Abstract
BACKGROUND
Brain cavernous angiomas with symptomatic hemorrhage (CASH) are uncommon but exact a heavy burden of neurological disability from recurrent bleeding, for which there is no proven therapy. Candidate drugs to stabilize the CASH lesion and prevent rebleeding will ultimately require testing of safety and efficacy in multisite clinical trials. Much progress has been made in understanding the epidemiology of CASH, and novel biomarkers have been linked to the biological mechanisms and clinical activity in lesions. Yet, the ability to enroll and risk-stratify CASH subjects has never been assessed prospectively at multiple sites. Biomarkers and other outcomes have not been evaluated for their sensitivity and reliability, nor have they been harmonized across sites.
OBJECTIVE
To address knowledge gaps and establish a research network as infrastructure for future clinical trials, through the Trial Readiness grant mechanism, funded by National Institute of Neurological Disorders and Stroke/National Institutes of Health.
METHODS
This project includes an observational cohort study to assess (1) the feasibility of screening, enrollment rates, baseline disease categorization, and follow-up of CASH using common data elements at multiple sites, (2) the reliability of imaging biomarkers including quantitative susceptibility mapping and permeability measures that have been shown to correlate with lesion activity, and (3) the rates of recurrent hemorrhage and change in functional status and biomarker measurements during prospective follow-up.
EXPECTED OUTCOMES
We propose a harmonized multisite assessment of enrollment rates of CASH, baseline features relevant to stratification in clinical trials, and follow-up assessments of functional outcomes in relation to clinical bleeds. We introduce novel biomarkers of vascular leak and hemorrhage, with firm mechanistic foundations, which have been linked to clinical disease activity. We shall test their reliability and validity at multiple sites, and assess their changes over time, with and without clinical rebleeds, hence their fitness as outcome instruments in clinical trials.
DISCUSSION
The timing cannot be more opportune, with therapeutic targets identified, exceptional collaboration among researchers and the patient community, along with several drugs ready to benefit from development of a path to clinical testing using this network in the next 5 years.
Keywords: Trial readiness, Cavernous angioma, Cerebral cavernous malformation (CCM), Symptomatic hemorrhage, Drug development, Biomarkers
ABBREVIATIONS
- BVMC
Brain Vascular Malformation Consortium
- CA
cavernous angioma
- CASH
cavernous angiomas with symptomatic hemorrhage
- CCM
cerebral cavernous malformation
- DCEQP
dynamic contrast enhanced quantitative perfusion
- DVA
developmental venous anomaly
- FDA
Food and Drug Administration
- FUBV
follow-up and baseline validation
- MRI
magnetic resonance imaging
- mRS
modified Rankin Scale
- NIH
National Institutes of Health
- NINDS
National Institute of Neurological Disorders and Stroke
- QoL
quality of life
- QSM
quantitative susceptibility mapping
- ROCK
Rho-Associated Coiled-Coil Kinase
- SH
symptomatic haemorrhage
- SWI
susceptibility weighted images
GENERAL INFORMATION
Cavernous Angioma: A Common Lesion, an Uncommon Disease
Cavernous angioma (CA) of the brain is also referred to in the literature as cerebral cavernous malformation (CCM), hemangioma, or cavernoma. The lesion consists of clustered, giant, blood-filled capillary spaces (“caverns”), lined by endothelium, and separated by an amorphous matrix lacking mature vessel wall angioarchitecture.1 CA occurs in a sporadic form, manifesting a solitary lesion or a cluster of lesions associated with a venous developmental anomaly.2 The disease is familial in 20% to 30% of cases manifesting as multiple lesions that develop over time throughout the brain.3 Magnetic resonance imaging (MRI) is the hallmark diagnostic modality for these lesions, revealing chronic and acute hemorrhage and tiny occult lesions (Figure 1). CAs are histologically identical in familial and sporadic cases, and harbor similar somatic mutations.4-6
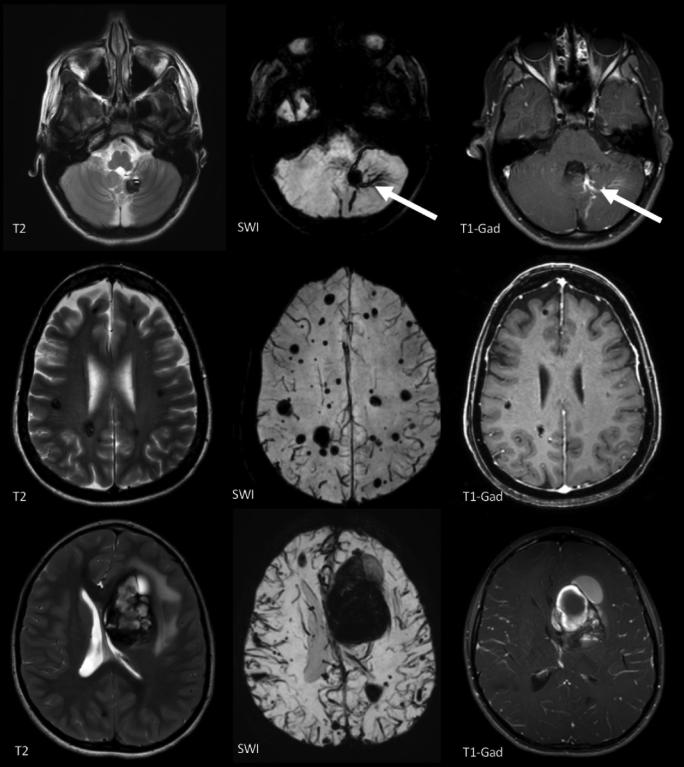
FIGURE 1.
Top row: MRI features of solitary CA, with “popcorn appearance” and surrounding “hemosiderin ring” on T2-weighted image (T2). The solitary CA is often clustered in association with a developmental venous anomaly (DVA, arrows), visualized on susceptibility weighted images (SWI), and T1 with gadolinium (T1-Gad). The SWI reveals no multifocal lesions elsewhere in the brain. Middle row: Autosomal dominant familial multifocal CAs, including multiple lesions on T2, and no DVA on SWI or T1-Gad. There are additional multifocal punctate lesions on SWI, not seen on conventional (T2 and T1) sequences. Bottom row: MRI of deep frontal CA with characteristic features of symptomatic hemorrhage on the same respective images.
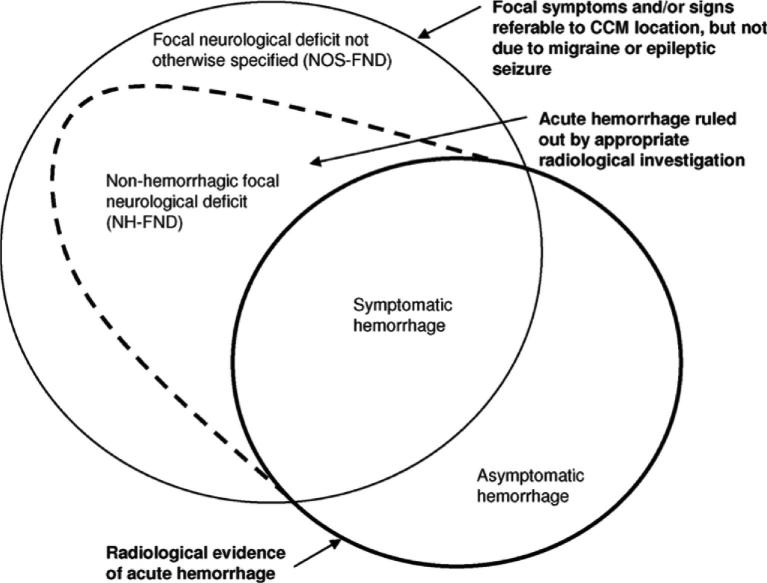
CAs are often detected incidentally, or in association with seizures or nonspecific symptoms.7-9 The natural history of such lesions is exceedingly benign, with <0.5% annual risk of clinically significant hemorrhage, and there is a consensus about these cases not needing interventions beyond symptom management and surveillance.10 But once a lesion has manifested a symptomatic hemorrhage (SH), its untreated clinical course is quite serious, with a 42% (CI 27-58) rate of recurrent bleeding or focal neurological deficit within 5 years.10-12Cavernous Angiomas with Symptomatic Hemorrhage (CASH) is hence a singular clinical entity, distinguishing lesions with a unique risk profile, impacting a patient's life, and meriting clinical intervention. The adjudicated definition of CASH13 requires diagnostic evidence of new lesional bleeding or hemorrhagic growth, in association with directly attributable symptoms (Figure 2).
FIGURE 2.
Spectrum of manifestations of CAs. The SH is a singular clinical event with distinct clinical implications in terms of future risk and deploying clinical interventions. The definition of SH has been rigorously adjudicated. Reproduced with permission from Stroke, Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA, Angioma Alliance Scientific Advisory B. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma Alliance Scientific Advisory Board. Stroke. Dec 2008;39(12):3222-3230.13
Lesions in the brainstem and deep brain locations are more likely than other CAs to bleed,14 rebleed,11 and cause severe disability.12,15 It is unclear if this greater propensity of SH simply reflects the clinical impact of bleeds in those locations, while lesions in less eloquent brain regions may bleed at similar rates but more likely without symptoms. Per current clinical practice and evidence-based guidelines, CAs are typically observed expectantly, and surgically excised only after significant clinical sequelae (typically 1 or more hemorrhagic strokes) particularly in deep or brainstem locations,10,16 with significant public health impact and cost of care.17
While brain surgery for lesion excision may benefit some patients, it can be associated with significant cost.18 Additionally, concerning are complications and morbidity with CA surgical excision of brainstem lesions, including a particularly alarming rate of surgical adverse events.16 In a nonrandomized population-based cohort study, lesion excision was associated with significantly worse functional outcomes and greater complications compared to conservative management.16 Stereotactic radiosurgery has been proposed, but there is controversy about its effectiveness and concern about complications and radiation-induced genesis of new CAs.19,20
The rates of development of new lesions, and of first SH in asymptomatic CAs, are far too low to allow meaningful testing or to compel primary prevention strategies for CASH. Cases with recent SH, where surgical resection is not undertaken (mostly in deep and brainstem locations), are the most likely to be followed expectantly per current evidence-based guidelines,10 with clinical equipoise for testing novel therapies aimed at preventing rebleeding. It would be desirable to develop a drug that stabilizes CASH and prevent recurrent bleeding. This would mitigate neurological sequelae of rebleeds and the complications of surgical resection in many patients. Based on current knowledge of natural history, the likelihood of therapeutic benefit for secondary prevention (symptomatic rebleed) would be greatest within 2 to 3 years after a SH.10-12
RATIONALE AND BACKGROUND INFORMATION
Candidate Therapeutics Under Development
Several candidate therapeutics have emerged in recent years, aimed at targeting signaling aberrations related to the loss of CCM gene function, and associated vascular permeability, angiogenic activity, and inflammatory response. Several drugs have already been shown to prevent lesion development or hemorrhage in preclinical experiments meeting the most stringent National Institute of Neurological Disorders and Stroke (NINDS) criteria of rigor and objectivity. Novel agents are being pursued by the pharmaceutical industry specifically for this disease and are currently at various stages of development. Other drugs in current clinical use for other applications are being explored for proof of concept effect, with the aim of potential repurposing. All these candidate drugs will ultimately require dose optimization and testing of safety and efficacy in multisite clinical trials (Table 1).
TABLE 1.
Candidate Therapeutics Implicated Mechanistically or Under Development for CAs.
| Targeted pathway/function | Drug(s) | Testing/proof of concept status | Development status |
|---|---|---|---|
| RhoA/ROCK | Fasudil26, 44 | Preclinicala | No current pathway for clinical development (side effects and expiration of patent) |
| Statins26 | Preclinicala | Ready for repurposing with IND exemption. Phase I-IIa proof of concept trial proposed | |
| ROCK-2 specific inhibitors (BA-1049) | Drug development stage (SBIR), preclinicala in progress | BioAxone committed to development for CASH indications in humans | |
| Inflammation | Tempol45 | Preclinical effect in acute models | Recursion Pharmaceuticals received Orphan Drug status, committed to development for CASH |
| Immuno-modulators (B-cell depletion)27,46 | Preclinicala | Potential repurposing of B-cell depletion biologics developed by several pharma companies for multiple sclerosis and other indications | |
| N-acetylcysteine47 | Mechanistic Studies | ||
| Avenanthramide48 | Mechanistic Studies | ||
| Platinum nanozymes49 | Mechanistic Studies | ||
| Vitamin D345,50 | Cohort studies showing aggressive CA disease in Vit D deficient subjects50 | Clinical trials not likely because of promiscuous use of Vit D in the population | |
| Angiogenesis/VEGF | Propranolol51,52 | Empiric case studies | Ready for repurposing with IND exemption. Plans for pragmatic exploratory trial |
| VEGF receptor inhibitor (SU5416 semaxanib)53 | Preclinical | Not likely to be pursued because of systemic side effects | |
| Endothelial-mesenchymal transition/β-catenin | Inhibitors of TGF-β and β-catenin (ex: Sulindac metabolites)54-56 | Preclinical | Sulindac proposal to the European Commission |
| Autophagy | mTOR inhibitors, autophagy inducers (ex: Torin 1, Rapamycin)57 | Preclinical | |
| DELTA-NOTCH | Recombinant DLL4,58 Sorafenib59 | Preclinical | |
| MEKK/ERK/KLF | BIX02189 (anti-MEK5),60 XMD17-109 (anti-ERK5)60 | Preclinical |
aIndicates that preclinical effects were shown (Fasudil, statins, B-cell depletion) or planned (BA-1049) in mouse models using contemporaneous randomized treatment assignment versus placebo, and blinded outcome assessment per NINDS guidelines.44
Opportunities in Clinical Research but Many Gaps in Trial Readiness
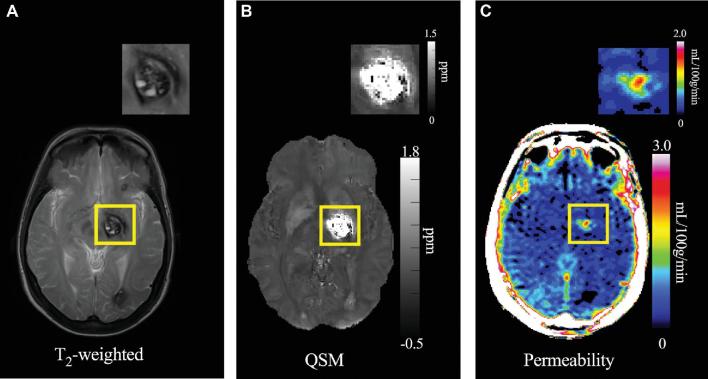
Much progress has been made in understanding the epidemiology and natural history of CAs, and several outcome assessment tools have been proposed, including an adjudicated definition of SH during clinical follow-up. Advanced MRI techniques have applied dynamic contrast-enhanced quantitative perfusion (DCEQP)21-23 and quantitative susceptibility mapping (QSM)23-25 to measure vascular leak and lesional iron content in CAs (Figure 3), and these have been linked to clinical activity in longitudinal follow-up, holding promise as sensitive biomarkers of lesional hemorrhage. There is a strong collaborative culture among major researchers in this disease, catalyzed by a very engaged patient advocacy and support group, the Angioma Alliance (www.angioma.org).
FIGURE 3.
A, T2-weighted image used to assess changes in size (diameter) and any new bleeding.22B, QSM image of the same CA shown with gray-scale map of QSM iron content (ppm). C, DCEQP permeability map of the same lesion with color scale Ki units in ml/100g/min.
However, the ability to screen and enroll cases with CASH, particularly those whose lesion has not been resected, has never been assessed at multiple sites, nor the spectrum of their baseline characteristics, including time from SH, hemorrhagic lesion location, disability status, and demographic features such as sex and age. Quality of life (QoL) has never been systematically evaluated in CAs, and it is unclear what functional status instruments best capture the clinical impact of CASH. Biomarkers of vascular permeability and iron leak in CAs have never been deployed or validated at multiple sites using uniform protocols. These and clinical outcomes instruments have not been harmonized, nor evaluated for their relative sensitivity and reliability, yet are essential to the planning of needed clinical trials. Without this information, clinical trials could use false assumptions and would likely fail.
STUDY GOALS AND OBJECTIVES
In response to these gaps in trial readiness, we proposed Specific Aim 1 to harmonize data entry, and to assess detection rates and baseline characteristics of CASH cases at multiple sites with high volume and clinical interest in CAs. Specific Aim 2 shall deploy rigorous tests of feasibility, accuracy, precision, and reproducibility of biomarker measurements at multiple sites, critical to their potential application as surrogate outcomes in clinical trials. Specific Aim 3 shall assess rates of clinical events versus changes in biomarkers and functional status/QoL measures during prospective follow-up.
STUDY DESIGN
This is an observational cohort study of CASH cases with no intervention. Five high-volume CA sites have been identified for this project. We are activating sites and preparing data structure in year 1, then will prospectively screen and enroll 200 CASH cases in years 2 to 5 at the 5 sites. We shall collect harmonized baseline data and follow a subset of approximately 120 cases with imaging biomarker validation studies to assess and compare clinical outcomes, biomarker outcomes, and functional status and patient-reported QoL outcomes for potential use in future clinical trials.
Inclusion Criteria
18 years of age and older
Diagnosed with a brain CA (single or multiple)
Had a SH within the past year (with demonstrated new lesional bleeding or hemorrhagic growth on diagnostic studies AND attributable new symptoms)
No prior treatment of the symptomatic lesion (after neurosurgical consultation).
Exclusion Criteria
Spinal CA as source of SH
Prior brain irradiation
Cases where verification of SH with clinical and imaging review cannot be accomplished
To be eligible for Aims 2 and 3, CASH cases enrolled in Aim 1 will be further excluded from follow-up and baseline validation (FUBV) for the following reasons:
Contraindication for administration of contrast agent or otherwise unwilling or unable to undergo research MRI studies
Pregnant or breastfeeding women
Homeless or incarcerated persons, or other reason a subject will be unable/unlikely to return for follow-up visits
METHODOLOGY
Knowledge Gaps Regarding the Prevalence of CASH and Baseline Characteristics
For planning and executing multisite clinical trials, it is unclear what fraction of CA patients would meet the likely trial eligibility criteria of SH within the prior year and an unresected lesion. That prevalence varied between 10% and 25% in the three largest ongoing CA research databases at US institutions, with different referral patterns and enrollment criteria (Table 2). This might vary further at other institutions, and when utilizing systematic screening. Prospective prevalence of CASH in systematic screening of CAs, and enrollment rates at multiple sites are not known. These data are essential for the proper design of clinical trials, including the number of prospective sites needed. It is also unclear how CASH cases might vary by age, sex, familial vs sporadic, lesion size and location, associated venous anomaly, past bleeds, and baseline disability. These features varied or were not assessed consistently in the 3 active prospective CA research databases in the US more importantly; the assessment of these features at multiple sites has never been harmonized or adjudicated. Further, it is unclear if functional status and QoL instruments capture the clinical impact of CASH. Inclusion/exclusion criteria and stratification for these characteristics will be important in clinical trials, as they will impact natural risk, and potentially the effect of putative therapies. These critical knowledge gaps will be addressed in Aim 1.
TABLE 2.
Variable Baseline CA Characteristics in the 3 Largest Ongoing Research Databases.
| Baseline characteristics | U Chicagoa | Mayo Rochesterb | BVMCc |
|---|---|---|---|
| Number of cerebral angioma (CA) cases | 245 | 115 | 322 |
| Age at first encounter (range) | 41.1 (0.13-93.5) | 45.5 (18.2-77.1) | 39.3 (0.4-84.9) |
| Sex M/F (ratio) | 76/169 (1/2.2) | 70/45 (1.6/1) | 118/199 (1/1.7) |
| Genotype sporadic/familial (ratio) | 151/94 (1.6/1) | 82/33 (2.5/1) | 0/322 (0/1) |
| Age at diagnosis (range) | 37.1 (0.3-93) | 43.4 | 31.9 (0.3-83.8) |
| Number of patients with past SH | 113 | 62 | 97 |
| Number of SHs(range) | 0.61 (0-14) | 0.3 (0-6) | |
| Number of CASH, SH within prior year, lesion unresected, age > 17 (% CA cases) | 42 (17) | 29 (25) | 31 (9.6) |
| Age at diagnosis of CASH (range) | 42.5 (21.3-71.3) | 45.1 (18.1-61.6) | 50.9 (19.7-84.9) |
| Sex M/F (ratio) | 16/26 (1/1.6) | 19/10 (1.9/1) | 13/18 (1/1.4) |
| Genotype sporadic/familial (ratio) | 28/14 (2/1) | 21/8 (2.65/1) | 0/31 (0/1) |
| Age at CA diagnosis (range) | 38.5 (7-72) | 46 (18.4-70.4) | 40.6 (12.2-83.8) |
| CASH lesion location (% of CASH cases) | Not collected | ||
| Brainstem | 23 (55) | 17 (58) | |
| Cerebellar | 2 (5) | 0 | |
| Deep supratentorial | 6 (14) | 4 (14) | |
| Lobar | 11 (26) | 7 (24) | |
| Associated venous anomaly (% of CASH cases) | 22 (52) | 11 (38) | 0 |
| CASH cases with mRS > 1 (%) | 11 (26%) | 19 (65%) | 17 (57%) |
aSubjects enrolled in biomarker studies 2013 to 2016, do not include other CA patients evaluated clinically without research enrollment.
bProspective Cohort study beginning in January 2015 where patients with CA identified clinically must consent to ongoing follow up.
cBrain Vascular Malformation Consortium (BVMC): Familial CA cases recruited in 2 phases: BVMC1 cases all had the same gene mutation in CCM1 (Q455X, aka “Common Hispanic Mutation”) and were recruited between 2009 and 2014; BVMC2 cases were recruited based on known genetic mutation in CCM1, CCM2, or CCM3 or having multifocal lesions, or family history, between 2014 and 2019.
Opportunities and Challenges With Novel Biomarkers Linked to Clinical Bleeding in CAs
Vascular leak is a fundamental feature of CAs, mediating hemorrhage and the associated accumulation of nonheme iron (including hemosiderin). Preclinical studies demonstrated rescue of vascular permeability and decreased lesional iron deposition with Rho-Associated Coiled-Coil Kinase (ROCK) inhibitors and statins,26 and B-cell depletion therapy27 in murine CA models recapitulating the human disease. The University of Chicago Neurovascular Program has implemented a novel MRI application of assessing iron deposition in human CAs using QSM.22-25 Mean lesional QSM was shown to reflect actual iron concentrations assessed by mass spectroscopy in resected human CA specimens.25 Researchers in Chicago and New Mexico optimized a second technique, DCEQP measure on MRI in human subjects, reflecting mechanistically postulated vascular hyperpermeability.21-23,28,29 The University of Chicago Neurovascular team used both QSM and DCEQP successfully in over 200 CA subjects and showed strong interobserver agreement in QSM and DCEQP measurements, stability of both measurements in clinically stable lesions, and reproducibility across MRI instrument platforms at the Chicago site.21,24 As predicted by the conservation of mass hypothesis, CA lesions with greater permeability (Ki) had higher lesional iron content (QSM),24 and lesional iron content was greater in older patients and in CAs with prior SH.24 More recent studies demonstrated a significant increase of mean lesional QSM and DCEQP Ki in human CA lesions manifesting interval SH or growth during longitudinal follow-up, while these did not change in stable lesions.23 There were tight, sensitive and specific thresholds of QSM and DCEQP increases in association with clinical events.23 Therefore, it is postulated that the QSM and DCEQP may be used as in Vivo measures of hemorrhagic activity and vascular leak, respectively, and may reflect sensitive, clinically meaningful therapeutic effect in human CAs. No other biomarkers have been as linked mechanistically to CA, nor associated as closely with clinical events.
However, the QSM validations and clinical correlations in CAs have been conducted at a single site to date, and DCEQP was applied to CAs at only 2 sites using slightly different protocols. The Food and Drug Administration (FDA)-approved image acquisition sequences of QSM and DCEQP have been implemented on different MRI instruments from major manufacturers, but their validation at multiple sites have not been reported, nor specifically in CAs. The feasibility, accuracy, precision, and reproducibility of biomarker measurements at multiple sites are critical to their potential application as outcomes instruments in clinical trials. We plan a rigorous approach to address these gaps in Aim 2.
DISCUSSION
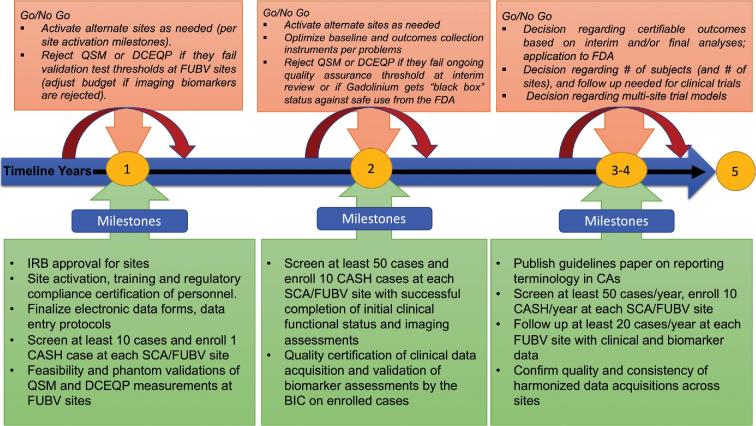
Potential drug development for CASH is very promising but also illustrates what past-NINDS Director Story Landis, PhD described as “an embarrassment of riches.” As with many rare neurological diseases, fundamental discoveries have identified cogent mechanistic targets for therapies, but their development into viable clinical solutions invariably meets obstacles and uncertainties in trial readiness. In this disease, there is a huge opportunity through clinical equipoise to target CAs with recent SH, where surgical resection of a lesion is not undertaken, with the aim of preventing recurrent bleeding within 2 to 3 years. There are unique collaborations among major experts and stakeholders in place, yet a critical “Valley of Death” awaits drug development in the absence of readiness for multisite clinical trials. Obstacles include a lack of harmonization of screening, baseline cohort characterization, prevalence rates and screen/enroll ratios, risk stratification, and the assessment of clinical, functional and biomarker outcomes at multiple sites. Further gaps include the unknown rate and sensitivity (fitness for purpose) of outcome parameters during follow-up, needed to postulate treatment effects. These obstacles and gaps are readily addressed by this current clinical readiness project (Figure 5). The timing cannot be more opportune, with therapeutic targets identified, and several drugs ready to benefit from a track to clinical testing in about 5 years.
FIGURE 5.
Project timeline, milestones, and Go/No Go decision points (SCA, screening and clinical assessment sites; FUBV, follow-up and biomarker validation sites).
We propose for the first time a harmonized multisite assessment of enrollment rates of CASH, baseline features relevant to stratification in clinical trials, and follow-up assessments of functional outcomes and QoL in relation to clinical bleeds. Without this information, clinical trials could use false assumptions and likely fail. We introduce novel biomarkers of vascular leak and hemorrhage, with firm mechanistic foundations, which have been linked to clinical disease activity. We shall test their reliability and validity at multiple sites, and assess their changes over time, with and without clinical rebleeds, hence their fitness as outcomes instruments in clinical trials. Such a comprehensive project has never been undertaken previously in this disease and will facilitate the design of clinical trials for emerging therapies. This project is a model of trial readiness that may be applied in other neurological diseases.
TRIAL STATUS
This trial readiness project is underway as of December 2017 and funded by the NINDS/National Institutes of Health (NIH) U01 NS104157 for 5 years (2017-2022).
FOLLOW-UP
Knowledge Gaps Regarding Follow-up and Outcomes Assessment
Reducing the rate of recurrent SH as a clinical outcome would decrease the burden of disease, since a single SH can lead to devastating neurological sequelae. This would be an approvable outcome, adjudicated, readily defined, and easy to measure. Yet there are many knowledge gaps and potential obstacles to planning clinical trials based on this primary outcome. Attempting to power a study based on recurrent SH would require at least a 1- to 2-years study and a significant number of patients (Table 3). The relative mix of lesions with different rebleed rates will greatly influence the sample size needed to show treatment effect based on this primary outcome. Limiting trials to brainstem lesions would enhance statistical power, but also restrict recruitment and prevent generalizability of a drug's effects to other CASH cases that could benefit. Other studies suggest an equally high rate of SH in deep supra-tentorial and possibly cerebellar CAs.14 The rates of recurrent SH in CASH trial candidates have never been assessed prospectively at multiple sites.
TABLE 3.
Power Calculation Models for Numbers of Patients Needed in Prospective Trials, Based on Different Rebleed Rates and Effect Size Assumptions, Assuming 1.5 years of Treatment/Follow-up.
| Calculated by Parexel, 90% power, 1-sided, Type 1 error 0.025, treatment allocation 1:1, dropout rate 10% | |||
|---|---|---|---|
| Annual rate of recurrent SH in control | Follow-up period | Reduction in SH rate | Sample size/group |
| 6% | 1.5 yr | 25% | 3341 |
| 1.5 yr | 50% | 720 | |
| 15% | 1.5 yr | 25% | 1161 |
| 1.5 yr | 50% | 253 | |
| 30% | 1.5 yr | 25% | 435 |
| 1.5 yr | 50% | 98 | |
Moderate to severe disability after SH has been reported in 11% to 22% of patients.10,30-32 A CA rebleed causes significantly greater disability than initial SH.15 The proportion of CASH cases with any functional disability varied between 26% and 65% at U Chicago, Mayo, and BVMC (Brain Vascular Malformation Consortium) cohorts. Only 2 studies assessed QoL in CAs, and both were limited to surgical patients. One study33 used Karnofsky performance scale, Patzold Rating, and SF-36, and the second34 used only SF-36. Newer QoL scores (eg, Neuro-QoL) have been validated and are more specific for stroke patients compared to SF-36,35-42 and easier to obtain than the Patzold Rating.43 No study to date has reported QoL measures or their changes over time in CASH patients observed without surgery. It will be useful to assess functional outcome and QoL measures at baseline and document their change over time with or without a symptomatic rebleed. One or more may be fit for use as a putative outcome in clinical trials of pharmacotherapies, and may even be useful in future surgical trials. As to the QSM and DCEQP biomarkers of lesional hemorrhage and permeability, even if they are successfully and reliably deployed at multiple sites, it remains unclear how they change during longitudinal follow-up in cases with CASH, and whether their changes are more sensitive than clinical events. The gaps of knowledge regarding outcome measures in clinical trials are addressed in Aim 3.
STATISTICAL ANALYSIS
Trial Modeling and Planning
The statistical analyses plan and sample size calculations for each of the three Aims are presented in the Supplemental Digital Content. Appropriate and complete statistical modeling of data collected by the screening and clinical assessment (SCA) and FUBV sites is imperative to the planning, development, and completion of a successful phase III interventional trial. The most critical component is the accurate assessment of the enrollment rates of CASH across the several sites, as well as the between-site variability in these estimates. Both within and between site variability, to be used to adequately power a phase III trial, will be calculated by developing random-effect models along with using appropriate link functions quantifying the rates of CASH in these models. As a follow-up step, in-depth data analyses will be performed using the demographic, imaging, clinical and biomarker data collected. These are applied to develop models assessing how predictive each of these factors is to the development of recurrent SH.
PROJECT MANAGEMENT/DATA MANAGEMENT/QUALITY ASSURANCE
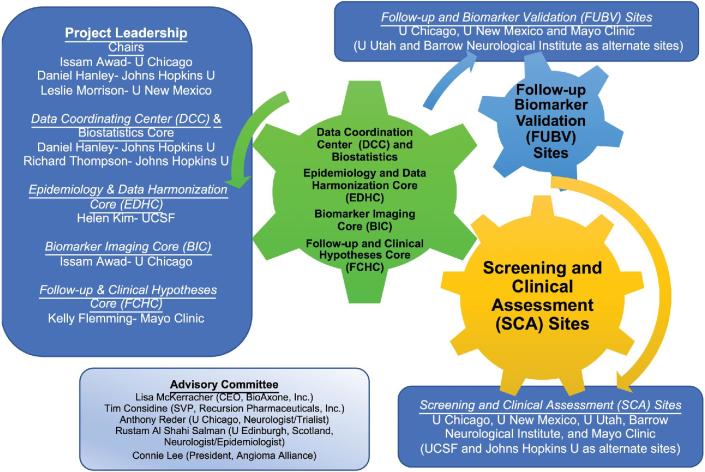
Leading CA research teams have been assembled (Figure 4) with expertise in the questions being tackled, including rigorous imaging validation and statistical approaches, and a data coordinating center with a proven record in trial planning and execution, forming the foundation of the future CA clinical research network. The project shall be overseen by a Central Institutional Review Board and implement an electronic data capture system allowing de-identified data entry and tracking. It includes data quality checks that will be executed at the time of data entry at each site and deploys robust features for data monitoring and security. Liaison was established with the US FDA Biomarker Qualification Program. An Advisory Committee with broad expertise will make suggestions prior to and during the progress of each aim, propose revisions, help us interpret emerging results, and ultimately approve and prioritize workable trial models.
FIGURE 4.
Leadership, scientific cores, clinical sites, and Advisory Committee of the CASH Trial Readiness project.
EXPECTED OUTCOMES OF THE STUDY
Understanding which factors are most predictive of recurrent SH will be crucial in developing a covariate adaptive randomization scheme for the phase III trial that ensures proper balance in these prognostic factors between treatment arms. Finally, statistical assessments of changes in lesional iron content and permeability biomarkers, QoL, and modified Rankin Scale (mRS) during follow-up of CASH patients will be performed. Understanding how these measures change during year 1 and 2 will help optimize the follow-up period in prospective trials, and develop hypotheses on how these measures are affected by treatment (as putative secondary or surrogate outcomes, or as potential composite outcomes).
DURATION OF THE PROJECT
We are activating sites and preparing data structure in year 1, then will prospectively screen and enroll 200 CASH cases in years 2 to 5 at the 5 sites.
ETHICS
All protocols will undergo Institutional Review Board review.
Disclosures
Name and address of the sponsor/funding agency: NINDS/NIH U01 NS104157 (2017-2022); NINDS; NIH; 9000 Rockville Pike; Bethesda, Maryland 20892. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental Digital Content. Methods. Statistical Methods and Sample Size Calculations. This details the sample size calculations per specific aim.
REFERENCES
- 1. Robinson JR Jr, Awad IA, Masaryk TJ, Estes ML. Pathological heterogeneity of angiographically occult vascular malformations of the brain. Neurosurgery. 1993;33(4):547-555; discussion 554-545. [DOI] [PubMed] [Google Scholar]
- 2. Abdulrauf SI, Kaynar MY, Awad IA. A comparison of the clinical profile of cavernous malformations with and without associated venous malformations. Neurosurgery. 1999;44(1):41-46; discussion 46-47. [DOI] [PubMed] [Google Scholar]
- 3. Gault J, Sain S, Hu LJ, Awad IA. Spectrum of genotype and clinical manifestations in cerebral cavernous malformations. Neurosurgery. 2006;59(6):1278-1285; discussion 1284-1275. [DOI] [PubMed] [Google Scholar]
- 4. Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet. 2009;18(5):919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gault J, Shenkar R, Recksiek P, Awad IA. Biallelic somatic and germ line CCM1 truncating mutations in a cerebral cavernous malformation lesion. Stroke. 2005;36(4):872-874. [DOI] [PubMed] [Google Scholar]
- 6. McDonald DA, Shi C, Shenkar R, et al. Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the CCM genes: evidence for a common biochemical pathway for CCM pathogenesis. Hum Mol Genet. 2014;23(16):4357-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75(5):709-714. [DOI] [PubMed] [Google Scholar]
- 8. Bos D, Poels MM, Adams HH, et al. Prevalence, clinical management, and natural course of incidental findings on brain MR images: the population-based Rotterdam Scan Study. Radiology. 2016;281(2):507-515. [DOI] [PubMed] [Google Scholar]
- 9. Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34(5):1163-1169. [DOI] [PubMed] [Google Scholar]
- 10. Akers A, Al-Shahi Salman R, Awad IA, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery. 2017;80(5):665-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horne MA, Flemming KD, Su IC, et al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Shahi Salman R, Hall JM, Horne MA, et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol. 2012;11(3):217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA Angioma Alliance Scientific Advisory Board. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Stroke. 2008;39(12):3222-3230. [DOI] [PubMed] [Google Scholar]
- 14. Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87(2):190-197. [DOI] [PubMed] [Google Scholar]
- 15. Robinson JR. Jr, Awad IA, Magdinec M, Paranandi L. Factors predisposing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery. 1993;32(5):730-736; discussion 735-736. [DOI] [PubMed] [Google Scholar]
- 16. Moultrie F, Horne MA, Josephson CB, et al. Outcome after surgical or conservative management of cerebral cavernous malformations. Neurology. 2014;83(7):582-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christensen MC, Morris S. Association between disability measures and short-term health care costs following intracerebral hemorrhage. Neurocrit Care. 2008;9(3):313-318. [DOI] [PubMed] [Google Scholar]
- 18. Revencu N, Vikkula M. Cerebral cavernous malformation: new molecular and clinical insights. J Med Genet. 2006;43(9):716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pham M, Gross BA, Bendok BR, Awad IA, Batjer HH. Radiosurgery for angiographically occult vascular malformations. Neurosurg Focus. 2009;26(5):1-9. [DOI] [PubMed] [Google Scholar]
- 20. Golden M, Saeidi S, Liem B, Marchand E, Morrison L, Hart B. Sensitivity of patients with familial cerebral cavernous malformations to therapeutic radiation. J Med Imaging Radiat Oncol. 2015;59(1):134-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mikati AG, Khanna O, Zhang L, et al. Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab. 2015;35(10):1632-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mikati AG, Tan H, Shenkar R, et al. Dynamic permeability and quantitative susceptibility: related imaging biomarkers in cerebral cavernous malformations. Stroke. 2014;45(2):598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girard R, Fam MD, Zeineddine HA, et al. Vascular permeability and iron deposition biomarkers in longitudinal follow-up of cerebral cavernous malformations. J Neurosurg. 2016:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan H, Zhang L, Mikati AG, et al. Quantitative susceptibility mapping in cerebral cavernous malformations: clinical correlations. AJNR. Am J Neuroradiol. 2016;37(7):1209-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan H, Liu T, Wu Y, et al. Evaluation of iron content in human cerebral cavernous malformation using quantitative susceptibility mapping. Invest Radiol. 2014;49(7):498-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shenkar R, Shi C, Austin C, et al. RhoA kinase inhibition with fasudil versus simvastatin in murine models of cerebral cavernous malformations. Stroke. 2017;48(1):187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi C, Shenkar R, Zeineddine HA, et al. B-Cell depletion reduces the maturation of cerebral cavernous malformations in murine models. J Neuroimmune Pharmacol. 2016;11(2):369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shenkar R, Shi C, Rebeiz T, et al. Exceptional aggressiveness of cerebral cavernous malformation disease associated with PDCD10 mutations. Genet Med. 2015;17(3):188-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hart BL, Taheri S, Rosenberg GA, Morrison LA. Dynamic contrast-enhanced MRI evaluation of cerebral cavernous malformations. Transl Stroke Res. 2013;4(5):500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83(1):56-59. [DOI] [PubMed] [Google Scholar]
- 31. Li D, Hao SY, Jia GJ, Wu Z, Zhang LW, Zhang JT. Hemorrhage risks and functional outcomes of untreated brainstem cavernous malformations. J Neurosurg. 2014;121(1):32-41. [DOI] [PubMed] [Google Scholar]
- 32. Dammann P, Jabbarli R, Wittek P, et al. Solitary sporadic cerebral cavernous malformations: risk factors of first or recurrent symptomatic hemorrhage and associated functional impairment. World Neurosurg. 2016;91:73-80. [DOI] [PubMed] [Google Scholar]
- 33. Dukatz T, Sarnthein J, Sitter H, et al. Quality of life after brainstem cavernoma surgery in 71 patients. Neurosurgery. 2011;69(3):689-695. [DOI] [PubMed] [Google Scholar]
- 34. Cornelius JF, Kurten K, Fischer I, Hanggi D, Steiger HJ. Quality of Life after surgery for cerebral cavernoma: brainstem versus nonbrainstem location. World Neurosurg. 2016;95:315-321. [DOI] [PubMed] [Google Scholar]
- 35. Cella D, Nowinski C, Peterman A, et al. The neurology quality-of-life measurement initiative. Arch Phys Med Rehabil. 2011;92(10):S28-S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hackett ML, Duncan JR, Anderson CS, Broad JB, Bonita R. Health-related quality of life among long-term survivors of stroke: results from the Auckland Stroke Study, 1991-1992. Stroke. 2000;31(2):440-447. [DOI] [PubMed] [Google Scholar]
- 37. Langton Hewer R. Rehabilitation after stroke. Q J Med. 1990;76(279):659-674. [PubMed] [Google Scholar]
- 38. Wade DT, Wood VA, Heller A, Maggs J, Langton Hewer R. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med. 1987;19(1):25-30. [PubMed] [Google Scholar]
- 39. Abubakar SA, Isezuo SA. Health related quality of life of stroke survivors: experience of a stroke unit. Int J Biomed Sci. 2012;8(3):183-187. [PMC free article] [PubMed] [Google Scholar]
- 40. Patel MD, McKevitt C, Lawrence E, Rudd AG, Wolfe CD. Clinical determinants of long-term quality of life after stroke. Age Ageing. 2007;36(3):316-322. [DOI] [PubMed] [Google Scholar]
- 41. Owolabi MO. Which is more valid for stroke patients: generic or stroke-specific quality of life measures? Neuroepidemiology. 2010;34(1):8-12. [DOI] [PubMed] [Google Scholar]
- 42. Williams LS, Weinberger M, Harris LE, Biller J. Measuring quality of life in a way that is meaningful to stroke patients. Neurology. 1999;53(8):1839-1839. [DOI] [PubMed] [Google Scholar]
- 43. Masur H. Skalen and Scores in der Neurologie. 2nd ed.Stuttgart, Baden-Wurtemberg: Thieme; 2000. [Google Scholar]
- 44. McDonald DA, Shi C, Shenkar R, et al. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke. 2012;43(2):571-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gibson CC, Zhu W, Davis CT, et al. Strategy for identifying repurposed drugs for the treatment of cerebral cavernous malformation. Circulation. 2015;131(3):289-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi C, Shenkar R, Kinloch A, et al. Immune complex formation and in situ B-cell clonal expansion in human cerebral cavernous malformations. J. Neuroimmunol. 2014;272(1-2):67-75. [DOI] [PubMed] [Google Scholar]
- 47. Goitre L, De Luca E, Braggion S, et al. KRIT1 loss of function causes a ROS-dependent upregulation of c-Jun. Free Radic Biol Med. 2014;68:134-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moglia A, Goitre L, Gianoglio S, et al. Evaluation of the bioactive properties of avenanthramide analogs produced in recombinant yeast. Biofactors. 2015;41(1):15-27. [DOI] [PubMed] [Google Scholar]
- 49. Moglianetti M, De Luca E, Pedone D, et al. Platinum nanozymes recover cellular ROS homeostasis in an oxidative stress-mediated disease model. Nanoscale. 2016;8(6):3739-3752. [DOI] [PubMed] [Google Scholar]
- 50. Girard R, Khanna O, Shenkar R, et al. Peripheral plasma vitamin D and non-HDL cholesterol reflect the severity of cerebral cavernous malformation disease. Biomarkers Med. 2016;10(3):255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leaute-Labreze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372(8):735-746. [DOI] [PubMed] [Google Scholar]
- 52. Zabramski JM, Kalani MY, Filippidis AS, Spetzler RF. Propranolol treatment of cavernous malformations with symptomatic hemorrhage. World Neurosurgery. 2016;88:631-639. [DOI] [PubMed] [Google Scholar]
- 53. DiStefano PV, Kuebel JM, Sarelius IH, Glading AJ. KRIT1 protein depletion modifies endothelial cell behavior via increased vascular endothelial growth factor (VEGF) signaling. J Biol Chem. 2014;289(47):33054-33065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bravi L, Malinverno M, Pisati F, et al. Endothelial cells lining sporadic cerebral cavernous malformation cavernomas undergo endothelial-to-mesenchymal transition. Stroke. 2016;47(3):886-890. [DOI] [PubMed] [Google Scholar]
- 55. Bravi L, Rudini N, Cuttano R, et al. Sulindac metabolites decrease cerebrovascular malformations in CCM3 -knockout mice. Proc Natl Acad Sci USA. 2015;112(27):8421-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maddaluno L, Rudini N, Cuttano R, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498(7455):492-496. [DOI] [PubMed] [Google Scholar]
- 57. Marchi S, Corricelli M, Trapani E, et al. Defective autophagy is a key feature of cerebral cavernous malformations. EMBO Mol Med. 2015;7(11):1403-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. You C, Sandalcioglu IE, Dammann P, Felbor U, Sure U, Zhu Y. Loss of CCM3 impairs DLL4-Notch signalling: implication in endothelial angiogenesis and in inherited cerebral cavernous malformations. J Cell Mol Med 2013;17(3):407-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wustehube J, Bartol A, Liebler SS, et al. Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Proc Natl Acad Sci USA. 2010;107(28):12640-12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou Z, Tang AT, Wong WY, et al. Cerebral cavernous malformations arise from endothelial gain of MEKK3–KLF2/4 signalling. Nature. 2016;532(7597):122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.