Abstract
Background and Objective:
Current studies give us inconsistent results regarding the inulin consumption in cancer patients. The results of to-date studies are summarized in this systematic review.
Methods:
Web of Science (Science citation index expanded), PubMed (Medline), Embase and CENTRAL Science direct, Google scholar, Scopus and Cochrane were searched. Cochrane Collaboration’s ‘Risk of Bias’ tool was used to assess the quality of included articles.
Results:
Our search yielded 2652 studies after the elimination of duplicates. Three randomized controlled trials (RCTs), reporting results from 197 patients, were eligible for inclusion in the present systematic review. Risk of bias in these studies was assessed as high and moderate.
Conclusion:
The available evidence is inconclusive regarding the effect of inulin and oligofructose on cancer outcomes. Nonetheless, possible inulin positive effects including improved stool consistency after abdomen radiotherapy and increased stool butyrate content which is involved in controlling tumor cells proliferation and apoptosis should not be denied. Further research is needed in this area before strong conclusions can be drawn.
Keywords: Inulin, Neoplasms, Dietary Supplements, Functional Food
INTRODUCTION
According to global cancer statistics in 2012, nearly 14.1 million new cases of cancer were diagnosed and the cancer mortality rate was 8.2 million worldwide.1 Globally, lung and breast cancer were the most prevalent cancers. The importance of healthy diet has been proven in cancer prevention and control, however, whether using dietary supplements during cancer treatment is effective for recovery from cancer, remains unclear.2 Fiber is one of the food components that has been studied a lot regarding its association with cancer. It has shown that each 5-g/d increase in fiber intake could reduce CRC-specific mortality by 18% (95% CI, 7%-28%; P = .002).3 Different types of fiber have different physical and chemical properties, all of which can affect digestion, appetite regulation, and energy consumption differently.4,5 In particular, there is growing interest in fermentable fibers, such as inulin-type fructans (ITFs), and the impact they have on the human body.6 Inulin is a carbohydrate highly available in the root of many Arsteracee such as chicory, and its hydrolysis by means of inulinase leads to a mixture of fructose and glucose.7,8 It is a naturally occurring polysaccharide composed of a mixture of oligomers and polymers containing 2 to 60 β-2-1 linked D-fructosemolecules, usually with an α-2-1 linked D-glucose end.9,10 Inulin exhibits a prebiotic effect when fermented under anaerobic conditions in the colon, preferentially stimulating the growth of bifidobacteria in the lower colon.11,12 Inulin also has potential health benefits, such as promoting immune system function, supporting the cardiovascular system, and increasing the absorption of minerals.13 Incorporating inulin or oligofructose in the diet of mice resulted in less tumor formation and growth as well as decreased metastases.14
Description of the intervention
This review explored the impact of inulin inulin-type fructans consumption in patients undergoing cancer treatments
Why it is important to do this review
No systematic review has been completed investigating inulin in cancer patients and the role of inulin in optimizing the management of cancer. This review will add to an overall understanding about the role of inulin in nutrition support in cancer patients. This review seeks to aid in developing an evidence-based approach to the medico-nutritional management of cancer patients, potentially justifying clinical recommendations and informing future research efforts.
METHODS
We sought to identify relevant studies published until December 2017 using a multifaceted strategy. We searched Web of Science (Science citation index expanded), PubMed (Medline), Embase and CENTRAL, Science direct, Google scholar, Scopus and Cochrane database to identify articles fulfilling our inclusion criteria using the following search terms: cancer OR neoplasm AND inulin OR supplement OR probiotics fiber OR prebiotic. We used two recent terms to find articles conducted on inulin-type fructans. We limited the search strategy to humans. We also electronically searched the content of two leading journals in cancer, European Journal of Cancer Prevention and Journal of Clinical Gastroenterology and also searched the content of Nutrition and Cancer, British Journal of Nutrition and European Journal of Cancer to find potentially relevant abstract publications or articles. We also hand-searched the reference lists of included trials and other systematic reviews of inulin to identify additional trials. Unpublished trials were sought through trial registries (http://www.controlled-trials.com and http://www.clinicaltrials.gov). No language restriction was applied.
Study selection
Two reviewers (RM, SMHMJ) independently screened abstract citations, retrieved articles and assessed trials for study inclusion. We included randomized controlled trials (RCTs) comparing inulin in cancer patients. Trials involving patients with any type of cancer or at any stage of cancer were eligible. No criteria were set for participant characteristics (age, gender, etc.).
Data abstraction and assessment of methodological quality
Two reviewers (RM, SMHMJ) independently screened all articles on title/abstract. Disagreements were solved by discussion. The reference lists of these articles were screened for additional studies. Final selection for inclusion was based on the assessment of the full-text article. We abstracted information regarding the trial’s methodological quality using the Cochrane’s criteria15 including method of randomization, allocation concealment, blinding, incomplete outcome data (loss of follow-up). We resolved disagreement among reviewers by consensus.
Our search yielded 2652 records after the elimination of duplicates. Three studies16-18 were qualified for inclusion in the present systematic review. Fig.1 shows the flow diagram for identification of relevant studies.
Fig.1.
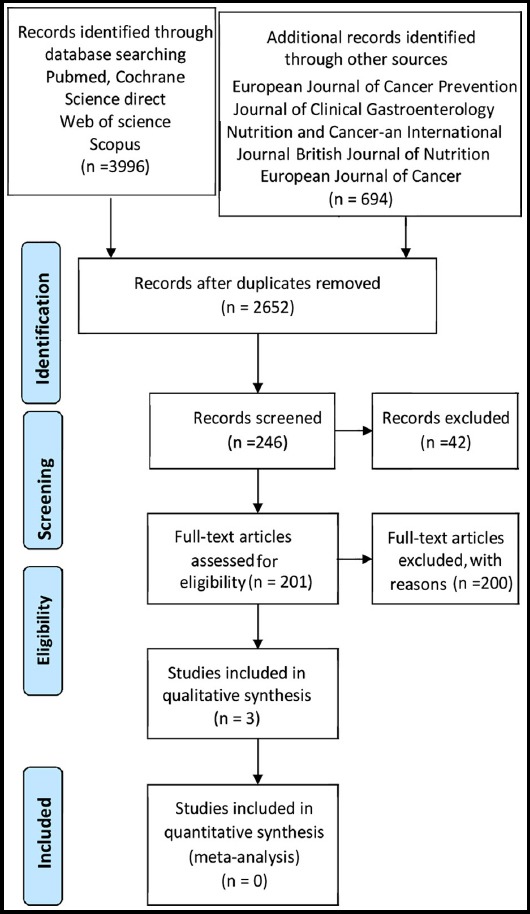
Flow diagram for identification of relevant studies.
RESULTS
Study identification and selection
Our search yielded 2652 records after the elimination of duplicates. Forty-two records were excluded through title and abstract screening and 204 studies were evaluated in more detail by obtaining full texts. We excluded 201 studies because they did not meet the study criteria. Three studies16-18 were qualified for inclusion in the present systematic review.
Study description and methodological quality
A summary of each study characteristics and results is given in Table-I. Limburg et al.17 evaluated the effects of possible effective agents for reducing colorectal cancer risk including sulindac, atorvastatin or prebiotic. Eighty-five patients with a history of previously resected colon cancer or advanced colorectal adenomas were randomized to one of the following groups for 6 months: atorvastatin (20mg/d); sulindac 300mg/d); oligofructose-enriched inulin (12g/d); or control. Percent change in number of rectal aberrant crypt foci (ACF) (precursor of colorectal adenoma) had no significant difference within and between groups compared to baseline values or control group. Biomarkers of proliferation (Ki67) and apoptosis (caspase-3) were not different between groups at the end of intervention. However, apoptosis increased in all arms.
Table-I.
Summary of included study characteristics.
| Reference | Design | Final Subject characteristics | Treatment | Results |
|---|---|---|---|---|
| Marie-Christine Boutron-Ruault, et al (2009)18 | RCT | 74 Subjects with small adenomas (<10 mm in diameter), larger adenomas, or no adenomas were recruited in nine French gastroenterology departments | All subjects received a 3-mo course of 5 g of sc-FOS twice daily | Butyrate was significantly increased in the adenoma groups. cholic acid, chenodeoxycholic acid, total primary bile acids and ursodeoxycholic acid increased and fecal lithocholic acid decreased in subjects without adenoma |
| Paul J. Limburg et al (2011)17 | RCT | 85 patients 40 years or older with a history of previously resected colon cancer | for 6 months Arm A : Atorvastatin 20 mg qd , N = 22 Arm B : Sulindac 150 mg bid , N = 21 Arm C : ORAFTI Synergy1 6 g bid , N = 20 Arm D : Control (maltodextrin) 6 g bid , N = 22 |
interventions with sulindac, atorvastatin, and ORAFTI Synergy1 did not yield significant reductions in rectal ACF number, as compared with control (maltodextrin) |
| P Garcia-Peris et al (2015)16 | RCT | 38 female gender, diagnosis of gynecological cancer requiring postoperative pelvic RT | from one week before to three weeks after RT. The prebiotic group received a mixture of fiber (6 gr inulin and 6 gr fos) received 6 g of Malt dextrin | bowel movements per month increased decrease in the number of days with watery stools |
RT = radiotherapy,FOS = fructo-oligosaccharide.
Garcia-Peris et al.16 conducted a 4-week randomized, double-blind, placebo-controlled trial on 38 gynecological cancer patients undergoing abdominal radiotherapy. Patients were randomly assigned to receive 12g/d of a mixture of inulin and fructo-oligosaccharide (prebiotic group) or placebo (12g/d maltodextrin). Number of days with watery stools decreased in prebiotic group (P=0.07) and increased in control group (P=0.07). At baseline, regarding quality-of-life (QOL), insomnia had the highest score in both groups. It changed to diarrhea at the end of study in placebo group, while remained unchanged in participants receiving prebiotic. However, groups did not differ in the score of QOL (overall or any of the items).
Boutron-Ruault et al.18 studied the effects of a 3-mo consumption of 10 g/d short-chain fructo-oligosaccharides in 74 patients with small, large or no colorectal adenomas. Biological parameters linked to colon cancer risk (short-chain fatty acids and fecal bile acids) were assessed. Mean fecal butyrate level significantly increased in those with adenoma (P=0.02). Fecal lithocholic acid significantly decreased in adenoma-free participants. However, colonic cell proliferation was not affected in any patients.
Risk of bias in included studies
The findings are presented in the ‘Risk of bias’ graph (Fig.2), which reviews the authors’ judgments about each risk of bias item shown as percentages across all included studies and the ‘Risk of bias’ summary (Fig.3), which reviews the authors’ judgments about each risk of bias item for each included study.
Fig.2.
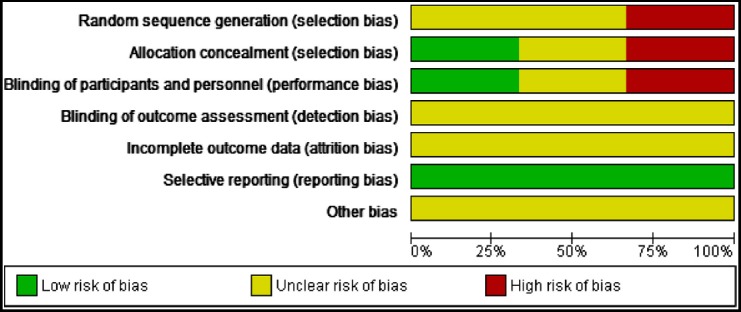
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Fig.3.
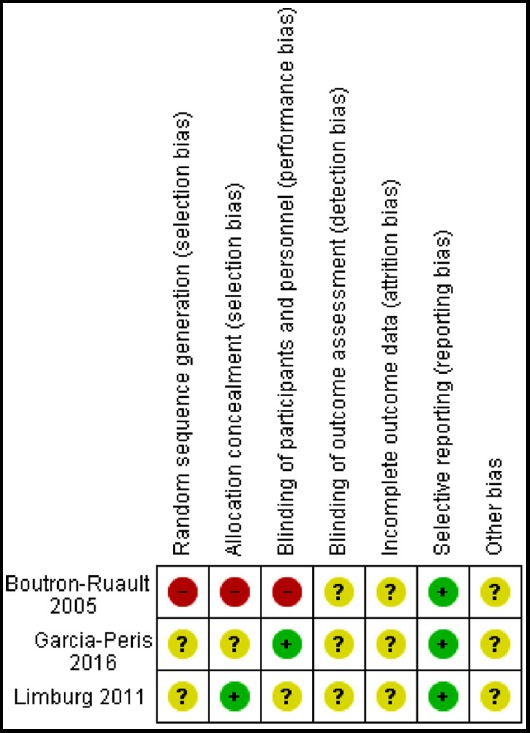
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
DISCUSSION
Inconclusive evidence of the effects of inulin and oligofructose on cancer outcomes in humans suggests a clear need for conducting large, well-designed and controlled RCTs to reach a firm conclusion.
Most studies evaluating inulin-type oligofructose roles in cancer have been focused on CRC. Animal experiments have demonstrated that inulin and oligofructose play roles as anti-carcinogenic and anti-metastatic agents, inhibit tumor growth, and potentiate cancer treatments in colorectal cancer.17 Increased number of bifidobacteria in the colon18,19 and its induced cell wall preparations20,21 are involved in the cancer growth inhibition effects of inulin and oligofructose. Furthermore, proliferative and apoptotic characteristics of these two prebiotics is related to decreased availability of glucose as an essential substrate for cancer cells.22-25 Additionally, in a review by Pool-Zobel26 on beneficial effects of inulin-type fructans in colorectal cancer, the proposed mechanisms were as follows: 1) decreasing exposure to genotoxic carcinogens in the gut or their genotoxic impacts; 2) growth inhibition; 3) gene expression modulation; and 4) lowering metastasis activities of colon tumor cells. Nevertheless, few studies on other types of cancer have shown that these non-digestible carbohydrates produce their anti-cancer effects by lowering serum glucose and fatty acids concentration required for cancer cells growth.
Two of three studies included in our review were conducted in colorectal cancer patients; one had null effects and the other showed an increase in fecal butyrate concentration after prebiotic supplementation. Supplementation of healthy adults who underwent colonoscopy with a mixture of inulin and oligofructose did not change cell proliferation;27 however, some limitations should be considered when interpreting the results. As authors pointed disagreements between endoscopic diagnosis of ACF and histologic evaluation, possible greater impacts of symbiotic vs. probiotics alone, and measuring biomarkers from rectum only could affect the observed null results. It has been postulated that protective properties of butyrate against colon cancer is related to inhibiting proliferation, inducing differentiation and apoptosis in transformed cells including cancer cells.28 Although butyrate increased in cancer patients in Boutron-Ruault study, cell proliferation remained unchanged. Perhaps if Limburg et al. assessed apoptosis and number of ACF, they might report similar results.
Advantages of inulin in the prevention of radiation enteritis in gynecologic cancer patients are consequences of intestinal flora recovery. Most studies in this area are limited to probiotics.29,30 Applying probiotics in patients with pelvic cancer reduce radiation-induced diarrhea in patients with pelvic cancer.30 It seems that the results are dependent on type of probiotics and the dose used.16
Limitations of the study
Several points should be considered while interpreting our results. Short study duration, small sample size, different dose of prebiotics, recruiting different patients, dissimilar biomarkers, and non-application of combination of biomarkers31 are propounded as presumable causes of finding inconsistent results. There are also some studies in mice models in which using inulin-type fructans has been resulted in enhanced proliferation of adenomas.32-34 Therefore, more studies are required to find real effects in humans.
CONCLUSION
No definite conclusion could be drawn. Nevertheless, possible positive effects of inulin and oligofructose should not be neglected. These effects include improved stool consistency after abdomen radiotherapy and increased stool butyrate content which is involved in controlling tumor cells proliferation and apoptosis. Future large, well-designed randomized controlled trials should be designed in order to discover inulin and oligofructose efficiency in controlling cancer outcomes by virtue of various appropriate biomarkers.
ACKNOWLEDGEMENTS
We would like to acknowledge Parvin Ebadi at the Ahvaz Jundishapur University of Medical Sciences assisted with searching journal abstracts.
Authors’ Contributions
SMHMJ and RM contributed to the protocol for this review.
SMHMJ, FAS and SMAN contributed in drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. doi:10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Norman HA, Butrum RR, Feldman E, Heber D, Nixon D, Picciano MF, et al. The role of dietary supplements during cancer therapy. J Nutr. 2003;133(11):3794S–3799S. doi: 10.1093/jn/133.11.3794S. doi:10.1093/jn/133.11.3794S. [DOI] [PubMed] [Google Scholar]
- 3.Song M, Wu K, Meyerhardt JA, Ogino S, Wang M, Fuchs CS, et al. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 2018;4(1):71–79. doi: 10.1001/jamaoncol.2017.3684. doi:10.1001/jamaoncol.2017.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21(3):411–418. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Virtamo J, Pietinen P, Huttunen J, Korhonen P, Malila N, Virtanen M, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation:a postintervention follow-up. JAMA. 2003;290(4):476–845. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Waterhouse AL, Chatterton NJ. Proton and Carbon NMR Chemical-shift Assignments for [β-d-Fruf-(2- 1)] 3-(2- 1)-α-d-Glcp (nystose) and [β-d-Fruf-(2- 1)] 4-(2- 1)-α-d-Glcp (1, 1, 1-kestopentaose) from Two-Dimensional NMR Spectral Measurements. Carbohydr Res. 1993;245(1):11–19. doi: 10.1016/0008-6215(93)80056-k. [DOI] [PubMed] [Google Scholar]
- 7.Giammona G, Mauro N, Scialabba C. Inulin for Cancer Therapy:Present and Perspectives. Int J Pharm Sci Rev Res. 2017;5 [Google Scholar]
- 8.Van der Zee M, Stoutjesdijk J, Van der Heijden P, De Wit D. Structure-biodegradation relationships of polymeric materials. 1. Effect of degree of oxidation on biodegradability of carbohydrate polymers. J Environ Polym Degrad. 1995;3(4):235–242. [Google Scholar]
- 9.Won CY, Chu CC. Inulin polysaccharide having pendant amino acids:synthesis and characterization. J Appl Polym Sci. 1998;70(5):953–963. [Google Scholar]
- 10.Hu Y, Zhang J, Yu C, Li Q, Dong F, Wang G, et al. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. Int J Biol Macromol. 2014;70:44–49. doi: 10.1016/j.ijbiomac.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Franck A, De Leenheer L. Biopolymers Online. KGaA, Weinheim: Wiley-VCH Verlag GmbH &Co; 2005. Inulin. [Google Scholar]
- 12.Jain AK, Sood V, Bora M, Vasita R, Katti DS. Electrosprayed inulin microparticles for microbiota triggered targeting of colon. Carbohydr Polym. 2014;112:225–234. doi: 10.1016/j.carbpol.2014.05.087. doi:10.1016/j.carbpol.2014.05.087. [DOI] [PubMed] [Google Scholar]
- 13.Sahiner N, Sagbas S. Multifunctional tunable p (inulin) microgels. Mater Sci Eng C. 2014;40:366–372. doi: 10.1016/j.msec.2014.04.028. doi:10.1016/j.msec.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Taper HS, Roberfroid MB. Possible adjuvant cancer therapy by two prebiotics-inulin or oligofructose. In vivo. 2005;19(1):201–204. [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br Med J (Clin Res Ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. doi:10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Peris P, Velasco C, Hernandez M, Lozano M, Paron L, De La Cuerda C, et al. Effect of inulin and fructo-oligosaccharide on the prevention of acute radiation enteritis in patients with gynecological cancer and impact on quality-of-life:a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2016;70(2):170. doi: 10.1038/ejcn.2015.192. doi:10.1038/ejcn.2015.192. [DOI] [PubMed] [Google Scholar]
- 17.Limburg PJ, Mahoney MR, Ziegler KLA, Sontag SJ, Schoen R, Benya RV, et al. Randomized phase II trial of sulindac, atorvastatin and prebiotic dietary fiber for colorectal cancer chemoprevention. Cancer Prev Res. 2011;0215 doi: 10.1158/1940-6207.CAPR-10-0215. doi:10.1158/1940-6207.CAPR-10-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutron-Ruault M-C, Marteau P, Lavergne-Slove A, Myara A, Gerhardt M-F, Franchisseur C, et al. Effects of a 3-mo consumption of short-chain fructo-oligosaccharides on parameters of colorectal carcinogenesis in patients with or without small or large colorectal adenomas. Nutr Cancer. 2005;53(2):160–168. doi: 10.1207/s15327914nc5302_5. [DOI] [PubMed] [Google Scholar]
- 19.Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108(4):975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Gibson G. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J Appl Microbiol. 1993;75(4):373–380. doi: 10.1111/j.1365-2672.1993.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsuyuki S, Yamazaki S, Akashiba H, Kamimura H, Sekine K, Toida T, et al. Tumor-suppressive effect of a cell wall preparation, WPG, from Bifidobacterium infantis in germfree and flora-bearing mice. Bifidobacteria and Microflora. 1991;10(1):43–52. [Google Scholar]
- 22.Sekine K, Watanabe-Sekine E, Ohta J, Toida T, Tatsuki T, Kawashima T, et al. Induction and activation of tumoricidal cells in vivo and in vitro by the bacterial cell wall of Bifidobacterium infantis. Bifidobacteria and Microflora. 1994;13(2):65–77. [Google Scholar]
- 23.Kok N, Roberfroid M, Robert A, Delzenne N. Involvement of lipogenesis in the lower VLDL secretion induced by oligofructose in rats. Br J Nutr. 1996;76(6):881–890. doi: 10.1079/bjn19960094. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita K, Kawai K, Itakura M. Effects of fructo-oligosaccharides on blood glucose and serum lipids in diabetic subjects. Nutr Res. 1984;4(6):961–966. [Google Scholar]
- 25.Fiordaliso M, Kok N, Desager J-P, Goethals F, Deboyser D, Roberfroid M, et al. Dietary oligofructose lowers triglycerides, phospholipids and cholesterol in serum and very low density lipoproteins of rats. Lipids. 1995;30(2):163–167. doi: 10.1007/BF02538270. [DOI] [PubMed] [Google Scholar]
- 26.Pool-Zobel BL. Inulin-type fructans and reduction in colon cancer risk:review of experimental and human data. Br J Nutr. 2005;93(S1):S73–S90. doi: 10.1079/bjn20041349. [DOI] [PubMed] [Google Scholar]
- 27.Langlands S, Hopkins M, Coleman N, Cummings J. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53(11):1610–1616. doi: 10.1136/gut.2003.037580. doi:10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pool-Zobel B, Van Loo J, Rowland I, Roberfroid M. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br J Nutr. 2002;87(S2):S273–S281. doi: 10.1079/BJNBJN/2002548. [DOI] [PubMed] [Google Scholar]
- 29.Giralt J, Regadera JP, Verges R, Romero J, de la Fuente I, Biete A, et al. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea:results from multicenter, randomized, placebo-controlled nutritional trial. Int J Radiat Oncol Biol Phys. 2008;71(4):1213–1219. doi: 10.1016/j.ijrobp.2007.11.009. doi:10.1016/j.ijrobp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial:impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33(5):761–767. doi: 10.1016/j.clnu.2013.10.015. doi:10.1016/j.clnu.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Clark MJ, Robien K, Slavin JL. Effect of prebiotics on biomarkers of colorectal cancer in humans:a systematic review -Nutr Rev. 2012;70(8):436–443. doi: 10.1111/j.1753-4887.2012.00495.x. doi:10.1111/j.1753-4887.2012.00495.x. [DOI] [PubMed] [Google Scholar]
- 32.Pajari AM, Rajakangas J, Päivärinta E, Kosma VM, Rafter J, Mutanen M. Promotion of intestinal tumor formation by inulin is associated with an accumulation of cytosolic β-catenin in Min mice. Int J Cancer. 2003;106(5):653–660. doi: 10.1002/ijc.11270. [DOI] [PubMed] [Google Scholar]
- 33.Misikangas M, Pajari A-M, Päivärinta E, Mutanen M. Promotion of adenoma growth by dietary inulin is associated with increase in cyclin D1 and decrease in adhesion proteins in Min/+mice mucosa. J Nutr Biochem. 2005;16(7):402–409. doi: 10.1016/j.jnutbio.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Misikangas M, Tanayama H, Rajakangas J, Lindén J, Pajari A-M, Mutanen M. Inulin results in increased levels of β-catenin and cyclin D1 as the adenomas increase in size from small to large in the Min/+mouse. Br J Nutr. 2008;99(5):963–970. doi: 10.1017/S0007114507853414. [DOI] [PubMed] [Google Scholar]


