroepiandrosterone sulfate
- DST
dexamethasone suppression test
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LDDST
low-dose dexamethasone suppression test
- LNSC
late-night salivary cortisol
- ODST
overnight dexamethasone suppression test
- T2DM
type 2 diabetes mellitus
- UFC
urinary-free cortisol
References and Notes
- 1. Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, Salcuni AS, Dolci A, Mendola M, Arosio M, Ambrosi B, Scillitani A, Ghigo E, Beck-Peccoz P, Terzolo M, Chiodini I. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827–834. [DOI] [PubMed] [Google Scholar]
- 2. Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396–405. [DOI] [PubMed] [Google Scholar]
- 3. Tauchmanovà L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, Fazio S, Lombardi G. Patients with subclinical Cushing’s syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87(11):4872–4878. [DOI] [PubMed] [Google Scholar]
- 4. Tsuiki M, Tanabe A, Takagi S, Naruse M, Takano K. Cardiovascular risks and their long-term clinical outcome in patients with subclinical Cushing’s syndrome. Endocr J. 2008;55(4):737–745. [DOI] [PubMed] [Google Scholar]
- 5. Morelli V, Eller-Vainicher C, Palmieri S, Cairoli E, Salcuni AS, Scillitani A, Carnevale V, Corbetta S, Arosio M, Della Casa S, Muscogiuri G, Spada A, Chiodini I. Prediction of vertebral fractures in patients with monolateral adrenal incidentalomas. J Clin Endocrinol Metab. 2016;101(7):2768–2775. [DOI] [PubMed] [Google Scholar]
- 6. Chiodini I. Clinical review: diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011;96(5):1223–1236. [DOI] [PubMed] [Google Scholar]
- 7. Barzon L, Scaroni C, Sonino N, Fallo F, Paoletta A, Boscaro M. Risk factors and long-term follow-up of adrenal incidentalomas. J Clin Endocrinol Metab. 1999;84(2):520–526. [DOI] [PubMed] [Google Scholar]
- 8. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 9. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(2):739–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ross EJ, Linch DC. Cushing’s syndrome—killing disease: discriminatory value of signs and symptoms aiding early diagnosis. Lancet. 1982;2(8299):646–649. [DOI] [PubMed] [Google Scholar]
- 11. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayo-Smith WW, Song JH, Boland GL, Francis IR, Israel GM, Mazzaglia PJ, Berland LL, Pandharipande PV. Management of incidental adrenal masses: a white paper of the ACR incidental findings committee. J Am Coll Radiol. 2017;14(8):1038–1044. [DOI] [PubMed] [Google Scholar]
- 13. Eldeiry LS, Garber JR. Adrenal incidentalomas, 2003 to 2005: experience after publication of the National Institutes of Health consensus statement. Endocr Pract. 2008;14(3):279–284. [DOI] [PubMed] [Google Scholar]
- 14. Chiodini I, Albani A, Ambrogio AG, Campo M, De Martino MC, Marcelli G, Morelli V, Zampetti B, Colao A, Pivonello R; ABC Group. Six controversial issues on subclinical Cushing’s syndrome. Endocrine. 2017;56(2):262–266. [DOI] [PubMed] [Google Scholar]
- 15. Altieri B, Muscogiuri G, Paschou SA, Vryonidou A, Della Casa S, Pontecorvi A, Fassnacht M, Ronchi CL, Newell-Price J. Adrenocortical incidentalomas and bone: from molecular insights to clinical perspectives [published correction appears in Endocrine. 2018;62(3): 517–518]. Endocrine. 2018;62(3):506–516. [DOI] [PubMed] [Google Scholar]
- 16. Bruno OD, Juárez-Allen L, Rossi MA, Longobardi V. In what clinical settings should Cushing’s syndrome be suspected? Medicina (B Aires). 2009;69(6):674–680. [PubMed] [Google Scholar]
- 17. Guignat L, Bertherat J. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline: commentary from a European perspective. Eur J Endocrinol. 2010;163(1):9–13. [DOI] [PubMed] [Google Scholar]
- 18. Scaroni C, Zilio M, Foti M, Boscaro M. Glucose metabolism abnormalities in Cushing syndrome: from molecular basis to clinical management. Endocr Rev. 2017;38(3):189–219. [DOI] [PubMed] [Google Scholar]
- 19. Steffensen C, Pereira AM, Dekkers OM, Jørgensen JO. Diagnosis of endocrine disease: prevalence of hypercortisolism in type 2 diabetes patients: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(6):R247–R253. [DOI] [PubMed] [Google Scholar]
- 20. Costa DS, Conceição FL, Leite NC, Ferreira MT, Salles GF, Cardoso CR. Prevalence of subclinical hypercortisolism in type 2 diabetic patients from the Rio de Janeiro Type 2 Diabetes Cohort Study. J Diabetes Complications. 2016;30(6):1032–1038. [DOI] [PubMed] [Google Scholar]
- 21. Cansu GB, Atılgan S, Balcı MK, Sarı R, Özdem S, Altunbaş HA. Which type 2 diabetes mellitus patients should be screened for subclinical Cushing’s syndrome? Hormones (Athens). 2017;16(1):22–32 [DOI] [PubMed] [Google Scholar]
- 22. Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27(3):193–202. [DOI] [PubMed] [Google Scholar]
- 23. Martins LC, Conceição FL, Muxfeldt ES, Salles GF. Prevalence and associated factors of subclinical hypercortisolism in patients with resistant hypertension. J Hypertens. 2012;30(5):967–973. [DOI] [PubMed] [Google Scholar]
- 24. Kann P, Laudes M, Piepkorn B, Heintz A, Beyer J. Suppressed levels of serum cortisol following high-dose oral dexamethasone administration differ between healthy postmenopausal females and patients with established primary vertebral osteoporosis. Clin Rheumatol. 2001;20(1):25–29. [DOI] [PubMed] [Google Scholar]
- 25. Tannenbaum C, Clark J, Schwartzman K, Wallenstein S, Lapinski R, Meier D, Luckey M. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87(10):4431–4437. [DOI] [PubMed] [Google Scholar]
- 26. Eller-Vainicher C, Cairoli E, Zhukouskaya VV, Morelli V, Palmieri S, Scillitani A, Beck-Peccoz P, Chiodini I. Prevalence of subclinical contributors to low bone mineral density and/or fragility fracture. Eur J Endocrinol. 2013;169(2):225–237. [DOI] [PubMed] [Google Scholar]
- 27. Chiodini I, Mascia ML, Muscarella S, Battista C, Minisola S, Arosio M, Santini SA, Guglielmi G, Carnevale V, Scillitani A. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med. 2007;147(8):541–548. [DOI] [PubMed] [Google Scholar]
- 28. Findling JW, Raff H, Aron DC. The low-dose dexamethasone suppression test: a reevaluation in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2004;89(3):1222–1226. [DOI] [PubMed] [Google Scholar]
- 29. Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem. 1997;34(3):222–229. [DOI] [PubMed] [Google Scholar]
- 30. Sasaki Y, Katabami T, Asai S, Fukuda H, Tanaka Y. In the overnight dexamethasone suppression test, 1.0 mg loading is superior to 0.5 mg loading for diagnosing subclinical adrenal Cushing’s syndrome based on plasma dexamethasone levels determined using liquid chromatography-tandem mass spectrometry. Endocr J. 2017;64(9):833–842. [DOI] [PubMed] [Google Scholar]
- 31. Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab. 1971;32(2):266–284. [DOI] [PubMed] [Google Scholar]
- 32. Guignard MM, Pesquies PC, Serrurier BD, Merino DB, Reinberg AE. Circadian rhythms in plasma levels of cortisol, dehydroepiandrosterone, delta 4-androstenedione, testosterone and dihydrotestosterone of healthy young men. Acta Endocrinol (Copenh). 1980;94(4):536–545. [DOI] [PubMed] [Google Scholar]
- 33. Sandouk Z, Johnston P, Bunch D, Wang S, Bena J, Hamrahian A, Kennedy L. Variability of late-night salivary cortisol in Cushing disease: a prospective study. J Clin Endocrinol Metab. 2018;103(3):983–990. [DOI] [PubMed] [Google Scholar]
- 34. Masserini B, Morelli V, Bergamaschi S, Ermetici F, Eller-Vainicher C, Barbieri AM, Maffini MA, Scillitani A, Ambrosi B, Beck-Peccoz P, Chiodini I. The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur J Endocrinol. 2009;160(1):87–92. [DOI] [PubMed] [Google Scholar]
- 35. Ceccato F, Barbot M, Albiger N, Antonelli G, Zilio M, Todeschini M, Regazzo D, Plebani M, Lacognata C, Iacobone M, Mantero F, Boscaro M, Scaroni C. Daily salivary cortisol and cortisone rhythm in patients with adrenal incidentaloma. Endocrine. 2018;59(3):510–519. [DOI] [PubMed] [Google Scholar]
- 36. Nunes ML, Vattaut S, Corcuff JB, Rault A, Loiseau H, Gatta B, Valli N, Letenneur L, Tabarin A. Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab. 2009;94(2):456–462. [DOI] [PubMed] [Google Scholar]
- 37. Sereg M, Toke J, Patócs A, Varga I, Igaz P, Szücs N, Horányi J, Pusztai P, Czirják S, Gláz E, Rácz K, Tóth M. Diagnostic performance of salivary cortisol and serum osteocalcin measurements in patients with overt and subclinical Cushing’s syndrome. Steroids. 2011;76(1-2):38–42. [DOI] [PubMed] [Google Scholar]
- 38. Raff H. Utility of salivary cortisol measurements in Cushing’s syndrome and adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(10):3647–3655. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Q, Dou J, Gu W, Yang G, Lu J. Reassessing the reliability of the salivary cortisol assay for the diagnosis of Cushing syndrome. J Int Med Res. 2013;41(5):1387–1394. [DOI] [PubMed] [Google Scholar]
- 40. Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: comparison of patients and healthy volunteers. Psychoneuroendocrinology. 2004;29(5):636–650. [DOI] [PubMed] [Google Scholar]
- 41. Weibel L, Spiegel K, Follenius M, Ehrhart J, Brandenberger G. Internal dissociation of the circadian markers of the cortisol rhythm in night workers. Am J Physiol. 1996;270(4 Pt 1):E608–E613. [DOI] [PubMed] [Google Scholar]
- 42. Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92(3):819–824. [DOI] [PubMed] [Google Scholar]
- 43. Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83(8):2681–2686. [DOI] [PubMed] [Google Scholar]
- 44. Bansal V, El Asmar N, Selman WR, Arafah BM. Pitfalls in the diagnosis and management of Cushing’s syndrome. Neurosurg Focus. 2015;38(2):E4. [DOI] [PubMed] [Google Scholar]
- 45. Mayo Clinic. Rochester 2018 Interpretive Handbook. Available at: www.mayocliniclabs.com/test-catalog/pod/MayoTestCatalog-Rochester--SortedByTestName-duplex-interpretive.pdf. Accessed 8 January 2019.
- 46. Raff H, Auchus RJ, Findling JW, Nieman LK. Urine free cortisol in the diagnosis of Cushing’s syndrome: is it worth doing and, if so, how? J Clin Endocrinol Metab. 2015;100(2):395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kelly CJ, Ogilvie A, Evans JR, Shapiro D, Wallace AM, Davies DL. Raised cortisol excretion rate in urine and contamination by topical steroids. BMJ. 2001;322(7286):594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yorke E, Atiase Y, Akpalu J, Sarfo-Kantanka O. Screening for Cushing syndrome at the primary care level: what every general practitioner must know. Int J Endocrinol. 2017;2017:1547358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loriaux DL. Diagnosis and differential diagnosis of Cushing’s syndrome. N Engl J Med. 2017;376(15):1451–1459. [DOI] [PubMed] [Google Scholar]
- 50. Yener S, Yilmaz H, Demir T, Secil M, Comlekci A. DHEAS for the prediction of subclinical Cushing’s syndrome: perplexing or advantageous? Endocrine. 2015;48(2):669–676. [DOI] [PubMed] [Google Scholar]
- 51. Fischli S, Jenni S, Allemann S, Zwahlen M, Diem P, Christ ER, Stettler C. Dehydroepiandrosterone sulfate in the assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2008;93(2):539–542. [DOI] [PubMed] [Google Scholar]
- 52. Dennedy MC, Annamalai AK, Prankerd-Smith O, Freeman N, Vengopal K, Graggaber J, Koulouri O, Powlson AS, Shaw A, Halsall DJ, Gurnell M. Low DHEAS: a sensitive and specific test for the detection of subclinical hypercortisolism in adrenal incidentalomas. J Clin Endocrinol Metab. 2017;102(3):786–792 [DOI] [PubMed] [Google Scholar]
- 53. Kreitschmann-Andermahr I, Psaras T, Tsiogka M, Starz D, Kleist B, Siegel S, Milian M, Kohlmann J, Menzel C, Führer-Sakel D, Honegger J, Sure U, Müller O, Buchfelder M. From first symptoms to final diagnosis of Cushing’s disease: experiences of 176 patients. Eur J Endocrinol. 2015;172(3):285–289. [DOI] [PubMed] [Google Scholar]
- 54. Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, Wass JAH, Chabre O, Pfeifer M, Feelders RA, Tsagarakis S, Trainer PJ, Franz H, Zopf K, Zacharieva S, Lamberts SWJ, Tabarin A, Webb SM; ERCUSYN Study Group. The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165(3):383–392. [DOI] [PubMed] [Google Scholar]
- 55. Bolland MJ, Holdaway IM, Berkeley JE, Lim S, Dransfield WJ, Conaglen JV, Croxson MS, Gamble GD, Hunt PJ, Toomath RJ. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin Endocrinol (Oxf). 2011;75(4):436–442. [DOI] [PubMed] [Google Scholar]
- 56. Cipoli DE, Martinez EZ, Castro M, Moreira AC. Clinical judgment to estimate pretest probability in the diagnosis of Cushing’s syndrome under a Bayesian perspective. Arq Bras Endocrinol Metabol. 2012;56(9):633–637. [DOI] [PubMed] [Google Scholar]
- 57. Valassi E, Franz H, Brue T, Feelders RA, Netea-Maier R, Tsagarakis S, Webb SM, Yaneva M, Reincke M, Droste M, Komerdus I, Maiter D, Kastelan D, Chanson P, Pfeifer M, Strasburger CJ, Tóth M, Chabre O, Tabarin A, Krsek M, Fajardo C, Bolanowski M, Santos A, Wass JAH, Trainer PJ; ERCUSYN Study Group. Diagnostic tests for Cushing’s syndrome differ from published guidelines: data from ERCUSYN. Eur J Endocrinol. 2017;176(5):613–624. [DOI] [PubMed] [Google Scholar]
- 58. Friedman TC, Ghods DE, Shahinian HK, Zachery L, Shayesteh N, Seasholtz S, Zuckerbraun E, Lee ML, McCutcheon IE. High prevalence of normal tests assessing hypercortisolism in subjects with mild and episodic Cushing’s syndrome suggests that the paradigm for diagnosis and exclusion of Cushing’s syndrome requires multiple testing. Horm Metab Res. 2010;42(12):874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Bucy C, Guignat L, Niati T, Bertherat J, Coste J. Health-related quality of life of patients with hypothalamic-pituitary-adrenal axis dysregulations: a cohort study. Eur J Endocrinol. 2017;177(1):1–8. [DOI] [PubMed] [Google Scholar]
- 60. Papoian V, Biller BM, Webb SM, Campbell KK, Hodin RA, Phitayakorn R. Patients’ perception on clinical outcome and quality of life after a diagnosis of Cushing syndrome. Endocr Pract. 2016;22(1):51–67. [DOI] [PubMed] [Google Scholar]
- 61. Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A; Endocrine Society. Endocrine Society. Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]