Abstract
Context
Previous studies have shown reduced placental levels of 11-β-hydroxysteroid dehydrogenase type 2 (11βHSD2) in preeclampsia (PE). However, it is unknown if the maternal cortisol-to-cortisone ratio is predictive of placental complications of pregnancy.
Objective
To determine the relationship between the maternal serum cortisol-to-cortisone ratio at different stages of pregnancy and the risk of PE or fetal growth restriction (FGR).
Design
Women from the Pregnancy Outcome Prediction Study experiencing PE (n = 194) or FGR (n = 185), plus a random sample of healthy controls (n = 279), were studied. Steroids were measured at ∼12, ∼20, ∼28, and ∼36 weeks of gestational age (wkGA). Separate analyses were performed for outcomes with term or preterm delivery. Associations were modeled using logistic regression.
Results
At 28 wkGA, the cortisol-to-cortisone ratio was negatively associated (OR per 1 SD increase, 95% CI)] with preterm PE (OR 0.33, 95% CI 0.19 to 0.57), term PE (OR 0.61, 95% CI 0.49 to 0.76), and preterm FGR (OR 0.50, 95% CI 0.29 to 0.85). At 36 wkGA, the cortisol-to-cortisone ratio was negatively associated with term PE (OR 0.42, 95% CI 0.32 to 0.55) but not term FGR (OR 1.07, 95% CI 0.87 to 1.31). Associations were not materially affected by adjustment for maternal characteristics.
Conclusions
A lower maternal serum cortisol-to-cortisone ratio precedes clinical manifestation of PE and preterm FGR by many weeks, despite previous reports of reduced levels of placental 11βHSD2 in these conditions. Our observations implicate enhanced maternal 11βHSD2 activity or reduced 11βHSD type 1 activity in the pathophysiology of PE.
A lower maternal cortisol-to-cortisone ratio precedes clinical diagnosis of preterm and term preeclampsia.
Preeclampsia (PE) and fetal growth restriction (FGR) are poorly understood conditions of pregnancy associated with substantial perinatal morbidity and mortality. PE is a disorder of placental origin, characterized by hypertension and proteinuria that develops during the antenatal or immediate postpartum period (1). It affects between 2% and 8% of pregnancies and is a major cause of direct maternal death worldwide (2, 3). It is often accompanied by FGR, defined as a fetus not reaching its genetically determined growth potential (4) as a result of poor placental function. Currently, there are no effective management options available, apart from delivery, and accurate early prediction of the disease is not possible.
One marker of placental function that might be affected in PE is the maternal serum cortisol-to-cortisone ratio (5). 11-β-Hydroxysteroid dehydrogenase type 2 (11βHSD2) is a steroid enzyme highly expressed in the placenta and with the metabolization of cortisol to cortisone, protects the fetus from exposure to excessive maternal cortisol during pregnancy, whereas 11βHSD type 1 11βHSD1 (11BHSD1) performs the reverse of 11βHSD2 and reactivates cortisol by converting cortisone to cortisol (6). Several studies have reported that both PE and FGR are associated with reduced levels of placental 11βHSD2 (7–15) and reduced expression and activity of placental 11βHSD1 have been demonstrated in cases of small for gestational age (SGA) (16, 17). Reduced 11βHSD2 activity would be expected to result in an increased maternal serum cortisol-to-cortisone ratio; hence, these findings suggest that the maternal serum cortisol-to-cortisone ratio may be used as a predictor or marker of disease. A small number of studies previously addressed this question and paradoxically, reported that women with PE exhibited lower cortisol-to-cortisone ratios, suggesting increased maternal 11βHSD2 activity (18–20). However, these analyses were limited to samples obtained following diagnosis and consisted of studies that had not included >14 cases of confirmed PE and 14 cases of confirmed FGR per study. The aim of the current study was to investigate the relationship between maternal cortisol and cortisone levels with PE and FGR before the onset of disease, using measurements taken at different time points in pregnancy in a large number of optimally characterized cases and controls.
Materials and Methods
Study design
We used a case-cohort design from the Pregnancy Outcome Prediction (POP) Study. Details of the study are provided elsewhere (21), but in brief, it was a prospective cohort study that collected blood, ultrasound scan, and pregnancy outcome data from 4212 nulliparous women at ∼12, ∼20, ∼28, and ∼36 weeks of gestational age (wkGA) between 14 January 2008 and 31 July 2012 (22, 23). The case cohort included 379 women with cases of PE and FGR and a random sample of the total cohort (n = 325) (24). Out of the 325 women in the random subcohort, 279 did not experience any adverse outcome and are referred to as “healthy.”
Definitions of outcomes studied
For this analysis, we studied women with PE or FGR and divided them into preterm and term subgroups based on gestational age at delivery (<37 wkGA and ≥37 wkGA, respectively). PE was diagnosed according to the American College of Obstetricians and Gynecologists (ACOG) 2013 Guidelines (25), and the cases in this analysis included all severe PE at term; nonsevere, nonsuperimposed PE at term; and all preterm PE. PE cases with preterm delivery were compared with all of the women in the subcohort who did not experience PE leading to preterm birth. Term PE cases were compared with women from the subcohort who delivered at term without experiencing any type of PE. Preterm FGR was defined as SGA <10th customized percentile (26) with delivery <37 wkGA. Preterm FGR cases were compared with women in the subcohort who did not deliver a preterm infant with FGR. We excluded preterm cases of PE from analyses of preterm FGR. FGR at term was defined as severe SGA (birth weight less than third customized percentile) or SGA with growth restriction (birth weight <10th customized percentile and reduced growth velocity of the abdominal circumference on serial ultrasound scans) with delivery ≥37 wkGA. Term FGR cases were compared with women in the subcohort who delivered an infant at term without FGR. In addition to preterm births, we excluded all types of term PE from analyses of term FGR. Exclusion of preterm births from the analysis of term outcome was done, as women delivering preterm are necessarily not at risk for complications at term, and the exclusion of PE from the analysis of FGR was made to separate better the two complications, which can be overlapping, especially at preterm gestational ages.
Outcomes were collected from paper-based hospital records and relevant electronic databases. Maternal characteristics [age, marital status, ethnicity, smoking, age at leaving full-time education (FTE)] were self-reported, except for height and body mass index (BMI), which were measured at the time of recruitment, and deprivation, which was measured using the Index of Multiple Deprivation 2007 (27), calculated from the woman’s postcode.
Steroid measurements
Steroid levels were measured in maternal serum at Metabolon, Inc. (Durham, NC) by nontargeted, ultra-HPLC and tandem mass spectrometry. Samples were run in batches of 36, and all batches contained an equal proportion of cases and controls. All samples from a given participant were run in the same batch. Peaks were quantified using area under the curve of primary mass spectrometry ions. Missing values were assumed to be the result of falling below the detection sensitivity and thus, were imputed with the minimum detection value based on each metabolite. In the entire POP Study, where 3196 samples were successfully processed, only one cortisol value and two cortisone values were missing (<0.1%). For this analysis, only one woman in the random subcohort had a missing cortisol and cortisone value, and this was at 12 wkGA. To adjust for instrument batch effects for each run day, the raw ion counts for each steroid were divided by the median value for the run day. Internal standards were not available for all metabolites, so values were not quantified in standard International System of Units. Hence, cortisol and cortisone levels were expressed as multiples of the median (MoM) (28).
Statistical analyses
The cortisol-to-cortisone ratio for a given sample was generated by division of the MoM for cortisol by the MoM for cortisone. Initial descriptive analyses were then conducted by the summarization of cortisol, cortisone, and the cortisol-to-cortisone ratio for each time of measurement (12, 20, 28, and 36 wkGA) in healthy pregnancies and women with PE or FGR. Linear regression analyses for cortisone against cortisol were performed in cases and controls, and P values for interaction between these groups were calculated. We excluded the 36-wkGA measurement from all the analyses of preterm delivery. The MoM values for the given hormone and their ratio at the given time of sampling were converted to Z scores, using the subcohort as the reference. Unadjusted logistic regressions were performed to analyze their associations with the outcomes. These analyses were repeated, adjusting for maternal characteristics (age at recruitment, age left FTE, height, BMI, smoking status, deprivation, ethnicity, marital status). Missing values were imputed using the mode for categorical variables and mean for continuous variables.
Time-to-event analyses (29) were then conducted for term PE from the 36-wkGA measurement onward and preterm PE from the 28-wkGA measurement onward. We studied the whole case-cohort sample, weighting the noncases of the comparator group by the inverse of the sampling fraction (30). We compared the cumulative incidence of PE between women with a cortisol-to-cortisone ratio in the first decile (using a threshold derived from the random subcohort) with women in the second to 10th deciles. Delivery without PE was considered a competing risk.
Finally, we performed a sensitivity analysis confined to women, where it was documented that they had not received steroids antenatally (either for fetal lung maturation or medical conditions, such as asthma). As the research database only started recording information on whether women had received steroids to accelerate fetal lung maturation from 27 November 2009 onward, the majority of women excluded in the sensitivity analysis were omitted because the data were absent, rather than they were documented as receiving steroids. Where the apparent statistical significance of an association between an outcome and the cortisol-to-cortisone ratio differed in the sensitivity analysis, we tested for an interaction between the ratio and antenatal steroid use to determine if there was a true difference between the groups or whether the P value became nonsignificant as a result of the reduced sample size in the subgroup.
Statistical software
All analyses were performed in Stata version 14.0 (StataCorp, College Station, TX).
Ethics approval
All patients gave their informed, written consent to participate, and the study was approved by the Cambridgeshire 2 Research Ethics Committee (Reference Number 07/H0308/163).
Results
Maternal characteristics
There were 878, 873, 854, and 591 women with steroid measurements at mean (±SD) gestational ages 89 (±6), 143 (±3), 198 (±3), and 254 (±3) days, respectively. These are reported as measurements at 12, 20, 28, and 36 wkGA. Maternal characteristics of the women studied are summarized in Table 1. There were 29 cases of preterm PE, 160 cases of PE at term, 25 cases of preterm FGR, and 165 cases of FGR at term in the case cohort for the main analysis. The sensitivity analysis included 12 cases of preterm PE, 123 cases of PE at term, 15 cases of preterm FGR, and 125 cases of FGR at term, and 251 women remained in the random subcohort.
Table 1.
Maternal Characteristics and Birth Outcomes in Women With Healthy Pregnancies, PE, and FGR
| Healthya | PEb | FGRc | |||
|---|---|---|---|---|---|
| Preterm | Term | Preterm | Term | ||
| n (% of case cohort) | 279 (30.2) | 29 (3.1) | 165 (17.9) | 25 (2.7) | 160 (17.3) |
| Maternal characteristics | |||||
| Age, y, mean (SD) | 30 (5) | 28 (6) | 30 (6) | 30 (5) | 30 (6) |
| Age left FTE, y, mean (SD) | 21 (4) | 20 (4) | 20 (4) | 20 (3) | 21 (4) |
| Missing, n (%) | 1 (0.3) | 1 (3.4) | 5 (3.0) | 0 (0.0) | 3 (1.9) |
| Height, cm, mean (SD) | 165 (6) | 162 (8) | 164 (6) | 165 (8) | 165 (7) |
| BMI, kg/m2, mean (SD) | 24.9 (4.7) | 29.7 (5.9) | 27.8 (6.3) | 25.0 (4.9) | 25.6 (5.0) |
| Booking MAP, mmHg, mean (SD) | 78 (9) | 87 (10) | 83 (10) | 81 (9) | 79 (8) |
| Missing, n (%) | 9 (3.2) | 0 (0.0) | 4 (2.4) | 1 (4.0) | 6 (3.8) |
| Married, n (%) | 203 (72.8) | 22 (75.9) | 105 (63.6) | 16 (64.0) | 98 (61.3) |
| Smoker, n (%) | 13 (4.7) | 1 (3.5) | 6 (3.6) | 3 (12.0) | 29 (18.1) |
| Alcohol, n (%) | 10 (3.6) | 0 (0.0) | 7 (4.2) | 1 (4.0) | 7 (4.4) |
| Deprivation quartile, n (%) | |||||
| 1 (lowest) | 60 (21.5) | 8 (27.6) | 41 (24.8) | 3 (12.0) | 42 (26.3) |
| 2 | 81 (29.0) | 2 (6.9) | 38 (23.0) | 6 (24.0) | 27 (16.9) |
| 3 | 57 (20.4) | 14 (48.3) | 40 (24.2) | 6 (24.0) | 41 (25.6) |
| 4 (highest) | 71 (25.4) | 5 (17.2) | 38 (23.0) | 9 (36.0) | 43 (26.9) |
| Missing | 10 (3.6) | 0 (0.0) | 8 (4.8) | 1 (4.0) | 7 (4.4) |
| White ethnicity, n (%) | 263 (94.3) | 26 (89.7) | 157 (95.2) | 21 (84.0) | 151 (94.4) |
| Nonwhite ethnicity, n (%) | 11 (3.9) | 2 (6.9) | 7 (4.2) | 4 (16.0) | 7 (4.4) |
| Missing | 5 (1.8) | 1 (3.4) | 1 (0.6) | 0 (0.0) | 2 (1.3) |
| Characteristics of delivery, n (%) | |||||
| Mode of delivery | |||||
| Vaginal | 140 (50.2) | 7 (24.1) | 36 (21.8) | 6 (24.0) | 94 (58.8) |
| Assisted vaginal | 58 (20.8) | 0 (0.0) | 51 (30.9) | 5 (20.0) | 30 (18.8) |
| Intrapartum caesarean | 54 (19.4) | 3 (10.3) | 55 (33.3) | 1 (4.0) | 16 (10.0) |
| Prelabor caesarean | 25 (9.0) | 19 (65.6) | 22 (13.3) | 13 (52.0) | 20 (12.5) |
| Missing | 2 (0.7) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Labor induced | 88 (31.5) | 7 (24.1) | 106 (64.2) | 4 (16.0) | 54 (33.8) |
Data are expressed as means (±SD) or n (%) with number of missing values below each characteristic. Where there is no missing category, data were complete.
Abbreviation: MAP, mean arterial blood pressure.
Women without FGR, PE, gestational diabetes, or spontaneous preterm delivery.
Diagnosed according to ACOG 2013 Guidelines (25) and divided into preterm (delivery <37 wkGA) and term (delivery ≥37 wkGA) outcomes. Cases of PE include all severe and nonsuperimposed, nonsevere PE at term, and all preterm PE.
Divided into preterm (delivery <37 wkGA) and term (delivery ≥37 wkGA) outcomes. Cases of FGR at term include severe SGA (birth weight less than third customized percentile) and SGA with growth restriction (birth weight <10th customized percentile and reduced growth velocity of the abdominal circumference on serial ultrasound scans) without PE. Cases of preterm FGR include SGA (birth weight <10th customized percentile) with preterm delivery and without PE. Maternal characteristics and the summary of cortisol and cortisone levels were summarized for cases and healthy women as a result of the small overlap in cases in the random subcohort.
Patterns of cortisol, cortisone, and cortisol-to-cortisone ratio throughout pregnancy
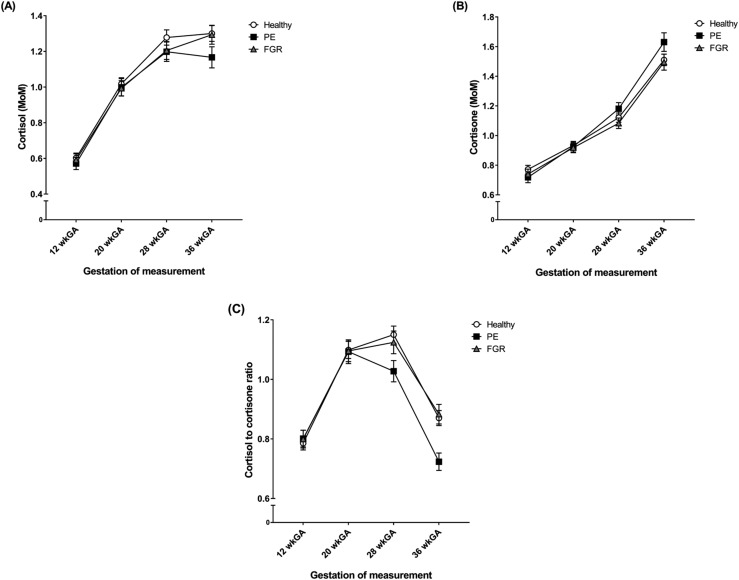
In healthy women, cortisol and cortisone both increased as pregnancy progressed [Fig. 1(A) and 1(B), respectively], whereas the cortisol-to-cortisone ratio showed an upward trend between 12 and 20 wkGA, plateaued until 28 wkGA, and then fell at 36 wkGA [Fig. 1(C)]. This pattern was similar in cases of FGR, but in PE, the cortisol-to-cortisone ratio started to decline at 28 wkGA onward [Fig. 1(C)].
Figure 1.
Mean with 95% CIs for (A) cortisol, (B) cortisone, and the (C) cortisol-to-cortisone ratio at 12, 20, 28, and 36 wkGA in healthy pregnancies, women that developed PE, and women who delivered an FGR infant. Cortisol and cortisone are reported as MoM. Healthy pregnancies included women who did not have FGR, PE, gestational diabetes, or spontaneous preterm delivery. Cases of PE were defined by the ACOG 2013 Guidelines (25) and included all severe and nonsevere, nonsuperimposed PE at term and all preterm PE. Cases of term FGR included severe SGA (birth weight less than third customized percentile at term) and SGA with growth restriction (birth weight <10th customized percentile at term with reduced growth velocity of the abdominal circumference on serial ultrasound scans) without PE. Cases of preterm FGR included SGA with preterm delivery (birth weight <10th customized percentile and delivery before 37 completed wkGA) without PE.
Associations among cortisol, cortisone, and cortisol-to-cortisone ratio with PE and FGR
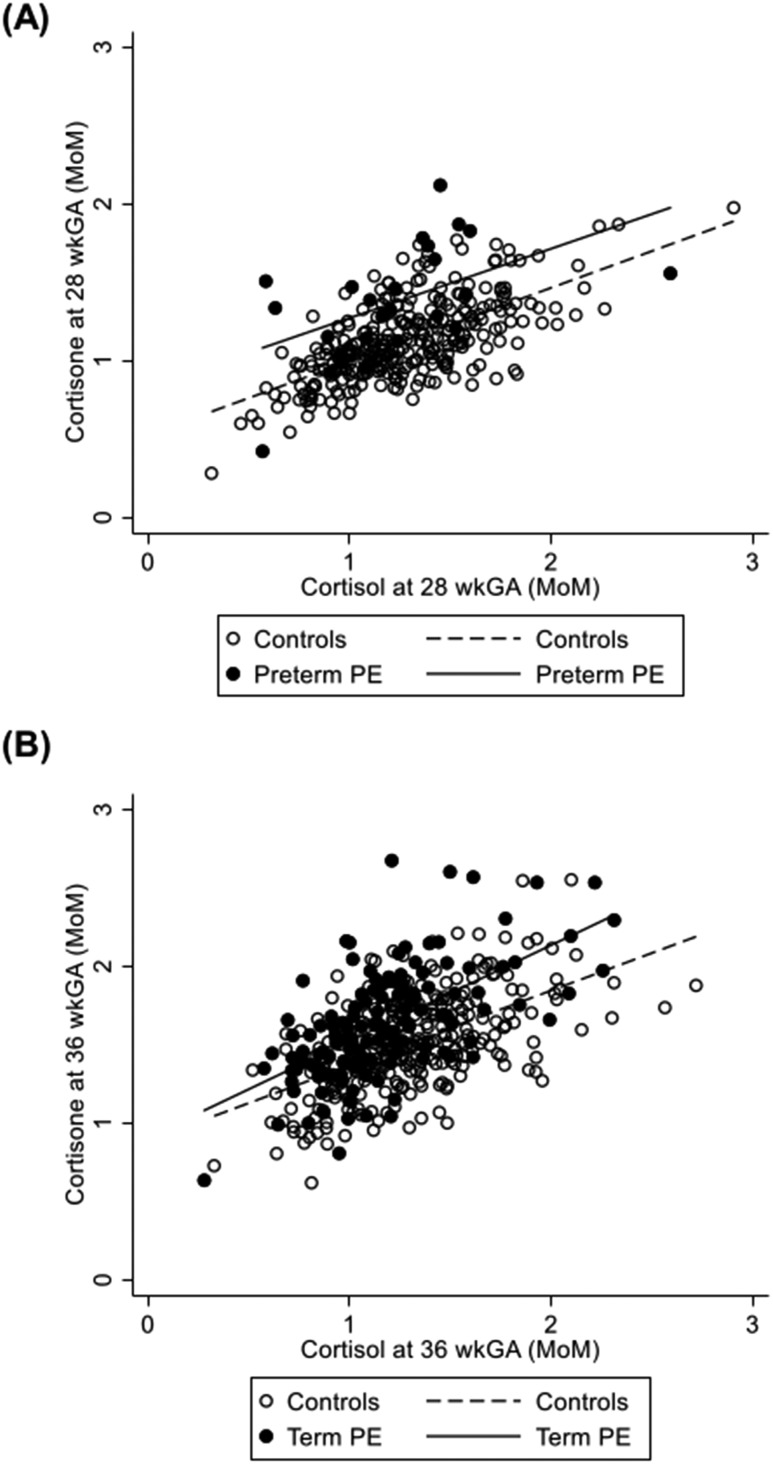
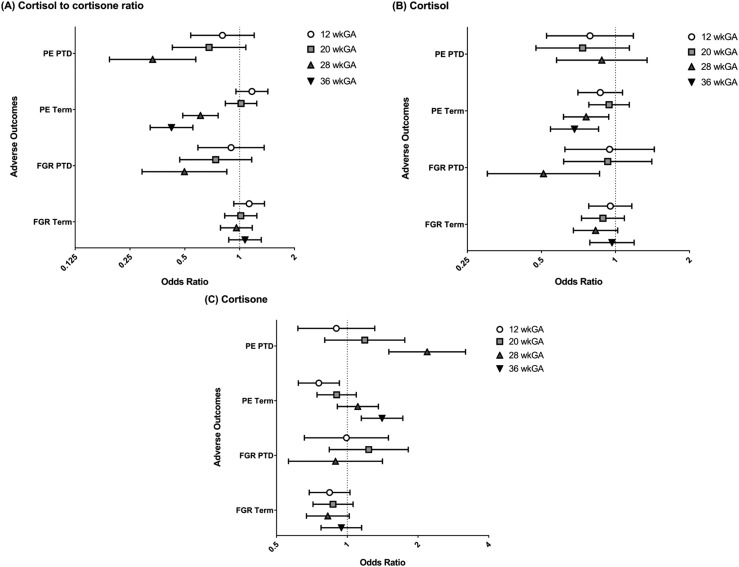
There was a positive association between cortisol and cortisone in cases and controls, but for a given level of cortisol, the cortisone level was greater in cases of preterm PE at 28 wkGA and term PE at 36 wkGA [Fig. 2(A) and 2(B), respectively]. The cortisol-to-cortisone ratio was negatively associated with PE [Table 2 and Fig. 3(A)] at 28 and at 36 wkGA, and the association was not materially affected by adjustment for maternal characteristics (Table 2). At 28 wkGA, the cortisol-to-cortisone ratio showed a negative association with the risk of preterm PE, term PE, and preterm FGR. At 36 wkGA, the cortisol-to-cortisone ratio was also negatively associated with term PE but not with term FGR [Fig. 3(A)]. The associations were broadly similar when the analysis was restricted to women who were not treated with steroids antenatally (Table 3). The association between the cortisol-to-cortisone ratio at 28 wkGA and preterm FGR was not statistically significant at an α of 0.05, but there was no evidence of an interaction between the groups with and without antenatal steroid use (P = 0.15 for the unadjusted analysis and P = 0.13 for the adjusted analysis). Hence, the apparent loss of statistical significance likely reflected reduced statistical power as a result of a smaller sample size.
Figure 2.
Scatter plots for cortisol vs cortisone at (A) 28 wkGA in cases of preterm PE and controls and (B) 36 wkGA in cases of term PE and controls. Cortisol and cortisone are reported as MoM. Cases of PE were defined by the ACOG 2013 Guidelines (25) and included all severe and nonsevere, nonsuperimposed PE at term and all preterm PE. Controls included women who did not have PE. Preterm delivery included pregnancies delivered <37 wkGA, and term delivery included pregnancies delivered ≥37 wkGA. For the measurement at 28 wkGA, the equation for the regression line in cases of preterm PE is y = 0.829 + 0.442 ⋅ x (P value for association = 0.01) and in controls, is y = 0.526 + 0.469 ⋅ x (P value for association < 0.001). The P value for interaction between preterm PE cases and controls at 28 wkGA is 0.80. For the measurement at 36 wkGA, the equation for the regression line in cases of term PE is y = 0.909 + 0.612 ⋅ x (P value for association <0.001) and in controls is y = 0.887 + 0.479 ⋅ x (P value for association < 0.001). The P value for interaction between term PE cases and controls at 36 wkGA is 0.10.
Table 2.
Unadjusted and Adjusted ORs (95% CI) for PE and FGR by 1 SD Higher Cortisol-to-Cortisone Ratio Measured at Different Time Points in Pregnancy
| Approximate Gestation of Measurement, wkGA | PEa | FGRb | ||||||
|---|---|---|---|---|---|---|---|---|
| Preterm | Term | Preterm | Term | |||||
| Unadjusted OR (95% CI) | Adjusted ORc (95% CI) | Unadjusted OR (95% CI) | Adjusted ORc (95% CI) | Unadjusted OR (95% CI) | Adjusted ORc (95% CI) | Unadjusted OR (95% CI) | Adjusted ORc (95% CI) | |
| 12 | 0.80 (0.54–1.20) n/N = 29/331 | 0.68 (0.44–1.04) n/N = 29/331 | 1.17 (0.95–1.43) n/N = 150/425 | 1.14 (0.92–1.41) n/N = 150/425 | 0.90 (0.59–1.36) n/N = 25/327 | 0.93 (0.60–1.45) n/N = 25/327 | 1.13 (0.93–1.37) n/N = 156/418 | 1.15 (0.94–1.41) n/N = 156/418 |
| 20 | 0.68 (0.43–1.08) n/N = 25/331 | 0.74 (0.46–1.21) n/N = 25/331 | 1.02 (0.83–1.24) n/N = 152/433 | 1.09 (0.88–1.34) n/N = 152/433 | 0.74 (0.47–1.16) n/N = 25/331 | 0.78 (0.49–1.24) n/N = 25/331 | 1.01 (0.83–1.24) n/N = 156/425 | 1.09 (0.88–1.35) n/N = 156/425 |
| 28 | 0.33 (0.19–0.57)d n/N = 24/328 | 0.34 (0.19–0.61)d n/N = 24/328 | 0.61 (0.49–0.76)d n/N = 151/430 | 0.61 (0.49–0.77)d n/N = 151/430 | 0.50 (0.29–0.85)e n/N = 21/325 | 0.50 (0.29–0.85)e n/N = 21/325 | 0.96 (0.79–1.17) n/N = 154/420 | 0.96 (0.78–1.19) n/N = 154/420 |
| 36f | 0.42 (0.32–0.55)d n/N = 134/409 | 0.42 (0.32–0.56)d n/N = 134/409 | 1.07 (0.87–1.31) n/N = 148/410 | 1.14 (0.92–1.41) n/N = 148/410 | ||||
Diagnosed according to ACOG Guidelines (25) and divided into preterm (delivery <37 wkGA) and term (delivery ≥37 wkGA) outcomes. Cases of PE include all severe and nonsuperimposed, nonsevere PE at term, and all preterm PE.
Divided into preterm (delivery <37 wkGA) and term (delivery ≥37 wkGA) outcomes. Cases of FGR at term include severe SGA (birth weight less than third customized percentile) and SGA with growth restriction (birth weight <10th customized percentile and reduced growth velocity of the abdominal circumference on serial ultrasound scans) without PE. Cases of preterm FGR include SGA (birth weight <10th percentile) with preterm delivery and without PE.
ORs adjusted for antenatal height, age, BMI, marital status, ethnicity, smoking, age at leaving FTE, and deprivation. In the analysis of preterm PE, the adjustments for ethnicity and smoking were omitted, as these variables predicted the outcome perfectly.
P < 0.001.
P < 0.05.
The 36-wkGA measurements have not been analyzed for preterm outcomes.
Figure 3.
Unadjusted ORs with 95% CIs at 12, 20, 28, and 36 wkGA for term PE, preterm PE, term FGR, and preterm FGR for (A) cortisol-to-cortisone ratio, (B) cortisol, and (C) cortisone. ORs are for cases referent to controls, associated with a 1-SD higher cortisol-to-cortisone ratio, cortisol, or cortisone value. Error bars represent the 95% CIs. Cases of PE were defined by the ACOG 2013 Guidelines (25) and included all severe and nonsevere, nonsuperimposed PE at term and all preterm PE. Cases of term FGR included severe SGA (birth weight less than third customized percentile at term), SGA with growth restriction (birth weight <10th customized percentile at term with reduced growth velocity of the abdominal circumference on serial ultrasound scans), and all cases of PE were excluded from analyses of term FGR. Cases of preterm FGR included SGA (birth weight <10th customized percentile) with preterm delivery, and cases of preterm PE were excluded from analyses of preterm FGR. Preterm delivery included pregnancies delivered <37 wkGA, and term delivery included pregnancies delivered ≥37 wkGA. PTD, preterm delivery.
Table 3.
Unadjusted and Adjusted ORs (95% CI) for PE and FGR by 1 SD Higher Cortisol-to-Cortisone Ratio Measured at Different Time Points in Pregnancy Confined to Women Who Were Documented As Not Having Received Steroids Antenatally
| Approximate Gestation of Measurement, wkGA | PEa | FGRb | ||||||
|---|---|---|---|---|---|---|---|---|
| Preterm | Term | Preterm | Term | |||||
| Unadjusted OR (95% CI) | Adjusted ORc (95% CI) | Unadjusted OR (95% CI) | Adjusted ORc (95% CI) | Unadjusted OR (95% CI) | Adjusted ORc (95% CI) | Unadjusted OR (95% CI) | Adjusted ORc (95% CI) | |
| 12 | 0.70 (0.37–1.33) n/N = 12/248 | 0.57 (0.28–1.16) n/N = 12/248 | 1.15 (0.91–1.44) n/N = 113/331 | 1.12 (0.88–1.43) n/N = 113/331 | 1.00 (0.59–1.68) n/N = 15/251 | 0.97 (0.56–1.69) n/N = 15/251 | 1.15 (0.93–1.42) n/N = 120/328 | 1.13 (0.90–1.42) n/N = 120/328 |
| 20 | 0.59 (0.26–1.32) n/N = 9/253 | 0.59 (0.25–1.41) n/N = 9/253 | 1.02 (0.81–1.29) n/N = 113/339 | 1.06 (0.84–1.35) n/N = 113/339 | 0.91 (0.52–1.61) n/N = 15/259 | 0.95 (0.52–1.73) n/N = 15/259 | 1.00 (0.79–1.26) n/N = 119/335 | 1.06 (0.83–1.36) n/N = 119/335 |
| 28 | 0.22 (0.08–0.66)e n/N = 9/246 | 0.10 (0.02–0.51)e n/N = 9/246 | 0.54 (0.41–0.71)d n/N = 112/330 | 0.54 (0.41–0.71)d n/N = 112/330 | 0.71 (0.37–1.35) n/N = 12/249 | 0.71 (0.35–1.41) n/N = 12/249 | 1.00 (0.80–1.26) n/N = 117/325 | 0.98 (0.77–1.25) n/N = 117/325 |
| 36f | 0.42 (0.31–0.57)d n/N = 99/315 | 0.41 (0.30–0.57)d n/N = 99/315 | 1.09 (0.86–1.39) n/N = 114/320 | 1.14 (0.89–1.47) n/N = 114/320 | ||||
Diagnosed according to ACOG Guidelines (24) and divided into preterm (delivery <37 wkGA) and term (delivery ≥37 wkGA) outcomes. Cases of PE included all severe and nonsuperimposed, nonsevere PE.
Divided into preterm (delivery <37 wkGA) and term (delivery ≥37 wkGA) outcomes. Cases of FGR at term include severe SGA (birth weight less than third customized percentile) and SGA with growth restriction (birth weight <10th customized percentile and reduced growth velocity of the abdominal circumference on serial ultrasound scans) without PE. Cases of preterm FGR include SGA (birth weight <10th percentile) with preterm delivery and without PE.
ORs adjusted for antenatal height, age, BMI, marital status, ethnicity, smoking, age at leaving FTE, and deprivation. In the analysis of preterm PE, the adjustments for ethnicity and smoking were omitted, as these variables predicted the outcome perfectly.
P < 0.001
P < 0.05
The 36-wkGA measurements have not been analyzed for preterm outcomes.
Cortisol was negatively associated with the risk of term PE from 28 wkGA onward [Fig. 3(B)], whereas the association between cortisone and the risk of term PE was negative in the first trimester and changed to positive in the third trimester [Fig. 3(C)]. A similar association was also seen with cortisone and preterm PE [Fig. 3(C)] but not cortisol [Fig. 3(B)]. The associations seen were stronger as pregnancy progressed for both the cortisol-to-cortisone ratio and cortisone in term and preterm PE [Fig. 3(A) and 3(C)]. Cortisol was negatively associated with preterm FGR at 28 wkGA [Fig. 3(B)], but there were no clear associations between cortisone and preterm FGR [Fig. 3(C)], and there was no clear association between cortisol or cortisone and term FGR [Fig. 3(B) and 3(C)].
Cumulative incidence of PE according to cortisol-to-cortisone ratio
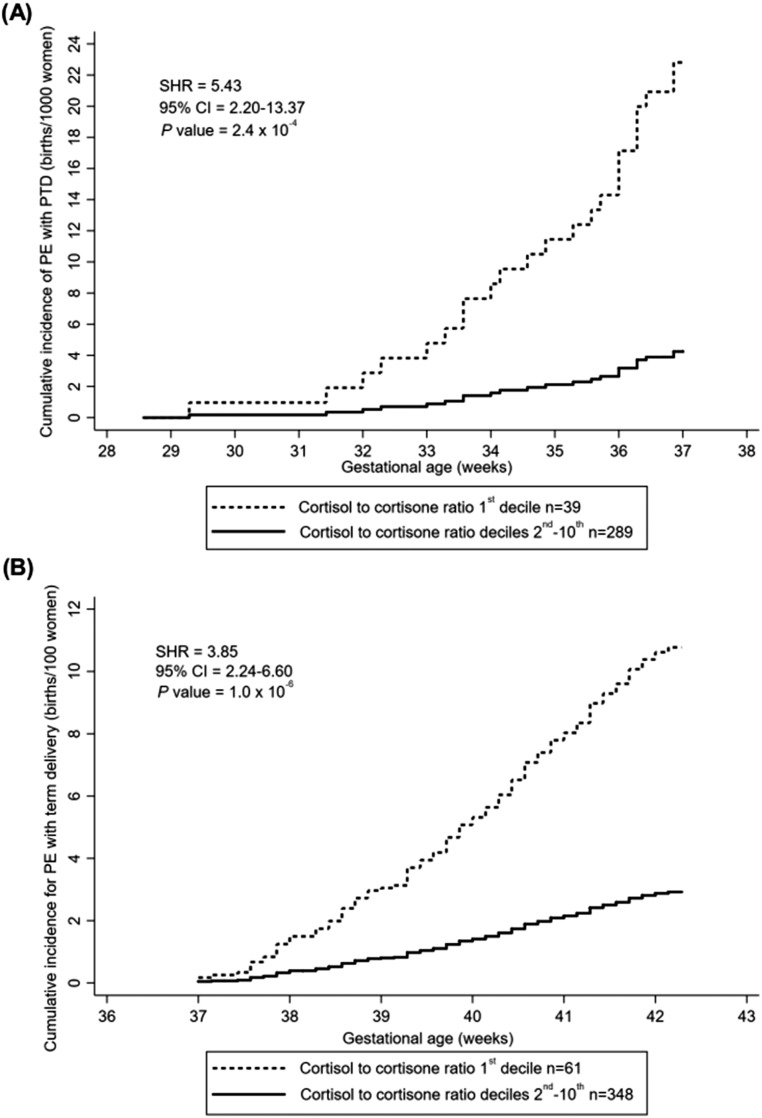
There was a higher cumulative incidence of preterm PE and term PE for women in the lowest decile of the cortisol-to-cortisone ratio compared with deciles two to 10 at the 28- and 36-wkGA measurements, respectively; ∼2.2% and 11% cases in the lowest decile vs 0.4% and 3% in deciles two to 10. For preterm PE, the curves started to deviate 2 weeks after the 28-wkGA measurement [Fig. 4(A)], and for term PE, the curves began to deviate at least 1 week after the 36 wkGA measurement [Fig. 4(B)]. Similar results were obtained when women treated with steroids antenatally were excluded.
Figure 4.
Cumulative incidence of PE comparing the first decile of the cortisol-to-cortisone ratio with the second through 10th deciles of cortisol-to-cortisone ratio from (A) 28 wkGA onward in cases of preterm PE and (B) 36 wkGA onward in cases of term PE. (A) Reported in births per 1000 women; (B) reported in births per 100 women. Cases of PE were defined by the ACOG 2013 Guidelines (25) and included all severe and nonsevere, nonsuperimposed PE at term and all preterm PE. Preterm delivery included pregnancies delivered <37 wkGA, and term delivery included pregnancies delivered ≥37 wkGA. Delivery without PE was considered as competing risk. PTD, preterm delivery; SHR, subhazard ratio.
Discussion
In this study, we found a clear negative association between the maternal serum cortisol-to-cortisone ratio and the risk of preterm and term PE. In women with term PE, the cortisol-to-cortisone ratio was significantly lower >8 weeks before delivery. In term PE, cortisol levels were lower at 28 wkGA, and cortisone levels were higher at 36 wkGA, and in preterm PE cortisone levels were higher at 28 wkGA. These changes could represent higher 11βHSD2 activity or lower 11βHSD1 activity in women with PE, which occur before the manifestation of clinical signs and symptoms.
Multiple studies have shown lower placental 11βHSD2 activity after vaginal and cesarean delivery in women with PE and FGR (7–15), but our work and that of other studies investigating maternal blood and urine cortisol-to-cortisone ratio in women with established disease (18–20) suggest that maternal systemic 11βHSD2 activity is increased. The observation of reduced 11βHSD2 activity in placentas from women with PE may reflect general placental failure and local regulation of cortisol metabolism by the feto-placental unit, which is independent of the mother. Aufdenblatten et al. (31) found that placental cortisol was almost completely inactivated in normotensive pregnancies, indicating an effective 11βHSD2 barrier from high levels of cortisol. This was in contrast to pregnancies with PE and low birth weight that had reduced placental 11βHSD2 activity and increased placental cortisol levels (32). Therefore, as 11βHSD2 is also expressed outside of the placenta, the association between PE and FGR with a lower cortisol-to-cortisone ratio could be related to extraplacental 11βHSD2 activity, such as maternal renal 11βHSD2 (33, 34).
PE is frequently accompanied by renal dysfunction, but impaired renal function and hypertension in diseases outside of pregnancy have been associated with decreased renal 11βHSD2 expression and activity (31, 35). Thus, maternal 11βHSD2 activity may increase as a compensatory response in PE, acting to reduce systemic cortisol. The systemic stress and inflammatory response that occur in PE (36, 37) may result in higher maternal cortisol secretion, which would suggest that 11βHSD2 activity increases to the extent that conversion of cortisol-to-cortisone exceeds cortisol secretion. However, cortisol secretion is not definitively known to rise in PE, and there is limited evidence that cortisol itself stimulates 11βHSD2 activity; exogenous corticosteroids have been shown to stimulate 11βHSD2 activity in bronchial epithelial cells (38), but the effects of cortisol on renal 11βHSD2 activity are not known, and as we observed a linear relationship between cortisol and cortisone levels in women with and without PE, it is likely that there are other factors responsible for stimulating 11βHSD2 action.
Another explanation for the changes observed in cortisol and cortisone levels might be reduced 11βHSD1 activity, as opposed to increased 11βHSD2 activity. There are studies showing reduced placental and chorionic 11βHSD1 activity in infants born SGA (birth weight <10th percentile) (10, 16) that could be a compensatory mechanism to reduce fetal cortisol exposure as a result of increased placental crossover of cortisol. We have been unable to identify any studies on the activity of placental 11BHSD1 from women with PE. Furthermore, 11βHSD1 is present in the chorio-decidua (39, 40), but it is unclear whether reduced 11βHSD1 activity at the feto-maternal interface would affect maternal cortisol and cortisone levels. Reduced maternal systemic 11βHSD1 activity is another possible explanation for the findings, but this is also yet to be investigated and would be an important area of future research.
Our study had several methodological strengths. First, this study looks at measurements of cortisol and cortisone in maternal serum preceding the onset of disease in both PE and FGR. Second, the case-cohort study design meant that we were able to study a large number of cases of PE and FGR. Moreover, as a result of the large size of the cohort and the availability of serial ultrasound scans, we were able to confine our analysis of FGR to either infants who were extremely SGA (less that third percentile) or infants <10th percentile with other features, indicating a likely pathological cause (preterm birth or reduced fetal growth velocity); many other studies have only studied SGA infants (<10th percentile), and a large proportion of these will be healthy. The case-cohort design also meant that we were able to compare cases of PE and FGR with a population representative of the whole cohort. This is more likely to demonstrate a true difference in participants, with and without the outcomes studied, than a case-control study; in case-control studies, the comparison group is often healthy, and differences seen may only reflect the lack of other outcomes in the control group rather than the outcome of interest in cases. Details of the methodology underlying case-cohort studies and potential statistical and reporting issues are described by Sharp et al. (41).
However, our study also has some limitations. Cortisol and cortisone were quantified as MoM rather than (SI) Units, so values cannot be directly compared with traditional measures of cortisol and cortisone in nanomoles per liter. This is because the metabolomics assay was untargeted, and internal standards were not available for all metabolites. However, the quantification method is accurate, and the ratio is unitless, so the results correctly reflect cortisol and cortisone levels measured using standard methods. Additionally, human cortisol secretion follows a circadian rhythm, which was not accounted for in this study (42). However, Kosicka et al. (19) controlled for diurnal variation by taking early-morning blood samples and drew the same conclusion that the maternal serum cortisol-to-cortisone ratio was reduced in PE. Cortisol secretion is influenced by external factors, including stress and anxiety (43). Although we adjusted for some characteristics, such as deprivation and marital and smoking status, it was difficult to quantify any other external stresses that the study participants may have been experiencing. However, confounding by maternal stress is unlikely to have caused the associations, as it would tend to be associated with increased rather than decreased cortisol levels. Furthermore, we also considered whether our findings could have been influenced by exogenous antenatal steroid use, and there were no clear differences in our results when these women were excluded. Whereas the association between the cortisol-to-cortisone ratio and preterm FGR at 28 wkGA, where cases of antenatal steroid use had been excluded, was not significant at an α of 0.05, the point estimates (OR) were within the 95% CIs of the analyses where they had not been excluded, and there was no evidence of an interaction between the ratio and exposure to steroids; thus, the higher P values reflected reduced statistical power from a smaller sample size.
In conclusion, this study demonstrates that a lower maternal serum cortisol-to-cortisone ratio precedes the clinical manifestation of PE and preterm FGR by many weeks, despite previous reports of elevated levels of placental 11βHSD2 in these conditions. Our observations implicate enhanced maternal 11βHSD2 activity in the pathophysiology of PE. However, further investigation into the potential use of the cortisol-to-cortisone ratio as a predictive marker of PE would be beneficial, given that there is no current accurate predictive test for the condition. In addition, research into maternal and placental 11βHSD1 and 11βHSD2 activity in women with PE and FGR, and links to fetal and newborn cortisol metabolism would be useful in gaining a deeper understanding of the pathophysiology of the diseases.
Acknowledgments
We are grateful to the participants involved in the POP Study. We thank Leah Bibby, Samudra Ranawaka, Katrina Holmes, Ryan Millar, and Josephine Gill for technical assistance during the study.
Financial Support: The POP Study was funded by the National Institute for Health Research (NIHR), Cambridge Comprehensive Biomedical Research Centre (women’s health theme), and a project grant from the Medical Research Council (United Kingdom; G1100221). The study was also supported by GE Healthcare (donation of two Voluson i ultrasound systems for the POP Study) and by the NIHR, Cambridge Clinical Research Facility, where all research visits took place. A.E.H. was an Academic Clinical Fellow funded by NIHR.
Author Contributions: G.C.S.S. had the original idea. N.A.J. performed the analyses and wrote the manuscript. A.E.H. performed the analyses, supervised the analysis, and contributed to the manuscript. U.S. assisted with statistical analyses, supervised the analysis, and contributed to the manuscript. E.C. assisted with data collection and contributed to the manuscript. G.C.S.S. and D.S.C.-J. conceived the study, supervised the analysis, and contributed to the manuscript.
Disclosure Summary: G.C.S.S. has no direct conflicts of interest in relationship to this work. His interests outside the area of this work are as follows: research support from GE Healthcare (supply of two diagnostic ultrasound systems), Roche (supply of equipment and reagents for biomarker studies, ∼£600,000 in value), GlaxoSmithKline (GSK; ∼£200,000), and Sera Prognostics (∼£90,000 in value). He has been paid to attend Advisory Boards by GSK and Roche. He has acted as a paid consultant to GSK and sat on a Data Safety and Monitoring Committee for a GSK vaccine trial. D.S.C.-J. has similarly received research support from GSK and Sera Prognostics, but this is outside the scope of the work described here. He has no other conflicts of interest. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- 11βHSD1
11-β-hydroxysteroid dehydrogenase type 1
- 11βHSD2
11-β-hydroxysteroid dehydrogenase type 2
- ACOG
American College of Obstetricians and Gynecologists
- BMI
body mass index
- FGR
fetal growth restriction
- FTE
full-time education
- MoM
multiples of the median
- PE
preeclampsia
- POP
Pregnancy Outcome Prediction
- SGA
small for gestational age
- wkGA
weeks of gestational age
References
- 1. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. [DOI] [PubMed] [Google Scholar]
- 2. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. [DOI] [PubMed] [Google Scholar]
- 3. Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith GCS. Universal screening for foetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2018;49:16–28. [DOI] [PubMed] [Google Scholar]
- 5. Kosicka K, Siemiątkowska A, Główka FK. 11β-Hydroxysteroid dehydrogenase 2 in preeclampsia. Int J Endocrinol. 2016;2016:5279462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Causevic M, Mohaupt M. 11beta-Hydroxysteroid dehydrogenase type 2 in pregnancy and preeclampsia. Mol Aspects Med. 2007;28(2):220–226. [DOI] [PubMed] [Google Scholar]
- 7. Cottrell EC, Seckl JR, Holmes MC, Wyrwoll CS. Foetal and placental 11β-HSD2: a hub for developmental programming. Acta Physiol (Oxf). 2014;210(2):288–295. [DOI] [PubMed] [Google Scholar]
- 8. Kajantie E, Dunkel L, Turpeinen U, Stenman UH, Wood PJ, Nuutila M, Andersson S. Placental 11 β-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab. 2003;88(1):493–500. [DOI] [PubMed] [Google Scholar]
- 9. Lazo-de-la-Vega-Monroy ML, Solís-Martínez MO, Romero-Gutiérrez G, Aguirre-Arzola VE, Wrobel K, Wrobel K, Zaina S, Barbosa-Sabanero G. 11 beta-Hydroxysteroid dehydrogenase 2 promoter methylation is associated with placental protein expression in small for gestational age newborns. Steroids. 2017;124:60–66. [DOI] [PubMed] [Google Scholar]
- 10. Struwe E, Berzl GM, Schild RL, Beckmann MW, Dörr HG, Rascher W, Dötsch J. Simultaneously reduced gene expression of cortisol-activating and cortisol-inactivating enzymes in placentas of small-for-gestational-age neonates. Am J Obstet Gynecol. 2007;197(1):43.e1–43.e6.1. [DOI] [PubMed] [Google Scholar]
- 11. Xiao X, Zhao Y, Jin R, Chen J, Wang X, Baccarelli A, Zhang Y. Fetal growth restriction and methylation of growth-related genes in the placenta. Epigenomics. 2016;8(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Z, Liu Q. Relationship between 11β-HSD2 mRNA and insulin sensitivity in term small-for-gestational age neonates after birth. Int J Clin Exp Pathol. 2015;8(1):928–932. [PMC free article] [PubMed] [Google Scholar]
- 13. Hu W, Wang H, Huang H. Analysis of gene expression and preliminary study of methylation about 11β-HSD2 gene in placentas of Chinese pre-eclampsia patients of Han ethnicity. J Obstet Gynaecol Res. 2015;41(3):343–349. [DOI] [PubMed] [Google Scholar]
- 14. McCalla CO, Nacharaju VL, Muneyyirci-Delale O, Glasgow S, Feldman JG. Placental 11 beta-hydroxysteroid dehydrogenase activity in normotensive and pre-eclamptic pregnancies. Steroids. 1998;63(10):511–515. [DOI] [PubMed] [Google Scholar]
- 15. Schoof E, Girstl M, Frobenius W, Kirschbaum M, Dörr HG, Rascher W, Dötsch J. Decreased gene expression of 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase in human placenta of patients with preeclampsia. J Clin Endocrinol Metab. 2001;86(3):1313–1317. [DOI] [PubMed] [Google Scholar]
- 16. Mericq V, Medina P, Kakarieka E, Márquez L, Johnson MC, Iñiguez G. Differences in expression and activity of 11beta-hydroxysteroid dehydrogenase type 1 and 2 in human placentas of term pregnancies according to birth weight and gender. Eur J Endocrinol. 2009;161(3):419–425. [DOI] [PubMed] [Google Scholar]
- 17. Struwe E, Berzl GM, Schild RL, Beckmann MW, Dörr HG, Rascher W, Dötsch J. Simultaneously reduced gene expression of cortisol-activating and cortisol-inactivating enzymes in placentas of small-for-gestational-age neonates. Am J Obstet Gynecol. 2007;197(1):43.e1–43.e6. [DOI] [PubMed] [Google Scholar]
- 18. Kosicka K, Siemiątkowska A, Krzyścin M, Bręborowicz GH, Resztak M, Majchrzak-Celińska A, Chuchracki M, Główka FK. Glucocorticoid metabolism in hypertensive disorders of pregnancy: analysis of plasma and urinary cortisol and cortisone. PLoS One. 2015;10(12):e0144343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kosicka K, Siemiątkowska A, Szpera-Goździewicz A, Krzyścin M, Bręborowicz GH, Główka FK. Increased cortisol metabolism in women with pregnancy-related hypertension. Endocrine. 2018;61(1):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vasku M, Kleine-Eggebrecht N, Rath W, Mohaupt MG, Escher G, Pecks U. Apparent systemic 11ß-dehydroxysteroid dehydrogenase 2 activity is increased in preeclampsia but not in intrauterine growth restriction. Pregnancy Hypertens. 2018;11:7–11. [DOI] [PubMed] [Google Scholar]
- 21. Pasupathy D, Dacey A, Cook E, Charnock-Jones DS, White IR, Smith GC. Study protocol. A prospective cohort study of unselected primiparous women: the Pregnancy Outcome Prediction Study. BMC Pregnancy Childbirth. 2008;8(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386(10008):2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaccioli F, Lager S, Sovio U, Charnock-Jones DS, Smith GCS. The Pregnancy Outcome Prediction (POP) Study: Investigating the relationship between serial prenatal ultrasonography, biomarkers, placental phenotype and adverse pregnancy outcomes. Placenta. 2017;59(Suppl 1):S17–S25. [Google Scholar]
- 24. Gong S, Sovio U, Aye ILMH, Gaccioli F, Dopierala J, Johnson MD, Wood AM, Cook E, Jenkins BJ, Koulman A, Casero RA Jr, Constância M, Charnock-Jones DS, Smith GCS. Placental polyamine metabolism differs by fetal sex, fetal growth restriction, and preeclampsia. JCI Insight. 2018;3(13):e120723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 26. Gardosi J. Customised assessment of fetal growth potential: implications for perinatal care. Arch Dis Child Fetal Neonatal Ed. 2012;97(5):F314–F317. [DOI] [PubMed] [Google Scholar]
- 27. Noble M, McLennan D, Wilkinson K, Whitworth A, Barnes H, Dibben C. The English Indices of Deprivation 2007. London: Department of Communities and Local Government; 2008. [Google Scholar]
- 28. Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81(16):6656–6667. [DOI] [PubMed] [Google Scholar]
- 29. Fine JP, Gray RJ. Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 30. Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime Data Anal. 2000;6(1):39–58. [DOI] [PubMed] [Google Scholar]
- 31. Mongia A, Vecker R, George M, Pandey A, Tawadrous H, Schoeneman M, Muneyyirci-Delale O, Nacharaju V, Ten S, Bhangoo A. Role of 11βHSD type 2 enzyme activity in essential hypertension and children with chronic kidney disease (CKD). J Clin Endocrinol Metab. 2012;97(10):3622–3629. [DOI] [PubMed] [Google Scholar]
- 32. Aufdenblatten M, Baumann M, Raio L, Dick B, Frey BM, Schneider H, Surbek D, Hocher B, Mohaupt MG. Prematurity is related to high placental cortisol in preeclampsia. Pediatr Res. 2009;65(2):198–202. [DOI] [PubMed] [Google Scholar]
- 33. Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. Cloning and tissue distribution of the human 11 β-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol. 1994;105(2):R11–R17. [DOI] [PubMed] [Google Scholar]
- 34. Diederich S, Quinkler M, Burkhardt P, Grossmann C, Bähr V, Oelkers W. 11beta-Hydroxysteroid-dehydrogenase isoforms: tissue distribution and implications for clinical medicine. Eur J Clin Invest. 2000;30(Suppl 3):21–27. [DOI] [PubMed] [Google Scholar]
- 35. Quinkler M, Zehnder D, Lepenies J, Petrelli MD, Moore JS, Hughes SV, Cockwell P, Hewison M, Stewart PM. Expression of renal 11beta-hydroxysteroid dehydrogenase type 2 is decreased in patients with impaired renal function. Eur J Endocrinol. 2005;153(2):291–299. [DOI] [PubMed] [Google Scholar]
- 36. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2):499–506. [DOI] [PubMed] [Google Scholar]
- 37. Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl):38–42. [DOI] [PubMed] [Google Scholar]
- 38. Suzuki S, Koyama K, Darnel A, Ishibashi H, Kobayashi S, Kubo H, Suzuki T, Sasano H, Krozowski ZS. Dexamethasone upregulates 11beta-hydroxysteroid dehydrogenase type 2 in BEAS-2B cells. Am J Respir Crit Care Med. 2003;167(9):1244–1249. [DOI] [PubMed] [Google Scholar]
- 39. Sun K, Yang K, Challis JR. Differential expression of 11 beta-hydroxysteroid dehydrogenase types 1 and 2 in human placenta and fetal membranes. J Clin Endocrinol Metab. 1997;82(1):300–305. [DOI] [PubMed] [Google Scholar]
- 40. Alfaidy N, Li W, MacIntosh T, Yang K, Challis J. Late gestation increase in 11beta-hydroxysteroid dehydrogenase 1 expression in human fetal membranes: a novel intrauterine source of cortisol. J Clin Endocrinol Metab. 2003;88(10):5033–5038. [DOI] [PubMed] [Google Scholar]
- 41. Sharp SJ, Poulaliou M, Thompson SG, White IR, Wood AM. A review of published analyses of case-cohort studies and recommendations for future reporting. PLoS One. 2014;9(6):e101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab. 1971;32(2):266–284. [DOI] [PubMed] [Google Scholar]
- 43. Kane HS, Dunkel Schetter C, Glynn LM, Hobel CJ, Sandman CA. Pregnancy anxiety and prenatal cortisol trajectories. Biol Psychol. 2014;100:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]