Abstract
BACKGROUND
Glioblastoma is the most common primary malignancy of the brain, with a dismal prognosis. Immunomodulation via checkpoint inhibition has provided encouraging results in non-CNS malignancies, but prediction of responders has proven to be challenging in glioblastoma patients.
OBJECTIVE
To determine the proportion of patients who have a measurable increase of interferon gamma levels in brain tumor tissue after their first dose of nivolumab, and to evaluate the safety of using brain tumor microdialysis to monitor for immune response while evaluating the safety of the combination of anti-programmed death 1 (PD-1) and anti-lymphocyte activation gene 3 (LAG-3) checkpoint inhibition.
METHODS
The study design is a single-center, nonrandomized phase 1 clinical trial. Up to 15 adult patients with recurrent glioblastoma will be enrolled with the goal of 10 patients completing the trial over an anticipated 18 mo. Patients will undergo biopsy; placement of microdialysis catheters and lumbar drains; treatment with anti-PD-1 checkpoint inhibition; comprehensive immune biomarker collection; tumor resection; and then treatment with anti-PD-1 and anti-LAG-3 checkpoint inhibition until progression.
EXPECTED OUTCOMES
We expect interferon gamma levels to increase in the brain as measured via microdialysis in treated patients. Based on published reports, microdialysis in this patient population is expected to be safe, and anti-LAG-3 and anti-PD-1 combined will likely have a similar side effect profile to other checkpoint inhibitor combinations.
DISCUSSION
The failure of recent trials of immune therapies in glioblastoma underscores the need to appropriately measure response in the treated tissue. This trial may provide insight on indicators of which patients will respond to immune therapy.
Keywords: Glioblastoma, Nivolumab, Microdialysis, Brain neoplasms, Biomarkers, Immunotherapy, Neuro-oncology
ABBREVIATIONS
- CRO
Contract Research Organization
- CSF
cerebral spinal fluid
- IFNg
interferon gamma
- IRB
Institutional Review Board
- LAG-3
lymphocyte activation gene 3
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
GENERAL INFORMATION
Title: Cytokine Microdialysis for Real-Time Immune Monitoring in Glioblastoma Patients Undergoing Checkpoint Blockade.
Study Dates: April 2018 to present.
Sponsor/Funding Agency: Intramural Research Program of the National Institute of Neurological Disorders and Stroke.
Avindra Nath, MD, Office of the Clinical Director, 10 Center Dr, 10/7C103, Bethesda, MD 20892.
Bristol-Myers Squibb (drug only support)–Bristol-Myers Squibb may review any manuscript prior to publication to ensure that it contains no violations of confidentiality and protects relevant intellectual property.
Center for Human Immunology, Autoimmunity, and Inflammation.
Registry: Clinicaltrials.gov: NCT03493932 (Registration Date: April 11, 2018).
Institutional Approvals: National Institutes of Health Combined Neurosciences Internal
Review Board number - 18-N-0077; Date of This Submission/Version: 03/21/18/v1.0; Food and Drug Administration Investigational New Drug Application number: 136039.
Investigators: Address: National Institutes of Health Clinical Center, Room 3D-20, 10 Center Drive, Bethesda, MD 20814.
Edjah K. Nduom, MD, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, Maryland, Phone: 301-480-0284, Twitter: @eknduom, Email: edjah.nduom@nih.gov.
RATIONALE AND BACKGROUND INFORMATION
Glioblastoma is the most common primary malignancy of the brain, and the prognosis with conventional therapy is poor, with a median survival of only 14.6 mo after standard chemotherapy and radiation.1 Recurrent glioblastoma treatment lacks high-quality evidence supporting its effectiveness.
Many years of study into the microenvironment of diffuse gliomas has shown that these lesions produce immunosuppressive factors, allowing these lesions to continue to proliferate unchecked,2 despite many having an influx of immune cells.3 To enhance immune responses to malignant tumors, investigators have recently developed monoclonal antibodies that target and inactivate immunosuppressive pathways.
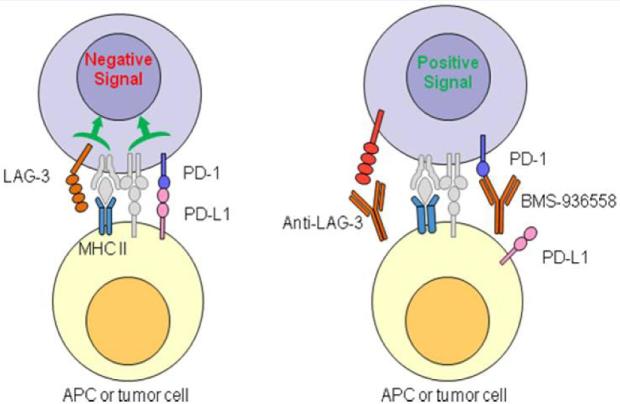
Programmed death 1 (PD-1) is a T-cell surface immunosuppressive receptor; it is activated by programmed death ligand 1 and 2 (PD-L1 and PD-L2).4 PD-L1 is upregulated in the tumor microenvironment of many tumors, and engagement of this ligand with its receptor, PD-1, reduces the cellular immune response within the tumor microenvironment.4,5 Targeting of the immunosuppressive checkpoint, PD-1, has provided initial evidence that immune therapy can be applied successfully in other cancers. Nivolumab, a monoclonal antibody targeting PD-1, was found to be efficacious and approved6 for advanced melanoma, as was pembrolizumab, another PD-1 inhibitor.7 Lymphocyte activation gene 3 (LAG-3) is another checkpoint present on activated T cells.8 LAG-3 and PD-1 are co-expressed on the surface of tumor infiltrating T-cells in ovarian cancer and glioblastoma patients, and these markers denote poorly functional CD8+ T effector cells.9,10 The efficacy of single agent checkpoint inhibition may be limited by compensatory upregulation of other checkpoints, and this may be overcome by using combinations of checkpoint inhibitors (Figure 1).11
FIGURE 1.
Model for Augmented T-cell Activity Mediated by Inhibition of LAG-3 and PD-1 (Courtesy, Bristol-Myers Squibb; BMS-986016 Investigator Brochure, Appendix C).
For the past few years, there has been widespread belief that checkpoint inhibitors, such as nivolumab and anti-LAG-3 therapy, could effectively target immunosuppression in the glioblastoma microenvironment, just as they have done in other systemic tumors. Preclinical models have supported this argument, with murine anti-PD-1, anti-PD-L1, and multi-checkpoint inhibitor treatments demonstrating their efficacy by increasing overall survival in murine models of gliomas.12-14
Yet, the successes of these therapeutics in treating diffuse glioma in humans are not guaranteed for numerous reasons. Most preclinical models rely on the murine immune system, which is very different from the human immune system.15 Human and nonhuman models have differing cytokine profiles and dynamics for cell infiltration and inflammation patterns. Accordingly, the results of these murine studies cannot be automatically applied to human patients.
Additionally, even in the successful trials of these checkpoint inhibitors in other cancers, these treatments seem to only be effective in a small subset of patients.16 Immune phenotyping has been attempted during checkpoint inhibitors trials in other cancers.17 To date, none of these studies has yielded an accurate way to predict an immune reaction in the brain in response to checkpoint inhibition.18
The evaluation of an immune response in glioblastoma is further complicated by the unique microenvironment of the brain. We have shown that immune cells can infiltrate the glioblastoma microenvironment,19,20 yet further research is necessary to determine if systemically administered checkpoint inhibitors impact the tumor microenvironment and evoke an immune effect. To be comprehensive, such an analysis needs to include the measurement of cytokine expression in the immune microenvironment.
Local cytokine expression has not been evaluated in glioblastoma patients undergoing checkpoint inhibition, even though there is evidence that inflammatory cytokines could signal an effective response to checkpoint inhibitors. A recent study in mice has shown that PD-1 blockade leads to an upregulation of interferon gamma (IFNg) production in circulating T lymphocytes responding to an infection.21 This agrees with previous research that shows that PD-L1 blockade upregulated IFNg production from T cells co-cultured with glioma tumor cells in vitro.22 Anti-PD-1 therapy has also been shown to increase IFNg expression in the tumor microenvironment in a preclinical model of adoptive cell transfer.23 Further, in recurrent glioblastoma patients, there is evidence that regulatory T cells in the systemic circulation of these patients release increasing amounts of IFNg in response to nivolumab.24
Our plan is to collect an unprecedented set of matched longitudinal samples directly from the tumor microenvironment of glioblastoma patients before and after treatment with a checkpoint inhibitor to determine if this systemic treatment has a local neuroinflammatory effect. We will be able to use the results of this data collection to better predict how the brain might react to immune therapeutics.
STUDY GOALS AND OBJECTIVES
Primary Goals
To determine the proportion of patients who have a measurable increase of IFNg levels in the brain tumor tissue after their first dose of nivolumab as compared to the pretreatment baseline as measured by cerebral microdialysis,
To evaluate the safety and feasibility of using brain tumor microdialysis to monitor response to immune modulators in patients with recurrent glioblastoma, and
To evaluate the safety of the combination of nivolumab and anti-LAG-3 antibody treatment.
Secondary Goals
To determine the change in IFNg production within the tumor microenvironment and in the rest of the body before and after therapy with nivolumab;
To evaluate the histologic response of the immune microenvironment of brain tumor tissue to the first dose of nivolumab;
To evaluate the clinical response (progression free survival, overall survival) of recurrent glioblastoma patients to treatment with BMS-986016 and nivolumab;
To describe the difference in survival between responders and nonresponders;
To examine the differences in the immune cells and secreted factors of the tumor environment as compared to the immune cells and secreted factors of the cerebral spinal fluid (CSF), blood, and bone marrow in response to this treatment.
STUDY DESIGN
This protocol is an open-label single-armed pilot study of the investigational off-label use of nivolumab and BMS-986016 in recurrent glioblastoma patients.
Study Populations
Eighteen or older, with first recurrence of their previously treated glioblastoma (see Table 1 for full Inclusion and Exclusion Criteria).
Accrual ceiling will be 15.
Patients will be recruited to the NIH Clinical Center in Bethesda, MD.
Target of 10 completed patients.
Withdrawals which do not complete the initial study procedures will be replaced up to the accrual ceiling of 15 patients (see Table 2 for Criteria for Withdrawal from Study).
TABLE 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Be 18 yr of age or older | 1. Have a bleeding disorder that cannot be corrected before invasive testing or surgery, or other medical conditions that would make surgery or general anesthesia unsafe, or severe immunodeficiency or systemic cancer not related to a brain lesion |
| 2. Have solitary recurrent glioblastoma that is amenable to surgical resection | 2. Are pregnant or breastfeeding. Pregnancy testing will be performed prior to study enrollment at each outpatient MRI scan |
| 3. Agree to undergo brain surgery | 3. Cannot have an MRI scan |
| 4. Are eligible for 03-N-0164 “Evaluation and Treatment of Neurosurgical Disorders” protocol | 4. Are claustrophobic |
| 5. Willing and able to appoint a durable power of attorney | 5. Are not able to lie on their back for up to 60 min |
| 6. Are not on steroids, or are on a stable (without an increase for the preceding 2 wk) or decreasing dose of steroids | 6. Have an absolute lymphocyte count less than 500 |
| 7. Are willing to use an effective method of contraception during the clinical study as defined on the consent and for 5 mo (for women) or 7 mo (for men) after the last dose of the study drug | 7. Have multiple brain tumors, evidence of carcinomatous meningitis, gliomatosis cerebri, or primary CNS lymphoma |
| 8. Have an unrelated autoimmune disease | |
| 9. Have a significant cardiac history, such as 2 or more MIs OR 2 or more coronary revascularization procedures | |
| 10. Have abnormal findings on ECG such as prolonged QT interval, T-wave abnormalities or arrhythmia. Abnormal findings on ECG will prompt an evaluation by a cardiologist prior to enrolment in the study | |
| 11. Are currently undergoing treatment with another therapeutic agent for glioblastoma | |
| 12. Have a history of having been treated with immune therapy | |
| 13. Have an ejection fraction <50% on screening echocardiogram | |
| 14. Have positive serology for HIV, HBV, and HCV | |
| 15. Have an active infection that requires systemic antibacterial, antiviral, or antifungal therapy <7 d prior to initiation of study drug therapy | |
| 16. Have a history of transfer of autologous or allogeneic T cells | |
| 17. Have cardiac Troponin T or I >2x the institutional upper limit of normal at screening | |
| 18. At the time of enrolment, lack of consent capacity due to cognitive impairment that would make them incapable of understanding the explanation of the procedures in this study. Cognitive capacity to consent will be determined at the time of enrollment. Patients with mental disorders or those patients who are cognitively impaired yet still retain consent capacity will not be excluded | |
| 19. Cannot speak English or Spanish fluently |
TABLE 2.
Criteria for Withdrawal From study
| Subjects must be withdrawn from the study at any time for the following reasons: | |
| 1. Subject's prerogative: The subject desires to discontinue participation in this study | |
| 2. Investigator's prerogative: The subject is unwilling or unable to comply with the protocol | |
| 3. The biopsy indicates that the patient does not have a true pathological recurrence of their tumor | |
| 4. The patient experiences a grade 3 or 4 toxicity during phase 1 of the trial which precludes the completion of the intensive care unit monitoring period | |
| 5. The patient experiences a toxicity consistent with the nivolumab prescribing information that requires discontinuation of nivolumab. Such toxicitieswill be deemed sufficient to discontinue both nivolumab and BMS-986016. Specifically, these toxicities are: | |
| a. Immune-mediated pneumonitis: grade 3 or 4 immune-mediated pneumonitis will prompt automatic discontinuation of study drugs | |
| b. Immune-mediated colitis: grade 4 immune-mediated colitis will prompt automatic discontinuation of study drugs | |
| c. Immune-mediated hepatitis: grade 3 or 4 immune-mediated hepatitis will prompt automatic discontinuation of study drugs | |
| d. Immune-mediated endocrinopathies: grade 4 immune-mediated hypophysitis; grade 3 or 4 immune-mediated adrenal insufficiency; and grade 4hyperglycemia will prompt automatic discontinuation of study drugs. Additionally, thyroid function will be followed, and thyroid hormonereplacement will be given as needed | |
| e. Immune-mediated nephritis and renal dysfunction: grade 4 serum creatinine elevation will prompt automatic discontinuation of study drugs | |
| f. Immune-mediated skin adverse reactions: grade 4 rash will prompt automatic discontinuation of study drugs | |
| g. Immune-mediated encephalitis: grade 4 encephalitis will prompt automatic discontinuation of study drugs | |
| h. Infusion reactions: grade 3 or 4 infusion reactions will prompt automatic discontinuation of study drugs | |
| i. In addition to the above, due to the report of myocarditis in 1 patient on the combination of BMS-986016 and nivolumab, grade 4 myocarditis will prompt automatic discontinuation of study drugs |
METHODOLOGY
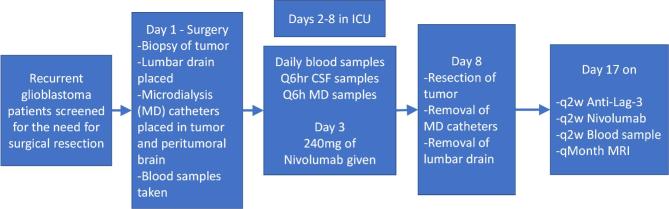
Patients will undergo a surgical procedure that includes stereotactic needle biopsy to confirm the diagnosis of recurrent glioblastoma (Figure 2 – study day 1). Any patient whose diagnosis of recurrent glioblastoma cannot be confirmed on frozen section will be removed from the study and replaced.
FIGURE 2.
Study schematic, demonstrating intensive immune monitoring of trial patients.
Following this confirmation, 2 microdialysis catheters will be implanted: one into the tumor and one into the surrounding brain (Figure 3). A lumbar intrathecal catheter will also be inserted to obtain serial samples of CSF after surgical biopsy. Finally, a bone marrow biopsy will be performed when possible. We will allow the inflammatory response to catheter implantation to subside by waiting 2 d before obtaining the baseline microdialysis and CSF samples. On study day 3, we will administer 240 mg of the PD-1 inhibitor, nivolumab. We will then obtain samples every 6 h from the microdialysis catheters. We will also obtain daily research blood samples and CSF. On study day 8, we will remove the microdialysis catheters and lumbar intrathecal catheter and perform a maximally safe surgical resection of the recurrent glioblastoma tumor. A second bone marrow biopsy will also be performed when possible. We will continue to enroll patients until we have 10 patients who have completed this initial monitoring period and surgical resection.
FIGURE 3.
Microdialysis catheter safely placed by study authors in a patient with recurrent glioblastoma at the NIH Clinical Center during a recently completed blood–brain barrier study.
On day 17 of the trial, each patient will receive 240 mg of nivolumab and 80 mg of an anti-LAG-3 antibody, BMS-986016. They will be treated with nivolumab and BMS-986016 every 2 wk until tumor progression or until the patient experiences an immune mediated toxicity that prevents further treatment. A research blood sample will be taken prior to treatment at each appointment 2 wk.
DISCUSSION
Recent failed trials of immune therapies in glioblastoma highlight the need to better understand the glioblastoma microenvironment to explain why these immune therapies have been ineffective against this tumor type. Specifically, rindopepimut, a vaccine targeted at the epidermal growth factor variant III, failed to improve progression-free survival in newly diagnosed glioblastoma patients during a randomized phase III trial, even though it demonstrated great initial promise in a phase II study.25 Additionally, nivolumab did not show efficacy as a single agent in recurrent glioblastoma when compared to bevacizumab in a randomized study.26,27 Without efforts to better understand these failures, we are doomed to repeat them in the future.
If successful, this trial could lead to a new way to determine whether a patient will respond to immune therapy. Our longitudinal sampling process has broad applicability to different immune therapeutics including checkpoint inhibition, adoptive cell therapy, and tumor vaccines. Once our pilot data have been validated, we could improve the therapeutic index of our immunotherapeutic clinical trials by enriching treatment groups for patients that show early signs of response. Conversely, patients who do not demonstrate markers of early response could be spared further ineffective therapy and be offered a different therapy based on the dynamic changes in their tumor microenvironment.
Beyond our primary objectives, we also believe that this pilot trial has the potential to answer many burning questions. Potential secondary gains could be a better understanding of how CSF samples reflect changes in the immune microenvironment of the brain – is there a reliable relationship between cytokine levels in the interstitial fluid and CSF, or are they completely different and unrelated? What is the relationship between the immune cell populations in the CSF to those found in multiplex IHC in the tumor and surrounding brain? Are there serum markers that predict intracranial inflammation better than CSF markers do? Is the timeline of response of these markers in the various compartments – microdialysate, CSF, and serum – similar or different after systemic immune stimulation?
While we feel that this protocol will provide critical information for the treatment of brain tumors, we also feel that the study has the potential to create generalizable knowledge that will have a high impact in the scientific community. This trial presents an opportunity to reinvent how we measure neuroinflammation.
TRIAL STATUS
At the time of manuscript submission, the status of the trial is recruiting.
SAFETY CONSIDERATIONS
Our trial design includes rigorous safety evaluations, including an in-depth review after each of the first 3 patients by a Safety Monitoring Committee. If it is determined at any point that performance of cerebral microdialysis for cytokines presents an undue risk to our patients, the pilot trial will be halted. At that time, the pilot would be reassessed and only reopened using less invasive means to obtain biomarkers of an immune response. These alternative means would include serial CSF collection and the use of pre- and posttreatment surgical specimens to determine pathological response.
The risk of placement and use of microdialysis catheters is reasonable in this patient group. The MD 71 microdialysis catheter has been used in multiple recent trials in glioma patients without any associated morbidity.28-30 The stereotactic biopsy performed as a part of the microdialysis implantation surgery carries a 1% risk of symptomatic hemorrhage.31
In clinical trials using nivolumab for other indications, the most common adverse events (graded using the Common Terminology Criteria for Adverse Events), regardless of causality, were fatigue, decreased appetite, diarrhea, nausea, cough, dyspnea, constipation, vomiting, rash, pyrexia, and headache. Common treatment-related adverse events included fatigue, rash, diarrhea, pruritus, decreased appetite, and nausea. Serious adverse effects were rare and included grade 3 or 4 pneumonitis, sarcoidosis, and elevated alanine aminotransferase levels.
While patients are at home, they will be instructed to contact us for any suspected adverse events. Generally, for any reported new symptom or adverse event, nonimmune events (such as disease progression) will be ruled out. If the adverse event is deemed to be related to nivolumab or BMS-986016, the side effects will be generally managed with administration of high-dose (2-4 mg/kg prednisolone equivalent) corticosteroids followed by a tapered dose and interruption of nivolumab therapy.
FOLLOW-UP
The patients will undergo a magnetic resonance imaging every 4 wk to detect signs of recurrence/pseudoprogression. It has been our clinical practice at the NIH Clinical Center to consider tissue sampling in patients when imaging changes and steroid challenges do not clearly identify whether a patient has recurrence or pseudoprogression due to an inflammatory response to checkpoint inhibition. At the completion of enrollment, we will analyze the CSF, microdialysis fluid, and blood for immune markers by proteomic analysis. We will measure changes in immune markers in the various compartments (brain tumor, brain, blood, CSF, bone marrow) before and after each patient's first dose of nivolumab.
DATA MANAGEMENT AND STATISTICAL ANALYSIS
Data Management
Research samples and data will be coded for storage and analysis. The codes will be maintained by the Principal Investigator and the study coordinator. Research data will not be used to make treatment decisions and will not be placed in the patient's chart.
Data and samples may be shared with collaborating laboratories at NIH or outside of NIH and/or submitted to NIH-designated repositories and databases. Coded data and samples will be shared with Bristol-Myers Squibb.
Statistical Analysis
The accrual ceiling for the study will be 15 patients with the target of 10 patients completing the study. Adequate patients will be recruited through an Institutional Review Board (IRB)-approved flyer. Table 3 indicates that a sample size of 10 subjects: (a) will have 94.5% power to reject the null hypothesis: P ≤ 0.1 when ≥6 subjects are found to have increased IFNg in 5 d after the treatment and (b) will have 93.0% power to reject the null hypothesis: P ≤ 0.4 when ≥9 subjects are found to have increased IFNg. Table 4 presents the 95% confidence limits for the observed binomial proportion 0.3 to 1.0 based on a sample size of 10 subjects. For a sample size of 10 subjects, if 7 subjects are observed with increased IFNg in 5 d, the interval of (0.35, 0.93) will have 95% probability to include the true proportion.
TABLE 3.
The Estimated Power of 10 Subjects for Different Null and Observed Proportions
| Did | Expected Proportion | Actual Alpha | Estimated Power |
|---|---|---|---|
| 0.1 | 0.9 | 0.0128 | >0.999 |
| 0.8 | 0.0128 | >0.999 | |
| 0.7 | 0.0128 | 0.989 | |
| 0.6 | 0.0128 | 0.945 (a) | |
| 0.2 | 0.9 | 0.00637 | 0.998 |
| 0.8 | 0.00637 | 0.967 | |
| 0.7 | 0.00637 | 0.85 | |
| 0.3 | 0.9 | 0.01059 | 0.987 |
| 0.8 | 0.01059 | 0.879 | |
| 0.4 | 0.9 | 0.01834 | 0.930 (b) |
TABLE 4.
95% Confidence Limits (CL) for Proportions With a Sample Size of 10 Subjects
| Observed proportion | 95% CL | |
|---|---|---|
| 1 | 0.6915 | 1 |
| 0.9 | 0.555 | 0.9975 |
| 0.8 | 0.4439 | 0.9748 |
| 0.7 | 0.3475 | 0.9333 (a) |
| 0.6 | 0.2624 | 0.8784 |
| 0.5 | 0.1871 | 0.8129 |
| 0.4 | 0.1216 | 0.7376 |
| 0.3 | 0.0667 | 0.6525 |
QUALITY ASSURANCE
The Principal Investigator will ensure that:
the protocol is being correctly followed,
changes to the protocol have been approved by the IRB,
accurate, complete, and current records are being maintained and are secure,
subject withdrawal or study failure is noted in the records, and
informed consent has been correctly documented.
Emmes (Rockville, Maryland) has been engaged by NINDS as the Contract Research Organization (CRO) to monitor this protocol. The PI/sponsor, via the CRO, will be responsible for providing adequate oversight of the investigation to ensure adequate protection of the rights, welfare, and safety of human subjects and the quality and integrity of the resulting data.
This protocol will undergo periodic review by the NINDS Quality Assurance Audit Committee. The protocol is a more than minimal risk study and will be monitored based on the agreed upon monitoring plan with the CRO, at least once yearly. Adverse events will be reported to the FDA and NIH Combined Neurosciences Institutional Review Board in accordance with federal guidelines.
EXPECTED OUTCOMES OF THE STUDY
Based on preclinical data, we expect to see a predictable increase in IFNg levels in the microdialysate after systemic treatment with a checkpoint inhibitor. However, we cannot know for sure that we will be able to accurately detect an increase in IFNg levels in recurrent glioblastoma patients by cerebral microdialysis. While IFNg has been reliably recovered by cerebral microdialysis in the past,28 there is a paucity of studies evaluating the response of the human cerebral microenvironment to immune interventions. For this reason, we will not only perform proteomic testing on the microdialysate, but we will be analyzing CSF, serum, and biopsy samples by this method as well. In the CSF and serum samples, there will be no concerns about accuracy of recovery based on the size of the cytokine being evaluated relative to microdialysis catheter pore size. Finally, as outlined above, our systematic sampling of tissue both before and after treatment with checkpoint inhibitors gives us an additional method to test whether the tumor has, in fact, responded to systematic immune modulation. We will use paired comparisons between the number of cells in the tumor microenvironment expressing CD4, CD8, CD3, PD-1, PD-L1, and LAG-3 before and after treatment with nivolumab to determine whether an immune response has occurred.
For evaluation of treatment response in recurrent glioblastoma patients undergoing checkpoint blockade, recent results suggest a median survival of 9 to 10 mo for recurrent glioblastoma patients treated with checkpoint inhibition.26,27 Our study is not powered to detect a significant difference in survival between responders and nonresponders. Accordingly, descriptive statistics will be used to evaluate median survival of treated patients.
DURATION OF THE PROJECT
After enrollment of the first patient, we anticipate that it will take a period of 18 mo to complete enrollment of up to 15 patients, 10 of which will have completed phase 1, the inpatient portion of the study. Thereafter, we anticipate 6 mo for completion of initial biomarker analysis and statistical review. We plan to complete longitudinal biomarker analysis, survival analysis, and final statistical review at 24 mo after completion of enrollment.
PROJECT MANAGEMENT
See Table 5 for list and description of all staff associated with study.
TABLE 5.
Investigators and Project Management
| Name | Affiliation | Project Management |
|---|---|---|
| Edjah K. Nduom | SNB/NINDS | He will obtain informed consent from subjects enrolling in the study. He will perform surgical procedures in this study, and will analyze study data |
| John D. Heiss, MD | SNB/NINDS | He will obtain informed consent from subjects enrolling in the study. He will perform surgical procedures in this study and will analyze study data |
| Prashant Chittiboina, MD, MPH | SNB/NINDS | He will obtain informed consent from subjects enrolling in the study. He will perform surgical procedures in this study and will analyze study data |
| Kareem A. Zaghloul, MD, PhD | SNB/NINDS | He will obtain informed consent from subjects enrolling in the study. He will perform surgical procedures in this study and will analyze study data |
| Mark Gilbert, MD | NOB/NCI | He will supervise the diagnosis and management of patients with brain tumors |
| Deric M. Park, MD | NOB/NCI | He will participate in the diagnosis and management of patients with brain tumors |
| Jing Wu, MD, PhD | NOB/NCI | She will participate in the diagnosis and management of patients with brain tumors |
| Sadhana Jackson, MD | NOB/NCI | She will obtain informed consent from subjects enrolling in the study, and she will help with microdialysis sample collection and data analysis |
| Gretchen C. Scott, BSN, RN | SNB/NINDS | She will organize outside medical records for review by the study team. In addition, she will record patient outcomes and adverse events that will be used in data and safety monitoring |
| Christina Piper Hayes, M, CRNP | SNB/NINDS | She will obtain informed consent from subjects enrolling in the study, monitor for adverse events, perform study assessments according to her credentialed qualifications, and complete CRFs |
| Sarah Benzo, BSN, RN | SNB/NINDS | She will organize outside medical records for review by the study team. In addition, she will record patient outcomes and adverse events that will be used in data and safety monitoring |
| Christopher S. Hourigan, MD, DPhil | Hematology Branch/NHLBI | He will perform and oversee bone marrow examinations and analyze study data |
| Amber J. Giles, PhD | NOB/NCI | She will analyze study data |
| John Lynes, MD | SNB/NINDS | He will analyze study data |
| Jinguo Chen, MD | CHI/NIAID | He will analyze study data |
SNB: Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health.
NOB: Neuro-Oncology Branch, National Cancer Institute, National Institutes of Health.
NHLBI: National Heart Lung and Blood Institute, National Institutes of Health.
ETHICS
This is a very demanding research protocol for patients. The additional surgical procedure introduces risk that the patients would not otherwise encounter in routine treatment of their disease. Given the risk, there is a concern that the study neurosurgeons could enroll patients who are not good surgical candidates for the purposes of quickly completing enrollment. For this reason, if any patient has not been directly referred to the study neurosurgeons as a good candidate for surgical resection, the patient's medical record will need to be reviewed by an outside neurosurgeon for suitability as a surgical candidate prior to enrollment. Furthermore, subjects will be counseled that they do not have to participate in this study to receive surgery at the NIH Clinical Center. They may also prefer to receive evaluation and surgical treatment under the care of their own physicians at an outside facility. Screened patients will also be informed of other treatment trials, including those at the NIH Clinical Center, local academic centers, and private institutions.
Capacity to consent will be determined at enrollment by the treating advanced medical practitioner. A lack of consent capacity due to cognitive impairment will preclude the patient from being fully enrolled into the trial (see Table 1). The NIH Ability to Consent Assessment Team will be consulted if there are any concerns regarding a patient's capacity to consent. Participants will receive a verbal explanation of the purposes, procedures, and risks of the study and of their rights as research participants. Participants will have the opportunity to review the written consent form and ask questions regarding this study prior to enrollment.
Disclosures
Funding and support came from: Intramural Research Program of the National Institute of Neurological Disorders and Stroke; Bristol-Myers Squib (drug support); Center for Human Immunology, Autoimmunity and Inflammation.
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015;17(suppl 7):vii9–vii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–5172. [DOI] [PubMed] [Google Scholar]
- 4. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pratt D, Dominah G, Lobel G, et al. Programmed Death Ligand 1 Is a Negative Prognostic Marker in Recurrent Isocitrate Dehydrogenase-Wildtype Glioblastoma. Neurosurgery. published online ahead of print: 2018. (doi: 10.1093/neuros/nyy268). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172(9):5450–5455. [DOI] [PubMed] [Google Scholar]
- 9. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107(17):7875–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woroniecka K, Chongsathidkiet P, Rhodin KE, et al. T Cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang RY, Francois A, McGray AR, Miliotto A, Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. OncoImmunology. 2017;6(1):e1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;(20):5290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 15. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. [DOI] [PubMed] [Google Scholar]
- 16. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015;3(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan AC, Heimberger AB, Khasraw M. Immune checkpoint inhibitors in gliomas. Curr Oncol Rep. 2017;19(4):23. [DOI] [PubMed] [Google Scholar]
- 19. Nduom EK, Wei J, Yaghi NK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ranjan S, Quezado M, Garren N, et al. Clinical decision making in the era of immunotherapy for high grade-glioma: report of four cases. BMC Cancer. 2018;18(1):v119.2–v119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Honda T, Egen JG, Lammermann T, Kastenmuller W, Torabi-Parizi P, Germain RN. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014;40(2):235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7467. [PubMed] [Google Scholar]
- 23. Peng WY, Liu CW, Xu CY, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72(20):5209–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lowther DE, Goods BA, Lucca LE, et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight. 2016;1(5):e85935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schuster J, Lai RK, Recht LD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17(6):854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reardon DA, Omuro A, Brandes AA, et al. OS10.3 randomized Phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol. 2017;19(suppl_3):iii21–iii21. [Google Scholar]
- 28. Portnow J, Badie B, Liu X, et al. A pilot microdialysis study in brain tumor patients to assess changes in intracerebral cytokine levels after craniotomy and in response to treatment with a targeted anti-cancer agent. J Neurooncol. 2014;118(1):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tabatabaei P, Visse E, Bergstrom P, Brannstrom T, Siesjo P, Bergenheim AT. Radiotherapy induces an immediate inflammatory reaction in malignant glioma: a clinical microdialysis study. J Neurooncol. 2017;131(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marcus HJ, Carpenter KL, Price SJ, Hutchinson PJ. In vivo assessment of high-grade glioma biochemistry using microdialysis: a study of energy-related molecules, growth factors and cytokines. J Neurooncol. 2010;97(1):11–23. [DOI] [PubMed] [Google Scholar]
- 31. Field M, Witham TF, Flickinger JC, Kondziolka D, Lunsford LD. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J Neurosurg. 2001;94(4):545–551. [DOI] [PubMed] [Google Scholar]