Abstract
Background
Insulin via continuous intravenous infusion (ICII) is a standard of care for treating patients with diabetic ketoacidosis (DKA). Once DKA is resolved, ICII is transitioned to subcutaneous therapy. However, recent guidelines recommend continuation of home dose subcutaneous basal insulin (HDBI) in patients with DKA. The objective of this study was to evaluate outcomes in patients who received early vs delayed HDBI.
Methods
This is a retrospective cohort study of patients ≥16 years old admitted to the medical intensive care unit between 1 July 2012 and 30 June 2015 with a primary diagnosis of DKA who received ICII and HDBI. Patients were stratified into early or delayed groups if they received HDBI before or after resolution of DKA, respectively. The primary outcome was incidence of transitional failure, defined as resumption of ICII or recurrence of DKA after initial ICII discontinuation.
Results
A total of 106 admissions were included for analysis; 33 (31.1%) received early HDBI. The incidence of transitional failure was similar between the early and delayed groups (OR, 0.60; 95% CI, 0.26 to 1.44; P = 0.72). In the early group, ICII duration was shorter at 13.8 hours [interquartile range (IQR), 10.1 to 16.5] vs 17.1 hours (IQR, 12.6 to 21.1; P = 0.04), with a trend toward lower rates of hypoglycemia (OR, 0.41; 95% CI, 0.16 to 1.05; P = 0.058).
Conclusion
There was no significant difference in incidence of transitional failure between early and delayed HDBI. Early HDBI was associated with a shorter duration of ICII and a trend toward less hypoglycemia. A prospective analysis is needed to confirm these findings.
Keywords: insulin, diabetes, glargine, detemir, isophane, diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is a serious, life-threatening complication of diabetes mellitus. DKA is responsible for more than 500,000 hospital days per year at an estimated annual cost of 2.4 billion USD [1]. Treatment of patients with DKA requires correction of dehydration, hyperglycemia, ketoacidosis, and electrolyte imbalances as well as identification of comorbid precipitating events [1].
Insulin via continuous intravenous infusion (ICII) is the standard of care for treating patients with DKA. Insulin promotes glucose utilization and suppresses generation of additional ketones, which helps to correct acidosis and other metabolic complications of DKA [2]. After DKA is resolved, ICII is transitioned to subcutaneous insulin therapy. If the patient was previously on a stable dose of basal insulin, that dose is typically resumed [1].
Guidelines from several organizations address insulin use in DKA management [1, 3–7]. Although guidelines from the Joint British Diabetes Societies and the British Society of Pediatric Endocrinology and Diabetes suggest considering the continuation of home dose subcutaneous basal insulin (HDBI) in patients being treated acutely for DKA [4–6], there is no mention of this practice in the American Diabetes Association, Canadian Diabetes Association, or International Society for Pediatric and Adolescent Diabetes guidelines [1, 3, 7]. The evidence cited for the recommendation to continue HDBI in the British guidelines consists of expert opinion and one small randomized controlled trial that showed a decrease in rebound hyperglycemia using an early weight-based dose of basal insulin [8]. Fewer than 50% of patients in this trial were being treated for DKA. Additional studies evaluating the role of early basal insulin in children [9, 10] and adults [11, 12] with DKA have been performed, but they also used weight-based dosing regimens instead of continuing HDBI. The objective of this study was to evaluate DKA treatment outcomes in patients who received early vs delayed HDBI.
1. Methods
This retrospective cohort study was conducted in a 562-bed rural academic medical center in Burlington, Vermont. Following institutional review board approval, all patients admitted with a diagnosis of DKA between 1 July 2012 and 30 June 2015 were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Patients aged 16 years or older admitted to the medical intensive care unit (ICU) who received ICII and HDBI were included. Patients were excluded if they required surgery within 48 hours of ICII discontinuation, were pregnant, had vasopressor-dependent shock, or had another indication for insulin therapy (e.g., hypertriglyceridemia). Patients who did not meet criteria for early or delayed HDBI and those for whom ICII was discontinued before resolution of DKA were also excluded. Resolution of DKA was defined as blood glucose ≤250 mg/dL and two of the following: anion gap ≤12 mEq/L, serum bicarbonate ≥15 mmol/L, or venous or arterial pH >7.3. This definition is based on criteria for resolution of DKA from the American Diabetes Association (ADA), with a different blood glucose threshold due to institutional guidelines [1]. Patients from outside hospitals were included if they met diagnostic criteria for DKA per the ADA on transfer [1]. Patients who were admitted on a home continuous subcutaneous delivery system were included if the system was discontinued on admission, if there was sufficient documentation in the medical record to calculate their home basal dose, and if inpatient HDBI was started as a long- or intermediate-acting subcutaneously delivered preparation. Multiple admissions per patient were allowed.
Basal insulin was considered HDBI if the first 24 hours of long- or intermediate-acting subcutaneously administered insulin was within 25% of the patient’s total daily home basal insulin dose. In the instance of premixed insulin, only the intermediate-acting component counted toward total daily basal dose. Early HDBI was defined as initiation of basal insulin within 24 hours of the initiation of ICII and before resolution of DKA. Delayed HDBI was defined as initiation of therapy with the above-mentioned insulins that did not meet early criteria and within 6 hours before, or any time after, discontinuation of ICII.
The primary outcome was incidence of transitional failure between groups. Transitional failure was defined as the resumption of ICII or recurrence of DKA between 2 and 12 hours after initial discontinuation of ICII. Up to 2 hours off ICII was allowed due to our institutional DKA protocol in cases of hypokalemia or hypoglycemia. Resumption of ICII before this 2-hour time point resulted in transitional failure if the original ICII order was discontinued and if there was clear documentation that a transition to HDBI was attempted. Resumption of ICII after the 2-hour point was allowed in cases where the original ICII order was not discontinued and there was clear documentation that the infusion was being held due to hypokalemia or hypoglycemia. Recurrence of DKA was defined as blood glucose ≥250 mg/dL and two of the following: calculated anion gap >12 mEq/L, serum bicarbonate <15 mmol/L, or venous or arterial pH <7.3. Our institutional DKA protocol is based on the ADA consensus statement on treatment of hyperglycemic crises in adult patients [1]. We target an initial hourly blood glucose decrease of 60 to 125 mg/dL and increase the ICII infusion rate if this target is not met. Our protocol does not address the transition from ICII to subcutaneous insulin; this is left to the discretion of the provider.
Secondary outcomes included incidence of rebound hyperglycemia, incidence of hypoglycemia, number of finger stick blood glucose (FSBG) measurements in the first 24 hours, time to anion gap ≤12 mEq/L, duration and maximum rate of ICII, and lengths of stay in the medical ICU and hospital. Rebound hyperglycemia was defined as any blood glucose level >200 mg/dL in the 24 hours after discontinuation of ICII. Patients in whom ICII was discontinued with blood glucose >200 mg/dL were not included in this outcome. Hypoglycemia was defined as any blood glucose <70 mg/dL during ICII therapy or in the 24 hours after discontinuation. Both venous and capillary samples were evaluated when assessing blood glucose.
Demographic information, baseline comorbid status using the age-adjusted Charlson Comorbidity Index [13, 14]; severity of illness as measured by the Simplified Acute Physiology Score II [15]; and information on inpatient management such as insulin, intravenous fluid, steroid, and antibiotic therapy, laboratory values, length of stay, inpatient mortality, and precipitating factors were collected by electronic and manual data extraction. The Charlson Comorbidity Index was calculated using ICD-9-CM codes and a coding algorithm developed by Quan et al. [14]. If a laboratory value was below or above the limit of detection (e.g., serum bicarbonate <5 mmol/L or β-hydroxybutyrate >9 mmol/L), the limit of detection was used. Resuscitation fluid was defined as the type of fluid that constituted the majority of the first 2 L of documented intravenous intake. Balanced crystalloid resuscitation included use of lactated Ringer’s, PlasmaLyteTM-A (Baxter, Deerfield, IL) or NormosolTM-R (Hospira, Inc., Lake Forest, IL) solutions.
Descriptive statistics were used to summarize demographic information, inpatient management, and initial laboratory values. For the primary and secondary outcomes, univariate analysis was used to determine unadjusted ORs and associated 95% CIs. Categorical variables were compared among groups using Fisher exact test. Continuous variables were compared among groups using a two-group t test or Mann-Whitney U test if data were not normally distributed. The significance threshold was set at P < 0.05. All analyses were completed using SPSS (SPSS Statistics for Windows, Version 22.0; IBM Corp., Armonk, NY).
2. Results
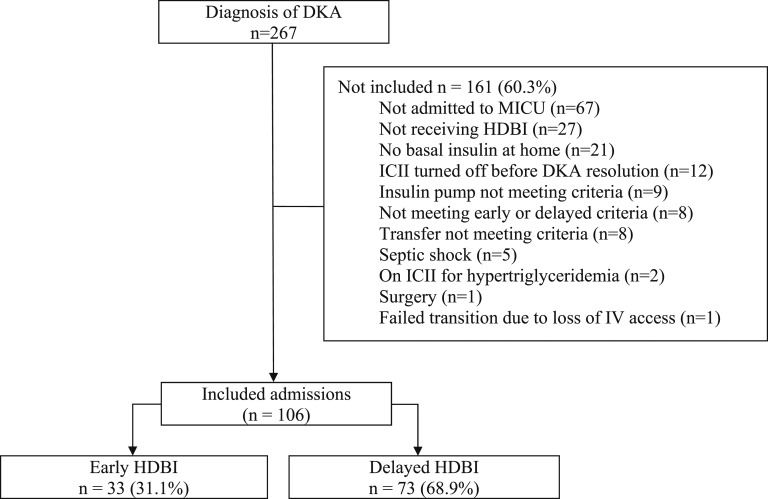
A search of hospital electronic health records yielded 267 DKA admissions. Of these, 106 (40%) admissions from 57 patients were included in the final analysis (Fig. 1). Admission to a unit other than medical ICU (42%) and not having received HDBI (16.7%) were the most common reasons for noninclusion. The median patient age within the cohort was 29 years (range, 18 to 92 years), and 36 (34%) were male. The groups were similar at baseline, with a higher body mass index and higher incidence of balanced crystalloid resuscitation in the early group. Additionally, a higher percentage of patients received early HDBI during the latter 2 years of data collection (Table 1).
Figure 1.
Flowchart of patients who met inclusion/exclusion criteria for the study population.
Table 1.
Demographic and Initial Laboratory Values
| Variable | Early (n = 33) | Delayed (n = 73) |
|---|---|---|
| Age, y | 31 (23–43) | 28 (23–49) |
| Male | 14 (42) | 22 (30) |
| White | 32 (97) | 72 (99) |
| BMI, kg/m2a | 25.9 (23.5–29.7) | 23.7 (21.5–26.2) |
| Charlson comorbidity index | 2 (1–3) | 2 (1–3.5) |
| Admission yeara | ||
| 2015 | 15 (45) | 7 (10) |
| 2014 | 15 (45) | 22 (30) |
| 2013 | 2 (6) | 31 (42) |
| 2012 | 1 (3) | 13 (18) |
| Home basal insulin | ||
| Insulin glargine | 27 (82) | 48 (66) |
| Insulin detemir | 1 (3) | 6 (8) |
| Intermediate-acting insulin | 4 (12) | 17 (23) |
| Insulin pump | 1 (3) | 2 (3) |
| Home TDD basal insulin, units | 36 (24.5–41) | 31 (20–43) |
| Documented noncompliance | 15 (45) | 26 (36) |
| Admission laboratory values | ||
| Anion gap, mEq/Lb | 28 (24–32) | 30 (24–35) |
| Bicarbonate, mEq/L | 9 (5–13.5) | 7 (5–11.5) |
| Glucose, mg/dL | 558 (444–743) | 503 (370–736) |
| pH | 7.25 (7.00–7.34) | 7.14 (7.04–7.27) |
| BHB, mmol/L | 7.4 (5.4–9) | 8.7 (6.3–9) |
| HbA1c, %b | 10.7 (9.5–12.4) | 11.5 (9.9–12.7) |
| Lactate, mmol/L | 2.0 (1.3–3.2) | 2.2 (1.6–3.4) |
Data are reported as n (%) or median (interquartile range).
Abbreviations: BHB, β-hydroxybutyrate; BMI, body mass index; SAPS II, Simplified Acute Physiology Score; TDD, total daily dose.
P < 0.05 by Fisher exact test or Mann-Whitney U test for categorical and continuous variables, respectively.
Normally distributed continuous variables were analyzed with a two-group t test.
A similar number of patients in the early and delayed groups received an intravenous insulin bolus prior to ICII initiation (55 vs 48%; P = 0.68). Early and delayed groups had similar initial ICII rates of 0.1 U/kg/h [interquartile range (IQR), 0.07 to 0.11] vs 0.09 U/kg/h (IQR, 0.05 to 0.1; P = 0.21), initial daily doses of basal insulin of 0.48 U/kg (IQR, 0.34 to 0.62) vs 0.44 U/kg (IQR, 0.31 to 0.59; P = 0.56), and maximum ICII rates of 0.13 U/kg/h (IQR, 0.1 to 0.19) vs 0.12 U/kg/h (IQR, 0.09 to 0.15; P = 0.32). Good separation of cohorts was achieved because patients in the early group received their first dose of basal insulin 2.5 hours (IQR, 0.4 to 6.4) after ICII initiation, whereas the delayed group received their first dose of basal insulin 15.6 hours after ICII initiation (IQR, 11.0 to 19.6; P < 0.001). Total volume administered in the first 24 hours was not significantly different between early and delayed groups at 6.6 L (IQR, 4.5 to 9.5) vs 6.8 L (IQR, 5.2 to 8.8; P = 0.82).
There was no significant difference in the rates of transitional failure between the early and delayed HDBI groups (6 vs 10%, P = 0.717) (Table 2). Most patients (89%) failed the transition due to resumption of ICII after initial discontinuation. No patient who resumed ICII had rebound DKA. Early HDBI was associated with less time on ICII and favorable trends toward reduced hypoglycemia, fewer interruptions of ICII due to hypoglycemia, and fewer FSBG checks. When severe hypoglycemia, defined as any blood glucose <40 mg/dL, was evaluated, there was no significant difference between the early and delayed groups (12 vs 10%; P = 0.74). Rebound hyperglycemia was similarly common between groups, with an 86% incidence in evaluable patients, and there was no significant difference in median time to hyperglycemia between the early and delayed groups (6.4 vs 4.2 hours; P = 0.15). There were no significant differences between groups in any other secondary outcomes. Due to a lower-than-expected primary outcome event rate, a preplanned multivariate analysis was not performed.
Table 2.
Comparison of Outcomes by Group
| Early (n = 33) | Delayed (n = 73) | ORa (95% CI)a | |
|---|---|---|---|
| Transitional failure | 2 (6) | 7 (10) | 0.61 (0.06–3.47) |
| Resume ICII | 2 (6) | 6 (8) | 0.72 (0.07–4.3) |
| DKA recurrence | 0 (0) | 1 (1) | — |
| Rebound hyperglycemiab | 26 (81) | 59 (88) | 0.59 (0.16–2.29) |
| All hypoglycemia | 11 (33) | 40 (55) | 0.41 (0.16–1.05) |
| Hypoglycemia on ICII | 6 (18) | 23 (32) | 0.48 (0.14–1.43) |
| ICII held for hypoglycemia | 2 (6) | 17 (23) | 0.21 (0.02–1.003) |
| P Valuec | |||
|---|---|---|---|
| Time to anion gap closure, h | 7.8 (4.8–12.5) | 9.1 (5.7–10.8) | 0.73 |
| Time on ICII, h | 13.8 (9.5–16.9) | 17.1 (12.4–21.2) | 0.04 |
| FSBG in first 24 h, n | 18 (15.5–20) | 20 (17.5–22) | 0.06 |
| Length of stay | |||
| ICU, h | 34.0 (20.4–48.9) | 33.3 (22.7–42.8) | 0.83 |
| Hospital, d | 2 (2–3) | 2 (2–3) | 0.90 |
Data are reported as n (%) or median (interquartile range).
Calculated using Fisher exact test.
Total evaluable patients: 32 in the early group, 67 in the delayed group.
P values were calculated using a Mann-Whitney U test.
3. Discussion
Guidelines from the Joint British Diabetes Societies Inpatient Care Group and the British Society for Pediatric Endocrinology and Diabetes suggest consideration be given to continuation of HDBI during the initial management of DKA. The evidence base for these recommendations consists of weight-based dosing of insulin glargine [8, 10–12]. No previous study has evaluated the impact of following these guideline recommendations.
Our retrospective evaluation of early vs delayed HDBI is an important study of continuing basal insulin at patients’ home doses in acute DKA. Early HDBI failed to show a significant difference in the rate of transitional failure. However, we demonstrated that use of early HDBI was associated with shorter duration of ICII and trends toward reduced incidence of hypoglycemia, fewer interruptions of ICII due to hypoglycemia, and fewer FSBG checks.
Previous studies evaluating early subcutaneous basal insulin in the acute management of adult patients with DKA receiving ICII have been performed [8, 11, 12]. Hsia et al. [8] performed a prospective randomized trial evaluating early insulin glargine in 61 patients with diabetes requiring ICII. Patients randomized to early insulin glargine received a dose of 0.25 U/kg within 12 hours of ICII initiation. Only 41% of the patients had DKA, with another 41% treated after solid organ transplantation. They observed high rates of rebound hyperglycemia in the control group, with 93.5% of patients with at least one glucose value >180 mg/dL during the 12-hour follow-up period. The rate of rebound hyperglycemia in the intervention group was significantly lower at 33%. Three asymptomatic hypoglycemic measurements occurred in two control patients and no patients in the intervention group. Houshyar et al. [12] executed a prospective randomized trial evaluating an early weight-based dose of insulin glargine in the management of 40 adult patients with DKA. Intervention patients received 0.4 U/kg of insulin glargine within 3 hours of ICII initiation. Notably, 40% of patients had newly diagnosed diabetes and baseline diabetes control was poor, with an average HbA1c of 12.5%. The authors noted trends toward faster recovery from acidosis and a reduction in the amount of required insulin. Rebound hyperglycemia, defined as the percentage of blood glucose readings in the first 24 hours >150 mg/dL, was significantly lower in the intervention group than in the control group (35% vs 51%). Hypoglycemia occurred in nine patients overall, with no difference between groups. Doshi et al. [11] studied the effect of dosing insulin glargine in the acute management of DKA in the emergency department. In this prospective pilot study, 40 patients were randomized to ICII alone (control group) or to ICII plus 0.3 U/kg insulin glargine (intervention group). There were no significant differences in outcomes, including time to closure of anion gap and hospital and ICU length of stay. Hypoglycemia, defined as blood glucose ≤60 mg/dL during 24 hours after anion gap closure, occurred in 13% of patients. Rebound hyperglycemia was not evaluated.
Two studies of early long-acting insulin have been performed in the pediatric population [9, 10]. Shankar et al. [9] retrospectively evaluated 12 children with DKA who received 0.3 U/kg of insulin glargine in the first 6 hours of management and compared them with 59 control subjects. All patients received ICII. They found a shorter time to acidosis correction, a shorter duration of ICII, and a trend toward shorter hospital stay. The authors noted no episodes of symptomatic or asymptomatic hypoglycemia in either group; the rate of rebound hyperglycemia was not assessed. Harrison et al. [10] also retrospectively evaluated 55 pediatric admissions aged 2 to 21 years who received >4 hours of coadministered subcutaneous glargine and ICII and compared them with 94 patients whose overlap was <4 hours. The duration of DKA and ICII was longer and hypokalemia more frequent in the patients with early coadministered glargine. Incidence of hypoglycemia was similar between groups. Neither study included information about home insulin dose.
Our finding of decreased duration of ICII is consistent with the literature [9, 12]. We were unable to replicate previous findings of decreased time to closure of anion gap and decreased rebound hyperglycemia [8, 9, 11]. We discovered high rates of hyperglycemia in both patient groups. However, our criterion for hyperglycemia was strict. Various other studies describe rebound hyperglycemia using percentages of elevated blood glucose readings and time in target glucose concentrations [12, 16]. Our rate of rebound hyperglycemia was similar to the control group studied by Hsia et al. [8], whose definition closely matched ours.
Higher-than-expected rates of hypoglycemia were also seen in our patient population, even with the trend favoring early HDBI. We evaluated the maximum rate of ICII in our population, but there was no difference between groups. It is possible that a shorter duration of ICII led to less opportunity for hypoglycemia; however, our study was not designed to investigate this association, and it remains hypothesis-generating only. All five trials of early insulin glargine with ICII in DKA described lower rates of hypoglycemia than our study, despite similar definitions [8–12]. Three of these studies detailed their insulin treatment protocol for DKA [9–11]. In all three protocols, insulin infusion was started at 0.1 U/kg/h, and the rate was not typically adjusted. This is in contrast to our DKA protocol, where the ICII rate was increased if the hourly decrease in blood glucose was <60 mg/dL. Our DKA protocol also targeted an initial hourly blood glucose decrease of 60 to 125 mg/dL, which is higher than suggested by the current ADA DKA guidelines [1].
Demographically, our patient population was broadly representative of patients with DKA with the exception that we enrolled only two nonwhite patients. This is representative of the population of Vermont [17]. Otherwise, our sample matches other studies in age distribution, sex dispersion, insurance coverage, and rate of nonadherence [1, 12, 18–21].
This study has several limitations. Due to its retrospective nature, patients were not randomized to receive early or delayed HDBI, and collected data were limited to those recorded in the electronic health record. This was a single-center study, and DKA protocols may vary between institutions. It is possible that inconsistent timing of HDBI administration, variable protocol compliance, and a nonstandard transition from ICII to subcutaneous insulin confounded our results. Although our DKA protocol remained consistent through the study period, practice change occurred, as evidenced by increasing usage of early HDBI and balanced crystalloid resuscitation in later years. Our study was also not designed to test for protocol compliance. Our providers do not order the protocol as a single order but select an order set in which doses can be modified or omitted. For example, the distribution of initial ICII rates indicates that some providers replace 0.1 U/kg/h with lower or higher rates of infusion. Finally, we were unable to account for carbohydrate intake in the early stages of DKA due to limitations in documentation. It may prove difficult to adequately power a study examining the benefits of early HDBI in patients with DKA. Doshi et al. [11] predicted a trial of 1120 patients would be needed to detect a treatment difference in time to closure of anion gap. In our trial, if the difference in transitional failure was the result of the intervention and not due to chance, a trial of 720 patients would be needed to detect a treatment difference, assuming a 5% significance level and 80% power.
In summary, we performed a retrospective chart review evaluating DKA treatment outcomes in patients who received early vs delayed HDBI. There was no significant difference in incidence of transitional failure between early and delayed HDBI. Early HDBI was associated with less time on ICII and with a trend toward lower rates of hypoglycemia. A large, multicenter prospective analysis is needed to confirm these exploratory findings.
Acknowledgments
We thank Allison M. Kaigle Holm, Senior Research Specialist, and Deirdre LaFrance, Senior Measurement Analyst, both at the Jeffords Institute for Quality & Operational Effectiveness, The University of Vermont Medical Center for research and data analytics support and Takamaru Ashikaga, Biostatistics Core Facility, The University of Vermont Cancer Center.
Financial Support: This work was supported by grants from the American College of Clinical Pharmacy (to S.R. and J.A.E.).
Disclosure Summary: S.R. and J.A.E. received support from the American College of Clinical Pharmacy Critical Care PRN through the Resident Research Grant. M.G. has worked as a consultant for Novo Nordisk and Sanofi, USA.
Glossary
Abbreviations:
- ADA
American Diabetes Association
- DKA
diabetic ketoacidosis
- FSBG
finger stick blood glucose
- HDBI
home dose subcutaneous basal insulin
- ICII
insulin via continuous intravenous infusion
- ICU
intensive care unit
- IQR
interquartile range
References and Notes
- 1. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gosmanov AR, Gosmanova EO, Dillard-Cannon E. Management of adult diabetic ketoacidosis. Diabetes Metab Syndr Obes. 2014;7:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goguen J, Gilbert J; Diabetes Canada Clinical Practice Guidelines Expert Committee. Hyperglycemic emergencies in adults. Can J Diabetes. 2018;42(Suppl 1):S109–S114. [DOI] [PubMed] [Google Scholar]
- 4. British Society for Paediatric Endocrinology and Diabetes. Guideline for the management of children and young people under the age of 18 years with diabetic ketoacidosis 2015. Available at: www.bsped.org.uk/media/1381/dkaguideline.pdf. Updated 26 August 2015. Accessed 20 April 2018. [DOI] [PubMed]
- 5. Joint British Diabetes Societies Inpatient Care Group. Joint British Diabetes Societies Inpatient Care Group for NHS Diabetes, 2013. The management of diabetic ketoacidosis in adults. 2nd ed. Available at: www.diabetes.org.uk/Documents/About%20Us/What%20we%20say/Management-of-DKA-241013.pdf. Updated September, 2013. Accessed 20 April 2018.
- 6. Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JA, Courtney CH, Hilton L, Dyer PH, Hamersley MS; Joint British Diabetes Societies. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabet Med. 2011;28(5):508–515. [DOI] [PubMed] [Google Scholar]
- 7. Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser N, Jain V, Lee WW, Mungai LN, Rosenbloom AL, Sperling MA, Hanas R; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl 20):154–179. [DOI] [PubMed] [Google Scholar]
- 8. Hsia E, Seggelke S, Gibbs J, Hawkins RM, Cohlmia E, Rasouli N, Wang C, Kam I, Draznin B. Subcutaneous administration of glargine to diabetic patients receiving insulin infusion prevents rebound hyperglycemia. J Clin Endocrinol Metab. 2012;97(9):3132–3137. [DOI] [PubMed] [Google Scholar]
- 9. Shankar V, Haque A, Churchwell KB, Russell W. Insulin glargine supplementation during early management phase of diabetic ketoacidosis in children. Intensive Care Med. 2007;33(7):1173–1178. [DOI] [PubMed] [Google Scholar]
- 10. Harrison VS, Rustico S, Palladino AA, Ferrara C, Hawkes CP. Glargine co-administration with intravenous insulin in pediatric diabetic ketoacidosis is safe and facilitates transition to a subcutaneous regimen. Pediatr Diabetes. 2017;18(8):742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doshi P, Potter AJ, De Los Santos D, Banuelos R, Darger BF, Chathampally Y. Prospective randomized trial of insulin glargine in acute management of diabetic ketoacidosis in the emergency department: a pilot study. Acad Emerg Med. 2015;22(6):657–662. [DOI] [PubMed] [Google Scholar]
- 12. Houshyar J, Bahrami A, Aliasgarzadeh A. Effectiveness of insulin glargine on recovery of patients with diabetic ketoacidosis: a randomized controlled trial. J Clin Diagn Res. 2015;9(5):OC01–OC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- 14. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 15. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. [DOI] [PubMed] [Google Scholar]
- 16. Kanji S, Singh A, Tierney M, Meggison H, McIntyre L, Hebert PC. Standardization of intravenous insulin therapy improves the efficiency and safety of blood glucose control in critically ill adults. Intensive Care Med. 2004;30(5):804–810. [DOI] [PubMed] [Google Scholar]
- 17. United States Census Bureau. QuickFacts Vermont; United States. Available at: www.census.gov/quickfacts/fact/table/VT,US/RHI225216. Accessed 20 April 2018.
- 18. Maldonado MR, Chong ER, Oehl MA, Balasubramanyam A. Economic impact of diabetic ketoacidosis in a multiethnic indigent population: analysis of costs based on the precipitating cause. Diabetes Care. 2003;26(4):1265–1269. [DOI] [PubMed] [Google Scholar]
- 19. Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med. 1997;157(6):669–675. [PubMed] [Google Scholar]
- 20. Kim S. Burden of hospitalizations primarily due to uncontrolled diabetes: implications of inadequate primary health care in the United States. Diabetes Care. 2007;30(5):1281–1282. [DOI] [PubMed] [Google Scholar]
- 21. Kitabchi AE, Murphy MB, Spencer J, Matteri R, Karas J. Is a priming dose of insulin necessary in a low-dose insulin protocol for the treatment of diabetic ketoacidosis? Diabetes Care. 2008;31(11):2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]